Abstract
Attention-deficit/hyperactivity disorder (ADHD) has been found to be highly comorbid in children and adolescents with obsessive-compulsive disorder (OCD). Some have proposed, however, that obsessive anxiety may cause inattention and executive dysfunction, leading to inappropriate ADHD diagnoses in those with OCD. If this were the case, these symptoms would be expected to decrease following successful OCD treatment. The present study tested this hypothesis and evaluated whether ADHD symptoms at baseline predicted OCD treatment response. Obsessive-compulsive and ADHD symptoms were assessed in 50 youth enrolled in a randomized controlled trial investigating selective serotonin reuptake inhibitor and cognitive behavioral treatment. Repeated-measures analysis of variance (RMANOVA) revealed that ADHD symptoms at baseline do not significantly predict treatment outcome. A multivariate RMANOVA found that OCD treatment response moderated change in inattention; participants who showed greater reduction in OCD severity experienced greater reduction in ADHD-inattentive symptoms, while those with less substantial reduction in obsessions and compulsions showed less change. These findings suggest that children and adolescents with OCD and inattention may experience meaningful improvements in attention problems following OCD treatment. Thus, in many youth with OCD, inattention may be inherently tied to obsessions and compulsions. Clinicians may consider addressing OCD in treatment before targeting inattentive-type ADHD.
1. Introduction
Attention-deficit/hyperactivity disorder (ADHD) has been reported to be highly comorbid in children and adolescents with obsessive-compulsive disorder (OCD), with a pooled prevalence estimate of 19% across 42 studies of youth with a primary diagnosis of OCD (Abramovitch, Dar, Mittelman, & Wilhelm, 2015). The high risk for ADHD in children with OCD is of particular concern when considering the detrimental effects of co-occurring ADHD and OCD. Specifically, children with both OCD and ADHD often have an earlier age of onset compared to those with OCD only, are more functionally impaired (Masi et al., 2006), perform worse in school (Geller et al., 2003), have higher rates of other comorbid conditions like major depression, tic disorders, and disruptive behavior disorders (Masi et al., 2006), and may experience poorer treatment outcomes (Storch et al., 2008; Walitza et al., 2008).
1.1. Diagnosis of ADHD in individuals with OCD
Although the number, types, and severity of ADHD symptoms are similar when individuals with OCD and ADHD are compared with those who only have ADHD (Geller et al., 2002), evidence from familial, clinical, and neuropsychological studies is beginning to indicate that the reported comorbidity rate may be an overestimation of the true prevalence of ADHD in OCD (Abramovitch, Dar, Hermesh, & Schweiger, 2012).
While there is evidence for shared genetic risk factors between OCD, ADHD, and tic disorders (Mathews & Grados, 2011; Pinto et al., 2016), there is also support for independent familial risk for OCD and ADHD in the absence of tics. Indeed, studies that have investigated ADHD prevalence in people with a primary diagnosis of OCD have generally found a higher prevalence when they included children with co-occurring tic disorders (Sheppard et al., 2010). First-degree family members of children with ADHD only and healthy children are equally unlikely to have OCD when compared to relatives of children with both OCD and ADHD (Geller et al., 2007a). Similar results were found in a study investigating children with OCD and ADHD, children with OCD only, and healthy controls; first-degree relatives of children with ADHD and OCD were significantly more likely to have ADHD than relatives of healthy controls and children with OCD only, while there was no significant difference in the rate of ADHD between the OCD only and healthy control groups (Geller et al., 2007b). Together, these two studies indicate that there are independent familial risk factors for developing ADHD and OCD.
Studies comparing cognition and self-reported ADHD symptoms in individuals with ADHD, with OCD, and healthy controls are beginning to suggest that ADHD-like inattention may be caused by obsessive-compulsive symptoms in those with OCD. For instance, while ADHD and OCD are both characterized by frontostriatal dysfunction compared with healthy controls, the proposed neurological pathways to these deficits are antithetical. Obsessive-compulsive disorder is linked with frontostriatal hyperactivity, perhaps reflecting the desire for high control over planning and certainty; ADHD is characterized by a lower activity in these regions, as inattention and executive dysfunction may be related to a lack of planning or inhibitory control (Abramovitch et al., 2012; Norman et al., in press). These findings support a recently proposed “executive overload” model of OCD, which posits that deficits in frontal functions are a consequence of repeated attempts to control thoughts, leading to exhaustion of the executive system (Abramovitch et al., 2012). Executive functioning deficits are also prevalent in youth with OCD (Abramovitch et al., 2015), and deficits across multiple domains of parent-reported executive functioning have been associated with increased obsessive-compulsive symptom severity in children with the disorder (McNamara et al., 2014). Exhaustion of the executive system caused by obsessive thinking could result in behaviors that are phenomenologically similar to symptoms of ADHD (e.g., distractibility, forgetfulness), but instead are related OCD. Indeed, Abramovitch and colleagues (2012; Abramovitch, Dar, Mittelman, & Schweiger 2013) found that while obsessions and compulsions may be linked with ADHD symptoms and executive dysfunction among individuals with OCD, the two constructs seem to be unrelated in people with subclinical levels of obsessive-compulsive symptoms. Finally, numerous studies have demonstrated that adults with OCD are less behaviorally impulsive than healthy controls (Abramovitch & McKay, 2016), though these results have not been replicated with children and adolescents. The frequency of hyperactive-impulsive symptoms in children with OCD may be different; obsessive-compulsive symptom severity has been linked with other externalizing behaviors like oppositionality and defiance (Lebowitz, Omer, and Leckman, 2011) and emotional dysregulation (McGuire et al., 2013; McNamara et al., 2014), which may in turn be associated with impulsive, hyperactive behavior.
These findings beg the question of whether diagnosed ADHD in children with OCD always represents true underlying ADHD pathology. It may be the case that obsessive-compulsive symptoms exacerbate inattention in many people with OCD, leading to inappropriate ADHD diagnoses (see Abramovitch, Dar, Mittelman, & Wilhelm, 2015 for a recent review). In childhood OCD, this may manifest as externalizing symptoms exacerbated by the presence of OCD. For instance, children with OCD may have difficulty staying in their seat during class or paying attention to their parents due to having repeated intrusive thoughts, rather than having underlying inattentive, hyperactive dispositions. To our knowledge, this hypothesis has never been tested in children with the disorder. Frontostriatal dysfunction tends to normalize following successful treatment of children and adolescents with OCD, and thus attentional abilities may improve as well (Freyer et al., 2011). If that is the case, ADHD-like symptoms may decrease following treatment that targets obsessive-compulsive symptoms, and that reduction would be expected to correspond with reductions in OCD severity.
1.2. ADHD and OCD treatment outcome
Previous research has also found that comorbid ADHD may interfere with success in OCD treatment (Geller et al., 2003; Storch et al., 2008). The first-line treatment recommended for children with OCD is cognitive behavioral therapy with exposure and response prevention (CBT-E/RP) in mild to moderate cases and in combination with selective serotonin reuptake inhibitors (SSRIs) in severe cases (Geller & March, 2012; Freeman et al., 2014). Children and adolescents with OCD and ADHD have been shown to have relatively poorer treatment outcomes in CBT-E/RP. Though the mechanisms have not been studied directly, children with elevated ADHD symptoms may experience poorer treatment outcomes because impulsive, hyperactive children may be more resistant to participating in challenging exposure exercises or resist engaging in compulsions. Further, inattentive children may not encode corrective information following exposures (Storch et al., 2008). Following successful inpatient treatment, those with both OCD and ADHD are more likely to experience a relapse of symptoms compared to patients with OCD without ADHD (Walitza et al., 2008). There have been mixed results with respect to whether children with both conditions respond poorly to SSRI treatment, with one study finding poorer treatment outcomes among those with ADHD and OCD (Geller et al., 2003), and another finding non-significant results (Masi et al., 2006). In the largest randomized controlled trial of combined CBT-SSRI treatment of children and adolescents with OCD to date, externalizing symptoms (an aggregate of ADHD, oppositional defiant disorder, and conduct disorder symptoms) predicted poorer treatment outcome (Garcia et al., 2010).
No study to date, however, has evaluated ADHD symptoms specifically as a predictor of combined CBT-E/RP and SSRI treatment. Though measuring comorbid ADHD symptoms as a continuous variable is common in psychiatric research and there is mounting evidence that the disorder can be appropriately classified on a “spectrum” (Asherson & Trzaskowski, 2015), OCD treatment outcome studies have typically examined ADHD or externalizing problems as a dichotomous diagnostic predictor or moderator. Measuring ADHD as a continuous variable may also capture subclinical symptoms that may also interfere with treatment.
1.3. The present study
If OCD truly contributes to ADHD-like inattention, these symptoms should be expected to decrease as obsessions and compulsions decline. In the present study, we test the hypothesis that attention problems will decrease following successful OCD treatment. We also examine whether other ADHD symptoms like impulsivity and hyperactivity decrease following OCD treatment. The second study aim is to evaluate whether baseline ADHD symptoms predict multimodal treatment outcome, measuring ADHD symptoms as continuous variable. It is hypothesized that fewer parent-rated ADHD symptoms at baseline predict superior treatment outcome.
2. Methods
2.1. Participants and design
The present study was a secondary analysis of a randomized controlled trial investigating multimodal treatment for OCD (Blinded for review). The trial involved three treatment groups undergoing combined CBT-E/RP+SSRI treatment. In each group, children received 18 consecutive weeks of medication management with a board-certified child and adolescent psychiatrist, and beginning at the fourth week, engaged in 14 consecutive weeks of CBT-E/RP. Treatment was delivered at two OCD specialty treatment centers in the Southeastern United States. Therapists were post-doctoral associates supervised by licensed psychologists trained and experienced in CBT-E/RP. In the first group, SSRI dosages could be increased up to 50mg per week if tolerated, reaching a maximum dose of 200mg as quickly as by the fifth week (RegSert; n = 17, 30%). In the second group, dosages could only be increased by 25mg per week when increases were tolerated, reaching the maximum possible dosage of 200mg no sooner than the ninth week (SlowSert; n = 21, 38%). In the last group, participants received matching pill placebo for the entire trial (Placebo; n = 18, 32%). Treating psychiatrists and psychologists were blind to group assignment. The study was approved by the institutional review boards at both sites. The purposes and benefits of the study were reviewed with each family, and parent consent and child assent were obtained prior to study involvement.
Inclusion criteria for the study were: 1) age 7-17 years old, 2) a primary diagnosis of OCD as determined by a licensed psychologist or psychiatrist supervising the administration of the Schedule for Affective Disorders and Schizophrenia for School-Age Children- Present and Lifetime Version (K-SADS-PL; Kaufman et al., 1997), 3) a score of at least 18 on the Children's Yale-Brown Obsessive Compulsive Scale (CYBOCS; Scahill et al., 1997), 4) English speaking and ability to read. Exclusionary criteria were: 1) prior adequate SSRI trial, 2) history of rheumatic fever, serious autoimmune disorder, or other serious medical condition, 3) inability to swallow study medication, 4) presence of suicidal ideation or history of suicide attempt in the past year, 5) pregnancy or having unprotected sex in females, 6) presence of comorbid DSM-IV psychosis, bipolar disorder, autism, anorexia, or substance abuse, and 7) taking psychiatric medication other than psychostimulants for ADHD. Of note, three children in the present study were on a stable regimen of psychostimulants. Eight (16%) qualified for an ADHD diagnosis per the cutoffs recommended for the parent-report ADHD measure described below, and nine (18%) met diagnostic criteria per the K-SADS-PL interview at baseline. Interestingly, there was diagnostic agreement between the K-SADS-PL and SNAP-IV on only four of these children.
Though 56 children were originally enrolled in the study, complete data were not available for six: one did not complete the ADHD measure at baseline, and five dropped out prior to beginning CBT and did not complete the ADHD measure at their final session. Of the remaining 50, 29 were male (58%) and 48 identified as Caucasian (96%), with one participant identifying as Black/African-American (2%) and one identifying as Asian (2%). Mean age among participants was 11.76 years old (SD=3.26). Among the 50 remaining participants, 15 (30%) were in the RegSert group, 19 (38%) were in the SlowSert group, and 16 (32%) were in the placebo group.1
2.2. Measures
2.2.1. Demographics
Demographic data, including participant age and gender, were collected via self-report at the beginning of the study.
2.2.2. Children's Yale-Brown Obsessive-Compulsive Scale (CYBOCS)
The CY-BOCS is a psychometrically validated, semi-structured interview of OC symptoms in children (Scahill et al., 1997; Storch et al., 2004). The CY-BOCS includes two five-item sections measuring both obsessions and compulsions, each section assessing time taken by symptoms, distress, functional impairment, control over symptoms, and resistance against symptoms. Each item is scored from 0-4, with higher numbers indicating increased severity. The two sections are combined to create a total score ranging from 0-40. The CYBOCS was administered at the first and last sessions of treatment.
2.2.3. Swanson, Nolan, and Pelham-IV (SNAP-IV)
The SNAP-IV is a psychometrically sound, parent-report measure of externalizing symptoms in childhood (Bussing et al., 2008; Swanson, Nolan, & Pelham, 1992). The measure consists of three subscales, including a nine-item inattention factor, a nine-item hyperactivity-impulsivity factor, and an eight-item oppositional/defiant factor. For the present study, only the inattention and hyperactivity-impulsivity items were evaluated. The two factors can be summed to create a total score reflecting total ADHD symptom severity. Parents completed the SNAP-IV at the first and last sessions of treatment.
2.3. Statistical Analysis
First, a series of independent samples t-tests were conducted to determine whether there were differences in scores of ADHD-inattention, ADHD-hyperactivity-impulsivity, obsessions, or compulsions.
Correlations between OCD severity and ADHD severity were then conducted to examine relationships between these variables both before and after treatment. The CYBOCS was used to determine obsession, compulsion, and total OCD severity, and the SNAP-IV was used to determine inattention, hyperactivity-impulsivity, and total ADHD severity scores.
Repeated-measures analysis of variance (RMANOVA) was used to determine whether ADHD symptoms at baseline significantly predict obsessive-compulsive symptom reduction. Attention-deficit hyperactivity disorder symptoms were measured using baseline SNAP-IV scores and OC symptoms were measured using the CYBOCS as a within-subjects factor. Treatment type (RegSert vs. SlowSert vs. Placebo) was included as a between-subjects factor.
Another RMANOVA was used to evaluate whether ADHD symptoms significantly changed during treatment, using SNAP-IV scores at baseline and end of treatment as a within-subjects factor. Change scores in CYBOCS (baseline CYBOCS-final CYBOCS) were used to determine whether changes in OC symptoms significantly moderated changes in ADHD symptoms. Children's Yale-Brown Obsessive-Compulsive Scale scores at baseline were also included as a covariate in the RMANOVA. Interactions between treatment type and time, and between CYBOCS change and time, were evaluated to determine whether reductions in ADHD symptoms varied by medication status (i.e., placebo vs. sertraline) and reduction of OC symptoms. Finally, a multivariate RMANOVA was used with the inattention and hyperactivity-impulsivity subscales of the SNAP-IV as dependent variables, rather than the SNAP-IV total score. This analysis was conducted to determine whether OC symptom reduction influenced change in different subtypes of ADHD symptoms. For all analyses, partial eta squared values (η2partial) between .01 and .09 were considered small, values between .09 and .25 were considered medium, and values above .25 were considered large (Cohen, 1988). Bonferroni adjustments were made to account for multiple comparisons in all analyses.
3. Results
3.1. Assessment of site differences
A series of paired samples t-tests revealed no differences in baseline OCD severity, inattention, or hyperactivity-impulsivity (p > .05 for all comparisons). As reported by [Blinded for review], there were no differences in treatment outcome across sites.
3.2. Correlations between obsessive-compulsive and ADHD symptoms
At baseline, the CYBOCS-obsessions and CYBOCS-compulsions subscales were highly positively correlated, r = .72, p < .001, as were the ADHD-inattentive and hyperactive-impulsive subscales, r = .51, p < .001. There were no significant correlations between any ADHD variable and any OCD variable at baseline. At the end of treatment, however, all these variables were significantly positivity correlated; inattention was significantly positively correlated with CYBOCS-obsessions severity, r = .32, p = .021, CYBOCS-compulsions severity, r = .31, p = .025, and CYBOCS total, r = .33, p = .33, p = .019. Similarly, hyperactivity-impulsivity was significantly positively correlated with CYBOCS-obsessions severity, r = .34, p = .015, CYBOCS-compulsions severity, r = .30, p = .0.030, and CYBOCS total, r = .33, p = .33, p = .018. A summary of CYBOCS and SNAP-IV scores before and after treatment can be found in Table 1, and the correlation coefficients between these variables at baseline and the end of treatment can be found in Tables 2 and 3, respectively.
Table 1. Descriptive statistics before and after treatment.
| Before treatment M (SD) | After treatment M (SD) | |
|---|---|---|
| Total OCD severity | 24.7 (4.6) | 14.5 (7.5) |
| Obsessions | 12.0 (2.5) | 7.1 (3.8) |
| Compulsions | 12.7 (2.4) | 7.4 (4.1) |
| Total ADHD symptoms | 18.7 (12.9) | 15.3 (11.7) |
| Inattention | 11.3 (8.2) | 9.6 (7.4) |
| Hyperactivity/Impulsivity | 7.4 (6.6) | 5.7 (5.7) |
Note: OCD=obsessive-compulsive disorder; ADHD=attention-deficit/hyperactivity disorder. OCD was measured with the Children's Yale-Brown Obsessive-Compulsive Scale; obsessions and compulsions were measured with its subscales. ADHD was measured with the Swanson, Nolan, and Pelham-IV; inattention and hyperactivity/impulsivity were measured using this measure's subscales.
Table 2. Pre-treatment correlations between OCD and ADHD variables.
| Total OCD severity | Obsessions | Compulsions | Total ADHD symptoms | Inattention | Hyperactivity/Impulsivity | |
|---|---|---|---|---|---|---|
| Total OCD severity | 1 | |||||
| Obsessions | .93* | 1 | ||||
| Compulsions | .93* | .72* | 1 | |||
| Total ADHD symptoms | .13 | .10 | .15 | 1 | ||
| Inattention | .04 | .03 | .05 | .90* | 1 | |
| Hyperactivity/Impul sivity | .21 | .16 | .22 | .84* | .51* | 1 |
p<.001
Note: OCD=obsessive-compulsive disorder; ADHD=attention-deficit/hyperactivity disorder. OCD was measured with the Children's Yale-Brown Obsessive-Compulsive Scale; obsessions and compulsions were measured with its subscales. ADHD was measured with the Swanson, Nolan, and Pelham-IV; inattention and hyperactivity/impulsivity were measured using this measure's subscales.
Table 3. Post-treatment correlations between OCD and ADHD variables.
| Total OCD severity | Obsessions | Compulsions | Total ADHD symptoms | Inattention | Hyperactivity/Impulsivity | |
|---|---|---|---|---|---|---|
| Total OCD severity | 1 | |||||
| Obsessions | .96*** | 1 | ||||
| Compulsions | .97*** | 1 | 1 | |||
| Total ADHD symptoms | .37** | .73** | .35* | 1 | ||
| Inattention | .33* | .32* | .31* | .92*** | 1 | |
| Hyperactivity/Impul sivity | .33* | .34* | .30* | .85*** | .57*** | 1 |
p<.05,
p<.01,
p<.001
Note: OCD=obsessive-compulsive disorder; ADHD=attention-deficit/hyperactivity disorder. OCD was measured with the Children's Yale-Brown Obsessive-Compulsive Scale; obsessions and compulsions were measured with its subscales. ADHD was measured with the Swanson, Nolan, and Pelham-IV; inattention and hyperactivity/impulsivity were measured using this measure's subscales.
3.2. Change in CYBOCS score as predictor of ADHD symptom reduction
Repeated-measures analyses of variance predicting SNAP-IV scores revealed a significant interaction between time and change in CYBOCS score, F(1, 45) = 7.37, p = .009, η2partial = .14, such that those with larger CYBOCS change scores experienced greater reductions in ADHD symptoms. Those in remission at the end of treatment (as defined by a 45% reduction CYBOCS score; Storch, Lewin, De Nadai, & Murphy, 2010) experienced a 5.3 point mean reduction in SNAP-IV scores (Mpre = 17.7, Mpost = 12.4), indicative of a 30.0% reduction in ADHD symptoms, while those not in remission only declined 1.6 points on the SNAP-IV on average (Mpre = 19.7, Mpost = 18.1), corresponding with a 8.1% reduction in symptoms. Neither baseline CYBOCS score nor treatment group significantly interacted with time to predict changes in SNAP-IV ratings. A summary of within-subjects effects are presented in Table 4.
Table 4. Repeated-measures ANOVA predicting SNAP-IV symptoms (n=50).
| F | η2 | |
|---|---|---|
| Time | 1.14 | .03 |
| Time*CYBOCSpre | 1.38 | .03 |
| Time*ACYBOCS | 7.37* | .14 |
| Time*Treatment Arm | 0.06 | <.01 |
p<01
Note: Time is represented by measurements taken before and after treatment; SNAP-IV = Swanson, Nolan, & Pelham-IV; CYBOCS = Children's Yale-Brown Obsessive-Compulsive Scale; CYBOCS_pre = CYBOCS score at baseline; ΔCYBOCS = CYBOCS at baseline minus CYBOCS at the end of treatment
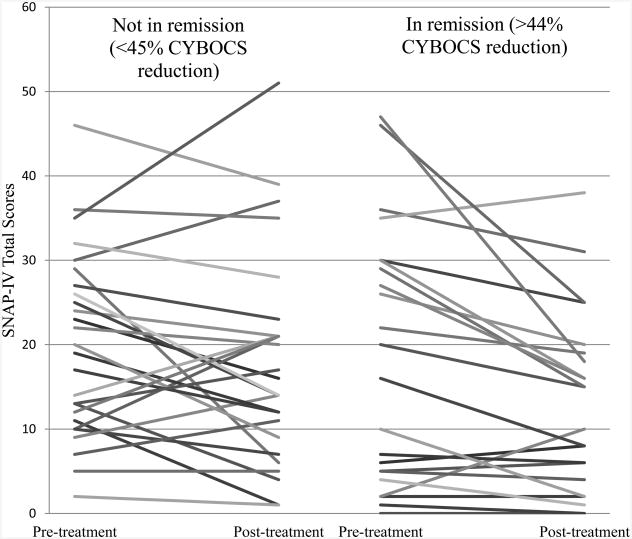
Figure 1 shows change in ADHD symptoms across treatment for each participant. Participants were divided into two groups based on whether or not they met “remission” status at the end of treatment, as indicated by at least a 45% reduction in CYBOCS scores. Inspection of this figure shows that among children in the “remission” group, 10 of 11 participants with substantial ADHD symptoms at the beginning of treatment (SNAP-IV total scores ≥ 20) showed ADHD symptom reduction after OCD treatment. Among those 11 participants, the average SNAP-IV reduction across treatment was 10.0 points (range of scale: 0-54). By contrast, there is no easily discernible pattern among those not in remission.
Figure 1. SNAP-IV scores before and after treatment broken up by OCD remission status at the end of treatment.
Note: SNAP-IV = Swanson Nolan and Pelham-IV; CYBOCS = Children's Yale-Brown Obsessive-Compulsive Scale
The above graph displays changes in SNAP-IV scores before and after treatment in each participant. Participants in the right half of the graph were classified as “in remission” at the end of treatment, while participants on the left half were “not in remission.”
3.3. Changes in inattention vs. hyperactivity-impulsivity
Multivariate analyses that included the SNAP-IV-inattention and SNAP-IV-hyperactivity-impulsivity subscales as dependent variables replicated the above results, revealing a non-significant multivariate effect of time but a significant aggregate effect of the time-change in CYBOCS interaction on inattention and hyperactivity-impulsivity symptoms, F(2, 44) = 4.24, p = .021, η2partial = .16. The interaction between time and CYBOCS change remained nonsignificant, as was the interaction between time and treatment arm. Univariate analyses indicate that there was a significant interaction between time and change in CYBOCS score on the SNAP-IV-inattention subscale, F(1) = 8.46, p = .006, η2partial = .16, though there was no relationship between the time-CYBOCS change score interaction and the hyperactivity-impulsivity subscale, F(1) = 2.56, p = .12, η2partial = .05. Those in remission at the end of treatment experienced an average 2.9 point reduction in the SNAP-IV-inattention subscale (Mpre = 10.4, Mpost = 7.5), while those not in remission only experienced a 0.3 point reduction on the SNAP-IV inattention scale Mpre = 12.1, Mpost = 11.8). Among the ten participants in remission at the end of treatment with substantial ADHD-inattentive symptoms at baseline, the average SNAP-IV-inattentive subscale reduction was 5.6 points. Univariate effects on each outcome (SNAP-IV-inattention subscale and SNAP-IV-hyperactivity-impulsivity subscale) are presented in Table 5.
Table 5. Multivariate repeated-measures ANOVA predicting SNAP-IV subscales (n=50).
| DV IV | F | η2 |
|---|---|---|
| SNAP-IV-inattentive | ||
| Time | 1.86 | .04 |
| Time* CYBOCSpre | 2.56 | .05 |
| Time* ACYBOCS | 8.46* * | .16 |
| Time* Treatment Arm | 0.19 | .01 |
| SNAP-IV-hyperactive/impulsive | ||
| Time | 0.16 | <.01 |
| Time* CYBOCSpre | 0.11 | <.01 |
| Time* ACYBOCS | 2.40 | .05 |
| Time* Treatment Arm | 0.07 | <.01 |
p<05; **p<01
Note: Time is represented by measurements taken before and after treatment; DV = dependent variable; IV = independent variable; SNAP-IV = Swanson, Nolan, & Pelham-IV; CYBOCS = Children's Yale-Brown Obsessive-Compulsive Scale; CYBOCS_pre = CYBOCS score at baseline; ΔCYBOCS = CYBOCS at baseline minus CYBOCS at the end of treatment
3.4. ADHD symptoms at baseline as predictor of treatment outcome
Repeated-measures analyses of variance analyses predicting CYBOCS scores revealed a large, significant main effect of time, F(1, 46) = 22.05, p < .001, η2partial = .32. The interaction between baseline ADHD symptoms and time was non-significant, F(1, 46) = 0.07, p = .79, η2partial = .002. The interaction between time and treatment arm was also non-significant, F(2, 46) = 0.94, p = .40, η2partial = .04, as reported in more detail by [Blinded for review]. Thus, all treatments appeared to reduce obsessive-compulsive symptoms, but the extent of improvement was no better in one treatment group than the other, nor was it influenced by ADHD symptoms at baseline.
4. Discussion
Results confirmed the hypothesis that ADHD-like inattention in children and adolescents with OCD is reduced among those who respond successfully to OCD treatment. The hypothesis that parent-rated ADHD symptoms would predict treatment outcome was unsupported. Children in the present study underwent an intervention that focused on obsessive-compulsive symptoms, and not secondary problems like inattention or hyperactivity. Thus, changes in secondary outcomes such as inattention might largely be attributed to the targeted OCD intervention. Further, treatment modality did not moderate this relationship (i.e., placebo and CBT-E/RP vs. SSRI and CBT-E/RP), indicating that this finding persists across psychological and multimodal forms of treatment. This result suggests that inattention symptoms observed in an assessment of a child with OCD may not indicate a comorbid ADHD diagnosis, but rather a downstream consequence of obsessional thinking. Our findings may reflect the recently proposed concept that clinical obsessions can overload the executive system, resulting in deficits like inattention (Abramovitch et al., 2012; Abramovitch et al., 2013) and extend this pattern of findings to a treatment-seeking group of children and adolescents, a group in which ADHD is especially likely to be diagnosed (Faraone, Biederman, & Mick, 2006). Results suggest that other ADHD symptoms such as impulsivity and hyperactivity, however, may not decrease following OCD treatment. That said, another interesting finding was that OCD severity, inattention, and hyperactivity-impulsivity were all correlated at the end of treatment, which will be discussed in more depth later in this section.
Depressive symptoms often remit following successful treatment of OCD (Anholt et al., 2011; Meyer et al., 2014), but the same has not been demonstrated with ADHD. These data indicate that inattentive-type ADHD symptoms decline with obsessive-compulsive symptoms as well. This notion may be somewhat counterintuitive to many mental health professionals, as ADHD is often thought of as representative of unchanging, inherent dysfunction in the brain. Practitioners and researchers alike may diagnose ADHD in children with OCD when they present with impairing levels of inattention. As this study and others report, this phenomenon may sometimes be a consequence of significant obsessive symptomology, rather than true ADHD pathology.
Results are also congruent with the recently proposed “executive overload” model of OCD (Abramovitch et al., 2012). Deficits in frontal/executive functions like attention, working memory, planning, and organization are commonly associated with ADHD, and are often thought to manifest as distractibility and inattention in children. That said, previous studies have failed to consistently find neurocognitive deficits that are hallmarks of ADHD such as inattention and executive dysfunction in children with OCD despite relatively consistent findings in the adult literature that point to the contrary (Abramovitch et al., 2015). Thus, it may be that the clinical features of ADHD, such as inattention during conversations or forgetfulness, appear before functional cognitive deficits are observed in lab-based tests of executive dysfunction among children and adolescents with OCD. Recently, researchers have characterized an “impulsive-compulsive” continuum of behavior (Hollander, 2005) and have used this concept to address the issue of executive dysfunction in both ADHD and OCD, considering that each disorder falls on opposite ends of this spectrum, resulting in similar cognitive deficits (Abramovitch, Dar, Mittelman, & Wilhelm, 2015). Frontostriatal dysfunction improves following successful psychotherapeutic treatment of adults with OCD (Freyer et al., 2011), and thus inattention may decrease as well. Future studies that incorporate functional measures of attention and neuroimaging with children and adolescents with OCD could investigate this hypothesis.
Clinicians may reflect on these findings when considering a diagnosis of ADHD in children with OCD. An incorrect diagnosis can result in unnecessarily placing children on psychostimulant medication, introducing the possibility of dependence and misuse (Wilens et al., 2008) and reduced desire to socially engage (Panksepp, 1998). Further, several cases studies have documented the exacerbation or even emergence of obsessive-compulsive symptoms following methylphenidate treatment (e.g., Coskun, 2011; Kouris, 1998). As noted by Abramovitch (2016), behavioral treatments for ADHD may also be contraindicated in many cases of OCD, as developing structured, compensatory strategies to manage inattention may resemble excessive or compulsive checking (e.g., setting up a calendar for school, organizing a backpack or notes at a child's desk). Misdiagnosis could also affect their attentional self-efficacy, putting them at risk for self-handicapping (i.e., considering that they will perform more poorly in academic and other cognitively-demanding areas of life due to some underlying attention disorder) and underachieving. Attention problems that are linked to OCD likely begin after obsessive-compulsive symptoms appear, and thus pinpointing whether inattention pre-dated obsessions and compulsions could improve diagnostic accuracy. When OCD symptomology clearly precedes attention problems, it may be useful to take a “response-to-intervention” approach to diagnosing ADHD in this population; when a child presents with a primary diagnosis of OCD and co-occurring inattention, it may be useful to initially target only obsessive-compulsive symptoms in treatment. If treatment is completed and successful, children may often also experience a clinically meaningful reduction in distractibility and attention problems. If inattentive ADHD symptoms remain despite an adequate trial of CBT-E/RP, SSRIs, or their combination, augmentation strategies such as ADHD-targeted behavioral management or psychostimulant treatment may be helpful. Assessing other ADHD symptoms may also be helpful in determining a diagnosis; if a child presents only with forgetfulness, distractibility, an inattentiveness, but does not appear overly impulsive or hyperactive, this may provide further evidence that ADHD-like symptoms are secondary to OCD pathology (Abramovitch, 2016). 2
As noted, however, some children do present with true ADHD and OCD, and elevated levels of these symptoms are especially likely among children with comorbid tic disorders (Mathews & Grados, 2011; Pinto et al., 2016). Even in these cases, however, children with comorbid OCD and tic disorders generally have a more severe presentation (Lebowitz et al., 2012) and have a stronger familial loading with each other than they do with ADHD (Pinto et al., 2016), which may in turn lead to more prevalent epiphenomena like inattention or executive dysfunction. In these more complex cases, a thorough assessment of the most concerning presenting problems should be conducted on an ongoing basis.
In contrast to previous studies, ADHD symptoms measured as a continuous variable at baseline did not predict treatment response (e.g., Geller et al., 2003; Storch et al., 2008). In the present study, the majority of children had subclinical ADHD symptoms at baseline, potentially limiting our ability to detect an effect of elevated ADHD symptomology on treatment response. In light of previous literature, this result may suggest that only severe levels of ADHD may impede treatment outcome. The present study used parent-reported ADHD symptoms, which are likely to capture inattention, distractibility, and other ADHD-like characteristics that are exacerbated by obsessive distress, rather than formally diagnosed ADHD. This methodological approach may have further contributed to the null results, as ADHD-like symptoms that are exacerbated by obsessive anxiety may not represent true ADHD-derived attentional dysfunction that would interfere with treatment. That said, psychologists, psychiatrists, and pediatricians often use parental impressions as a primary assessment tool when working with children with suspected ADHD.
Another surprising finding was that neither inattention nor hyperactivity/impulsivity symptoms were correlated with obsessive-compulsive symptom severity at baseline, but that all were significantly positively correlated at the end of treatment. One hypothesis for this result is that ADHD symptoms at baseline may be caused by obsessive overload on executive functioning, but they may also reflect other comorbidities common in pediatric OCD such as depression or disruptive behavior disorders (Geller, Biederman, Griffin, Jones, & Lefkowitz, 1996). At the end of treatment then, upon completion of an intensive multimodal outpatient treatment protocol, these more significantly impaired comorbid children who had poorer treatment outcomes were also the patients still exhibiting secondary symptoms such as inattention, impulsivity, etc. The finding that hyperactivity-impulsivity was correlated with obsessions and compulsions at the end of treatment comes in contrast with many studies that have shown that adults with OCD are generally less impulsive than healthy controls (Abramovitch & McKay, 2016). Children with OCD may present with different challenges, as agitation from chronic obsessional distress may contribute to related problems like emotional dysregulation (McGuire et al, 2013; McNamara et al., 2014) and oppositionality (Lebowitz, Omer, & Leckman, 2011) which can also contribute to secondary symptoms like hyperactivity and impulsivity. Indeed, children with OCD may be compulsive in some contexts (e.g., rigidly adhering to rituals) and impulsive in other contexts (e.g., difficulty waiting their turn). More research comparing children with OCD and children without OCD is needed to confirm and extend the current study finding.
Even though our pediatric sample findings are consistent with recent conclusions about the nature of ADHD symptoms in adults with OCD (Abramovitch et al., 2012; 2013), our results are preliminary in nature and limited by a small sample size and relatively few children diagnosed with ADHD per diagnostic interview (n = 9/50). Studying a larger number of children who meet diagnostic criteria for both conditions would allow us to draw stronger conclusions regarding changes in clinically meaningful attention problem in children with primary OCD. Children did not receive a diagnostic interview at the end of treatment, limiting our ability to test whether any of the children diagnosed with ADHD at the beginning of the study no longer met DSM-IV diagnostic criteria for the disorder. The study would have further benefitted from functional measures of attention, more comprehensive assessments of ADHD symptoms (e.g., a clinical interview at the end of treatment, other self-report measures), and more frequent measurements of ADHD symptoms throughout treatment in order to assess change more reliably.
Future research might replicate these findings with a larger sample using more comprehensive measures of ADHD. Cross-sectional studies comparing children with ADHD, children with OCD, and healthy controls on various measures of internalizing and externalizing symptoms could provide another avenue of inquiry to determine the reliability of ADHD diagnoses in children with OCD. Future research could also investigate whether the pattern of findings described in this study occur in children with other internalizing and obsessive-compulsive related disorders; it may be the case that ADHD-like inattention improves following successful treatment of generalized anxiety, depression, or other internalizing problems as well. Future studies may also investigate whether other externalizing problems decrease following successful OCD treatment, including both general oppositionality and OCD-specific defiance (e.g., demanding parents participate in rituals; Lebowitz, Omer, & Leckman, 2011). Finally, research should continue refining current first-line psychiatric assessment approaches, as relying too heavily on symptom checklists may be insufficient diagnostic tools.
5. Conclusions
Attention-deficit/hyperactivity disorder-like inattention and distractibility decline following successful OCD treatment, regardless of modality (i.e., CBT-E/RP alone or in combination with SSRIs). This is the first study to investigate whether ADHD-like problems are linked with obsessive-compulsive symptoms among children and adolescents with OCD, and echoes results found in the adult literature. Our findings suggest that attention problems are often tied to obsessions and compulsions in OCD; clinicians may wish to target obsessive-compulsive symptoms first when treating a child or adolescent with co-occurring OCD and clinically significant attention problems when OCD-onset clearly precedes ADHD-like inattention.
Highlights.
OCD treatment response corresponds with decreased ADHD-like inattention symptoms
Inattention may be tied to obsessions and compulsions in children and adolescents with OCD
ADHD symptoms measured continuously at baseline did not predict OCD treatment outcome
Acknowledgments
Role of Funding Sources: This research was supported by grant 5UO1 MH078594 from the NIMH (ClinicalTrials.gov Identifier: NCT00382291). Pfizer provided medication and placebo at no cost. The current manuscript does not necessarily reflect the official views of the National Institute of Mental Health or the National Institutes of Health. During the design of this study, the NIMH offered critiques to improve the study methodology, and monitored the progress of the trial. Neither the NIMH nor Pfizer contributed to the analyses or implications of the current study. Pfizer was annually notified about the progress of the study but was not involved in the design or implementation of any part of the study.
Dr. Storch receives grant support from the National Institutes of Health, the Fulbright Scholar Program, the Agency for Healthcare Research & Quality, and All Children's Hospital. He is also a consultant for Ruijin Hospital.
Dr. Bussing has received research support from the National Institutes of Health and Pfizer.
Dr. Murphy discloses research support from AstraZeneca Neuroscience (iMED), Otsuka Pharmaceuticals, Shire Pharmaceuticals, Roche Pharmaceuticals, Sunovion Pharmaceuticals, and Pfizer.
Dr. Wayne Goodman has participated as a site PI and/or co-I for several NIH sponsored trials, including the R01 that funded this trial.
This study was a secondary analysis from a randomized control trial funded by 5UO1 MH078594 from the NIMH (ClinicalTrials.gov Identifier: NCT00382291).
Footnotes
Due to a pharmacy error during the trial, 9 of 56 families enrolled in the study were excluded in analyses by Storch et al., 2013. The present analysis used all families with data available at the beginning of the trial and at their final session of treatment, which included the 9 affected by the pharmacy error, resulting in a final sample size of 50 child-parent dyads.
For a more thorough discussion of the assessment and treatment of co-occurring OCD and attention problems, please refer to Abramovitch, 2016.
Contributors: Mr. Guzick generated hypotheses for the present study, formulated the plan of analysis, and wrote the initial draft of the manuscript. Dr. McNamara assisted with conceptualizing the study and interpreting the results. Dr. Reid contributed to data management of the trial and assisted in study conceptualization and statistical analyses. Ms. Balkhi also assisted in study conceptualization and analyses. Dr. Bussing assisted with hypothesis formulation and design of the current study. Dr. Geffken also helped conceptualize the current study, including the hypotheses, analyses, and implications. Drs. Storch, Bussing, Murphy, and Goodman designed the randomized controlled trial, acquired and managed grant funding, and oversaw data collection. All authors reviewed and revised the manuscript and approved the final version.
Conflict of Interest: All other authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramovitch A. Misdiagnosis of ADHD in individuals diagnosed with obsessive-compulsive disorder: Guidelines for practitioners. Current Treatment Options in Psychiatry. 2016;3(3):225–234. [Google Scholar]
- Abramovitch A, Abramowitz JS, Mittelman A, Stark A, Ramsey K, Geller DA. Research Review: Neuropsychological test performance in pediatric obsessive– compulsive disorder–a meta-analysis. Journal of Child Psychology and Psychiatry. 2015 doi: 10.1111/jcpp.12414. [DOI] [PubMed] [Google Scholar]
- Abramovitch A, Dar R, Hermesh H, Schweiger A. Comparative neuropsychology of adult obsessive-compulsive disorder and attention deficit/hyperactivity disorder: Implications for a novel executive overload model of OCD. Journal of Neuropsychology. 2012;6(2):161–191. doi: 10.1111/j.1748-6653.2011.02021.x. [DOI] [PubMed] [Google Scholar]
- Abramovitch A, Dar R, Mittelman A, Schweiger A. Don't judge a book by its cover: ADHD-like symptoms in obsessive compulsive disorder. Journal of Obsessive-Compulsive and Related Disorders. 2013;2(1):53–61. [Google Scholar]
- Abramovitch A, Dar R, Mittelman A, Wilhelm S. Comorbidity between attention deficit/hyperactivity disorder and obsessive-compulsive disorder across the lifespan: a systematic and critical review. Harvard Review of Psychiatry. 2015;23(4):245. doi: 10.1097/HRP.0000000000000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramovitch A, McKay D. Behavioral Impulsivity in Obsessive–Compulsive Disorder. Journal of Behavioral Addictions. 2016;5(1):1–3. doi: 10.1556/2006.5.2016.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholt GE, Aderka IM, van Balkom AJ, Smit JH, Hermesh H, de Haan E, van Oppen P. The impact of depression on the treatment of obsessive–compulsive disorder: results from a 5-year follow-up. Journal of Affective Disorders. 2011;135(1):201–207. doi: 10.1016/j.jad.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Asherson P, Trzaskowski M. Attention-deficit/hyperactivity disorder is the extreme and impairing tail of a continuum. Journal of the American Academy of Child and Adolescent Psychiatry. 2015;54249(4) doi: 10.1016/j.jaac.2015.01.014. [DOI] [PubMed] [Google Scholar]
- Bussing R, Fernandez M, Harwood M, Hou W, Garvan CW, Eyberg SM, Swanson JM. Parent and teacher SNAP-IV ratings of attention deficit hyperactivity disorder symptoms psychometric properties and normative ratings from a school district sample. Assessment. 2008;15(3):317–328. doi: 10.1177/1073191107313888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale, New Jersey: Erlbaum; 1988. [Google Scholar]
- Coşkun M. Methylphenidate induced obsessive-compulsive symptoms treated with sertraline. Bulletin of Clinical Psychopharmacology. 2011;21(3):274–274. [Google Scholar]
- Faraone SV, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychological Medicine. 2006;36(02):159–165. doi: 10.1017/S003329170500471X. [DOI] [PubMed] [Google Scholar]
- Freeman J, Garcia A, Frank H, Benito K, Conelea C, Walther M, Edmunds J. Evidence base update for psychosocial treatments for pediatric obsessive-compulsive disorder. Journal of Clinical Child & Adolescent Psychology. 2014;43(1):7–26. doi: 10.1080/15374416.2013.804386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyer T, Kloppel S, Tuscher O, Kordon A, Zurowski B, Kuelz A, et al. Voderholzer U. Frontostriatal activation in patients with obsessive-compulsive disorder before and after cognitive behavioral therapy. Psychological Medicine. 2011;41(1):207–216. doi: 10.1017/S0033291710000309. [DOI] [PubMed] [Google Scholar]
- Garcia AM, Sapyta JJ, Moore PS, Freeman JB, Franklin ME, March JS, Foa EB. Predictors and Moderators of Treatment Outcome in the Pediatric Obsessive Compulsive Treatment Study (POTS I) J Am Acad Child Adolesc Psychiatry. 2010;49(10):1024–1033. doi: 10.1016/j.jaac.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Faraone SV, Cradock K, Hagermoser L, Zaman N, et al. Spencer TJ. Attention-deficit/hyperactivity disorder in children and adolescents with obsessive-compulsive disorder: fact or artifact? Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41(1):52–58. doi: 10.1097/00004583-200201000-00011. [DOI] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Griffin S, Jones J, Lefkowitz TR. Comorbidity of juvenile obsessive-compulsive disorder with disruptive behavior disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35(12):1637–1646. doi: 10.1097/00004583-199612000-00016. [DOI] [PubMed] [Google Scholar]
- Geller DA, Coffey B, Faraone S, Hagermoser L, Zaman NK, Farrell CL, et al. Biederman J. Does comorbid attention-deficit/hyperactivity disorder impact the clinical expression of pediatric obsessive-compulsive disorder? CNS Spectrums. 2003;8(4):259–264. doi: 10.1017/s1092852900018472. [DOI] [PubMed] [Google Scholar]
- Geller DA, March J. Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(1):98–113. doi: 10.1016/j.jaac.2011.09.019. [DOI] [PubMed] [Google Scholar]
- Geller D, Petty C, Vivas F, Johnson J, Pauls D, Biederman J. Examining the relationship between obsessive-compulsive disorder and attention-deficit/hyperactivity disorder in children and adolescents: a familial risk analysis. Biological Psychiatry. 2007a;61(3):316–321. doi: 10.1016/j.biopsych.2006.03.083. [DOI] [PubMed] [Google Scholar]
- Geller D, Petty C, Vivas F, Johnson J, Pauls D, Biederman J. Further evidence for co-segregation between pediatric obsessive compulsive disorder and attention deficit hyperactivity disorder: a familial risk analysis. Biological Psychiatry. 2007b;61(12):1388–1394. doi: 10.1016/j.biopsych.2006.09.026. [DOI] [PubMed] [Google Scholar]
- Hollander E. Obsessive–compulsive disorder and spectrum across the life span. International Journal of Psychiatry in Clinical Practice. 2005;9(2):79–86. doi: 10.1080/13651500510018347. [DOI] [PubMed] [Google Scholar]
- Ivarsson T, Melin K, Wallin L. Categorical and dimensional aspects of co-morbidity in obsessive-compulsive disorder (OCD) European Child & Adolescent Psychiatry. 2008;17(1):20–31. doi: 10.1007/s00787-007-0626-z. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Kiddie-Sads-present and Lifetime version (K-SADS-PL) Pittsburgh: University of Pittsburgh, School of Medicine; 1997. [Google Scholar]
- Lebowitz ER, Motlagh MG, Katsovich L, King RA, Lombroso PJ, Grantz H, et al. Coffey BJ. Tourette syndrome in youth with and without obsessive compulsive disorder and attention deficit hyperactivity disorder. European Child & Adolescent Psychiatry. 2012;21(8):451–457. doi: 10.1007/s00787-012-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz ER, Omer H, Leckman JF. Coercive and disruptive behaviors in pediatric obsessive-compulsive disorder. Depression and Anxiety. 2011;28(10):899–905. doi: 10.1002/da.20858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouris S. Methylphenidate-induced obsessive-compulsiveness. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37(2):135. doi: 10.1097/00004583-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Masi G, Millepiedi S, Mucci M, Bertini N, Pfanner C, Arcangeli F. Comorbidity of obsessive-compulsive disorder and attention-deficit/hyperactivity disorder in referred children and adolescents. Comprehensive Psychiatry. 2006;47(1):42–47. doi: 10.1016/j.comppsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Mathews CA, Grados MA. Familiality of Tourette syndrome, obsessive-compulsive disorder, and attention-deficit/hyperactivity disorder: heritability analysis in a large sib-pair sample. Journal of the American Academy of Child & Adolescent Psychiatry. 2011;50(1):46–54. doi: 10.1016/j.jaac.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire JF, Small BJ, Lewin AB, Murphy TK, De Nadai AS, Phares V, et al. Storch EA. Dysregulation in pediatric obsessive compulsive disorder. Psychiatry Research. 2013;209(3):589–595. doi: 10.1016/j.psychres.2013.04.003. [DOI] [PubMed] [Google Scholar]
- McNamara JP, Reid AM, Balkhi AM, Bussing R, Storch EA, Murphy TK, et al. Geffken GR. Self-regulation and other executive functions relationship to pediatric OCD severity and treatment outcome. Journal of Psychopathology and Behavioral Assessment. 2014;36(3):432–442. [Google Scholar]
- Meyer JM, Mcnamara JPH, Reid AM, Storch EA, Geffken GR, Bussing R, et al. Prospective relationship between obsessive-compulsive and depressive symptoms during multimodal treatment in pediatric obsessive-compulsive disorder. Child Psychiatry and Human Development. 2014;45(2):163–72. doi: 10.1007/s10578-013-0388-4. [DOI] [PubMed] [Google Scholar]
- Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, Rubia K. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: A comparative meta-analysis. JAMA Psychiatry. 73:E1–E11. doi: 10.1001/jamapsychiatry.2016.0700. in press. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Attention deficit hyperactivity disorders, psychostimulants, and intolerance of childhood playfulness: A tragedy in the making? Current Directions in Psychological Science. 1998;7(3):91–98. [Google Scholar]
- Pinto R, Monzani B, Leckman JF, Rück C, Serlachius E, Lichtenstein P, Mataix-Cols D. Understanding the covariation of tics, attention-deficit/hyperactivity, and obsessive-compulsive symptoms: A population-based adult twin study. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2016 doi: 10.1002/ajmg.b.32436. [DOI] [PubMed] [Google Scholar]
- Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, et al. Leckman JF. Children's Yale-Brown obsessive compulsive scale: reliability and validity. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(6):844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- Sheppard B, Chavira D, Azzam A, Grados MA, Umaña P, Garrido H, Mathews CA. ADHD prevalence and association with hoarding behaviors in childhood-onset OCD. Depression and Anxiety. 2010;27(7):667–674. doi: 10.1002/da.20691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Lewin AB, De Nadai AS, Murphy TK. Defining treatment response and remission in obsessive-compulsive disorder: a signal detection analysis of the children's yale-brown obsessive compulsive scale. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(7):708–717. doi: 10.1016/j.jaac.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Larson MJ, Geffken GR, Lehmkuhl HD, Jacob ML, et al. Goodman WK. Impact of comorbidity on cognitive-behavioral therapy response in pediatric obsessive-compulsive disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(5):583–592. doi: 10.1097/CHI.0b013e31816774b1. [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Geffken GR, Soto O, Sajid M, Allen P, et al. Goodman WK. Psychometric evaluation of the Children's Yale–Brown Obsessive-Compulsive Scale. Psychiatry Research. 2004;129(1):91–98. doi: 10.1016/j.psychres.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Swanson J, Nolan W, Pelham WE. The SNAP-IV rating scale. Irvine, CA: University of California at Irvine; 1992. [Google Scholar]
- Walitza S, Zellmann H, Irblich B, Lange KW, Tucha O, Hemminger U, et al. Warnke A. Children and adolescents with obsessive-compulsive disorder and comorbid attention-deficit/hyperactivity disorder: preliminary results of a prospective follow-up study. Journal of Neural Transmission. 2008;115(2):187–190. doi: 10.1007/s00702-007-0841-2. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, et al. Fusillo S. Misuse and diversion of stimulants prescribed for ADHD: A systematic review of the literature. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(1):21–31. doi: 10.1097/chi.0b013e31815a56f1. [DOI] [PubMed] [Google Scholar]