Abstract
Background
The prognostic value of occurrence of ischemic stroke in a patient despite aspirin treatment (aspirin treatment failure) is not known. Our objective was to determine if aspirin treatment failure predicts recurrent ischemic stroke and/or death.
Methods
We performed a post-hoc analysis of data from the National Institute of Neurological Disorders and Stroke (NINDS) intravenous recombinant tissue plasminogen activator (rt-PA) trial and the Trial of ORG 10172 in Acute Stroke Treatment (TOAST). Multivariate analysis was used to calculate the odds ratio (OR) of recurrent stroke and recurrent stroke or death for aspirin treatment failure patients for the duration of available follow-up (3 months for TOAST patients; 12 months for NINDS rt-PA trial patients).
Results
The rate of aspirin treatment failure was 40% and 35% among 1275 patients and 624 patients recruited in the TOAST and NINDS rt-PA trials, respectively. The risk of stroke and death at 3 months and 1 year was not higher among patients classified as aspirin treatment failures among the TOAST (OR 1.1; 95% confidence interval [CI] 0.8–1.6; P =.7) or NINDS rt-PA trial patients (OR 0.8; 95% CI 0.6–1.3; P =.4), respectively. In subgroup analysis, aspirin treatment failure was not found to be associated with recurrent stroke or with the combined endpoint of stroke and death among categories defined by etiologic subtype, including those with large artery atherosclerosis.
Conclusions
In a post-hoc analysis of 2 randomized ischemic stroke trials, aspirin treatment failure was not found to be associated with an increased risk of recurrent stroke or death.
Keywords: Aspirin resistance, aspirin treatment failure, ischemic stroke
Aspirin treatment failure is a clinical term that refers to the recurrence of thromboembolic events in patients treated with aspirin.1 Aspirin use has been shown to reduce the risk of serious vascular events (nonfatal myocardial infarction, nonfatal stroke, or vascular death) by approximately 25%.2 If the analysis is restricted to patients with cerebrovascular disease, the relative risk reduction is 13%.3,4 Among patients with vascular disease, the annual risk of a serious vascular event is in the 4% to 8% range and aspirin can be expected to prevent at least 10 to 20 such events for every 1000 patients treated for 1 year.2,5 Subsequently, 30 to 60 vascular events should be expected to occur among every 1000 patients on aspirin over the course of a year (treatment failure). The pathophysiologic basis of ischemic events that occur during treatment with aspirin is unknown, but several factors, including aspirin resistance, platelet hyperaggregability, and reduced aspirin bioavailability have been proposed.6 There is a lot of variability in the manner that aspirin resistance and aspirin treatment failure are defined, and the terms are sometimes used interchangeably. Despite the lack of a concrete putative mechanism and uniform definitions, aspirin treatment failure is widely believed to be associated with a higher likelihood of future events, and it therefore affects management decisions. We undertook this study to examine whether aspirin treatment failure among ischemic stroke patients is associated with a higher risk of recurrent ischemic stroke and death.
Methods
We analyzed the data from 2 prospective, randomized controlled stroke trials, the Trial of ORG 10172 in Acute Stroke Treatment (TOAST)7 and the National Institute of Neurological Disorders and Stroke (NINDS) recombinant tissue plasminogen activator (rt-PA) trial.8 The TOAST dataset was acquired from the investigators following permission from the NINDS, and for the NINDS rt-PA trial, the public access data files available from National Technical Information Services (Springfield, VA) were used. The design and primary results of the trial have been presented in detail in previous publications.8–10
Briefly, TOAST tested the efficacy of the low–molecular weight heparinoid ORG 10172 (daparinoid) in acute ischemic stroke. The study randomized 1281 patients over the course of 6 years. Classification of stroke subtype was based on a central-blinded evaluation assessing the clinical findings and the results of brain imaging and ancillary diagnostic tests. Categories were large artery atherosclerosis, cardioembolism, small artery occlusion, other determined cause, and undetermined cause.11 A 7-day course of daparinoid or placebo was administered within 24 hours from stroke onset. The main outcome measure was the rate of favorable outcome at 3 months, defined as the combination of a Glasgow Outcome Scale score of I or II and a modified Barthel Index ≥12 at 3 months or 7 days. Patients were assessed daily during the acute treatment period and had a follow-up examination at 3 months.
The NINDS rt-PA stroke trial was a randomized trial of intravenous (IV) rt-PA for acute ischemic stroke. The trial was conducted in 2 parts. First, 291 patients were randomized to receive either placebo or 0.9 mg/kg IV rt-PA within 3 hours of stroke onset. The goal was to test the efficacy of rt-PA at 24 hours after stroke onset, but clinical data were also collected at 3 months. Subsequently, 333 patients were randomized to test the effect of rt-PA on favorable outcome at 3 months measured by a global outcome scale derived from the combination of 4 existing scales (modified Rankin Scale [mRS], Glasgow Outcome Scale, Barthel Index, and National Institutes of Health Stroke Scale [NIHSSS] score). Clinical data were collected at baseline, 24 hours, and 3 months after randomization. In a follow-up study,9 outcome data on all 624 patients were collected by means of telephone interviews at 6 and 12 months after stroke onset. Stroke subtypes were determined based on the TOAST criteria.11
Statistical Analysis
We sought to evaluate the effect of aspirin treatment failure on outcome by the end of available follow-up after the initial stroke. The patients who had been taking aspirin at the time of their stroke (at least 7 days earlier in TOAST, and before randomization in NINDS) and would therefore be characterized as aspirin treatment failures were compared to those who were not taking aspirin. The outcome measures were occurrence of any stroke and the combined endpoint of stroke and/or and death at 3 months for the TOAST patients and at 1 year for the NINDS rt-PA trial patients.
For each study, we compared the distribution of demographics, cardiovascular risk factors, severity (NIHSS score on admission), and etiology of stroke between the patients who had previously been taking aspirin and those who had not taken aspirin. Data are presented as percent or mean ± SD. The Chi-square test was used for comparing categorical data, and the t test was used for continuous data. The effect of previous aspirin use on outcomes was evaluated using multivariate logistic regression. For this analysis, we considered all variables that differed between the 2 groups and included those that had an association with outcome variables of P <.20 by univariate comparison in the final model. Stratified analysis was then performed according to the etiology of ischemic stroke. For this secondary analysis, our model only adjusted for age and initial stroke severity (NIHSS score) for the TOAST-recruited patients and for initial NIHSS score for NINDS rt-PA trial–recruited patients because of sample size limitations.
Results
The TOAST trial randomized 1281 patients. Follow-up for 3 months was available for 1275 patients, of whom 509 had been taking aspirin for at least 1 week before their stroke. The NINDS trial randomized 624 patients. Of those patients, 216 had taken aspirin before stroke onset. In both trials, the patients who had been taking aspirin were mostly men and white, were older, had a higher prevalence of cardiovascular risk factors with the exception of cigarette smoking, and had similar baseline stroke severity and frequency of stroke subtypes as the “aspirin-naïve” patients (Tables 1 and 2).
Table 1.
Patient demographics, cardiovascular risk factors, and stroke subgroups based on etiology according to the TOAST study
| Patients taking aspirin before recruitment* | Patients not taking aspirin | P value | |
|---|---|---|---|
| No. of patients | 509 | 766 | |
| Age, y (mean ± SD) | 67 ± 10 | 64 ± 12 | <.0001 |
| Women | 189 (37%) | 313 (41%) | .02 |
| Race/ethnicity | |||
| White | 357 (71%) | 440 (57%) | <.0001 |
| African American | 101 (20%) | 189 (25%) | |
| Hispanic | 38 (7%) | 95 (12%) | |
| Asian | 5 (1%) | 29 (4%) | |
| Other | 8 (2%) | 13 (2%) | |
| Mean NIHSS score at baseline (± SD) | 7.9 ± 5.2 | 8.8 ± 5.8 | .13 |
| Active trial treatment | 250 (49%) | 384 (50%) | .72 |
| History of stroke | 144 (28%) | 85 (11%) | <.0001 |
| History of atrial fibrillation† | 64 (13%) | 35 (5%) | <.0001 |
| History of myocardial infarction | 153 (30%) | 79 (10%) | <.0001 |
| Congestive heart failure | 65 (13%) | 53 (7%) | .0005 |
| Diabetes | 161 (32%) | 210 (27%) | .2 |
| Hypertension | 362 (71%) | 483 (63%) | .01 |
| Hypercholesterolemia | 157 (31%) | 134 (17%) | <.0001 |
| Cigarette smoking | 176 (35%) | 321 (42%) | .03 |
| Etiology of stroke‡ | |||
| Large vessel disease | 255 (33%) | 159 (32%) | .15 |
| Cardioembolic | 94 (12%) | 81 (16%) | |
| Lacunar | 311 (41%) | 209 (41%) | |
| Other | 104 (14%) | 55 (11%) | |
Abbreviations: NIHSS, National Institutes of Health Stroke Scale; SD, standard deviation; TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
Aspirin treatment failure patients are compared to patients who were not on aspirin. Data taken from the Trial of ORG 10172 in Acute Stroke Treatment.
Aspirin intake for a minimum of 7 days before randomization.
Data only partially available.
Data missing for 8 patients.
Table 2.
Patient demographics, cardiovascular risk factors, and stroke subgroups based on etiology according to the NINDS intravenous recombinant tissue plasminogen trial
| Patients taking aspirin before recruitment | Patients not taking aspirin | P value | |
|---|---|---|---|
| No. of patients | 216 | 408 | |
| Age, y (mean ± SD) | 69 ± 11 | 66 ± 12 | .0005 |
| Women | 85 (39%) | 177 (43%) | .33 |
| Race/ethnicity | |||
| White | 154 (71%) | 249 (61%) | .04 |
| African American | 44 (20%) | 125 (31%) | |
| Hispanic | 5 (1%) | 7 (2%) | |
| Asian | 15 (7%) | 22 (5%) | |
| Other | 2 (1%) | 5 (1%) | |
| Mean NIHSS score at baseline (± SD) | 14.4 ± 7 | 15 ± 7.2 | .31 |
| Active trial treatment | 127 (59%) | 185 (45%) | <.0001 |
| History of stroke | 47 (22%) | 36 (9%) | <.0001 |
| History of atrial fibrillation | 50 (23%) | 65 (16%) | .03 |
| History of myocardial infarction | 60 (29%) | 71 (18%) | .002 |
| Congestive heart failure | 36 (17%) | 63 (15%) | .69 |
| Diabetes | 53 (25%) | 78 (19%) | .11 |
| Hypertension | 155 (72%) | 253 (62%) | .01 |
| Hypercholesterolemia | 59 (33%) | 82 (24%) | .05 |
| Cigarette smoking | 58 (27%) | 157 (38%) | .004 |
| Etiology of stroke | |||
| Large vessel disease | 36 (17%) | 89 (22%) | .37 |
| Cardioembolic | 91 (42%) | 150 (38%) | |
| Lacunar | 23 (11%) | 38 (10%) | |
| Other | 65 (30%) | 123 (31%) | |
Abbreviations: NIHSS, National Institutes of Health Stroke Scale; NINDS, National Institute of Neurological Disorders and Stroke; SD, standard deviation.
Aspirin treatment failure patients are compared to patients who were not on aspirin. Data from the NINDS intravenous recombinant tissue plasminogen study.
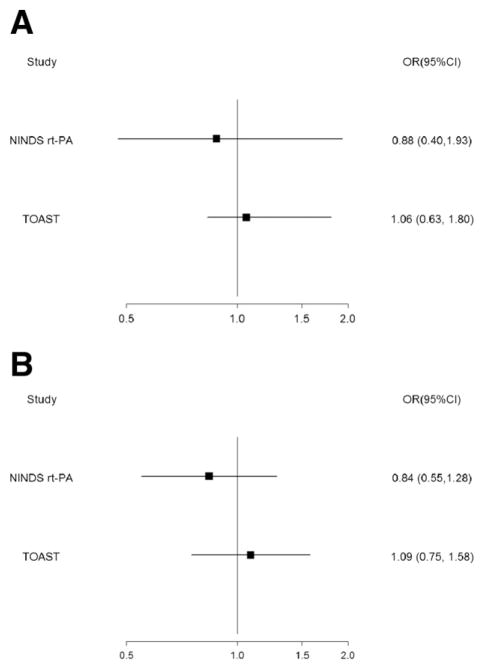
In univariate analysis, there was no difference in the risk of stroke between the aspirin treatment failure and “aspirin-naïve” patients in either the TOAST (P =.55) at 3 months or the NINDS rt-PA (P = .93) trial at 1 year. This finding was confirmed in multivariate analysis. After adjusting for age, gender, baseline NIHSS score, history of previous stroke, congestive heart failure, and hypertension, aspirin failure among the TOAST-recruited patients was not associated with stroke recurrence at 3 months (odds ratio [OR] 1.06; 95% confidence interval [CI] 0.63–1.80; P = .82; Fig 1). The same lack of association was observed among the NINDS rt-PA trial–recruited patients after adjusting for age, baseline NIHSS score, history of previous stroke, hypertension, cigarette smoking, and coronary artery disease (OR 0.88; 95% CI 0.40–1.93; P =.74; Fig 1).
Figure 1.
(A) Stroke. (B) Stroke and/or death. Effect of aspirin treatment failure on stroke and stroke and death at 3 months (TOAST) and 1 year (NINDS rt-PA trial). OR, odds ratio; CI, confidence interval; TOAST, trial of ORG 10172 in acute stroke treatment; NINDS rt-PA, National Institute of Neurological Disorders and Stroke recombinant tissue plasminogen trial.
Univariate analysis revealed no association between aspirin treatment failure with regard to the combined endpoint of stroke and death in the patients recruited in TOAST (P = .54) and those recruited in the NINDS rt-PA trial (P =.61). Multivariate analysis was performed adjusting for the same variables for each study as listed above. The association between aspirin treatment failure and stroke and/or death was not significant among the TOAST patients within 3 months (OR 1.09; 95% CI 0.75–1.58; P =.67) or the NINDS rt-PA patients (OR 0.84; 95% CI 0.55–1.28; P =.42) within 1 year (Fig 1).
In subgroup analysis (Tables 3 and 4), no association between aspirin treatment failure and both outcome measures could be identified in any of the stroke subtypes defined by stroke etiology. In multivariate analysis, we adjusted for patient age and baseline NIHSS score for the TOAST-recruited patients and for baseline NIHSS score for the NINDS rt-PA–recruited patients. Aspirin treatment failure was not associated with stroke or the combined stroke and death endpoint in any of the stroke subtypes, including large artery atherosclerosis, in either trial (Tables 3 and 4).
Table 3.
Association between aspirin treatment failure and endpoints according to stroke subtype: Analysis of data from the Trial of ORG 10172 in Acute Stroke Treatment
| Event rate | Univariate analysis | Multivariate analysis* | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| Patients taking aspirin before ischemic stroke† | Patients not taking aspirin before ischemic stroke | P value | OR (95% CI) | P value | |
| Stroke within 3 months | |||||
| Large vessel disease | 9/159 (6%) | 8/255 (3%) | .21 | 1.7 (0.65–4.7) | .27 |
| Cardiomebolic | 4/81 (5%) | 4/94 (4%) | 1 | 1.2 (0.29–4.9) | .82 |
| Small vessel disease | 13/209 (6%) | 14/311 (5%) | .42 | 1.4 (0.63–3.0) | .43 |
| Stroke or death within 3 months | |||||
| Large vessel disease | 23/159 (14%) | 32/255 (13%) | .66 | 1.2 (0.68–2.3) | .47 |
| Cardioembolic | 10/81 (12%) | 16/94 (17%) | .40 | 0.73 (0.30–1.8) | .50 |
| Small vessel disease | 19/209 (9%) | 19/311 (6%) | .23 | 1.5 (0.79–3.0) | .21 |
Abbreviations: CI, confidence interval; OR, odds ratio.
Adjusted for patient age and National Institutes of Health Stroke Scale score at baseline.
Aspirin intake for a minimum of 7 days before onset of ischemic stroke.
Table 4.
Association between aspirin treatment failure and endpoints according to stroke subtype: Analysis of data from the NINDS intravenous recombinant tissue plasminogen trial
| Event rate | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| Patients taking aspirin before ischemic stroke | Patients not taking aspirin before ischemic stroke | P value | OR (95% CI) | P value | |
| Stroke within 1 year | |||||
| Large vessel disease | 1/36 (3%) | 4/89 (4%) | 1 | 0.64 (0.7–6.0) | .69 |
| Cardioembolic | 8/91 (9%) | 12/150 (8%) | .81 | 1.1 (0.43–2.8) | .86 |
| Small vessel disease | 0/23 (0%) | 1/38 (3%) | —* | —* | —* |
| Stroke or death within 1 year | |||||
| Large vessel disease | 8 (22%) | 28 (31%) | .38 | 0.57 (0.22–1.5) | .24 |
| Cardioembolic | 34 (37%) | 60 (30%) | .79 | 0.97 (0.54–1.7) | .91 |
| Small vessel disease | 1 (4%) | 5 (13%) | .39 | —* | —* |
Abbreviations: CI, confidence interval; NINDS, National Institute of Neurological Disorders and Stroke; OR, odds ratio.
Analysis not possible.
Discussion
Aspirin treatment failure among ischemic stroke patients is widely believed to be associated with a higher likelihood of future ischemic events. In this post-hoc analysis, however, we did not observe an increase in the risk of recurrent stroke or death among patients who failed aspirin therapy.
Aspirin resistance, broadly defined as an inadequate protective effect of aspirin, is the phenomenon most commonly implicated as a cause of aspirin treatment failure. Aspirin resistance can be assessed by a variety of methods that measure platelet aggregation ex vivo or by measuring levels of thromboxane A2 metabolites. Numerous studies have found aspirin resistance to correlate with higher risk of subsequent events among patients with cardiovascular disease,12 peripheral vascular disease,13 and stroke.14,15 This finding has been confirmed for cardiac patients in several metaanalyses.16
Still, the clinical significance, and even the true prevalence of aspirin resistance remains a subject of debate.17 The prevalence of aspirin resistance among vascular patients has been reported at widely divergent rates.18,19 Differences in the techniques used account for part of the variability in the results. Using different assays, Lordkipanidze et al20 found that the prevalence rates of aspirin resistance changed from 2.8% to 59.5% for the same cohort of 201 patients with stable coronary artery disease. Studies have used different aspirin dosing regimens,21 even though aspirin resistance has been shown to be dose-dependent and reversible in some individuals by using higher aspirin doses.21,22 Patient noncompliance is frequent,23 and there has been no uniform definition of aspirin resistance.24 Finally, it appears that aspirin resistance is a dynamic phenomenon that can develop in the course of treatment even though initially absent,25,26 and be brought on by conditions that are associated with higher platelet activation, such as inflammation.24
Until now, very few studies have reported on aspirin treatment failure in the context of the prevention of recurrent stroke. Most of the available data on the clinical significance of aspirin treatment failure stem from post-hoc analyses from 3 major multicenter, double-blind, randomized stroke trials: Warfarin-Aspirin Symptomatic Intracranial Disease (WASID),27 Warfarin-Aspirin Recurrent Stroke Study Group (WARSS),28 and Ticlopidine Aspirin Stroke Study Group (TASS).29 In TASS, the rate of recurrent stroke was estimated for 3034 patients during a 2- to 6-year period after randomization to aspirin or ticlopidine. The risk of recurrent stroke was higher (12%) in the 1297 patients who had previously been treated with aspirin. Post-hoc analysis also revealed that patients who had been taking aspirin at the time of their qualifying event were among those who were most likely to benefit from ticlopidine.30
WARSS randomized 2206 patients with different stroke etiologies to aspirin or warfarin and followed them for 2 years. Post-hoc analysis revealed higher rates of stroke and death among the patients who were on aspirin at the time of randomization.31
WASID enrolled 569 patients with symptomatic intracranial disease.27 The patients received either aspirin or warfarin and were followed up for a mean of 1.8 years. Forty six percent of those patients were on an antiplatelet agent at the time of their qualifying event. In post-hoc analysis, aspirin treatment failure did not predict future stroke (OR 1.01; 95% CI 0.58–1.77; P =.97) or future stroke or vascular death (OR 0.86; 95% CI 0.55–1.34; P =.51).32
Based on these data, one could reason that patients with symptomatic intracranial disease form a subset where aspirin treatment failure does not predict future stroke. However, our results revealed that aspirin treatment failure was not associated with stroke or stroke and death at 1 year among the NINDS rt-PA trial–recruited patients and at 3 months among the TOAST-recruited patients, regardless of stroke etiology. Both studies in our analysis included patients immediately after stroke onset, unlike TASS, WARSS, and WASID. This recruitment paradigm allows for the ascertainment of early recurrent stroke or death. Because the rates of early stroke recurrence are substantial, especially among patients with large vessel disease,33,34 the current analysis is likely to have produced more accurate data.
Our study has a number of limitations. First, we do not know the proportion of patients who continued aspirin treatment following the initial treatment failure and those who were started on a different antithrombotic treatment. The fact that the TOASTand NINDS rt-PA trials were conducted in the 1990s was advantageous to our purpose because treatment options were more limited at that time, and therefore variability in the treatment of the patients during the follow-up period should have been relatively low. We could also not account for the effect that potential risk factor modifications and use of other medications could have modified the outcome of some of the patients. Our study is a secondary analysis of completed randomized trials. Data on some important variables may be missing, so we cannot exclude the possibility that unmeasured confounding could explain some of our findings. Because of strict inclusion and exclusion criteria in both studies, there is concern that the studied patients might not be representative of the overall ischemic stroke patient population. The relatively small number of patients within various subgroups is another reason to be cautious with extrapolating our results to the general population of patients with ischemic stroke. The length of available follow-up for the TOAST patients was relatively limited, so it is conceivable that a significant number of events were not recorded. However, the only available data on early stroke recurrence in aspirin treatment failure patients revealed that 50% of the events occurred within 36 days from the initial stroke.35 Despite those limitations, the information this study provides may be useful to clinicians discussing prognosis with stroke patients who have been taking aspirin and may help guide the design of future clinical trials.
Many questions remain unanswered regarding the significance of aspirin resistance and treatment failure. Future studies should further address the following questions: (1) How prevalent and reliable a finding is aspirin resistance? (2) Does aspirin resistance predict aspirin treatment failure? (3) Does aspirin treatment failure predict future clinical events?
At this time, aspirin remains the single most cost effective and widely used agent for the prevention of atherothrombotic ischemic events. There are currently not sufficient data to support changing antiplatelet regimens or favoring endovascular procedures in aspirin treatment failure patients.
Acknowledgments
Dr. Lakshminarayan is on the adverse events committee for CVRx and AGA Medical, both device manufacturers. She is funded by National Institutes of Health Grant K23NS051377, is a coinvestigator for the Centers for Disease Control and Prevention Grant U58DP000857, and received support from an American Academy of Neurology fellowship (#84500-2002). Dr. Adams has received financial support from NMT Medical, Schering-Plough, Medtronic, and Merck. Dr. Qureshi has received funding from National Institutes of Health RO-1-NS44976-01A2 (medication provided by ESP Pharma), the American Heart Association Established Investigator Award 0840053N, and the Minnesota Medical Foundation, Minneapolis, MN.
Footnotes
Drs. Georgiadis, Cordina, Vazquez, Tariq, and Suri have no conflicts of interest to disclose.
References
- 1.Bhatt DL, Topol EJ. Scientific and therapeutic advances in antiplatelet therapy. Nat Rev Drug Discov. 2003;2:15–28. doi: 10.1038/nrd985. [DOI] [PubMed] [Google Scholar]
- 2.Antiplatelet Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Algra A, van Gijn J. Aspirin at any dose above 30 mg offers only modest protection after cerebral ischaemia. J Neurol Neurosurg Psychiatry. 1996;60:197–199. doi: 10.1136/jnnp.60.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Algra A, van Gijn J. Cumulative meta-analysis of aspirin efficacy after cerebral ischaemia of arterial origin. J Neurol Neurosurg Psychiatry. 1999;66:255. doi: 10.1136/jnnp.66.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrono C, Coller B, FitzGerald GA, et al. Platelet-active drugs: The relationships among dose, effectiveness, and side effects: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):234S–264S. doi: 10.1378/chest.126.3_suppl.234S. [DOI] [PubMed] [Google Scholar]
- 6.Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. 2006;367:606–617. doi: 10.1016/S0140-6736(06)68040-9. [DOI] [PubMed] [Google Scholar]
- 7.Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. JAMA. 1998;279:1265–1272. [PubMed] [Google Scholar]
- 8.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 9.Kwiatkowski TG, Libman RB, Frankel M, et al. Effects of tissue plasminogen activator for acute ischemic stroke at one year. National Institute of Neurological Disorders and Stroke Recombinant Tissue Plasminogen Activator Stroke Study Group. N Engl J Med. 1999;340:1781–1787. doi: 10.1056/NEJM199906103402302. [DOI] [PubMed] [Google Scholar]
- 10.Patel SC, Levine SR, Tilley BC, et al. Lack of clinical significance of early ischemic changes on computed tomography in acute stroke. JAMA. 2001;286:2830–2838. doi: 10.1001/jama.286.22.2830. [DOI] [PubMed] [Google Scholar]
- 11.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 12.Gum PA, Kottke-Marchant K, Welsh PA, et al. A prospective, blinded determination of the natural history of aspirin resistance among stable patients with cardiovascular disease. J Am Coll Cardiol. 2003;41:961–965. doi: 10.1016/s0735-1097(02)03014-0. [DOI] [PubMed] [Google Scholar]
- 13.Mueller MR, Salat A, Stangl P, et al. Variable platelet response to low-dose ASA and the risk of limb deterioration in patients submitted to peripheral arterial angioplasty. Thromb Haemost. 1997;78:1003–1007. [PubMed] [Google Scholar]
- 14.Grotemeyer KH. Effects of acetylsalicylic acid in stroke patients. Evidence of nonresponders in a subpopulation of treated patients. Thromb Res. 1991;63:587–593. doi: 10.1016/0049-3848(91)90085-b. [DOI] [PubMed] [Google Scholar]
- 15.Grotemeyer KH, Scharafinski HW, Husstedt IW. Two-year follow-up of aspirin responder and aspirin non responder. A pilot-study including 180 post-stroke patients. Thromb Res. 1993;71:397–403. doi: 10.1016/0049-3848(93)90164-j. [DOI] [PubMed] [Google Scholar]
- 16.Snoep JD, Hovens MM, Eikenboom JC, et al. Association of laboratory-defined aspirin resistance with a higher risk of recurrent cardiovascular events: A systematic review and meta-analysis. Arch Intern Med. 2007;167:1593–1599. doi: 10.1001/archinte.167.15.1593. [DOI] [PubMed] [Google Scholar]
- 17.Hjemdahl P. Aspirin resistance testing not ready for “prime time”. Heart. 2009;95:1220–1222. doi: 10.1136/hrt.2008.159954. [DOI] [PubMed] [Google Scholar]
- 18.Buchanan MR, Schwartz L, Bourassa M, et al. Results of the BRAT study—A pilot study investigating the possible significance of ASA nonresponsiveness on the benefits and risks of ASA on thrombosisin patientsundergoing coronary artery bypass surgery. Can J Cardiol. 2000;16:1385–1390. [PubMed] [Google Scholar]
- 19.Tantry US, Bliden KP, Gurbel PA. Overestimation of platelet aspirin resistance detection by thrombelastograph platelet mapping and validation by conventional aggregometry using arachidonic acid stimulation. J Am Coll Cardiol. 2005;46:1705–1709. doi: 10.1016/j.jacc.2005.05.090. [DOI] [PubMed] [Google Scholar]
- 20.Lordkipanidze M, Pharand C, Schampaert E, et al. A comparison of six major platelet function tests to determine the prevalence of aspirin resistance in patients with stable coronary artery disease. Eur Heart J. 2007;28:1702–1708. doi: 10.1093/eurheartj/ehm226. [DOI] [PubMed] [Google Scholar]
- 21.Tantry US, Mahla E, Gurbel PA. Aspirin resistance. Prog Cardiovasc Dis. 2009;52:141–152. doi: 10.1016/j.pcad.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Helgason CM, Tortorice KL, Winkler SR, et al. Aspirin response and failure in cerebral infarction. Stroke. 1993;24:345–350. doi: 10.1161/01.str.24.3.345. [DOI] [PubMed] [Google Scholar]
- 23.Cotter G, Shemesh E, Zehavi M, et al. Lack of aspirin effect: Aspirin resistance or resistance to taking aspirin? Am Heart J. 2004;147:293–300. doi: 10.1016/j.ahj.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Sweeny JM, Gorog DA, Fuster V. Antiplatelet drug ‘resistance’. Part 1: Mechanisms and clinical measurements. Nat Rev Cardiol. 2009;6:273–282. doi: 10.1038/nrcardio.2009.10. [DOI] [PubMed] [Google Scholar]
- 25.Helgason CM, Bolin KM, Hoff JA, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke. 1994;25:2331–2336. doi: 10.1161/01.str.25.12.2331. [DOI] [PubMed] [Google Scholar]
- 26.Pulcinelli FM, Pignatelli P, Celestini A, et al. Inhibition of platelet aggregation by aspirin progressively decreases in long-term treated patients. J Am Coll Cardiol. 2004;43:979–984. doi: 10.1016/j.jacc.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 27.Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 28.Mohr JP, Thompson JL, Lazar RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 29.Hass WK, Easton JD, Adams HP, Jr, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med. 1989;321:501–507. doi: 10.1056/NEJM198908243210804. [DOI] [PubMed] [Google Scholar]
- 30.Grotta JC, Norris JW, Kamm B. Prevention of stroke with ticlopidine: Who benefits most? TASS Baseline and Angiographic Data Subgroup. Neurology. 1992;42:111–115. doi: 10.1212/wnl.42.1.111. [DOI] [PubMed] [Google Scholar]
- 31.Mohr JP. Evidence of aspirin and warfarin failure in the WARRS study. Presented at the 27th International Stroke Conference; San Antonio, TX. February 7–9, 2002. [Google Scholar]
- 32.Turan TN, Maidan L, Cotsonis G, et al. Failure of antithrombotic therapy and risk of stroke in patients with symptomatic intracranial stenosis. Stroke. 2009;40:505–509. doi: 10.1161/STROKEAHA.108.528281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology. 2004;62:569–573. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 34.Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–924. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 35.Thijs VN, Albers GW. Symptomatic intracranial atherosclerosis: Outcome of patients who fail antithrombotic therapy. Neurology. 2000;55:490–497. doi: 10.1212/wnl.55.4.490. [DOI] [PubMed] [Google Scholar]