Abstract
Quality and efficacy of two locally-manufactured generic albendazole (ABZ) products (Curex and Royal Drug) used for de-worming children in Nepal since 1999 were tested against the originator product (GSK). The study conducted disintegration and dissolution testing and a randomised controlled clinical trial comparing cure rates (CR) and egg reduction rates (ERR) for Ascaris lumbricoides, Trichuris trichiura and hookworm infections. Stool samples from 1277 children were examined before and 21 days after treatment. For A. lumbricoides GSK's (97.0%) and Royal Drug's (95.0 %) product provided significantly higher CR than Curex's (82.6%); however, all products provided ERR higher than 90%. For T. trichiura Curex's product showed significantly lower ERR (63.2%). For hookworm, GSK's product performed significantly better (CR 74.3%, ERR 87.1%) than Royal Drug's (CR 53.3%, ERR 80.8%) and Curex's (CR 50.7%, ERR 73.1%). Only GSK's product passed both disintegration and dissolution. Both generic products failed dissolution. Curex's product showed poor disintegration. Despite its lower efficacy the cheaper Curex's product achieved good results in controlling morbidity due to soil-transmitted helminth (STH) infections. This study shows that cost-effectiveness of drugs used in mass de-worming campaigns should not be inferred on the basis of one single quality testing parameter.
Keywords: Soil-transmitted helminths, ABZ, dissolution, efficacy, Nepal
Introduction
STH infections have been an enduring public health problem in Nepal (Dreyfuss et al., 2000; Khanal and Walgate, 2002; Navitsky et al., 1998). Various large scale de-worming interventions have been conducted to control the problem. Since 1998, as part of school feeding programme, about half a million primary-school-aged children have been de-wormed twice a year with locally-manufactured generic ABZ tablets (Royal Drugi) given as a single 400 mg dose. Following this intervention an important reduction in the prevalence of infection (from 74% to 51%) and in the proportion of heavy-intensity infections (from 9.3% to 2.1%) was demonstrated (Bordignon and Shakia, 2003; Khanal and Walgate, 2002). In addition, comparison of haemoglobin levels in schools covered and not covered by the school feeding programme showed a significant reduction of the percentage of anaemic children (from 50% to 11%) in the treated group (Bordignon and Shakia, 2003). In 1999, de-worming was integrated with the national biannual vitamin A capsule distribution. As part of the programme, about two million children aged 13-59 months have been de-wormed twice a year (WHO/UNICEF, 2004). An impressive reduction of anaemia (from 47% to 11%) has been recently demonstrated as a major outcome of this intervention (Pandey et al., 2004). The Ministry of Health in 2001 initiated de-worming of pregnant women (after the 1st trimester), targeting about one million pregnant women each year. Potential benefits of this intervention on maternal anaemia and infant mortality has been recently demonstrated (Christian et al., 2004). Anthelminthic treatment is also routinely offered at primary heath care facilities to all those suspected to be infected. The government of Nepal plans to expand de-worming of school-age children nationwide covering a total of 3.5 million children and to initiate de-worming adolescents.
Since the inception of biannual de-worming of pre-school children in 1999, millions of ABZ tablets have been procured from different local suppliers. Quality of the procured products was not routinely tested. On one occasion a batch of ABZ used in a school feeding programme (Royal Drug) failed quality testing conducted according to the United States Pharmacopeia (USP) XXIV (The United States Pharmacopeia, 2000) due to inadequate dissolution, although it complied with the requirements of the Indian Pharmacopoeia (IP) 1996, which do not include dissolution for ABZ chewable tablets (Indian Pharmacopoeia, 1996). Another local brand (Curexii) of generic ABZ for the national de-worming in pre-schoolchildren initially purchased by UNICEF and subsequently directly procured by the Ministry of Health of Nepal never underwent dissolution testing nor efficacy monitoring.
In order to assess the quality and efficacy of generic local de-worming tablets used in Nepal, the Ministry of Health with the support of WHO conducted quality control tests and an efficacy trial. The quality control assessment involved testing two locally manufactured generic ABZ products (Royal Drug and Curex) and the imported innovator product (GSKiii) according to the full monograph requirements of both the IP 1996 and the USP XXIV. A randomised single-blind trial was also carried out in school-age children in rural Nepal to compare the efficacy of generic and originator products in the treatment of infections caused by Ascaris lumbricoides, Trichuris trichiura and the hookworms.
The outcomes of both the quality testing and the efficacy study are compared and discussed in the present paper.
Material and Methods
Laboratory quality control assessment
ABZ 400mg tablets from the three different sources were tested at the Royal Drug Research Laboratory, which is the official quality control laboratory of the Ministry of Health of Nepal. All three products were tested for disintegration (IP), dissolution (USP) and for active substance content (both USP XXIV and IP 1996 assays). No additional testing was conducted. The analytical methods used were those of the mentioned compendial references (The United States Pharmacopeia, 2000; Indian Pharmacopoeia, 1996). Reference substances were purchased from the USP.
Efficacy trial
We conducted a community-based, randomised, single-blind trial on the efficacy of generic and originator ABZ (ABZ). The study was carried out among primary school-age children in Nepal. The rural region of Syanja was selected for known high prevalence for STH infections, relatively good accessibility of the surrounding villages, and security reasons.
One the day prior to the scheduled treatment date, children eligible to participate in the trial were visited at home and their parents or guardian were given a consent explanatory letter, in local language. They were also given a container in which to bring a fresh stool sample the following day. On the collection day, the field Team visited every household and enrolled the eligible children. ABZ tablets were pre-packed in opaque sealed envelopes according to random numbers, and the unique identification number labelled on the envelope was reported on the record form and on the pot containing the stool sample.
After having collected the stool sample, children were administered the drug under supervision. Children were randomly allocated to three groups: children receiving GSK's ABZ 400 mg (Group A), children receiving Royal Drug's ABZ 400 mg (Group B), and children receiving Curex's ABZ 400 mg (Group C).
Children were not admitted to the trial if they were too sick (e.g. severe diarrhoea, severe anaemia, high fever), did not have parental/guardian’s permission to participate, or did not provide a stool sample.
Stool samples were taken to the field laboratory and processed the same day by the Kato-Katz technique to assess prevalence and intensity of A. lumbricoides, T. trichiura and hookworm infections, according to WHO criteria (WHO, 1994).
A random sample of 10% of the Kato-Katz smears were read in duplicate by two independent technicians in order to evaluate the accuracy of the diagnosis and the precision of the egg counts. Re-examination of slides was performed if the readings showed a difference in egg count greater than 10%. In case of hookworm a new smear was made and read again independently by two technicians.
Twenty one days after treatment, all children investigated at baseline and tested positives for STH infections were re-visited and another stool sample was collected. Children who failed to produce a stool sample were followed up the next day.
Statistical analysis
Data were entered and analysed using the EpiInfo package. Egg counts before and after treatment allowed calculation of cure rate (CR) and egg reduction rate (ERR), the study primary end points, in all treatment groups.
CR were calculated as the proportion of children with egg counts >0 before treatment that were cured after treatment. ERR, were calculated as the proportion of mean egg counts reduced by the treatment.
Proportions were compared using standard χ2 tests. Means were compared using analysis of variance by ANOVA tests if Bartlett’s test of heterogeneity indicated homogeneity of variances, and by the Kruskal-Wallis test if Bartlett’s test was significant at the 5% level.
Helminth infections were divided into categories of intensity to represent the egg counts distribution in the study population before and after treatment. These categories were used to classify light, moderate and heavy infections according to WHO criteria (Montresor et al., 2002).
Ethical considerations
This study involved drug products that were approved for marketing in Nepal by the national regulatory authority, the Department of Drug Administration. These products were used by qualified health professionals for their approved indications in the population involved in the study. Field teams were instructed to collect information on adverse events occurred in treated children. The purpose of the trial was explained to the parents or guardians of the children eligible to participate in the study in a written consent form and any question arising was answered by the study team. Consent of parents and guardians was obtained prior to the enrolment of their children. Children still infected at the end of the study period were offered additional anthelminthic treatment. Feedback was given to all staff and MoH officers involved in the study through an official report. Ethical clearance was sought and obtained from the MoH Nepal, being this study an evaluation of the ongoing national helminth control programme. The results of laboratory testing presented in this paper were not known before treatment was administered. Since all products were freely available in Nepal, it was not considered necessary to await testing results before starting treatment.
Results
Laboratory quality control testing
Both the USP and IP are recognized by the Nepalese authorities. Results of laboratory testing for dissolution, disintegration, and active compound, on the three products according to the US and Indian Pharmacopoeias are reported in Table 1. All products have the required content of active ingredient. One of the three products, Curex's, has performed very poorly at the disintegration test, while the other two have shown a good disintegration time of less than 15 minutes. All three drugs fulfilled the Indian Pharmacopoeia monograph that does not require to test dissolution for ABZ chewable tablets. However, dissolution tests were conducted for all the three drugs. Royal Drug's and Curex's ABZ failed dissolution tests according to the US Pharmacopoeia that requires that 80% of the labeled amount be dissolved in 30 minutes. The originator's product passed the dissolution test.
Table 1. Laboratory analysis of albendazole drugs according to the US Pharmacopoeia (USP) and Indian Pharmacopoeia (IP).
| Drug Albendazole 400 mg |
Batch N | Quantity (mg/tablet) | % Active Ingredient | Disintegration time (minutes) | % Dissolution | Dissolution | Remarks |
|---|---|---|---|---|---|---|---|
| Zentel 400 (GSK) |
48907G100 | 397.6 (USP) 394.5 (IP) |
99.2 (USP) 98.7 (IP) |
6.7 (IP) | 84.8 (USP) | Passed | Chewable |
| RDZ-400 (Royal Drug Ltd Nepal) |
T-53 | 401.3 (USP) 394.0 (IP) |
100.4 (USP) 98.7 (IP) |
11.8 (IP) | 10.3 (USP) | Failed | Chewable |
| Azol 400 (Curex Ltd Nepal) |
61 | 413.8 (USP) 415.8 (IP) |
103.5 (USP) 103.9 (IP) |
> 1 hour (IP) | 0.27 (USP) | Failed | Chewable |
Efficacy trial
A total of 2140 children were screened at baseline and 1506 (70.4%) were found positive for A. lumbricoides or T. trichiura or hookworm infections. Out of 1506 positive children, 1277 (84.8% of those treated) were re-examined at 21 days follow up. The 229 children lost at follow-up were evenly distributed in the three treatment groups and their characteristics, including prevalence of STH infections, did not differ from children that were followed up (data not shown).
Analysis has been done on the 1277 positive children for which data at baseline and at 21 days follow up were available. The mean age was 11.6 (SD 2.9) and 53.4% were boys.
CR and ERR before and 21 days after treatment with the three products are presented in Table 2. CR for A. lumbricoides were significantly better in children treated with GSK's and Royal Drug's ABZ; however, ERRs were similar for the three products and were over 90%. For T. trichiura Curex's ABZ showed ERR significantly lower than the other two products. For hookworm infections, GSK's ABZ performed significantly better than the other two products in terms of both CR and ERR.
Table 2. Prevalence (% Pos), intensity (EPG), Cure Rate (CR), Egg Reduction Rate (ERR) before and 21 days after treatment with albendazole GSK, albendazole Royal Drug and albendazole Curex.
| Drug Albendazole 400 mg |
N | Day 0 | Day 21 | CR | ERR (95%CI) | |||
|---|---|---|---|---|---|---|---|---|
| % Pos | EPG# | % Pos | EPG | |||||
| Ascaris | GSK | 429 | 31.5 | 13 | 0.9 | 0.0 | 97.0 | 92.6 (89.2, 95.0) |
| Royal Drug | 419 | 33.9 | 17 | 1.7 | 0.1 | 95.0 | 93.8 (90.9, 95.8) | |
| Curex | 429 | 36.1 | 19 | 6.3 | 0.6 | 82.6 * ^ | 91.9 (88.0, 94.4) | |
| Total | 1277 | 33.8 | 16 | |||||
| Trichuris | GSK | 429 | 80.7 | 79 | 62.2 | 22 | 28.6 | 71.7 (64.4, 77.5) |
| Royal Drug | 419 | 80.0 | 75 | 61.6 | 21 | 26.6 | 71.4 (64.6, 77.1) | |
| Curex | 429 | 78.3 | 60 | 60.6 | 21 | 28.0 | 63.2 (53.7, 70.8) § @ | |
| Total | 1277 | 79.6 | 71 | |||||
| Hookworms | GSK | 429 | 49.0 | 13 | 14.2 | 0.9 | 74.3 | 87.1 (83.3, 90.1) |
| Royal Drug | 419 | 50.1 | 15 | 24.6 | 2.0 | 53.3 * | 80.8 (75.6, 84.9) § | |
| Curex | 429 | 47.8 | 12 | 25.9 | 2.4 | 50.7 * | 73.1 (66.2, 78.6) * ^ | |
| Total | 1277 | 48.9 | 13 | |||||
Expressed as geometric mean
different from Albendazole GSK p<0.001
different from Albendazole GSK p<0.05
different from Albendazole RD <0.001
different from Albendazole RD <0.05
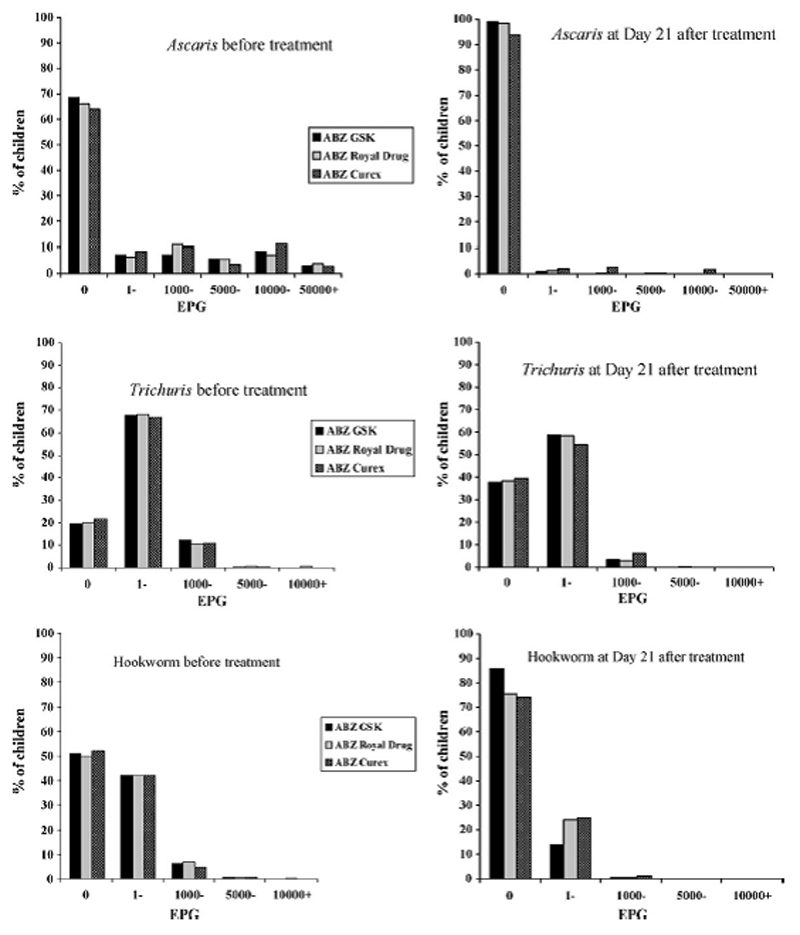
To test whether the changes in efficacy were related to the initial intensity of infection, ERR and CR were analysed for the different infection intensity (eggs per gram - EPG) groups at baseline. Results confirmed an equivalent performance of GSK's and Royal Drug's products for A. lumbricoides infection, both higher than Curex's. However, differences were statistically significant only in light infections. GSK's ABZ had significantly better efficacy than the other compounds for T. trichiura and hookworm infections at low intensities. GSK's product performed better than Curex's in all STH infections at low intensities (data not shown). Data on intensity of infection by EPG groups before and after treatment with the three products are shown in Figure 1.
Figure 1.
Distribution of A. lumbricoides, T. trichiura and hookworm egg counts before and after treatment with ABZ GSK, ABZ Royal Drug and ABZ Curex.
Discussion
The present study is unique because it associates a clinical trial comparing the efficacy of three preparations of ABZ 400 mg with quality testing of the same batches against two different pharmacopoeial standards.
Our results indicate, (with different degrees of significance) a descending gradient of efficacy from GSK's product to Royal Drug's and then Curex's for the treatment of STH infections in Nepal with particularly significant difference for hookworm infection.
Disintegration of GSK's and Royal Drug's tablets was satisfactory while Curex's did not disintegrate even in an hour. No correlation of disintegration time with in vitro dissolution was found in a previous study (Galia et al., 1999), however the disintegration performance of Curex's product, which is poorer than that of the other two products, is accompanied by a poorer clinical performance. Dissolution of ABZ tablets has been shown, in other studies, to vary between the innovator product and several generic drugs (Galia et al., 1999). Our results confirm that dissolution of GSK's ABZ meets the requirements of the USP, and show poor dissolution for the two generics from Nepal, with values (0-10%) even lower than those (32-64%) observed in other studies (Galia et al., 1999). Different dissolution characteristics of solid oral dosage forms are the result of one or more formulation factors such as, for example, particle size of the active ingredient, excipients, and differences in the manufacturing process (Solvang and Finholt, 1970). Consistently adequate release of poorly soluble drugs like ABZ can only be guaranteed by careful formulation and Good Manufacturing Practices, which are not yet fully implemented in Nepal (WHO assessment of drug regulatory situation in Nepal, unpublished report).
Our study aimed at providing information useful for mass treatment campaigns and we did not gather sufficient data to discuss tablet formulation and its biopharmaceutical implications. However, our results seem to suggest that the observed differences in dissolution could be the most probable reason for the different drug efficacy patterns. Lower dissolution results in lower amounts of ABZ becoming available for its anthelminthic action in the gastrointestinal tract. The fact that some of the children might have not chewed the tablets could explain the lower therapeutic efficacy of the drugs with lower disintegration and dissolution, but this needs to be demonstrated.
If effectively chewing the tablets were confirmed to be the main reason for the different efficacy that we have observed, then making sure that all children chew their tablet before swallowing could be a possible way to overcome the problem. However, despite the fact the personnel supervising the de-worming activities can be instructed to ensure that children chew their tablet, this is difficult to implement when large numbers of children are treated at the same time.
Cost-effectiveness is a critical aspect that health authorities should carefully consider when planning de-worming programmes. Over a four-years period, eight de-worming rounds were conducted in Nepal with different locally manufactured products (including Curex's ABZ which has shown lower efficacy in this trial) and an important reduction in STH morbidity was achieved (Pandey et al., 2004). Around 6.8 million tablets of Curex's ABZ are procured every year at a total cost of approximately US$81,600. At the time of this study, local purchasing price of one tablet of ABZ was US$0.012 for Curex's and US$0.017 for Royal Drug's. GSK's ABZ has never been used in de-worming campaigns in Nepal, therefore, no information is available on the price that would have been paid for large quantities. Its price in street pharmacies in Nepal at the time of the study was US$0.2. Information from other programmes indicate that the price of one tablet of GSK's ABZ could be as low as US$0.02 (Fenwick, personal communication). If 6.8 million tablets had been procured at Royal Drug or GSK prices for large quantities, the total cost of drugs for a year would have been respectively US$115,600 or US$136,000, i.e. about 41%. or 66% more than with Curex's product.
One could assume that a better clinically performing drug could give a higher health benefit and perhaps permit to run de-worming campaigns only once/year instead of every six months halving the yearly costs of the drug and of its distribution (although this still needs to be demonstrated).
The results of this study trigger questions on the importance of certain criteria used for planning mass treatment campaigns with anthelminthic drugs. The extremely poor performance of Curex's ABZ in quality tests would lead to the conclusion that it is unsuitable for use in a campaign. Yet, it has shown a good degree of effectiveness that, although inferior to the other two drugs, challenges the relevance or reliability of quality testing, as currently done, as a major decision criterion for inclusion of a specific product in de-worming campaigns.
Finally, the observed difference in the efficacy between the two generic brands is a source of concern. Both products have gone through the same assessment process by the national regulatory authority. It is therefore important for the Department of Drug Administration to consider the results presented here and re-assess the products concerned in the interest of public health in Nepal.
This trial suggests that dissolution is an important parameter in assessing drug quality of oral solid forms, including chewable tablets. However, this study shows that cost-effectiveness of drugs used in mass de-worming campaigns can never be presumed on the basis of one single quality testing parameter.
Acknowledgements
The present study would not have been possible without the hard work and dedication of the Field Team composed by Mr Rabindra Shrestha and Mr Makame Kombo (supervisors), Mrs Narayani Subedi, Mr Shyam Sunder Sedai, Mr Hari Om Shrestha, Mr Suresh Maharjan, Mr Amrit Sapkota, Mr Anil KC, Mr Rajesh Shrestha and Mr Krishna P. Dakal. Special thanks go to Mr Rajani Shrestha, Royal Drug Laboratory. Drugs used in this trial were donated by GSK through WHO, by Royal Drug through WFP and by the Ministry of Health of Nepal. Precious logistic and technical support was given to this study from Dr P.O. Blomquist, UNICEF Country Office, by Dr K. Wagner, WHO Representative Office in Nepal, by Mr. Ram K. Shrestha, National Vitamin A Program, NTAG. This study was co- funded by the Parasitic Diseases and Vector Control and by the Drug Management and Policy, World Health Organization, Geneva.
Footnotes
Royal Drug
Curex
GSK
References
- Bordignon GP, Shakya DR. A de-worming programme in Nepal supported by the World Food Programme. In: Crompton DWT, Montresor A, Nesheim MC, Savioli L, editors. Controlling Diseases due to helminth infections. World Health Organization; Geneva: 2003. pp. 87–92. [Google Scholar]
- Christian P, West KP. Antenatal anthelmintic treatment, birthweight, and infant survival in rural Nepal. Lancet. 2004;364:981–983. doi: 10.1016/S0140-6736(04)17023-2. [DOI] [PubMed] [Google Scholar]
- Dreyfuss ML, Stoltzfus RJ, Shrestha JB, Pradhan EK, LeClerq SC, Khatry SK, Shrestha SR, Katz J, Albonico M, West KP., Jr Hookworms, malaria and vitamin A deficiency contribute to anemia and iron deficiency among pregnant women in the plains of Nepal. J Nutr. 2000;30(10):2527–2536. doi: 10.1093/jn/130.10.2527. [DOI] [PubMed] [Google Scholar]
- Galia E, Horton J, Dressman JB. ABZ generics – a comparative in vitro study. Pharm Res. 1999;16(12):1871–1875. doi: 10.1023/a:1018907527253. [DOI] [PubMed] [Google Scholar]
- Indian Pharmacopoeia. Government of India, Ministry of Health & Family Welfare. The Controller of Publications, Civil Lines, Delhi -110 054; 1996. [Google Scholar]
- Khanal P, Walgate R. Nepal de-worming programme ready to go worldwide. Bull World Health Organ. 2002;80:423–424. [PMC free article] [PubMed] [Google Scholar]
- Navitsky RC, Dreyfuss ML, Shresta J, Kahtry SK, Stoltzfus RJ, Albonico M. Ancylostoma duodenale is responsible for hookworm infections among pregnant women in the rural plains of Nepal. J Parasitol. 1998;84(3):647–651. [PubMed] [Google Scholar]
- Montresor A, Crompton DWT, Gyorkos TW, Savioli L. Helminth control in school age children. World Health Organization; Geneva: 2002. [Google Scholar]
- Pandey S, Mathema P, Okamura K, Blomquist PO, Shresta R. Reduced anaemia brings brighter future for preschool children in Nepal. De-worming Impact Evaluation Study. International Nutritional Anaemia Consultative Group; Lima, Peru: 2004. Nov, 2004. [Google Scholar]
- Solvang S, Finholt P. Effect of tablets processing and formulation factors on dissolution rate of the active ingredient in human gastric juice. J Pharm Sci. 1970;59:49–52. doi: 10.1002/jps.2600590106. [DOI] [PubMed] [Google Scholar]
- The United States Pharmacopeia, USP 24, January 2000, United States Pharmacopeial Convention Inc., 12601 Twinbrook Parkway, Rockville, MD 20852.
- WHO. Bench Aids for the diagnosis of intestinal parasites. Geneva: 1994. [Google Scholar]
- WHO. How to add de-worming to vitamin A distribution. Geneva: 2004. WHO/CDS/CPE/2004.11. [Google Scholar]