Abstract
Purpose of review
To review studies of behavioral economic interventions (financial incentives, choice architecture modifications, or commitment devices) to prevent type 2 diabetes mellitus (T2DM) among at-risk patients or improve self-management among patients with T2DM.
Recent findings
We found 15 studies that used varied study designs and outcomes to test behavioral economic interventions in clinical, workplace, or health plan settings. Of four studies that focused on prevention of T2DM, two found that financial incentives increased weight loss and completion of a fasting blood glucose test, and two choice architecture modifications had mixed effects in encouraging completion of tests to screen for T2DM. Of 11 studies that focused on improving self-management of T2DM, four of six tests of financial incentives demonstrated increased engagement in recommended care processes or improved biometric measures, and three of five tests of choice architecture modifications found improvements in self-management behaviors.
Summary
Though few studies have tested behavioral economic interventions for prevention or treatment of T2DM, those that have suggest such approaches have potential to improve patient behaviors and should be tested more broadly.
Keywords: behavioral economics, diabetes, self-management, prevention, interventions, scoping review
INTRODUCTION
The prevention and treatment of type 2 diabetes mellitus (T2DM) both have clearly defined clinical strategies that can help patients reduce their risk for future T2DM-related health problems. Patients with prediabetes -- who have not yet developed T2DM but have elevated blood glucose levels that put them at high risk for developing T2DM[1–3] -- can significantly reduce their risk for T2DM through two main strategies. First, they could participate in a Diabetes Prevention Program (DPP) with the goals to lose 7% of their body weight and get at least 150 minutes of moderate physical activity per week. Second, they could take metformin.[4–6] For patients who already have T2DM, weight loss[7] and regular physical activity,[8] an array of pharmacotherapies,[9] and numerous disease management programs[10] can help them optimize their disease control and reduce their risk for future complications. All of these strategies are either already widely available in clinical settings or are rapidly being disseminated through community partnerships.[11,12]
This widespread availability of efficacious strategies to prevent and treat T2DM has great potential to reduce the public health burden of T2DM.[13] However, the real world impact of these strategies is limited by low levels of patient engagement. In the case of prevention of T2DM, even when at-risk patients are aware of their elevated risk, few are consistently engaged in strategies to help them prevent or delay the onset of T2DM.[14–17] For individuals diagnosed with T2DM, while overall levels of glycemic control have increased in the United States in recent years, good glycemic control remains elusive for many,[18] and levels of recommended weight loss and physical activity are low in this population.[19]
One key contributor to this gulf between the widespread availability of efficacious strategies to prevent and treat T2DM and patients’ engagement in these strategies may be cognitive biases representing errors in mental processing that can keep individuals from taking steps that will help them achieve their long-term goals. In recent years such cognitive biases have been elucidated by the field of behavioral economics, which combines elements of neoclassical economics and psychology to improve the understanding and shaping of human behavior.[20] One important cognitive bias that may hinder engagement in strategies to prevent and treat T2DM is individuals’ tendency to overvalue near-term costs and benefits in their decision making.[21] These “present-biased preferences” can impede behaviors to prevent and treat T2DM because such behaviors often require individuals to exhibit high levels of self-control[22] by enduring certain and immediate inconveniences (e.g., decreasing pleasurable intake of high-caloric foods or injecting insulin) in return for uncertain and distant benefits (e.g., reducing long-term risk for complications from T2DM). Other examples of cognitive biases that can influence individuals’ decisions to engage in strategies to prevent and treat T2DM include their excessive optimism about experiencing a positive outcome in the future (e.g., expecting to win a state lottery after buying one ticket)[23] and their perception of losses of a given magnitude as being more painful than the enjoyment gained from otherwise equivalent gains (e.g., regretting losing $10 more than enjoying winning $10).[24]
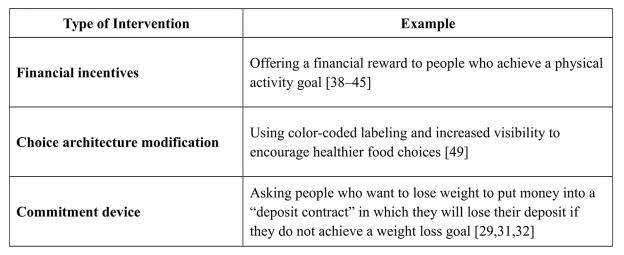
A range of interventions have been developed and tested that seek to overcome cognitive biases that can impede engagement in behaviors to achieve long-term goals (Figure 1). One such approach is the use of financial incentives to provide an immediate benefit for engaging in behaviors with certain and immediate inconveniences but uncertain and distant benefits. These types of incentives have been shown to promote weight loss,[25–37] physical activity,[38–45] and medication adherence.[46–48] Other approaches grounded in insights from behavioral economics with potential applications to the prevention and treatment of T2DM include choice architecture modifications (i.e., restructuring choice environments to systematically influence decisions)[49–52] and commitment devices (i.e., opportunities for individuals to commit their future selves to a course of action in order to achieve a long-term goal).[32,53–56]
Figure 1.
Categories of Behavioral Economic Interventions
Such behavioral economic approaches offer new opportunities to reduce the public health burden of T2DM by surmounting the cognitive biases that can limit patients’ engagement in evidence-based strategies to prevent and treat T2DM. The objective of our review was to identify behavioral economic strategies that have been tested to promote prevention and treatment of T2DM and suggest promising directions for future research in this area.
METHODS
Data Sources and Search Strategy
In January 2017 we conducted a comprehensive search of MEDLINE and PsycINFO, a key database of behavioral and social science research, for patient-oriented studies published after January 1, 2000 that used a behavioral economic strategy to prevent T2DM or to improve T2DM self-management.
Numerous behavioral economic strategies have been used to encourage lifestyle change. To ensure that we captured these diverse interventions in our review, a research librarian first conducted exploratory searches in MEDLINE and Google Scholar to find key articles and citations that related to behavioral economics and health promotion. Using this information, the research librarian then worked with two authors (AF and JK) to generate a broad search using controlled vocabulary (e.g., MeSH terms) and keywords to identify behavioral economic approaches (e.g., choice architecture, commitment devices, loss aversion, nudges, incentives, or reinforcements) used in interventions that were tested among individuals with elevated risk for developing T2DM (e.g., prediabetes or glucose intolerance) or existing T2DM. Because of great heterogeneity in intervention approaches, populations, and outcomes, and because to our knowledge there have been no previous efforts to summarize the use of patient-oriented behavioral economic strategies to improve T2DM prevention and treatment, our aim was to map this literature through a scoping review.[57]
Study Eligibility and Selection Criteria
We used the PICOTS (Population, Intervention, Comparator, Outcome, Time, Setting) framework to identify relevant studies (Table 1). Studies were eligible for inclusion if they evaluated a patient-facing behavioral economic intervention to either prevent T2DM (including both uptake of screening and modification of behavioral risk factors) or improve T2DM self-management among individuals with T2DM. We did not limit our review to any particular study design (e.g., randomized controlled trials) because several studies used an observational approach (e.g., a cohort study) to evaluate a behavioral economic intervention. We included studies with diverse outcomes given significant heterogeneity among study objectives and outcome measures. Outcomes for prevention of T2DM included weight loss and having a screening test for T2DM. Outcomes for T2DM self-management included medication adherence, home self-monitoring, use of recommended clinical monitoring, physical activity, enrollment in a health promotion program, and glycemic control. We excluded studies of provider-focused interventions such as financial incentives directed at providers (i.e., pay-for-performance) because these have been previously summarized.[58] We also excluded studies that used behavioral economic approaches to promote weight loss and physical activity without the stated aim of encouraging prevention of T2DM or improved T2DM self-management, as this literature is less directly related to the target populations for this review and has already been summarized elsewhere.[25,43,59] Finally, we excluded articles that were not published in English. The reference lists of articles meeting eligibility criteria were manually screened and electronic citation tracking was performed using Scopus. Additional search details are available from the authors upon request.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Population | |
|
|
| Intervention | |
|
|
| Comparator | |
|
|
| Outcomes | |
|
|
| Timing | |
|
|
| Setting | |
|
|
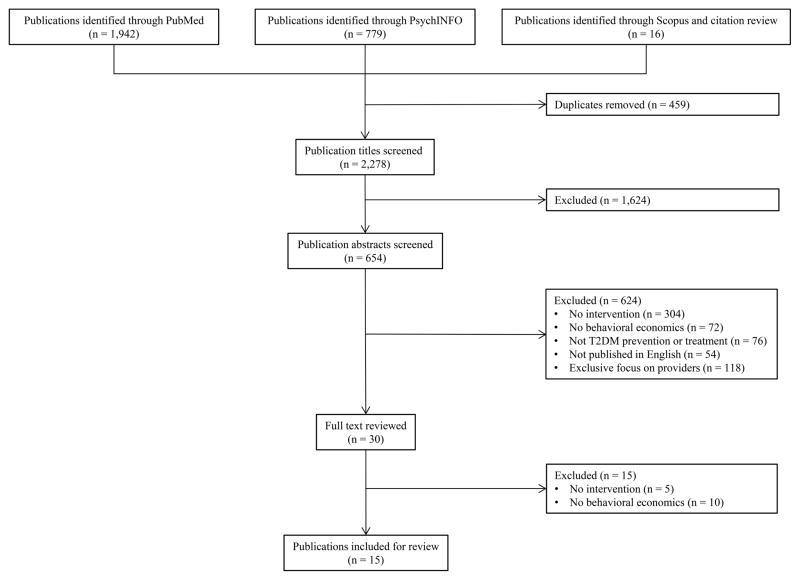
Using this search strategy, one author (AF) reviewed titles and abstracts for eligibility. Full text articles were reviewed in detail when the title and abstract contained insufficient information to determine study eligibility. All discrepancies were resolved through consensus discussion with a second author (JK). The full texts of 30 articles were reviewed in detail. Of these 30 articles, 15 publications met our eligibility criteria and were included in the final sample for our review (Figure 2).
Figure 2.
Article Flow Chart
Data Abstraction
One author (AF) abstracted the data related to the following: (1) study design and purpose; (2) behavioral economic approach; (3) participant characteristics, and (4) outcome measures. A second author (DH) reviewed each article to validate the abstracted data.
RESULTS
Fifteen publications met our eligibility criteria. These included four studies that tested behavioral economic strategies to improve the prevention of T2DM (Table 2) and 11 studies that tested behavioral economic strategies to improve the treatment of T2DM (Table 3).
Table 2.
Behavioral Economics Interventions Targeting T2DM Prevention
| Faghri, USA[60] | Mehrotra, South Africa[61] | Park, England[62] | Sallis, England[63] | |
|---|---|---|---|---|
| REACH | ||||
| Enrolled | 99 | 1,317,654 | 116 | 3,511 |
| Analyzed | 73 | 1,317,654 | 116 | 3,511 |
| Study population | Long-term nursing home facility employees | Members of private health plan in South Africa | Patients of general practice clinics | Patients of general practice clinics |
| Study design | 3-arm cluster randomized controlled trial
|
Prospective cohort study | 2-arm randomized controlled trial
|
Single-arm quasi-randomized controlled trial |
| Recruitment | Workplace announcements; newsletters; posters; flyers | NA | NA | Members eligible for a health check |
| Screening | All employees screened for eligibility | NA | Medical record review | NA |
| Eligibility | Non-diabetic; BMI ≥25kg/m^2; ADA Diabetes risk score ≥8 | Voluntary program available to all health plan members | Non-diabetic; aged 40–69 years; high risk of T2DM based on Cambridge risk score | Age 40 to 74 years |
| PARTICIPANT CHARACTERISTICS | ||||
| Mean age in years (SD) | IG: 45 (11) NIG: 49 (11) |
NR | Gain frame: 57.6 (7.7) Loss frame: 59.0 (7.0) |
Control: 53.1 (9.8) Intervention: 52.8 (9.8) |
| Mean BMI at baseline (SD) | IG: 36.7 (7.7) NIG: 33.9 (5.8) |
NR | Gain frame: 61% (n=35) ≥30 Loss frame: 68% (n=40) ≥30 |
NR |
| Gender (% female) | IG: 91 NIG: 89 |
Enrollees: 50.4 Non-enrollees: 52.3 |
Gain frame: 18 (32) Loss frame: 22 (37) |
Control: 939 (53.5) Intervention: 894 (50.9) |
| Race (% Caucasian) | IG: 40 NIG: 55 |
NR | NR | NR |
| Ethnicity (% Hispanic) | IG: 2.9 NIG: 6.3 |
NR | NR | NR |
| INTERVENTION CHARACTERISTICS | ||||
| Strategy | Financial incentives | Financial incentives | Message-framing | Behavioral science influenced invitations |
| Program details | Standard incentive: received $10 for every 1.0lbs lost per week if BMI ≥25 or 1.5 lbs lost per week if BMI ≥30. Standard incentive plus deposit: same incentive as standard + deposit up to $80 (based on weight loss target) which was met dollar-for-dollar if participants met goal | Voluntary reward program; participants pay monthly rate ($17/individual, $21/family); earn points for healthy behaviors (e.g. smoking cessation, preventive care); greater points lead to greater discounts on goods and services (e.g. movie tickets, flights). Additional points earned for normal cholesterol and glucose levels. | Invitations with loss frame message (e.g., “If have diabetes, but are not detected early, your diabetes may lead to more complications) or gain frame message (e.g., “If your diabetes is detected early, you can receive early and more effective treatment) used to invited individuals to participate in T2DM screening. |
Control group: received standard letters from national primary prevention program Intervention group: received a letter with 4 modifications: (1) simplified sentences; (2) clear action steps (e.g., call to book an appointment); (3) personalization (e.g., “you are due for a health check); (4) action reminder (e.g., tear-off slip to place on the refrigerator) |
| Duration | 16-weekly sessions | 6 years (2005 to 2011) | NR | 1 time screening event |
| Primary outcome(s) | IG participants lost 2.3 kg more than those in the NIG* | Incentive program participants were more likely to receive preventive care services than non-participants, including blood glucose testing (OR 1.51)* | 82% (n=95) of patients attended T2DM screening; there was no significant difference in the overall rate of T2DM screening between loss framed group and gain framed group | 33.5% (n=588) of individuals who received the intervention letter attended T2DM screening compared with 29.3% (n=514) of those who received the standard letter (OR 1.26, 95% CI 1.09–1.47) |
p < 0.05
NA = not applicable, NR = not reported
Table 3.
Behavioral Economics Interventions Targeting T2DM Management
| Austin, USA[64] | Gopalan (1), South Africa[70] | Gopalan (2), USA[74] | |
|---|---|---|---|
| REACH | |||
| Enrolled | 2,565 | 3,906 | 177 |
| Analyzed | 2,565 | 3,665 | 177 |
| Study population | Academic medical center patients | Health plan members | Academic Internal Medicine practices |
| Study design | Quasi-experimental study | 5-arm randomized controlled trial:
|
3-arm randomized controlled trial: “T2DM report card” used to reflect an individual’s glycemic control using:
|
| Recruitment | Eligible participants contacted by study team | Eligible participants contacted by study team | Eligible participants contacted by study team |
| Screening | Physical referral or medical record review | Billing / pharmacy codes for T2DM or oral T2DM medications | Medical record review |
| Eligibility | T2DM; no HbA1c or LDL-C within 1 year | Age ≥18 years; T2DM | Age ≥18 years; T2DM; HbA1c >8% |
| PARTICIPANT CHARACTERISTICS | |||
| Mean age in years (SD) | Control: 58.6 Intervention: 63.7 |
(1) through (5)
refer to study arms, defined above (1) 55.9 (10.9); (2) 55.2 (10.7) ; (3) 55.0 (10.8); (4) 55.4 (10.0); (5) 56.0 (10.6) |
Control: 56.5 Grade: 57.8 Face: 55.0 |
| Mean baseline HbA1c (%) | NR | NR | Control: 10.2 Grade: 9.7 Face: 9.8 |
| Gender (% female) | Control: 53.2 Intervention: 49.6 |
(1) through (5)
refer to study arms, defined above (1) 19.7; (2) 20.2; (3) 19.8; (4) 19.1; (5) 20.6 |
Control: 29.3 Grade: 22.4 Face: 27.9 |
| Race (% Caucasian) | NR | NR | Control: 3.5 Grade: 3.4 Face: 6.6 |
| Ethnicity (% Hispanic) | Control: 4.8 Intervention: 2.6 |
NR | NR |
| INTERVENTION CHARACTERISTICS | |||
| Strategy | Financial incentives and patient reminders | Message-framing | Message-framing |
| Program details | Participants notified by postal letter that they were past due for lab testing; $6 gas cards offered as reward for completing test | 5 e-mail messaging strategies (detailed in “study design”) used to encourage participants to join HF program; 1 initial e-mail and 2 reminder e-mails | Glycemic control shown using 1 of 3 strategies in letters sent via postal mail: (1) HbA1c; (2) letter grade; (3) face/emotion |
| Duration | 3 months | 1 month | 6 months |
| Primary outcomes | Increased HbA1c screening frequency among intervention vs. control group in 2-years after intervention (3.34 vs. 2.69 screening tests)* | All message groups had higher HF enrollment rates than the control group*; enrollment rates were highest in the EAC arm | HbA1c at 6 months did not differ significantly between groups. |
| Grady, USA[71] | Hochberg, Israel[72] | Huntsman, USA[65] | |
|---|---|---|---|
| REACH | |||
| Enrolled | 180 | 27 | 60 |
| Analyzed | 155 | 27 | 50 |
| Study population | Diabetes outpatient facility | NR | Community health clinic; predominantly Hispanic/Latino population |
| Study design | Randomized controlled trial | Randomized controlled trial | 3-arm randomized controlled trial:
|
| Recruitment | Advertisements in local newspapers | NR | Patients recruited during routine medical visits |
| Screening | Certified diabetes educator assessed cognitive and physical ability to perform foot care. | NR | Screening of medical records performed so eligible patients notified at regular visit. |
| Eligibility | Age ≥18 years; T2DM for > 5 years; no known foot problems | T2DM; sedentary lifestyle | Age ≥18 years; insulin-dependent type 1 or type 2 diabetes; limited financial access to glucose monitoring system |
| PARTICIPANT CHARACTERISTICS | |||
| Mean age in years (SD) | 61.2 (11.4) | Control: 55.1 (3.6) Intervention: 58.7 (2.1) |
NR |
| Mean baseline HbA1c (%) | NR | Control: 8.7 Intervention: 7.7 |
NR |
| Gender (% female) | 58.7 | Control: 14 Intervention: 40 |
NR |
| Race (% Caucasian) | NR | NR | NR |
| Ethnicity (% Hispanic) | NR | NR | NR |
| INTERVENTION CHARACTERISTICS | |||
| Strategy | Message-framing | Message-framing | Financial incentives |
| Program details | Foot care educational video adapted to gain-framed (i.e. positive outcome of good foot care) and loss-framed (i.e. negative outcome of poor foot care) versions; participant behavior was assessed at 0-, 3-, and 6-months. |
Control group: weekly SMS reminders to exercise Intervention: personalized SMS messages (1 to 7 per week) to encourage physical activity through positive and negative feedback with and without social component |
Participants in all 3 study arms (detailed above) instructed to measure blood glucose 2/day; glucometer data reviewed by study team at 30 days; incentive group rewarded with gift cards based on the number of measurements (maximum $50). |
| Duration | 1-time intervention, 10-minutes in duration | 3 months | 30 days |
| Primary outcomes | Foot care behavioral scores increased in both groups; gain-framed messages led to sustained positive behavior change* | Intervention participants increased minutes of walking/day compared to the control participants* | No significant differences between groups in adherence to self-monitoring of blood glucose |
| Long, USA[69] | Misra-Hebert, USA[68] | Myers, USA[73] | |
|---|---|---|---|
| REACH | |||
| Enrolled | 118 | 1,092 | NR |
| Analyzed | 118 | 1,092 | 239 |
| Study population | African American veterans | Academic medical center Employee Health Program (EHP) members | Adults with T2DM |
| Study design | 3-arm randomized controlled trial | Retrospective cohort study; 2-year cohorts used to analyze effect of specific incentives (2008–09, 2009–10, 2010–11, 2011–12) | Randomized controlled trial |
| Recruitment | Eligible participants contacted by study team | NA | Eligible participants contacted by study team |
| Screening | Medical record review | EHP claims data linked with medical record data | Medical record review |
| Eligibility | T2DM; HbA1c ≥8% on 2 occasions; age 50–70 years; black/African American | T2DM | T2DM, Age ≥18 years |
| PARTICIPANT CHARACTERISTICS | |||
| Mean age in years (SD) | Control: 60(4) Peer mentor group: 60 (5) Financial incentive group: 59 (5) |
NR | 58.63 (11.96) |
| Mean baseline HbA1c (%) | Control: 9.9 (1.6) Peer mentor group: 9.8 (1.8) Financial incentive group: 9.5 (1.2) |
2008–09: 7.52 (1.82) 2009–10: 7.35 (1.57) 2010–11: 7.33 (1.6) 2011–12: 7.31 (1.69) |
NR |
| Gender (% female) | Control: 8 Peer mentor group: 0 Financial incentive group: 10 |
2008–09: 73 2009–10: 72 2010–11: 71 2011–12: 71 |
52 |
| Race (% Caucasian) | 0 | 2008–09: 62 2009–10: 61 2010–11: 58 2011–12: 58 |
70 |
| Ethnicity (% Hispanic) | 0 | NR | 12 |
| INTERVENTION CHARACTERISTICS | |||
| Strategy | Financial incentives | Financial incentives | Message-framing |
| Program details | Peer mentor group: mentor had controlled T2DM and was trained in motivational interviewing; weekly mentor-mentee telephone calls encouraged. Financial incentives: $100 for 1 point HbA1c decrease or $200 for 2 point decrease or HbA1c ≤ 6.5% at 6 months | 2008–09: fixed $100 incentive to participate in a disease management program 2010: fixed $300 incentive 2011–12: up to 30% health insurance premium discount based on participation and achievement of clinical goals (HbA1c < 7%) |
Participants received health messages framed by valence (gains vs losses) crossed with temporal proximity (immediate vs distal) in four frames, surveys sent via mail |
| Duration | 6 months | 4 years | 1 time intervention |
| Primary outcomes | Change in HbA1c at 6-months: Peer support: −1.07 (95% CI −1.84 to −0.31)* Incentive: −0.45 (95% CI −1.23 to 0.32) |
The change in HbA1c was not significant between EHP members and non-employee comparison group | There were no significant differences in the intention to increase physical activity among frames |
| Raiff, USA[66] | Sen, USA[67] | |
|---|---|---|
| REACH | ||
| Enrolled | 3 | 75 |
| Analyzed | 3 | 75 |
| Study population | T2DM patients of a local endocrinology clinic. | Primary care medical home patients |
| Study design | Multiple baseline design | 3-arm randomized controlled trial:
|
| Recruitment | Participants recruited during routine office visits | Eligible participants contacted by study team |
| Screening | Medical record review | Medical record review |
| Eligibility | T2DM; age 30–75 years; oral T2DM treatment requiring ≥2 daily doses; missed ≥ 1 dose in past 7 days; HbA1c 7–10% | Age18–80 years; HbA1c ≥7.5% |
| PARTICIPANT CHARACTERISTICS | ||
| Mean age in years (SD) | 45 | Control: 54.1 (2.0) Low-incentive: 54.7 (2.1) High-incentive: 54.3 (1.9) |
| Mean baseline HbA1c (%) | NR | Control: 9.3 (0.3) Low-incentive: 9.3 (0.4) High-incentive: 9.8 (0.3) |
| Gender (% female) | 33 | Control: 61 Low-incentive: 67 High-incentive: 65 |
| Race (% Caucasian) | 33 | Control: 25 Low-incentive: 29 High-incentive: 27 |
| Ethnicity (% Hispanic) | NR | NR |
| INTERVENTION CHARACTERISTICS | ||
| Strategy | Financial incentives and patient reminders | Financial incentives |
| Program details | Baseline phase: electronic medication dispenser used to monitor medication adherence; $10 for completion (regardless of adherence) Treatment phase: text message reminders if medication was not taken within user-specified timeframe; financial reward for adherence started at $1.00 and increased by $0.20/day; $1.50 bonus for every 3 days of adherence; non-adherence reset reward to $1.00 | All participants received 3 devices for self-monitoring: (1) glucometer; (2) blood pressure cuff; (3) digital scale; device data electronically transferred to study website; incentive-arm participants selected a 2-digit number between 1 to 99 at start of the study; the study’s website randomly selected a number within this range each morning and participants could win if they had used all devices on the day prior |
| Duration | Baseline: 8–12 days; Treatment: 21–23 days | 12-weeks |
| Primary outcomes | Daily medication adherence rates increased during the treatment phase compared to the baseline phase* | Low- and high-incentive participants had significantly higher rates of device adherence than control participants (low incentive: 81%, high incentive: 77%, control: 58%)* Adherence declined upon discontinuation of the incentive, particularly among high-incentive participants |
p < 0.05
NR = not reported
Behavioral Economic Strategies to Improve Prevention of T2DM
Of the four studies focused on prevention of T2DM, two tested the use of different forms of financial incentives. In the first incentive study, 99 overweight or obese employees from four US long-term care facilities who had elevated risk for T2DM were enrolled in a group-level randomized trial in which they participated in a 16-week weight loss program.[60] In the program, all participants received a personalized weight loss and physical activity consultation that established weekly weight loss goals. Employees of two of the four facilities were randomized to receive financial incentives for losing weight that they could receive as either (1) a fixed payment per pound of weight lost or (2) an opportunity to commit their own money for each pound of weight they hoped to lose that was matched dollar-for-dollar by the investigators but forfeited when goals were not achieved. The other two facilities served as controls. Immediately after the 16-week program, employees in the incentivized facilities lost an average of 5.0 pounds more and had healthier eating scores compared to employees in the non-incentivized facilities. After a 12-week follow-up only the healthier eating difference was sustained. The authors did not find evidence of differences in outcomes between the two incentive approaches, but noted their study was not powered to evaluate their comparative effectiveness. The second incentive study was a cohort study of 4,186,047 members of a private health insurance plan in South Africa in which enrollees could choose to pay roughly 20 US dollars per month to join a voluntary incentive program.[61] In this program, enrollees could receive points for healthy behaviors such as receiving clinical preventive services (e.g., fasting glucose testing), visiting a gym, or buying healthier foods. These points accumulated and could then be exchanged for discounts on a variety of consumer goods and services. Nearly two-thirds (65.5%) of plan members joined the incentive program, which led to an estimated 4.7% increase in fasting glucose testing relative to plan members who did not join the incentive program.
The other two studies focused on prevention of T2DM tested different ways of framing information about opportunities to participate in health screenings that aimed to prevent T2DM and related chronic conditions. In the first framing study, 116 non-diabetic patients of two general practices in the UK who were found to be at elevated risk for T2DM were randomized to receive by mail one of two invitations to be screened for T2DM: (1) a gain frame (“if your diabetes is detected early, you can receive earlier and more effective treatment”) or (2) a loss frame (“if you have diabetes but are not detected early, your diabetes may lead to more complications”).[62] There were no differences in screening uptake between the two arms. In the second framing study, 3,511 patients of four general practices of the National Health Service in the UK were randomized to receive by mail one of two invitations to participate in a cardiovascular risk assessment (an NHS Health Check) that in part aimed to prevent or delay the onset of T2DM.[63] The control invitation used a standard informational NHS Health Check invitation template. The intervention invitation featured four manipulations that leveraged specific behavioral science insights: simplification, behavioral specificity, enhanced personal salience, and implementation intentions. These manipulations of the standard invitation template led to a statistically significant 4.2% increase in the percentage of patients who attended an NHS Health Check.
Behavioral Economic Strategies to Improve Treatment of T2DM
Of the 11 studies focused on treatment of T2DM, six tested different forms of financial incentives. Two studies tested gift cards for completing recommended processes of care. One quasi-experimental study of 1,157 patients with T2DM who were due for hemoglobin A1c (HbA1c) and low-density lipoprotein cholesterol (LDL-C) testing found that a reminder letter combined with a $6 gas card increased the mean number of HbA1c tests over two years from 2.7 to 3.3.[64] A three-arm pilot randomized trial of 60 insulin-dependent diabetic patients found that neither a gift card worth $10 for every 10 glucometer measurements nor pain-free lancets increased self-monitoring of blood glucose over 30 days compared to usual care.[65]
Two studies tested other forms of financial incentives to encourage adherence to recommended care processes. The first study was a single-arm nonconcurrent multiple baseline trial which found that graduated daily financial incentives for medication adherence worth up to $84.10 combined with text messages improved medication adherence by nearly 40% over three weeks among three patients who were prescribed oral medications for their T2DM.[66] The second study was a six month, three-arm randomized trial that tested whether 12 weeks of lottery incentives with daily values of $2.80 and $1.40, respectively, could increase daily home wireless device monitoring of blood glucose, blood pressure, and weight among patients with T2DM.[67] Both incentives significantly increased daily home self-monitoring over 12 weeks by approximately the same amount, but only the smaller incentive led to higher levels of daily self-monitoring 12 weeks after the incentives ended.
Two studies tested financial incentives that were partially or completely based on achieving diabetes care targets. The first study was a retrospective cohort analysis of 2,103 employees with T2DM who received either a $100 incentive to participate in an employer-sponsored disease management program, a $300 incentive to participate in the disease management program, or a 30% health insurance premium discount that was tied to both disease management program participation and achievement of HbA1c, LDL-C, and blood pressure targets.[68] Overall these financial incentives led to modest declines in HbA1c levels, systolic blood pressure, LDL-C, and weight over five years compared to 2,672 commercially insured non-employee patients of the same primary care physicians. The other study was a three-arm randomized trial of 118 predominantly African American Veterans with T2DM and persistently poor glycemic control. Participants received $100 for decreasing HbA1c by one point and $200 for decreasing HbA1c by two points or to 6.5%. Financial incentives did not improve HbA1c levels over six months while the study arm receiving a peer mentoring program without financial incentives decreased HbA1c levels by an average of 1.1 points.[69]
The other five studies examined different ways to frame information to encourage better self-management of T2DM. Four of these studies focused on improving behaviors that are key components of T2DM self-management. The first study was a randomized trial among 3,906 diabetic members of a South African health insurance plan who chose to participate in a voluntary wellness program and found that a T2DM-specific message, a recommendation from a peer with T2DM, a physician’s recommendation, and a T2DM-specific message with a choice architecture modification called enhanced active choice[52] all increased enrollment in an incentivized healthy food program at 1 month, with little difference in gains achieved among the different approaches.[70] The second study randomized 180 patients with longstanding T2DM to view a gain-framed or loss-framed educational video about proper foot care and found that the gain-framed video increased reported recommended foot care behaviors six months later more than the loss-framed video.[71] The third study randomized 27 sedentary patients with T2DM, a pedometer, and personal physical activity plan to receive repeated text messages that either did (treatment) or did not (control) adapt based on an individual’s walking behaviors and found that the adaptive messages were significantly more effective in increasing the amount and pace of their walking.[72] The fourth study was a randomized trial that used a two-by-two design to test gain vs. loss and immediate vs. distal frames to increase physical activity among 239 patients with T2DM and found no differences in immediate intentions to increase physical activity among the four arms.[73] Just one study examined the effects of message framing on glycemic control. In this study, 177 patients with poorly controlled T2DM were randomized to one of three “report cards” that communicated current glycemic control as either a letter grade ranging from A to F, faces with an emotion, or an HbA1c value, and found no differences in glycemic control or perceptions of control at six months.[74]
DISCUSSION
Our scoping review identified 15 studies that have tested different behavioral economic approaches to enhance the prevention and treatment of T2DM. The vast majority of these studies focused on treatment rather than prevention, and most tested financial incentives, which to date have likely been the most widely tested behavioral economic strategy to encourage healthy behaviors, rather than choice architecture modifications or commitment devices.
The eight studies of financial incentives we identified illustrate that financial incentives have potential in the short-term to encourage behaviors that are integral to the successful prevention and self-management of T2DM. Yet the varied incentive designs, populations, and outcomes in these studies preclude the ability to draw firm conclusions about where, when, and how incentives might work best to promote healthy behaviors in different contexts and among different populations. One important conclusion that can be drawn from these studies, however, is that in future research there are a number of ways in which financial incentives could better support the prevention and treatment of T2DM. First, many of the financial incentives that were tested in this context offered rewards that were infrequent and provided long after the behavior they were seeking to encourage. Such rewards could more effectively promote engagement in recommended behaviors by being more frequent and immediately after the targeted behavior. Second, although financial incentives for healthy behaviors have become nearly ubiquitous in large US workplaces,[75,76] such incentives are typically offered either for a one-time behavior (e.g., completion of a health risk appraisal) or for a limited period of time. Therefore, incentives might be most cost-effective if they are used to encourage participation in time-limited programs known to have long-term benefits (e.g., DPPs[5,77]) or to enhance engagement in programs that help individuals form new habits (e.g., daily self-monitoring of glucose or weight) that may carry forward into the future even when those behaviors are not being directly incentivized. Third, though financial incentives have been shown to encourage a range of healthy behaviors that are integral to the prevention and self-management of T2DM, these changes are often modest and not well sustained after removal of the incentives. Financial incentives could potentially be more effective by integrating insights from behavioral economics with principles of other evidence-based psychological approaches such as self-determination theory.[78,79] For example, incentives could be reinforced with tailored messages that link incentives to people’s aspirations in life as well as link recommended behaviors to these aspirations. In this way, financial incentives could be more salient and cultivate greater autonomous motivation to prevent and treat T2DM, resulting in sustained engagement in recommended behaviors after incentives end.[80,81]
We identified seven studies that tested different message framing strategies to encourage prevention and self-management of T2DM. Those that were effective either integrated multiple behavioral science insights into messages that encouraged a specific one-time behavior (e.g., enrollment in a healthy eating program) or used an alternative modality (e.g., video or repeated text messages) that afforded a higher messaging “dose” to encourage longitudinal behaviors that required a greater degree of effort. Messages that were ineffective either used simple gain vs. loss framing or targeted only proximal mediators (e.g., perceptions or intentions) of engagement in the target behavior. These findings highlight the promise of messaging strategies which leverage novel behavioral insights. They also underscore the importance of ensuring messages to encourage T2DM prevention and treatment are of a dose that is proportionate to the effort required to engage in the targeted behavior.
One promising behavioral economic strategy that was applied in a limited way in just one of the studies we identified was the use of a commitment device.[60] This strategy of committing one’s self to a future course of action and then applying supports to encourage adherence to that course of action[55,82–84] has been shown to improve a range of behaviors that can be influenced by cognitive biases such as savings,[85] academic performance,[83] tobacco use,[54,86] and weight loss.[29,32]. This strategy could have important applications in both T2DM prevention and treatment, for example by inviting individuals to commit to taking evidence-based steps to prevent T2DM or to improve their self-management of T2DM.
This review has several limitations. First, we only included interventions described in the peer-reviewed literature and therefore may not have captured interventions sponsored by employers or health plans that have not been reported in journals. Second, because the field of behavioral economics draws on psychology and other behavioral sciences to overcome the limitations of neoclassical economic theory it is perhaps less of a discrete field than other social science disciplines. As a result, there are no commonly accepted definitions of what constitutes a behavioral economic intervention. Thus our team had to rely on our own knowledge of this literature to classify interventions as using a behavioral economic approach. Third, we conducted a scoping review which aimed to map out the use of behavioral economic strategies in the context of preventing and treating T2DM. Consistent with the focus of this type of review we did not systematically rate the quality of evidence generated from each study. However, we have strived to describe the studies we reviewed in sufficient detail for readers to understand their respective methodologic strengths and limitations.
CONCLUSIONS
Behavioral economic approaches have great potential to overcome the present-biased preferences that can hinder patients’ engagement in healthy behaviors, including proven strategies to prevent and treat T2DM. Our scoping review identified a number of studies that illustrate this potential and highlight key future directions for research into behavioral economic strategies to improve the prevention and treatment of T2DM.
Acknowledgments
We are grateful for the assistance of Judy Smith of the Taubman Health Sciences Library at the University of Michigan for her assistance with developing and refining the search strategy for our scoping review. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government. Support was provided by the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service. Dr. Kullgren is a VA HSR&D Career Development awardee at the Ann Arbor VA. This study also received support from the National Institute of Diabetes and Digestive and Kidney Diseases (Grant Number P30DK092926 (MCDTR)).
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Jeffrey T. Kullgren has received consulting fees from SeeChange Health and HealthMine, and speaking honoraria from AbilTo, Inc. Dina Hafez, Michele Heisler, and Allison Fedewa declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease?: A meta-analysis of prospective studies. Arch Intern Med. 2004;164:2147–55. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM. Pre-Diabetes, Metabolic Syndrome, and Cardiovascular Risk. J Am Coll Cardiol. 2012;59:635–43. doi: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated Hemoglobin, Diabetes, and Cardiovascular Risk in Nondiabetic Adults. N Engl J Med. 2010;362:800–11. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 5.Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. The Lancet. 2009;374:1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care. 2015;38:1161–72. doi: 10.2337/dc14-1630. [DOI] [PubMed] [Google Scholar]
- 8.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065–79. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Standards of Medical Care in Diabetes-2017: Summary of Revisions. Diabetes Care. 2017;40:S4–5. doi: 10.2337/dc17-S003. [DOI] [PubMed] [Google Scholar]
- 10.Pimouguet C, Le Goff M, Thiébaut R, Dartigues JF, Helmer C. Effectiveness of disease-management programs for improving diabetes care: a meta-analysis. CMAJ. 2011;183:E115–27. doi: 10.1503/cmaj.091786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vojta D, Sa JD, Prospect T, Stevens S. Effective Interventions For Stemming The Growing Crisis Of Diabetes And Prediabetes: A National Payer’s Perspective. Health Aff (Millwood) 2012;31:20–6. doi: 10.1377/hlthaff.2011.0327. [DOI] [PubMed] [Google Scholar]
- 12.Vojta D, Koehler TB, Longjohn M, Lever JA, Caputo NF. A Coordinated National Model for Diabetes Prevention: Linking Health Systems to an Evidence-Based Community Program. Am J Prev Med. 2013;44:S301–6. doi: 10.1016/j.amepre.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States 2014. Atlanta, GA: US Department of Health and Human Services; 2014. [Internet] Available from: https://www.cdc.gov/diabetes/data/statistics/2014StatisticsReport.html. [Google Scholar]
- 14.Yang K, Lee Y-S, Chasens ER. Outcomes of Health Care Providers’ Recommendations for Healthy Lifestyle Among U. Adults with Prediabetes Metab Syndr Relat Disord. 2011;9:231–7. doi: 10.1089/met.2010.0112. [DOI] [PubMed] [Google Scholar]
- 15.Okosun IS, Lyn R. Prediabetes awareness, healthcare provider’s advice, and lifestyle changes in American adults. Int J Diabetes Mellit [Internet] 2010 [cited 2012 Aug 29]; Available from: http://www.sciencedirect.com/science/article/pii/S1877593410001074.
- 16.Ruge T, Nyström L, Lindahl B, Hallmans G, Norberg M, Weinehall L, et al. Recruiting High-Risk Individuals to a Diabetes Prevention Program: How hard can it be? Diabetes Care. 2007;30:e61–e61. doi: 10.2337/dc06-2466. [DOI] [PubMed] [Google Scholar]
- 17.Gopalan A, Lorincz IS, Wirtalla C, Marcus SC, Long JA. Awareness of Prediabetes and Engagement in Diabetes Risk–Reducing Behaviors. Am J Prev Med. 2015;49:512–9. doi: 10.1016/j.amepre.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in Prevalence and Control of Diabetes in the U ., 1988–1994 and 1999–2010. Ann Intern Med. 2014;160:517. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Bolge SC, Lopez JMS, Zhu VJ, Stang PE. Changes in Body Weight Among People With Type 2 Diabetes Mellitus in the United States, NHANES 2005–2012. Diabetes Educ. 2016;42:336–45. doi: 10.1177/0145721716640096. [DOI] [PubMed] [Google Scholar]
- 20.Loewenstein G, Brennan T, Volpp KG. Asymmetric paternalism to improve health behaviors. JAMA. 2007;298:2415–7. doi: 10.1001/jama.298.20.2415. [DOI] [PubMed] [Google Scholar]
- 21.O’Donoghue T, Rabin M. Doing It Now or Later. Am Econ Rev. 1999;89:103–24. [Google Scholar]
- 22.Loewenstein G. Adv Behav Econ. Princeton, NJ: Princeton University Press; 2004. Out of Control: Visceral Influences on Behavior; pp. 689–723. [Google Scholar]
- 23.Svenson O. Are we all less risky and more skillful than our fellow drivers? Acta Psychol (Amst ) 1981;47:143–8. [Google Scholar]
- 24.Kahneman D, Tversky A. Prospect Theory: An Analysis of Decision under Risk. Econometrica. 1979;47:263–91. [Google Scholar]
- 25.Jeffery RW. Financial Incentives and Weight Control. Prev Med. 2012;55:S61–7. doi: 10.1016/j.ypmed.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffery RW, Wing RR, Thorson C, Burton LR, Raether C, Harvey J, et al. Strengthening behavioral interventions for weight loss: A randomized trial of food provision and monetary incentives. J Consult Clin Psychol. 1993;61:1038–45. doi: 10.1037//0022-006x.61.6.1038. [DOI] [PubMed] [Google Scholar]
- 27.Jeffery RW, Gerber WM, Rosenthal BS, Lindquist RA. Monetary contracts in weight control: Effectiveness of group and individual contracts of varying size. J Consult Clin Psychol. 1983;51:242–8. doi: 10.1037//0022-006x.51.2.242. [DOI] [PubMed] [Google Scholar]
- 28.Jeffery RW, Bjornson-Benson WM, Rosenthal BS, Kurth CL, Dunn MM. Effectiveness of monetary contracts with two repayment schedules on weight reduction in men and women from self-referred and population samples. Behav Ther. 1984;15:273–9. [Google Scholar]
- 29.Volpp KG, John LK, Troxel AB, Norton L, Fassbender J, Loewenstein G. Financial incentive–based approaches for weight loss: A randomized trial. JAMA. 2008;300:2631–7. doi: 10.1001/jama.2008.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kullgren JT, Troxel AB, Loewenstein G, Asch DA, Norton LA, Wesby L, et al. Individual-Versus Group-Based Financial Incentives for Weight Loss: A Randomized, Controlled Trial. Ann Intern Med. 2013;158:505–14. doi: 10.7326/0003-4819-158-7-201304020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.John LK, Loewenstein G, Troxel AB, Norton L, Fassbender JE, Volpp KG. Financial incentives for extended weight loss: a randomized, controlled trial. J Gen Intern Med. 2011;26:621–6. doi: 10.1007/s11606-010-1628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kullgren JT, Troxel AB, Loewenstein G, Norton LA, Gatto D, Tao Y, et al. A Randomized Controlled Trial of Employer Matching of Employees’ Monetary Contributions to Deposit Contracts to Promote Weight Loss. Am J Health Promot. 2016;30:441–52. doi: 10.1177/0890117116658210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leahey TM, Subak LL, Fava J, Schembri M, Thomas G, Xu X, et al. Benefits of adding Small Financial Incentives or Optional Group Meetings to a Web-based Statewide Obesity Initiative. Obesity. 2015;23:70. doi: 10.1002/oby.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leahey T, Rosen J. DietBet: A Web-Based Program that Uses Social Gaming and Financial Incentives to Promote Weight Loss. JMIR Serious Games [Internet] 2014 doi: 10.2196/games.2987. [cited 2016 Dec 26];2. Available from: http://games.jmir.org/2014/1/e2/ [DOI] [PMC free article] [PubMed]
- 35.Finkelstein EA, Linnan LA, Tate DF, Birken BE. A Pilot Study Testing the Effect of Different Levels of Financial Incentives on Weight Loss Among Overweight Employees. J Occup Environ Med. 2007;49:981–9. doi: 10.1097/JOM.0b013e31813c6dcb. [DOI] [PubMed] [Google Scholar]
- 36.Cawley J, Price JA. A case study of a workplace wellness program that offers financial incentives for weight loss. J Health Econ. 2013;32:794–803. doi: 10.1016/j.jhealeco.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 37.Lahiri S, Faghri PD. Cost-effectiveness of a workplace-based incentivized weight loss program. J Occup Environ Med. 2012;54:371–7. doi: 10.1097/JOM.0b013e318247a394. [DOI] [PubMed] [Google Scholar]
- 38.Charness G, Gneezy U. Incentives to Exercise. Econometrica. 2009;77:909–31. [Google Scholar]
- 39.Patel MS, Asch DA, Rosin R, Small DS, Bellamy SL, Heuer J, et al. Framing Financial Incentives to Increase Physical Activity Among Overweight and Obese Adults: A Randomized, Controlled Trial. Ann Intern Med. 2016;164:385–94. doi: 10.7326/M15-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel MS, Volpp KG, Rosin R, Bellamy SL, Small DS, Fletcher MA, et al. A Randomized Trial of Social Comparison Feedback and Financial Incentives to Increase Physical Activity. Am J Health Promot. 2016;30:416–24. doi: 10.1177/0890117116658195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patel MS, Asch DA, Rosin R, Small DS, Bellamy SL, Eberbach K, et al. Individual Versus Team-Based Financial Incentives to Increase Physical Activity: A Randomized, Controlled Trial. J Gen Intern Med. 2016;31:746–54. doi: 10.1007/s11606-016-3627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pope L, Harvey J. The efficacy of incentives to motivate continued fitness-center attendance in college first-year students: a randomized controlled trial. J Am Coll Health. 2014;62:81–90. doi: 10.1080/07448481.2013.847840. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell MS, Goodman JM, Alter DA, John LK, Oh PI, Pakosh MT, et al. Financial incentives for exercise adherence in adults: systematic review and meta-analysis. Am J Prev Med. 2013;45:658–67. doi: 10.1016/j.amepre.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 44.Strohacker K, Galárraga O, Emerson J, Fricchione SR, Lohse M, Williams DM. Impact of Small Monetary Incentives on Exercise in University Students. Am J Health Behav. 2015;39:779–86. doi: 10.5993/AJHB.39.6.5. [DOI] [PubMed] [Google Scholar]
- 45.Royer H, Stehr MF, Sydnor JR. Incentives, Commitments and Habit Formation in Exercise: Evidence from a Field Experiment with Workers at a Fortune-500 Company [Internet] National Bureau of Economic Research; 2012. Nov, Report No.: 18580. Available from: http://www.nber.org/papers/w18580. [Google Scholar]
- 46.Volpp KG, Loewenstein G, Troxel AB, Doshi J, Price M, Laskin M, et al. A test of financial incentives to improve warfarin adherence. BMC Health Serv Res. 2008;8:272. doi: 10.1186/1472-6963-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kimmel SE, Troxel AB, Loewenstein G, Brensinger CM, Jaskowiak J, Doshi JA, et al. Randomized trial of lottery-based incentives to improve warfarin adherence. Am Heart J. 2012;164:268–74. doi: 10.1016/j.ahj.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reese PP, Kessler JB, Doshi JA, Friedman J, Mussell AS, Carney C, et al. Two Randomized Controlled Pilot Trials of Social Forces to Improve Statin Adherence among Patients with Diabetes. J Gen Intern Med. 2016;31:402–10. doi: 10.1007/s11606-015-3540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thorndike AN, Sonnenberg L, Riis J, Barraclough S, Levy DE. A 2-Phase Labeling and Choice Architecture Intervention to Improve Healthy Food and Beverage Choices. Am J Public Health. 2012;102:527–33. doi: 10.2105/AJPH.2011.300391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Downs JS, Loewenstein G, Wisdom J. Strategies for Promoting Healthier Food Choices. Am Econ Rev. 2009;99:159–64. doi: 10.1257/aer.99.2.159. [DOI] [PubMed] [Google Scholar]
- 51.Liu PJ, Wisdom J, Roberto CA, Liu LJ, Ubel PA. Using Behavioral Economics to Design More Effective Food Policies to Address Obesity. Appl Econ Perspect Policy. 2014;36:6–24. [Google Scholar]
- 52.Keller PA, Harlam B, Loewenstein G, Volpp KG. Enhanced active choice: A new method to motivate behavior change. J Consum Psychol. 2011;21:376–83. [Google Scholar]
- 53.Ariely D, Wertenbroch K. Procrastination, Deadlines, and Performance: Self-Control by Precommitment. Psychol Sci. 2002;13:219–24. doi: 10.1111/1467-9280.00441. [DOI] [PubMed] [Google Scholar]
- 54.Giné X, Karlan D, Zinman J. Put Your Money Where Your Butt Is: A Commitment Contract for Smoking Cessation. Am Econ J Appl Econ. 2010;2:213–35. [Google Scholar]
- 55.Halpern SD, Asch DA, Volpp KG. Commitment contracts as a way to health. BMJ. 2012;344:e522–e522. doi: 10.1136/bmj.e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rogers T, Milkman KL, Volpp KG. Commitment devices: Using initiatives to change behavior. JAMA. 2014;311:2065–6. doi: 10.1001/jama.2014.3485. [DOI] [PubMed] [Google Scholar]
- 57.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. [Google Scholar]
- 58.Lorincz IS, Lawson BCT, Long JA. Provider and Patient Directed Financial Incentives to Improve Care and Outcomes for Patients with Diabetes. Curr Diab Rep. 2013;13:188–95. doi: 10.1007/s11892-012-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paul-Ebhohimhen V, Avenell A. Systematic review of the use of financial incentives in treatments for obesity and overweight. Obes Rev. 2008;9:355–67. doi: 10.1111/j.1467-789X.2007.00409.x. [DOI] [PubMed] [Google Scholar]
- 60•.Faghri PD, Li R. Effectiveness of Financial Incentives in a Worksite Diabetes Prevention Program. Open Obes J. 2014;6:1–12. doi: 10.2174/1876823720140107001. This study tested financial incentives for weight loss among employees at high risk for developing T2DM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mehrotra A, An R, Patel DN, Sturm R. Impact of a Patient Incentive Program on Receipt of Preventive Care. Am J Manag Care. 2014;20:494–501. [PMC free article] [PubMed] [Google Scholar]
- 62.Park P, Simmons RK, Prevost AT, Griffin SJ. A Randomized Evaluation of Loss and Gain Frames in an Invitation to Screening for Type 2 Diabetes: Effects on Attendance, Anxiety and Self-rated Health. J Health Psychol. 2010;15:196–204. doi: 10.1177/1359105309344896. [DOI] [PubMed] [Google Scholar]
- 63•.Sallis A, Bunten A, Bonus A, James A, Chadborn T, Berry D. The effectiveness of an enhanced invitation letter on uptake of National Health Service Health Checks in primary care: a pragmatic quasi-randomised controlled trial. BMC Fam Pract [Internet] 2016 doi: 10.1186/s12875-016-0426-y. [cited 2017 Feb 13];17. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4806508/ This study tested whether an enhanced invitation letter using insights from behavioral science could increase attendance at National Health Service Health Checks in the UK. [DOI] [PMC free article] [PubMed]
- 64.Austin S, Wolfe B. The effect of patient reminders and gas station gift cards on patient adherence to testing guidelines for diabetes. Wis Med J. 2011;110:132–7. [PubMed] [Google Scholar]
- 65.Huntsman MAH, Olivares FJ, Tran CP, Billimek J, Hui EE. Pain Reduction and Financial Incentives to Improve Glucose Monitoring Adherence in a Community Health Center. PLOS ONE. 2014;9:e114875. doi: 10.1371/journal.pone.0114875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raiff BR, Jarvis BP, Dallery J. Text-message reminders plus incentives increase adherence to antidiabetic medication in adults with type 2 diabetes. J Appl Behav Anal. 2016;49:947–53. doi: 10.1002/jaba.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67•.Sen AP, Sewell TB, Riley EB, Stearman B, Bellamy SL, Hu MF, et al. Financial Incentives for Home-Based Health Monitoring: A Randomized Controlled Trial. J Gen Intern Med. 2014;29:770–7. doi: 10.1007/s11606-014-2778-0. This study tested whether financial incentives could increase adherence to home self-monitoring of blood glucose, blood pressure, and weight among patients with poorly controlled T2DM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68•.Misra-Hebert AD, Hu B, Taksler G, Zimmerman R, Rothberg MB. Financial Incentives and Diabetes Disease Control in Employees: A Retrospective Cohort Analysis. J Gen Intern Med. 2016;31:871–7. doi: 10.1007/s11606-016-3686-2. This study examined whether financial incentives could increase participation in disease management and improve measures of disease control among employees with T2DM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Long JA, Jahnle EC, Richardson DM, Loewenstein G, Volpp KG. Peer Mentoring and Financial Incentives to Improve Glucose Control in African American Veterans: A Randomized, Controlled Trial. Ann Intern Med. 2012;156:416–24. doi: 10.1059/0003-4819-156-6-201203200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70•.Gopalan A, Paramanund J, Shaw PA, Patel D, Friedman J, Brophy C, et al. Randomised controlled trial of alternative messages to increase enrolment in a healthy food programme among individuals with diabetes. BMJ Open. 2016;6:e012009. doi: 10.1136/bmjopen-2016-012009. This study compared the effectiveness of T2DM-focused messaging strategies at increasing enrollment in a healthy food program among adults with T2DM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Grady JL, Entin EB, Entin EE, Brunyé TT. Using message framing to achieve long-term behavioral changes in persons with diabetes. Appl Nurs Res. 2011;24:22–8. doi: 10.1016/j.apnr.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 72•.Hochberg I, Feraru G, Kozdoba M, Mannor S, Tennenholtz M, Yom-Tov E. Encouraging Physical Activity in Patients With Diabetes Through Automatic Personalized Feedback via Reinforcement Learning Improves Glycemic Control. Diabetes Care. 2016;39:e59–60. doi: 10.2337/dc15-2340. This study examined whether a mobile phone application with a learning algorithm could improve adherence to exercise in patients with T2DM. [DOI] [PubMed] [Google Scholar]
- 73.Myers R. Moderating the effectiveness of messages to promote physical activity in type 2 diabetes. Grad Theses Diss [Internet] 2010 Available from: http://scholarcommons.usf.edu/etd/1719.
- 74•.Gopalan A, Tahirovic E, Moss H, Troxel AB, Zhu J, Loewenstein G, et al. Translating the Hemoglobin A1C with More Easily Understood Feedback: A Randomized Controlled Trial. J Gen Intern Med. 2014;29:996–1003. doi: 10.1007/s11606-014-2810-4. This study tested the effect of two alternative communication formats of HbA1c values on improving glycemic control among patients with poorly controlled T2DM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.19th Annual Towers Watson/National Business Group on Health Employer Survey on Purchasing Value in Health Care [Internet] Towers Watson; 2014. [cited 2014 Nov 10]. Available from: http://www.towerswatson.com/en-US/Insights/IC-Types/Survey-Research-Results/2014/05/full-report-towers-watson-nbgh-2013-2014-employer-survey-on-purchasing-value-in-health-care. [Google Scholar]
- 76.Claxton G, Rae M, Long M, Damico A, Whitmore H, Foster G. Health Benefits In 2016: Family Premiums Rose Modestly, And Offer Rates Remained Stable. Health Aff (Millwood) 2016;35:1908–17. doi: 10.1377/hlthaff.2016.0951. [DOI] [PubMed] [Google Scholar]
- 77.Group TDPPR. The 10-Year Cost-Effectiveness of Lifestyle Intervention or Metformin for Diabetes Prevention: An intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723–30. doi: 10.2337/dc11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Deci EL, Ryan RM. The “What” and “Why” of Goal Pursuits: Human Needs and the Self-Determination of Behavior. Psychol Inq. 2000;11:227–68. [Google Scholar]
- 79.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55:68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 80.Kullgren JT, Williams GC, An LC. Patient-centered financial incentives for health: Can employers get change for their dollars? Healthcare. 2013;1:82–5. doi: 10.1016/j.hjdsi.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 81.Kullgren JT, Williams GC, Resnicow K, An LC, Rothberg A, Volpp KG, et al. The promise of tailoring incentives for healthy behaviors. Int J Workplace Health Manag. 2016;9:2–16. doi: 10.1108/IJWHM-12-2014-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thaler Richard H. Toward a Positive Theory of Consumer Choice. J Econ Behav Organ. 1980:39–60. [Google Scholar]
- 83.Ariely D, Wertenbroch K. Procrastination, Deadlines, and Performance: Self-Control by Precommitment. Psychol Sci. 2002;13:219–24. doi: 10.1111/1467-9280.00441. [DOI] [PubMed] [Google Scholar]
- 84.Rogers T, Milkman KL, Volpp KG. Commitment devices: using initiatives to change behavior. JAMA. 2014;311:2065–6. doi: 10.1001/jama.2014.3485. [DOI] [PubMed] [Google Scholar]
- 85.Strotz RH. Myopia and inconsistency in dynamic utility maximization. Rev Econ Stud. 1955;23:165–80. [Google Scholar]
- 86.Halpern SD, French B, Small DS, Saulsgiver K, Harhay MO, Audrain-McGovern J, et al. Randomized Trial of Four Financial-Incentive Programs for Smoking Cessation. N Engl J Med. 2015 doi: 10.1056/NEJMoa1414293. [DOI] [PMC free article] [PubMed] [Google Scholar]