Abstract
Previous research has shown that there is considerable overlap in the neural networks mediating successful memory encoding and retrieval. However, little is known about how the relevant human brain regions interact during these distinct phases of memory or how such interactions are affected by memory deficits that characterize mild cognitive impairment (MCI), a condition that often precedes dementia due to Alzheimer’s disease. Here we employed multivariate Granger causality analysis using autoregressive modeling of inferred neuronal time series obtained by deconvolving the hemodynamic response function from measured blood oxygenation level- dependent (BOLD) time series data, in order to examine the effective connectivity between brain regions during successful encoding and/or retrieval of object location associations in MCI patients and comparable healthy older adults. During encoding, healthy older adults demonstrated a left hemisphere dominant pattern where the inferior frontal junction, anterior intraparietal sulcus (likely involving the parietal eye fields), and posterior cingulate cortex drove activation in most left hemisphere regions and virtually every right hemisphere region tested. These regions are part of a frontoparietal network that mediates top-down cognitive control and is implicated in successful memory formation. In contrast, in the MCI patients, the right frontal eye field drove activation in every left hemisphere region examined, suggesting reliance on more basic visual search processes. Retrieval in the healthy older adults was primarily driven by the right hippocampus with lesser contributions of the right anterior thalamic nuclei and right inferior frontal sulcus, consistent with theoretical models holding the hippocampus as critical for the successful retrieval of memories. The pattern differed in MCI patients, in whom the right inferior frontal junction and right anterior thalamus drove successful memory retrieval, reflecting the characteristic hippocampal dysfunction of these patients. These findings demonstrate that neural network interactions differ markedly between MCI patients and healthy older adults. Future efforts will investigate the impact of cognitive rehabilitation of memory on these connectivity patterns.
1. Introduction
The field of cognitive psychology has long held that the mental processes invoked during the encoding and retrieval of memories are inexorably linked (see [1] for review). In support of this idea, functional neuroimaging research has revealed substantial overlap in the brain regions mediating the successful encoding and retrieval of memories [2–4] . This finding is often referred to as the “reinstatement hypothesis” since it conveys the importance of re-activating the same brain regions during both phases of memory. Rugg and colleagues [5] proposed a neuroanatomical model wherein the encoding-related neocortical representations of a stimulus are bound into a memory by the hippocampus. The successful retrieval of this memory depends on the re-activation of at least a subset of these previously engaged regions, which is facilitated by the hippocampus. In many respects, this model is consonant with the multiple trace theory of episodic memory proposed by Moscovitch and colleagues [6].
Extensive evidence suggests that, relative to their younger counterparts, cognitively intact older adults show greater lateral prefrontal (PFC) activation that is typically viewed as compensatory in nature [7] and is present even after controlling for potential vascular confounds [8]. Greater effective connectivity has also been reported between PFC subregions as well as between the PFC and medial temporal lobe (MTL) structures in older relative to younger adults [9, 10]. Thus, both activation and connectivity analyses suggest that older adults rely comparatively more on the PFC during memory encoding and retrieval. This shift may reflect increased top-down cognitive control over memory-related processes, perhaps compensating for age-related decline in hippocampal structure and function [11].
Mild cognitive impairment (MCI) is generally believed to be a precursor to dementia, particularly of the Alzheimer’s type [12]. In contrast to the normal patterns of learning and memory described above, patients with MCI experience characteristic memory deficits that are typically attributed to medial temporal lobe (e.g., hippocampal) dysfunction [12, 13]. However, we [14] and others [15] have reported widespread hypoactivation in patients with MCI, suggesting network-level dysfunction. Further, correlational analyses between activation magnitude and behavioral performance have revealed marked shifts in the brain regions associated with new memories in MCI patients relative to controls [14, 16]. Such findings led us to hypothesize that MCI patients rely on more basic cognitive processes (e.g., focusing on physical properties of stimuli) while learning new information, at the expense of more effective “top-down” strategies that are at least partially dependent on the PFC [14]. This interpretation is consonant with both behavioral [17–19] and neuroimaging [20] findings of impaired top-down control in patients with MCI. Thus, memory deficits in MCI may arise following the combination of hippocampal dysfunction and loss of the previously noted, age-appropriate upregulation of the PFC; a possibility supported by previous research [21].
Effective connectivity studies capture directionality of influence and are an ideal method for assessing this possible shift in cognitive processing. However, most of the existing work on connectivity in MCI has relied on functional connectivity analyses (which do not include directionality information) using data acquired during the resting state. Such data may not accurately reflect active cognitive processes such as learning and memory, especially given recent findings of poor relationships between measures of functional and effective connectivity in patients with MCI [22]. Of the few effective connectivity studies in MCI patients, Neufang and colleagues [22] demonstrated significantly reduced connectivity, when compared to healthy controls, between frontal and parietal regions known to be involved in top-down processing. The current study is, to the best of our knowledge, the first to examine effective connectivity during memory encoding and retrieval in patients with MCI and comparable healthy controls. Given the literature reviewed above, we predicted that patients with MCI, when compared with healthy older adults, would demonstrate reduced PFC connectivity during encoding (i.e., the loss of top- down processing) and that the hippocampus would play a reduced role during retrieval given the characteristic dysfunction in this structure.
2. Materials and Methods
2.1 Participants
A total of 34 right-handed older adults, of whom 18 were MCI patients and 16 were healthy older controls, completed functional magnetic resonance imaging (fMRI) scanning as they encoded and recalled object-location associations (OLAs). Their baseline neuropsychological (see Table 1) and encoding-related fMRI data were reported earlier [14]. Each of the MCI patients had been diagnosed with amnestic MCI according to Petersen’s criteria [23] at a consensus conference, prior to being included in our study. Specifically, they presented with subjective memory complaints and showed objective evidence of memory impairment but intact everyday functioning. The healthy older controls (HOC) were recruited from a longitudinal registry maintained by the Emory Alzheimer’s Disease Research Center and from the general community. These individuals were free of subjective and objective memory impairments and were independent in activities of daily living. General exclusion criteria included a history of neurologic disease (e.g. dementia, stroke, epilepsy, traumatic brain injury), psychiatric disorders (e.g. severe depression, bipolar disorder, schizophrenia), and current or past alcohol or drug abuse.
Table 1.
Demographic, neuropsychological, brain volumetrics, and performances on the experimental OL task for the healthy older controls (HOC) and MCI groups. Standard deviations are provided in parentheses.
| HOC (n=16) | MCI (n=18) | t(32) | p-value | |
|---|---|---|---|---|
| Age | 72.1 (7.3) | 71.2 (8.5) | 0.33 | .74 |
| Education (years) | 16.1 (2.7) | 17.1 (2.1) | 1.19 | .25 |
| MMSE | 27.8 (1.97) | 26.7 (2.3) | 1.47 | .15 |
| RBANS Indices (Standard Scores) | ||||
| Immediate Memory* | 105.8 (13.5) | 87.1 (13.2) | 4.1 | <.001 |
| Visuospatial/construction | 99.5 (14.4) | 94.9 (16.8) | 0.84 | .41 |
| Language* | 103.3 (15.2) | 92.2 (7.1) | 2.77 | .009 |
| Attention | 110.4 (11.8) | 105.9 (11.8) | 1.11 | .28 |
| Delayed Memory* | 103.8 (8.9) | 74.9 (15.1) | 6.68 | <.001 |
| Total Score* | 106.5 (14.4) | 87.8 (10.4) | 4.39 | <.001 |
| Trails A (T-scores) | 49.0 (8.7) | 45.8 (12.1) | 0.87 | .39 |
| Trails B (T-scores) | 50.4 (9.0) | 47.7 (6.7) | 0.98 | .34 |
| GDS (raw scores) | 1.1 (1.9) | 1.6 (1.9) | 0.74 | .46 |
| FAQ* (raw scores) | 0.4 (0.7) | 3.6 (3.9) | 3.07 | .005 |
| Brain Volumetrics (% intracranial volume; ICV) | ||||
| Cortical gray | 30.1 (2.1) | 29.1 (1.6) | 1.57 | .13 |
| Lateral ventricles | 2.2 (0.8) | 2.8 (1.0) | 1.95 | .06 |
| Inferior lateral ventricles | 0.16 (0.05) | 0.19 (0.07) | 1.73 | .09 |
| Hippocampus (Total) | 0.49 (0.06) | 0.46 (0.06) | 1.39 | .18 |
| Left | 0.24 (0.03) | 0.23 (0.03) | 1.0 | .32 |
| Right | 0.25 (0.03) | 0.24 (0.04) | 1.5 | .14 |
| Amygdala | 0.23 (0.03) | 0.21 (0.05) | 1.70 | .10 |
| Object-Location Association Test | ||||
| Novel accuracy (% correct)* | 48.6 (11.3) | 37.3 (10.6) | 3.0 | .005 |
| Novel reaction time (in seconds) | 2.72 (.64) | 2.78 (.50) | 0.3 | .79 |
| Repeated accuracy (% correct) | 99.2 (1.6) | 95.9 (9.5) | 1.3 | .19 |
| Repeated reaction time (in seconds) | 0.94 (.16) | 1.0 (.32) | 0.6 | .55 |
Significant differences are indicated by * (via t-tests).
MMSE = mini-mental state exam; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; GDS = Geriatric Depression Scale; FAQ = Functional Activities Questionnaire. Brain volumes were obtained from NeuroQuant®. Table modified from Hampstead et al., (2011), Neuropsychologia, 49, 2349–2361.
As previously reported [14], all participants completed a brief neuropsychological screening protocol at the time of enrollment, which was typically within one month before fMRI scanning. The protocol consisted of the Mini Mental Status Exam (MMSE) [24], the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [25], the Trail Making Test, the Geriatric Depression Scale (GDS) [26], and the Functional Activities Questionnaire (FAQ) [27]. The RBANS includes a series of tests that comprise 5 larger indices (Immediate Memory, Visuospatial/construction, Attention, Language, Delayed Memory) as well as a Total score. Multiple studies have demonstrated the utility of the RBANS for diagnosing MCI and Alzheimer’s dementia [28–30]. The resulting neuropsychological data were use to verify that MCI patients had not progressed to dementia or reverted to “normal” (i.e., ensuring persistent impairment) and that HOC were not demonstrating deficits relative to historic expectations. As seen in Table 1, the groups were comparable in regard to demographic (i.e., age, education) and most cognitive variables (aside from learning, memory, and semantic fluency). The groups also demonstrated comparable brain volumes (see 2.4 Volumetric analysis), thereby mitigating concerns about partial volume effects confounding the imaging data. Each participant provided informed written consent. Emory University’s Institutional Review Board and the Research and Development Committee of the Atlanta VAMC approved the study.
2.2 fMRI scanning paradigm
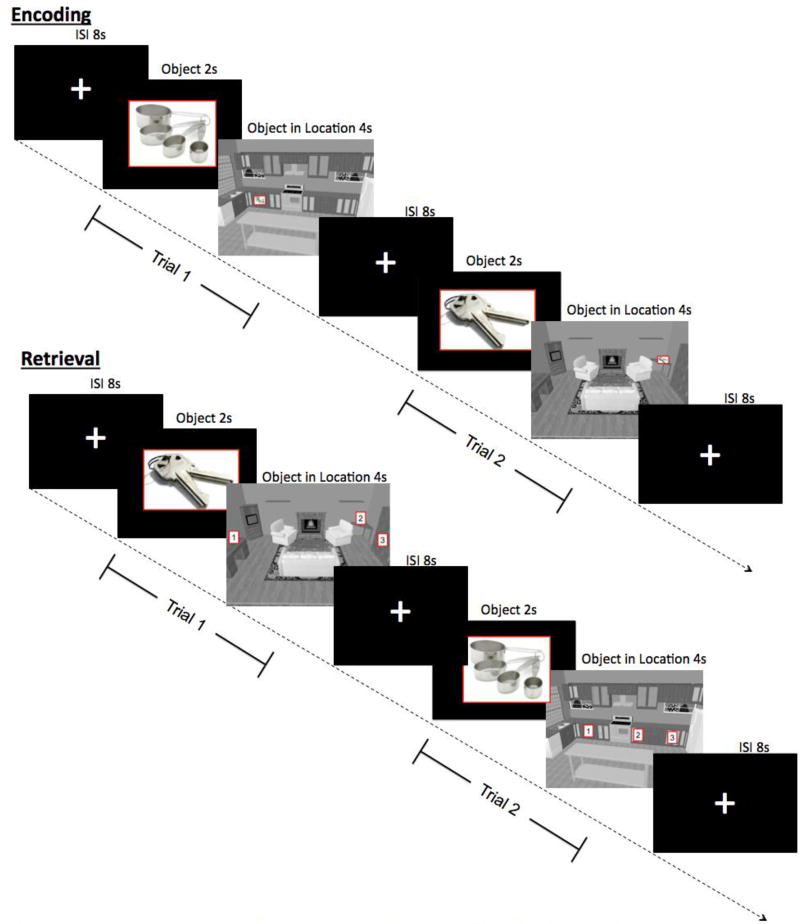
The OLA task was described in detail earlier [14]. Briefly, participants were instructed to learn and remember 92 OLAs. The objects were selected because they were highly concrete, familiar, imageable, and frequently used in everyday life. We created two versions of each of 9 rooms that are encountered in daily life (bathroom, bedroom, dining room, garage, kitchen, laundry room, living room, office, recreational room). In each room, we identified five locations that spanned the width and, to the extent possible, height of the room and then pseudorandomly placed 5 objects within each room such that any of the objects could have reasonably appeared in any of the locations. This resulted in 90 OLAs that were each shown once during fMRI scanning (see below) and are referred to as the “novel stimuli” hereafter. The remaining two OLAs were created by placing a single object within an additional kitchen and living room. These two stimuli were presented multiple times during scanning, which controlled for low-level perceptual processes and general task familiarity; they are referred to as the “repeated stimuli” hereafter.
The same design was used for both the encoding and retrieval scans, which were separated by a 1-hour delay. During each of 5 functional runs, participants viewed 18 novel and 9 repetitions of the repeated stimuli in a “slow” event-related design. Each 6s trial consisted of a 2s object-only phase (to allow for object identification) followed immediately by the object in its location for 4s. Trials were separated by 8s ISIs to allow return of signal to baseline. Six 10s baseline periods were pseudorandomly distributed in each run to allow for estimation of baseline signal. Total run length was 438s. The order of runs was randomized for each participant.
During encoding, participants were instructed to remember the object’s location. During retrieval, participants selected the object’s location from among 3 choices, each of which was an actual target location within that room, using an fMRI-compatible response pad. This design was intended to promote recollection (i.e., requiring the retrieval of the unique object-location pair) over familiarity (i.e., recalling that something belonged within a location without clear memory for the actual pairing).
2.3 fMRI scanning parameters
MR scans were performed on a Siemens Trio 3T MRI scanner (Siemens Medical Solutions, Malvern, PA), using a 12-channel head coil. For blood oxygenation level-dependent (BOLD) contrast, T2*-weighted functional images were acquired using a single- shot, gradient-recalled, echo-planar imaging (EPI) sequence with the following parameters: repetition time (TR) 2000ms, echo time (TE) 30ms, field of view (FOV) 220mm, flip angle (FA) 90°, 29 axial slices of 4mm thickness, in-plane resolution 3.4×3.4mm, and in-plane matrix 64×64. These images were co-registered with high-resolution anatomic images acquired using a 3D MPRAGE sequence (TR 2300ms, TE 3.9ms, inversion time (TI) 1100ms, FA 8°) consisting of 176 sagittal slices of 1mm thickness (FOV 256mm, in-plane resolution 1×1mm, in-plane matrix 256×256).
2.4 Volumetric analysis
As previously described [14], we obtained volumetric measurements from the high-resolution anatomic images using NeuroQuant®, which is a fully automated program that has been validated against other segmentation procedures and found sensitive to volumetric changes in mild AD [31] and MCI [30]. This program provides volumes for three medial temporal lobe regions (hippocampus, amygdala, inferior lateral ventricles). We used the percent of intracranial volume (%ICV) values for each of these regions since this method reduces measurement variability more than other commonly used methods [32]. These values are shown in Table 1; importantly, there were no significant differences between the groups.
2.5 Functional image analyses
As described earlier in detail [14], image processing and analysis were performed using Brain-Voyager QX v2.4 (Brain Innovation, Maastricht, The Netherlands). Functional runs were motion-corrected in real time using Siemens 3D-PACE (prospective acquisition motion correction). For each subject, the functional images were realigned to the first image of the series. Images were pre-processed using trilinear-sinc interpolation for intra-session alignment of functional volumes, sinc interpolation for slice scan time correction, and high-pass temporal filtering to two cycles per run to remove slow drifts in the data. They were then co- registered with anatomic images and transformed into Talairach space. For group analysis, transformed data were spatially smoothed with an isotropic Gaussian kernel of 4mm (full-width half-maximum) and normalized across runs and subjects using the default z-baseline normalization option in BrainVoyager (based on data where the predictor values are at or near zero (≤0.1)).
We coded each novel trial as either correct or incorrect based on whether the participant successfully recalled the unique OLA pairing during the retrieval scan. Incorrect trials were excluded from subsequent analyses. The HOC performed significantly better than chance (t(15)=5.4, p<0.001; range = 34.45 – 72.22% correct) while the MCI did not (t(17)=1.58, p=0.132; range = 21.11 – 65.56% correct). However, both groups responded significantly faster on correct than incorrect trials during the retrieval scan (HOC: t(15)=6.67, p<0.001; MCI: t(17)=2.34, p=0.032) thereby suggesting differences in cognitive processing between trials with correct responses and those with incorrect responses. There was no significant relationship between accuracy and reaction time differences between trials with incorrect and correct responses in either group (HOC: r=0.114, p=0.674; MCI: r=−0.155, p=0.54). Given the low accuracy of the MCI group, we performed additional analyses of the fMRI data. These results, illustrated in Supplemental Figures 1–3, clearly demonstrate that there were, in fact, significant differences between correct and incorrect trials on both contrast- and connectivity-based analyses.
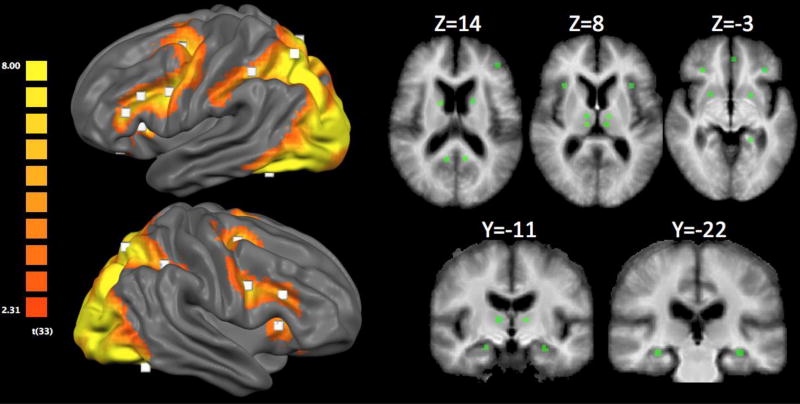
As stated above, our primary aim was to examine the patterns of connectivity between brain regions during memory encoding and retrieval. Therefore, we used a general linear model (GLM) treating participants as a random factor, and performed a conjunction analysis to identify brain regions more active for the novelcorrect than the repeated trials (i.e., novelcorrect > repeated) during both encoding and retrieval in the entire sample (i.e., both healthy controls and MCI patients). The resulting FDR-corrected (FDR q≤0.05) activation map (Fig. 2) revealed bilateral activations in brain regions associated with the ventral visual stream (e.g., fusiform gyrus), dorsal visual stream (e.g., superior parietal lobule), lateral frontoparietal cognitive control network (e.g., inferior frontal gyrus, intraparietal sulcus), and medial temporal lobe (e.g., hippocampal body). We selected a total of 29 regions of interest (ROIs) from this contrast (Table 2), using previous meta-analytic findings of areas that are involved in successful encoding and retrieval [4] to guide our selection.
Figure 2.
Surface rendering of the activation map from the conjunction analysis. Results are FDR corrected at p<0.05. White squares represent regions selected for subsequent Granger causality analysis. Subcortical ROIs can be visualized by the green squares in the volume-based slices to the right.
Table 2.
Areas included in GCA analyses and the contrast from which they were identified.
| Peak Talairach Coordinates |
|||||
|---|---|---|---|---|---|
| Area number for GCA |
x | y | z | t-max | |
| 1 | Rostral prefrontal cortex (lateral)* | −39 | 38 | 17 | 7.94 |
| 2 | Superior frontal gyrus (medial) | −5 | 8 | 47 | 7.42 |
| 3 | Anterior cingulate cortex (dorsal)* | −11 | 20 | 32 | 8.26 |
| 4 | Rostral medial prefrontal cortex+ | −6 | 54 | 25 | 4.64 |
| 5 | Ventromedial prefrontal cortex+ | −2 | 41 | −6 | 4.46 |
| 6 | Superior frontal sulcus (frontal eye fields) | −24 | −4 | 52 | 8.04 |
| 7 | Inferior frontal sulcus | −41 | 18 | 25 | 7.38 |
| 8 | Inferior frontal gyrus | −47 | 27 | 19 | 9.48 |
| 9 | Posterior orbital gyrus | −32 | 29 | −4 | 5.66 |
| 10 | Inferior frontal junction | −43 | 2 | 28 | 9.90 |
| 11 | Insula | −32 | 17 | 6 | 7.17 |
| 12 | Caudate nucleus* | −17 | 5 | 13 | 7.28 |
| 13 | Putamen* | −20 | 5 | −4 | 8.50 |
| 14 | Anterior thalamic nuclei | −12 | −13 | 9 | 6.25 |
| 15 | Dorsomedial thalamic nuclei | −11 | −18 | 4 | 7.20 |
| 16 | Temporal pole+ | −47 | 12 | −22 | 5.49 |
| 17 | Middle temporal gyrus+ | −61 | −18 | −7 | 4.53 |
| 18 | Hippocampus (anterior)+ | −26 | −10 | −13 | 3.34 |
| 19 | Hippocampus (body) | −29 | −22 | −7 | 4.69 |
| 20 | Parahippocampal gyrus | −21 | −40 | −5 | 8.32 |
| 21 | Fusiform gyrus | −38 | −55 | −16 | 9.91 |
| 22 | Retrosplenial cortex | −9 | −52 | 10 | 8.55 |
| 23 | Posterior cingulate cortex (dorsal)* | −11 | −30 | 38 | 8.53 |
| 24 | Intraparietal sulcus (anterior) | −36 | −44 | 40 | 7.21 |
| 25 | Superior parietal lobule | −17 | −67 | 47 | 10.91 |
| 26 | Rostral prefrontal cortex (lateral)* | 30 | 40 | 19 | 7.79 |
| 27 | Superior frontal gyrus (medial) | 6 | 9 | 46 | 7.92 |
| 28 | Anterior cingulate cortex (dorsal)* | 6 | 20 | 32 | 10.74 |
| 29 | Superior frontal sulcus (frontal eye fields) | 24 | −5 | 52 | 7.52 |
| 30 | Inferior frontal sulcus | 40 | 20 | 23 | 5.64 |
| 31 | Posterior orbital gyrus | 27 | 30 | −4 | 4.76 |
| 32 | Inferior frontal junction | 41 | 3 | 30 | 7.97 |
| 33 | Insula | 30 | 18 | 8 | 5.42 |
| 34 | Caudate nucleus* | 16 | 2 | 14 | 8.88 |
| 35 | Putamen* | 18 | 5 | −4 | 10.51 |
| 36 | Anterior thalamic nuclei | 9 | −13 | 7 | 6.62 |
| 37 | Dorsomedial thalamic nuclei | 9 | −18 | 8 | 6.65 |
| 38 | Hippocampus (anterior) | 21 | −9 | −12 | 3.96 |
| 39 | Hippocampus (body) | 27 | −22 | −11 | 5.64 |
| 40 | Parahippocampal gyrus | 22 | −34 | −10 | 9.86 |
| 41 | Fusiform gyrus | 36 | −54 | −16 | 9.94 |
| 42 | Retrosplenial cortex | 7 | −52 | 10 | 7.50 |
| 43 | Posterior cingulate cortex (dorsal)* | 8 | −29 | 34 | 8.77 |
| 44 | Intraparietal sulcus (anterior) | 33 | −46 | 39 | 4.90 |
| 45 | Superior parietal lobule | 18 | −67 | 46 | 10.59 |
Note:
denotes ROIs that were more active during encoding;
denotes ROIs that were uniquely active during retrieval.
All unmarked ROIs were selected from the conjunction analysis. Coordinates represent the peak (tmax) within each ROI.
In order to ensure that areas preferentially involved in encoding or retrieval were also included in the connectivity analyses, we directly compared the novelcorrect trials during encoding and retrieval (i.e., encoding novelcorrect > retrieval novelcorrect). The resulting FDR-corrected activation map (FDR q≤0.05) revealed 6 areas active during encoding but not retrieval: the anterior hippocampus, other temporal lobe structures (e.g., middle temporal gyrus; temporal pole), and medial PFC (see Table 2). Ten additional regions were selected because they were uniquely associated with retrieval (e.g., caudate, putamen, cingulate cortex, rostral PFC). We again referred to meta-analytic findings [4] whenever possible.
In all then, a total of 45 ROIs were identified and submitted to the effective connectivity analyses described below. All ROIs were cubes of 5 mm per side, centered on the peaks of activation. Although deactivations are important, it is less clear how to interpret effective connectivity in relation to deactivations as compared to activations; thus, deactivations were not considered in this initial study.
2.6 Effective connectivity analyses
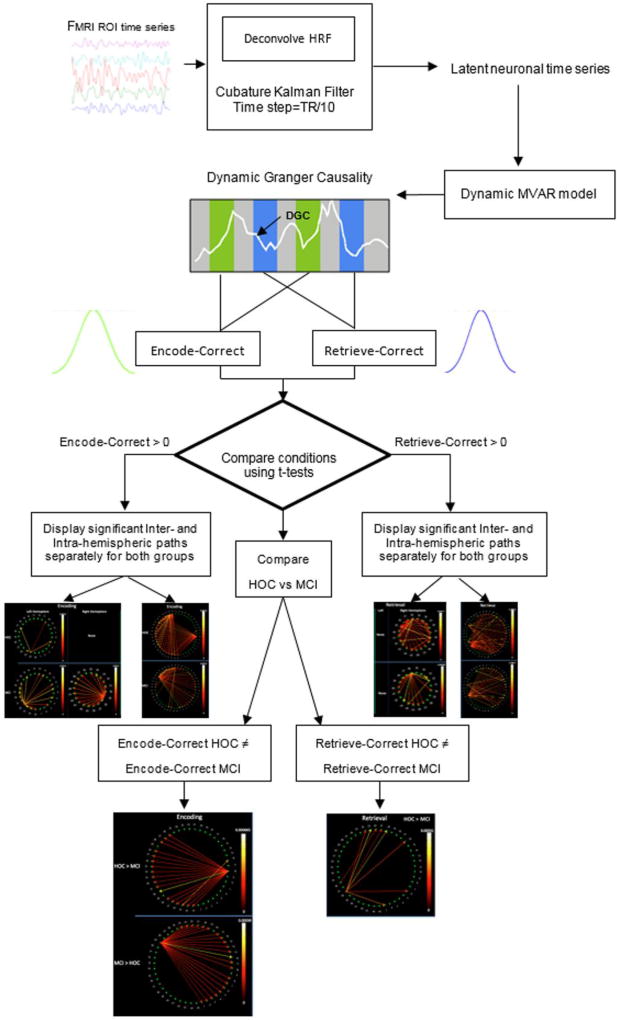
Granger causality analysis (GCA) is an exploratory method that can be used to study directional influences between different brain regions. The principle underlying GCA is that, if past values of time series “A” help predict the future values of time series “B”, then a causal influence from time series A to time series B can be inferred [33]. GCA was performed on latent neuronal time series obtained by blind deconvolution of the hemodynamic response function (HRF) from the measured BOLD time series, as described in detail in recent publications [34–38]. Such hemodynamic deconvolution removes the smoothing effect of the HRF and also its inter-subject and inter-regional variability [39], leading to improved estimation of effective connectivity [40, 41] and avoiding potential confounds due to HRF variability across regions within individuals, and between individuals (whether normal or pathologic). The BOLD time series were averaged across voxels contained in each ROI, normalized separately for each run and participant by dividing by the grand mean for that run, and concatenated across all runs and participants to form a single vector per ROI. The resulting data were deconvolved using a cubature Kalman filter-based method [42]. Then, a dynamic multivariate autoregressive (MVAR) model (of order 5, obtained from the Akaike [43] and Bayesian [44] information criteria) was applied to the set of hidden neuronal variables obtained after deconvolution. The model is considered dynamic because its coefficients were allowed to vary as a function of time. Both recent simulations [45] and experimental results [46] indicate that MVAR modeling methodology is very reliable for making inferences about path directionality and significance. The underlying model used in deconvolution is formulated in continuous time, and hence, can be sampled at any arbitrary resolution. As Havlicek et al showed [42], sampling the latent neural variables at TR/10 provides the best reconstruction of neural events. Since the independent variables in our model were not significantly correlated with the error term, there was no endogeneity bias. Also, the deconvolved time series were input to the model without having to artificially slice the time series, by permitting the MVAR model coefficients to vary as a function of time to obtain dynamic GC time series. Time points (or TRs) associated with those trials in the encoding and retrieval phases that corresponded to correctly remembered stimuli were identified and the effective connectivity values corresponding to those periods were extracted from the dynamic GC time series (for each path) and pooled into encoding and retrieval connectivity samples, respectively (Fig. 3). Effective connectivity was first evaluated within each participant group (i.e., healthy older adults, MCI patients) during encoding and retrieval by performing a one sample Welch’s t-test (for each path) using group- level connectivity samples aggregated across all subjects in the group. We then compared group- level connectivity samples from both groups using a two sample Welch’s t-test. The paths whose p-values obtained from the t-tests were smaller than 0.05 (FDR-corrected) were then interpreted. For convenience, we refer to “sources” and “targets” of “paths”, or “drivers” and “recipients” of connectivity, recognizing that a path does not necessarily imply a direct projection from one ROI to another. The overall flow of our analyses can be seen in Figure 3. Although all ROIs were entered into the analyses at once, we present the findings separately for intra- and inter- hemispheric connectivity, for convenience. In the figures below, drivers are qualitatively identified as those source nodes with the highest number of outputs. Between-group differences were evaluated using t-tests to obtain paths whose weights differed significantly. All analyses used an FDR-corrected p<0.05.
Figure 3.
Analytic flow of the study.
3. Results
3.1 Effective connectivity during encoding
HOC
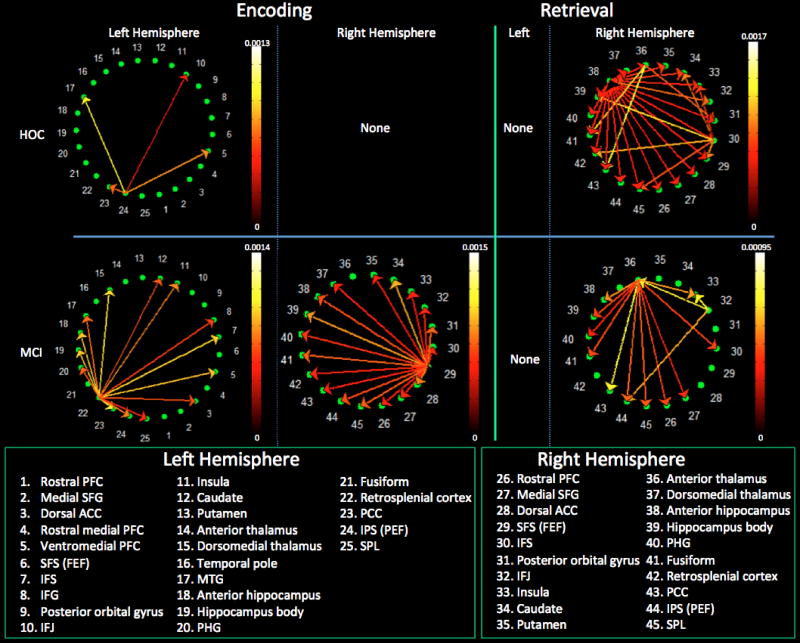
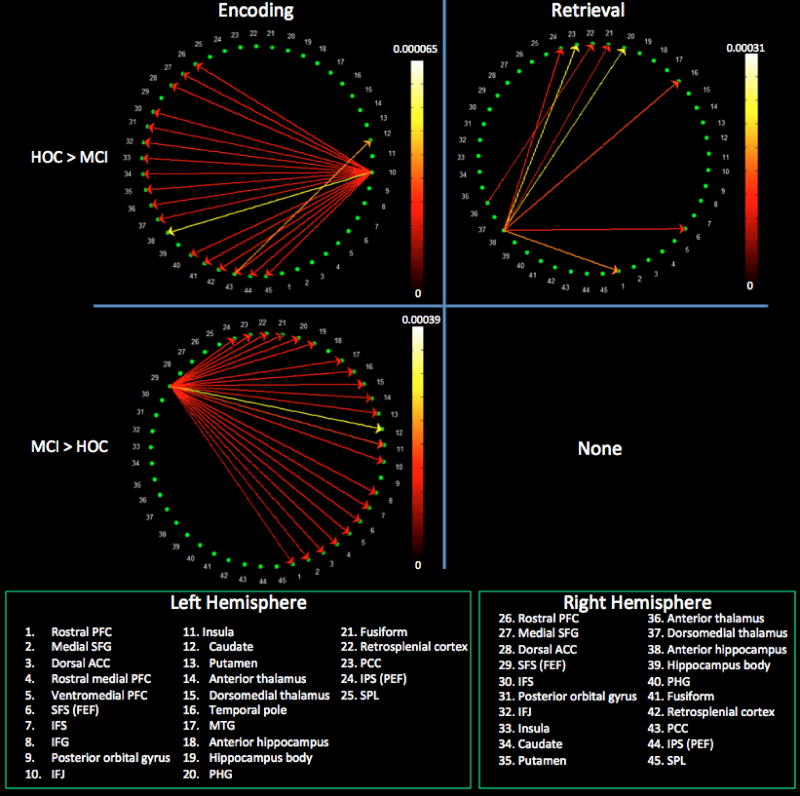
The left anterior intraparietal sulcus (IPS) emerged as the only intrahemispheric source of significant paths; these paths targeted the left inferior frontal junction (IFJ – the meeting place of the inferior frontal sulcus and precentral sulcus), ventromedial PFC, posterior cingulate cortex (PCC), and middle temporal gyrus (MTG) (see Figure 4). This same left IPS region, as well as the left IFJ and left PCC, emerged as the primary sources of interhemispheric paths (Figure 5). The PCC was the only right hemisphere source that reached statistical significance, with its effect on the left caudate.
Figure 4.
Intrahemispheric connectivity for the healthy controls (top row) and MCI patients (bottom row) during encoding (left columns) and retrieval (right columns). Reference bars represent the FDR corrected p ≤ 0.05. Abbreviated region names are included; see Table 2 for unabbreviated list.
Figure 5.
Interhemispheric connectivity in healthy controls (top row) and MCI patients (bottom row) during encoding (left) and retrieval (right). Reference bars represent the FDR corrected p ≤ 0.05. Abbreviated region names are included; see Table 2 for unabbreviated list.
MCI
Within the left cerebral hemisphere, the retrosplenial cortex (RSC) was the primary source, with paths emanating from it that targeted the PFC, parietal, and temporal neocortex as well as the hippocampus (Figure 4). A single primary driver, located at the junction of the superior frontal and precentral sulci, drove connectivity in the right hemisphere. This region corresponds to the location of the frontal eye fields (FEF) and is known to be responsible for voluntary attentional shifts [47, 48]. These same two regions (i.e., left RSC and right FEF) were the sole drivers of interhemispheric connectivity, with the right FEF being the most influential (Figure 5).
HOC vs. MCI
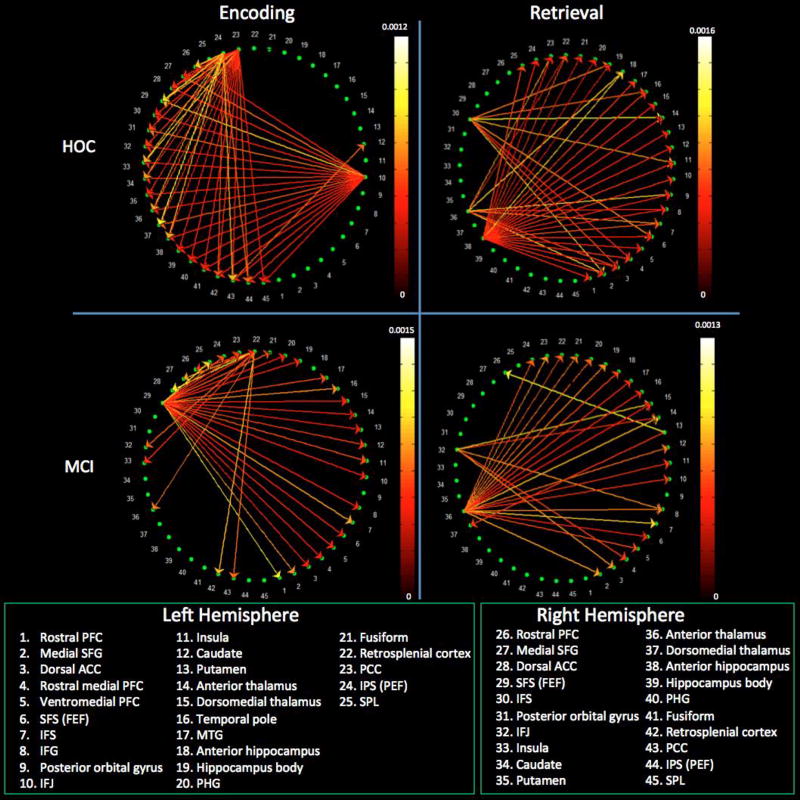
As is clear from the within-group analyses above, healthy controls and MCI patients demonstrated substantially different patterns of connectivity. When we performed a between-group comparison of connectivity, healthy controls demonstrated significantly greater weights for paths originating in the left IFJ and driving all right hemisphere ROIs as well as for a path from the right dorsal PCC to the left caudate. Conversely, MCI patients demonstrated significantly greater weights for paths arising in the right FEF and targeting virtually all right and left hemisphere ROIs (Figures 6 & 7).
Figure 6.
Intrahemispheric connectivity differences between healthy controls and MCI patients during encoding and retrieval. Reference bars represent the FDR corrected p ≤ 0.05. Abbreviated region names are included; see Table 2 for unabbreviated list.
Figure 7.
Interhemispheric connectivity differences between healthy controls and MCI patients during encoding and retrieval. Reference bars represent the FDR corrected p ≤ 0.05. Abbreviated region names are included; see Table 2 for unabbreviated list.
3.2 Effective connectivity during retrieval
HOC
Three right hemisphere sources accounted for both intra- (Figure 4) and interhemispheric connectivity (Figure 5). The anterior hippocampus (i.e., head) was the primary driver as it interacted with virtually all ROIs regardless of cerebral hemisphere. The inferior frontal sulcus (IFS) and anterior thalamus were also significant drivers. There were no significant sources in the left hemisphere.
MCI
There were no significant paths within the left hemisphere. In the right hemisphere, the anterior thalamus and, to a lesser extent, the right IFJ were the primary sources of paths (Figure 4). These same two regions were the primary drivers of interhemispheric connectivity, with the right anterior thalamus playing a central role (Figure 5). The left putamen demonstrated significant interhemispheric driving of the right dorsomedial thalamus and rostral PFC.
HOC vs. MCI
Relative to MCI, healthy controls demonstrated significantly greater weights for paths originating in the right anterior hippocampus and driving the left IPS, PCC, fusiform gyrus, parahippocampal cortex, temporal pole, FEF, and rostral PFC. Greater path weight was also evident from the right anterior thalamus to the left PCC. There were no areas with stronger connectivity in the MCI compared to the control group (Figures 6 & 7).
4. Discussion
The current study is the first to examine differences in task-related effective connectivity during memory encoding and retrieval as well as how connectivity is affected by MCI. We included only trials in which stimuli were successfully encoded and recalled in order to avoid confounding successful memory formation with mere attempts; notably, both MCI patients and controls exhibited significantly faster correct responses compared to incorrect responses. The two primary goals were to 1) examine whether activated regions interact differently during memory encoding and retrieval and 2) examine how the patterns of connectivity are affected by MCI.
Our findings in cognitively intact older adults revealed that the phase of memory has a substantial impact on the pattern of effective connectivity. Successful encoding was largely associated with paths arising in three left hemisphere regions (the IFJ, IPS, and PCC) whereas retrieval was associated with paths arising from three right hemisphere regions (anterior hippocampus, anterior thalamus, and IFS). From a global standpoint, this pattern supports the hemispheric asymmetry in encoding and retrieval model (HERA; [49, 50]), which holds that the left hemisphere preferentially mediates encoding while the right mediates retrieval. One of the key weaknesses of the HERA model is that the nature of the task can bias the overall pattern of activation such that verbal processing is more likely to engage the left hemisphere while visuospatial processing is more likely to engage the right hemisphere [51–53]. However, the current study used an OLA task that is both theoretically and empirically (see Table 2 and [14]) mediated by both cerebral hemispheres, thus minimizing intrinsic bias towards one or the other hemisphere. Thus, our findings support the HERA model by demonstrating that brain regions interact differently based on the phase of memory (i.e., encoding vs. retrieval). The loci of the particular regions driving phase-specific differences in the healthy controls are also meaningful given the larger body of research reviewed earlier. Specifically, neocortical areas drove encoding-related activation whereas the hippocampus was the primary driver of retrieval-related activation; findings that support the model proposed by Rugg and colleagues [5]. This pattern differed significantly, however, in those with MCI as is discussed below.
4.1 Effective connectivity during encoding
In cognitively intact older adults, the primary drivers during encoding (i.e., the IFJ, IPS, and PCC) are all part of a commonly identified frontoparietal network that is involved in adaptive cognitive control [54] and working memory [55]. Our data suggest that the left anterior IPS, a region also known as the parietal eye field (PEF), played a critical role early in the learning process, which is consonant with its role in goal-directed (i.e., top-down) attentional processing (see [56] for review). This region drove activation within the ventrolateral PFC, which has long been associated with deeper levels of processing [57] and successful memory encoding [58, 59]. Specifically, our data suggest that the IFJ and PCC received information from the left anterior IPS via intrahemispheric paths; these three regions then modulated activation in all right hemisphere regions. Previous research has shown that the IFJ modulates top-down attentional processing [60] and adaptively drives the use of critical brain regions in a task- specific manner [61, 62].The PCC is known to be involved in self-referential [63] and semantic processing [64], both of which enhance the salience of memories. Such personally-relevant, semantic processing is consonant with this region’s role in recollective (i.e., high-confidence) memories [65]. Together, these findings suggest that cognitively intact older adults rely on top- down, elaborative processing during successful memory encoding of OLAs.
In contrast, MCI patients demonstrated a markedly different pattern of effective connectivity that was primarily driven by the right FEF and to a lesser degree by the left RSC. The FEF mediates oculomotor saccades and evidence has emerged for its role in voluntary shifts of spatial attention (see [56] for review). Considering these findings within the context of the OLA task, cognitively intact older adults may have approached the task with the active goal of associating the object and location, a cognitive set that engaged the PEF (i.e., goal-directed attentional processing) and other regions described above. Conversely, MCI patients may have relied on a more basic, FEF-mediated, visual search process that reflects the loss of top-down control; a possibility supported by previous findings [17–19].
While the implications are less clear, the shift from PCC in controls to RSC in MCI may also be functionally meaningful since midline posterior regions (e.g., precuneus, PCC, RSC) are affected early in the course of AD [66]. The severity of such compromise is associated with learning and memory impairment [67, 68]. In fact, there is evidence of functional connectivity disruption of the RSC in MCI [69], suggesting that this region is dysfunctional. However, findings from the resting state may not capture the entirety of the RSC’s response to disease. Our data, acquired during actual task performance, revealed that MCI patients were more reliant on the left RSC during successful encoding. This finding may reflect the compensatory coordination of task-related brain areas and is consistent with the RSC’s extensive reciprocal connections with other structures of the Papez/Delay-Brion circuit as well as key neocortical regions that include the PEF [70]. Although the exact cognitive processes remain unclear, this region has been implicated in episodic memory, navigation, and imagining and planning for future events [70]. It has extensive reciprocal connections with the anterior thalamic nuclei, which is intriguing given the increased involvement of these nuclei during retrieval (see below). In fact, prior research has shown that hippocampally-based spatial learning is dependent on the integrity of the RSC and anterior thalamic nuclei [71]. Although our data suggest that the hippocampal dysfunction in MCI patients resulted in a compensatory upregulation of functionally related areas, future studies should evaluate how methodological factors such as the nature of scan acquisition (i.e., task vs. rest), task (e.g., learning vs. memory), and analytic method (e.g., functional vs. effective connectivity), influence such findings.
4.2 Effective connectivity during retrieval
In cognitively intact older adults, successful memory retrieval was primarily mediated by the right hippocampus with the right anterior thalamic nuclei and right IFS playing relatively lesser roles. These findings support previous models of memory wherein the hippocampus is central to the recollection of episodic memories [5, 6]. It is interesting to note that the anterior hippocampal ROI was significantly more active during encoding than recall (see Table 2), yet it was the primary driver of retrieval-based activation – at least in healthy older adults. We posit that, because the anterior hippocampus plays a vital role in associative binding during encoding [72], it must help coordinate the recall of the individual components of the memory during retrieval. However, our data also support Aggleton’s [71] recent call to consider changes within the larger memory-related network in those with learning and memory deficits. Specifically, MCI patients relied on an altered network during successful memory retrieval that was characterized by intrahemispheric input from the right IFJ to the right anterior thalamic nuclei, which then drove activation in most of the other regions included. A recent meta-analysis revealed that the right IFJ monitors task-concurrent versus non-concurrent responding [73] and error monitoring [74]. This type of monitoring presumably becomes more necessary as the salience of, or confidence in, memories decline – as would be expected with the hippocampal dysfunction observed in these MCI patients (see Figures 6 & 7 and [14, 75]). The anterior thalamic nuclei were previously shown to be critical for hippocampally-based spatial processing [71] though other work raises the possibility that this is at least partially due to unique contributions from the mamillary bodies via the mamillothalamic tract [76]. Regardless, the thalamus has dense reciprocal connections with virtually every other brain region and our data raise the possibility that patients capitalized on this extensive innervation via compensatory reliance on the anterior thalamic nuclei. An alternative explanation is that patients were less efficient in retrieving the location of a given object, which then necessitated more extensive cognitive “searching” mediated by the IFJ. Future studies could explicitly examine these possibilities by examining connectivity during high- versus low-confidence trials.
4.3 Potential limitations
Several methodological factors warrant discussion, the first of which relates to behavioral differences between the groups. While acquiring the data, we provided extensive practice and ensured that all participants understood the instructions before they entered the scanner. We also reminded patients of the instructions between each functional run. As with the previously reported activation analyses [14], we chose to limit analyses to only stimuli that were successfully encoded/retrieved. This approach eliminated confounding factors that are associated with subsequent forgetting (see meta-analysis [77]) but resulted in a different number of trials being considered for each participant (typically fewer for MCI than controls). This concern may be mitigated by our previous findings that MCI patients demonstrated a comparable pattern of activation as did healthy controls, albeit of a lesser magnitude [14]. We also used Welch’s t-test, which is designed for samples of unequal size and unequal variance [78]. As a result, the difference in the number of included trials should not affect the connectivity results. Although accuracy for the location of novel objects was not significantly different than chance in the MCI group, three pieces of data mitigate concerns that correct trials were due merely to guessing. First, MCI patients were significantly faster to respond on correct than incorrect trials. The relationship between reaction time and accuracy was not significant, which indicates that the high performing individuals do not account for the previously noted differences. Second, MCI patients demonstrated a more extensive pattern of activation during incorrectly than correctly encoded trials, especially within the right hemisphere (Supplemental Figure 1), suggesting inefficient or “noisy” processing. In contrast, patients demonstrated greater activation during correctly retrieved trials, possibly suggesting more contextually rich (multi-modal) memories. Third, there were substantial differences in effective connectivity for correct and incorrect trials (Supplemental Figures 2 & 3). Regardless, future studies should attempt to match the number of trials across groups, especially considering the relatively poor overall performances in both groups, though this may require substantial modifications (e.g., fewer stimuli, longer encoding duration) that could in turn present other issues (e.g., ceiling performance, boredom in controls). An alternative approach for future studies is to correlate behavioral performance and path weights, which could replicate and extend our current results. Although we attempted above to consider likely explanations for the observed differences in effective connectivity between MCI and controls, we should emphasize that it remains uncertain whether the differences reflect pathologic or compensatory processes in MCI. It is interesting to note that patterns of interhemispheric connectivity were especially different between the groups, which suggests that additional study of these interactions may be fruitful.
Unlike other effective connectivity methods that require a priori models and that use a limited number of brain regions [79–82], our approach was largely data-driven and used a large number of areas. As with any approach it is possible that we omitted critical nodes in our analyses. However, our findings in controls are remarkably consistent with theoretical models of hemispheric specialization [50, 83] and medial temporal lobe functioning [5, 6], providing some reassurance that appropriate regions were included. The results obtained by the application of GCA to fMRI data could theoretically be confounded because of slower temporal sampling relative to faster neuronal processes as well as the variability of the hemodynamic response, which can be partially of non-neural origin. However, recent studies employing theoretical arguments [84], empirical simulations, and experimental manipulations have shown the validity and utility of the application of GCA to fMRI. Although Smith et al [85], based on simulations of various connectivity methods, argued against using GCA, many simulations reported both before [86, 87] and after [45, 88–90] this study have shown the validity of the application of GCA to fMRI data. However, it is noteworthy that simulations make restrictive assumptions, and hence it is not surprising that some of them do not agree with each other. In this context, experimental studies become important. Abler et al [91] used a simple auditory-motor paradigm in one of the earlier experimental studies and demonstrated, using fMRI data acquired with a relatively long TR of 2440 ms, that GCA correctly inferred causal influences from the auditory cortex to the motor cortex, which was expected in this paradigm. In addition, they argued that significant Granger causality obtained from slowly sampled fMRI data may correctly reflect corresponding neural connectivity, but the reverse may not always be true, i.e. absence of significant Granger causality obtained from slowly sampled fMRI data does not always imply a corresponding lack of causal influence at the neural level. This caveat must be considered when interpreting results obtained using the application of GCA to fMRI data acquired with longer TRs. Experimental studies in the recent past demonstrated, using deconvolved fMRI data, that neural delays lesser than the acquisition TR can be inferred using GCA [40, 46, 92]. By using intra-cerebral EEG as ground truth, David et al [40] showed that directional connectivity inferred from both GCA (applied to deconvolved fMRI data) and dynamic causal modeling (DCM) matched that obtained by intra-cerebral EEG. The convergence of evidence from these different methods provides face validity to the application of GCA to deconvolved fMRI. In the current study, our TR was reasonable with respect to previous studies (such as the one by Abler et al) and the potential confounding effects of HRF variability were accounted for by deconvolving it out of the observed fMRI data. Given these factors, we believe the current analyses to be valid. However, it will be important for future studies to replicate our findings using both GCA and other analytic methods with more favorable acquisition parameters (e.g., faster sampling and/or higher field strength) or other approaches such as magnetoencephalography or electroencephalography.
4.4 Conclusions and clinical implications
Taken as a whole, our findings indicate that MCI patients demonstrate a loss of top-down cognitive control during encoding as well as a loss of hippocampally-driven retrieval when compared to cognitively intact older adults. These differences are especially striking given the relatively mild clinical status of those with MCI (i.e., Delayed Memory Index ~ 1.7 SD below the mean but comparable brain volumes as the HOC). Our findings are particularly meaningful since we previously reported that cognitive rehabilitation techniques that require top-down processing improved long-term retention [93, 94]. These previously described behavioral changes were accompanied by increased activation in the left frontoparietal control network during encoding [95] as well as the hippocampus during both encoding and retrieval [75]. Thus, our previous data suggest that relatively simple cognitive interventions can mitigate the maladaptive changes described above, at least under certain circumstances. We plan to explore potential changes in effective connectivity associated with such interventions. The present findings may also help inform the selection of neurostimulation methods and pharmacologic agents that target cognitive control processes.
Supplementary Material
Figure 1.
Examples of stimuli and design of the encoding (top) and retrieval (bottom) runs.
Acknowledgments
This work was supported by the Emory Alzheimer’s Disease Research Center, National Institute on Aging (Grant 2P50AG025688) and Department of Veterans Affairs (B6366W to BMH), and was presented at the 2013 Annual meeting of the Society for Neuroscience in San Diego, CA, USA. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None
References
- 1.Roediger HL, Gallo DA, Geraci L. Processing approaches to cognition: The impetus from the levels-of-processing framework. Memory. 2002;10(5–6):319–332. doi: 10.1080/09658210224000144. [DOI] [PubMed] [Google Scholar]
- 2.Nyberg L, et al. Reactivation of encoding-related brain activity during memory retrieval. Proc Natl Acad Sci U S A. 2000;97(20):11120–4. doi: 10.1073/pnas.97.20.11120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wheeler ME, Petersen SE, Buckner RL. Memory's echo: vivid remembering reactivates sensory-specific cortex. Proc Natl Acad Sci U S A. 2000;97(20):11125–9. doi: 10.1073/pnas.97.20.11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spaniol J, et al. Event-related fMRI studies of episodic encoding and retrieval: meta-analyses using activation likelihood estimation. Neuropsychologia. 2009;47(8–9):1765–79. doi: 10.1016/j.neuropsychologia.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 5.Rugg MD, et al. Encoding-retrieval overlap in human episodic memory: A functional neuroimaging perspective. In: Sossin WS, et al., editors. Essence of Memory. 2008. pp. 339–352. [DOI] [PubMed] [Google Scholar]
- 6.Moscovitch M, et al. Functional neuroanatomy of remote episodic, semantic and spatial memory: a unified account based on multiple trace theory. J Anat. 2005;207(1):35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis SW, et al. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18(5):1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu P, et al. Age-related differences in memory-endcoding fMRI responses after accounting for decline in vascular reactivity. NeuroImage. 2013;78:415–425. doi: 10.1016/j.neuroimage.2013.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook IA, et al. Aging and brain activation with working memory tasks: an fMRI study of connectivity. International Journal of Geriatric Psychiatry. 2007;22(4):332–342. doi: 10.1002/gps.1678. [DOI] [PubMed] [Google Scholar]
- 10.Waring JD, Addis DR, Kensinger EA. Effects of aging on neural connectivity underlying selective memory for emotional scenes. Neurobiology of Aging. 2013;34:451–467. doi: 10.1016/j.neurobiolaging.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gutchess AH, et al. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial-temporal activity. Journal of Cognitive Neuroscience. 2005;17(1):84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- 12.Albert MS, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: Recommendations from the National Institute on Aging and Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR, Jr, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55(4):484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hampstead BM, et al. Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object location associations. Neuropsychologia. 2011;49:2349–2361. doi: 10.1016/j.neuropsychologia.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machulda MM, et al. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61(4):500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Protzner AB, et al. Network interactions explain effective encoding in the context of medial temporal damage in MCI. Hum Brain Mapp. 2011;32(8):1277–89. doi: 10.1002/hbm.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchens RL, et al. Relationship between control beliefs, strategy use, and memory performance in amnestic mild cognitive impairment and healthy aging. Journals of Gerontology, Series B: Psychological Sciences and Social Sciences. 2013 doi: 10.1093/geronb/gbt016. [DOI] [PubMed] [Google Scholar]
- 18.Perry R, Hodges J. Dissociation between top-down attentional control and the time course of visual attention as measured by attentional dwell time in patients with mild cognitive impairment. European Journal of Neuroscience. 2003;18:221–226. doi: 10.1046/j.1460-9568.2003.02754.x. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro F, Guerreiro M, DeMendonca A. Verbal learning and memory deficits in mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2007;29(2):187–197. doi: 10.1080/13803390600629775. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, et al. Functional degeneration in dorsal and ventral attention systems in amnestic mild cognitive impairment and Alzheimer’s disease: An fMRI study. Neuroscience Letters. 2015;585:160–165. doi: 10.1016/j.neulet.2014.11.050. [DOI] [PubMed] [Google Scholar]
- 21.Dannhauser TM, et al. The functional anatomy of divided attention in amnestic mild cognitive impairment. Brain. 2005;128(6):1418–1427. doi: 10.1093/brain/awh413. [DOI] [PubMed] [Google Scholar]
- 22.Neufang S, et al. Predicting effective connectivity from resting-state networks in healthy elderly and patients with prodromal Alzheimer’s disease. Human Brain Mapping. 2014;35:954–963. doi: 10.1002/hbm.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of International Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 24.Folstein M, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive status of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Randolph C. Repeatable battery for the assessment of neuropsychological status manual. San Antonio: The Psychological Corporation; 1998. [Google Scholar]
- 26.Yesavage JA, T B, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale - A preliminary report. Journal of Psychiatric Research. 1983;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 27.Pfeffer RI, K T, Harrah CH, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 28.Duff K, et al. Utility of the RBANS in detecting cognitive impairment associated with Alzheimer's disease: Sensitivity specificity, and positive and negative predictive powers. Archives of Clinical Neuropsychology. 2008;23(5):603–612. doi: 10.1016/j.acn.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duff K, et al. Diagnostic Accuracy of the RBANS in Mild Cognitive Impairment: Limitations on Assessing Milder Impairments. Archives of Clinical Neuropsychology. 2010;25(5):429–441. doi: 10.1093/arclin/acq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.England HB, Gillis MM, Hampstead BM. RBANS Memory Indices Are Related to Medial Temporal Lobe Volumetrics in Healthy Older Adults and Those with Mild Cognitive Impairment. Archives of Clinical Neuropsychology. 2014;29(4):322–328. doi: 10.1093/arclin/acu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brewer JB, et al. Fully-automated quantification of regional brain volumes for improved detection of focal atrophy in Alzheimer's disease. American Journal of Neuroradiology. 2009;30:578–580. doi: 10.3174/ajnr.A1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold JJ, S L. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15:79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CWJ G. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424–438. [Google Scholar]
- 34.Sathian K, Deshpande G, Stilla R. Neural changes with tactile learning reflect decision-level reweighting of perceptual readout. The Journal of Neuroscience. 2013;33(12):5387–5398. doi: 10.1523/JNEUROSCI.3482-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lacey S, et al. Spatial imagery in haptic shape perception. Neuropsychologia. 2014;60:144–158. doi: 10.1016/j.neuropsychologia.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grant MM, et al. Influence of early life stress on intra- and extra-amygdaloid causal connectivity. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hutcheson NL, et al. Effective connectivity during episodic memory retrieval in schizophrenia participants before and after antipsychotic medication. Human Brain Mapping. 2015;36(4):1442–1457. doi: 10.1002/hbm.22714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheelock M, et al. Threat-related learning relies on distinct dorsal prefrontal cortex network connectivity. NeuroImage. 2014;102:904–912. doi: 10.1016/j.neuroimage.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Handwerker DA, Ollinger JM, D'Esposito M. Variation of BOLD hemodynamic responses across subjects and brain regions and their effects on statistical analyses. NeuroImage. 2004;21(4):1639–1651. doi: 10.1016/j.neuroimage.2003.11.029. [DOI] [PubMed] [Google Scholar]
- 40.David O, et al. Identifying Neural Drivers with Functional MRI: An Electrophysiological Validation. PLoS biology. 2008;6(122):2683–2697. doi: 10.1371/journal.pbio.0060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryali S, et al. Estimation of functional connectivity in fMRI data using stability selection-based sparse partial correlation with elastic net penalty. NeuroImage. 2012;59(4):3852–3861. doi: 10.1016/j.neuroimage.2011.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Havlicek M, et al. Dynamic modeling of neuronal responses in fMRI using cubature Kalman filtering. NeuroImage. 2011;56(4):2109–2128. doi: 10.1016/j.neuroimage.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akaike H. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 1974;19(6):716–723. [Google Scholar]
- 44.Schwarz G. Estimating the Dimension of a Model. Ann. Statist. 1978;6(2):461–464. [Google Scholar]
- 45.Wen X, Rangarajan G, Ding M. Is Granger Causality a Viable Technique for Analyzing fMRI Data? PloS One. 2013;8(7) doi: 10.1371/journal.pone.0067428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katwal SB, et al. Measuring relative timings of brain activities using fMRI. NeuroImage. 2013;66:436–448. doi: 10.1016/j.neuroimage.2012.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 48.Amiez C, Petrides M. Anatomical organization of the eye fiends in the human and non-human primate frontal cortex. Progress in Neurobiology. 2009;89(2):220–230. doi: 10.1016/j.pneurobio.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Tulving E, et al. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A. 1994;91(6):2016–20. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nyberg L, Cabeza R, Tulving E. PET studies of encoding and retrieval: The HERA model. Psychon Bull Rev. 1996;3(2):135–148. doi: 10.3758/BF03212412. [DOI] [PubMed] [Google Scholar]
- 51.Kelley WM, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20(5):927–36. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- 52.McDermott KB, et al. Set- and code-specific activation in frontal cortex: an fMRI study of encoding and retrieval of faces and words. J Cogn Neurosci. 1999;11(6):631–40. doi: 10.1162/089892999563698. [DOI] [PubMed] [Google Scholar]
- 53.Lee AC, et al. Asymmetric frontal activation during episodic memory: the effects of stimulus type on encoding and retrieval. Neuropsychologia. 2000;38(5):677–92. doi: 10.1016/s0028-3932(99)00094-9. [DOI] [PubMed] [Google Scholar]
- 54.Dosenback NUF, et al. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baddeley A. Working memory: Looking back and looking forward. Nature Reviews Neuroscience. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 56.Mendendorp WP, et al. Parietofrontal circuits in goal-oriented behavior. European Journal of Neuroscience. 2011;33:2017–2027. doi: 10.1111/j.1460-9568.2011.07701.x. [DOI] [PubMed] [Google Scholar]
- 57.Nyberg L. Levels of processing: A view from functional brain imaging. Memory. 2002;10(5/6):345–348. doi: 10.1080/09658210244000171. [DOI] [PubMed] [Google Scholar]
- 58.Kuhl BA, Bainbridge WA, Chun MM. Neural reactivation reveals mechanisms for updating memory. Journal of Neuroscience. 2012;32(10):3453–3461. doi: 10.1523/JNEUROSCI.5846-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bergmann H, et al. Distinct neural correlates of associative working memory and long-term memory encoding in the medial temporal lobe. NeuroImage. 2012;63:989–977. doi: 10.1016/j.neuroimage.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 60.Kim H. Involvement of the dorsal and ventral attention networks in oddball stimulus processing: A meta-analysis. Human Brain Mapping. 2014;35(5):2265–2284. doi: 10.1002/hbm.22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baldauf D, Desimone R. Neural mechanisms of object-based attention. Science. 2014;344:424–427. doi: 10.1126/science.1247003. [DOI] [PubMed] [Google Scholar]
- 62.Xu Y. Inferior frontal junction biases perception through neural synchrony. 2014;18(9):447–448. doi: 10.1016/j.tics.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson S, et al. Neural correlates of self-reflection. Brain. 2002;125:1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- 64.Binder J, et al. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huijbers W, et al. Imagery and retrieval of auditory and visual information: Neural correlates of successful and unsuccessful performance. Neuropsychologia. 2011;49(7):1730–1740. doi: 10.1016/j.neuropsychologia.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 66.Jack DR. Alzheimer Disease: New concepts on its neurobiology and the clinical role imaging will play. Radiology. 2012;263(2):343–360. doi: 10.1148/radiol.12110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Walhovd KB, et al. Multimodal imaging in mild cognitive impairment: Metabolism, morphometry, and diffusion of the temporal-parietal memory network. NeuroImage. 2009;45(1):215–223. doi: 10.1016/j.neuroimage.2008.10.053. [DOI] [PubMed] [Google Scholar]
- 68.Walhovd KB, et al. Multi-modal imaging predicts memory performance in normal aging and cognitive decline. Neurobiology of Aging. 2010;31(7):1107–1121. doi: 10.1016/j.neurobiolaging.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network - Anatomy, function, and relevance to disease. In: Kingstone A, Miller AB, editors. Annals of the New York Academy of Sciences. 2008. pp. 1–38. [DOI] [PubMed] [Google Scholar]
- 70.Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nature Reviews Neuroscience. 2009;10:792–802. doi: 10.1038/nrn2733. [DOI] [PubMed] [Google Scholar]
- 71.Aggleton JP. Looking beyond the hippocampus: Old and new neurological targets for understanding memory disorders. Proceedings of the Royal Society B. 2014;281 doi: 10.1098/rspb.2014.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chua EF, et al. Evidence for a specific role of the anterior hippocampal region in successful associative encoding. Hippocampus. 2007;17(11):1071–1080. doi: 10.1002/hipo.20340. [DOI] [PubMed] [Google Scholar]
- 73.Cieslik EC, et al. Three key regions for supervisory attentional control: Evidence from neuroimaging meta-analyses. Neuroscience and Biobehavioral Reviews. 2015;48:22–34. doi: 10.1016/j.neubiorev.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.King JA, et al. Post-error behavioral adjustments are facilitated by activation and suppression of task-relevant and task-irrelevant information processing. The Journal of Neuroscience. 2010;30(38):12759–12769. doi: 10.1523/JNEUROSCI.3274-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hampstead BM, et al. Mnemonic strategy training partially restores hippocampal activity in patients with mild cognitive impairment. Hippocampus. 2012;22(8):1652–1658. doi: 10.1002/hipo.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vann SD. Dismantling the Papez circuit for memory in rats. ELIFE. 2013;2 doi: 10.7554/eLife.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim H. Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. NeuroImage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 78.Ruxton GD. The unequal variance t-test is an underused alternative to Student's t-test and the Mann-Whitney U test. Behavioral Ecology. 2006;17(4):688–690. [Google Scholar]
- 79.Friston KJ, Harrison L, W P. Dynamic causal modeling. NeuroImage. 2003;19(4):1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- 80.Stephan KE, Roebroeck A. A short history of causal modeling of fMRI data. NeuroImage. 2012;62(2):856–863. doi: 10.1016/j.neuroimage.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 81.Lohmann G, et al. Critical comments on dynamic causal modeling. NeuroImage. 2012;59(3):2322–2329. doi: 10.1016/j.neuroimage.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 82.McCormick C, et al. Hippocampal-neocortical networks differ during encoding and retrieval of relational memory: functional and effective connectivity analyses. Neuropsychologia. 2010;48(11):3272–3281. doi: 10.1016/j.neuropsychologia.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 83.Tulving E, et al. Hemispheric encoding/retrieval asymmetry in episodic memory: positron emission tomography findings. Proc Natl Acad Sci U S A. 1994;91(6):2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Davey CE, et al. The equivalence of linear Gaussian connectivity techniques. Human Brain Mapping. 2013;34(9):1999–2014. doi: 10.1002/hbm.22043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith SM, et al. Network modeling methods for fMRI. NeuroImage. 2011;54(2):875–891. doi: 10.1016/j.neuroimage.2010.08.063. [DOI] [PubMed] [Google Scholar]
- 86.Deshpande G, Sathian K, Hu X. Effect of hemodynamic variability on Granger causality analysis of fMRI. NeuroImage. 2010;52(3):884–896. doi: 10.1016/j.neuroimage.2009.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schippers MB, Renken R, Keysers C. The effects of intra- and inter-subject variability of hemodynamic response on group level Granger causality analysis. NeuroImage. 2011;57(1):22–36. doi: 10.1016/j.neuroimage.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 88.Wang HE, et al. A systematic framework for functional connectivity measures. Fronteers of Neuroscience. 2014;8 doi: 10.3389/fnins.2014.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vinck M, et al. How to detect the Granger-causal flow direction in the presence of additive noise? NeuroImage. 2015;108:301–318. doi: 10.1016/j.neuroimage.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 90.Smith JF, et al. Identifying effective connectivity parameters in simulated fMRI: A direct comparison of switching linear dynamic system, stochastic dynamic causal, and multivariate autoregressive models. Fronteers of Neuroscience. 2013;7(70) doi: 10.3389/fnins.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abler B, et al. Investigating directed influences between activated brain areas in a motor-response task using fMRI. Magnetic Resonance Imaging. 2006;24(2):181–185. doi: 10.1016/j.mri.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 92.Ryali S, et al. Multivariate dynamical systems models for estimating causal interactions in fMRI. NeuroImage. 2011;54(2):807–823. doi: 10.1016/j.neuroimage.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hampstead BM, et al. Explicit memory training leads to improved memory for face-name pairs in patients with mild cognitive impairment: Results of a pilot investigation. Journal of the International Neuropsychological Society. 2008;14(5):883–889. doi: 10.1017/S1355617708081009. [DOI] [PubMed] [Google Scholar]
- 94.Hampstead BM, et al. Mnemonic strategy training improves memory for object location associations in both healthy elderly and patients with amnestic mild cognitive impairment: a randomized, single-blind study. Neuropsychology. 2012;26(3):385–399. doi: 10.1037/a0027545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hampstead BM, et al. Activation and effective connectivity changes following explicit memory training for face name pairs in patients with mild cognitive impairment: A pilot study. Neurorehabilitation and Neural Repair. 2011;25(3):201–222. doi: 10.1177/1545968310382424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.