Abstract
Background
Atrial fibrillation (AF) is an important, independent risk factor for stroke and is estimated to cause a 5-fold increase in ischemic stroke risk. The aim of this article is to describe the changing epidemiology of AF in the United States and to assess the implications for stroke prevention and treatment.
Review Summary
AF prevalence is increasing in the general population. This is likely due to the aging of the population, the improvements in coronary care and the rising prevalence of AF risk factors such as diabetes. Risk factors such as rheumatic heart disease and hypertension have decreased in prevalence over the past few decades. However, novel risk factors such as obesity and possibly the metabolic syndrome have been identified and these have the potential to further increase AF prevalence. The utilization of warfarin has improved and this is reflected in falling ischemic stroke rates in the AF population. There is evidence for an increased incidence of anticoagulant associated intraparenchymal hemorrhages during the 1990s.
Conclusions
Although the decline in stroke rates in AF is laudable, the rising prevalence of AF, the changing profile of risk factors, and the recent plateauing of warfarin use indicate that stroke in AF patients will continue to be a significant public health problem.
Keywords: epidemiology, atrial fibrillation, stroke
Atrial fibrillation (AF) is an important, independent risk factor for stroke and is estimated to cause a 5-fold increase in ischemic stroke risk. This risk is not homogeneous; it varies with the presence of comorbid conditions and can be substantially reduced by prophylactic anticoagulation. Various epidemiological studies indicate that the prevalence of AF is increasing in the United States. In this article we describe the changing epidemiology of AF in the United States, and assess the implications for stroke prevention and treatment.
The burden of atrial fibrillation in the United States population is increasing.
EPIDEMIOLOGY OF AF
AF is the most common sustained cardiac arrhythmia, with an estimated prevalence of 0.4% to 1% in the general US population.1–3 AF is a disease of older adults; its age-specific prevalence is highest in people 85 years and older (11%–12%) and substantially lower in those 55 years and younger (0.1%–0.2%).2–5 AF is more prevalent in men than in women, both overall and within various age categories.2 However, the total number of patients with AF among men and women may be the same because there are a greater number of elderly women as compared with elderly men.2 A survey of hospital discharges has found that 55% of hospitalized patients discharged with a primary diagnosis of AF were women.6 AF is more prevalent in whites than in blacks.6,7
The burden of AF in the US population is increasing. The Anticoagulation and Risk Factors in Atrial Fibrillation study estimated that 2.3 million adults in the United States had AF in the year 2000.2 This is likely an underestimate because many patients with paroxysmal AF remain asymptomatic, hence undetected.5,7,8 Investigators for the Anticoagulation and Risk Factors in Atrial Fibrillation study projected a 2.5-fold increase in the number of adults with AF over the next 50 years (Fig. 1). Some community-based studies have projected a much higher AF disease burden, in the range of 15 to16 million adults by the year 2050.9 These projections of increased disease burden, ie, an increase in the absolute number of AF cases, are largely based on the anticipated aging of the US population. Moreover, some epidemiological studies indicate that, even after age adjustment, the prevalence rate of AF in the population is increasing. For example, in the Framingham Heart Study, the age-adjusted prevalence in men aged 65 to 84 years increased significantly from 3.2% in 1968–1970 to 9.1% in 1987–1989.10 In the Rochester Epidemiology Project, the age-adjusted prevalence of AF increased over 30 years (1960–1989) in both men and women.11 This study also identified a rising trend in various comorbidities associated with AF such as hypertension and heart failure. The rising AF prevalence is likely multifactorial, reflecting complex interactions between the aging US population and secular trends in the prevalence of major risk factors for AF.12
FIGURE 1.
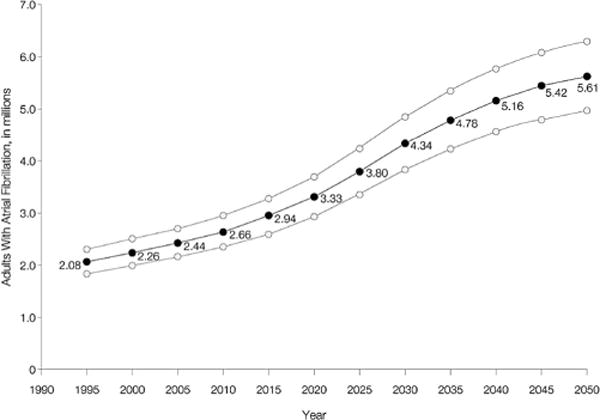
Projected number of adults with atrial fibrillation in the United States between 1995 and 2050. Upper and lower curves represent the upper and lower scenarios based on sensitivity analyses. Reprinted with permission from JAMA. 2001;285:2370–2375. Copyright © 2001, American Medical Association. All rights reserved.
The incidence of AF ranges from less than 1 per 1000 patient-years in those under 40 years of age up to 19.2 per 1000 patient-years in those over 65 years of age.1,6,13,14 The age-specific incidence of AF from different population studies is shown in Figure 2. In every age decade, the incidence is greater in men than in women. The differential higher incidence in men remains even after stratification for the presence of cardiovascular disease.13,15 Longitudinal studies conducted over a span of 2 or 3 decades demonstrate a rising incidence of AF in the general population.9 Survival trends in AF are reported to be either unchanged or improved.16–18 The combination of a rising incidence and a static or improving survival rate likely contributes to the observed trends in AF prevalence.
FIGURE 2.
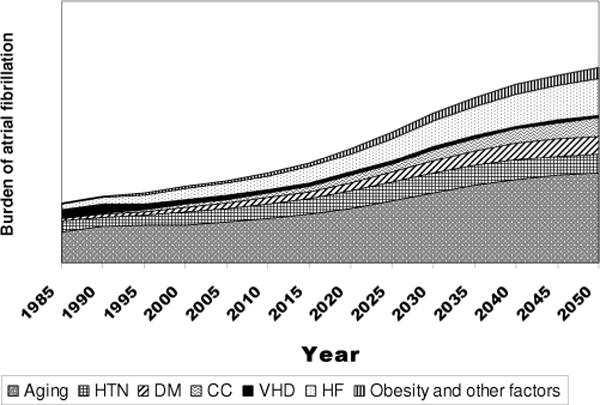
Conceptual model of population trends in atrial fibrillation (AF) risk factors underlying the anticipated increase in AF burden in future decades. HTN = hypertension, DM = diabetes mellitus, CC = improved coronary care, VHD = valvular heart disease, HF = heart failure.
RISK FACTORS FOR AF
Population-based studies have identified several independent risk factors for chronic or recurrent AF. These include prior myocardial infarction (MI),14,15 coronary artery disease,13,14 congestive heart failure,15 valvular heart disease,13–15 cardiomyopathy,14 and hypertension.14,15 Other risk factors demonstrated in epidemiological studies are advanced age, male gender, and diabetes.15 Paroxysmal AF has been reported in male endurance athletes.19 Less common risk factors that are associated particularly with transient AF include binge alcohol abuse,20,21 the immediate postoperative period after cardiac surgery,22 and hyperthyroidism.23 Recently, some authors have reported novel risk factors including obesity,24 metabolic syndrome,25 obstructive sleep apnea, and inflammation,26 as well as psychosocial risk factors, such as anger and hostility.27 The secular trends in the major risk factors for AF likely interact in complex ways, contributing to its increased prevalence.
Ischemic heart disease is a significant risk factor for AF. The Cardiovascular Health Study found coronary artery disease to be an independent risk factor in a general population of men and women over 65 years of age, (relative risk 1.5).13 Prior MI has been shown to be an independent risk factor in men; some epidemiological studies report a relative risk as high as 3.6, even after adjustment for other variables.14 Advances in medical and surgical treatment of coronary artery disease have led to improved survival after acute MI.28,29 Hence more patients are surviving with “sicker hearts” which increases the substrate for subsequent development of AF. In the Framingham study, for instance, a significant rising trend in AF prevalence was observed in men but not in women.10 This was attributed in part to improved survival after MI, a condition that is more common in men than in women.
Valvular heart disease is an independent risk factor for AF. Its adjusted relative risk is between 2 and 3.13 The association is reported to be stronger in women than in men (odds ratios, 3.4 and 1.8, respectively).15 The prevalence of valvular disease in the AF population is modest in the range of 5% to 9%.2,15 Furthermore, mitral stenosis, a major risk factor for AF has declined in prevalence in the US population. This is due to declines in rheumatic fever and rheumatic heart disease.30 The decline in rheumatic valvular heart disease in the United States implies that most future cases of AF will be nonrheumatic AF in contrast to parts of Asia where rheumatic valvular heart disease is the presumed etiology for more than half of prevalent AF cases.31
Like AF, heart failure is a disease of the elderly and shares many of its risk factors (eg, prior MI, hypertension, diabetes).6 The prevalence of heart failure is increasing. Contributing factors are an aging population and the improved survival after MI.32 The rising prevalence of heart failure has implications for AF. In the Framingham study, the odds ratio for patients with heart failure developing incident AF was significant, in the range of 4 to 6. Because the prevalence of heart failure in this population was not very high in this population (approximately 3% range for both men and women), the population attributable risk was modest (10%–12%).15 Nevertheless, with data suggesting an increase in the prevalence of heart failure, this condition may become a significant contributor to AF risk (Fig. 3).
FIGURE 3.
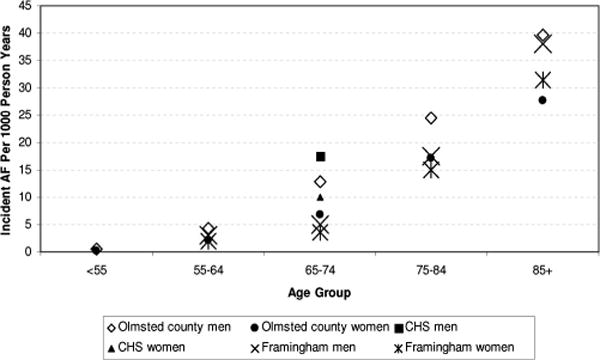
Age-specific atrial fibrillation incidence in various epidemiological studies.9,13,15 CHS = Cardiovascular Health Study.
Hypertension is another major risk factor for the development of AF. Although the actual adjusted relative risk is modest (1.5), the population attributable risk of hypertension for AF is high, estimated at 14%.15 Studies monitoring the prevalence of hypertension in the general US population showed a marked decline in its age-adjusted prevalence from the 1970s to the 1988–1994 time period, followed by a rising prevalence in 1999–2000.33,34 The current prevalence is estimated to be 31% (up from 24% in the late 1980s); this represents 35 million women and 30 million men.34 This rise is offset by an increased awareness of hypertension and early detection and treatment.35 Thus, the overall role of hypertension in incident AF may have diminished in the late 1990s when compared with the 1970s. Nevertheless, hypertension continues to be a highly prevalent condition, affecting nearly 1 in 3 adults.6 As such it likely continues to contribute substantially to the risk of AF.
Epidemiological studies show that the prevalence of risk factors for atrial fibrillation is increasing in the United States population.
In addition to the cardiovascular risk factors discussed above, metabolic conditions such as diabetes mellitus15,36 and more recently obesity24 are independent risk factors for AF. Their contribution seems to be modest. For example, the odds ratio associated with diabetes as contributing to AF risk is 1.4 in men and 1.6 in women.15 Obesity is associated with a 4% increased risk of AF for every unit increase in body mass index (BMI).24 Nevertheless, rapid increases in the prevalence of diabetes and obesity are cause for concern. In 2001, diabetes prevalence among US adults was 7.9%, a 61% increase from 1990.37 The prevalence of obesity (BMI ≥30) among US adults increased by 74% from 1991 to 2001 (12%–21%).37 These trends have implications for AF prevalence.
In summary, various epidemiological studies show that the prevalence of risk factors for AF is increasing in the US population. This can translate into an increased incidence of AF. Existing studies do not show any dramatic improvements in the survival of AF patients in the United States. Hence its rising prevalence is likely due to increased incidence. Although the prevalence of many of the risk factors for AF is increasing, (diabetes mellitus, obesity, heart failure, prior MI), and the population is aging, some risk factors such as valvular heart disease are becoming less prevalent. Hypertension prevalence and control have also improved since the 1970s. Finally, there is some indication that the rise in AF prevalence cannot be explained entirely by the changing profile of its risk factors.26
STROKE RISK IN AF PATIENTS
AF is an important independent risk factor for stroke, and is estimated to cause a 5-fold increase in ischemic stroke risk.38 This risk, however, is not homogeneous; it varies from <2%/yr in otherwise healthy patients in the 50 to 59 age range to over 10% in the elderly especially in the presence of other comorbidities. Table 1 lists the demographic, clinical, and echocardiographic features identified as increasing the ischemic stroke risk in patients with AF. These risk factors have implications for treatment.
Current evidence and guidelines support treatment with aspirin in young atrial fibrillation patients at low risk for thromboembolism (<2%/year). In high risk patients (>4%/year) anticoagulation with warfarin is recommended.
TABLE 1.
Risk Factors for Ischemic Stroke in Patients With Atrial Fibrillation
| Demographic |
| Age39–42 |
| Female39,40,43 |
| Clinical |
| Noncardiac |
| Hypertension—history,40–42 systolic BP >16040,41 |
| Diabetes39,40 |
| Prior thromboembolism (ischemic stroke, TIA, peripheral embolism)40–42 |
| Asymptomatic strokes on computed tomography44 |
| Cardiac |
| Rheumatic mitral disease especially stenosis45 |
| Coronary artery disease1 |
| Heart failure46 |
| Echocardiographic47–53 |
| Mitral annulus calcification |
| Left atrial enlargement |
| Left ventricular dysfunction |
| Ventricular wall thickness |
| Left atrial spontaneous echo contrast |
| Left atrial appendage or chamber reduction in flow velocity |
| Proximal aortic atheromata |
| Left atrial thrombus |
Reprinted with permission from Neurologist. 1998;4:235–258.54
In the 1990s multiple clinical trials demonstrated that warfarin effectively reduced the risk of ischemic stroke by about 60% to 65% in unselected patients with nonvalvular (by convention and for the purpose of risk stratification nonvalvular AF excludes AF in conjunction with mitral stenosis and prosthetic valves. It does not exclude mitral regurgitation, tri-cuspid regurgitation, or other valvular disease) AF.55–58 The effectiveness of aspirin was limited to about 20% risk reduction. Due to the cost, risk, and disutility of taking warfarin, the practical imperative was to assign warfarin to those at highest risk of ischemic stroke. The presence of rheumatic heart disease, especially rheumatic mitral stenosis, is a strong risk factor for stroke in patients with AF and a clear indication for anticoagulation.1 Rheumatic fever and its sequelae of rheumatic mitral stenosis are declining in prevalence in the United States, but they continue to be a significant problem in developing countries.31 Current evidence and guidelines support treatment with aspirin in young AF patients at low risk for thromboembolism (<2%/yr). In high risk patients (>4%/yr) anticoagulation with warfarin is recommended in the absence of bleeding complications or contraindications to anticoagulation.55 In AF patients at moderate risk (2%–4%/yr), the choice will depend on patient’s and physician’s attitudes toward stroke risk, the risks associated with anticoagulation, and the inconvenience of anti-coagulation which requires frequent monitoring.
Numerous schemes are available for stroke risk stratification of AF patients based on their burden of risk factors. Table 2 summarizes the major schemes which have been independently validated in literature.39,46,59–61 Intended to guide clinical management, these schemes use a subset of the risk factors in Table 1. All schemes in Table 2 give considerable weight to prior cerebral ischemia, and all of them use a history of hypertension or elevated blood pressure and advanced age in their risk calculations. Sex is used in all schemes except that put forth by the Atrial Fibrillation Investigators (AFI) and the CHADS2 (acronym based on Congestive heart failure, Hypertension, Age >75, Diabetes, Ischemic stroke/transient ischemic attack [TIA]) classification.46,59 Diabetes mellitus is a factor in all except the Stroke Prevention in Atrial Fibrillation Investigators (SPAF III) computation though a subsequent (and as yet un-validated) modification of this scheme has included diabetes mellitus in risk stratification.40,60 Heart failure or ventricular dysfunction enters in the CHADS2, SPAF, and American College of Chest Physicians61 schemes but not in the AFI or Framingham stratification. The CHADS2, AFI, and Framingham schemes are based only on clinical features whereas the American College of Chest Physicians and SPAF classifications allow echocardiographic variables. A very recent systematic review that used data pooled from multiple AF studies identified prior cerebral ischemia, history of hypertension, age >75 years, and diabetes mellitus as independent clinical risk factors for stroke in AF.67 This analysis suggested that the evidence was inconclusive as to whether heart failure or coronary artery diseases were independent risk factors. Whether this will lead to further modification of the stratification schemes shown in Table 2 remains to be seen. The plethora (a comprehensive review of stroke risk stratification schemes in AF is under review; personal communication Hart RG) of available risk stratification schemes, (only a subset of which are shown in Table 2), offers different patient management recommendations. This can lead to variance in clinical practice. The latest version of the American Heart Association guidelines on the management of AF has recommended anticoagulation with warfarin for those with 1 high risk factor (prior ischemic stroke, TIA or embolism, mitral stenosis, prosthetic valves), or more than 1 moderate risk factor (age ≥75, hypertension, heart failure, left ventricular ejection fraction ≤35%, diabetes mellitus). For those with 1 moderate risk factor, the recommended therapy is either aspirin or warfarin; and for those with AF, but no risk factors, aspirin is recommended.1 These American Heart Association guideline recommendations are similar though not identical with the CHADS2 classification and have not been independently validated to date.
TABLE 2.
Stroke Risk Stratification Schemes in Patients With Atrial Fibrillation and Estimated Ischemic Stroke Rates on Validation Cohorts of Atrial Fibrillation Patients on Aspirin Alone
| Schemes and Stroke Rates | Stroke Risk Category
|
||
|---|---|---|---|
| Low Risk | Moderate Risk | High Risk | |
| CHADS2,59 1 point CHF, HTN, age >75, DM; 2 points prior ischemic stroke, TIA | Score 0 | Score 1 or 2 | >2 |
| Stroke rate*62 | 0.8 | 2.7 | 5.3 |
| Stroke rate†62 | 0.8 | 2.2–4.5 | 8.6–13.7 |
| AFI46 | Not moderate or high risk | Age >65 yr; not high risk | HTN, DM, prior cerebral ischemia |
| Stroke rate*62 | 0.9 | 1.7 | 3.5 |
| Stroke rate†62 | NR | NR | 6.1 |
| Framingham39, prior cerebral ischemia = 6 points; DM = 4; female = 6; age 0–10 points; BP <120 = 0; 120–139 = 1; 140–159 = 2; 160–179 = 3; >179 = 4 | ≤7 | 7–13 | >13 |
| Stroke rate*62 | 1.4 | 3.2 | 4.2 |
| Stroke rate†62 | NR | NR | 7.9 |
| SPAF60 | Not moderate or high risk | HTN; no high risk features | Prior cerebral ischemia, women >75 yr; recent HF or LV dysfunction by echo, SBP >160 |
| Stroke rate*62,63 | 1.1 | 2.7, 3.6 | 3.6 |
| Stroke rate†62 | NR | NR | 6.5 |
| ACCP61 | Not moderate or high risk | Age 65–75, CAD, DM, Thyrotoxicosis | More than 1 moderate risk factor, prior cerebral ischemia, HTN, HF or LV dysfunction by echo, age >75 |
| Stroke rate*62 | 0.5 | 1.0 | 3.0 |
| Stroke rate†62 | NR | NR | 5.1 |
AFI indicates Atrial Fibrillation Investigators; SPAF, Stroke Prevention in Atrial Fibrillation; ACCP, American College of Chest Physicians; NR, not reported; CAD, coronary artery disease; HTN, hypertension; DM, diabetes mellitus; HF, heart failure; LV, left ventricle. All validation cohorts reported consist of atrial fibrillation (AF) patients using aspirin. For estimates from other validation cohorts comprised in part of AF patients not on any anti-thrombotic therapy (and hence at a higher risk) or patients on aspirin and fixed dose warfarin.39,59,64–66
Primary ischemic stroke prevention.
Validation cohort includes patients with prior ischemic stroke or TIA.
The observed association between the risk factors discussed above and stroke risk raises the obvious question as to whether risk factor modification (eg, treatment of diabetes) could lead to reduced ischemic stroke rates in AF patients. There is insufficient evidence to conclude that treatment of diabetes or heart failure would result in a lowered ischemic stroke risk in AF patients. The PROGRESS (the Perindopril Protection Against Recurrent Stroke Study) trial data suggest that blood pressure reduction in AF patients with a history of ischemic stroke may be protective against recurrent strokes, even when baseline blood pressure is not high enough to qualify as “hypertension” by conventional criteria.68
TRENDS IN WARFARIN USE AND EFFECT ON STROKE RATES
Many studies have documented the under-use of warfarin in AF patients.69,70 Few studies have examined temporal trends in warfarin use and concomitant change in either ischemic or hemorrhagic stroke rates in AF patients. Our recent study, examining these trends, found that warfarin use rates increased from 25% in 1992 to 56% in 2002 in the general Medicare population (Fig. 4).4 Concomitant with increased warfarin use, the ischemic stroke rates among AF patients decreased significantly over the study years. Of note, the hemorrhagic stroke rates did not increase significantly in this population, despite the substantial increase in warfarin use, suggesting that the fall in ischemic stroke rates may in part be due to improved hypertension control or other factors. Another community-based study found that post-AF ischemic stroke decreased from 1980 to 2000 and that both aspirin and warfarin use rates increased.71 Systolic blood pressure also declined during the study period. Hence, the observed ischemic stroke trends could not be attributed to antithrombotic therapy alone. Despite the promising trends in its use, warfarin use has plateaued recently and this therapy continues to be under-used in the US population (Fig. 4). For example, we found that up to 36% of patients considered to be at moderate or high risk for stroke were not anticoagulated despite the absence of contraindications.4
A case-control study comparing atrial fibrillation patients on warfarin with and without intracranial hemorrhage concluded that advanced age (>85 years) and supra-therapeutic levels of the international normalized ratio (international normalized ratio >3.5) were associated with an increased risk of intracranial hemorrhage.
FIGURE 4.
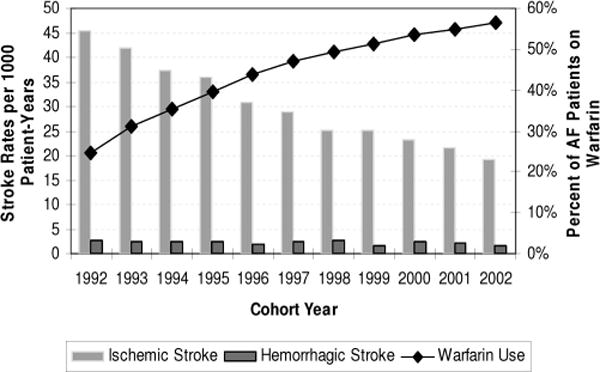
Trends in warfarin use and stroke rates in atrial fibrillation patients in the General Medicare population. Reprinted with permission from Stroke. 2006;37:1969–1974.
Warfarin use has been associated with an increased risk of intracranial hemorrhage (ICH); intraparenchymal hemorrhages (IPH) accounted for 70% of anticoagulant associated ICH and subdural hematomas accounted for the remainder.72 A case-control study comparing AF patients on warfarin with and without ICH concluded that advanced age (>85 years) and supra-therapeutic levels of the international normalized ratio (international normalized ratio >3.5) were associated with an increased risk of ICH. Various analyses of groups of anticoagulated patients have concluded that intensity of anticoagulation, advanced patient age, and cerebral atrophy are risk factors for subdural hematomas with minor trauma being a frequent precipitant.72 Similar analyses for IPH have shown that advanced age,73–75 intensity of anticoagulation,73 hypertension (especially systolic), and prior ischemic stroke72 are risk factors. The rates of anticoagulant associated IPH in clinical trials have ranged from 0.3% to 1.8%/yr.55 As mentioned earlier, our analysis of warfarin use and hemorrhagic stroke rates from 1992 to 2002 among AF patients in the general Medicare population did not show a significant increase in the rate of IPH despite a substantial increase in warfarin use rates (Fig. 4). A recent population-based study, however, found that the incidence of anticoagulant associated IPH quintupled in the 1990s rising from 0.8 to 4.4 per 100,000 persons over the span of a decade.76 As the use of warfarin in AF becomes more prevalent—prompted by the diffusion of trial evidence into community practice and the changing risk factor profile of AF patients—further increases in anticoagulant associated ICH can be expected. More population-based surveillance studies are needed to monitor this trend.
CONCLUSIONS
The prevalence of AF is increasing in the general US population. This is likely due to the aging of the population, improvements in coronary care, and the rising prevalence of risk factors for AF such as diabetes. Risk factors such as rheumatic heart disease and hypertension have decreased in prevalence over the past few decades. However, novel risk factors such as obesity and possibly the metabolic syndrome have been identified. These have the potential to further increase AF prevalence. The use of warfarin has improved and this is reflected in falling ischemic stroke rates in the AF population. One population-based study has shown an increased incidence of anticoagulant associated IPH during the 1990s. Although the decline in stroke rates in AF is laudable, the rising prevalence of AF, the changing profile of risk factors, and the recent plateauing of warfarin use indicate that stroke in AF patients will continue to be a significant public health problem.
Acknowledgments
The authors acknowledge AM Weber-Main for her critical review and editing of manuscript drafts.
Supported by an NINDS/NIH career development award K23NS051377 (to K.L.) and Astra Zeneca (to C.A.H.).
Footnotes
Kamakshi Lakshminarayan, David C. Anderson, and Adnan I. Qureshi report no conflicts of interest.
Charles A. Herzog has served as a consultant to Medtronic and Guidant corporations.
References
- 1.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: full text. A report of the American college of Cardiology/American heart association task force on practice guidelines and the European society of cardiology committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation) developed in collaboration with the European heart rhythm association and the heart rhythm society. Europace. 2006;8:651–745. doi: 10.1093/europace/eul097. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 3.Chugh SS, Blackshear JL, Shen WK, et al. Epidemiology and natural history of atrial fibrillation: clinical implications. J Am Coll Cardiol. 2001;37:371–378. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 4.Lakshminarayan K, Solid CA, Collins AJ, et al. Atrial fibrillation and stroke in the general Medicare population: a 10-year perspective (1992 to 2002) Stroke. 2006;37:1969–1974. doi: 10.1161/01.STR.0000230607.07928.17. [DOI] [PubMed] [Google Scholar]
- 5.Furberg CD, Psaty BM, Manolio TA, et al. Prevalence of atrial fibrillation in elderly subjects (the cardiovascular health study) Am J Cardiol. 1994;74:236–241. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 6.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics–2007 update: a report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 7.Blackshear JL, Kopecky SL, Litin SC, et al. Management of atrial fibrillation in adults: prevention of thromboembolism and symptomatic treatment. Mayo Clin Proc. 1996;71:150–160. doi: 10.4065/71.2.150. [DOI] [PubMed] [Google Scholar]
- 8.Page RL, Wilkinson WE, Clair WK, et al. Asymptomatic arrhythmias in patients with symptomatic paroxysmal atrial fibrillation and paroxysmal supraventricular tachycardia. Circulation. 1994;89:224–227. doi: 10.1161/01.cir.89.1.224. [DOI] [PubMed] [Google Scholar]
- 9.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted county, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 10.Wolf PA, Benjamin EJ, Belanger AJ, et al. Secular trends in the prevalence of atrial fibrillation: the Framingham study. Am Heart J. 1996;131:790–795. doi: 10.1016/s0002-8703(96)90288-4. [DOI] [PubMed] [Google Scholar]
- 11.Tsang TS, Petty GW, Barnes ME, et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol. 2003;42:93–100. doi: 10.1016/s0735-1097(03)00500-x. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Census Bureau. U.S. interim projections by age, sex, race, and Hispanic origin. Available at: http://www.census.gov/ipc/www/usinterimproj.
- 13.Psaty BM, Manolio TA, Kuller LH, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 14.Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba follow-up study. Am J Med. 1995;98:476–484. doi: 10.1016/S0002-9343(99)80348-9. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin EJ, Levy D, Vaziri SM, et al. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 16.Miyasaka Y, Barnes ME, Bailey KR, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol. 2007;49:986–992. doi: 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 17.Khairallah F, Ezzedine R, Ganz LI, et al. Epidemiology and determinants of outcome of admissions for atrial fibrillation in the United States from 1996 to 2001. Am J Cardiol. 2004;94:500–504. doi: 10.1016/j.amjcard.2004.04.068. [DOI] [PubMed] [Google Scholar]
- 18.Stewart S, MacIntyre K, Chalmers JW, et al. Trends in case-fatality in 22968 patients admitted for the first time with atrial fibrillation in Scotland, 1986–1995. Int J Cardiol. 2002;82:229–236. doi: 10.1016/s0167-5273(01)00626-x. [DOI] [PubMed] [Google Scholar]
- 19.Hoogsteen J, Schep G, Van Hemel NM, et al. Paroxysmal atrial fibrillation in male endurance athletes. A 9-year follow-up. Europace. 2004;6:222–228. doi: 10.1016/j.eupc.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Thornton JR. Atrial fibrillation in healthy non-alcoholic people after an alcoholic binge. Lancet. 1984;2:1013–1015. doi: 10.1016/s0140-6736(84)91109-7. [DOI] [PubMed] [Google Scholar]
- 21.Ettinger PO, Wu CF, De La Cruz C, Jr, et al. Arrhythmias and the “holiday heart”: alcohol-associated cardiac rhythm disorders. Am Heart J. 1978;95:555–562. doi: 10.1016/0002-8703(78)90296-x. [DOI] [PubMed] [Google Scholar]
- 22.Aranki SF, Shaw DP, Adams DH, et al. Predictors of atrial fibrillation after coronary artery surgery. Current trends and impact on hospital resources. Circulation. 1996;94:390–397. doi: 10.1161/01.cir.94.3.390. [DOI] [PubMed] [Google Scholar]
- 23.Woeber KA. Thyrotoxicosis and the heart. N Engl J Med. 1992;327:94–98. doi: 10.1056/NEJM199207093270206. [DOI] [PubMed] [Google Scholar]
- 24.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation (see comment) JAMA. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 25.Korantzopoulos P, Kokkoris S, Papaioannides D. The association of metabolic syndrome with atrial fibrillation: an emerging epidemiological and pathophysiological hypothesis. Cardiology. 2005;104:148–149. doi: 10.1159/000087636. [DOI] [PubMed] [Google Scholar]
- 26.Gersh BJ, Teresa SM, Tsang ME, et al. The changing epidemiology of non-valvular atrial fibrillation: the role of novel risk factors. Eur Heart J. 2005;7S:C5–C11. [Google Scholar]
- 27.Eaker ED, Sullivan LM, Kelly-Hayes M, et al. Anger and hostility predict the development of atrial fibrillation in men in the Framingham Offspring Study. Circulation. 2004;109:1267–1271. doi: 10.1161/01.CIR.0000118535.15205.8F. [DOI] [PubMed] [Google Scholar]
- 28.National Registry of Myocardial Infarction. Available at: www.nrmi.org/nrmi-data.html.
- 29.McGovern PG, Jacobs DR, Jr, Shahar E, et al. Trends in acute coronary heart disease mortality, morbidity, and medical care from 1985 through 1997: the Minnesota heart survey. Circulation. 2001;104:19–24. doi: 10.1161/01.cir.104.1.19. [DOI] [PubMed] [Google Scholar]
- 30.Dajani AS. Current status of nonsuppurative complications of group A streptococci. Pediatr Infect Dis J. 1991;10:S25–S27. doi: 10.1097/00006454-199110001-00006. [DOI] [PubMed] [Google Scholar]
- 31.Vora A. Management of atrial fibrillation in rheumatic valvular heart disease. Curr Opin Cardiol. 2006;21:47–50. doi: 10.1097/01.hco.0000198985.78508.55. [DOI] [PubMed] [Google Scholar]
- 32.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): developed in collaboration with the American College of Chest Physicians and the International Society For Heart And Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 33.Briefel RR, Johnson CL. Secular trends in dietary intake in the united states. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 34.Fields LE, Burt VL, Cutler JA, et al. The burden of adult hypertension in the United States 1999 to 2000: a rising tide (see comment) Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 35.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 36.Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. 2005;105:315–318. doi: 10.1016/j.ijcard.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 37.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 38.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 39.Wang TJ, Massaro JM, Levy D, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA. 2003;290:1049–1056. doi: 10.1001/jama.290.8.1049. [DOI] [PubMed] [Google Scholar]
- 40.Hart RG, Pearce LA, McBride R, et al. Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I–III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke. 1999;30:1223–1229. doi: 10.1161/01.str.30.6.1223. [DOI] [PubMed] [Google Scholar]
- 41.Moulton AW, Singer DE, Haas JS. Risk factors for stroke in patients with nonrheumatic atrial fibrillation: a case-control study. Am J Med. 1991;91:156–161. doi: 10.1016/0002-9343(91)90008-l. [DOI] [PubMed] [Google Scholar]
- 42.Stollberger C, Chnupa P, Kronik G, et al. Transesophageal echocardiography to assess embolic risk in patients with atrial fibrillation. ELAT Study Group. Embolism in left atrial thrombi. Ann Intern Med. 1998;128:630–638. doi: 10.7326/0003-4819-128-8-199804150-00004. [DOI] [PubMed] [Google Scholar]
- 43.Fang MC, Singer DE, Chang Y, et al. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. Circulation. 2005;112:1687–1691. doi: 10.1161/CIRCULATIONAHA.105.553438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silent brain infarction in nonrheumatic atrial fibrillation. EAFT study group. European atrial fibrillation trial. Neurology. 1996;46:159–165. doi: 10.1212/wnl.46.1.159. [DOI] [PubMed] [Google Scholar]
- 45.Wolf PA, Dawber TR, Thomas HE, Jr, et al. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology. 1978;28:973–977. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 46.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 47.Echocardiographic predictors of stroke in patients with atrial fibrillation: a prospective study of 1066 patients from 3 clinical trials. Arch Intern Med. 1998;158:1316–1320. doi: 10.1001/archinte.158.12.1316. [DOI] [PubMed] [Google Scholar]
- 48.Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. The stroke prevention in atrial fibrillation investigators committee on echocardiography. Ann Intern Med. 1998;128:639–647. doi: 10.7326/0003-4819-128-8-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 49.Uzui H, Lee JD, Shimizu H, et al. Echocardiographic and hematological variables as a risk factor for stroke in chronic nonvalvular atrial fibrillation. J Cardiol. 1998;32:15–20. [PubMed] [Google Scholar]
- 50.Zabalgoitia M, Halperin JL, Pearce LA, et al. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke prevention in atrial fibrillation III investigators. J Am Coll Cardiol. 1998;31:1622–1626. doi: 10.1016/s0735-1097(98)00146-6. [DOI] [PubMed] [Google Scholar]
- 51.Mitusch R, Lange V, Stierle U, et al. Transesophageal echocardiographic determinants of embolism in nonrheumatic atrial fibrillation. Int J Card Imaging. 1995;11:27–34. doi: 10.1007/BF01148951. [DOI] [PubMed] [Google Scholar]
- 52.Aronow WS, Ahn C, Kronzon I, et al. Association of mitral annular calcium with new thromboembolic stroke at 44-month follow-up of 2,148 persons, mean age 81 years. Am J Cardiol. 1998;81:105–106. doi: 10.1016/s0002-9149(97)00854-0. [DOI] [PubMed] [Google Scholar]
- 53.Aronow WS, Ahn C, Kronzon I, et al. Risk factors for new thromboembolic stroke in patients > or = 62 years of age with chronic atrial fibrillation. Am J Cardiol. 1998;82:119–121. doi: 10.1016/s0002-9149(98)00247-1. [DOI] [PubMed] [Google Scholar]
- 54.Anderson DC, Koller RL, Asinger RW, et al. Atrial fibrillation and stroke: epidemiology, pathophysiology and management. Neurologist. 1998;4:235–258. [Google Scholar]
- 55.Hart RG, Halperin JL, Pearce LA, et al. Lessons from the stroke prevention in atrial fibrillation trials. Ann Intern Med. 2003;138:831–838. doi: 10.7326/0003-4819-138-10-200305200-00011. [DOI] [PubMed] [Google Scholar]
- 56.Secondary prevention in non-rheumatic atrial fibrillation after transient ischaemic attack or minor stroke. EAFT (European Atrial Fibrillation Trial) study group. Lancet. 1993;342:1255–1262. [PubMed] [Google Scholar]
- 57.Ezekowitz MD, Bridgers SL, James KE, et al. Warfarin in the prevention of stroke associated with nonrheumatic atrial fibrillation. Veterans affairs stroke prevention in nonrheumatic atrial fibrillation investigators. N Engl J Med. 1992;327:1406–1412. doi: 10.1056/NEJM199211123272002. [DOI] [PubMed] [Google Scholar]
- 58.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 59.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the national registry of atrial fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 60.Stroke Prevention in Atrial Fibrillation Investigators. Risk factors for thromboembolism during aspirin therapy in patients with atrial fibrillation: the stroke prevention in atrial fibrillation study. J Stroke Cerebrovasc Dis. 1995;5:147–157. doi: 10.1016/S1052-3057(10)80166-1. [DOI] [PubMed] [Google Scholar]
- 61.Albers GW, Dalen JE, Laupacis A, et al. Antithrombotic therapy in atrial fibrillation. Chest. 2001;119:194S–206S. doi: 10.1378/chest.119.1_suppl.194s. [DOI] [PubMed] [Google Scholar]
- 62.Gage BF, van Walraven C, Pearce L, et al. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004;110:2287–2292. doi: 10.1161/01.CIR.0000145172.55640.93. [DOI] [PubMed] [Google Scholar]
- 63.Patients with nonvalvular atrial fibrillation at low risk of stroke during treatment with aspirin: stroke prevention in atrial fibrillation III study. The SPAF III writing committee for the stroke prevention in atrial fibrillation investigators. JAMA. 1998;279:1273–1277. [PubMed] [Google Scholar]
- 64.Lip GY, Lane D, Van Walraven C, et al. Additive role of plasma von willebrand factor levels to clinical factors for risk stratification of patients with atrial fibrillation. Stroke. 2006;37:2294–2300. doi: 10.1161/01.STR.0000236840.00467.84. [DOI] [PubMed] [Google Scholar]
- 65.Pearce LA, Hart RG, Halperin JL. Assessment of three schemes for stratifying stroke risk in patients with nonvalvular atrial fibrillation. Am J Med. 2000;109:45–51. doi: 10.1016/s0002-9343(00)00440-x. [DOI] [PubMed] [Google Scholar]
- 66.Feinberg WM, Kronmal RA, Newman AB, et al. Stroke risk in an elderly population with atrial fibrillation. J Gen Intern Med. 1999;14:56–59. doi: 10.1046/j.1525-1497.1999.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stroke Risk in Atrial Fibrillation Working Group. Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007;69:546–554. doi: 10.1212/01.wnl.0000267275.68538.8d. [DOI] [PubMed] [Google Scholar]
- 68.Arima H, Hart RG, Colman S, et al. Perindopril-based blood pressure-lowering reduces major vascular events in patients with atrial fibrillation and prior stroke or transient ischemic attack. Stroke. 2005;36:2164–2169. doi: 10.1161/01.STR.0000181115.59173.42. [DOI] [PubMed] [Google Scholar]
- 69.Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160:41–46. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 70.Brass LM, Krumholz HM, Scinto JD, et al. Warfarin use following ischemic stroke among Medicare patients with atrial fibrillation. Arch Intern Med. 1998;158:2093–2100. doi: 10.1001/archinte.158.19.2093. [DOI] [PubMed] [Google Scholar]
- 71.Miyasaka Y, Barnes ME, Gersh BJ, et al. Time trends of ischemic stroke incidence and mortality in patients diagnosed with first atrial fibrillation in 1980 to 2000: report of a community-based study. Stroke. 2005;36:2362–2366. doi: 10.1161/01.STR.0000185927.63746.23. [DOI] [PubMed] [Google Scholar]
- 72.Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke. 1995;26:1471–1477. doi: 10.1161/01.str.26.8.1471. [DOI] [PubMed] [Google Scholar]
- 73.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897–902. doi: 10.7326/0003-4819-120-11-199406010-00001. [DOI] [PubMed] [Google Scholar]
- 74.Bleeding during antithrombotic therapy in patients with atrial fibrillation. The stroke prevention in atrial fibrillation investigators. Arch Intern Med. 1996;156:409–416. [PubMed] [Google Scholar]
- 75.Landefeld CS, Goldman L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. Am J Med. 1989;87:144–152. doi: 10.1016/s0002-9343(89)80689-8. [DOI] [PubMed] [Google Scholar]
- 76.Flaherty ML, Kissela B, Woo D, et al. The increasing incidence of anticoagulant-associated intracerebral hemorrhage. Neurology. 2007;68:116–121. doi: 10.1212/01.wnl.0000250340.05202.8b. [DOI] [PubMed] [Google Scholar]


