Abstract
The recognition of Cerebrovascular Disease (CVD) at earlier clinical stages may favor the control of vascular risk factors and prevention of dementia. However, operational criteria for symptomatic phases at non-dementia stages are often difficult, as the current criteria normally require the evidence of extensive subcortical disease.
Objective
To identify the neuroimaging profile of Vascular Mild Cognitive Impairment (VaMCI), the impact of those aspects over cognition and the neuropsychological tests that distinguished VaMCI from other groups.
Methods
Searches were performed in Scopus, ISI and PsycINFO, using the following key terms: "vascular mild cognitive impairment" OR "vascular cognitive impairment no dementia" OR "vascular cognitive impairment not demented" OR "subcortical mild cognitive impairment".
Results
Of 249 papers, 20 studies were selected. Ten of those included only patients with severe White Matter Hyperintensities (WMH), whereas 10 others admitted subjects with moderate-to-severe WMH. Both groups showed poor performances in Executive Function (EF) tasks in comparison to normal controls and other diagnostic groups. Among EF tests, those assessing "complex" EF abilities consistently distinguished VaMCI from other groups, regardless of the severity of WMH. VaMCI subjects with severe or moderate-to-severe WMH showed cognitive deficits in comparison with other groups. "Complex" EF tests were the most useful in differentiating those patients from the other groups.
Conclusion
The occurrence of VaMCI may be associated with the presence of CVD at moderate levels; the detection of vascular damage at earlier stages may allow the adoption of therapeutic actions with significant effect-sizes.
Keywords: cerebrovascular disorders, vascular dementia, cerebral infarction, neurological diagnostic techniques
Abstract
O reconhecimento precoce da Doença Cerebrovascular (DCV) pode permitir o controle de fatores de risco e a prevenção de demência. Contudo, critérios operacionais em seus estágios sintomáticos não-demenciais apresentam problemas, já que critérios atuais requerem a presença de extensa doença isquêmica subcortical.
Objetivo
Identificar o perfil de neuroimagem do Comprometimento Cognitivo Leve Vascular (CCLV), o impacto destes aspectos sobre a cognição e os testes neuropsicológicos que distinguem CCLV de outros grupos.
Métodos
Foram realizadas buscas no Scopus, ISI e PsycINFO, usando a estratégia: "vascular mild cognitive impairment" OR "vascular cognitive impairment no dementia" OR "vascular cognitive impairment not demented" OR "subcortical mild cognitive impairment".
Resultados
De 249 artigos, 20 foram selecionados. 10 destes incluíram apenas pacientes com hiperintensidades de substância branca (HSB) graves, enquanto 10 outros admitiram pacientes com HSB moderadas-a-graves. Ambos os grupos apresentaram desempenho pobre em tarefas de Função Executiva (FE) em comparação com controles normais e outras categorias diagnósticas. Dentre os testes de FE, aqueles que avaliam FE "complexas" diferiram consistentemente CCLV de outros grupos, independentemente da gravidade de HSB. Sujeitos com CCLV e HSB graves ou moderadas-a-graves apresentaram dificuldades cognitivas quando comparados aos demais grupos. Testes que avaliam FE "complexa" foram os mais úteis na diferenciação destes pacientes dos outros grupos.
Conclusão
A ocorrência de VaMCI pode estar associada à presença de HSB moderadas; a detecção precoce do dano vascular permitiria a adoção de medidas terapêuticas com tamanhos de efeito significativos.
Keywords: transtornos cerebrovasculares, demência vascular, infarto cerebral, técnicas de diagnóstico neurológico
INTRODUCTION
Vascular Cognitive Impairment (VCI) is an umbrella concept which comprises a continuum of vascular-related cognitive impairment, from high-risk preclinical conditions ("brain-at-risk") to Vascular Dementia (VaD). Intermediate stages are commonly referred as Vascular Mild Cognitive Impairment (VaMCI) or Vascular Cognitive Impairment No-Dementia (Va-CIND).1 Recent operational criteria, such as the 2011 American Heart Association (AHS)/American Stroke Association (ASA) scientific statement on vascular contributions to cognitive impairment, suggested that the relationship between CVD and cognitive changes could be characterized whether through the evidence of cognitive deficits succeeding a clinical stroke or through identifying vascular lesions on neuroimaging deemed severe enough to explain the cognitive impairment.2
More detailed neuroimaging criteria have been described in the 2014 International Society for Vascular Behavioral and Cognitive Disorders (VASCOG) statement for diagnosis of Vascular Cognitive Disorders (VCD). In this document, CVD was evidenced by the presence of one of the following changes:
[1] extensive and confluent subcortical White Matter Hyperintensities (WMH);
[2] large-vessel infarcts: 1 (for Mild VCD) or ≥2 (for Major VCD);
[3] 1 strategically placed infarct (in the thalamus or basal ganglia);
[4] >2 lacunar infarcts outside the brainstem or at least 1 lacune combined with extensive WMH; and
(5) intracerebral hemorrhages: ≥2 or 1 strategically placed.3
The VASCOG statement represented a more comprehensive neuroimaging criterion in comparison to the AHA/ASA recommendations and a substantial change in relation to the Erkinjuntti's neuroimaging criteria for Subcortical Ischemic VaD (2000), in which extensive and confluent WMH or moderate WMH combined with at least 5 lacunes was required to characterize CVD.4 Nonetheless, the persistence in the new criteria of the need for extensive and confluent WMH contrasted with some studies, which have suggested that moderate WMH with less than 5 lacunes could account for cognitive impairments.5 As indicated by several studies, mild WMH is highly prevalent among normal elderly individuals and has not been significantly associated with cognitive changes.6
One possible advantage in identifying CVD in its mildest clinical (VaMCI) and neuroimaging (moderate subcortical WMH and less than 5 lacunes) stages is the fact that progression of vascular damage might be preventable. Early detection might allow the adoption of disease-modifying therapies that could prevent the progression of vascular lesions; therefore, it might interrupt the advance of cognitive impairment that could result in VaD. Finally, recent diagnostic criteria for Va-CIND overlap with the ASA/AHA criteria for VaMCI,7 thus the term VaMCI has been used in this review to refer to both constructs.
According to the above pondering, a systematic review was undertaken aiming:
[1] to assess the neuroimaging profile of individuals classified as VaMCI in clinical studies;
[2] to determine whether different neuroimaging criteria impact over cognitive findings, and
[3] to identify neuropsychological tests that could distinguish VaMCI from normal controls or other diagnostic groups across studies using different criteria for CVD. The authors hypothesized that the choice of establishing the threshold of brain vascular lesions into moderate or severe stages of WMH may account for divergent cognitive findings among studies.
METHODS
Data search and selection. Studies were found through searches in Scopus, ISI Web Of Knowledge and PsycINFO, using the following key terms, in all fields and published in any date: "vascular mild cognitive impairment" OR "vascular cognitive impairment no dementia" OR "vascular cognitive impairment not demented" OR "subcortical mild cognitive impairment". This search strategy was augmented with hand searches of reference lists of included studies. More articles were obtained from directly contacting authors for relevant papers.
After the searches were performed, articles were included if they were: clinical studies, which included neuroimaging data from individuals with VaMCI; that compared cognitive performances between VaMCI and other diagnostic groups [VaD, AD, non-vascular MCI (non-VaMCI)] or normal controls; and written in English, French, Spanish or Portuguese.
The authors have excluded studies that: classified individuals as VaMCI based solely on clinical/ neuropsychological aspects (e.g., studies in which the cognitive deficits were judged to have vascular cause through clinical features, such as stepwise progression, sudden onset, gait disturbances, focal neurological signs or those that applied only an ischemic score to identify the presence of cerebrovascular disease); did not assess subjects with MCI, defined as those presenting cognitive impairments that do not fulfill criteria for dementia; did not acknowledge a detailed neuroimaging criterion for the diagnosis of VaMCI (e.g., cognitive impairment considered associated with vascular lesions through subjective evaluation from an expert); did not compare cognitive performances between VaMCI and controls or other diagnostic groups; or included subjects with cortical infarction or cortical atrophy suggestive of large-vessel or neurodegenerative diseases. The current study followed the standard protocols of PRISMA statement.8
Data extraction. Data were extracted from full-texts by one author (FKS) and reviewed by a second author (EE). Divergences were furtherly discussed among the entire team of authors.
RESULTS
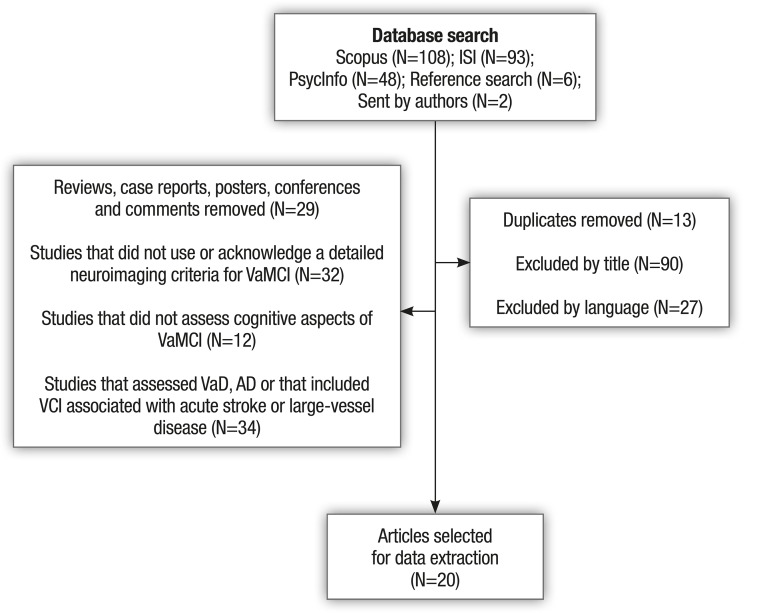
Of a total of 249 retrieved papers, 20 studies were selected for data extraction. Figure 1 summarizes the stages of data search and selection.
Figure 1.
Flow-chart describing the process of study selection.
Clinical criteria for MCI. Participants in the studies presented objective cognitive deficits and preserved functional status. Mild differences included articles that identified those with cognitive impairments based on performances in screening tests for cognitive deficits (e.g., MMSE ≥ 24, CDR= 0.5, Clock Drawing Test scores lower than 2/6).9-14 Cognitive impairment was defined as performances 1 to 2 standards deviations (between the 16th and the 2nd percentile) below mean normative values, in some studies.15-21 Few studies, all of them prior to 2009, required impairment in memory for diagnosis of MCI;17,22,23 however, most papers did not include any specific cognitive domain or proposed dysexecutive symptoms as typically associated with VaMCI.
Neuroimaging criteria for subcortical vascular disease. Ten of the studies classified subcortical CVD as the presence of white-matter changes compatible with severe WMH and/or at least 5 subcortical lacunes. Five of those followed the criteria proposed by Erkinjuntti et al. (2000) for Binswanger's Disease, which requires the presence of severe WMH, periventricular lesions larger than 10 mm and deep WMH equal or over 25 mm of diameter.11,15,16,24,25 A modified version of the Computerized Tomography (CT) criterion for Subcortical Vascular Dementia proposed by Erkinjuntti et al. (2000) was applied in two of the studies. CVD, in those cases, was represented by patchy or diffuse leukoaraiosis and at least one lacunar infarct on neuroimaging.9,22 Evidence of extensive WMH, defined as lesions larger than 3 mm of diameter in the semioval center and larger than 5 mm in the deep gray nuclei, was the criterion used in one study.26 Other methods for identification of individuals with severe WMH included semiautomatic white-matter volumetry techniques. Nordahl et al. (2005) classified individuals with WMH extending for more than 19.375% of total white-matter volume as presenting severe WMH.23 Moretti et al. (2008) computed the presence of CVD by counting voxels corresponding to WMH and identifying those individuals whose lesions corresponded to values over the fourth quartile of volume damage.10 Table 1 illustrates those findings.
Table 1.
Studies that included severe (largely confluent) WMH and/or at least 5 lacunes for diagnosis of SVD.
| Author, year | N | Groups | Clinical criteria for MCI | Neuroimaging criteria for SVD | Neuropsychological tests |
|---|---|---|---|---|---|
| Frisoni et al., 2002 | 64 | VaMCI, VaD, non-VaMCI | Dysexecutive syndrome + memory impairment + unimpaired complex ADL | Patchy WMH or diffuse symmetrical WMH + 1 lacunar infact | WCST, Category fluency, Letter fluency, Token test, Corsi test, Digit span, Prose recall |
| Galluzzi et al., 2005 | 43 | VaMCI, non-VaMCI | MMSE ≥ 24, CDR=0.5 | Patchy WMH or diffuse symmetrical WMH + 1 lacunar infact | WCST, Category fluency, Letter fluency, Corsi test, Digit Span, Prose recall |
| Nordahl et al., 2005 | 42 | NC, VaMCI, non-VaMCI | Memory complaints, poor perfomances in Memory tasks, preserved global cognitive performances, unimpaired ADL | WMH extension above the 75th percentile (WMH above 19.375% of total white matter volume) | MMSE, Wechsler Memory Scale Revised, Memory Assessment Scales List Learning, BNT, Block design, Digit Span, Category fluency |
| Shim et al., 2008 | 57 | NC, VaMCI, non-VaMCI | Objectively measured cognitive decline + unimpaired ADL | Severe WMH, periventricular WMH > 10mm, deep WMH ≥ 25 mm | MMSE, 12-word list from HVLT, Digit span, Rey-Osterrieth Complex Figure Test, BNT, Stroop, Category fluency, Letter Fluency, Go-No Go, Luria Loop test |
| Moretti et al., 2008 | 116 | VaMCI, atrophic MCI, “cholinergic” MCI | Cognitive complaints + MMSE between 24 and 27, or (MMSE of 28 or higher + Clock Drawing Test of 2/6 or worse) + unimpaired ADL | Number of voxels corresponding to WMH above the upper quartile | Rey word list immediate and delayed recall, Trail Making Test A, B and B-A, Clock Drawing Test, Raven matrices, Inverted motor learning, Rey-Osterrieth Complex Figure Test, Category fluency, Letter Fluency, Token test |
| Fernández et al., 2011 | 53 | NC, VaMCI, non-VaMCI | Petersen (2001), Frisoni (2002) | Extensive WMH (WMH>3 in semiovale nuclei or >5 mm in deep grey nuclei) or diffuse symmetrical WMH + 1 lacunar infact | MMSE, CERAD (Category fluency, BNT, Word list memory test, constructional praxis, TMT A and B), Digit Span, Abstraction, Letter fluency |
| Bella et al., 2011 | 20 | NC, VaMCI | Not demented (DSM-IV), MMSE ≥ 24 | Severe WMH, periventricular WMH > 10 mm, deep WMH ≥ 25 mm | MMSE, Stroop |
| Kim et al., 2012 | 48 | VaMCI, VaD | Subjective cognitive complaints; objective cognitive decline below the 1 SD on neuropsychological tests; normal general cognitive function; normal ADL; not demented | Severe WMH, periventricular WMH > 10 mm, deep WMH ≥ 25 mm | MMSE, Digit span, Rey-Osterrieth Complex Figure Test, Seoul Verbal Learning Test, Controlled Oral Word Association Test, Stroop |
| Lee et al., 2014 | 207 | VaMCI, non-VaMCI | Subjective cognitive complaints, normal ADL, cognitive performance < 16th percentile on tests, absence of dementia, focal neurological symptoms/signs | Severe WMH, periventricular WMH > 10 mm, deep WMH ≥ 25 mm | MMSE, Digit span, Rey-Osterrieth Complex Figure Test, Seoul Verbal Learning Test, Controlled Oral Word Association Test, Stroop |
| Sheorajpanday et al., 2014 | 57 | VaMCI, non-VaMCI | First presentation of cognitive decline, age ≥ 55 years, intact ADL, not VaD (NINDS-AIREN), presumed vascular cause | Severe WMH, periventricular WMH > 10 mm, deep WMH ≥ 25 mm | MMSE, Wechsler Memory Scale III, Wechsler Adult Intelligence Scale III, TMT A and B, Rey-Osterrieth Complex Figure Test, Digit spam, Category fluency, Letter fluency, Raven matrices |
Moderate WMH and/or less than 5 lacunes were deemed sufficient to characterize CVD in ten of the studies. Overall, individuals that scored 2 or more in the modified-Fazekas Scale, corresponding to the presence of moderate periventricular WMH ("smooth halo") with beginning confluent deep WMH, were selected for those studies. Identification of at least 2 lacunar infarcts was an alternative criterion for diagnosis of moderately severe cerebrovascular disease. Table 2 depicts those results.
Table 2.
Studies that included moderate (beginning confluent; smooth halo) WMH and/or less than 5 lacunes required for diagnosis of SVD.
| Author, year | N | Groups | Clinical criteria for MCI | Neuroimaging criteria for SVD | Neuropsychological tests |
|---|---|---|---|---|---|
| Norlund et al., 2007 | 180 | NC, VaMCI, non-VaMCI | Subjective and objective cognitive impairment for more than 6 months not demented. | Moderate WMH or 2 or more lacunes | Visual Object and Space Perception, Assessment of Subtle Language Deficits, Parallel Serial Mental Operations, TMT |
| Gainotti et al., 2008 | 142 | NC, VaMCI, non-VaMCI | Long-term Memory performance < 2 scores from cutoff, no cognitive impairment in non-memory domains, preserved functional status | 2 or more subcortical infarcts (below 2 cm of size) or 1 subcortical infact + periventricularWMH of any size | RAVLT, Rey-Osterreith Complex Figure, Digit and Spatial Span, phonological and categorical verbal fluency, Raven's Standard Progressive Matrices, Multiple Features Targets Cancellation, Vill's test for temporal rule induction, Stroop interference test |
| Zhou et al., 2009a | 156 | NC, VaMCI, non-VaMCI | Cognitive impairment + CDR=0.5 + unimpaired ADL | Wahlund scale ≥ 2 or more than 2 lacunes | MMSE, Digit Span Backwards and Forward, WHO-UCLA AVLT, Rey-Osterreith Complex Figure, Stroop, Semantic Verbal Fluency, WAIS-RC, California Card Sorting Test, CDT |
| Zhou et al., 2009b | 160 | NC, VaMCI | Cognitive impairment + CDR=0.5 + unimpaired ADL | Wahlund scale ≥ 2 or more than 2 lacunes | MMSE, Digit Span Backwards and Forward, WHO-UCLA AVLT, Rey-Osterreith Complex Figure, Stroop, Semantic Verbal Fluency, WAIS-RC, California Card Sorting Test, CDT |
| Norlund et al., 2011 | 216 | VaMCI, non-VaMCI, VaMCI and non-VaMCI with and without biological markers | Cognitive complaint+ objective cognitive decline + not demented + unimpaired ADL | Moderate WMH or 2 or more lacunes | Digit Symbol, TMT, Digit Span, RAVLT, Wechsler's Logical Memory, Rey-Osterreith Complex Figure, Visual Object and Space Perception, Block Design, Token Test, Boston Naming, Semantic Verbal Fluency, Parallel Serial Mental Operations, Dual Task, Stroop, Wisconsin Card Sorting Test, Cognitive Estimation Test |
| Marra et al., 2011 | 135 | VaMCI, non-VaMCI | subjective and objective cognitive deficits (worse than 1.67 SD from normal values) and normal functional status | Fazekas ≥ 2 or more than 3 lacunes; or Periventricular WMH grade 1 + 2 or more lacunes | Rey's Auditory Verbal Learning Task, Rey-Osterrieth complex figure, Stroop, Multiple Features target cancellation, Phonological and Semantic Verbal Fluency, Raven's Progressive Matrices |
| Villeneuve et al., 2011 | 72 | NC, MCI with WMH and MCI without WMH | Subjective cognitive complaint + cognitive performance 1.5 SD below normative values + preserved ADL | Wahlund ≥ 2 | Mémoria computerized battery, BEM-144, RL/RI word recall Task, Rey-Osterrieth complex figure, Stroop, WAIS-III, Boston Naming Test, Benton judgment of line orientation test |
| Yi et al., 2012 | 54 | NC, VaMCI | Subjective cognitive complaints, objective cognitive impairments, not demented (DSM-IV), normal ou near-normal functionbal status, CDR= 0.5, MMSE≥ 24. | Moderate to severe WMH in at least 1 region with a Wahlund rating scale score ≥2 and/or multiple pericentricular and deep lacunes | MMSE, AVLT |
| Sudo et al., 2013 | 36 | NC, VaMCI | Impairment of 1.5 SD below the mean on 1 or more cognitive tests in relation to normative values, preserved or mildly impaired functional activities, (FAQ <5) | Moderate or severe degree of WMH on Fazekas scale and hippocampal atrophy ≤1 on de Leon score (none or questionable atrophy) | MMSE, CAMCOG, CDT, TMT, Semantic Verbal Fluency, Boston Naming Test |
| Brookes et al., 2015 | 503 | VaMCI, CVD without cognitive impairment | scoring ≤1.5 SD of the normal mean on a given test | lacunar infarcts or lacunar infarcts with leukoaraiosis (Fazekas≥2) | Brief Memory and Executive Test (BMET), MMSE, MoCA |
Cognitive performances and neuroimaging criteria. Although the choice of neuropsychological tests varied across studies, cognitive assessment in most cases included tasks that measured executive function (EF), memory, language and visuospatial/ visuoconstructive abilities. Table 3 summarizes the main affected cognitive abilities in the selected studies. EF has been divided into 3 components, following studies that performed a latent variable approach of multiple EF measures: "shifting" (switching between tasks), "inhibition" (deliberate overriding of prepotent responses) and "working memory/updating" (monitoring and rapidly changing new contents).27 Tests categorized as "less specific EF tests" included tasks that assessed multiple EF dimensions (e.g., Clock Drawing Test, Verbal Fluency etc.), instead of measuring one single aspect of it.28 Matching between neuropsychological tests and cognitive domains was made in accordance with evidences in the literature.21,28-40 Table 4 summarizes the correspondence between cognitive domains and neuropsychological tests used in the studies.
Table 3.
Summary of cognitive findings in the selected studies according with the neuroimaging criteria for CVD.
| Criteria for CVD | Articles | Affected cognitive functions in studies | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Shifting | Inhibition | Working memory/ updating | Less specific EF tasks | Visuospatial / Visuoconstructive abilities | Memory | Language | Global cognition | ||
| Severe WMH and/or or ≥ 5 lacunes | Frisoni et al., 2002 | VaMCI≠VaD** | Non-VaMCI≠VaMCI* VaMCI≠VaD** | n.s. | Non-VaMCI≠VaMCI* | - | n.s. | n.s. | VaMCI≠VaD** |
| Galluzzi et al., 2005 | n.s. | Non-VaMCI≠VaMCI* | n.s. | Non-VaMCI≠VaMCI* | - | n.s. | - | n.s. | |
| Nordahl et al., 2005 | - | - | n.s. | NC≠VaMCI** | NC≠VaMCI** Non-VaMCI≠VaMCI** | NC≠VaMCI** NC≠non-VaMCI** | n.s. | NC≠VaMCI** NC≠non-VaMCI** | |
| Shim et al., 2008 | - | n.s. | n.s. | Non-VaMCI≠VaMCI* | Non-VaMCI≠VaMCI** | Non-VaMCI≠VaMCI* | Non-VaMCI≠VaMCI* | n.s. | |
| Moretti et al., 2008 | Non-VaMCI≠VaMCI* | - | - | n.s. | Non-VaMCI≠VaMCI* | Non-VaMCI≠VaMCI* | n.s. | - | |
| Fernández et al., 2011 | NC≠VaMCI* | - | n.s. | NC≠VaMCI* NC≠non-VaMCI* | n.s. | NC≠VaMCI* NC≠non-VaMCI* | n.s. | NC≠VaMCI* NC≠non-VaMCI* | |
| Bella et al. 2011 | - | NC≠VaMCI* | - | - | - | - | - | n.s. | |
| Kim et al., 2012 | - | n.s. | VaMCI≠VaD** | VaMCI≠VaD** | VaMCI≠VaD** | VaMCI≠VaD** | VaMCI≠VaD** | VaMCI≠VaD** | |
| Lee et al., 2014 | - | n.s. | n.s. | n.s. | n.s. | Non-VaMCI≠VaMCI** | n.s. | Non-VaMCI≠VaMCI* | |
| Sheorajpanday et al., 2014 | n.s. | - | Non-VaMCI≠VaMCI** | Non-VaMCI≠VaMCI** | n.s. | n.s. | n.s. | n.s. | |
| Moderate or severe WMH and/or <5 lacunes | Norlund et al., 2007 | NC≠VaMCI** Non-VaMCI≠VaMCI* | NC≠VaMCI* | NC≠VaMCI** Non-VaMCI≠VaMCI* | NC≠VaMCI** | NC≠VaMCI* Non-VaMCI≠VaMCI* | NC≠VaMCI** | NC≠VaMCI** Non-VaMCI≠VaMCI* | NC≠VaMCI* NC≠non-VaMCI* |
| Gainotti et al., 2008 | - | NC≠VaMCI* | n.s. | n.s. | NC≠VaMCI* | NC≠VaMCI** Non-VaMCI≠VaMCI** | NC≠VaMCI** Non-VaMCI≠VaMCI** | NC≠VaMCI* | |
| Zhou et al., 2009a | - | NC≠VaMCI** | NC≠VaMCI** | NC≠VaMCI** Non-VaMCI≠VaMCI** | NC≠VaMCI** | NC≠VaMCI** Non-VaMCI≠VaMCI** | - | NC≠VaMCI** | |
| Zhou et al., 2009b | - | NC≠VaMCI** | NC≠VaMCI* | NC≠VaMCI** | NC≠VaMCI** | NC≠VaMCI** | - | NC≠VaMCI** | |
| Norlund et al., 2011 | n.s. | n.s. | n.s. | n.s. | n.s. | Non-VaMCI≠VaMCI* | n.s. | n.s. | |
| Marra et al., 2011 | - | n.s. | n.s. | n.s. | Non-VaMCI≠VaMCI** | Non-VaMCI≠VaMCI** | Non-VaMCI≠VaMCI** | n.s. | |
| Villeneuve et al., 2011 | - | NC≠VaMCI* | - | NC≠VaMCI* | NC≠VaMCI* | NC≠VaMCI* | NC≠VaMCI* | NC≠VaMCI* | |
| Yi et al., 2012 | - | - | - | - | - | NC≠VaMCI** | - | NC≠VaMCI** | |
| Sudo et al., 2013 | NC≠VaMCI* | - | n.s. | n.s. | NC≠VaMCI* | n.s. | - | NC≠VaMCI* | |
| Brookes et al., 2015 | CVD≠VaMCI** | - | CVD≠VaMCI** | - | - | CVD≠VaMCI** | - | CVD≠VaMCI** | |
p<0.05;
p<0.01
Table 4.
Cognitive domains and corresponding neuropsychological tasks.
| Cognitive functions | Tests | |
|---|---|---|
| Executive Function (EF) | Shifting | Wisconsin Card Sorting Test (WMST): perseveration, Trail-Making Test (TMT) B, Dual task, Number-Letter sequencing |
| Inhibition | WCST: non-perseverative errors and categories, Go/No go, Fist/Edge/Palm sequence, Stroop test | |
| Working Memory/Update | Digit Span forward and backwards, Corsi test, Parallel Serial Mental Operations, CAMCOG: Working Memory Subtest, Number and Letter sequencing. | |
| Less specific EF tests | Category and Letter verbal fluency, Luria loop, Raven matrices, Barcelona test (Abstraction subtest), CAMCOG: Abstraction subtest, COWAT, Digit-Symbol substitution test, Cognitive estimation test, WAIS-III(picture interpretation and arrangement, Clock Drawing Test/CLOX 1 (spontaneous drawing), California Card Sorting Test | |
| Visuospatial/visuoconstructive abilities | Block design, Rey figure: copy, TMT A, Visual Object and Space Perception, Lines cancellation test, Clock Drawing Test/CLOX 2. (copy), Multiple Features Target Cancellation | |
| Memory | Prose recall, Babcock Story Recall test, Wechsler Memory Scale-Revised, Memory Assessment Scales, Hopkins Verbal Learning Test, Rey figure: recall and recognition, CAMCOG: Memory subtest, Five-item memory test | |
| Language | Token test, Boston Naming test, Assessment of Subtle Language Deficits | |
| Global Cognition | MMSE, CAMCOG, BMET | |
MMSE: Mini-Mental State Examination; CAMCOG: Cambridge Cognitive Examination part of the Cambridge Examination for. Mental Disorders of the Elderly (CAMDEX).; BMET: Brief Memory and Executive Test; WAIS-III: Wechsler Adult Intelligence Scale, 3rd Edition.
Studies using the severe WMH and/or more than 5 lacunes criteria evidenced significant differences among VaMCI, VaD and controls in EF, Memory and Visuospatial/ Visuoconstructive tasks. Tests that measured "impure" and unspecific EF dimensions, labeled herein as "less specific EF tasks", consistently distinguished VaMCI from the other groups, while Working Memory Tasks appear to be less sensitive for detection of VaMCI. As expected, performances in Memory tests identified non-VaMCI from VaMCI, but also differentiated VaMCI from controls in some studies. Global cognitive measures were more accurate in distinguishing VaMCI from controls and VaD than from non-VaMCI.
When moderate-to-severe WMH and/or less than 5 lacunes were used as criteria for CVD, EF, Memory, Visuospatial abilities tests, as well as Global Cognitive assessment, differentiated VaMCI from controls in most studies. Memory and Language tests were accurate measures in distinguishing VaMCI from non-VaMCI. Among EF dimensions, Inhibition and unspecific EF tests consistently detected VaMCI from controls in the selected studies.
DISCUSSION
The idea that VCI comprises a spectrum of different stages of vascular-related cognitive impairment may suggest that dementia can be preceded by subtle cognitive changes associated with CVD.41 However, the boundaries of vascular burden that mark the earliest clinical stages of CVD still need to be defined. The importance of establishing the milder pathological clinical phase of VCI resides in the fact that early identification of cognitive decline associated with CVD might allow adequate control of vascular risk factors, so as to prevent progression to dementia. In this perspective, the adoption of the neuroimaging criteria proposed by Erkinjuntti et al. for Binswanger Disease (2000) identified cases in which white-matter injury is already extensive, that may limit the effect-sizes of prophylactic actions. The present article reviewed data suggestive of expressive cognitive changes associated with moderate-to-severe WMH and less than 5 lacunes. Identification of those subjects might allow more effective actions in preventing progression of cognitive decline.
Studies using either severe or moderate-to-severe CVD criteria demonstrated that EF performances could distinguish VaMCI from non-VaMCI, VaD and normal controls. Global and "impure" EF tasks, comprising instruments that assess multiple and complex EF abilities, such as planning, reasoning, decision-making and abstract thinking, appear to be more sensitive in discriminating VaMCI from controls than specific and "pure" EF measures, even in the group with moderate WMH. Data from functional neuroimaging studies suggested that those "higher level" EF may recruit diverse areas in the prefrontal, parietal, medial and superior temporal cortices, and subcortical structures (amygdala, thalamus and cerebellum).42,43 These findings indicate that complex EF may result from the fine integration of many different cortical areas and subcortical regions, which depends on an extensive and delicate network of neural projections.44 Moderate white-matter changes, represented by periventricular smooth halo and beginning confluent deep WMH on neuroimaging, may be sufficient to interrupt segments of inter-cortical and/or cortical-subcortical loops, leading to disconnection of areas associated with complex EF.45
On the other hand, data on the accuracy of more specific EF measures in distinguishing controls, VaMCI and non-VaMCI appeared to be inconsistent, as observed in relation to shifting tasks. Performances in inhibition tasks were significantly worse in VaMCI subjects than in controls in most of the studies with moderate-to-severe CVD. This finding might suggest an early impairment of inhibitory control in VCI patients, which is in line with a previous prospective study.46 Interconnections among prefrontal cortex, subcortical regions and posterior areas might be interrupted in those patients, leading to loss of prefrontal inhibitory inputs over cortical-subcortical networks associated with task-irrelevant distracters.47,48 Among the severe CVD group, only two studies performed a similar analysis, showing conflicting results. Furthermore, working memory tasks were consistently inaccurate in differentiating VaMCI from non-VaMCI in most studies. Reports of impairments in working memory in amnestic MCI are abundant in the literature; thus, both Vascular and amnestic MCI might share, through different pathological mechanisms, similar prefrontal and cingulate dysfunction associated with working memory abilities.49
Non-executive cognitive domains were also tested in the studies. As expected, episodic memory tasks were more impaired in "atrophic" MCI than in VaMCI, in most of the studies. Yet, the finding that episodic memory performances were significantly poorer in VaMCI than in controls may highlight the role of the prefrontal cortex for the retrieval of information. Recent evidence suggested that left prefrontal cortex may participate in the recall process through the use of environmental cues and the ability to inhibit irrelevant memories during a task.50 Also, not surprisingly, impairments in visuospatial and visuoconstructive abilities were more prominent in VaMCI than in non-VaMCI and controls. Those alterations have been associated with CVD in different studies.51,52 Finally, screening tests (MMSE) and global cognitive assessment instruments (CAMCOG, BMET) identified VaMCI from controls in many studies and also from non-VaMCI in a smaller number of articles. Differently from longer neuropsychological batteries, many studies reported ceiling-effects for MMSE in samples comprising single-domain MCI subjects. However, evidence suggested that it may present similar accuracy in detecting multidomain impairments as compared with the Montreal Cognitive Assessment (MoCA) and the Addenbrooke's Cognitive Examination-Revised (ACE-R).53
Some other issues should be addressed. Despite slight variations, specially related to the instruments used to detect cognitive impairment and to the degree of deviation from normal cognition necessary to characterize the disorder, the clinical criteria proposed by Petersen et al. for MCI (2001) were adopted almost unchanged by most of the authors.54 This fact might indicate that, albeit past criticisms were directed to the disorder's construct validity, the use of the clinical entity described by Petersen et al. has largely prevailed among clinical studies.55 Conversely, other operational criteria have shown to be not optimal to identify MCI associated with CVD. Salvadori et al. (2015) reported that the criteria proposed by Winblad et al. (2004) might overlook non-amnestic MCI presentations.52
There are limitations in this review that need to be commented. Different terminologies used to describe periventricular and deep WMH and imprecise expressions (e.g., "patchy WMH", "diffuse WMH", "smooth halo" and "caps"), present in different criteria make it difficult to compare lesion loads across studies. Furthermore, the characterization of periventricular/deep WMH itself has been object of divergence by some authors, who adopted different distances between the ventricle's margin and the lesion to define it as "periventricular" or "deep".56,57 Moreover, tasks classified as assessing a specific aspect of EF may not be pure measures of that process, since they commonly require other EF and non-EF features. Models of EF as a unique or multiple constructs have been proposed and there is no agreement regarding neuropsychological tests that may thoroughly assess all of its aspects. Further studies using confirmatory factor-analysis of EF measures may allow the establishment of cognitive batteries comprising tests that evaluate complementary processes of EF.
The present review evidenced that the choice of neuroimaging criteria to characterize CVD in MCI subjects did not result in groups with different cognitive profiles. One possible hypothesis is the complex nature of subcortical disease, in which vascular and non-vascular (e.g., Alzheimer's disease, multiple sclerosis) events often interact, ultimately resulting in WM disconnection and cognitive impairment.58,59 In addition, as suggested by Pasi et al. (2015), that may also be due to the fact that cognitive tests may lose their accuracy in distinguishing groups of patients once certain degree of vascular lesions is reached.60
In conclusion, evidence in the literature suggested that the use of moderate-to-severe WMH and less than 5 lacunar infarcts as the earliest pathological neuroimaging presentation of CVD appear to be appropriate. Future operational criteria for VCI, especially for VaMCI, should place more emphasis in the clinical relevance of the early diagnosis. As mentioned, this measure may allow early intervention over risk-factors, with opportune effect in preventing progression to VaD.
Funding Statement
Support. Conselho Nacional de Pesquisa (CNPq) for the support to Jerson Laks, who is a Researcher 2 of this council. Fundação de Apoio à Pesquisa do Estado do Rio de Janeiro (FAPERJ): APQ1 (Proc. 111.327/2014).
Footnotes
This study was conducted at the Instituto de Psiquiatria, Universidade Federal do Rio de Janeiro
Disclosure: The authors report no conflits of interest
Support. Conselho Nacional de Pesquisa (CNPq) for the support to Jerson Laks, who is a Researcher 2 of this council. Fundação de Apoio à Pesquisa do Estado do Rio de Janeiro (FAPERJ): APQ1 (Proc. 111.327/2014).
Authors contributions. Design of the study: FKS, EE, JL. Analysis of the data: FKS, EE. Intellectual contribution to the writing of the manuscript: FKS, GSA, LEV, CT, DMM, EE. Manuscript written by: FKS. Interim and final revision: EE, GSA, JL.
REFERENCES
- 1.Garrett KD, Browndyke JN, Whelihan W, et al. The neuropsychological profile of vascular cognitive impairment-no dementia: comparison to patients at risk for cerebrovascular disease and vascular dementia. Arch Clin Neuropsych. 2004;19:745–757. doi: 10.1016/j.acn.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachdev P, Kalaria R, O'Brien J, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord. 2014;28:206–218. doi: 10.1097/WAD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erkinjuntti T, Inzitari D, Pantoni L, et al. Research criteria for subcortical vascular dementia in clinical trials. J Neural Transmission. 2000;59(Suppl 1):23–30. doi: 10.1007/978-3-7091-6781-6_4. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt R, Ropele S, Ferro J, et al. Diffusion-weighted imaging and cognition in the Leukoaraiosis and Disability in the Elderly Study. Stroke. 2010;41:e402–e408. doi: 10.1161/STROKEAHA.109.576629. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry. 2006;14:724–733. doi: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Q, Zhou Y, Wang Y, Dong K, Wang Y. A new diagnostic algorithm for vascular cognitive impairment: the proposed criteria and evaluation of its reliability and validity. Chin Med J. 2010;123:311–319. [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700–b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galluzzi S, Sheu CF, Zanetti O, Frisoni GB. Distinctive clinical features of mild cognitive impairment with subcortical cerebrovascular disease. Dement Geriatr Cogn Disord. 2005;19:196–203. doi: 10.1159/000083499. [DOI] [PubMed] [Google Scholar]
- 10.Moretti DV, Pievani M, Fracassi C, et al. Brain vascular damage of cholinergic pathways and EEG markers in mild cognitive impairment. J Alzheimers Dis. 2008;15:357–372. doi: 10.3233/jad-2008-15302. [DOI] [PubMed] [Google Scholar]
- 11.Bella R, Ferri R, Pennisi M, et al. Enhanced motor cortex facilitation in patients with vascular cognitive impairment-no dementia. Neurosci Lett. 2011;503:171–115. doi: 10.1016/j.neulet.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 12.Zhou A, Jia J. A screen for cognitive assessments for patients with vascular cognitive impairment no dementia. Int J Geriatr Psychiatry. 2009;24:1352–1357. doi: 10.1002/gps.2265. [DOI] [PubMed] [Google Scholar]
- 13.Zhou A, Jia J. Different cognitive profiles between mild cognitive impairment due to cerebral small vessel disease and mild cognitive impairment of Alzheimer's disease origin. J Int Neuropsychol Soc. 2009;15:898–905. doi: 10.1017/S1355617709990816. [DOI] [PubMed] [Google Scholar]
- 14.Yi L, Wang J, Jia L, et al. Structural and functional changes in subcortical vascular mild cognitive impairment: a combined voxel-based morphometry and resting-state fMRI study. PLoS One. 2012;7:e44758. doi: 10.1371/journal.pone.0044758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SH, Kang HS, Kim HJ, et al. The effect of ischemic cholinergic damage on cognition in patients with subcortical vascular cognitive impairment. J Geriatr Psychiatry Neurol. 2012;25:122–127. doi: 10.1177/0891988712445089. [DOI] [PubMed] [Google Scholar]
- 16.Lee MJ, Seo SW, Na DL, et al. Synergistic effects of ischemia and β-amyloid burden on cognitive decline in patients with subcortical vascular mild cognitive impairment. JAMA Psychiatry. 2014;71:412–422. doi: 10.1001/jamapsychiatry.2013.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gainotti G, Ferraccioli M, Vita MG, Marra C. Patterns of neuropsychological impairment in MCI patients with small subcortical infarcts or hippocampal atrophy. J Int Neuropsychol Soc. 2008;14:611–619. doi: 10.1017/S1355617708080831. [DOI] [PubMed] [Google Scholar]
- 18.Marra C, Ferraccioli M, Vita MG, Quaranta D, Gainotti G. Patterns of cognitive decline and rates of conversion to dementia in patients with degenerative and vascular forms of MCI. Curr Alzheimer Res. 2011;8:24–31. doi: 10.2174/156720511794604552. [DOI] [PubMed] [Google Scholar]
- 19.Villeneuve S, Massoud F, Bocti C, Gauthier S, Belleville S. The nature of episodic memory deficits in MCI with and without vascular burden. Neuropsychologia. 2011;49:3027–3035. doi: 10.1016/j.neuropsychologia.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Sudo FK, Alves CE, Alves GS, et al. White matter hyperintensities, executive function and global cognitive performance in vascular mild cognitive impairment. Arq Neuropsiquiatr. 2013;71:431–436. doi: 10.1590/0004-282X20130057. [DOI] [PubMed] [Google Scholar]
- 21.Brookes RL, Hollocks MJ, Khan U, Morris RG, Markus HS. The Brief Memory and Executive Test (BMET) for detecting vascular cognitive impairment in small vessel disease: a validation study. BMC Med. 2015;13:51–51. doi: 10.1186/s12916-015-0290-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frisoni GB, Galluzzi S, Bresciani L, Zanetti O, Geroldi C. Mild cognitive impairment with subcortical vascular features: clinical characteristics and outcome. J Neurol. 2002;249:1423–1432. doi: 10.1007/s00415-002-0861-7. [DOI] [PubMed] [Google Scholar]
- 23.Nordahl CW, Ranganath C, Yonelinas AP, DeCarli C, Reed BR, Jagust WJ. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia. 2005;43:1688–1697. doi: 10.1016/j.neuropsychologia.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shim YS, Yoon B, Shon YM, Ahn KJ, Yang DW. Difference of the hippocampal and white matter microalterations in MCI patients according to the severity of subcortical vascular changes: neuropsychological correlates of diffusion tensor imaging. Clin Neurol Neurosurg. 2008;110:552–561. doi: 10.1016/j.clineuro.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 25.Sheorajpanday RV, Mariën P, Nagels G, Weeren AJ, Saerens J, van Putten MJ, De Deyn PP. Subcortical vascular cognitive impairment, no dementia: EEG global power independently predicts vascular impairment and brain symmetry index reflects severity of cognitive decline. J Clin Neurophysiol. 2014;31:422–428. doi: 10.1097/WNP.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 26.Fernández PJ, Campoy G, García Santos JM, et al. Is there a specific pattern of attention deficit in mild cognitive impairment with subcortical vascular features? Evidence from the Attention Network Test. Dement Geriatr Cogn Disord. 2011;31:268–275. doi: 10.1159/000327165. [DOI] [PubMed] [Google Scholar]
- 27.Miyake A, Friedman NP. The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Curr Dir Psychol Sci. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder HR, Miyake A, Hankin BL. Advancing understanding of executive function impairments and psychopathology: bridging the gap between clinical and cognitive approaches. Front Psychol. 2015;6:328–328. doi: 10.3389/fpsyg.2015.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vakil E, Blachstein H. Rey Auditory-Verbal Learning Test: structure analysis. J Clin Psychol. 1993;49:883–890. doi: 10.1002/1097-4679(199311)49:6<883::aid-jclp2270490616>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Greve KW, Farrell JF, Besson PS, Crouch JA. A psychometric analysis of the California Card Sorting Test. Arch Clin Neuropsychol. 1995;10:265–278. [PubMed] [Google Scholar]
- 31.Chapman LL, White DA, Storandt M. Prose recall in dementia. A comparison of delay intervals. Arch Neurol. 1997;54:1501–1504. doi: 10.1001/archneur.1997.00550240053012. [DOI] [PubMed] [Google Scholar]
- 32.Cappa SF, Binetti G, Pezzini A, Padovani A, Rozzini L, Trabucchi M. Object and action naming in Alzheimer's disease and frontotemporal dementia. Neurology. 1998;50:351–355. doi: 10.1212/wnl.50.2.351. [DOI] [PubMed] [Google Scholar]
- 33.Saxton J, Ratcliff G, Munro CA, et al. Normative data on the Boston Naming Test and two equivalent 30-item short forms. Clin Neuropsychol. 2000;14:526–534. doi: 10.1076/clin.14.4.526.7204. [DOI] [PubMed] [Google Scholar]
- 34.Ferber S, Karnath HO. How to assess spatial neglect-line bisection or cancellation tasks? J Clin Exp Neuropsychol. 2001;23:599–607. doi: 10.1076/jcen.23.5.599.1243. [DOI] [PubMed] [Google Scholar]
- 35.Peña-Casanova J, Monllau A, Böhm P, et al. Grupo NORMACODEM Correlations between cognition and function in Alzheimer's disease: based on the abbreviated Barcelona Test (a-BT) Neurologia. 2005;20:4–8. [PubMed] [Google Scholar]
- 36.Shin MS, Park SY, Park SR, Seol SH, Kwon JS. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nat Protoc. 2006;1:892–899. doi: 10.1038/nprot.2006.115. [DOI] [PubMed] [Google Scholar]
- 37.Piccardi L, Iaria G, Ricci M, Bianchini F, Zompanti L, Guariglia C. Walking in the Corsi test: which type of memory do you need? Neurosci Lett. 2008;432:127–131. doi: 10.1016/j.neulet.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, et al. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009;15:438–450. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- 39.Paula JJ, Bertola L, Nicolato R, Moraes EN, Malloy-Diniz LF. Evaluating language comprehension in Alzheimer's disease: the use of the Token test. Arq Neuropsiquiatr. 2012;70:435–440. doi: 10.1590/s0004-282x2012000600010. [DOI] [PubMed] [Google Scholar]
- 40.Marra C, Gainotti G, Scaricamazza E, Piccininni C, Ferraccioli M, Quaranta D. The Multiple Features Target Cancellation (MFTC): an attentional visual conjunction search test. Normative values for the Italian population. Neurol Sci. 2013;34:173–180. doi: 10.1007/s10072-012-0975-3. [DOI] [PubMed] [Google Scholar]
- 41.Meyer JS, Xu G, Thornby J, Chowdhury MH, Quach M. Is Mild cognitive impairment prodromal of vascular dementia like Alzheimer's disease. Stroke. 2002;33:1981–1985. doi: 10.1161/01.str.0000024432.34557.10. [DOI] [PubMed] [Google Scholar]
- 42.Noveck IA, Goel V, Smith KW. The neural basis of conditional reasoning with arbitrary content. Cortex. 2004;40(4-5):613–622. doi: 10.1016/s0010-9452(08)70157-6. [DOI] [PubMed] [Google Scholar]
- 43.Gold JI, Shadlen MN. The Neural Basis of Decision Making. Ann Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 44.Liang P, Wang Z, Yang Y, Jia X, Li K. Functional disconnection and compensation in mild cognitive impairment: evidence from DLPFC connectivity using resting-state fMRI. PLoS One. 2011;6:e22153. doi: 10.1371/journal.pone.0022153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malloy P, Correia S, Stebbins G, Laidlaw DH. Neuroimaging of white matter in aging and dementia. Clin Neuropsychol. 2007;21:73–109. doi: 10.1080/13854040500263583. [DOI] [PubMed] [Google Scholar]
- 46.Jokinen H, Kalska H, Ylikoski R, et al. Longitudinal cognitive decline in subcortical ischemic vascular disease--the LADIS Study. Cerebrovasc Dis. 2009;27:384–391. doi: 10.1159/000207442. [DOI] [PubMed] [Google Scholar]
- 47.Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta Psychol (Amst) 1999;101(2-3):159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 48.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004 Apr;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Migo EM, Mitterschiffthaler M, O'Daly O, et al. Alterations in working memory networks in amnestic mild cognitive impairment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2015;22:106–127. doi: 10.1080/13825585.2014.894958. [DOI] [PubMed] [Google Scholar]
- 50.Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- 51.Rasquin SM, Lodder J, Visser PJ, Lousberg R, Verhey FR. Predictive accuracy of MCI subtypes for Alzheimer's disease and vascular dementia in subjects with mild cognitive impairment: A 2-year follow-up study. Dement Geriatr Cogn Disord. 2005;19:113–119. doi: 10.1159/000082662. [DOI] [PubMed] [Google Scholar]
- 52.Salvadori E, Poggesi A, Valenti R, et al. Operationalizing mild cognitive impairment criteria in small vessel disease: The vascular mild cognitive impairment-Tuscany study. Alzheimers Dement. 2015 doi: 10.1016/j.jalz.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery after TIA and stroke. Stroke. 2012;43:464–469. doi: 10.1161/STROKEAHA.111.633586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 55.Gauthier S, Touchon J. Mild cognitive impairment is not a clinical entity and should not be treated. Arch Neurol. 2005;62:1164–1166. doi: 10.1001/archneur.62.7.1164. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt R, Fazekas F, Kleinert G, et al. Magnetic resonance imaging signal hyperintensities in the deep and subcortical white matter. A comparative study between stroke patients and normal volunteers. Archiv Neurol. 1992;49:825–827. doi: 10.1001/archneur.1992.00530320049011. [DOI] [PubMed] [Google Scholar]
- 57.Scheltens P, Barkhof F, Leys D, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114:7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- 58.Nordlund A, Göthlin M, Wallin A. Vascular disease, Alzheimer's disease biomarkers and cognition in mild cognitive impairment: additive or synergetic effects? Dement Geriatr Cogn Disord. 2011;32:250–256. doi: 10.1159/000334653. [DOI] [PubMed] [Google Scholar]
- 59.Nordlund A, Rolstad S, Klang O, Lind K, Hansen S, Wallin A. Cognitive profiles of mild cognitive impairment with and without vascular disease. Neuropsychology. 2007;21:706–712. doi: 10.1037/0894-4105.21.6.706. [DOI] [PubMed] [Google Scholar]
- 60.Pasi M, Salvadori E, Poggesi A, et al. White matter microstructural damage in small vessel disease is associated with Montreal cognitive assessment but not with mini mental state examination performances: vascular mild cognitive impairment Tuscany study. Stroke. 2015;46:262–264. doi: 10.1161/STROKEAHA.114.007553. [DOI] [PubMed] [Google Scholar]