Abstract
Three-component coupling of Fischer carbene complexes, enyne aldehyde hydrazones, and electron-deficient alkynes leads to simple benzoate derivatives in a process involving the formation of an N-aminopyrrole derivative, Diels-Alder reaction, and nitrene extrusion. The products are readily converted into isoquinolones through reaction with primary amines. The reaction proceeds best with highly substituted and electron-rich pyrroles even though these are the sterically least favorable substrates, and this reactivity trend is supported by a computational study.
Graphical abstract

Introduction
Diels-Alder reactions that result in the direct preparation of benzene ring systems (benzannulation) are comparatively rare1 and include pyrone-alkyne cycloadditions,2 cyclopentadienone-alkyne cycloadditions,3 and the cycloaddition of furans with alkynes followed by C-O bond cleavage.4 Diverse multinuclear aromatic ring systems (e.g. F-both rings aromatic, Scheme 1) are readily accessed through the three-component coupling of 2-alkynylbenzaldehyde hydrazones (A-aromatic), Fischer carbene complexes (B), and electron-deficient alkynes (D).5 A key event in this process is the Diels-Alder reaction of an isoindole system (C-10π-aromatic) with electron-deficient alkynes followed by nitrene extrusion.6 Isoindoles readily undergo Diels-Alder reactions due to an embedded o-xylylene substructure.7 Simple pyrrole derivatives lack this structural feature and undergo Diels-Alder reactions only with tremendous difficulty.8 Lewis acid activation of this Diels-Alder reaction routinely results in undesired Michael addition due to the high nucleophilicity of the pyrrole system.9 In a limited study, highly substituted 1-aminopyrrole systems undergo thermal Diels-Alder reactions accompanied by a nitrene/diazene extrusion process that directly results in an aromatic system.10 Only a limited number of synthetic routes exist for 1-aminopyrrole synthesis, including: (1) condensation of unsymmetrical dialkylhydrazine derivatives with 1,4-dicarbonyl compounds,11 (2) lithiation/alkylation of simpler 1-aminopyrroles,12 or (3) N-amination of simple pyrroles.13 The focus of this manuscript is the synthesis of simple benzenes through use of simple pyrroles derived from the coupling of non-aromatic enyne hydrazone systems and Fischer carbene complexes,14 which vastly extends the structural options for the aminopyrrole reacting partner. The overall reaction process is surprisingly facile and efficient given that it lacks the o-quinone dimethide (or o-xylylene) activation.
Scheme 1.

Isoindole versus pyrrole-mediated preparation of benzene rings through three-component coupling
The reaction in Scheme 1 employing nonaromatic reactants represents a convergent three-component coupling approach leading to substituted benzene rings. The initial reaction forming the pyrrole is accompanied by an exocyclic carbon-carbon formation event, and thus the net result is a highly versatile three-component coupling resulting from the formation of three carbon-carbon bonds. The reaction currently under development is ideally suited to the preparation of highly substituted benzoic acid/phthalate derivatives and isoquinolone15,16 derivatives. Examples of medicinally-important compounds that possess these structural features are depicted in Figure 1 and include a PARP-1 phthalimide-norsesquiterpene alkaloid inhibitor,17,18 substituted phthalate β-lactamase inhibitors,19 and the structurally unique natural product nigelactone.20
Figure 1.
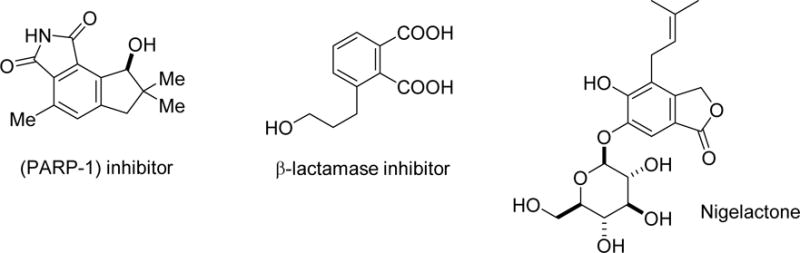
Medicinally-important substituted benzoic acid and isoquinolone derivatives.
Results and Discussion
A survey of the three component coupling of carbene complexes, enyne-aldehyde hydrazones, and alkyne dienophiles is presented in Table 1. Initially, reactions employing previously-synthesized21 aminopyrrole-ketones (6) and DMAD were investigated (entries 1–3) in order to evaluate the critical Diels-Alder and nitrene extrusion steps of the design. Diels-Alder reactions were initially performed at high concentration in toluene solvent using purified pyrrole-ketones at 0.1–0.3M concentration in refluxing toluene for 24 h. This process was high yielding for entries 1–2 and the TLC (silica gel) of the reaction showed no other mobile products. The reaction in entry 3 proceeded in lower yield, however the most obvious competing process, intramolecular Diels-Alder reaction resulting in 8,22 was not observed. The monosubstituted pyrrole system in entry 4 was completely unreactive to DMAD, regardless of whether the ketone or enol ether (expected to be more reactive) was employed in the Diels-Alder reaction event. The failure of the reaction in entry 4 shows that activation of the pyrrole by electron-donating alkyl and aryl groups is more important than favorable sterics.
Table 1.
Synthesis of substituted benzenes through three-component coupling of enyne hydrazones, Fischer carbene complexes, and electron-deficient alkynes.

| ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | R4 | R5 | EWG | Methoda | Yield |
| 1 | H | Ph | n-Bu | Me | COOMe | COOMe | C | 92% (7a) |
| 2 | Ph | H | n-Bu | Me | COOMe | COOMe | C | 93% (7b) |
| 3 | Allyl | H | n-Bu | 3-buten-1-yl | COOMe | COOMe | C | 57% (7c) |
| 4 | H | H | n-Bu | Me | COOMe | COOMe | B | 0% |
| 5 | R1,2 = −(CH2)4− | n-Bu | 2-thienyl | COOMe | COOMe | A | 62% (5e) | |
| 6 | R1,2 = −(CH2)4− | TMS | n-Bu | COOMe | COOMe | B | 64% (7f) | |
| 7 | R1,2 = −(CH2)4− | TMS | Me | COOEt | −CH=CHCOOEt | B | 58% (7g) | |
| 8 | R1,2 = −(CH2)3− | TMS | n-Bu | COOMe | COOMe | B | 70% (7h) | |
| 9b | R1,2 = −(CH2)4− | TMS | Me | COOMe | COOMe | B (D) | 58 % (54%) (7i) | |
| 10 | R1,2 = −(CH2)4− | n-Bu | Me | COOMe | COOMe | D | 60% (7j) | |
| 11 | R1,2 = −(CH2)3− | TMS | Me | COOMe | COOMe | D | 50% (7k) | |
Methods: A – no enol ether hydrolysis and Diels-Alder reaction was conducted after filtration and dioxane removal from the carbene reaction; B – enol ether hydrolysis after Diels-Alder reaction, the Diels-Alder reaction was conducted after filtration and dioxane removal from carbene reaction; C – the yield is for the Diels-Alder/nitrene extrusion step only using isolated pyrrole-ketone (6); D – one-pot reaction with enol ether hydrolysis at the end.
The yield in parentheses refers to the reaction using conditions D.
Next, the three-component coupling was investigated using highly-substituted (R1,2 ≠ H) enyne hydrazones. Since the pyrrole enol ether derivatives had previously shown air sensitivity,14 the crude product from the carbene complex enyne-hydrazone coupling was purified only through filtration and solvent removal, followed by treatment of the crude product with DMAD in dioxane (at higher concentration than the carbene alkene coupling), followed by enol ether hydrolysis.23 Under these conditions, the highly substituted and electron-rich systems of entries 5–8 readily underwent the Diels-Alder reaction to afford the phthalate systems. The thienyl system of entry 5 was very resistant to acid-catalyzed hydrolysis and was thus isolated without the hydrolysis step. The Diels-Alder reaction employing the unsymmetrical ethyl propiolate dimer as dienophile in entry 7 proceeded similarly, and afforded a single regioisomer24 as the exclusive product. The reaction in entry 9 was also investigated as a one-pot reaction omitting the solvent evaporation step, and there were only minor differences in the final product yields. Since the one-pot reaction was far more operationally convenient, the one-pot sequential addition reaction was employed for the remaining compound in entries 10–11. The overall yields for these processes were in the same range as the operationally less convenient reactions in the other entries.
Some preliminary examinations of the reactivity profile for the products in Table 1 were conducted (see Scheme 2). The condensation reaction of the benzene derivatives from Table 1 (7) with benzylamine led to the isoquinolone systems (9). Attempted formation of m-cyclophanes (10, 11)25 through attempted RCM of the pyrrole 6c or the benzene product 7c were both unsuccessful.26
Scheme 2.

Synthetic transformations of phthalate esters from Table 1
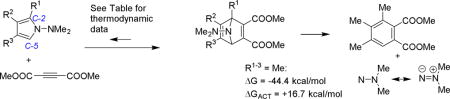
To gain further insight into the limitations encountered with the monosubstituted pyrrole derivative, computational studies of the reaction between 1-dimethylaminopyrroles and DMAD were conducted. The reaction free energies, free energies of activation, and transition state asynchronicity are depicted in Table 2. Selected transition states are depicted in Figure 2. In all cases, the Diels-Alder step of these reactions are endergonic. The least endergonic process involves the unsubstituted pyrrole system in entry 1, which can be attributed to a steric effect, however kinetically this process occurs with a higher activation energy than the trisubstituted pyrrole system in entry 2. The monosubstituted aminopyrrole system in entry 3 is both the kinetically and thermodynamically least favorable process. The trisubstituted electron rich pyrrole in entry 2 engages in a kinetically and thermodynamically more favorable Diels-Alder reactions compared to monosubstituted 1-aminopyrroles. Examination of the transition state structures for the processes is very revealing. The transition-state structure in entry 2 is a classical concerted but very slightly asynchronous Diels-Alder transition state, where bond making to carbon-5 of the pyrrole (1.55 Angstroms) is only slightly more advanced than bond making to carbon-2 (1.58 Angstroms). There are only minimal differences between product and transition state in this reaction. However, the transition state structures in entries 1 and 3 are very different. These transition states are very asynchronous (despite the highly symmetrical nature of both reactants in entry 1) and features bond lengths in the forming C-C bonds of 1.83 Angstroms and 2.95 Angstroms, and is thus more indicative of a stepwise reaction. In the trisubstituted pyrrole system, the transition state for the nitrene extrusion step was also calculated. The overall Diels-Alder nitrene extrusion sequence is highly exergonic (ΔG = −29.1 kcal/mol for the system in entry 2). The nitrene extrusion process is kinetically more facile than the Diels-Alder step, which suggests that isolation/observation of the azanorbornadiene intermediate will be difficult. In a previous manuscript using a carboethoxypyrrole substrate,10a the authors attributed transient NMR signals to an azanorbornene intermediate.
Table 2.
Optimized Transition States from the Reactions in Entries 1 and 2
| ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | ΔG (kcal/mol) | ΔGACT (kcal/mol) | TS Asynchronicity |
| 1 | H | H | H | +8.40 | +27.01 | 1.12 |
| 2 | Me | Me | Me | +15.21 | +22.67 | 0.03 |
| 3 | Me | H | H | +19.28 | +34.24 | 0.89 |
TS Asynchronicity = long forming sigma bond minus short sigma bond in the transition state in Angstroms.
Figure 2.

Transition state structures for the reaction in Table 2, entries 1 and 2
Summary and Conclusions
In summary, the three-component sequential coupling of carbene complexes, enyne-hydrazones, and highly electron-deficient alkynes readily leads to diverse highly substituted phthalate derivatives. Although the process is less energetically favorable than previous studies that involved highly activated aminoisoindoles, the yields are comparable to those from that investigation. The synthetic versatility of the aminopyrrole synthesis from enyne hydrazone-Fischer carbene complex coupling significantly expands the synthesis options for this compound class, and their facile participation in Diels-Alder reactions even at the non-optimal concentrations of the carbene complex-alkyne coupling reactions provides an operationally simple method for the synthesis of highly substituted phthalate and isoquinoline derivatives.
Supplementary Material
Benzannulation via coupling of Fischer carbenes, enyne hydrazones, alkyne dienophiles
Benzene synthesis via pyrrole Diels-Alder reactions accompanied by nitrene extrusion
Transformation of phthalate-benzylketone three-component adducts to isoquinolones
Acknowledgments
This work was supported by the National Institutes of Health (5SC3GM111131).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kotha S, Misra S, Halder S. Tetrahedron. 2008;64:10775–10790. [Google Scholar]
- 2.Afarinkia K, Vinader V, Nelson TD, Posner GH. Tetrahedron. 1992;48:9111–9171. [Google Scholar]
- 3.Recent examples:; a Wang H, Yu S. J Am Chem Soc. 2015;137:13792–13795. doi: 10.1021/jacs.5b09878. [DOI] [PubMed] [Google Scholar]; b Vo TH, Perera U, Gayani E, Shekhirev M, Mehdi Pour M, Kunkel DA, Lu H, Gruverman A, Sutter E, Cotlet M, Nykypanchuk D, Zahl P, Enders A, Sinitskii A, Sutter P. Nano Lett. 2015;15:5770–5777. doi: 10.1021/acs.nanolett.5b01723. [DOI] [PubMed] [Google Scholar]; c Kaur S, Bhalla V, Kumar M. ACS Appl Mater Interfaces. 2015;7:16617–16624. doi: 10.1021/acsami.5b04179. [DOI] [PubMed] [Google Scholar]; d Shan L, Liu D, Li H, Xu X, Shan B, Xu JB, Miao Q. Adv Mater. 2015;27:3418–3423. doi: 10.1002/adma.201500149. [DOI] [PubMed] [Google Scholar]
- 4.a Mahdjour S, Harche-Kaid M, Haidour A, Chahboun R, Alvarez-Manzaneda E. Org Lett. 2016;18:5964–5967. doi: 10.1021/acs.orglett.6b03121. [DOI] [PubMed] [Google Scholar]; Although gold-catalyzed phenol synthesis from furans and alkynes does not involve a Diels-Alder reaction, then net transformation is similar. For a recent example, see:; b Ibanez S, Poyatos M, Dawe LN, Gusev D, Peris E. Organometallics. 2016;35:2747–2758. [Google Scholar]
- 5.Duan S, Sinha-Mahapatra DK, Herndon JW. Org Lett. 2008;10:1541–1544. doi: 10.1021/ol800242n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpino LA, Padykula RE, Barr DE, Hall FH, Krause JG, Dufresne RF, Thoman CJ. J Org Chem. 1988;53:2565–2572. [Google Scholar]
- 7.For a recent leading reference, see:; Lin C, Zhen L, Cheng Y, Du HJ, Zhao H, Wen X, Kong LY, Xu QL, Sun H. Org Lett. 2015;17:2684–2687. doi: 10.1021/acs.orglett.5b01078. [DOI] [PubMed] [Google Scholar]
- 8.a Ding X, Nguyen ST, Williams JD, Peet NP. Tetrahedron Lett. 2014;55:7002. doi: 10.1016/j.tetlet.2014.10.114. [DOI] [PMC free article] [PubMed] [Google Scholar]; B Domingo LR, Perez P, Ortega DE. J Org Chem. 2013;78:2462. doi: 10.1021/jo3027017. [DOI] [PubMed] [Google Scholar]
- 9.a Noland WE, Lee CK. J Org Chem. 1980;45:4573–4582. [Google Scholar]; b Avalos M, Babiano R, Bravo JL, Cintas P, Jiménez JL, Palacios JC, Silva MA. Green Chem. 2001;3:26–29. [Google Scholar]
- 10.a Schultz AG, Shen M. Tetrahedron Lett. 1979;20:2969–2972. [Google Scholar]; b Schultz AG, Shen M, Ravichandran R. Tetrahedron Lett. 1981;22:1767–1770. [Google Scholar]; c Schultz AG, Shen M. Tetrahedron Lett. 1981;22:1775–1778. [Google Scholar]
- 11.a Schultz AG, Shen M. Tetrahedron Lett. 1979;20:2965–2968. [Google Scholar]; b Hart H, Lai CY, Nwokogu GC, Shamouilian S. Tetrahedron. 1987;43:5203–5224. [Google Scholar]
- 12.Martinez GR, Grieco PA, Srinivasan CV. J Org Chem. 1981;46:3760–3761. [Google Scholar]
- 13.Davis L, Olsen GE, Klein JT, Kapples KJ, Huger FP, Smith CP, Petko WW, Cornfeldt M, Effland RC. J Med Chem. 1996;39:582–587. doi: 10.1021/jm950644v. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Herndon JW. Org Lett. 2003;5:2043–2045. doi: 10.1021/ol034414j. [DOI] [PubMed] [Google Scholar]
- 15.For recent citations to the synthesis and uses of isoquinolones, see:; a Shi L, Yu K, Wang B. Chem Commun. 2015;51:17277–17280. doi: 10.1039/c5cc05977a. [DOI] [PubMed] [Google Scholar]; b Matsubara T, Ilies L, Nakamura E. Chem Asian J. 2016;11 doi: 10.1002/asia.201501095. [DOI] [PubMed] [Google Scholar]; c Wang H, Yu S. Org Lett. 2015;17:4272–4275. doi: 10.1021/acs.orglett.5b01960. [DOI] [PubMed] [Google Scholar]; d Shaikh AC, Shinde DR, Patil NT. Org Lett. 2016;18:1056–1059. doi: 10.1021/acs.orglett.6b00175. [DOI] [PubMed] [Google Scholar]
- 16.For a comprehensive review, see:; Glushkov VA, Shklyaev YV. Chem Heterocycl Compd. 2001;37:663–687. [Google Scholar]
- 17.Kashinath K, Jadhav PD, Reddy DS. Org Biomol Chem. 2014;12:4098–4103. doi: 10.1039/c4ob00300d. [DOI] [PubMed] [Google Scholar]
- 18.For a mini-review of related stuctures, see:; Kashinath K, Reddy DS. Org Biomol Chem. 2015;13:970–973. doi: 10.1039/c4ob02143f. [DOI] [PubMed] [Google Scholar]
- 19.Hiraiwa Y, Morinaka A, Fukushima T, Kudo T. Bioorg Med Chem Lett. 2009;19:5162–5165. doi: 10.1016/j.bmcl.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Feng Y, Liu YM, Liu QH, Lei YJ. Heterocycles. 2012;85:3015–3019. [Google Scholar]
- 21.Zhang Y, Herndon JW. Org Lett. 2003;5:2043–2045. doi: 10.1021/ol034414j. [DOI] [PubMed] [Google Scholar]
- 22.This is an important pathway for isoelectronic furan and isobenzofuran systems. For a recent review, see:; Herndon JW, Dhakal B. ARKIVOC. 2016;2016:276–306. doi: 10.3998/ark.5550190.p009.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.This step was performed because enol ethers have previously been problematic in long acquisition-time 13C NMR spectra and in acquiring high-resolution mass spectra from external facilities, presumably due to decomposition during shipment.
- 24.This regiochemistry is counter-intuitive in that the conjugated ester is acting as the controlling group over the directly-attached ester group, however this regiochemistry is consistent with that observed in the previous manuscipt5 and consistent with computational predictions; Dai M, Sarla D, Yu M, Danishefsky SJ, Jones GO, Houk KN. J Am Chem Soc. 2007;129:645–657. doi: 10.1021/ja065762u. [DOI] [PubMed] [Google Scholar]
- 25.There is experimental precedent for this ring system; Tobe Y, Ueda K, Kakiuchi K, Odaira Y, Kai Y, Kasai N. Tetrahedron. 1986;42:1851–1858. [Google Scholar]
- 26.To our knowledge, the smallest m-cyclophane ring formed through RCM is an 11-membered ring in a conformationally-biased system, see:; Kotha S, Chinnam AK, Shirbhate ME. J Org Chem. 2015;80:9141–9146. doi: 10.1021/acs.joc.5b01433. [DOI] [PubMed] [Google Scholar]; For a review on cyclophane syntheses, often via metathesis, see:; Kotha S, Shirbhate ME, Waghule GT. Beilstein J Org Chem. 2015;11:1274–1331. doi: 10.3762/bjoc.11.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


