Abstract
Due to the critical role of stereochemistry in determining properties such as biological activity, as well as growing interest in sustainability, there is a strong impetus to develop catalytic and enantioselective methods for synthesis. Alkylboranes are an important family of target compounds, serving as useful intermediates, as well as endpoints (medicines such as Velcade™), in fields such as pharmaceutical science and organic chemistry. Because C–B bonds can be transformed into a wide variety of C–X bonds (e.g., X = C, N, O, and halogen) with stereochemical fidelity, the ability to generate enantioenriched alkylboranes in which boron is bound to a stereogenic carbon represents an extremely powerful tool in synthesis, providing access to an enormous array of valuable classes of chiral molecules, including alcohols, amines, and alkyl halides. Despite progress in recent years, there is still a need for more versatile methods for the catalytic asymmetric synthesis of enantioenriched alkylboranes. In this report, we describe a novel approach toward this objective, specifically, the use of a chiral nickel catalyst to achieve stereoconvergent couplings of readily available racemic α-haloboranes with organozinc reagents under mild conditions. We demonstrate that this method provides straightforward access to a diverse array of enantioenriched alkylboranes, and we highlight the remarkable utility of these compounds in synthesis.
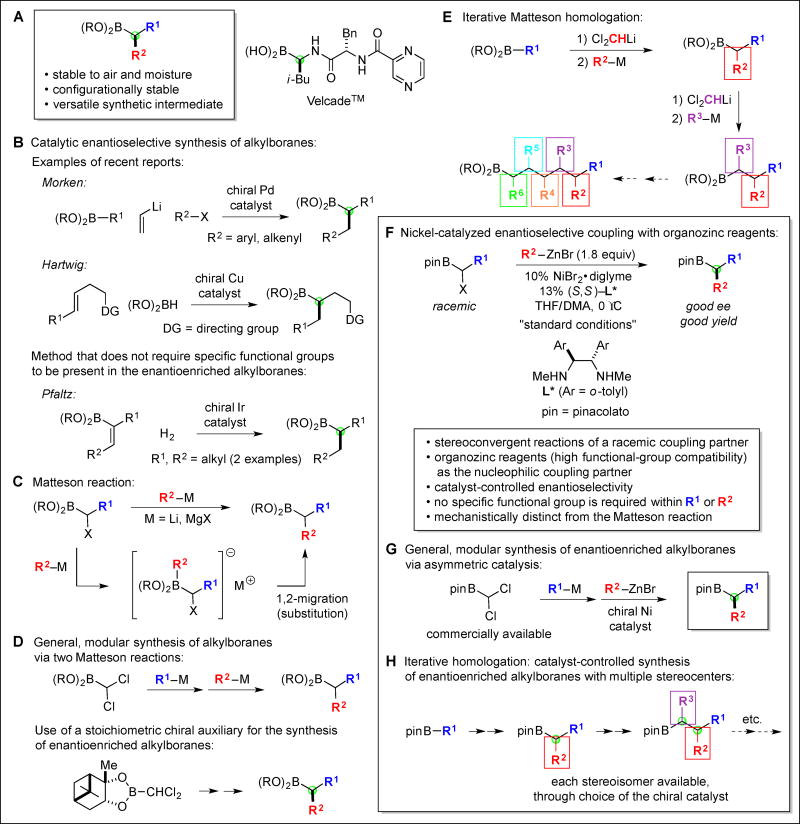
Organoboron compounds play an important role in fields ranging from materials science to biochemistry to organic synthesis (1, 2); for example, in organic chemistry, they serve as products or as reaction partners in powerful transformations such as the hydroboration of olefins (3) and the Suzuki cross-coupling (4). Although impressive progress has been made in organoboron chemistry during the past decades, tremendous opportunities remain, including expanding their role in enantioselective synthesis. For instance, the development of methods for the asymmetric synthesis of alkylboranes wherein boron is attached to a stereogenic carbon (e.g., Fig. 1A) is an important objective, given their significance both as endpoints (e.g., Velcade™) (5, 6) and as versatile precursors to a wide range of other valuable families of molecules, including enantioenriched amines and alcohols (1, 7, 8). In particular, alkylboronate esters (Fig. 1A) simultaneously possess desirable aspects of stability (including air, moisture, and configurational) and of reactivity (stereospecific conversion of the C–B bond to C–C, C–N, C–O, C–halogen, and other C–heteroatom bonds).
Fig. 1. Alkylboranes.
(A)–(E) Background. (F)–(H): This study.
Whereas early efforts to synthesize enantioenriched alkylboranes focused primarily on the use of stoichiometric chiral auxiliaries to control the stereochemistry of the product (8), recent investigations have increasingly focused on exploiting chiral catalysts. A number of important advances have been described, although virtually all methods furnish chiral alkylboranes that must contain a specific functional group in a specific position, for example an aryl, alkenyl, or a directing group (Fig. 1B) (9–11).
Matteson has developed a powerful, versatile strategy for the synthesis of alkylboranes, via the coupling of α-haloboranes with organolithium or organomagnesium reagents (Fig. 1C) (12). This reaction proceeds through initial addition of the organometallic nucleophile to the electrophilic boron, followed by a 1,2-migration (substitution with inversion) to form the desired carbon–carbon bond. The Matteson reaction serves as the foundation for a general, modular method for the synthesis of alkylboranes, including an enantioselective process that employs a stoichiometric chiral auxiliary (Fig. 1D). Moreover, the reaction can be applied in an iterative procedure that affords homologated alkylboranes (Fig. 1E).
Thus, Matteson couplings of α-haloboranes with organometallic nucleophiles represent a highly effective approach to the synthesis of alkylboranes. Nevertheless, areas for improvement persist. For example, it would be attractive to exploit a chiral catalyst, rather than a stoichiometric chiral auxiliary, to control enantioselectivity; of course, this is essential for the synthesis at will of any of the possible stereoisomers in the iterative strategy illustrated in Fig. 1E. Furthermore, it is desirable to employ nucleophilic coupling partners other than highly reactive organolithium and organomagnesium reagents, since they limit the range of functional groups that can be present. In this report, we address these challenges by achieving a Matteson-like coupling in a mechanistically distinct way, specifically, we utilize a chiral nickel catalyst to cross-couple racemic α-haloboranes with organozinc reagents, thereby generating alkylboranes with high enantioselectivity (Fig. 1F–1H).
We have recently established that, through the use of a nickel catalyst, a wide range of alkyl electrophiles can be coupled with a diverse array of organometallic nucleophiles, often with high levels of enantioselectivity (13–15); these cross-couplings proceed through organonickel intermediates that are generated and consumed via elementary steps such as oxidative addition, transmetalation, and reductive elimination (16). In pursuing a transition-metal-catalyzed variant of the Matteson coupling, we chose to focus on the use of organozinc reagents as the nucleophilic coupling partner (Negishi-type reactions), because they can be generated under mild conditions, they do not require a stoichiometric activator (unlike, for example, typical Suzuki cross-couplings) (4), and they are compatible with a broad spectrum of functional groups (17).
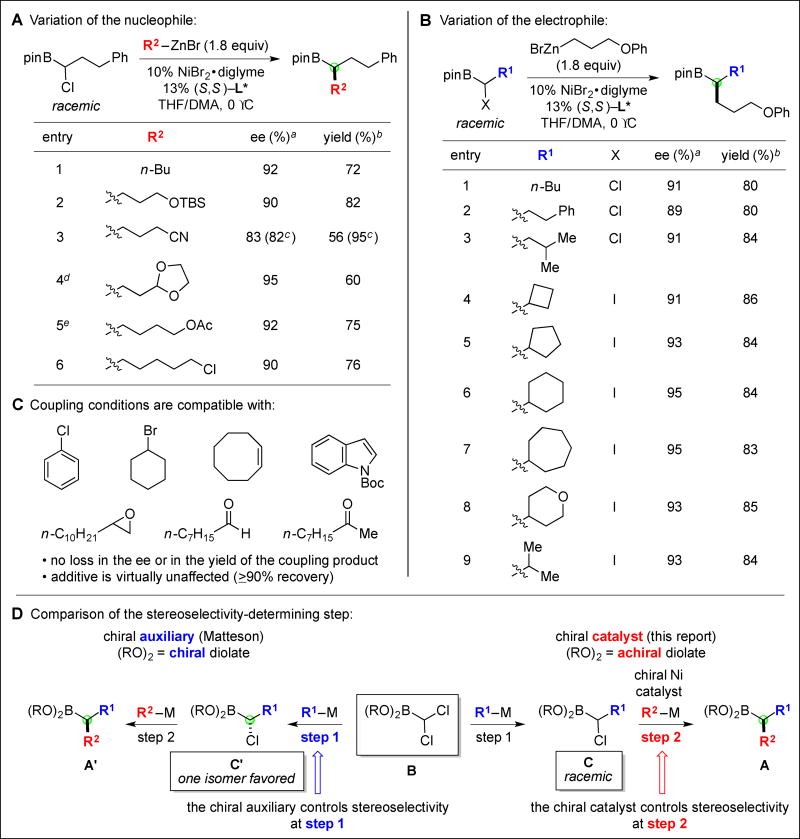
Whereas treatment of the α-chloroborane depicted in Fig. 2A with n-BuMgBr results in a rapid reaction (complete consumption of the electrophile within 15 minutes at room temperature in THF/DMA), replacement of n-BuMgBr with n-BuZnBr leads to no coupling after 24 hours. However, through the addition of an appropriate nickel catalyst (NiBr2•diglyme and a chiral 1,2-diamine), the coupling of the previously unreactive organozinc reagent can be achieved even at 0 °C (entry 1 of Fig. 2A); importantly, this catalyst not only accomplishes the construction of a new carbon–carbon bond, it does so with very good enantioselectivity (92% enantiomeric excess (ee)) from a racemic mixture of the electrophile. Furthermore, this new method is versatile: a wide array of α-haloboranes and organozinc reagents can be coupled under mild conditions with good ee (Fig. 2A–B). Interestingly, essentially no desired product is observed in the absence of the diamine, consistent with a ligand-accelerated process (18). Although we have previously applied chiral nickel/diamine catalysts to stereoconvergent Suzuki cross-couplings of racemic alkyl electrophiles (14), this is the first time that they have proved to be the ligands of choice for corresponding Negishi cross-couplings.
Fig. 2. Nickel-catalyzed asymmetric synthesis of alkylboranes.
(A)–(B) Variation in the coupling partners. (C) Functional-group compatibility. (D) Comparison of the enantioselectivity-determining step using a chiral auxiliary versus a chiral catalyst. (All data are the average of two experiments. aDetermined after oxidation to the alcohol. bYield of purified product. cThe α-iodoborane was used. dReaction temperature: 10 °C. eCatalyst loading: 12% NiBr2•diglyme, 16% L*.).
As illustrated in Fig. 2A, a variety of organozinc reagents can be employed as the nucleophilic coupling partner, including functionalized substrates that bear a silyl ether, a cyano group, an acetal, an ester, or a primary alkyl chloride, furnishing the target alkylborane with very good enantioselectivity from a racemic α-haloborane. The combination of good ee and good yield establishes that both enantiomers of the electrophile are being converted to the enantioenriched alkylborane product (i.e., this is a stereoconvergent reaction, not a simple kinetic resolution).
Similarly, an array of α-haloboranes serve as suitable electrophilic coupling partners (Fig. 2B), including sterically demanding compounds (entries 4–9); in the latter cases, due to the sensitivity of the reaction to steric hindrance, it is advantageous to employ a more reactive α-iodo, rather than an α-chloro, borane. When the coupling depicted in entry 2 is conducted on a larger scale (1.3 g of purified product), a lower catalyst loading can be used to generate the desired alkylborane with comparable ee and yield (3.0% NiBr2•diglyme/3.6% L*; 90% ee, 77% yield). To determine the compatibility of the new process with various functional groups, we have carried out the cross-coupling illustrated in entry 8 in the presence of a range of compounds (1.0 equiv in individual experiments), and we have established that the ee and the yield of the product are essentially unaffected, as is the additive (Fig. 2C) (19, 20); of course, organolithium and organomagnesium reagents would react with functional groups such as secondary alkyl bromides, epoxides, aldehydes, and ketones.
As a consequence of the mechanistic dichotomy between the Matteson reaction and this nickel-catalyzed cross-coupling, there is a divergence between which step of the modular asymmetric synthesis leads to the stereoselective formation of product A (Fig. 2D). In the Matteson approach using a stoichiometric chiral auxiliary, the two chlorines of electrophile B are diastereotopic due to the chiral diolate ligand, and their differential reactivity in the 1,2-migration of the tetravalent boron intermediate (for an outline of the mechanism of the Matteson reaction, see Fig. 1C) results in the stereoselective formation of compound C’ and then A’. In contrast, for the asymmetric nickel-catalyzed cross-coupling, racemic α-haloborane C is converted by the chiral nickel/diamine catalyst into product A in an enantioconvergent reaction, likely through a radical generated from homolytic cleavage of the C–Cl bond (16).
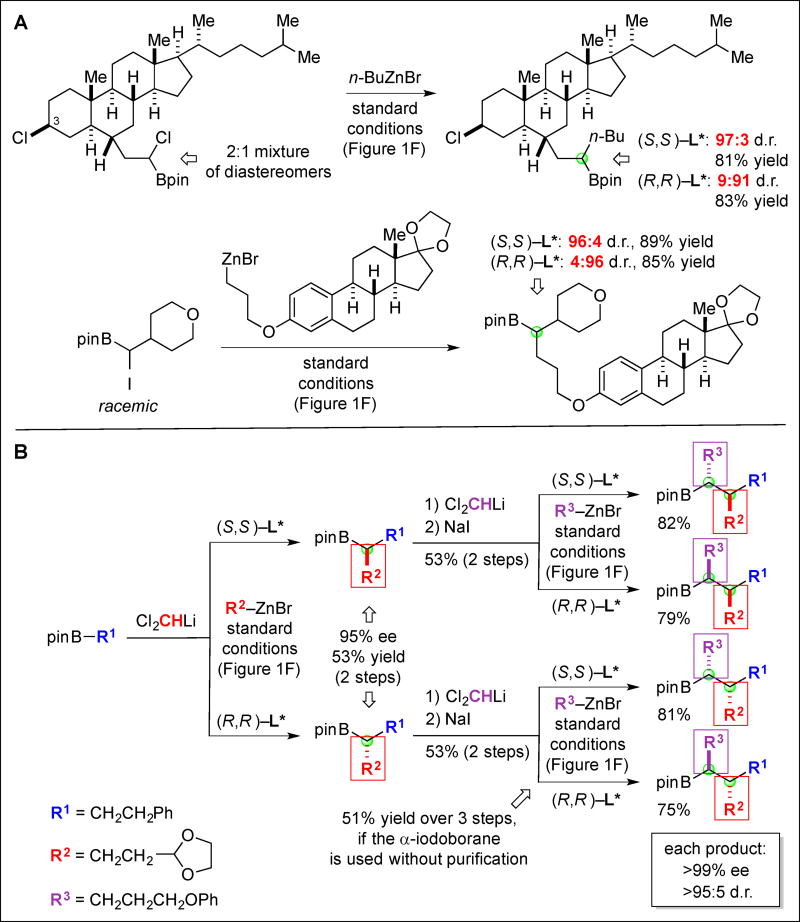
We have applied this nickel-catalyzed method for the stereoselective synthesis of alkylboranes to more complex partners. For example, under our standard conditions a derivative of cholesterol serves as a suitable electrophile (top of Fig. 3A; selective reaction of the chloride α to boron, rather than the chloride in the 3 position) and a derivative of estrone functions as an effective nucleophile (bottom of Fig. 3A), leading to each of the desired coupling products with high stereoselectivity and in good yield. In both cases, the stereochemistry of the chiral ligand (L*), rather than that of the substrate, is the predominant determinant of the stereochemistry α to boron.
Fig. 3. Catalyst-controlled stereoselectivity in the asymmetric synthesis of alkylboranes.
(A) Complex coupling partners. (B) Iterative homologation.
We have also demonstrated that our method can be exploited in an iterative homologation process (Fig. 3B). Thus, in contrast to a sequence of Matteson reactions employing a stoichiometric chiral auxiliary, this new nickel-catalyzed asymmetric coupling can provide access to any of the four possible diastereomers of the target alkylborane with excellent stereoselectivity from a single starting material, simply through choice of the appropriate enantiomer of the nickel/L* catalyst for each key carbon–carbon bond-forming process. As for the cross-couplings illustrated in Fig. 3A, the configuration of the chiral catalyst, not that of the coupling partners, primarily dictates the stereochemistry of the products in Fig. 3B. This approach complements the recent elegant work of Aggarwal (21).
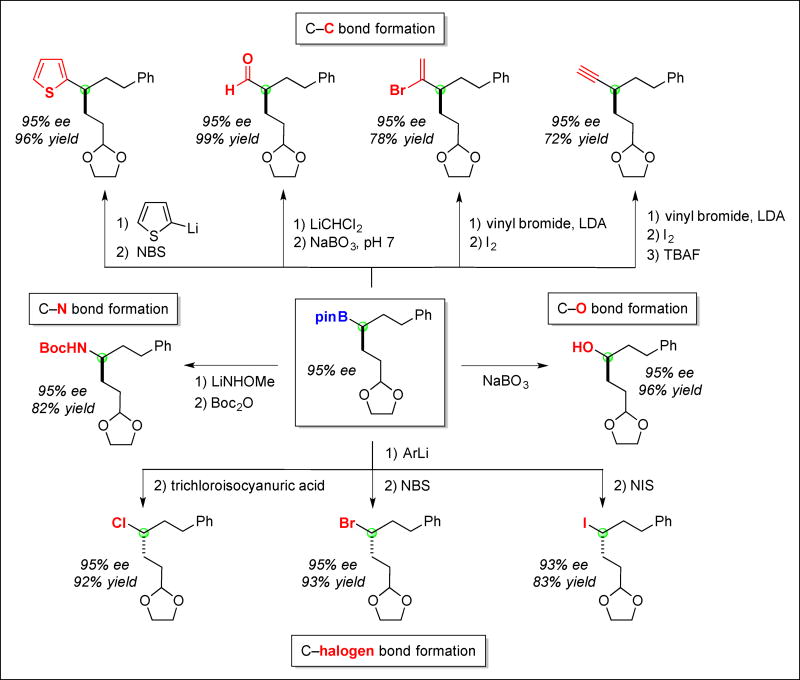
As indicated at the outset, enantioenriched alkylboranes are extremely versatile intermediates in organic synthesis that can be converted into other important families of compounds with preservation of the ee at the boron-bound carbon (1, 7, 8, 22–25); several illustrative examples are provided in Fig. 4. Thus, C–C, C–N, C–O, and C–halogen bond formation can be achieved in good yield, affording access to a wide array of functional groups that are common in valuable synthesis targets, including bioactive molecules (e.g., heterocycles, aldehydes, amines, alcohols, and alkyl halides), all with little or no erosion in enantiomeric excess. The diversity of useful compounds that can be readily synthesized by exploiting this method for the catalytic asymmetric synthesis of alkylboranes compares favorably to other catalytic enantioselective processes that have been developed to date.
Fig. 4. Enantioenriched alkylboranes as versatile intermediates.
Conversion to diverse families of organic molecules via C–C, C–N, C–O, and C–halogen bond formation. (ArLi = (3,5-bis(trifluoromethyl)phenyl)lithium).
Thus, we have developed a versatile, modular strategy for the catalytic asymmetric synthesis of enantioenriched alkylboranes, from readily available coupling partners, that is not limited to products that contain specific functional groups in specific positions. With the aid of a nickel catalyst, α-haloboranes are coupled with organozinc reagents by a pathway that is mechanistically distinct from the original Matteson coupling, thereby enabling the use of nucleophiles that have improved functional-group compatibility as compared with the organolithium and organomagnesium reagents used in Matteson reactions; moreover, by employing a chiral diamine ligand, the first catalytic asymmetric couplings of this type can be achieved (Fig. 1F–G). Exploiting a chiral catalyst (in contrast to a stoichiometric chiral auxiliary) can enable the highly stereoselective synthesis of each of the possible alkylborane diastereomers that are produced in an iterative homologation strategy through catalyst, rather than substrate, control (Fig. 1H). By providing straightforward access to a very wide array of alkylboranes, and thereby to many diverse families of enantioenriched organic compounds through subsequent functionalization (Fig. 4), this catalytic asymmetric method may have a substantial impact on the many fields that benefit from ready access to chiral molecules.
Supplementary Material
Supplementary Materials:
Materials and Methods
Supplementary Text
Tables S1 to S7
X-Ray Diffraction Data
References (26–39)
Spectral Data
Acknowledgments
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences: R01–GM62871), the Alexander von Humboldt Foundation (fellowship for J. S.), the German National Merit Foundation (fellowship for M. S.), the David S. Koons SURF Endowment (fellowship for A. T. L.), and the Gordon and Betty Moore Foundation (Caltech Center for Catalysis and Chemical Synthesis). We thank J. M. Ahn, L. M. Henling and M. K. Takase (Caltech X-Ray Crystallography Facility), N. D. Schley, M. Shahgholi (Caltech Mass Spectrometry Facility), D. G. VanderVelde (Caltech NMR Facility), and S. C. Virgil (Caltech Center for Catalysis and Chemical Synthesis) for assistance and helpful discussions. Experimental procedures and characterization data are provided in the supplementary materials.
References and Notes
- 1.Hall DG, editor. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials. 1 and 2 Wiley–VCH; 2011. [Google Scholar]
- 2.Miyaura N, Yamamoto Y. In: Comprehensive Organometallic Chemistry III. Crabtree RH, Mingos DMP, editors. Vol. 9. Elsevier; 2007. Chapter 5. [Google Scholar]
- 3.Brown HC. Hydroboration. Benjamin/Cummings; 1980. [Google Scholar]
- 4.Suzuki A. Angew. Chem. Int. Ed. 2011;50:6722–6737. doi: 10.1002/anie.201101379. [DOI] [PubMed] [Google Scholar]
- 5.Smoum R, Rubinstein A, Dembitsky VM, Srebnik M. Chem. Rev. 2012;112:4156–4220. doi: 10.1021/cr608202m. [DOI] [PubMed] [Google Scholar]
- 6.Ni N, Wang B. In: Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials. Hall DG, editor. Vol. 2. Wiley–VCH; 2011. Chapter 13. [Google Scholar]
- 7.Leonori D, Aggarwal VK. Angew. Chem. Int. Ed. 2015;54:1082–1096. doi: 10.1002/anie.201407701. [DOI] [PubMed] [Google Scholar]
- 8.Matteson DS. Stereodirected Synthesis with Organoboranes. Springer; 1995. [Google Scholar]
- 9.Zhang L, et al. Science. 2016;351:70–74. doi: 10.1126/science.aad6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xi Y, Hartwig JF. J. Am. Chem. Soc. 2016;138:6703–6706. doi: 10.1021/jacs.6b02478. [DOI] [PubMed] [Google Scholar]
- 11.Ganić A, Pfaltz A. Chem. Eur. J. 2012;18:6724–6728. doi: 10.1002/chem.201200246. [DOI] [PubMed] [Google Scholar]
- 12.Matteson DS. In: Boronic Acids: Preparation, Applications in Organic Synthesis and Medicine. Hall DG, editor. Wiley–VCH; 2005. Chapter 8. [Google Scholar]
- 13.Fischer C, Fu GC. J. Am. Chem. Soc. 2005;127:4594–4595. doi: 10.1021/ja0506509. [DOI] [PubMed] [Google Scholar]
- 14.Wilsily A, Tramutola F, Owston NA, Fu GC. J. Am. Chem. Soc. 2012;134:5794–5797. doi: 10.1021/ja301612y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang Y, Fu GC. J. Am. Chem. Soc. 2015;137:9523–9526. doi: 10.1021/jacs.5b04725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schley ND, Fu GC. J. Am. Chem. Soc. 2014;136:16588–16593. doi: 10.1021/ja508718m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu S, Kamada H, Kim EH, Oda A, Negishi E-i. In: Metal-Catalyzed Cross-Couplings and More. de Meijere A, Bräse S, Oestreich M, editors. Vol. 1. Wiley–VCH; 2014. Chapter 3. [Google Scholar]
- 18.Berrisford DJ, Bolm C, Sharpless KB. Angew. Chem. Int. Ed. 1995;34:1059–1070. [Google Scholar]
- 19.Bissember AC, Levina A, Fu GC. J. Am. Chem. Soc. 2012;134:14232–14237. doi: 10.1021/ja306323x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins KD, Glorius F. Nat. Chem. 2013;5:597–601. doi: 10.1038/nchem.1669. [DOI] [PubMed] [Google Scholar]
- 21.Burns M, et al. Nature. 2014;513:183–188. doi: 10.1038/nature13711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Noble A, Myers EL, Aggarwal VK. Angew. Chem. Int. Ed. 2016;55:4270–4274. doi: 10.1002/anie.201600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonet A, Odachowski M, Leonori D, Essafi S, Aggarwal VK. Nat. Chem. 2014;6:584–589. doi: 10.1038/nchem.1971. [DOI] [PubMed] [Google Scholar]
- 24.Mlynarski SN, Karns AS, Morken JP. J. Am. Chem. Soc. 2012;134:16449–16451. doi: 10.1021/ja305448w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larouche-Gauthier R, Elford TG, Aggarwal VK. J. Am. Chem. Soc. 2011;133:16794–16797. doi: 10.1021/ja2077813. [DOI] [PubMed] [Google Scholar]
- 26.Denmark SE, Su X, Nishigaichi Y, Coe DM, Wong K-T, Winter SBD, Choi JY. J. Org. Chem. 1999;64:1958–1967. doi: 10.1021/jo9820723. [DOI] [PubMed] [Google Scholar]
- 27.Cordier CJ, Lundgren RJ, Fu GC. J. Am. Chem. Soc. 2013;135:10946–10949. doi: 10.1021/ja4054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lou S, Fu GC. Org. Synth. 2010;87:330–338. [PMC free article] [PubMed] [Google Scholar]
- 29.Binder JT, Cordier CJ, Fu GC. J. Am. Chem. Soc. 2012;134:17003–17006. doi: 10.1021/ja308460z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonet A, Odachowski M, Leonori D, Essafi S, Aggarwal VK. Nat. Chem. 2014;6:584–589. doi: 10.1038/nchem.1971. [DOI] [PubMed] [Google Scholar]
- 31.Xi Y, Hartwig JF. J. Am. Chem. Soc. 2016;138:6703–6706. doi: 10.1021/jacs.6b02478. [DOI] [PubMed] [Google Scholar]
- 32.Matteson DS, Majumdar D. J. Am. Chem. Soc. 1980;102:7588–7590. [Google Scholar]
- 33.Wang Y, Noble A, Myers EL, Aggarwal VK. Angew. Chem. Int. Ed. 2016;55:4270–4274. doi: 10.1002/anie.201600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mlynarski SN, Karns AS, Morken JP. J. Am. Chem. Soc. 2012;134:16449–16451. doi: 10.1021/ja305448w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabalka GW, Shoup TM, Goudgaon NM. J. Org. Chem. 1989;54:5930–5933. [Google Scholar]
- 36.Larouche-Gauthier R, Elford TG, Aggarwal VK. J. Am. Chem. Soc. 2011;133:16794–16797. doi: 10.1021/ja2077813. [DOI] [PubMed] [Google Scholar]
- 37.Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H. J. Appl. Cryst. 2009;42:339–341. doi: 10.1107/S0021889811041161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheldrick GM. Acta Cryst. A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 39.Hoye TR, Jeffrey CS, Shao F. Nat. Protoc. 2007;2:2451–2458. doi: 10.1038/nprot.2007.354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials:
Materials and Methods
Supplementary Text
Tables S1 to S7
X-Ray Diffraction Data
References (26–39)
Spectral Data