Summary
The essential transition metal copper is important in lipid metabolism, redox balance, iron mobilization and many other critical processes in eukaryotic organisms. Genetic diseases where copper homeostasis is disrupted, including Menkes Disease and Wilson Disease, indicate the importance of copper balance to human health. The severe consequences of insufficient copper supply are illustrated by Menkes Disease, caused by mutation in the X-linked ATP7A gene encoding a protein that transports copper from intestinal epithelia into the bloodstream and across the blood-brain barrier. Inadequate copper supply to the body due to poor diet quality or malabsorption can disrupt several molecular level pathways and processes. Though much of the copper distribution machinery has been described and consequences of disrupted copper handling have been characterized in human disease as well as animal models, physiological consequences of sub-optimal copper due to poor nutrition or malabsorption have not been extensively studied. Recent work indicates that insufficient copper may be important in a number of common diseases including obesity, ischemic heart disease, and metabolic syndrome. Specifically, marginal copper deficiency (CuD) in has been reported as a potential etiologic factor in diseases characterized by disrupted lipid metabolism such as non-alcoholic fatty-liver disease (NAFLD). In this review, we discuss the available data suggesting that a significant portion of the North American population may consume insufficient copper, the potential mechanisms by which CuD may promote lipid biosynthesis, and the interaction between CuD and dietary fructose in the etiology of NAFLD.
Keywords: copper deficiency, non-alcoholic fatty-liver disease, fructose, oxidative stress, inflammation, fatty acid biosynthesis
Introduction
Mammals acquire copper from dietary sources with typical high copper foods including organ meats, shellfish, seeds, beans and ready-to-eat cereals with potential contributions from copper pipes that supply drinking water (1). It is clear that copper balance is important in health, and since copper is acquired from food, poor diet quality or malabsorption can have health consequences related to copper insufficiency. Because of its labile redox potential, copper homeostasis and control are paramount to cell survival; thus in vivo copper is almost always bound to specific proteins or other small molecules.
Mammalian copper homeostasis and delivery to cuproenzymes is accomplished by the action of copper-specific membrane transporters as well as intracellular copper chaperones and has been reviewed elsewhere (2, 3). Copper is mobilized from epithelial cells through active transport into the hepatic portal circulation, where copper is most likely bound to albumin and transcuperin (3, 4). The liver is the central regulatory organ of copper homeostasis, and this organ also modulates lipid and carbohydrate metabolism. In hepatic cells, copper is either utilized for cellular processes including respiration in the mitochondria and radical detoxification or it is actively transported into the trans-Golgi network via ATP7B. Excess copper can be sequestered by metallothioneins as well as exported via ATP7B, which traffics to endo/lysosome-derived compartments and the apical (bile canilicular) membrane (5, 6). Copper can exit hepatic cells via the basolateral membrane as a cofactor in the ferroxidase ceruloplasmin (7). Copper mobilized to the blood can be taken up by other tissues including the brain, kidney, heart, connective tissue and pancreas. Thus, copper distribution can be described as a diphasic process where copper uptake into the liver is phase 1 and distribution to other organs via the circulatory system is phase 2. Excess copper is removed from hepatocytes by secretion into bile where is can be eliminated in feces.
Disruption of the copper distribution machinery in cells impacts copper-dependent biochemical processes including electron transport via cytochrome C oxidase (8), radical detoxification by copper-zinc superoxide dismutase (CuZnSOD) (9), but also connective tissue formation (lysyl oxidase) (10), neurotransmitter synthesis (peptidylglycine alpha-amidating monooxidase) (11), iron mobilization (ceruloplasmin and hephaestin) (7). As the central point of copper balance, insufficient copper supply in the liver may have both local and systemic consequences.
Liver specific deletion of the high-affinity copper transporter Ctr1 in mouse (Ctr1hep/hep) results in only a 48–79% decrease in hepatic copper (at 2 and 10 months), which suggests alternate import routes (12). This liver-specific inactivation of Ctr1 also reduced activity of CuZnSOD and cytochrome C oxidase (12), indicating that decreased Cu supply impacts specific cuproenzymes in the liver. This study did not report iron retention in the liver; however, intestine-specific deletion of Ctr1 (Ctr1int/int) resulted in hepatic iron hyperaccumulation with iron deposits in Kupffer cells (13), suggesting attenuation of iron mobilization and implicating decreased hephaestin activity. Taken together, these results suggest that insufficient Cu in the liver may have direct effects on metabolic capacity through decreased CCO activity as well as increased oxidative stress due to both iron accumulation and decreased CuZnSOD activity.
As indicated in the Ctr1int/int mouse, iron homeostasis is known to be linked to copper homeostasis, as is zinc homeostasis. The key interaction between copper and iron is the role of copper in ferroxidases ceruloplasmin and hephaestin, which oxidize iron for mobilization, accelerating the reaction and avoiding the risk of radical generation in auto-oxidation (14). The mechanistic interaction of copper and zinc is less well understood, but it is clear that high zinc consumption inhibits copper absorption, as illustrated by acquired hypocupremia associated with excess zinc consumption (38,39). It is important to note that dysregulation in one of these metals may lead to dysregulation of the others. All three metals are important in antioxidant defense, immune defense and other critical processes on both cellular and organismal levels (15). These three key metals are often incorporated into antioxidant enzymes such as superoxide dismutase, catalase, and glutathione. Alterations in homeostasis of these metals often lowers cell ability to regulate redox conditions resulting in cellular damage and increased ALS, ALT and ALP levels. The severity of many metabolic syndrome (MetS) related disorders is gauged by elevated levels of these circulating biomarkers of increased oxidative stress.
Key biochemical and molecular processes impacted by dietary copper deficiency have been studied in several animal models (16–20); however, the potential roles for insufficient copper in human diseases are less well understood. Frank copper deficiency is uncommon in clinical practice, but it is occasionally reported. Current research aims to understand how marginal copper deficiency that does not meet a clinically deficient threshold might be important in specific human metabolic conditions, particularly those related to MetS including dyslipidemia and non-alcoholic fatty-liver disease.
Copper deficiency in humans
Copper deficiency (CuD) has been typically defined by measured serum concentration (21). Depending on the laboratory, the lower limit of normal is between 70–80 μg/dL. Because at least 70% of serum copper is incorporated within ceruloplasmin, whose synthesis depends on copper availability, serum ceruloplasmin concentration is also decreased in this condition. Potential causes of copper deficiency and their associated serum copper concentrations are noted in Table 1 (22–25). Among the causes, Menkes disease is the most studied genetic disorder related to copper deficiency. Menkes Disease is an X-linked disease involving abnormal ATP7A, with clinical manifestations including kinky hair formation, neural degeneration, failure to thrive and death in infancy, though other ATP7A-associated conditions have been reported with less severe phenotypes (26). Despite low brain copper in Menkes disease, liver copper is not markedly different than control patients. In addition to those described in for Menkes Disease, some of the wide range of clinical manifestations of copper deficiency and the putative cuproenzymes involved include iron overload and anemia due to ceruloplasmin inactivation (27); neurodegenerative disease (amyotropic lateral sclerosis) and Cu/Zn superoxide dismutase (28); neutropenia (29); myocardial fibrosis, cardiovascular disease and vascular abnormalities due to lysyl oxidase deficiency with altered collagen crosslinking (29, 30); and blood clotting disorders with Factor V in blood clotting (31). Though the liver is central in modulating copper balance, to our best knowledge, no studies have examined cirrhosis or other chronic liver disease as a potential cause of systemic copper deficiency.
Table 1.
Clinical Copper deficiency in humans and observed serum copper concentrations
| Causes of copper deficiency | Mechanism | Approximate serum copper in humans (micrograms/deciliter) |
|---|---|---|
| Menkes’ disease | Abnormal ATP7A leading to “maldistribution” of copper and deficiency in certain organs, i.e. brain | Very low |
| Nephrotic syndrome | Renal copper andceruloplasminloss | 65 |
| Protein-losing enteropathy | GI copper andceruloplasminloss | 42 |
| Celiac Disease | GI copper andceruloplasminloss and decreased absorption | 40 |
| Excessive dietary fructose | Impaired duodenal Ctr1 expression, decreased absorption | Unknown |
| Excessive dietary zinc | Prevents copper absorption | 14 |
| Gastric or R-Ybypass surgery | Decreased copper absorption | 59 |
Is the Western diet adequate in copper?
Despite the infrequent observation of acute copper deficiency, it is possible that a significant number of people consume insufficient copper for optimal health. Klevay asserted in a 2011 review that the Western diet is often inadequate in copper and pointed out that copper content in food has declined in recent decades, while also highlighting the limitations of our current knowledge about optimal copper consumption (32). Data that became available when the National Health and Nutrition Examination and Survey (NHANES) began reporting serum copper levels appears to support the assertion that at least a portion of Americans are copper deficient: the 2011–2012 data release reported 102 of the 2594 serum specimens (3.93%) had values <80 micrograms/dL of total copper. Low serum copper in this subset of the population may be related to above-mentioned causes; however, insufficient dietary copper intake is a plausible contributing factor. The Recommended Daily Intake (RDI) of Cu for adult men and women in North America is 0.9 mg (33) and is calculated as 130% of the Estimated Average Requirement (EAR). The current EAR of 0.7 mg/day as specified by the Food and Nutrition Board [2001] is defined as the intake level where 50% of the population will not be deficient (34). Several key biomarkers were to calculate the EAR for Cu, as well as the RDI, including plasma Cu and ceruloplasmin, erythrocyte SOD activity, and platelet copper concentration and cytochrome C oxidase activity in a set of depletion-repletion studies (33). Analysis of the distribution of average copper intake from NHANES 2011–12 (https://www.cdc.gov/nchs/nhanes/) reveals that 29% of respondents did not meet the RDI (Figure 1). Further, 14% of specimens fell below the Estimated Average Requirement (EAR) for copper. These data indicate that a significant portion of the population might consume insufficient copper. A retrospective examination of exposure-response studies was recently re-analyzed to develop a generalized linear model of copper nutrition and propose an optimum intake for humans (35). This model indicates an optimum copper intake of 2.6 mg/day, much greater than the U.S. RDI and supporting a hypothesis that though the current RDI is sufficient to prevent clinical copper deficiency in most of the population, it might not be sufficient for optimal health.
Figure 1. Distribution of 2-day average copper intakes in NHANES 2011–12.
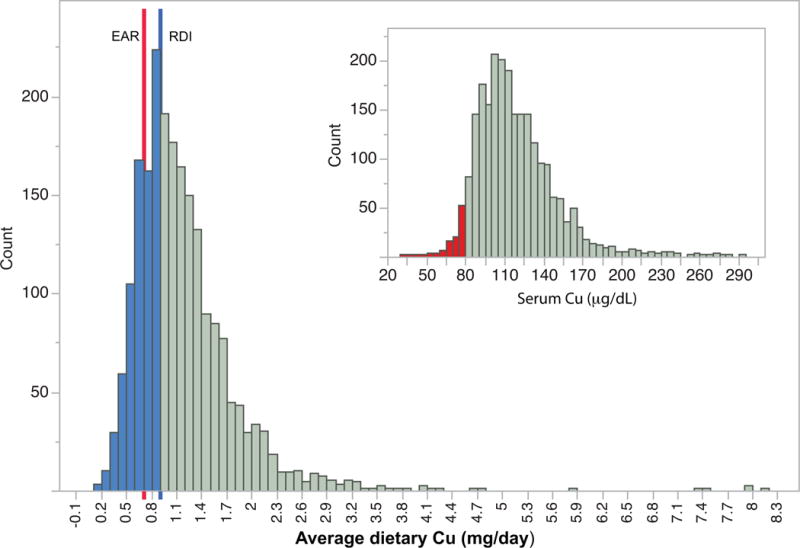
The blue line indicates the U.S. RDI, values below the U.S. RDI are shaded in blue. The red line indicates the EAR. Inset: distribution of serum copper in NHANES 2011–12. Samples below 80 micrograms/deciliter are in red.
Insufficient dietary copper increases lipid synthesis
Marginal CuD has recently gained attention in a number of diseases including Alzheimer’s Disease, ischemic heart disease (IHD), non-alcoholic fatty-liver disease (NAFLD) and obesity (22, 56, 36). Many of these clinical manifestations have been linked with alterations in lipid metabolism and ultimately dyslipidemia (37, 38), including abnormal cholesterol metabolism in Alzheimer’s Disease (recently reviewed here: (39)). Altered plasma lipid profiles are currently under study as predictive biomarkers of cardiovascular risk in Type 2 Diabetes Mellitis (40). The link between mild CuD and hypercholesterolemia in humans was known as early as 1984 (41). In experiments with a rat model of mild CuD, total cholesterol and plasma levels rose to increase in bioavailable cholesterol by 60% (42). The biochemical mechanism by which CuD promotes increased circulating cholesterol is not well defined; both increased cholesterol synthesis and decreased cholesterol degradation have been proposed (43, 44), while it is possible that both are involved in CuD-induced dyslipidemia. Curiously, pathogenic lipid accumulation in mouse models promotes hepatic CuD as demonstrated in obese ob/ob mice (45) and a high-fat diet-induced NAFLD model (46). In the latter case, increased liver expression of copper transporters Atp7A and Atp7B was observed, suggesting a potential mechanism by which lipid accumulation can promote hepatic CuD.
Fatty acid and cholesterol regulatory factors that are potentially modulated by CuD are sterol regulatory element-binding proteins 1 and 2 (SREBP-1 and SREBP-2). Nuclear localization of mature SREBP-1 is increased the livers of rats fed low-copper diets, but no change in the DNA binding site is observed (47). Both isoforms of SREBP-1 (SREBP-1a and SREBP-1c) are predominantly involved in regulation of fatty acid synthesis (FAS), while SREBP-2 is important in the modulation of cholesterol biosynthesis (48). The increase in nuclear localization of mature SREBP-1 and accompanying decrease in membrane-bound (ER-localized SREBP-1) is correlated with a 400% increase in FAS and an 80% decrease in cholesterol 7 alpha hydroxylase (CYP7A1) a key enzyme in cholesterol degradation (47). Nuclear localization of both SERBP-1 and SERBP-2 also promote expression of proprotein convertase subtilisin/kexin type 9 (PCSK9), which enhances degradation of LDL receptors resulting in reduced hepatic LDL uptake (49).
The exact mechanism that links CuD to increased nuclear localization of SREBPs has yet to be identified; however, increased insulin secretion is an appealing possibility as CuD has been linked with increased insulin secretion in fa/fa Zucker Rats (50). This change is thought to be due to lowered synthesis of prostaglandin endoperoxide synthase (PGHS) due to damage from oxidative stress, whereby decreased levels of PGHS slow arachidonic acid degradation stimulating lipoxygenase insulin stimulatory pathways (50). Increased insulin has been shown to downregulate hepatic insulin-induced gene 2a (INSIG-2a), while decreased INSIG-2a levels promote COP II transport of the SCAP-SREBP complex from the ER leading to an increase in fatty acid synthesis; this route of activation circumvents the canonical high cholesterol regulatory mechanism of liver x receptor (LXR) inhibition by polyunsaturated fatty acids and down-regulation of SREBP-1c mRNA levels (51).
A second potential modulatory point for CuD in lipid metabolism is in the acyl CoA pathway. A transcriptomic approach identified multiple acyl CoA-related enzymes downregulated in the intestine of a CuD rat model (52): Acyl CoA synthetase (Acs11) and carnitine-palmitoyltransferase (Cpta1) are downregulated in low copper conditions, reducing mitochondrial and peroxisomal beta-oxidation of fatty acids as well as carnitine-bound transport of fatty acids; l-3-hydroxyacyl CoA dehydrogenase (Hadhb) is also downregulated by low copper, reducing the catalysis of L-3-hydroxyacyl CoA oxidation by NAD+; CuD also downregulates fatty acid translocase CD36, which is located in the plasma membrane and also acts as a scavenger receptor on macrophages, whose downregulation decreases the availability of intracellular fatty acids. Together, attenuation of fatty acid oxidation, reduced production of acetyl-CoA and energy would lead to an accumulation of cytoplasmic fatty acid.
Cu deficiency and dietary fructose are linked in processes that may promote non-alcoholic fatty-liver disease
Although it has been understood for several decades that CuD promotes lipogenesis in animal models as well as in humans, CuD has only recently implicated as factor in the pathogenesis of non-alcoholic fatty-liver disease (NAFLD). NAFLD prevalence doubled from 1988–2008, and is estimated to affect 20–40% of the US population, with variability between specific demographic groups (53). Aigner and co-workers identified low hepatic copper in NAFLD patients, reporting that hepatic steatosis was inversely correlated with hepatic Cu (54). This study also analyzed a rat model of NAFLD and revealed a histopathological response consistent with NAFLD in rats fed a CuD diet, supporting the link between CuD and hepatic dyslipidemia. Several other groups have reported NAFLD-like symptoms in animal models fed diets deficient in copper, including transcriptomics analysis that found increased expression of transcripts associated with inflammation, fibrosis and hepatic stellate cell activation, all consistent with NAFLD progression (23, 55, 56). More recently, a second clinical study confirmed the inverse correlation between hepatic copper content and the extent of steatosis (57), but also revealed the important finding that the correlation only held in patients withoutMetS. This observation suggests that the relationship between CuD and hepatic lipid metabolism may involve additional factors, including diet components known to contribute to MetS.
NAFLD patients presenting with mild inflammation and any degree of fibrosis are at highest risk for progression to more serious non-alcoholic steatohepatitis (NASH) (58), while NASH progression is also associated with the development of other MetS pathologies that increase cardiovascular disease risk and insulin resistance, the precursor state to type II diabetes (59, 60). However, the mechanisms behind the progression of NAFLD to NASH have as yet remained elusive, highlighting the need to identify triggers for inflammation and fibrosis in NAFLD models and in clinical settings. Thus, CuD, with its role in promoting lipogenesis, as well as the interaction of CuD with other nutrients associated with NAFLD, particularly fructose, are emerging as important areas of study.
High fructose diets induce MetS, NAFLD, and insulin resistance in rodent models (23, 61, 62) and promote hepatic lipid storage in humans (63, 64), with induction of disease in short timeframes. The mean caloric contribution of fructose in the US diet is estimated at 10.2%, with 1/4 of adolescents consuming at least 15% of calories as fructose (65). NHANES 2011–12 data indicates a median sugar consumption of 23% of calories with an upper quartile of sugar consumption over 30% of calories. Several animal studies have used diet-induced NAFLD to investigate the interaction between dietary sugars and copper. These experiments indicated that either fructose and sucrose can exacerbate diet-induced CuD, while the pathological effects of dietary CuD and sugar consumption appear to be additive (23, 55, 56). One study found that even modest fructose intake (3% w/v in drinking water) was sufficient to induce liver damage (55); however, this study used a purified AIN76A diet containing 50% sucrose. Tallino and co-workers reported mild NAFLD-like pathology from either a copper-deficient diet or high sugar (30% sucrose w/w, based on AIN76A), with high sugar exacerbating CuD and both pro-inflammatory and pro-fibrotic transcriptional activation in response to high sucrose or low Cu, including specific Th2 and Th17 immune cell activation consistent with NAFLD progression (56). It is not yet known whether the levels of fructose reported in dietary studies promote CuD in humans, though the upper quartile of sugar consumption reported in NHANES 2011–12 is similar to the 30% w/w sucrose diet reported by Tallino and co-workers.
As noted above, mouse models of NAFLD using lipogenic diets or ob/ob mice promote hepatic CuD, and it is possible that the link between CuD and fructose consumption in rodent models is via fructose-induced lipogenesis. Specifically, fructose metabolism occurs primarily in the liver and initially converted to fructose-1-phosphate by fructokinase, followed by conversion to dihydroxyacetone phosphate and glyceraldehyde 3-phosphate by aldolase B and entry into the glycolytic pathway. This step bypasses the phosphofructokinase rate-limiting that regulates glycolytic substrate entry from glucose. Fructose-derived intermediates enter the tricarboxylic acid (TCA) cycle as pyruvate. Excess TCA intermediates promote citrate export to the cytoplasm as substrate for lipogenesis. The higher level of de novo lipogenesis from fructose as compared to glucose feeding was illustrated in a rat model and revealed a 5-fold excess contribution to circulating triglycerides from fructose (66). Extensive discussion of fructose metabolism in the liver and its potential role in de-novo lipogenesis is presented by Nomura and Yamanouchi (67).
However, the interaction of CuD and fructose in NAFLD may have a second mechanism in enhanced oxidative damage: fructose metabolism can directly increase concentrations of uric acid as part of the recovery from rapid depletion of intercellular phosphate and ATP (68); as uric acid can A) act as a source of intracellular oxidative stress, and B) is related to increases in intracellular citrate which feeds directly into de novo fatty acid synthesis pathways (69). One caveat to this hypothesis is that uric acid can act as an both an antioxidant and a pro-oxidant, scavenging radicals and transition metal ions in serum, but also promoting oxidative stress in various cell types (70, 71).
Thus, as a result of fructose metabolism, the lipotoxicity from increased fatty acids coupled with oxidative stress from uric acid may initiate inflammation and subsequent fibrosis, while the lipid accumulation can also promote CuD. This line of reasoning is supported by clinical research as well as animal models of NASH: increased consumption of fructose has been clinically associated with hyperuricemia and lower steatosis/higher fibrosis stage (72), and evidence that fructose is directly responsible for inflammation in NASH comes from studies of fructose-fed wild-type and fructokinase knockout mice: wild type mice exhibited increased expression of the cytokine tumor necrosis factor alpha (TNFα) and increased fibrosis, and this process was attenuated by fructokinase knockout (73). Fructose may play an indirect role in increasing oxidative stress by the induction of CuD, suppressing radical detoxification by CuZnSOD but also promoting iron retention and associated oxidative stress. This assertion is supported by the observation of Kupffer cell iron deposits in NAFLD patients with enhanced apoptosis (74), reminiscent of the iron deposits reported in Kupffer cells of the Ctr1int/int mouse (13). Loss of CuZnSOD and hepatic fibrosis were recently linked in the CuZnSOD mouse with observations of extensive collagen deposition and liver histology characterized by extensive advanced glycation end products (AGEs), which would inhibit collagen degradation (75). This observation supports the hypothesis that CuD may have a role in hepatic fibrosis in addition to promotion of lipogenesis. One question that remains unclear, however, is that CuD is also associated with decreased lysyl oxidase activity, suggesting that fibrosis development would be less severe in this condition. For example, inhibition of lysyl oxidase in a carbon tetrachloride-induced mouse model of fibrosis resulted in decreased collagen stabilization, suggesting a role for the enzyme in fibrosis progression (76). However, despite a ½ to 2/3 reduction of lysyl oxidase activity by dietary CuD in weanling rats, there were minimal effects on connective tissue maturation (77), suggesting that collagen crosslinking may be retained despite insufficient dietary Cu.
The above studies indicate that CuD may be a significant factor in NAFLD that is linked with fructose as well as other factors in a complex disease etiology. We have indicated proposed mechanisms of NAFLD pathology and emphasized the significant roles for CuD in the disease as a schematic (Figure 2). Although the supporting data is drawn from a number of experimental studies, the interconnected pathways suggest that future studies may necessitate integrated or systems approaches to understand how inadequate Cu impacts health and if this essential metal should be more often considered in diagnosis as well as intervention in disease.
Figure 2. Schematic representation of factors in NAFLD etiology.
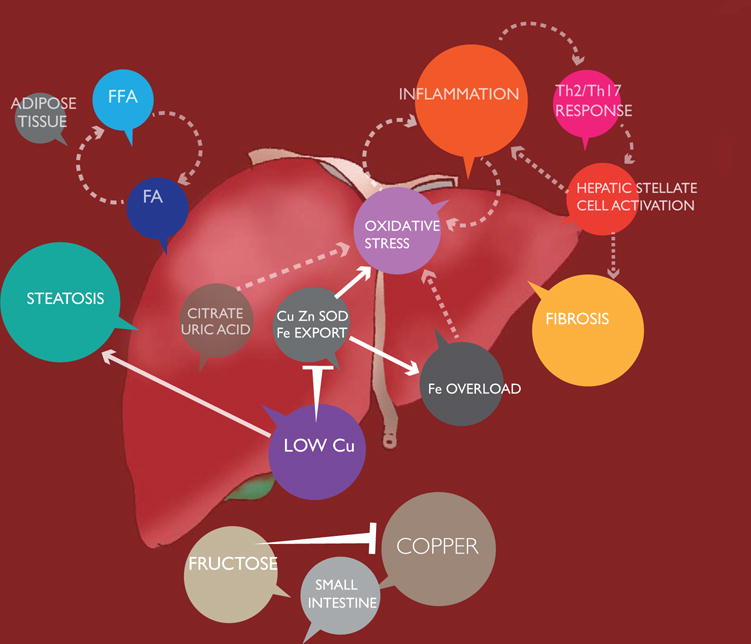
Interactions between Cu deficiency and fructose may contribute to NASH. While obesity and associated lipotoxicity of liberated free fatty acids are commonly attributed as drivers of NASH after the development of steatosis, copper deficiency and fructose consumption may promote lipogenesis, oxidative stress, inflammation and hepatic stellate cell activation, compounding the progression of NAFLD. Abbreviations: FA, fatty acids; FFA, free fatty acids.
Conclusion
Though acute copper deficiency is rarely reported, the importance of this micronutrient to human health cannot be understated. Sub-optimal levels of copper have been shown to promote dyslipidemia and increase oxidative stress in both animal models as well as in humans in studies that span several decades. Dietary survey data indicate that a significant portion of the U.S. population does not meet recommended copper consumption levels, leading to the hypothesis that alterations in lipid metabolism due to insufficient dietary copper may contribute to the incidence and/or severity of metabolic diseases including NAFLD. In addition, CuD and its physiological effects may interact with high dietary fructose intake, presenting overlapping and potentially synergistic effects on the physiology and pathology of diseases including non-alcoholic fatty-liver disease. However much remains unknown particularly about the mechanisms behind these clinical manifestations. We propose that future work should focus on clearly linking MetS related diseases with alteration in lipid metabolism caused by CuD. One potential route of investigation is alterations that affect SREBP localization like altered insulin secretion and INSIG-2a regulation. Other areas of investigation are the mechanism(s) behind downregulation of acyl CoA pathway genes (Acs11) and (Cpta1) or discovering novel mechanism of alteration in cholesterol homeostasis due to CuD.
Acknowledgments
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103395. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the NIH. We thank Sarah Brunjes-Hall for assistance with figure design.
References
- 1.Pennington JAT, Schoen SA, Salmon GD, Young B, Johnson RD, Marts RW. Composition of Core Foods of the U.S. Food Supply, 1982–1991: III Copper, Manganese, Selenium, and Iodine. J Food Compos Anal. 1995;8:171–217. [Google Scholar]
- 2.Nevitt T, Ohrvik H, Thiele DJ. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim Biophys Acta. 2012;1823:1580–1593. doi: 10.1016/j.bbamcr.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 4.Gordon DT, Leinart AS, Cousins RJ. Portal copper transport in rats by albumin. Am J Physiol. 1987;252:E327–333. doi: 10.1152/ajpendo.1987.252.3.E327. [DOI] [PubMed] [Google Scholar]
- 5.Polishchuk EV, Concilli M, Iacobacci S, Chesi G, Pastore N, Piccolo P, Paladino S, Baldantoni D, van IJzendoorn SCD, Chan J, Chang CJ, Amoresano A, Pane F, Pucci P, Tarallo A, Parenti G, Brunetti-Pierri N, Settembre C, Ballabio A, Polishchuk RS. Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis. Dev Cell. 2014;29:686–700. doi: 10.1016/j.devcel.2014.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nyasae LK, Schell MJ, Hubbard AL. Copper directs ATP7B to the apical domain of hepatic cells via basolateral endosomes. Traffic Cph Den. 2014;15:1344–1365. doi: 10.1111/tra.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terada K, Kawarada Y, Miura N, Yasui O, Koyama K, Sugiyama T. Copper incorporation into ceruloplasmin in rat livers. Biochim Biophys Acta. 1995;1270:58–62. doi: 10.1016/0925-4439(94)00072-x. [DOI] [PubMed] [Google Scholar]
- 8.Nair PM, Mason HS. Reconstitution of cytochrome C oxidase from a copper-depleted enzyme and Cu. J Biol Chem. 1967;242:1406–1415. [PubMed] [Google Scholar]
- 9.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 10.Gacheru SN, Trackman PC, Shah MA, O’Gara CY, Spacciapoli P, Greenaway FT, Kagan HM. Structural and catalytic properties of copper in lysyl oxidase. J Biol Chem. 1990;265:19022–19027. [PubMed] [Google Scholar]
- 11.Eipper BA, Perkins SN, Husten EJ, Johnson RC, Keutmann HT, Mains RE. Peptidyl-alpha-hydroxyglycine alpha-amidating lyase. Purification, characterization, and expression. J Biol Chem. 1991;266:7827–7833. [PubMed] [Google Scholar]
- 12.Kim H, Son HY, Bailey SM, Lee J. Deletion of hepatic Ctr1 reveals its function in copper acquisition and compensatory mechanisms for copper homeostasis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G356–364. doi: 10.1152/ajpgi.90632.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nose Y, Kim BE, Thiele DJ. Ctr1 drives intestinal copper absorption and is essential for growth, iron metabolism, and neonatal cardiac function. Cell Metab. 2006;4:235–244. doi: 10.1016/j.cmet.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Collins JF, Prohaska JR, Knutson MD. Metabolic crossroads of iron and copper. Nutr Rev. 2010;68:133–147. doi: 10.1111/j.1753-4887.2010.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Ann Nutr Metab. 2007;51:301–323. doi: 10.1159/000107673. [DOI] [PubMed] [Google Scholar]
- 16.Matak P, Zumerle S, Mastrogiannaki M, El Balkhi S, Delga S, Mathieu JRR, Canonne-Hergaux F, Poupon J, Sharp PA, Vaulont S, Peyssonnaux C. Copper deficiency leads to anemia, duodenal hypoxia, upregulation of HIF-2α and altered expression of iron absorption genes in mice. PloS One. 2013;8:e59538. doi: 10.1371/journal.pone.0059538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klevay LM. Dietary cholesterol lowers liver copper in rabbits. Biol Trace Elem Res. 1988;16:51–57. doi: 10.1007/BF02795333. [DOI] [PubMed] [Google Scholar]
- 18.Prohaska JR. Impact of copper limitation on expression and function of multicopper oxidases (ferroxidases) Adv Nutr Bethesda Md. 2011;2:89–95. doi: 10.3945/an.110.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broderius M, Mostad E, Wendroth K, Prohaska JR. Levels of plasma ceruloplasmin protein are markedly lower following dietary copper deficiency in rodents. Comp Biochem Physiol Toxicol Pharmacol CBP. 2010;151:473–479. doi: 10.1016/j.cbpc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lassi KC, Prohaska JR. Rapid alteration in rat red blood cell copper chaperone for superoxide dismutase after marginal copper deficiency and repletion. Nutr Res N Y N. 2011;31:698–706. doi: 10.1016/j.nutres.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Cartwright GE, Wintrobe MM. THE QUESTION OF COPPER DEFICIENCY IN MAN. Am J Clin Nutr. 1964;15:94–110. doi: 10.1093/ajcn/15.2.94. [DOI] [PubMed] [Google Scholar]
- 22.Danks DM. Copper deficiency in humans. Annu Rev Nutr. 1988;8:235–257. doi: 10.1146/annurev.nu.08.070188.001315. [DOI] [PubMed] [Google Scholar]
- 23.Song M, Schuschke DA, Zhou Z, Chen T, Pierce WM, Wang R, Johnson WT, McClain CJ. High Fructose Feeding Induces Copper Deficiency in Sprague-Dawley rats: A Novel Mechanism for Obesity Related Fatty Liver. J Hepatol. 2012;56:433–440. doi: 10.1016/j.jhep.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gletsu-Miller N, Broderius M, Frediani JK, Zhao VM, Griffith DP, Davis SS, Sweeney JF, Lin E, Prohaska JR, Ziegler TR. Incidence and prevalence of copper deficiency following roux-en-y gastric bypass surgery. Int J Obes 2005. 2012;36:328–335. doi: 10.1038/ijo.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar N, Ahlskog JE, Gross JB. Acquired hypocupremia after gastric surgery. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2004;2:1074–1079. doi: 10.1016/s1542-3565(04)00546-4. [DOI] [PubMed] [Google Scholar]
- 26.Kaler SG. Inborn errors of copper metabolism. Handb Clin Neurol. 2013;113:1745–1754. doi: 10.1016/B978-0-444-59565-2.00045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris ZL, Durley AP, Man TK, Gitlin JD. Targeted gene disruption reveals an essential role for ceruloplasmin in cellular iron efflux. Proc Natl Acad Sci U S A. 1999;96:10812–10817. doi: 10.1073/pnas.96.19.10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnson MA, Macdonald TL, Mannick JB, Conaway MR, Gaston B. Accelerated s-nitrosothiol breakdown by amyotrophic lateral sclerosis mutant copper,zinc-superoxide dismutase. J Biol Chem. 2001;276:39872–39878. doi: 10.1074/jbc.M102781200. [DOI] [PubMed] [Google Scholar]
- 29.Lazarchick J. Update on anemia and neutropenia in copper deficiency. Curr Opin Hematol. 2012;19:58–60. doi: 10.1097/MOH.0b013e32834da9d2. [DOI] [PubMed] [Google Scholar]
- 30.Klevay LM. Cardiovascular disease from copper deficiency–a history. J Nutr. 2000;130:489S–492S. doi: 10.1093/jn/130.2.489S. [DOI] [PubMed] [Google Scholar]
- 31.Mann KG, Lawler CM, Vehar GA, Church WR. Coagulation Factor V contains copper ion. J Biol Chem. 1984;259:12949–12951. [PubMed] [Google Scholar]
- 32.Klevay LM. Is the Western diet adequate in copper? J Trace Elem Med Biol Organ Soc Miner Trace Elem GMS. 2011;25:204–212. doi: 10.1016/j.jtemb.2011.08.146. [DOI] [PubMed] [Google Scholar]
- 33.Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US); Washington (DC): 2001. [online] http://www.ncbi.nlm.nih.gov/books/NBK222310/ (Accessed January 7, 2017) [PubMed] [Google Scholar]
- 34.Cockell KA, Bertinato J, L’Abbé MR. Regulatory frameworks for copper considering chronic exposures of the population. Am J Clin Nutr. 2008;88:863S–6S. doi: 10.1093/ajcn/88.3.863S. [DOI] [PubMed] [Google Scholar]
- 35.Chambers A, Krewski D, Birkett N, Plunkett L, Hertzberg R, Danzeisen R, Aggett PJ, Starr TB, Baker S, Dourson M, Jones P, Keen CL, Meek B, Schoeny R, Slob W. An exposure-response curve for copper excess and deficiency. J Toxicol Environ Health B Crit Rev. 2010;13:546–578. doi: 10.1080/10937404.2010.538657. [DOI] [PubMed] [Google Scholar]
- 36.Klevay LM. Alzheimer’s disease as copper deficiency. Med Hypotheses. 2008;70:802–807. doi: 10.1016/j.mehy.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y, Mahley RW. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol Dis. 2014;72(Pt A):3–12. doi: 10.1016/j.nbd.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chouinard-Watkins R, Plourde M. Fatty acid metabolism in carriers of apolipoprotein E epsilon 4 allele: is it contributing to higher risk of cognitive decline and coronary heart disease? Nutrients. 2014;6:4452–4471. doi: 10.3390/nu6104452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xue-shan Z, Juan P, Qi W, Zhong R, Li-hong P, Zhi-han T, Zhi-sheng J, Gui-xue W, Lu-shan L. Imbalanced cholesterol metabolism in Alzheimer’s disease. Clin Chim Acta Int J Clin Chem. 2016;456:107–114. doi: 10.1016/j.cca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 40.Alshehry ZH, Mundra PA, Barlow CK, Mellett NA, Wong G, McConville MJ, Simes J, Tonkin AM, Sullivan DR, Barnes EH, Nestel PJ, Kingwell BA, Marre M, Neal B, Poulter NR, Rodgers A, Williams B, Zoungas S, Hillis GS, Chalmers J, Woodward M, Meikle PJ. Plasma Lipidomic Profiles Improve on Traditional Risk Factors for the Prediction of Cardiovascular Events in Type 2 Diabetes Mellitus. Circulation. 2016;134:1637–1650. doi: 10.1161/CIRCULATIONAHA.116.023233. [DOI] [PubMed] [Google Scholar]
- 41.Klevay LM, Inman L, Johnson LK, Lawler M, Mahalko JR, Milne DB, Lukaski HC, Bolonchuk W, Sandstead HH. Increased cholesterol in plasma in a young man during experimental copper depletion. Metabolism. 1984;33:1112–1118. doi: 10.1016/0026-0495(84)90096-9. [DOI] [PubMed] [Google Scholar]
- 42.Lei KY. Alterations in plasma lipid, lipoprotein and apolipoprotein concentrations in copper-deficient rats. J Nutr. 1983;113:2178–2183. doi: 10.1093/jn/113.11.2178. [DOI] [PubMed] [Google Scholar]
- 43.Valsala P, Kurup PA. Investigations on the mechanism of hypercholesterolemia observed in copper deficiency in rats. J Biosci. 1987;12:137–142. [Google Scholar]
- 44.Klevay LM. IHD from copper deficiency: a unified theory. Nutr Res Rev. 2016;29:172–179. doi: 10.1017/S0954422416000093. [DOI] [PubMed] [Google Scholar]
- 45.Church SJ, Begley P, Kureishy N, McHarg S, Bishop PN, Bechtold DA, Unwin RD, Cooper GJS. Deficient copper concentrations in dried-defatted hepatic tissue from ob/ob mice: A potential model for study of defective copper regulation in metabolic liver disease. Biochem Biophys Res Commun. 2015;460:549–554. doi: 10.1016/j.bbrc.2015.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heffern MC, Park HM, Au-Yeung HY, Van de Bittner GC, Ackerman CM, Stahl A, Chang CJ. In vivo bioluminescence imaging reveals copper deficiency in a murine model of nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2016;113:14219–14224. doi: 10.1073/pnas.1613628113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tang Z, Gasperkova D, Xu J, Baillie R, Lee JH, Clarke SD. Copper deficiency induces hepatic fatty acid synthase gene transcription in rats by increasing the nuclear content of mature sterol regulatory element binding protein 1. J Nutr. 2000;130:2915–2921. doi: 10.1093/jn/130.12.2915. [DOI] [PubMed] [Google Scholar]
- 48.Ye J, DeBose-Boyd RA. Regulation of cholesterol and fatty acid synthesis. Cold Spring Harb Perspect Biol. 2011 doi: 10.1101/cshperspect.a004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schulz R, Schlüter KD, Laufs U. Molecular and cellular function of the proprotein convertase subtilisin/kexin type 9 (PCSK9) Basic Res Cardiol. 2015;110:4. doi: 10.1007/s00395-015-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller GD, Keen CL, Stern JS, Uriu-Hare JY. Copper deficiency and arachidonic acid enhance insulin secretion in isolated pancreatic islets from lean (FaFa) Zucker rats. Pancreas. 1998;17:390–396. doi: 10.1097/00006676-199811000-00010. [DOI] [PubMed] [Google Scholar]
- 51.Yabe D, Komuro R, Liang G, Goldstein JL, Brown MS. Liver-specific mRNA for Insig-2 down-regulated by insulin: implications for fatty acid synthesis. Proc Natl Acad Sci U S A. 2003;100:3155–3160. doi: 10.1073/pnas.0130116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tosco A, Fontanella B, Danise R, Cicatiello L, Grober OMV, Ravo M, Weisz A, Marzullo L. Molecular bases of copper and iron deficiency-associated dyslipidemia: a microarray analysis of the rat intestinal transcriptome. Genes Nutr. 2010;5:1–8. doi: 10.1007/s12263-009-0153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2011;9:524–530.e1. doi: 10.1016/j.cgh.2011.03.020. quiz e60. [DOI] [PubMed] [Google Scholar]
- 54.Aigner E, Strasser M, Haufe H, Sonnweber T, Hohla F, Stadlmayr A, Solioz M, Tilg H, Patsch W, Weiss G, Stickel F, Datz C. A role for low hepatic copper concentrations in nonalcoholic Fatty liver disease. Am J Gastroenterol. 2010;105:1978–1985. doi: 10.1038/ajg.2010.170. [DOI] [PubMed] [Google Scholar]
- 55.Song M, Schuschke DA, Zhou Z, Chen T, Shi X, Zhang J, Zhang X, Pierce WM, Johnson WT, Vos MB, McClain CJ. Modest fructose beverage intake causes liver injury and fat accumulation in marginal copper deficient rats. Obes Silver Spring Md. 2013;21:1669–1675. doi: 10.1002/oby.20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tallino S, Duffy M, Ralle M, Cortes MP, Latorre M, Burkhead JL. Nutrigenomics analysis reveals that copper deficiency and dietary sucrose up-regulate inflammation, fibrosis and lipogenic pathways in a mature rat model of nonalcoholic fatty liver disease. J Nutr Biochem. 2015 doi: 10.1016/j.jnutbio.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stättermayer AF, Traussnigg S, Aigner E, Kienbacher C, Huber-Schönauer U, Steindl-Munda P, Stadlmayr A, Wrba F, Trauner M, Datz C, Ferenci P. Low hepatic copper content and PNPLA3 polymorphism in non-alcoholic fatty liver disease in patients without metabolic syndrome. J Trace Elem Med Biol Organ Soc Miner Trace Elem GMS. 2017;39:100–107. doi: 10.1016/j.jtemb.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, Ratziu V. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550–556. doi: 10.1016/j.jhep.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 59.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet Lond Engl. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 60.Vanni E, Bugianesi E, Kotronen A, De Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis Off J Ital Soc Gastroenterol Ital Assoc Study Liver. 2010;42:320–330. doi: 10.1016/j.dld.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 61.Francini F, Castro MC, Schinella G, García ME, Maiztegui B, Raschia MA, Gagliardino JJ, Massa ML. Changes induced by a fructose-rich diet on hepatic metabolism and the antioxidant system. Life Sci. 2010;86:965–971. doi: 10.1016/j.lfs.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Gaynes BI, Watkins JB. Comparison of glucose, sorbitol and fructose accumulation in lens and liver of diabetic and insulin-treated rats and mice. Comp Biochem Physiol B. 1989;92:685–690. doi: 10.1016/0305-0491(89)90250-2. [DOI] [PubMed] [Google Scholar]
- 63.Lecoultre V, Egli L, Carrel G, Theytaz F, Kreis R, Schneiter P, Boss A, Zwygart K, Lê KA, Bortolotti M, Boesch C, Tappy L. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obes Silver Spring Md. 2013;21:782–785. doi: 10.1002/oby.20377. [DOI] [PubMed] [Google Scholar]
- 64.Theytaz F, Noguchi Y, Egli L, Campos V, Buehler T, Hodson L, Patterson BW, Nishikata N, Kreis R, Mittendorfer B, Fielding B, Boesch C, Tappy L. Effects of supplementation with essential amino acids on intrahepatic lipid concentrations during fructose overfeeding in humans. Am J Clin Nutr. 2012;96:1008–1016. doi: 10.3945/ajcn.112.035139. [DOI] [PubMed] [Google Scholar]
- 65.Vos MB, Kimmons JE, Gillespie C, Welsh J, Blanck HM. Dietary fructose consumption among US children and adults: the Third National Health and Nutrition Examination Survey. Medscape J Med. 2008;10:160. [PMC free article] [PubMed] [Google Scholar]
- 66.Park OJ, Cesar D, Faix D, Wu K, Shackleton CH, Hellerstein MK. Mechanisms of fructose-induced hypertriglyceridaemia in the rat. Activation of hepatic pyruvate dehydrogenase through inhibition of pyruvate dehydrogenase kinase. Biochem J. 1992;282(Pt 3):753–757. doi: 10.1042/bj2820753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nomura K, Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J Nutr Biochem. 2012;23:203–208. doi: 10.1016/j.jnutbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, Schreiner G, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Sautin YY, Johnson RJ. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J Biol Chem. 2012;287:40732–40744. doi: 10.1074/jbc.M112.399899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, Ishimoto T, Sautin YY, Lanaspa MA. Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes. 2013;62:3307–3315. doi: 10.2337/db12-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kang DH, Ha SK. Uric Acid Puzzle: Dual Role as Anti-oxidantand Pro-oxidant. Electrolytes Blood Press E BP. 2014;12:1–6. doi: 10.5049/EBP.2014.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waring WS, Webb DJ, Maxwell SR. Systemic uric acid administration increases serum antioxidant capacity in healthy volunteers. J Cardiovasc Pharmacol. 2001;38:365–371. doi: 10.1097/00005344-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 72.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM, Nonalcoholic Steatohepatitis Clinical Research Network Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatol Baltim Md. 2010;51:1961–1971. doi: 10.1002/hep.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishimoto T, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Orlicky DJ, Cicerchi C, McMahan RH, Abdelmalek MF, Rosen HR, Jackman MR, MacLean PS, Diggle CP, Asipu A, Inaba S, Kosugi T, Sato W, Maruyama S, Sánchez-Lozada LG, Sautin YY, Hill JO, Bonthron DT, Johnson RJ. High-fat and high-sucrose (western) diet induces steatohepatitis that is dependent on fructokinase. Hepatol Baltim Md. 2013;58:1632–1643. doi: 10.1002/hep.26594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maliken BD, Nelson JE, Klintworth HM, Beauchamp M, Yeh MM, Kowdley KV. Hepatic reticuloendothelial system cell iron deposition is associated with increased apoptosis in nonalcoholic fatty liver disease. Hepatol Baltim Md. 2013;57:1806–1813. doi: 10.1002/hep.26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sakiyama H, Fujiwara N, Yoneoka Y, Yoshihara D, Eguchi H, Suzuki K. Cu,Zn-SOD deficiency induces the accumulation of hepatic collagen. Free Radic Res. 2016;50:666–677. doi: 10.3109/10715762.2016.1164856. [DOI] [PubMed] [Google Scholar]
- 76.Liu SB, Ikenaga N, Peng ZW, Sverdlov DY, Greenstein A, Smith V, Schuppan D, Popov Y. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J Off Publ Fed Am Soc Exp Biol. 2016;30:1599–1609. doi: 10.1096/fj.14-268425. [DOI] [PubMed] [Google Scholar]
- 77.Rucker RB, Romero-Chapman N, Wong T, Lee J, Steinberg FM, McGee C, Clegg MS, Reiser K, Kosonen T, Uriu-Hare JY, Murphy J, Keen CL. Modulation of lysyl oxidase by dietary copper in rats. J Nutr. 1996;126:51–60. doi: 10.1093/jn/126.1.51. [DOI] [PubMed] [Google Scholar]


