Abstract
Colorectal cancer (CRC) is highly prevalent worldwide, and despite notable progress in treatment still leads to significant morbidity and mortality. The use of nanoparticles as a drug delivery system has become one of the most promising strategies for cancer therapy. Targeted nanoparticles could take advantage of differentially expressed molecules on the surface of tumor cells, providing effective release of cytotoxic drugs. Several efforts have recently reported the use of diverse molecules as ligands on the surface of nanoparticles to interact with the tumor cells, enabling the effective delivery of antitumor agents. Here, we present recent advances in targeted nanoparticles against CRC and discuss the promising use of ligands and cellular targets in potential strategies for the treatment of CRCs.
Keywords: : cancer therapy, colorectal cancer (CRC), controlled release, drug delivery, targeted nanoparticles
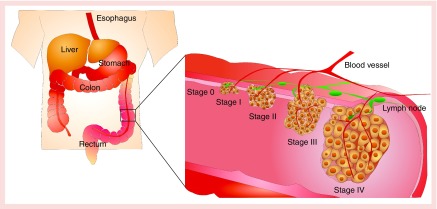
Colorectal cancer (CRC) is the fourth most widely diagnosed cancer worldwide and manifests as a malignant neoplasm in the mucosa of the colon or the rectum [1,2]. Based on the progression of cancer cells, the American Joint Committee on Cancer (AJCC) has classified CRC into five stages (Figure 1). Stage 0 is considered 100% cured after a surgical resection. The standard treatment for stages I–IIC is also surgical resection, with 5-year survival in the range of 37–74%. Patients diagnosed in advanced stages (stages IIIA–IV) receive adjuvant chemotherapy following surgical resection, but their survival rate decreases to 6% due to the high risk of metastasis and the recurrence (Table 1) [3,4].
Figure 1. . Stages of colorectal cancer according to the American Joint Committee on Cancer.
Stage 0; tumor confined to mucosa (carcinoma in situ). Stage I: tumor invades submucosa and muscularis propria. Stage II: tumor invades through the muscularis propria into pericolorectal tissues (IIA), then penetrates to the surface of the visceral peritoneum (IIB), then directly invades or is adherent to other organs or structures (IIC). Stage III: tumor invades muscularis propria with metastases in 1–3 regional lymph nodes or nearby tissue, or invades submucosa with metastases in 4–6 regional lymph nodes (IIIA). Then tumor penetrates to the surface of the visceral peritoneum with metastases in 1–3 regional lymph nodes or nearby tissue, or invades through the muscularis propria into pericolorectal tissues with metastases in 4–6 regional lymph nodes, or invades muscularis propria with metastases in 7 or more regional lymph nodes (IIIB). Then tumor penetrates to the surface of the visceral peritoneum with metastases in 4–6 regional lymph nodes, or invades through the muscularis propria into pericolorectal tissues with metastases in 7 or more regional lymph nodes, or directly invades or is adherent to other organs or structures with metastases in one or more regional lymph nodes (IIIC). Stage IV: metastasis confined to one organ or site (e.g., liver, lung, ovary, nonregional node).
Table 1. . Stages, treatment options and survival rate for colorectal cancer.
| Stage (TNM criteria) | Standard treatment option | 5-year observed survival rate |
|---|---|---|
| Stage 0 |
Surgical resection |
Considered curative |
| Stage I |
Surgical resection |
74% |
| Stage IIA |
Surgical resection |
67% |
| Stage IIB |
Surgical resection |
59% |
| Stage IIC |
Surgical resection |
37% |
| Stage IIIA |
Surgical resection Adjuvant chemotherapy |
73%† |
| Stage IIIB |
Surgical resection Adjuvant chemotherapy |
46%† |
| Stage IIIC |
Surgical resection Adjuvant chemotherapy |
28% |
| Stage IV – liver metastasis |
Surgical resection Local ablation Neoadjuvant chemotherapy Intra-arterial chemotherapy Adjuvant chemotherapy |
6% |
| Stage IV and recurrent CRC cancer | Surgical resection Adjuvant chemotherapy |
6% |
A high cellular heterogeneity characterizes CRC due to several genetic and biological alterations, which are responsible for the high variability between of tumors [5]. Despite the broad repertory of biomarkers of CRC described later as targets for advanced nanoparticles; recently, a tremendous effort by the Cancer Genome Atlas Network has been made for characterizing the molecular genetics of CRC in 224 samples [6]. By genome sequence analysis, the samples were classified into nonhypermutated and hypermutated types of cancer. Among the nonhypermutated tumors, the most frequently mutated genes were APC (81%), TP53 (60%), KRAS (31%), TTN (31%), PIK3CA (18%), FBXW7 (11%) and SMAD4 (10%), etc.; whereas the most commonly hypermutated tumors were CVR2A (63%), APC (51%), TGFBR2 (51%), MSH3 (40%) and MSH6 (40%) among others [6]. Nonhypermutated tumors (∼84%) exhibited a high frequency of DNA somatic copy number alterations with a microsatellite stable, and hypermutated tumors (∼16%) showed either microsatellite instability due to defective mismatch repair (∼13%) or DNA polymerase epsilon proofreading mutations (∼3%) [7]. The integrative analysis of the molecular genetics of CRC provides many insights into the biology of CRC and identifies potential therapeutic targets.
Adjuvant chemotherapy, usually used in stages III–IV, includes a variety of chemotherapeutics intended to slow tumor growth and improve life expectancy. Despite the highly efficient chemotherapeutic agents used to treat CRC, their low specificity often produces a range of dose-limiting side effects including hair loss, nausea and vomiting [8]. In an effort to minimize side effects, current therapeutic protocols involve the coadministration of different chemotherapeutic agents in a series of cycles. The number of doses, frequency and duration of cycles depend on the needs and general state of each patient [9]. In addition, fluctuations in plasma levels of drugs over chemotherapeutic cycles can encourage the development of drug resistance in tumor cells [10]. When added to the high financial cost of treatment, such interventions can significantly decrease quality of life for CRC patients [11,12].
Over the last few years, several types of drug-loaded nanoparticles in the size range of 20–400 nm (i.e., liposomes, dendrimers, polymeric nanoparticles and micelles) have made a strong impact on drug delivery for chemotherapy [13–16]. In fact, such systems are among the most promising developments in nanomedicine, which has grown exponentially: from simple nanoparticles loaded with drugs to multifunctional nanoparticles targeted to specific cancer cells through binding to unique cell-surface proteins [17–19]. Targeted nanoparticles exploit antigens differentially expressed on the surface of cancer cells, such as integrin [17,20] and folic acid receptors [21,22] and a number of such nanoparticles are currently undergoing clinical development [23]. There have been major advances in the use of nanoparticles as therapeutic platforms for the treatment of prostate [24,25], ovarian [26], breast [27,28] and lung cancers [29–31]. Nevertheless, despite the high morbidity and mortality associated with CRC, the clinical development of nanoparticles for treatment remains limited. In this review, we describe the state of the art in nanoparticles for CRC and discuss the tools available for future applications of such therapeutic strategies.
Current adjuvant chemotherapy against colorectal cancer
As stated above, much early-stage CRC is potentially curable by surgical resection [32]. Although adjuvant chemotherapy clearly benefits patients with stages III and IV disease, its use in stage II is not usually indicated because of the curative effects of resection [33,34]. For many years, the only cytotoxic drug used in adjuvant chemotherapy for treatment of CRC was fluoropyrimidine 5-fluorouracil (5-FU), an analog of thymine that inhibits DNA replication [35]. Due to the variable gastrointestinal absorption of 5-FU, its preferred route of administration is intravenous (iv.) [36]. In addition, the discomfort associated with iv. administration of 5-FU prompted the development of more effective and less expensive oral formulations, which can be classified into three groups: 5-FU prodrugs such as Tegafur and Capecitabine, 5-FU prodrugs combined with dihydropyrimidine dehydrogenase inhibitor and 5-FU combined with a dihydropyrimidine dehydrogenase inhibitor [37].
Since the early 2000s, other drugs have also come into use. For example, irinotecan inhibits the enzyme topoisomerase I, hindering the uncoiling of DNA during replication [38]; and oxaliplatin forms cross-linking DNA, preventing transcription and replication [39]. However, in addition to cancer cells, cytotoxic drugs also kill healthy cells that grow and divide quickly such as white blood cells, red blood cells and platelets; for this reason some of these cytotoxic drugs are administered with leucovorin, a vitamin that strengthens the production of blood cells and improves treatment efficiency [40]. The drug combinations currently used in adjuvant chemotherapy for CRC are presented in Table 2. Another family of therapeutic agents for treatment of CRC are monoclonal antibodies, which can be directed against molecules on the surface or in the environment of tumor cells [41]. Two monoclonal antibodies are licensed for use in humans: bevacizumab binds to VEGF-A, which inhibits the formation of blood vessels, reducing tumor vascularization and inhibiting tumor growth [42,43]; and cetuximab binds to the extracellular domain of the EGFR to block ligand-induced receptor signaling [44].
Table 2. . Adjuvant chemotherapy for colorectal cancer.
|
A. Drug combinations in adjuvant chemotherapy for stage III colorectal cancer | ||
|---|---|---|
| Regimen | Scheme | Ref. |
| FOLFOX4 regimen |
Oxaliplatin (85 mg/m2) administered iv. as a 2-h infusion on day 1; leucovorin (200 mg/m2) administered iv. as a 2-h infusion on day 1 and day 2; followed by a loading dose of 5-FU (400 mg/m2) bolus administered iv., then 5-FU (600 mg/m2) administered iv. as a 22-h continuous infusion on day 1 and day 2, repeat every 2 weeks |
[45,46] |
| FU/levamisole regimen |
5-FU (450 mg/m2) bolus administered iv. daily for 5 days, then weekly 28 days later plus levamisole (50 mg) administered orally three-times/days for 3 days, repeat every 2 weeks |
[47] |
| Mayo Clinic or North Central Cancer Treatment Group (NCCTG) regimen |
5-FU (450 mg/m2)-leucovorin (20 mg/m2) bolus administered iv. daily for 5 days, repeat every 4 weeks |
[48] |
| Roswell Park Memorial Institute (RPMI) or National Surgical Adjuvant Breast and Bowel Project (NSABP) regimen | 5-FU (500 mg/m2)-leucovorin (500 mg/m2) bolus administered iv. weekly for 6 weeks, repeat every 8 weeks | [49] |
|
B. Drugs combinations in adjuvant chemotherapy for stage IV colorectal cancer | ||
|---|---|---|
| Regimen | Scheme | Ref. |
| FOLFOX4 regimen |
Oxaliplatin (85 mg/m2) administered iv. as a 2-h infusion on day 1; leucovorin (200 mg/m2) administered iv. as a 2-h infusion on days 1 and 2; followed by a loading dose of 5-FU (400 mg/m2) bolus administered iv., then 5-FU (600 mg/m2) administered iv. as a 22-h continuous infusion on days 1 and 2, repeat every 2 weeks |
[45,46] |
| FOLFOX6 regimen |
Oxaliplatin (85–100 mg/m2) administered iv. as a 2-h infusion on day 1; leucovorin (400 mg/m2) administered iv. as a 2-h infusion on day 1; followed by a loading dose of 5-FU (400 mg/m2) bolus administered iv. on day 1, then 5-FU (2,400–3,000 mg/m2) administered iv. as a 46-h continuous infusion, repeat every 2 weeks |
[50] |
| FOLFIRI regimen |
Irinotecan (180 mg/m2) administered iv. as a 2-h infusion on day 1; leucovorin (400 mg/m2) administered iv. as a 2-h infusion on day 1; followed by a loading dose of 5-FU (400 mg/m2) bolus administered iv. on day 1, then 5-FU (2,400–3,000 mg/m2) administered iv. as a 46-h continuous infusion, repeat every 2 weeks |
[51] |
| FUFOX regimen |
Oxaliplatin (50 mg/m2) plus leucovorin (500 mg/m2) plus 5-FU (2000 mg/m2) administered iv. as a 22-h continuous infusion on days 1, 8, 22 and 29, repeat every 36 days |
[52] |
| FUOX regimen |
5-FU (2,250 mg/m2) administered iv. as a 48-h continuous infusion on days 1, 8, 15, 22, 29 and 36 plus oxaliplatin (85 mg/m2) on days 1, 15 and 29, repeat every 6 weeks |
[53] |
| XELOX regimen |
Oral capecitabine (1,000 mg/m2) two-times/days for 14 days plus oxaliplatin (130 mg/m2) on day 1, repeat every 3 weeks |
[54] |
| IFL (or Saltz) regimen |
Irinotecan (125 mg/m2), 5-FU (500 mg/m2) bolus administered iv. and leucovorin (20 mg/m2) bolus administered iv. weekly for 4 weeks, repeat every 6 weeks |
[55] |
| Douillard regimen |
Irinotecan (180 mg/m2) administered iv. as a 2-h infusion on day 1; leucovorin (200 mg/m2) administered iv. as a 2-h infusion on days 1 and 2; followed by a loading dose of 5-FU (400 mg/m2) bolus administered iv., then 5-FU (600 mg/m2) administered iv. as a 22-h continuous infusion on days 1 and 2, repeat every 2 weeks |
[56] |
| AIO or German AIO regimen | Irinotecan (100 mg/m2) administered iv. as a 2-h infusion on day 1; leucovorin (500 mg/m2) administered iv. as a 2-h infusion on day 1; followed by 5-FU (2000 mg/m2) administered iv. as a 24-h continuous infusion weekly, repeat every 13 weeks | [57] |
5-FU: 5-fluorouracil; AIO: Arbeitsgemeinschaft Internische Onkologie.
Since its approval, the combination of monoclonal antibodies with cytotoxic drugs has become first-line treatment for CRC, extending both progression-free survival and overall survival [42,58–61]. However, despite the improvements in treatments involving adjuvant chemotherapy and biological agents, drug resistance remains a major challenge and general side effects (e.g., fatigue, hair loss, nausea and vomiting, diarrhea or constipation, anemia, immunosuppression and bleeding) have prompted researchers to explore advanced strategies based on nanotechnology, either to improve the pharmacological properties of classic chemotherapeutics or to specifically target tumor tissue and reduce side effects.
Nanoparticles in colorectal cancer therapy
The development of therapeutic strategies for cancer treatment based on nanoparticles has generated substantial advances in pharmacology, decreasing the side effects of cytotoxic drugs and improving their efficacy, solubility, pharmacokinetics and biodistribution. Over the last 50 years, several nanoparticles of diverse shapes, sizes and chemical natures have shown high efficacy in encapsulating different types of anticancer cargo, including siRNA [62], antibiotics [63] and chemotherapeutics [26]. These first-generation anticancer nanoparticles reach the tumor tissue passively, taking advantage of the enhanced permeation and retention effect offered by the vascular and lymphatic drainage of tumors; this allows the extravasation and accumulation of nanoparticles within cancer cells and improves therapeutic efficacy [64]. Liposome-based platforms are the most well established and were the first nanocarriers approved by US FDA for use in humans [65]. They are vesicles composed of a phospholipid bilayer with an internal and external aqueous phase that supports the encapsulation of both hydrophilic and hydrophobic drugs.
Liposome-based nanoproducts currently under clinical study for the treatment of CRC include CPX-1, LE-SN38 and Thermodox; CPX-1 (Irinotecan HCl: Floxuridine) has completed Phase II clinical trials [66]. One study focused on patients with advanced CRC who were already receiving chemotherapy including oxaliplatin or irinotecan [67]. Other researchers evaluated the liposome formulation LE-SN38 in HT-29 tumor-bearing mice; tumor growth was inhibited by 51, 79 and 90% after 10 days of treatment using doses of 10, 20 and 40 mg/kg (respectively) compared with the drug-free liposome group [68]. However, assessment of the effects of LE-SN38 in patients with metastatic CRC after progression on oxaliplatin (Phase II of clinical trial) showed that the drug did not slow cancer progression in patients treated with LE-SN38 35 mg/m2 every 21 days for a minimum of 2 cycles [69]. Thermodox, another liposomal strategy in clinical trials, involves the use of thermally sensitive liposomal doxorubicin as an adjuvant therapy with radiofrequency thermal ablation in the treatment of recurrent or refractory colorectal liver metastases [70]. Although a study comparing Thermodox to radiofrequency thermal ablation monotherapy has been terminated, the results still are not available [71]. At present, several agents under preclinical development have shown promising in vitro results with potential applications for CRC, including oxaliplatin-loaded long-circulating liposomes (PEG-liposomal L-oHP) [72], liposomal curcumin [73] and doxorubicin-encapsulated liposome [74].
Since the 1980s, studies by Langer and coworkers have shown that biodegradable and noncytotoxic polymers such as poly(d,l-lactide-co-glycolide) and their derivates [75], polycaprolactone [76,77] and chitosan [78] offer a versatile platform for the development of nanoparticles and drug delivery. Polymeric nanoparticles are spherical, with a hydrophobic core and a hydrophilic shell formed by the self-assembly of biocompatible amphiphilic block copolymers through aqueous or microencapsulation methods. They are considerably more stable than liposomes, permit the efficient encapsulation of drugs of different chemical natures and allow sustained release in response to changes in temperature or pH [23,79–80].
At present, several formulations for cancer therapy in clinical trials have already shown outstanding pharmacokinetic performance. For instance, NK105 (PEG-P[Asp]-paclitaxel) showed an area under the curve significantly higher than paclitaxel alone between 0 and 48 h after iv. administration (191,000 ± 32,100 vs 1500 ± 108 ng·h/ml, respectively) [81]. Other formulations in clinical trials such as NK911 (PEG-P[Asp]-doxorubicin) have shown high accumulation in solid tumors in mice [16], and SP1049C (Pluronic L61, F127–doxorubicin) exhibited notable single-agent activity in patients with adenocarcinoma of the esophagus and gastroesophageal junction with high efficacy and fewer side effects compared with drug alone [82]. Assays in mice model of metastasis have shown that an in vivo gene delivery formulation comprising a core of high-molecular-weight linear polyethylenimine complexed with DNA and surrounded by a shell of polyethyleneglycol-modified (PEGylated) low-molecular-weight linear polyethylenimine are selectively transfected in neoplastic cells. However, only a small fraction of those cells expressed the transgene [83].
Polymeric nanoparticles continue to be a popular subject of study in cancer therapy because they are a strong platform with which to encapsulate both hydrophilic and hydrophobic drugs [84–86]. However, most drugs are released into the extracellular matrix; their effectiveness depends on diffusion through the tissue, and low in vivo specificity also limits their application [87]. Thus, recent site-specific targeting of nanoparticles is a promising advancement in cancer treatment research. One successful approach is the BIND-014 technology, which consists of docetaxel-loaded polymeric nanoparticles capable of recognizing prostate cancer through targeting against PSMA, a tumor antigen on prostate cancer cells and the vasculature of most nonprostate solid tumors. BIND-014 is currently in Phase II clinical trials for non-small-cell lung cancer and metastatic castration-resistant prostate cancer, having already demonstrated significant antitumor activity at a lower dose than conventional docetaxel in subjects with advanced or metastatic non-small-cell lung cancer [88].
Targeted nanoparticles for colorectal cancer therapy
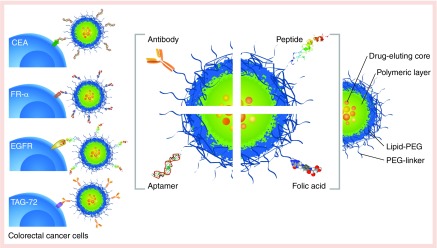
The conjugation of ligands such as antibodies, fragments of antibodies, peptides, aptamers and other small molecules on the surface of nanoparticles for the purpose of cell recognition has yielded a new generation of nanoparticles for cancer therapy with enhanced in vivo specificity (Figure 2). The incorporation of these ligands is usually achieved by chemical modification during nanoparticle synthesis or through chemical bonding between ligands and polymers before synthesis [89,90].
Figure 2. . Targeted nanoparticle strategy for colorectal cancer.
The most common CRC biomarkers overexpressed in the cellular membrane and the typical molecules/ligands used on the surface of nanoparticles in targeting strategies: FR-α, EGFR, TAG-72.
CEA: Carcinoembryonic antigen; CRC: Colorectal cancer; EGFR: EGF receptor; FR-α: Folate receptor-α; TAG-72: Tumor-associated glycoprotein.
Targeted nanoparticles are those that contain ligands on their surface and are capable of specifically recognizing cells. In applications against cancer, the promise of targeted nanoparticles is based on the fact that tumors express and/or overexpress some biomarkers, which can be used as targets for drug delivery. For example, Graf et al. described cisplatin prodrug-loaded poly(D,L-lactic-co-glycolic acid)-block-polyethylene glycol nanoparticles targeted with a cyclic pentapeptide c(RGDfK) that bind to the integrin receptor, which is highly upregulated in tumor-associated endothelial cells during angiogenesis [17].
One recent study used immunohistochemistry to analyze the expression of four biomarkers in mucosal and CRC tissues from 280 patients [91]. Carcinoembryonic antigen (CEA) was the marker most consistently overexpressed, that is, expressed in CRC 98.8% more than in normal tissue, followed by tumor-associated glycoprotein-72 at 79%, folate receptor-α at 37.1% and EGFR at 32.8%. This work supports the application of CEA as a potential cellular target for future development of targeted nanoparticles in the treatment of CRC [91] (see Figure 2). Markers have also been identified: IGF-1R [92], apolipoprotein A1 [93], the transmembrane receptor tyrosine kinase EphA4 [94], the receptor for hyaluronic acid-mediated motility [95] and α2 integrin [96].
Though the application of monoclonal antibodies (mAb) to CRC-targeting nanoparticles is still an emerging field, there are already many mAbs in preclinical and clinical development [59,97–100]. For example, the humanized A33 mAb (huA33 mAb) has shown great promise in clinical trials as an immunotherapeutic biological agent and also as a targeting ligand for CRC cells of polymer capsules formed by the layer-by-layer method [101,102]. As shown in Table 3, using targeted nanoparticles as a drug delivery system based on mAb is now one of the main approaches for CRC therapy under preclinical development. However, the major limitation of mAb is their large size and complexity, posing a challenge to their conjugation on the surface of nanoparticles [103,104].
Table 3. . Targeted nanoparticles for colorectal cancer under preclinical development.
| Formulation | Ligand | Target | Cell population | Ref. |
|---|---|---|---|---|
| Nanosized maghemite particle |
Antibody |
CEA |
High CEA-expressing cell line (LS174T) and a low CEA-expressing cell line (HCT116) |
[105] |
| Dextran- and PEG-coated superparamagnetic iron oxide nanoparticles (abf-SPION) |
scFv |
CEA |
LS174T, a CEA-expressing (CEA+ve) cancer cell line and A375M, a CEA-negative (CEA-ve) cancer cell line |
[106] |
| Dye-doped silica nanoparticles conjugated with polyamidoamine dendrimers |
Humanized anti-CEA monocolonal antibody A5B7 |
CEA |
LS174T, LoVo and HCT116 cells and murine xenografts model |
[107] |
| Conatumumab (AMG 655)-coated nanoparticles |
Antibody |
DR5 |
HCT116 cancer cells |
[108] |
| Photosensitizer meso-Tetra(N-methyl-4-pyridyl) porphine tetra tosylate chitosan/alginate nanoparticles |
Antibody |
DR5 |
HCT116 cancer cells |
[109] |
| Polymer capsules formed by the LbL technique |
Humanized A33 monoclonal antibody (huA33 mAb) |
A33 antigen |
LIM1215 cells (antigen-expressing) SW480 (nonantigen-expressing) |
[102] |
| Gold and iron oxide HNPs |
scFv |
A33 antigen |
Colorectal cancer cell lines (SW1222 and HT 29 cells) |
[110] |
| Poly(lactide- coglycolide) NP loaded with camptothecin |
Antibody |
Fas receptor (CD95/Apo-1) |
HCT116 cells |
[111] |
| Chitosan nanoparticles loaded with 5-ALA |
Folic acid |
FR |
HT29 and Caco-2 colorectal cancer cell lines overexpressing folate receptor |
[112] |
| FA-CS conjugates nanoparticles |
Folic acid |
FR |
HT-29 cancer cells |
[113] |
| HPMA-copolymer-doxorubicin conjugates |
Peptide GE11 |
EGFR |
HT29, SW480 and A431 cell lines |
[114] |
| T22-empowered protein-only nanoparticles |
18-mer peptide T22 (T22-GFP-H6) |
CXCR4 |
HeLa cells |
[115] |
| Chitosan nanoparticles encapsulating oxaliplatin (L-OHP) |
HA |
HA receptor |
Colon cancer (HT-29) in C57BL mice |
[116] |
| MSN |
Coated with poly-(L-lysine) and HA |
CD44 receptor |
HCT-116 cancer cells |
[117] |
| rHDL nanoparticles loaded with siRNA | Apo A-I | SR-B1 | Model colorectal cancer metastasis in mice (HCT116 cells) | [118] |
5-ALA: 5-aminolaevulinic acid; Apo A-1: Apolipoprotein A-I; CEA: Carcinoembryonic antigen; CXCR4: CXC chemokine receptor 4; DR5: Death receptor 5; FA-CS: Folate-chitosan; FR: Folate receptor; HA: Hyaluronic acid; HNP: Hybrid nanoparticle; LbL: layer-by-layer; MSN: Mesoporous silica nanoparticle; NP: Nanoparticle; rHDL: Reconstituted HDL; ScFv: Single-chain Fv antibody fragment; SR-B1: Scavenger receptor type B1.
Peptides also represent a promising targeting alternative, given their small size and ease of attachment to nanoparticles. However, the use of peptides for CRC, for example, the tumor necrosis factor-related apoptosis-inducing ligand [119], and the peptide RPMrel (CPIEDRPMC) [120] as a targeting ligand, has not yet been well explored. One study has shown high cellular uptake of HPMA-copolymer-DOX conjugate with the oligopeptide GE11 in CRC cells that overexpress EGFR, achieving selective release of doxorubicin [114].
The differential expression of FRα has been associated with several types of cancers including CRC [121]. Cell lines such as Caco-2 and HT29, which overexpress the folate receptor, selectively internalize nanoparticles conjugated with folate on their surface (i.e., functionalized) [113]. Sharma et al. described a multifunctional nanosystem based on methotrexate-loaded guar gum nanoparticles functionalized with folic acid (MTX-FA-GGNP), which released methotrexate at colonic pH (6.8) and displayed preferential in vivo uptake in colon tissue [122].
Imaging & detection in colorectal cancer
Nanoparticles may also be used to facilitate early diagnosis and monitor the efficacy of therapy. The design of nanoparticles could incorporate different contrast agents (e.g., radioactive, superparamagnetic or fluorescent), targeting groups and biocompatible coatings [123]. Since small molecular-weight gadolinium and metal chelate-based contrast agents have disadvantages such as low tissue specificity, rapid clearance and nonspecific extracellular distribution, nanotechnology may be used to modify such contrast agents to improve the sensitivity and specificity of CRC diagnostics [124]. Recently, He et al. described lectin core/shell nanoparticles formulated with iron oxide magnetite and gold (lectin−Fe2O3#Au NP), which allowed dual-modality imaging, that is, T2-weighted MR and x-ray CT in nude mice bearing colorectal tumor (SW620) [125]. Another strategy that showed outstanding in vivo effectiveness by MRI, low cytotoxicity and extraordinary fluorescence stability was based on nanoparticles formulated with a core of superparamagnetic iron oxide nanocrystals, conjugated with quantum dots and targeted with a monoclonal antibody binding to CEA-related cell-adhesion molecules [126].
Near-infrared fluorescent (NIRF) endoscopic detection is a novel approach that may increase the sensitivity and specificity of surveillance colonoscopy of patients with CRC. Studies in a mouse model of colitis-associated cancer monitored by NIRF endoscopy showed high efficiency in the detection of dysplastic foci within chronically inflamed colons [127]. Yang et al. reported the application of folic acid-conjugated chitosan nanoparticles loaded with 5-aminolaevulinic acid in NIRF endoscopy for CRC cells. The 5-aminolaevulinic acid is a precursor in heme group synthesis and is rapidly converted to the fluorophore protoporphyrin IX in normal cells. In cancer cells protoporphyrin IX accumulates intracellularly, because the degradation metabolism is slower than in normal cells, allowing its use in NIRF endoscopy and specifically on CRC cells, which overexpress the folate receptor [112].
Cancer stem cells in colorectal cancer
The biological basis of the recurrence of CRC after surgical treatment, chemotherapy and/or radiation still is not understood. Some authors suggest that factors like the stage of development of cancer, the age of the patient and the treatment received are a critical factor for the relapsed of patients; however, still the literature is controversial.
The cancer cells are recognized for a high rate of proliferation, and cellular division that promotes to boosting the number of mutations. The effect of cytotoxic drugs over the cancer cells generates a selective pressure that stresses the progeny and induces novel drug resistant mutants, which are responsible for relapses.
Interestingly, the relapse of those types of cancers that involve the drug resistant phenomenon is experienced shortly after the treatment; however, some tumors manifest relapse long time after the surgery or pharmacological treatment (months or years, and even when the treatment has been stopped). In those cases of cancer, the drug-resistant effect does not explain the relapse.
Several hypotheses are under investigation, and the common point of view is centered in the fact of the tumors are composed by clonal subpopulations of cancer cells, which differs in its growth rate, immunological characteristics, the ability to metastasize, the expression of proteins and sensitivity to treatments [128]. The authors also suppose a hierarchical interaction between the subpopulations of clones, which promotes the tumor progression. In this sense, it has been demonstrated the existence of cancer stem cells (CSCs) in various types of cancers including leukemia and CRC [129,130]. In this context, if the chemotherapeutic drugs affect the viability of cells under a highly rate of division, the CSCs, that are characterized by a slow proliferation rate, could promote the recurrence after a long time of the treatment.
Recent investigations have reported that CSCs in CRC could be characterized according to cellular markers such as CD44, CD133, CD166 and EpCAM [131–133]. At the signaling level, it has been proposed that both, WNT/β-catenin and NOTCH/HES1 pathways, are involved in the regulation of CSCs, and the self-renewal and maintenance of CSCs in CRC, respectively [134–136]. At present, several experimental drugs targeting to CSCs in combination with conventional chemotherapeutic drugs are in clinical trials for CRC and others type of cancers. The BBI608 targeted to STAT-3, and BBI503 targeted to Nanog and multiple kinases, have been developed by Boston Biomedical and currently are in Phase III on a clinical trial.
In summary, it seems to be that the elimination of all CSCs is critical to eradicating cancer and that failure to do so might be responsible for the occurrence of relapses and/or metastases frequently observed in the clinical management of CRC patients. Consequently, an adequate isolation and a profound identification of CSCs in CRC is essential for a better understanding of their role in the tumorigenesis process and the development of CSC-specific therapies.
Conclusion
Traditional cytotoxic drugs for CRC cause several side effects in part because of current therapeutic protocols, which are based on a series of cycles of administration. Recent trends in nanomedicine include the use of combined therapy of cytotoxic drugs loaded in targeted nanocarriers, which allows sustained release and site-specific delivery, reducing or even eliminating cycles, depending on the efficiency of the strategy. The combined therapy can also include the use of DNA [137] or RNA [138] to assemble the nanoparticles that leading to the expression or knockdown of genes enhancing effectiveness of the cytotoxic drugs.
The deeper understanding gained in recent years of manipulating the physicochemical properties of nanoparticles for in vivo application bodes well for their use in treatment of CRC. Relevant parameters include optimal size to avoid the immune system response and clearance by glomerular filtration in the kidneys (range: 10–100 nm) [139,140] and the best shape to encourage longer circulation time and faster uptake by cancer cells (spherical) [141]. Moreover, other relevant parameters include the optimal surface charge to promote cellular binding and prevent complement activation (range: 0 to -10 mV) [142] and sufficient density of targeting ligands on the surface of nanoparticles to optimize tissue-specific targeting (range: 0.5–5%) [88].
On the other hand, optimizing the targeting technology for CRC therapy still faces several challenges, including the correlation of biomarkers with the early stages in the development of CRC and the identification of novel highly specific molecular targets. Thus far, peptides, aptides, aptamers and small molecules are the most attractive tools for targeting, given their small size, high affinity and ease of conjugation on the surface of nanoparticles; they hold great promise for future drug development and medical translation.
Future perspective
The future perspective of targeted nanoparticles is brilliant because several of current promising formulations under preclinical and clinical developments for CRC soon and after overtaking the high standards of safety and efficacy for patients required by the FDA could be in the market. A future challenge is to implement systems or protocols to determine the molecular expression profile of tumors from patients with CRC and classify them according to the genetic profile, stage of development of tumor and putative targeting molecules. All above will support the rational administration of precise-targeted nanoformulations containing the most effective drug combination.
Executive summary.
Colorectal cancer & adjuvant chemotherapy
Colorectal cancer (CRC) is the fourth most widely diagnosed cancer worldwide and despite notable progress in treatment still leads to significant morbidity and mortality.
Based on the progression of cancer the standard treatment involves surgical resection, and adjuvant chemotherapy; however, since stages IIC–IV the survival rate over 5 years is lower than 50% due to the high risk of metastasis and the recurrence.
Despite the highly efficient chemotherapeutic agents used to treat CRC, drug resistance remains a major challenge and general side effects.
Targeted nanoparticles for colorectal cancer
The development of therapeutic strategies based on nanoparticles as a drug delivery system has become one of the most brilliant strategies for cancer therapy.
The conjugation of ligands on the surface of nanoparticles for the purpose of cell recognition has yielded a new generation of nanoparticles (targeted nanoparticles).
Targeted nanoparticles could take advantage of differentially expressed molecules on the surface of tumor cells, providing an adequate release of cytotoxic drugs.
Since contrast agents have disadvantages such as low tissue specificity, rapid clearance and nonspecific extracellular distribution, targeted nanoparticles may be used to modify such contrast agents to improve the sensitivity and specificity of CRC diagnostics.
Future perspective
The near future of precise-targeted nanoformulations containing the most effective drug combination for CRC is just around the corner due to the promising advances in this field of research.
Footnotes
Financial & competing interests disclosure
OC Farokhzad acknowledges NIH support from grants HL127464, CA151884 and EB015419; and by the David Koch-Prostate Cancer Foundation Award in Nanotherapeutics. C Vilos acknowledges support from FONDECYT regular grant no. 1161438, UNAB Regular Grant DI-695-15/R, MECESUP PMI-UAB1301, and the Basal Program for Centers of Excellence, grant FB0807 CEDENNA, CONICYT. The authors declare the following competing financial interest(s): OC Farokhzad has financial interests in BIND Therapeutics, Selecta Biosciences, Tarveda Therapeutics and Placon Therapeutics, which are developing nanoparticle technologies for medical applications. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 3.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. Springer; NY, USA: 2010. Colon and rectum; pp. 143–164. [Google Scholar]
- 4.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant Tumours. International Union Against Cancer, Wiley-Blackwell Chichester; Sussex, UK; NJ, USA: 2010. Colon and rectum; p. 309. [Google Scholar]
- 5.Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu. Rev. Med. 2015;66:83–95. doi: 10.1146/annurev-med-051513-102539. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muller MF, Ibrahim AE, Arends MJ. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016 doi: 10.1007/s00428-016-1956-3. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chabner BA, Roberts TG., Jr Timeline: chemotherapy and the war on cancer. Nat. Rev. Cancer. 2005;5(1):65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 9.Field KM, Kosmider S, Jefford M, Jennens R, Green M, Gibbs P. Chemotherapy treatments for metastatic colorectal cancer: is evidence-based medicine in practice? J. Oncol. Pract. 2008;4(6):271–276. doi: 10.1200/JOP.0852002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill BT, Moran E, Etievant C, et al. Low-dose twice-daily fractionated X-irradiation of ovarian tumor cells in vitro generates drug-resistant cells overexpressing two multidrug resistance-associated proteins, P-glycoprotein and MRP1. Anticancer Drugs. 2000;11(3):193–200. doi: 10.1097/00001813-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Emmert M, Pohl-Dernick K, Wein A, et al. Palliative treatment of colorectal cancer in Germany: cost of care and quality of life. Eur. J. Health Econ. 2013;14(4):629–638. doi: 10.1007/s10198-012-0408-5. [DOI] [PubMed] [Google Scholar]
- 12.Quach C, Sanoff HK, Williams GR, Lyons JC, Reeve BB. Impact of colorectal cancer diagnosis and treatment on health-related quality of life among older Americans: a population-based, case-control study. Cancer. 2015;121(6):943–950. doi: 10.1002/cncr.29125. [DOI] [PubMed] [Google Scholar]
- 13.Deeken JF, Slack R, Weiss GJ, et al. A Phase I study of liposomal-encapsulated docetaxel (LE-DT) in patients with advanced solid tumor malignancies. Cancer Chemother. Pharmacol. 2013;71(3):627–633. doi: 10.1007/s00280-012-2048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mcgowan I, Gomez K, Bruder K, et al. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 gel (VivaGel) in sexually active young women (MTN-004) AIDS. 2011;25(8):1057–1064. doi: 10.1097/QAD.0b013e328346bd3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang K, Wen Y, Wang C. Clinical application of anticancer nanoparticles targeting metastasis foci of cervical lymph nodes in patients with oral carcinoma. Hua xi kou qiang yi xue za zhi. 2003;21(6):447–450. [PubMed] [Google Scholar]
- 16.Matsumura Y, Hamaguchi T, Ura T, et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer. 2004;91(10):1775–1781. doi: 10.1038/sj.bjc.6602204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graf N, Bielenberg DR, Kolishetti N, et al. alpha(V)beta(3) integrin-targeted PLGA-PEG nanoparticles for enhanced anti-tumor efficacy of a Pt(IV) prodrug. ACS Nano. 2012;6(5):4530–4539. doi: 10.1021/nn301148e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J. Natl. Cancer Inst. 2009;101(19):1308–1324. doi: 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muguruma N, Ito S, Bando T, et al. Labeled carcinoembryonic antigen antibodies excitable by infrared rays: a novel diagnostic method for micro cancers in the digestive tract. Intern. Med. 1999;38(7):537–542. doi: 10.2169/internalmedicine.38.537. [DOI] [PubMed] [Google Scholar]
- 20.Benezra M, Phillips E, Overholtzer M, et al. Ultrasmall integrin-targeted silica nanoparticles modulate signaling events and cellular processes in a concentration-dependent manner. Small. 2014;11(14):1721–1732. doi: 10.1002/smll.201402331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krais A, Wortmann L, Hermanns L, et al. Targeted uptake of folic acid-functionalized iron oxide nanoparticles by ovarian cancer cells in the presence but not in the absence of serum. Nanomedicine. 2014;10(7):1421–1431. doi: 10.1016/j.nano.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Landmark KJ, Dimaggio S, Ward J, et al. Synthesis, characterization, and in vitro testing of superparamagnetic iron oxide nanoparticles targeted using folic Acid-conjugated dendrimers. ACS Nano. 2008;2(4):773–783. doi: 10.1021/nn800034w. [DOI] [PubMed] [Google Scholar]
- 23.Kamaly N, Xiao Z, Valencia PM, Radovic-Moreno AF, Farokhzad OC. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 2012;41(7):2971–3010. doi: 10.1039/c2cs15344k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ki MH, Kim JE, Lee YN, et al. Chitosan-based hybrid nanocomplex for siRNA delivery and its application for cancer therapy. Pharm. Res. 2014;31(12):3323–3334. doi: 10.1007/s11095-014-1422-3. [DOI] [PubMed] [Google Scholar]
- 25.Hoang B, Ernsting MJ, Murakami M, Undzys E, Li SD. Docetaxel-carboxymethylcellulose nanoparticles display enhanced anti-tumor activity in murine models of castration-resistant prostate cancer. Int. J. Pharm. 2014;471(1):224–233. doi: 10.1016/j.ijpharm.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vilos C, Morales FA, Solar PA, et al. Paclitaxel-PHBV nanoparticles and their toxicity to endometrial and primary ovarian cancer cells. Biomaterials. 2013;34(16):4098–4108. doi: 10.1016/j.biomaterials.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 27.Cao B, Yang M, Zhu Y, Qu X, Mao C. Stem cells loaded with nanoparticles as a drug carrier for in vivo breast cancer therapy. Adv. Mater. 2014;26(27):4627–4631. doi: 10.1002/adma.201401550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Jin M, Shao S, et al. Small-sized polymeric micelles incorporating docetaxel suppress distant metastases in the clinically-relevant 4T1 mouse breast cancer model. BMC Cancer. 2014;14(1):329. doi: 10.1186/1471-2407-14-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Tang Z, Lin J, et al. Synergistic antitumor effects of doxorubicin-loaded carboxymethyl cellulose nanoparticle in combination with endostar for effective treatment of non-small-cell lung cancer. Adv. Healthc. Mater. 2014;3(11):1877–1888. doi: 10.1002/adhm.201400108. [DOI] [PubMed] [Google Scholar]
- 30.Emami J, Pourmashhadi A, Sadeghi H, Varshosaz J, Hamishehkar H. Formulation and optimization of celecoxib-loaded PLGA nanoparticles by the Taguchi design and their in vitro cytotoxicity for lung cancer therapy. Pharm. Dev. Technol. 2014:1–10. doi: 10.3109/10837450.2014.920360. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 31.Han Y, Zhang P, Chen Y, Sun J, Kong F. Co-delivery of plasmid DNA and doxorubicin by solid lipid nanoparticles for lung cancer therapy. Int. J. Mol. Med. 2014;34(1):191–196. doi: 10.3892/ijmm.2014.1770. [DOI] [PubMed] [Google Scholar]
- 32.Bokey EL, Moore JW, Chapuis PH, Newland RC. Morbidity and mortality following laparoscopic-assisted right hemicolectomy for cancer. Dis. Colon Rectum. 1996;39(Suppl. 10):S24–S28. doi: 10.1007/BF02053802. [DOI] [PubMed] [Google Scholar]
- 33.Zaniboni A, Labianca R. Gruppo Italiano Per Lo Studio E La Cura Dei Tumori Del D. Adjuvant therapy for stage II colon cancer: an elephant in the living room? Ann. Oncol. 2004;15(9):1310–1318. doi: 10.1093/annonc/mdh342. [DOI] [PubMed] [Google Scholar]
- 34.Schrag D, Rifas-Shiman S, Saltz L, Bach PB, Begg CB. Adjuvant chemotherapy use for medicare beneficiaries with stage II colon cancer. J. Clin. Oncol. 2002;20(19):3999–4005. doi: 10.1200/JCO.2002.11.084. [DOI] [PubMed] [Google Scholar]
- 35.Noordhuis P, Holwerda U, Van Der Wilt CL, et al. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 2004;15(7):1025–1032. doi: 10.1093/annonc/mdh264. [DOI] [PubMed] [Google Scholar]
- 36.Fraile RJ, Baker LH, Buroker TR, Horwitz J, Vaitkevicius VK. Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res. 1980;40(7):2223–2228. [PubMed] [Google Scholar]
- 37.Lamont EB, Schilsky RL. The oral fluoropyrimidines in cancer chemotherapy. Clin. Cancer Res. 1999;5(9):2289–2296. [PubMed] [Google Scholar]
- 38.Conti JA, Kemeny NE, Saltz LB, et al. Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. J. Clin. Oncol. 1996;14(3):709–715. doi: 10.1200/JCO.1996.14.3.709. [DOI] [PubMed] [Google Scholar]
- 39.Woynarowski JM, Chapman WG, Napier C, Herzig MC, Juniewicz P. Sequence- and region-specificity of oxaliplatin adducts in naked and cellular DNA. Mol. Pharmacol. 1998;54(5):770–777. doi: 10.1124/mol.54.5.770. [DOI] [PubMed] [Google Scholar]
- 40.International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet. 1995;345(8955):939–944. [PubMed] [Google Scholar]
- 41.Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo . Nature. 1993;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 42.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 43.Willett CG, Boucher Y, Di Tomaso E, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 2004;10(2):145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendelsohn J. Targeting the epidermal growth factor receptor for cancer therapy. J. Clin. Oncol. 2002;20(Suppl. 18):S1–S13. [PubMed] [Google Scholar]
- 45.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004;350(23):2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 46.André T1, Boni C, Mounedji-Boudiaf L, et al. Multicenter international study of oxaliplatin/5-fluorouracil/leucovorin in the adjuvant treatment of colon cancer (MOSAIC) investigators. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004;350:2343–2451. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 47.Moertel CG, Fleming TR, Macdonald JS, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann. Intern. Med. 1995;122(5):321–326. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 48.O'Connell MJ. A Phase III trial of 5-fluorouracil and leucovorin in the treatment of advanced colorectal cancer. A Mayo Clinic/North Central Cancer Treatment Group study. Cancer. 1989;63(Suppl. 6):1026–1030. doi: 10.1002/1097-0142(19890315)63:6+<1026::aid-cncr2820631307>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 49.Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J. Clin. Oncol. 1993;11(10):1879–1887. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 50.Maindrault-Goebel F, Louvet C, Andre T, et al. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur. J. Cancer. 1999;35(9):1338–1342. doi: 10.1016/s0959-8049(99)00149-5. [DOI] [PubMed] [Google Scholar]
- 51.Andre T, Louvet C, Maindrault-Goebel F, et al. CPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOR. Eur. J. Cancer. 1999;35(9):1343–1347. doi: 10.1016/s0959-8049(99)00150-1. [DOI] [PubMed] [Google Scholar]
- 52.Moehler M, Hoffmann T, Hildner K, Siebler J, Galle PR, Heike M. Weekly oxaliplatin, high-dose folinic acid and 24h-5-fluorouracil (FUFOX) as salvage therapy in metastatic colorectal cancer patients pretreated with irinotecan and folinic acid/5-fluorouracil regimens. Z. Gastroenterol. 2002;40(12):957–964. doi: 10.1055/s-2002-36156. [DOI] [PubMed] [Google Scholar]
- 53.Zhao G, Gao P, Yang KH, Tian JH, Ma B. Capecitabine/oxaliplatin as first-line treatment for metastatic colorectal cancer: a meta-analysis. Colorectal Dis. 2010;12(7):615–623. doi: 10.1111/j.1463-1318.2009.01879.x. [DOI] [PubMed] [Google Scholar]
- 54.Cassidy J, Tabernero J, Twelves C, et al. XELOX (capecitabine plus oxaliplatin): active first-line therapy for patients with metastatic colorectal cancer. J. Clin. Oncol. 2004;22(11):2084–2091. doi: 10.1200/JCO.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 55.Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N. Engl. J. Med. 2000;343(13):905–914. doi: 10.1056/NEJM200009283431302. [DOI] [PubMed] [Google Scholar]
- 56.Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355(9209):1041–1047. doi: 10.1016/s0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 57.Weh HJ, Wilke HJ, Dierlamm J, et al. Weekly therapy with folinic acid (FA) and high-dose 5-fluorouracil (5-FU) 24-hour infusion in pretreated patients with metastatic colorectal carcinoma. A multicenter study by the Association of Medical Oncology of the German Cancer Society (AIO) Ann. Oncol. 1994;5(3):233–237. doi: 10.1093/oxfordjournals.annonc.a058799. [DOI] [PubMed] [Google Scholar]
- 58.Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J. Clin. Oncol. 2003;21(1):60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 59.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized Phase III study. J. Clin. Oncol. 2008;26(12):2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 60.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: results from the Eastern Cooperative Oncology Group Study E3200. J. Clin. Oncol. 2007;25(12):1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 61.Kabbinavar FF, Schulz J, Mccleod M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized Phase II trial. J. Clin. Oncol. 2005;23(16):3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 62.Rudzinski WE, Palacios A, Ahmed A, Lane MA, Aminabhavi TM. Targeted delivery of small interfering RNA to colon cancer cells using chitosan and PEGylated chitosan nanoparticles. Carbohydr. Polym. 2016;147:323–332. doi: 10.1016/j.carbpol.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 63.Wang M, Gartel AL. Combination with bortezomib enhances the antitumor effects of nanoparticle-encapsulated thiostrepton. Cancer Biol. Ther. 2012;13(3):184–189. doi: 10.4161/cbt.13.3.18875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011;63(3):170–183. doi: 10.1016/j.addr.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 65.Loira-Pastoriza C, Todoroff J, Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv. Drug Deliv. Rev. 2014;75:81–91. doi: 10.1016/j.addr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 66.2012. https://clinicaltrials.gov/ct2/show/NCT00361842 Clinical trials database: NCT00361842.
- 67.2012. https://clinicaltrials.gov/ct2/show/NCT00361842 Clinical trials database: NCT00361842.
- 68.Lei S, Chien PY, Sheikh S, Zhang A, Ali S, Ahmad I. Enhanced therapeutic efficacy of a novel liposome-based formulation of SN-38 against human tumor models in SCID mice. Anticancer Drugs. 2004;15(8):773–778. doi: 10.1097/00001813-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 69.2006. https://clinicaltrials.gov/ct2/show/NCT00311610 ClinicalTrials.gov, Identifier: NCT00311610.
- 70.Chen KJ, Chaung EY, Wey SP, et al. Hyperthermia-mediated local drug delivery by a bubble-generating liposomal system for tumor-specific chemotherapy. ACS Nano. 2014;8(5):5105–5115. doi: 10.1021/nn501162x. [DOI] [PubMed] [Google Scholar]
- 71.Celsion. Phase 2 Study of Thermodox as Adjuvant Therapy With Thermal Ablation (RFA) in Treatment of Metastatic Colorectal Cancer(mCRC) (ABLATE) ClinicalTrials.gov, Identifier: NCT01464593. 2011 [Google Scholar]
- 72.Yang C, Liu HZ, Fu ZX, Lu WD. Oxaliplatin long-circulating liposomes improved therapeutic index of colorectal carcinoma. BMC Biotechnol. 2011;11:21. doi: 10.1186/1472-6750-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li L, Ahmed B, Mehta K, Kurzrock R. Liposomal curcumin with and without oxaliplatin: effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007;6(4):1276–1282. doi: 10.1158/1535-7163.MCT-06-0556. [DOI] [PubMed] [Google Scholar]
- 74.Cay O, Kruskal JB, Nasser I, Thomas P, Clouse ME. Liver metastases from colorectal cancer: drug delivery with liposome-encapsulated doxorubicin. Radiology. 1997;205(1):95–101. doi: 10.1148/radiology.205.1.9314969. [DOI] [PubMed] [Google Scholar]
- 75.Choi KC, Bang JY, Kim C, et al. Antitumor effect of adriamycin-encapsulated nanoparticles of poly(DL-lactide-co-glycolide)-grafted dextran. J. Pharm. Sci. 2009;98(6):2104–2112. doi: 10.1002/jps.21588. [DOI] [PubMed] [Google Scholar]
- 76.Li R, Li X, Xie L, et al. Preparation and evaluation of PEG-PCL nanoparticles for local tetradrine delivery. Int. J. Pharm. 2009;379(1):158–166. doi: 10.1016/j.ijpharm.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 77.Wang J, Peng CA. Anticancer effectiveness of polymeric drug nanocarriers on colorectal cancer cells. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011;2011:3249–3252. doi: 10.1109/IEMBS.2011.6090883. [DOI] [PubMed] [Google Scholar]
- 78.Ji AM, Su D, Che O, et al. Functional gene silencing mediated by chitosan/siRNA nanocomplexes. Nanotechnology. 2009;20(40):405103. doi: 10.1088/0957-4484/20/40/405103. [DOI] [PubMed] [Google Scholar]
- 79.Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable controlled-release polymers and polymeric nanoparticles: mechanisms of controlling drug release. Chem. Rev. 2016;116(4):2602–2663. doi: 10.1021/acs.chemrev.5b00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release. 2001;70(1):1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- 81.Emoto S, Yamaguchi H, Kishikawa J, Yamashita H, Ishigami H, Kitayama J. Antitumor effect and pharmacokinetics of intraperitoneal NK105, a nanomicellar paclitaxel formulation for peritoneal dissemination. Cancer Sci. 2012;103(7):1304–1310. doi: 10.1111/j.1349-7006.2012.02274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Valle JW, Armstrong A, Newman C, et al. A phase 2 study of SP1049C, doxorubicin in P-glycoprotein-targeting pluronics, in patients with advanced adenocarcinoma of the esophagus and gastroesophageal junction. Invest. New Drugs. 2011;29(5):1029–1037. doi: 10.1007/s10637-010-9399-1. [DOI] [PubMed] [Google Scholar]
- 83.Yang J, Hendricks W, Liu G, et al. A nanoparticle formulation that selectively transfects metastatic tumors in mice. Proc. Natl Acad. Sci. USA. 2013;110(36):14717–14722. doi: 10.1073/pnas.1313330110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi WI, Kamaly N, Riol-Blanco L, et al. A solvent-free thermosponge nanoparticle platform for efficient delivery of labile proteins. Nano Lett. 2014;14(11):6449–6455. doi: 10.1021/nl502994y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kamaly N, Fredman G, Fojas JJ, et al. Targeted Interleukin-10 nanotherapeutics developed with a microfluidic chip enhance resolution of inflammation in advanced atherosclerosis. ACS Nano. 2016;10(5):5280–5292. doi: 10.1021/acsnano.6b01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yameen B, Vilos C, Choi WI, et al. Drug delivery nanocarriers from a fully degradable peg-conjugated polyester with a reduction-responsive backbone. Chemistry (Easton) 2015;21(32):11325–11329. doi: 10.1002/chem.201502233. [DOI] [PubMed] [Google Scholar]
- 87.Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 2006;5(8):1909–1917. doi: 10.1158/1535-7163.MCT-06-0141. [DOI] [PubMed] [Google Scholar]
- 88.Hrkach J, Von Hoff D, Mukkaram Ali M, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012;4(128):128ra139. doi: 10.1126/scitranslmed.3003651. [DOI] [PubMed] [Google Scholar]
- 89.Arruebo M, Valladares M, González-Fernández Á. Antibody-conjugated nanoparticles for biomedical applications. J. Nanomater. 2009;2009:1–24. [Google Scholar]
- 90.Yu DH, Lu Q, Xie J, Fang C, Chen HZ. Peptide-conjugated biodegradable nanoparticles as a carrier to target paclitaxel to tumor neovasculature. Biomaterials. 2010;31(8):2278–2292. doi: 10.1016/j.biomaterials.2009.11.047. [DOI] [PubMed] [Google Scholar]
- 91.Tiernan JP, Perry SL, Verghese ET, et al. Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br. J. Cancer. 2013;108(3):662–667. doi: 10.1038/bjc.2012.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oshima T, Akaike M, Yoshihara K, et al. Clinicopathological significance of the gene expression of matrix metalloproteinase-7, insulin-like growth factor-1, insulin-like growth factor-2 and insulin-like growth factor-1 receptor in patients with colorectal cancer: insulin-like growth factor-1 receptor gene expression is a useful predictor of liver metastasis from colorectal cancer. Oncol. Rep. 2008;20(2):359–364. [PubMed] [Google Scholar]
- 93.Yu B, Li SY, An P, et al. Comparative study of proteome between primary cancer and hepatic metastatic tumor in colorectal cancer. World J. Gastroenterol. 2004;10(18):2652–2656. doi: 10.3748/wjg.v10.i18.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oshima T, Akaike M, Yoshihara K, et al. Overexpression of EphA4 gene and reduced expression of EphB2 gene correlates with liver metastasis in colorectal cancer. Int. J. Oncol. 2008;33(3):573–577. [PubMed] [Google Scholar]
- 95.Zlobec I, Terracciano L, Tornillo L, et al. Role of RHAMM within the hierarchy of well-established prognostic factors in colorectal cancer. Gut. 2008;57(10):1413–1419. doi: 10.1136/gut.2007.141192. [DOI] [PubMed] [Google Scholar]
- 96.Van Der Bij GJ, Oosterling SJ, Bogels M, et al. Blocking alpha2 integrins on rat CC531s colon carcinoma cells prevents operation-induced augmentation of liver metastases outgrowth. Hepatology. 2008;47(2):532–543. doi: 10.1002/hep.22013. [DOI] [PubMed] [Google Scholar]
- 97.Conaghan P, Ashraf S, Tytherleigh M, et al. Targeted killing of colorectal cancer cell lines by a humanised IgG1 monoclonal antibody that binds to membrane-bound carcinoembryonic antigen. Br. J. Cancer. 2008;98(7):1217–1225. doi: 10.1038/sj.bjc.6604289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oukkal M, Djilat K, Hadjam RM, et al. [Treatment of advanced and/or metastatic colorectal cancer with bevacizumab in combination with oxaliplatin-based chemotherapy (Folfox7 regimen)] Bull. Cancer. 2010;97(4):469–474. doi: 10.1684/bdc.2010.1088. [DOI] [PubMed] [Google Scholar]
- 99.Cassidy J, Clarke S, Diaz-Rubio E, et al. Randomized Phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J. Clin. Oncol. 2008;26(12):2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 100.Douillard JY, Siena S, Cassidy J, et al. Randomized, Phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J. Clin. Oncol. 2010;28(31):4697–4705. doi: 10.1200/JCO.2009.27.4860. [DOI] [PubMed] [Google Scholar]
- 101.Chong G, Lee FT, Hopkins W, et al. Phase I trial of 131I-huA33 in patients with advanced colorectal carcinoma. Clin. Cancer Res. 2005;11(13):4818–4826. doi: 10.1158/1078-0432.CCR-04-2330. [DOI] [PubMed] [Google Scholar]
- 102.Cortez C, Tomaskovic-Crook E, Johnston AP, et al. Influence of size, surface, cell line, and kinetic properties on the specific binding of A33 antigen-targeted multilayered particles and capsules to colorectal cancer cells. ACS Nano. 2007;1(2):93–102. doi: 10.1021/nn700060m. [DOI] [PubMed] [Google Scholar]
- 103.Brennan FR, Shaw L, Wing MG, Robinson C. Preclinical safety testing of biotechnology-derived pharmaceuticals: understanding the issues and addressing the challenges. Mol. Biotechnol. 2004;27(1):59–74. doi: 10.1385/MB:27:1:59. [DOI] [PubMed] [Google Scholar]
- 104.Weinberg WC, Frazier-Jessen MR, Wu WJ, et al. Development and regulation of monoclonal antibody products: challenges and opportunities. Cancer Metastasis Rev. 2005;24(4):569–584. doi: 10.1007/s10555-005-6196-y. [DOI] [PubMed] [Google Scholar]
- 105.Da Paz MC, Santos Mde F, Santos CM, et al. Anti-CEA loaded maghemite nanoparticles as a theragnostic device for colorectal cancer. Int. J. Nanomedicine. 2012;7:5271–5282. doi: 10.2147/IJN.S32139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vigor KL, Kyrtatos PG, Minogue S, et al. Nanoparticles functionalized with recombinant single chain Fv antibody fragments (scFv) for the magnetic resonance imaging of cancer cells. Biomaterials. 2010;31(6):1307–1315. doi: 10.1016/j.biomaterials.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 107.Tiernan JP, Ingram N, Marston G, et al. CEA-targeted nanoparticles allow specific in vivo fluorescent imaging of colorectal cancer models. Nanomedicine (Lond.) 2015;10(8):1223–1231. doi: 10.2217/nnm.14.202. [DOI] [PubMed] [Google Scholar]
- 108.Fay F, Mclaughlin KM, Small DM, et al. Conatumumab (AMG 655) coated nanoparticles for targeted pro-apoptotic drug delivery. Biomaterials. 2011;32(33):8645–8653. doi: 10.1016/j.biomaterials.2011.07.065. [DOI] [PubMed] [Google Scholar]
- 109.Abdelghany SM, Schmid D, Deacon J, et al. Enhanced antitumor activity of the photosensitizer meso-Tetra(N-methyl-4-pyridyl) porphine tetra tosylate through encapsulation in antibody-targeted chitosan/alginate nanoparticles. Biomacromolecules. 2013;14(2):302–310. doi: 10.1021/bm301858a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kirui DK, Rey DA, Batt CA. Gold hybrid nanoparticles for targeted phototherapy and cancer imaging. Nanotechnology. 2010;21(10):105105. doi: 10.1088/0957-4484/21/10/105105. [DOI] [PubMed] [Google Scholar]
- 111.Mccarron PA, Marouf WM, Quinn DJ, et al. Antibody targeting of camptothecin-loaded PLGA nanoparticles to tumor cells. Bioconjug. Chem. 2008;19(8):1561–1569. doi: 10.1021/bc800057g. [DOI] [PubMed] [Google Scholar]
- 112.Yang SJ, Lin FH, Tsai KC, et al. Folic acid-conjugated chitosan nanoparticles enhanced protoporphyrin IX accumulation in colorectal cancer cells. Bioconjug. Chem. 2010;21(4):679–689. doi: 10.1021/bc9004798. [DOI] [PubMed] [Google Scholar]
- 113.Li P, Wang Y, Zeng F, Chen L, Peng Z, Kong LX. Synthesis and characterization of folate conjugated chitosan and cellular uptake of its nanoparticles in HT-29 cells. Carbohydr. Res. 2011;346(6):801–806. doi: 10.1016/j.carres.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 114.Kopansky E, Shamay Y, David A. Peptide-directed HPMA copolymer-doxorubicin conjugates as targeted therapeutics for colorectal cancer. J. Drug Target. 2011;19(10):933–943. doi: 10.3109/1061186X.2011.632011. [DOI] [PubMed] [Google Scholar]
- 115.Unzueta U, Cespedes MV, Ferrer-Miralles N, et al. Intracellular CXCR4(+) cell targeting with T22-empowered protein-only nanoparticles. Int. J. Nanomedicine. 2012;7:4533–4544. doi: 10.2147/IJN.S34450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jain A, Jain SK, Ganesh N, Barve J, Beg AM. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomedicine. 2010;6(1):179–190. doi: 10.1016/j.nano.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 117.Gary-Bobo M, Brevet D, Benkirane-Jessel N, et al. Hyaluronic acid-functionalized mesoporous silica nanoparticles for efficient photodynamic therapy of cancer cells. Photodiagnosis Photodyn. Ther. 2012;9(3):256–260. doi: 10.1016/j.pdpdt.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 118.Shahzad MM, Mangala LS, Han HD, et al. Targeted delivery of small interfering RNA using reconstituted high-density lipoprotein nanoparticles. Neoplasia. 2011;13(4):309–319. doi: 10.1593/neo.101372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cao L, Du P, Jiang SH, Jin GH, Huang QL, Hua ZC. Enhancement of antitumor properties of TRAIL by targeted delivery to the tumor neovasculature. Mol. Cancer Ther. 2008;7(4):851–861. doi: 10.1158/1535-7163.MCT-07-0533. [DOI] [PubMed] [Google Scholar]
- 120.Kelly KA, Jones DA. Isolation of a colon tumor specific binding peptide using phage display selection. Neoplasia. 2003;5(5):437–444. doi: 10.1016/s1476-5586(03)80046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shia J, Klimstra DS, Nitzkorski JR, et al. Immunohistochemical expression of folate receptor alpha in colorectal carcinoma: patterns and biological significance. Hum. Pathol. 2008;39(4):498–505. doi: 10.1016/j.humpath.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 122.Sharma M, Malik R, Verma A, et al. Folic acid conjugated guar gum nanoparticles for targeting methotrexate to colon cancer. J. Biomed. Nanotechnol. 2013;9(1):96–106. doi: 10.1166/jbn.2013.1474. [DOI] [PubMed] [Google Scholar]
- 123.Mulder WJ, Griffioen AW, Strijkers GJ, Cormode DP, Nicolay K, Fayad ZA. Magnetic and fluorescent nanoparticles for multimodality imaging. Nanomedicine (Lond.) 2007;2(3):307–324. doi: 10.2217/17435889.2.3.307. [DOI] [PubMed] [Google Scholar]
- 124.Weissleder R, Tung CH, Mahmood U, Bogdanov A., Jr In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999;17(4):375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- 125.He X, Liu F, Liu L, Duan T, Zhang H, Wang Z. Lectin-conjugated FeO2Au3core@shell nanoparticles as dual mode contrast agents for <i>in vivo</i> detection of tumor. Mol. Pharm. 2014;11(3):738–745. doi: 10.1021/mp400456j. [DOI] [PubMed] [Google Scholar]
- 126.Larson DR, Zipfel WR, Williams RM, et al. Water-soluble quantum dots for multiphoton fluorescence imaging in vivo . Science. 2003;300(5624):1434–1436. doi: 10.1126/science.1083780. [DOI] [PubMed] [Google Scholar]
- 127.Gounaris E, Martin J, Ishihara Y, et al. Fluorescence endoscopy of cathepsin activity discriminates dysplasia from colitis. Inflamm. Bowel Dis. 2013;19(7):1339–1345. doi: 10.1097/MIB.0b013e318281f3f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Heppner GH, Miller BE. Tumor heterogeneity: biological implications and therapeutic consequences. Cancer Metastasis Rev. 1983;2(1):5–23. doi: 10.1007/BF00046903. [DOI] [PubMed] [Google Scholar]
- 129.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 130.O'brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 131.Palma S, Zwenger AO, Croce MV, Abba MC, Lacunza E. From molecular biology to clinical trials: toward personalized colorectal cancer therapy. Clin. Colorectal Cancer. 2016;15(2):104–115. doi: 10.1016/j.clcc.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 132.Huang EH, Hynes MJ, Zhang T, et al. Aldehyde dehydrogenase 1 is a marker for normal and malignant human colonic stem cells (SC) and tracks SC overpopulation during colon tumorigenesis. Cancer Res. 2009;69(8):3382–3389. doi: 10.1158/0008-5472.CAN-08-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dalerba P, Dylla SJ, Park IK, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc. Natl Acad. Sci. USA. 2007;104(24):10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vermeulen L, De Sousa EMF, Van Der Heijden M, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010;12(5):468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y, Krivtsov AV, Sinha AU, et al. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327(5973):1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sikandar SS, Pate KT, Anderson S, et al. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 2010;70(4):1469–1478. doi: 10.1158/0008-5472.CAN-09-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chou LY, Zagorovsky K, Chan WC. DNA assembly of nanoparticle superstructures for controlled biological delivery and elimination. Nat. Nanotechnol. 2014;9(2):148–155. doi: 10.1038/nnano.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Shu Y, Pi F, Sharma A, et al. Stable RNA nanoparticles as potential new generation drugs for cancer therapy. Adv. Drug Deliv. Rev. 2014;66:74–89. doi: 10.1016/j.addr.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat. Biotechnol. 2007;25(10):1165–1170. doi: 10.1038/nbt1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Choi HS, Ipe BI, Misra P, Lee JH, Bawendi MG, Frangioni JV. Tissue- and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett. 2009;9(6):2354–2359. doi: 10.1021/nl900872r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li Y, Kroger M, Liu WK. Shape effect in cellular uptake of PEGylated nanoparticles: comparison between sphere, rod, cube and disk. Nanoscale. 2015;7(40):16631–16646. doi: 10.1039/c5nr02970h. [DOI] [PubMed] [Google Scholar]
- 142.Salvador-Morales C, Zhang L, Langer R, Farokhzad OC. Immunocompatibility properties of lipid-polymer hybrid nanoparticles with heterogeneous surface functional groups. Biomaterials. 2009;30(12):2231–2240. doi: 10.1016/j.biomaterials.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]