Abstract
Objective
Complementary and alternative medicine (CAM), or integrative medicine, has become increasingly popular among patients with head and neck cancer. Despite its increasing prevalence, many patients feel uncomfortable discussing such therapies with their physicians, and many physicians are unaware and underequipped to evaluate or discuss their use with patients. The aim of this manuscript is to use recent data to outline the decision-making inherent to integrative medicine utilization among patients with head and neck cancer, to discuss the ethical implications inherent to balancing integrative and conventional approaches to treatment, and to highlight available resources to enhance head and neck cancer providers’ understanding of integrative medicine.
Data Sources
Randomized controlled trials involving integrative medicine or CAM treatment for cancer patients.
Review Methods
Trials were drawn from a systematic PubMed database search categorized into cancer prevention, treatment, and symptom management.
Conclusions
Integrative medicine is gaining popularity for the management of cancer and is most commonly used for symptom management. A number of randomized controlled trials provide data to support integrative therapies, yet physicians who treat head and neck cancer may be faced with ethical dilemmas and practical barriers surrounding incorporation of integrative medicine.
Implications for Practice
In the management of head and neck cancer, there is an increasing demand for awareness of, dialogue about, and research evaluating integrative medicine therapies. It is important for otolaryngologists to become aware of integrative therapy options, their risks and benefits, and resources for further information in order to effectively counsel their patients.
Introduction
Complementary and alternative medicine (CAM) encompasses “a group of diverse medical and health care interventions, practices, products, or disciplines that are not generally considered part of conventional medicine”.1 These modalities are used by 38% of adult patients in the United States and prevalence continues to increase.2 In recent years, “integrative medicine” has replaced “complementary and alternative medicine” as the preferred terminology. Integrative medicine focuses on a collaborative approach to patient care that involves “bringing conventional and complementary approaches together in a coordinated way”3 and suggests a more cohesive and patient-centered treatment.
As the use of integrative therapies is becoming increasingly common among patients with cancer, physicians must become adept at helping patients navigate these therapies. However, despite its increasing popularity among patients, integrative medicine has been slow to gain acceptance in the mainstream healthcare system, in part due to a lack of awareness among clinicians of research and clinical trials demonstrating the efficacy of integrative therapies4, the barriers to paying for integrative therapies, and a paucity of resources to guide clinicians in finding integrative medicine resources and providers.
Despite its overall growing presence in Western medicine, a role for integrative medicine has not been fully established among head and neck cancer providers. For patients and providers alike, key considerations in using integrative therapies include assessing the potential risks and benefits of the therapy, available evidence for specific therapies, and whether patients intend to use such therapy as an alternative treatment or in a complementary/integrative manner.5–7 Given this complex and challenging landscape, clinicians may benefit from an increased awareness of how integrative medicine can enhance the patient-doctor interaction and potentially improve patient experiences. Herein we present index patients with head and neck cancer who used integrative medicine in order to guide a discussion of how clinicians might better understand the types of and rationale for integrative medicine as they navigate the inherent practical and ethical challenges. We also offer resources clinicians can use to help guide them in determining the evidence for as well as the potential harms, interactions, and contraindications of integrative therapies.
Methods
Articles were identified via a formal PubMed database search specifying all randomized controlled trials published within the past ten years investigating integrative medicine or CAM for cancer patients and/or head and neck cancer patients. Trials were assessed by the authors and sorted into categories of cancer prevention, cancer treatment, or symptom management.
Illustrative deidentified case examples were chosen to exemplify the inherent issues. The University of Michigan Institutional Review Board has determined that publication of de-identified case studies does not require formal review or approval.
Discussion
Integrative Medicine Use in Head and Neck Cancer
Several types of integrative therapies, including natural products, mind-body practices, naturopathy, and Ayurveda, are commonly used among patients3. Biologically-based therapies such as herbal medicines, medicinal teas, and vitamins/minerals are the most common integrative therapies used by patients with head and neck cancer, followed by mind-body interventions such as visualization.6,8 Patients tend to use integrative medicine in regard to cancer in one of three separate paradigms: 1) prevention of cancer, 2) intended treatment of cancer, and 3) mitigation of side effects associated with cancer and conventional treatments.8,9 While scientific evidence is generally limited with regard to the efficacy of integrative therapies for cancer prevention and treatment, there is a wealth of data supporting integrative therapies for symptom management. In fact, there are over 200 randomized controlled trials focusing on integrative therapies for symptom management of breast cancer alone.10
While prevalence of integrative medicine usage varies greatly across studies, systematic reviews suggest that approximately one-third to one-half of cancer patients are using some form of integrative therapy11,12. Specifically in head and neck cancer, rates of use are more varied, ranging from 6–79%.6,9,13 This variation may be a function of methodology of data collection as well as timing; notably, integrative medicine use in head and neck cancer patients may increase dramatically after diagnosis and initiation of treatment.8
It may be difficult for patients and physicians to know where to find reliable information regarding the efficacy of these therapies, especially because the preponderance of internet-based information is of questionable veracity and accuracy.13 There is an increasing demand for rigorous integrative medicine data and clinical trials in cancer in order for physicians to provide better recommendations to their patients.14
Cancer Prevention
A 45-year old woman is referred to the head and neck oncology clinic for worsening white lesions in the oral buccal mucosa. She has no previous tobacco or alcohol use. Her past medical history is otherwise unremarkable, but she does have an uncle who was a heavy smoker and developed larynx cancer. On exam she appears to have benign oral leukoplakia. She is relieved, but asks about any data for dietary supplements that may help in preventing oral cancer.
Integrative therapies for cancer prevention have generated interest from patients and researchers alike. A number of natural compounds commonly used for cancer prevention have demonstrable anti-cancer effects in both in vitro and in vivo translational studies, while others are backed by in testimonials or individual case studies. Curcumin, an active compound in turmeric, has been shown to have anti-cancer properties in both in vitro and in vivo studies.15–17 A number of antioxidants have been shown to display cancer preventive properties as well by reducing oxidative free radical production in cells.18,19 Common antioxidants utilized include green tea (active agent: EGCG), grape seed extract (resveratrol), and pomegranates (polyphenols), which have been shown to have anti-cancer properties.20
Other dietary factors may play a role in cancer prevention. Prospective epidemiologic studies have identified that pretreatment low fruit intake predicts worse survival in head and neck cancer patients21 while diets rich in whole foods such as fruits, vegetables, whole grains, fish, and poultry are associated with better prognosis.22 Diets rich in soy may also aid in head and neck cancer prevention: soy isoflavones, particularly genistein, have been shown to inhibit proliferation of head and neck cancer cells in vitro, and may act as a chemopreventive agent in various human carcinomas.23,24 Additionally, an epidemiologic study suggested a possible decreased thyroid cancer risk associated with consumption of soy-based foods.25
There have been some early phase clinical trials which have demonstrated modest efficacy of agents in premalignant oral cavity lesions, including retinoic acid and green/black tea (Table I).26 However, despite encouraging preclinical data, demonstrating a clinically significant and durable preventative intervention in patients has been difficult. The challenges of such studies in regards to head and neck cancer prevention are the relatively low overall incidence of head and neck cancer (necessitating an extremely large cohort) and the high potential of confounding patient dietary and lifestyle factors. Specific patient populations at significantly elevated risk for head and neck cancers (e.g. patients with Fanconi anemia or BRCA2 mutations) may prove to be ideal populations in which to validate chemoprevention agents. However, such studies have not been performed to date. As such, currently there are no clear evidence-based recommendations for head and neck cancer chemoprevention (dietary or otherwise).
Table I.
Selected Randomized Controlled Trials for Integrative Medicine for Head and Neck Cancer Prevention
| Study | Intervention | Outcome measured | Findings |
|---|---|---|---|
| Sankaranarayanan (1997)55 | Vitamin A extract vs. β-carotene | Chemoprevention of oral premalignant lesions | Vitamin A and β-carotene administration both induced regression of oral premalignant lesions more effectively than placebo |
| Hong (1986)56 | 13-cRA vs. placebo | Chemoprevention of oral premalignant lesions | 13-cRA significantly decreased size of oral premalignant lesions |
| Hong (1990)57 | 13-cRA vs. placebo | Chemoprevention of second primary tumors | 13-cRA prevents second primary tumor development |
| Tsao (2009)26 | Green tea extract vs. placebo | Chemoprevention of oral premalignant lesions | No significant difference between groups |
| Mayne (2001)58 | Β-carotene vs. placebo | Chemoprevention of second primary tumors | No decrease or delay in second primary tumor development |
| Dash (2012)59 | Tea | Chemoprevention | Inconclusive, high dropout rate |
| Halder (2005)60 | Black tea | Chemoprevention of oral premalignant lesions | Clinical improvement shown among group administered black tea |
| Li (1999)61 | Tea | Chemoprevention of oral premalignant lesions | Tea consumption led to a decrease in size of premalignant lesions as well as pathological differences |
| Mallery (2014)62 | Black raspberry gel | Chemoprevention of oral premalignant lesions (OPL) | Topical application of black raspberry gel is effective in decreasing size and histologic grade of oral premalignant lesions. |
| Shin (2015)63 | Green tea polyphenon E | Maximum tolerated dose of green tea polyphenon E with erlotinib for chemoprevention of premalignant lesions | Ongoing clinical trial |
| Goodin (2009)64 | Green tea | Efficacy of green tea lozenge on prevalence, size, and severity of oral leukoplakia | No study results posted |
Cancer Treatment
A 53 year-old male smoker with advanced stage laryngeal squamous cell carcinoma is treated with concurrent chemoradiotherapy with curative intent. He presents for follow-up shortly after completion of treatment with persistent/recurrent cancer. The patient is counseled that a salvage laryngectomy represents his only option for curative intent. He is hesitant to lose his voice box and wants to explore other options, including a trip to Central America for alternative therapy with a traditional healer. He is counseled about the risks of disease progression and mortality associated with delay of standard treatment, but is not ready to consider surgery until he has explored his alternatives.
Integrative medicine has long been considered for curative treatment of cancer, both as a monotherapy and as an adjuvant therapy. It is important to note that patients who seek out alternative therapy and forego conventional treatment comprise a small minority (<5%) of patients using integrative medicine. This small cohort is often motivated to use alternative medicine due to distrust of and/or dissatisfaction with the conventional healthcare system as well as a strong desire for control.27 For physicians, awareness of this underlying distrust for conventional medicine may allow for a useful starting point for discussions with such patients.
More commonly, patients seek out integrative therapy as a supplement to standard medical care. Indeed, current NIH-funded clinical studies are investigating integrative medical therapies in a variety of cancer treatment paradigms to supplement current standard of care.28
Interestingly, many chemotherapeutic agents currently being used in Western medicine for cancer treatment are derived from natural compounds.29 For example, (−)-gossypol, a therapeutic derived from cottonseeds and previously used as an herbal Chinese compound, has been shown to enhance radiation sensitivity in vitro30 as well as inhibit tumor growth in in vivo models of head and neck cancer.31 Mistletoe extract historically has been used as an adjuvant cancer therapeutic in many European countries, with in vitro studies demonstrating cytotoxic effects on cancer cells and anecdotal evidence of a clinical effect in laryngeal cancer.32,33 Other commonly used natural compounds have been demonstrated to have potential as anti-cancer agents in in vitro studies, including curcumin (turmeric) and genistein (soy). 24,34,35
Clinical trials studying integrative products as treatment for head and neck cancer have been limited (Table II). The most robust study to date involved mistletoe extract as an adjuvant therapy after surgery/radiotherapy, which did not demonstrate an effect on survival.36 Notably, our institution is currently conducting a clinical trial examining the potential benefit of adding (−)-gossypol (AT101) to chemoradiation for advanced larynx cancer.37 Further clinical trials advancing promising agents from in vitro/in vivo studies may uncover novel adjuvant therapeutic agents in the near future.
Table II.
Selected Randomized Controlled Trials for Integrative Medicine for Head and Neck Cancer Treatment
| Study | Intervention | Outcome measured | Findings |
|---|---|---|---|
| Pisters (2001)65 | Green tea extract | Maximum-tolerated dose of green tea extract (Phase I Trial) | Maximum-tolerated dose was 4.2 g/m2 once daily or 1.0 g/m2 three times a day. |
| Steuer-Vogt (2001)36 | Mistletoe extract | Efficacy of mistletoe preparation as adjuvant therapy after surgery/radiation | Mistletoe had no effect on survival rates, cellular immune response, or quality of life |
| Worden (2015)37 | (−)-gossypol (AT-101) | Efficacy of AT-101 and chemotherapy for organ preservation in advanced laryngeal cancer | Ongoing clinical trial |
Symptom Management
A 62 year-old woman with tonsil cancer is treated with trans-oral robotic resection and neck dissection. Postoperative adjuvant radiation therapy is recommended. She develops local treatment toxicity, including neck pain, stiffness and xerostomia. She is committed to finishing her course of radiation, but asks if any additional options exist for helping her with her troublesome symptoms.
Patients most often use integrative medicine to counteract side effects of cancer and/or conventional treatment. Frequent thematic responses from a survey of head and neck cancer patients pursuing integrative medicine therapies at MD Anderson included to “improve emotional well-being, hope, and optimism”; and to do “everything to fight the disease”.38 Head and neck cancer therapies are often associated with significant local and systemic morbidity; thus, patients may be drawn to integrative therapies that may ameliorate or temporize bothersome side effects.14
A variety of studies support the efficacy of various integrative therapies for symptom management during chemotherapy and radiation for head and neck cancer, including honey for mucositis and calendula cream for skin toxicity after radiotherapy for head and neck cancer39,40 While encouraging, many of these studies are limited by small sample sizes.
Acupuncture has repeatedly been shown to effectively manage numerous cancer- and radiation-related symptoms, including xerostomia, pain, nausea/vomiting, pain after neck dissection for head and neck cancer, as well as provide pain relief from cancer symptoms (Table III).41–45 These promising results show that acupuncture may provide significant symptom relief for patients with head and neck cancer and can be considered as a therapeutic option. Additionally, an ongoing Phase III clinical trial is studying the effect of acupuncture on radiation-induced xerostomia, although the results of this study are not yet available.46
Table III.
Selected Randomized Controlled Trials for Integrative Medicine for Symptom Management in Head and Neck Cancer
| Study | Intervention | Outcome measured | Findings |
|---|---|---|---|
| Barton (2010)66 | Ginseng | Symptom management – fatigue, toxicity | No calculable results, only high doses of ginseng showed some improvements |
| Alimi (2003)42 | Acupuncture | Symptom management – cancer pain | Pain intensity after 2 months significantly decreased for patients in acupuncture group |
| Chen (2008)67 | Acupuncture vs. orally-administered pain medication | Symptom management – cancer pain | Both methods effectively controlled pain; total effective rate of acupuncture group was better than medication group (p<0.05) |
| Jayachandran (2012) 68 | Honey | Symptom management – oral mucositis | Significant reduction in mucositis in group with honey treatment |
| Simcock (2012)45 | Acupuncture | Symptom management – radiation-induced xerostomia | Significant reduction in symptoms of dry mouth in acupuncture treatment group |
| Pfister (2010)41 | Acupuncture | Symptom management – pain and dysfunction after neck dissection, xerostomia | Significant reduction in pain, dysfunction, and xerostomia in acupuncture treatment group |
| Sahebjamee (2015)69 | Aloe vera | Symptom management – radiation-induced mucositis | Aloe vera was equally effective as the control mouthwash and could be used as an alternative |
| Tsujimoto (2015)70 | L-glutamine | Symptom management –chemoradiotherapy-induced mucositis | L-glutamine decreased severity of mucositis |
| Palatty (2014)71 | Sandalwood oil and turmeric-based cream | Symptom management – prevention of radiodermatitis after radiation therapy | Sandalwood oil/turmeric-based cream effective in preventing radiodermatitis |
| Lu (2012)72 | Acupuncture | Symptom management – dysphagia after chemoradiation | Inconclusive results |
| Meng (2012)73 | Acupuncture | Symptom management – radiation-induced xerostomia | Acupuncture led to significant reduction of xerostomia and improved quality of life |
| You (2009)74 | Indigowood root extract | Symptom management – radiation-induced mucositis | Extract reduced severity of mucositis, anorexia, and difficulty swallowing |
| Su (2004)75 | Aloe vera | Symptom management – prevention of radiation-related mucositis | No significant improvement after aloe vera treatment |
Additional integrative interventions, including visualization, meditation, and prayer may increase quality of life for patients with head and neck cancer by reducing psychological distress in cancer patients.47 In addition, yoga and meditation are routinely recommended for anxiety and mood disorders related to cancer.10 These mind-body practices are unlikely to interfere with conventional treatments and have the potential to provide comfort. In sum, there is robust evidence validated from reliable clinical trials supporting acupuncture and other integrative modalities in alleviating symptoms from cancer pain and treatment-associated toxicities.
Shared Decision-Making
As awareness and use of integrative medicine continues to increase, head and neck cancer providers may struggle to balance their ethical obligations while safely advising their patients about their options. Despite being used for centuries, integrative therapies remain poorly understood and underutilized in the modern Western healthcare system. This lack of awareness of integrative medicine research combined with a historical deficiency of educational material in most medical school curricula creates a population of physicians with little knowledge about or confidence in such therapies, despite a globally increasing interest in these therapies.48 This sets a difficult stage for the patient-physician interaction with regard to discussions about integrative medicine. For example, many patients feel that their physicians are unequipped or unwilling to discuss integrative medicine and therefore avoid disclosing their plans or behaviors to medical professionals.48 Not only does such nondisclosure create potential for patient harm through unintended therapeutic interactions, potential adverse side effects, and lack of regulated production or dosing, but it can also create an atmosphere of distrust in the patient-physician relationship.
An overriding theme in all venues of cancer care involves a strong patient-physician relationship with an emphasis on conversation and shared decision-making. As such, when patients with head and neck cancer show interest in integrative medicine, it is crucial that physicians take time to discuss these options. Physicians should consider screening for and opening dialogue in regard to integrative medicine use, as this can build trust between patient and physician as well as create an atmosphere of open-mindedness to integrative therapies.
Patients may have a variety of philosophical motivations for pursuing integrative medicine. For example, many patients use integrative medicine not because of a dissatisfaction with conventional medicine, but because the ideas behind integrative medicine are more aligned with their philosophical beliefs about health.27 By discussing goals of care with patients, physicians can create a more individualized treatment regimen.
When discussing integrative medicine with patients, physicians must be careful to avoid acting in a paternalistic manner. Since knowledge about these therapies is generally limited among conventional healthcare providers and patients, education and evidence-based research or appropriate referral to an integrative medicine provider is vital before beginning an integrative medicine regimen. Disclosing any physician bias regarding alternative therapies to patients is important6; however, physicians should refrain from passing judgment on patients for using these therapies and instead focus on having a clear discussion that leaves room for both patient and physician education.
The ultimate obligation of any physician is to avoid and mitigate patient harm. However, this ethical obligation becomes complicated when physicians and patients disagree about what constitutes the proper course of treatment. Since there is limited awareness about integrative therapies for head and neck cancer, a patient’s desire to use integrative medicine can provide a unique opportunity for physicians and patients to collaboratively explore these therapies and develop a treatment plan that is safe, effective, and patient-centered. In order to safeguard the health of patients, physicians are obliged to ensure that their patient’s use of integrative medicine is safe and monitored.
Despite the potential benefits of integrative therapies, it is imperative that physicians consider potential unintended effects that these therapies may have on standard treatment/cancer prognoses and advise their patients appropriately. A common patient misconception about herbal medicine is that natural compounds are always safe. If physicians believe that an integrative therapy has the potential to negatively impact patient health, they are obliged to discuss this with patients and possibly recommend a safer/more effective therapy. Rather than attempt to discredit or steer patients away from integrative therapies altogether, physicians might recommend treatments that are known to be safe and effective, as well as advise patients about harmful therapies that should be avoided.49 For example, cytochrome P450 inhibitors/activators such as St. John’s Wort have significant effects drug metabolism, and thus effects on chemotherapy response.50 Additionally, some agents generally considered to be safe in most circumstances, may be deleterious in specific instances. As a case in point, high-dose antioxidants have been shown to reduce tumor control during radiation and decrease survival18.
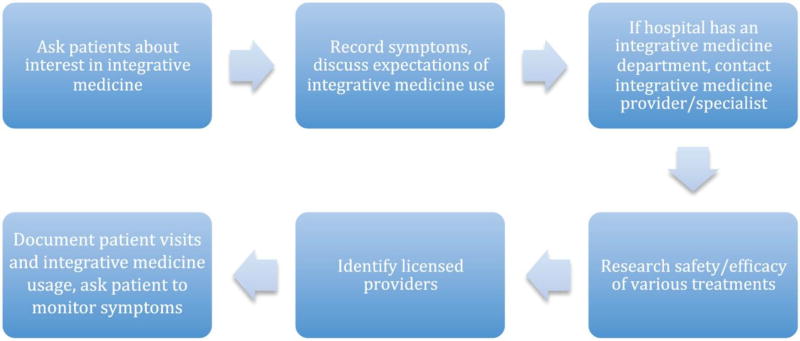
Eisenberg51 proposes a step-by-step strategy for physicians to advise patients who are interested in integrative medicine that emphasizes communication and teamwork between doctor and patient, as well as patient safety. His strategy involves questioning patients about current symptoms and closely monitoring these symptoms throughout the course of treatment. Next, physicians should discuss patients’ expectations and motivations for using integrative medicine and work together to research the safety and efficacy of each therapy. If patients still show interest, physicians should work with them to identify a licensed integrative medicine provider and brainstorm questions to ask during the initial visit. Many tertiary care centers are establishing departments of integrative medicine in general, and for cancer patients specifically. Finally, Eisenberg recommends carefully documenting patient visits and scheduling follow-ups to monitor the patient’s well-being throughout the course of integrative medicine treatments.
In order to integrate such therapeutic modalities into head and neck cancer management, physician and patient education must increase synergistically (Figure 1). Furthermore, the incorporation of research-based integrative medicine programs into oncology departments can optimize patient access to safe and effective integrative medicine.52 Open discussion between physician and patient is key in the joint effort to treat cancer in a more interdisciplinary, holistic manner.
Figure 1.
Approach to discussion of integrative medicine with head and neck cancer patients.
National Positions on Integrative Medicine
Integrative medicine is undeniably beginning to play a more important role in Western medicine. As such the Institute of Medicine has stated that “integrating CAM into traditional systems should be a high priority for research.”53 In 1998, the National Cancer Institute founded the Office of Cancer Complementary and Alternative Medicine (OCCAM) specifically to fund robust basic and clinical research into the potential for integrative medicine therapies for cancer. Additionally, the National Institute of Health (NIH) established the National Center for Complementary and Integrative Health (NCCIH; formerly known as the National Center for Complementary and Alternative Medicine).54 Both of these organizations conduct and fund a multitude of integrative medicine research, but also represent the necessity of increased clinically sound research about such therapies.
Integrative medicine programs have been or are being established in hospitals throughout the country and are frequently incorporated into cancer centers. Indeed, there has been a movement toward involving integrative medicine counselors as part of comprehensive cancer treatment planning and delivery.52 Additionally, there has been an expansion of interest in integrative medicine by private institutions as well as national organizations, which will aid in the assimilation of integrative medicine into the mainstream.54
Integrative Medicine Resources
The array of potential integrative therapy options can be daunting for both physicians and patients. Fortunately, there are publically available resources that can provide lists of commonly used integrative medicines, peer-reviewed research investigating their therapeutic effects, and potential drug side effects and drug interactions. Among those providing such resources are the NIH (NCCIH division), Memorial Sloan Kettering Cancer Center, and the Natural Medicines (Table IV).
Table IV.
Integrative Medicine Resources
| Source | Site | Overview of CAM? |
Herbal Information? |
Other CAM Information? |
Provider Information? |
Links to new research? |
|---|---|---|---|---|---|---|
| NIH | https://nccih.nih.gov/ | Yes | Yes | Yes | Yes | Yes |
| MSKCC | https://www.mskcc.org/cancer-care/treatments/symptom-management/integrative-medicine | Yes | Yes | Yes | Yes | Yes |
| Natural Medicines | https://naturalmedicines.therapeuticresearch.com | No | Yes | No | No | Yes |
| Consumer Lab | https://www.consumerlab.com | No | Yes | No | Yes | Yes |
| OCCAM | http://cam.cancer.gov/health_camaz.html | Yes | Yes | Yes | Yes | Yes |
| NCI CAM PDQ | http://www.cancer,gov/about-cancer/treatment/cam/hp | Yes | Yes | Yes | No | Yes |
Implications for Practice
The use of integrative medicine is prevalent among patients with head and neck cancer and is employed in three main paradigms: cancer prevention, cancer treatment, and management of side effects associated with cancer treatment. Patients most frequently seek out integrative medicine for hope, a sense of control in the treatment of their cancer, and symptom management. Using integrative medicine for cancer prevention and symptom management generally does not impose a significant risk to patient health, but awareness of potential interactions and risks are necessary for both the provider and patient. Barriers to care include patient discomfort in disclosing integrative medicine use, physician discomfort in discussing and providing recommendations on integrative therapies, and the relative lack of randomized clinical trials regarding the efficacy of integrative therapies in HNSCC. Although only a small cohort of patients choose to forego recommended clinical cancer-directed therapies in favor of alternative cancer treatments, such cases engender ethical dilemmas that are difficult for patients and clinicians alike. There is a need for greater awareness of, dialogue about, and research evaluating integrative medicine therapies for head and neck cancer management.
Acknowledgments
A.C.B. is a T32 research fellow funded on an NIH T-32 Training Grant (T32 DC005356).
Footnotes
The authors have no disclosures or conflicts of interest.
References
- 1.NCCAM Strategic Plan 2011–2015. [Accessed August 11, 2015];NIH Natl Cent Complement Integr Heal. 2011 https://nccih.nih.gov/about/plans/2011/introduction.htm.
- 2.Barnes PM, Bloom B, Nahin RL. National health statistics reports; no 12. Hyattsville, MD: National Center for Health Statistics; 2008. Complementary and alternative medicine use among adults and children: United States, 2007. [PubMed] [Google Scholar]
- 3.Complementary, Alternative, or Integrative Health: What’s In a Name? [Accessed August 11, 2015];NIH Natl Cent Complement Integr Heal. 2015 https://nccih.nih.gov/health/integrative-health.
- 4.Milden SP, Stokols D. Physicians’ attitudes and practices regarding complementary and alternative medicine. Behav Med. 2004;30(2):73–82. doi: 10.3200/BMED.30.2.73-84. [DOI] [PubMed] [Google Scholar]
- 5.The Use of Complementary and Alternative Medicine in the United States. [Accessed August 11, 2015];NIH Natl Cent Complement Integr Heal. 2008 https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm.
- 6.Miller MC, Pribitkin EA, Difabio T, Keane WM. Prevalence of complementary and alternative medicine use among a population of head and neck cancer patients: a survey-based study. Ear Nose Throat J. 2010;89(10):E23–7. doi: 10.1177/014556131008901005. [DOI] [PubMed] [Google Scholar]
- 7.Trinidade A, Shakeel M, Hurman D, Hussain A. Traditional and complementary and alternative medicines make for unwilling bedfellows in the management of cancer: a case report with a tragic outcome. J Laryngol Otol. 2011;125(11):1193–5. doi: 10.1017/S0022215111001794. [DOI] [PubMed] [Google Scholar]
- 8.Molassiotis A, Ozden G, Platin N, et al. Complementary and alternative medicine use in patients with head and neck cancers in Europe. Eur J Cancer Care (Engl) 2006;15(1):19–24. doi: 10.1111/j.1365-2354.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 9.Warrick PD, Irish JC, Morningstar M, Gilbert R, Brown D, Gullane P. Use of alternative medicine among patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1999;125(5):573–9. doi: 10.1001/archotol.125.5.573. [DOI] [PubMed] [Google Scholar]
- 10.Greenlee H, Balneaves LG, Carlson LE, et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. J Natl Cancer Inst Monogr. 2014;2014(50):346–58. doi: 10.1093/jncimonographs/lgu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst E, Cassileth BR. The prevalence of complementary/alternative medicine in cancer: a systematic review. Cancer. 1998;83(4):777–82. doi: 10.1002/(SICI)1097-0142(19980815)83:4<777::AID-CNCR22>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Horneber M, Bueschel G, Dennert G, Less D, Ritter E, Zwahlen M. How many cancer patients use complementary and alternative medicine: a systematic review and metaanalysis. Integr Cancer Ther. 2012;11(3):187–203. doi: 10.1177/1534735411423920. [DOI] [PubMed] [Google Scholar]
- 13.Asher BF, Seidman M, Snyderman C. Complementary and alternative medicine in otolaryngology. Laryngoscope. 2001;111(8):1383–9. doi: 10.1097/00005537-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Hendershot KA, Dixon M, Kono SA, Shin DM, Pentz RD. Patients’ perceptions of complementary and alternative medicine in head and neck cancer: a qualitative, pilot study with clinical implications. Complement Ther Clin Pract. 2014;20(4):213–8. doi: 10.1016/j.ctcp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008;52(9):1010–30. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- 16.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LoTempio MM, Veena MS, Steele HL, et al. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res. 2005;11(19 Pt 1):6994–7002. doi: 10.1158/1078-0432.CCR-05-0301. [DOI] [PubMed] [Google Scholar]
- 18.Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100(11):773–83. doi: 10.1093/jnci/djn148. [DOI] [PubMed] [Google Scholar]
- 19.Khan N, Afaq F, Mukhtar H. Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid Redox Signal. 2008;10(3):475–510. doi: 10.1089/ars.2007.1740. [DOI] [PubMed] [Google Scholar]
- 20.Zlotogorski A, Dayan A, Dayan D, Chaushu G, Salo T, Vered M. Nutraceuticals as new treatment approaches for oral cancer-II: Green tea extracts and resveratol. Oral Oncol. 2013;49(6):502–6. doi: 10.1016/j.oraloncology.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. J Clin Oncol. 2009;27(12):1969–75. doi: 10.1200/JCO.2008.18.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arthur AE, Peterson KE, Rozek LS, et al. Pretreatment dietary patterns, weight status, and head and neck squamous cell carcinoma prognosis. Am J Clin Nutr. 2013;97(2):360–8. doi: 10.3945/ajcn.112.044859.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarkar FH, Li Y. Soy isoflavones and cancer prevention. Cancer Invest. 2003;21(5):744–57. doi: 10.1081/CNV-120023773. [DOI] [PubMed] [Google Scholar]
- 24.Alhasan SA, Pietrasczkiwicz H, Alonso MD, Ensley J, Sarkar FH. Genistein-induced cell cycle arrest and apoptosis in a head and neck squamous cell carcinoma cell line. Nutr Cancer. 1999;34(1):12–9. doi: 10.1207/S15327914NC340102. [DOI] [PubMed] [Google Scholar]
- 25.Horn-Ross PL, Hoggatt KJ, Lee MM. Phytoestrogens and thyroid cancer risk: the San Francisco Bay Area thyroid cancer study. Cancer Epidemiol Biomarkers Prev. 2002;11(1):43–9. [PubMed] [Google Scholar]
- 26.Tsao AS, Liu D, Martin J, et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev Res. 2009;2(11):931–41. doi: 10.1158/1940-6207.CAPR-09-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Astin JA. Why patients use alternative medicine: results of a national study. JAMA. 1998;279(19):1548–53. doi: 10.1001/jama.279.19.1548. [DOI] [PubMed] [Google Scholar]
- 28.Overview: What NCCIH Funds. [Accessed January 11, 2016];Natl Cent Complement Integr Heal. 2015 https://nccih.nih.gov/grants/whatnccihfunds/overviewfunds.htm.
- 29.Newman DJ, Cragg GM. Natural Products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75(3):311–35. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zerp SF, Stoter TR, Hoebers FJ, et al. Targeting anti-apoptotic Bcl-2 by AT-101 to increase radiation efficacy: data from in vitro and clinical pharmacokinetic studies in head and neck cancer. Radiat Oncol. 2015;10:158. doi: 10.1186/s13014-015-0474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolter KG, Wang SJ, Henson BS, et al. (-)-gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia. 2006;8(3):163–72. doi: 10.1593/neo.05691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klingbeil MF, Xavier FC, Sardinha LR, et al. Cytotoxic effects of mistletoe (Viscum album L.) in head and neck squamous cell carcinoma cell lines. Oncol Rep. 2013;30(5):2316–22. doi: 10.3892/or.2013.2732. [DOI] [PubMed] [Google Scholar]
- 33.Shakeel M, Trinidade A, Geider S, Ah-See KW. The case for mistletoe in the treatment of laryngeal cancer. J Laryngol Otol. 2014;128(3):302–6. doi: 10.1017/S0022215114000103. [DOI] [PubMed] [Google Scholar]
- 34.Abuzeid WM, Davis S, Tang AL, et al. Sensitzation of head and neck cancer to cisplatin through the use of a novel curcumin analog. Arch Otolarygnol Head Neck Surg. 2011;137(5):499–507. doi: 10.1001/archoto.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khafif A, Lev-Ari S, Vexler A, et al. Curcumin: A potential radio-enhancer in head and neck cancer. Laryngoscope. 2009;119(10):2019–26. doi: 10.1002/lary.20582. [DOI] [PubMed] [Google Scholar]
- 36.Steuer-Vogt MK, Bonkowsky V, Ambrosch P, et al. The effect of an adjuvant mistletoe treatment programme in resected head and neck cancer patients. Eur J Cancer. 2001;37(1):23–31. doi: 10.1016/S0959-8049(00)00360-9. [DOI] [PubMed] [Google Scholar]
- 37.ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); Comprehensive Cancer Center, University of Michigan. Chemotherapy AND Bcl-xL Inhibitor (AT-101) For Organ Preservation In Adults With Advanced Laryngeal Cancer. 2000- [cited 16 Feb 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT01633541?term=NCT01633541&rank=1. NLM Identifier: NCT01633541. [Google Scholar]
- 38.Frenkel M, Cohen L, Peterson N, Palmer JL, Swint K, Bruera E. Integrative medicine consultation service in a comprehensive cancer center: findings and outcomes. Integr Cancer Ther. 2010;9(3):276–83. doi: 10.1177/1534735410378663. [DOI] [PubMed] [Google Scholar]
- 39.Cho HK, Jeong YM, Lee HS, Lee YJ, Hwang SH. Effects of honey on oral mucositis in patients with head and neck cancer: A meta-analysis. Laryngoscope. 2015;125(9):2085–92. doi: 10.1002/lary.25233. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Juresic E, Barton M, Shafiq J. Management of skin toxicity during radiation therapy: a review of the evidence. J Med Imaging Radiat Oncol. 2010;54(3):264–79. doi: 10.1111/j.1754-9485.2010.02170.x. [DOI] [PubMed] [Google Scholar]
- 41.Pfister DG, Cassileth BR, Deng GE, et al. Acupuncture for pain and dysfunction after neck dissection: results of a randomized controlled trial. J Clin Oncol. 2010;28(15):2565–70. doi: 10.1200/JCO.2009.26.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alimi D, Rubino C, Pichard-Leandri E, Fermand-Brule S, Dubreuil-Lemaire ML, Hill C. Analgesic effect of auricular acupuncture for cancer pain: a randomized, blinded, controlled trial. J Clin Oncol. 2003;21(22):4120–6. doi: 10.1200/jco.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Gillett J, Ientile C, Hiscock J, Plank A, Martin JM. Complementary and alternative medicine use in radiotherapy: what are patients using? J Altern Complement Med. 2012;18(11):1014–20. doi: 10.1089/acm.2011.0334. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization. [Accessed April 30, 2016];Acupuncture: Review and Analysis of Reports on Controlled Clinical Trials. 2002 http://www.who.int/iris/handle/10665/42414#sthash.BJdMfFsJ.dpuf.
- 45.Simcock R, Fallowfield L, Monson K, et al. ARIX: A randomised trial of acupuncture v oral care sessions in patients with chronic xerostomia following treatment of head and neck cancer. Ann Oncol. 2013;24(3):776–83. doi: 10.1093/annonc/mds515. [DOI] [PubMed] [Google Scholar]
- 46.M.D. Anderson Cancer Center. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); Acupuncture in Treating Dry Mouth Caused By Radiation Therapy in Patients With Head and Neck Cancer. 2000- [cited 16 Feb 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT01141231?term=NCT01141231&rank=1 NLM Identifier: NCT01141231. [Google Scholar]
- 47.Baider L, Uziely B, De-Nour AK. Progressive muscle relaxation and guided imagery in cancer patients. Gen Hosp Psychiatry. 1994;16(5):340–7. doi: 10.1016/0163-8343(94)90021-3. [DOI] [PubMed] [Google Scholar]
- 48.Frenkel M, Ben Arye E. The growing need to teach about complementary and alternative medicine: questions and challenges. Acad Med. 2001;76(3):251–4. doi: 10.1097/00001888-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Amin M, Glynn F, Rowley S, et al. Complementary medicine use in patients with head and neck cancer in Ireland. Eur Arch Otorhinolaryngol. 2010;267(8):1291–7. doi: 10.1007/s00405-010-1223-1. [DOI] [PubMed] [Google Scholar]
- 50.Weiger WA, Smith M, Boon H, et al. Advising Patients Who Seek Complementay and Alternative Therapies for Cancer. Ann Intern Med. 2002;137(11):889–903. doi: 10.7326/0003-4819-137-11-200212030-00010. [DOI] [PubMed] [Google Scholar]
- 51.Eisenberg DM. Advising patients who seek alternative medical therapies. Ann Intern Med. 1997;127(1):61–9. doi: 10.7326/0003-4819-127-1-199707010-00010. [DOI] [PubMed] [Google Scholar]
- 52.Ben-Arye E, Schiff E, Zollman C, et al. Integrating complementary medicine in supportive cancer care models across four continents. Med Oncol. 2013;30(2):511. doi: 10.1007/s12032-013-0511-1. [DOI] [PubMed] [Google Scholar]
- 53.Complementary and Alternative Medicine in the United States. Washington, DC: National Academies Press (US); 2005. Institute of Medicine (US) Committee on the Use of Complementary and Alternative Medicine by the American Public. http://www.ncbi.nlm.nih.gov/books/NBK83799/ [PubMed] [Google Scholar]
- 54.White JD. The National Cancer Institute’s perspective and agenda for promoting awareness and research on alternative therapies for cancer. 2002;8(5):545–50. doi: 10.1089/107555302320825048. [DOI] [PubMed] [Google Scholar]
- 55.Sankaranarayanan R, Mathew B, Varghese C, et al. Chemoprevention of oral leukoplakia with vitamin A and beta carotene: an assessment. Oral Oncol. 1997;33(4):231–6. doi: 10.1016/s0964-1955(97)00010-9. [DOI] [PubMed] [Google Scholar]
- 56.Hong WK, Endicott J, Itri LM, et al. 13-cis-retinoic acid in the treatment of oral leukoplakia. N Engl J Med. 1986;315(24):1501–5. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 57.Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N Engl J Med. 1990;323(12):795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 58.Mayne ST, Cartmel B, Baum M, et al. Randomized trial of supplemental beta-carotene to prevent second head and neck cancer. Cancer Res. 2001;61(4):1457–63. [PubMed] [Google Scholar]
- 59.Dash C, Chung FL, Rohan JA, et al. A six-month crossover chemoprevention clinical trial of tea in smokers and non-smokers: methodological issues in a feasibility study. BMC Complement Altern Med. 2012;12:96. doi: 10.1186/1472-6882-12-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Halder A, Raychowdhury R, Ghosh A, De M. Black tea (Camellia sinensis) as a chemopreventive agent in oral precancerous lesions. J Environ Pathol Toxicol Oncol. 2005;24(2):141–4. doi: 10.1615/JEnvPathToxOncol.v24.i2.70. [DOI] [PubMed] [Google Scholar]
- 61.Li N, Sun Z, Han C, Chen J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc Soc Exp Biol Med. 1999;220(4):218–24. doi: 10.1046/j.1525-1373.1999.d01-37.x. [DOI] [PubMed] [Google Scholar]
- 62.Mallery S, Tong M, Shumway B, et al. Topical application of a mucoadhesive freeze-dried black raspberry gel induces clinical and histologic regression and reduces loss of heterozygosity events in premalignant oral intraepithelial lesions: results from a multicentered, placebo-controlled clinical trial. Clin Cancer Res. 2014;20(7):1910–24. doi: 10.1158/1078-0432.CCR-13-3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Winship Cancer Institute, Emory University. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); Phase I Chemoprevention Trial With Green Tea Polyphenon E & Erlotinib in Patients With Premalignant Lesions of the Head & Neck. 2000- [cited 16 Feb 2016]. Available from: https://clinicaltrials.gov/ct2/show/NCT01116336?term=green+tea+head+and+neck+cancer&rank=1. NLM Identifier: NCT01116336. [Google Scholar]
- 64.University of Medicine and Dentistry of New Jersey. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); A Phase II Trial to Assess the Effects of Green Tea in Oral Leukoplakia. 2000- [cited 16 Feb 2016]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT00176566?term=green+tea+head+and+neck+cancer&rank=2. NLM Identifier: NCT00176566. [Google Scholar]
- 65.Pisters KM, Newman RA, Coldman B, et al. Phase I trial of oral green tea extract in adult patients with solid tumors. J Clin Oncol. 2001;19(6):1830–8. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- 66.Barton DL, Soori GS, Bauer BA, et al. Pilot study of Panax quinquefolius (American ginseng) to improve cancer-related fatigue: a randomized, double-blind, dose-finding evaluation: NCCTG trial N03CA. Support Cancer Care. 2010;18(2):179–87. doi: 10.1007/s00520-009-0642-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen ZJ, Guo YP, Wu ZC. Observation on the therapeutic effect of acupuncture at pain points on cancer pain. Zhongguo Zhen Jiu (Chinese Acupunct Moxibustion) 2008;28(4):251–3. [PubMed] [Google Scholar]
- 68.Jayachandran S, Balaji N. Evaluating the effectiveness of topical application of natural honey and benzydamine hydrochloride in the management of radiation mucositis. Indian J Palliat Care. 2012;18(3):190–5. doi: 10.4103/0973-1075.105689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahebjamee M, Mansourian A, Mohammad M, et al. Comparative efficacy of aloe vera and benzydamine mouthwashes on radiation-induced oral mucositis: a triple-blind, randomised, controlled clinical trial. Oral Health Prev Dent. 2015;13(4):309–15. doi: 10.3290/j.ohpd.a33091. [DOI] [PubMed] [Google Scholar]
- 70.Tsujimoto T, Yamamoto Y, Wasa M, et al. L-glutamine decreases the severity of mucositis induced by chemoradiotherapy in patients with locally advanced head and neck cancer: a double-blind, randomized, placebo-controlled trial. Oncol Rep. 2015;33(1):33–9. doi: 10.3892/or.2014.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palatty PL, Azmidah A, Rao S, et al. Topical application of a sandal wood oil and turmeric based cream prevents radiodermatitis in head and neck cancer patients undergoing external beam radiotherapy: a pilot study. Br J Radiol. 2014;87(1038):20130490. doi: 10.1259/bjr.20130490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu W, Wayne PM, Davis RB, et al. Acupuncture for dysphagia after chemoradiation in head and neck cancer: rationale and design of a randomized, sham-controlled trial. Contemp Clin Trials. 2012;33(4):700–11. doi: 10.1016/j.cct.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meng Z, Kay Garcia M, Hu C, et al. Sham-controlled, randomised, feasibility trial of acupuncture for prevention of radiation-induced xerostomia among patients with nasopharyngeal carcinoma. Eur J Cancer. 2012;48(11):1692–9. doi: 10.1016/j.ejca.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.You WC, Hsieh CC, Huang JT. Effect of extracts from indigowood root (Isatis indigotica Fort.) on immune responses in radiation-induced mucositis. J Altern Complement Med. 2009;15(7):771–8. doi: 10.1089/acm.2008.0322. [DOI] [PubMed] [Google Scholar]
- 75.Su CK, Mehta V, Ravikumar L, et al. Phase II double-blind randomized study comparing oral aloe vera versus placebo to prevent radiation-related mucositis in patients with head-and-neck neoplasms. Int J Radiat Oncol Biol Phys. 2004;60(1):171–7. doi: 10.1016/j.ijrobp.2004.02.012. [DOI] [PubMed] [Google Scholar]