Abstract
For designing single-arm phase II trials with time-to-event endpoints, a sample size formula is derived for the modified one-sample log-rank test under the proportional hazards model. The derived formula enables new methods for designing trials that allow a flexible choice of the underlying survival distribution. Simulation results showed that the proposed formula provides an accurate estimation of sample size. The sample size calculation has been implemented in an R function for the purpose of trial design.
Keywords: contiguous alternative, one-sample log-rank test, proportional hazards model, time-to-event, single-arm phase II trial, sample size
1 Introduction
A time-to-event endpoint, such as event-free survival or overall survival, is often the primary endpoint for cancer clinical trials. In pediatric oncology, single-arm phase II trials with time-to-event endpoints are often conducted with limited numbers of patients. Various statistical methods have been proposed for designing randomized phase III trials with time-to-event endpoints (e.g., by George and Desu, 1977; Lachin, 1981; Rubenstein et al., 1981; Schoenfeld, 1983; Lakatos, 1988; Barthel et al. 2006; and many others). However, the literature on designing single-arm phase II trials with time-to-event endpoints is relatively scarce. The current practice for designing such trials is limited to using a parametric maximum likelihood test under the exponential model or a naive approach based on dichotomizing the event time at a landmark time point (Owzar and Jung, 2008). Trial design under the exponential model may not be reliable and the naive approach is inefficient.
Recently, Kwak and Jung (2014) proposed a two-stage phase II survival trial design using the one-sample log-rank test (OSLRT) (Breslow, 1975; Woolson, 1981; and Finkelstein et al., 2003). However, simulation results showed that Kwak and Jung’s design is conservative and underpowered. To correct the power and conservativeness of the OSLRT, Wu (2015) proposed a modified one-sample log-rank test (MOSLRT) for single-arm phase II survival trial designs. The MOSLRT preserves the type I error well and provides adequate power for trial design for a class of common parametric survival distributions. However, all the parametric survival distributions make strong assumptions about the shape of the hazard functions and are difficult to validate for historical data. In this paper, formulae for the number of events and sample size are derived under the proportional hazards model. The derived formula for the number of events is an analog version of the Schoenfeld formula for a two-arm randomized phase III trial using the two-sample log-rank test (Schoenfeld, 1983). Trial design based on the proposed sample size formula offers great flexibility in choosing of the underlying survival distribution, which could be a parametric survival distribution, a non-parametric Kaplan-Meier curve, or a spline version of the survival distribution (Kooperberg and Stone, 1992; Bantis et al., 2012; Anderson et al., 2013).
The rest of the paper is organized as follows. The MOSLRT is introduced in section 2. The formulae for the number of events and sample size are derived in section 3. Parameter setting for trial design is discussed in section 4. Simulations are conducted to study the performance of the proposed methods in section 5. An example is given in section 6 to illustrate the single-arm phase II survival trial designs. Concluding remarks are made in section 7.
2 Test Statistics
Let S0(t) denote the survival function under the null hypothesis that is chosen for a single-arm phase II trial design. Let S(t) denote the survival function of the experimental treatment. Consider the following proportional hazards model:
| (1) |
where δ(> 0) is the hazard ratio. The hypothesis of improvement in survival with the experimental treatment is
| (2) |
Testing this hypothesis is equivalent to testing the difference between the survival distributions with the experimental treatment and under the null hypothesis. Thus, the OSLRT can be used. However, the OSLRT is conservative, as shown by Kwak and Jung (2014); Sun et al. (2010); and Wu (2015). Recently, Wu (2015) proposed a MOSLRT that preserves the type I error well and provides adequate power for study design. To introduce the MOSLRT, assume that during the accrual phase of the trial, n subjects are enrolled in the study. Let Ti and Ci denote the event time and censoring time, respectively, of the ith subject. We assume that the event time Ti and censoring time Ci are independent and that {Ti, Ci, i = 1, …, n} are independent and identically distributed. Then, the observed event time and event indicator are Xi = Ti ∧Ci and Δi = I(Ti ≤ Ci), respectively, for the ith subject. On the basis of the observed data {Xi, Δi, i = 1,…, n}, we define as the observed number of events and as the expected number of events (asymptotically), where Λ0(t) = − log S0(t) is the cumulative hazard function under the null hypothesis. Then, the MOSLRT is defined by
To study the asymptotic distribution, we formulate it using counting-process notation. Specifically, let Ni(t) = ΔiI{Xi ≤ t} and Yi(t) = I{Xi ≥ t} be the failure and at-risk processes, respectively, then
Thus, the counting-process formulation of the MOSLRT is given by , where
and
Under the null hypothesis H0, by the strong law of large numbers, and , where G(t) is the survival distribution of the censoring time C. Then, , and (Wu, 2015). Therefore, by the counting process central limit theorem (Fleming and Harrington, 1991), L is asymptotically standard normal distributed. Hence, we reject the null hypothesis H0 with one-sided type I error α if , where z1−α is the 100(1 − α) percentile of the standard normal distribution.
3 Sample Size Formulae
Traditionally, sample size is often derived under the fixed alternative. However, when the asymptotic distribution of the test statistics is difficult to derive under the fixed alternative, contiguous alternatives (Lin et al., 1999) can also be considered by assuming that the alternative value of the testing parameter decreases to the null value at the rate of n−1/2, where n is the sample size. For example, under the proportional hazard model, the null hypothesis of interest is H0 : γ = 0 and the fixed alternative hypothesis of interest is H1 : γ = γ1, where γ = − log(δ) is the negative hazard ratio and γ1 > 0. The contiguous alternatives of interest are , which converges to the null hypothesis H0 : γ = 0 as sample size n goes to infinity. Here, we will first derive a formula for the number of events under the contiguous alternatives. The proportional hazard model (1) is equivalent to λ(t) = e−γλ0(t), where λ0(t) and λ(t) are the hazard functions under the null hypothesis and the experimental treatment, and γ = − log(δ) > 0. To derive the formula, we consider a sequence of contiguous alternatives , where b < ∞. Under the H1n, as shown in Appendix 1, is approximately normal distributed with mean
and unit variance, where is the probability of failure under the null hypothesis, which can be shown as
Therefore, the study power 1 − β under the contiguous alternatives H1n satisfies the following:
Thus, d = np0, the expected number of events under the null hypothesis, satisfies the equation
Solving for d, we obtain
| (3) |
which gives the expected number of events under the null hypothesis. To calculate the sample size of the trial, let p1 be the probability of failure under the alternative, which is given by
where S1(t) = [S0(t)]δ and Λ1(t) = δΛ0(t). Then, the required sample size for the trial is given by d1/p1, where d1 is the number of events under the alternative. However, we don’t know for the d1, and we have only derived number of events d under the null hypothesis. The d/p0 is the sample size under the null which underestimates the sample size required under the alternative, where p0 is the probability of failure under the null. As d/p1 > d1/p1, thus, d/p1 overestimates the required sample size. Let P be the average probabilities of failure under the null and alternative, that is
then, a reasonable estimate of the required sample size is n = d/P which can be calculated by
| (4) |
For the purpose of comparison, the sample size formula for the MOSLRT under the fixed alternative H1 (Wu, 2015) is also given as follows:
| (5) |
where ω = v1 − v0, , and , with v0, v1, v00, and v01 being given by the following equations:
4 Parameter Setting for Trial Design
For trial design using sample size formula (4), we first consider one of the following common parametric survival distributions: Weibull, gamma, Gompertz, log-normal, or log-logistic. The design parameters of the underlying survival distribution S(t) under the null hypothesis can be set as follows. Let S(x) be the survival probability of S(t) at a landmark time point x, S0(x) be the level of S(x) at which investigators are no longer interested in the experimental treatment, and S1(x)(> S0(x)) be the level of S(x) at which investigators consider the experimental treatment is promising. Then the hypothesis of (2) is equivalent to the following hypothesis:
and the trial is powered at the alternative S(x) = S1(x). Here, the shape parameter of the underlying survival distribution is assumed to be known from historical data. Thus, the scale parameter (Table 1) for each distribution can be determined by the value of S0(x), which is given as follows:
Weibull , with λ0 = − log S0(x)/xκ,
Log-normal , with μ0 = log(x) − σΦ−1(1 − S0(x)),
Gompertz , with θ0 = −γ log S0(x)/(eγx − 1),
Gamma S0(t) = 1 − Ik(λ0t), with ,
Log-logistic S0(t) = 1/(1 + λ0tp), with λ0 = (1/S0(x) − 1)/xp.
The hazard ratio can be calculated by
and the survival distribution under the alternative is given by S1(t) = [S0(t)]δ. To calculate the probabilities p0 and p1 for formula (4), we assume that subjects are recruited with a uniform distribution over the accrual period ta and followed for a period of tf and that no subject is lost to follow-up. Thus, the censoring distribution is a uniform distribution over [tf, ta + tf]. That is, the censoring survival distribution G(t) = 1 if t ≤ tf; = (ta + tf − t)/ta if tf ≤ t ≤ ta + tf; = 0 otherwise. Hence, the probabilities of failure p0 and p1 can be calculated by the following integration:
where S1(t) = [S0(t)]δ. If S0(t) is a spline version of the survival distribution, then pi can also be calculated by numerical integration. If S0(t) is a Kaplan-Meier curve, then pi can be calculated numerically using Simpson’s rule as follows:
The proposed sample size formula can also incorporate lost to follow-up in the sample size calculation. For example, let C1 be the loss to follow-up time and C2 be the administrative censoring time, then the overall censoring time is C = C1 ∧ C2, where C1 and C2 are independent. Thus, the overall censoring distribution is G(t) = P(C > t) = P(C1 > t)P(C2 > t) = G1(t)G2(t). It is often assumed that the loss to follow-up distribution is an exponential G1(t) = e−ηt, and administrative censoring distribution G2(t) is uniform. Therefore the sample size formula (4) can be calculated by numerical integrations. For non-uniform accrual, once the accrual distribution is specified, the sample size can be calculated as well.
Table 1.
Various parametric distributions used for single-arm phase II trial designs.
| Surv. function | Density | Parameter | Cumu. hazard | Hazard | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Dist. | S(t) | f(t) | Scale | Shape | Λ(t) | λ(t) | |||
| WB(λ, κ) |
|
|
λ | κ | λtκ | κλtκ−1 | |||
| GM(λ, k) | 1 − Ik (λt) |
|
λ | k | − log S(t) |
|
|||
| LN(μ, σ2) |
|
|
μ | σ | − log S(t) |
|
|||
| LG(λ, p) |
|
|
λ | p | log(1 + λtp) |
|
|||
| GZ(θ, γ) |
|
|
θ | γ |
|
θeγt | |||
Footnote: abbreviation Dist.: distribution; Surv.: survival; Cumu.: cumulative
5 Simulation studies
We first investigated whether formula (4) would give an accurate sample size estimation. We calculated sample sizes under various hazard ratios δ = 1.2−1–2.0−1, with powers of 80%, 85%, and 90% and a type I error of 5%. The accuracy was assessed by simulations performed under the Weibull distribution. The Weibull shape parameter κ was set to 0.5, 1 and 2 to reflect a decreasing, constant and increasing hazard function, and the median survival time under the null was set to m0 = 1. We assumed that subjects were recruited with a uniform distribution over the accrual period ta = 3 (years) and followed for tf = 1 (year) and that no subject was lost to follow-up; that is, only administrative censoring was considered in the trial. Under these assumptions, the number of events and sample sizes were calculated, and empirical powers and type I errors were estimated based on 100,000 simulation runs (Table 2). All simulated empirical powers and type I errors were close to the nominal levels. Additional sample size calculations were conducted under the Weibull model for various combinations of accrual period ta, follow-up time tf and landmark time point x for survival probability S0(x) under null which varies from 0.2 to 0.7 and a 10% increasing survival probability S1(x) under alternative to mimic a variety of real trial design. Detail for the set up of the design parameters were given in Table 2. Simulations were conducted to estimate the empirical type I error and power for the corresponding sample size based on 100,000 runs. The empirical type I errors and powers were close to the nominal levels for all scenarios. Thus, the formula (4) did provide an accurate estimation of the sample size for trial design.
Table 2.
Number of events (d) and sample sizes (n) were calculated from formulae (3) and (4) for various of hazard ratios (δ) under the Weibull distribution, with nominal type I error of 0.05 and power of 80%, 85%, and 90%. The empirical type I errors and powers were estimated based on 100,000 simulation runs.
| Power | Design | κ = 0.5 | κ = 1 | κ = 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||||
| δ−1 | d | n |
|
|
n |
|
|
n |
|
|
|||||||
| 90% | 1.2 | 258 | 415 | 0.051 | 0.904 | 338 | 0.051 | 0.901 | 285 | 0.049 | 0.902 | ||||||
| 1.3 | 125 | 205 | 0.051 | 0.903 | 166 | 0.052 | 0.902 | 139 | 0.049 | 0.901 | |||||||
| 1.4 | 76 | 128 | 0.052 | 0.904 | 103 | 0.051 | 0.904 | 85 | 0.049 | 0.900 | |||||||
| 1.5 | 53 | 90 | 0.053 | 0.907 | 72 | 0.051 | 0.904 | 59 | 0.050 | 0.901 | |||||||
| 1.6 | 39 | 68 | 0.052 | 0.905 | 54 | 0.052 | 0.902 | 44 | 0.049 | 0.900 | |||||||
| 1.7 | 31 | 54 | 0.052 | 0.904 | 43 | 0.052 | 0.906 | 35 | 0.050 | 0.903 | |||||||
| 1.8 | 25 | 45 | 0.055 | 0.908 | 36 | 0.052 | 0.908 | 29 | 0.049 | 0.905 | |||||||
| 1.9 | 21 | 38 | 0.054 | 0.902 | 30 | 0.052 | 0.904 | 24 | 0.049 | 0.900 | |||||||
| 2.0 | 18 | 33 | 0.054 | 0.903 | 26 | 0.053 | 0.903 | 21 | 0.050 | 0.903 | |||||||
|
| |||||||||||||||||
| 85% | 1.2 | 217 | 349 | 0.051 | 0.854 | 284 | 0.051 | 0.853 | 240 | 0.049 | 0.852 | ||||||
| 1.3 | 105 | 172 | 0.051 | 0.854 | 140 | 0.051 | 0.856 | 116 | 0.049 | 0.852 | |||||||
| 1.4 | 64 | 107 | 0.054 | 0.856 | 86 | 0.051 | 0.855 | 71 | 0.050 | 0.853 | |||||||
| 1.5 | 44 | 75 | 0.053 | 0.855 | 60 | 0.052 | 0.856 | 49 | 0.049 | 0.853 | |||||||
| 1.6 | 33 | 57 | 0.054 | 0.858 | 46 | 0.051 | 0.860 | 37 | 0.050 | 0.853 | |||||||
| 1.7 | 26 | 46 | 0.053 | 0.861 | 36 | 0.052 | 0.856 | 29 | 0.049 | 0.854 | |||||||
| 1.8 | 21 | 38 | 0.053 | 0.861 | 30 | 0.052 | 0.861 | 24 | 0.049 | 0.855 | |||||||
| 1.9 | 18 | 32 | 0.054 | 0.858 | 26 | 0.052 | 0.865 | 20 | 0.050 | 0.851 | |||||||
| 2.0 | 15 | 28 | 0.055 | 0.859 | 22 | 0.053 | 0.859 | 17 | 0.049 | 0.848 | |||||||
|
| |||||||||||||||||
| 80% | 1.2 | 186 | 300 | 0.052 | 0.806 | 244 | 0.051 | 0.805 | 206 | 0.050 | 0.806 | ||||||
| 1.3 | 90 | 148 | 0.052 | 0.805 | 120 | 0.051 | 0.807 | 100 | 0.048 | 0.805 | |||||||
| 1.4 | 55 | 92 | 0.051 | 0.806 | 74 | 0.052 | 0.807 | 61 | 0.049 | 0.803 | |||||||
| 1.5 | 38 | 65 | 0.052 | 0.808 | 52 | 0.052 | 0.811 | 43 | 0.050 | 0.812 | |||||||
| 1.6 | 28 | 49 | 0.055 | 0.809 | 39 | 0.052 | 0.810 | 32 | 0.048 | 0.808 | |||||||
| 1.7 | 22 | 39 | 0.053 | 0.809 | 31 | 0.052 | 0.810 | 25 | 0.049 | 0.808 | |||||||
| 1.8 | 18 | 33 | 0.054 | 0.818 | 26 | 0.053 | 0.818 | 21 | 0.049 | 0.815 | |||||||
| 1.9 | 16 | 28 | 0.055 | 0.816 | 22 | 0.053 | 0.816 | 17 | 0.048 | 0.801 | |||||||
| 2.0 | 13 | 24 | 0.056 | 0.813 | 19 | 0.053 | 0.814 | 15 | 0.048 | 0.809 | |||||||
Next, we conducted simulations to compare the sample size formulae (4) and (5). In simulations, the survival distributions were taken as Weibull, gamma, Gompertz, log-normal and log-logistic (Table 1). The parameters of the survival distribution under the null were set as follows: the shape parameter of each distribution was set to 0.5, 1, and 2; the survival probabilities at a landmark time point x = 2 under the null were set to S0(x) = 0.2 – 0.7 and under the alternative were set to S1(x) = 0.35 – 0.8, with same accrual and censoring distributions as before. Given a nominal type I error of 5% and power of 80%, the required sample sizes based on formulae (4) and (5) were calculated for each design scenario. For each calculated sample size, 100,000 random samples were generated from the corresponding distribution to estimate the empirical type I error and power (Tables 3 and 4). The simulation results showed that the empirical powers were close to the nominal level of 80% for all scenarios. Thus, sample size formulae (4) and (5) both gave an accurate estimation of sample size. The results also showed that the sample sizes calculated by formula (4) under the contiguous alternatives (Table 3) were almost identical to that calculated by formula (5) under the fixed alternative (Table 4). Furthermore, the MOSLRT controlled the type I error well when the survival probability under the null was low (S0(x) < 0.5) and was slightly more liberal when the survival probability under the null (S0(x) ≥ 0.5) was high.
Table 3.
Sample sizes (n) were calculated from formula (4) for various of accrual period (ta), follow-up time (tf), landmark time point (x), and survival probabilities under null and alternative for the Weibull distribution with nominal type I error of 0.05 and power of 80%. The empirical type I errors and powers were estimated based on 100,000 simulation runs.
| Design | κ = 0.5 | κ = 1 | κ = 2 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| (ta, tf, x) | S0(x), S1(x) | n |
|
|
n |
|
|
n |
|
|
||||||
| (1,1,1) | 0.2, 0.3 | 90 | .050 | .806 | 85 | .050 | .808 | 79 | .050 | .808 | ||||||
| 0.3, 0.4 | 115 | .052 | .806 | 106 | .052 | .808 | 95 | .050 | .806 | |||||||
| 0.4, 0.5 | 128 | .052 | .808 | 115 | .052 | .805 | 99 | .050 | .806 | |||||||
| 0.5, 0.6 | 129 | .053 | .807 | 113 | .053 | .805 | 93 | .052 | .808 | |||||||
| 0.6, 0.7 | 118 | .054 | .807 | 102 | .053 | .806 | 79 | .052 | .806 | |||||||
| 0.7, 0.8 | 95 | .055 | .803 | 81 | .055 | .808 | 60 | .054 | .810 | |||||||
|
| ||||||||||||||||
| (1,2,1) | 0.2, 0.3 | 83 | .050 | .807 | 76 | .049 | .804 | 73 | .048 | .801 | ||||||
| 0.3, 0.4 | 103 | .051 | .809 | 90 | .050 | .807 | 83 | .047 | .803 | |||||||
| 0.4, 0.5 | 111 | .051 | .806 | 93 | .049 | .808 | 80 | .049 | .800 | |||||||
| 0.5, 0.6 | 109 | .053 | .806 | 86 | .051 | .807 | 69 | .049 | .807 | |||||||
| 0.6, 0.7 | 97 | .053 | .806 | 73 | .051 | .808 | 52 | .050 | .808 | |||||||
| 0.7, 0.8 | 77 | .056 | .808 | 55 | .054 | .808 | 35 | .050 | .819 | |||||||
|
| ||||||||||||||||
| (2,2,2) | 0.2, 0.3 | 90 | .051 | .807 | 85 | .050 | .810 | 79 | .050 | .803 | ||||||
| 0.3, 0.4 | 115 | .050 | .805 | 106 | .051 | .808 | 95 | .050 | .805 | |||||||
| 0.4, 0.5 | 128 | .052 | .807 | 115 | .052 | .808 | 99 | .050 | .806 | |||||||
| 0.5, 0.6 | 129 | .053 | .809 | 113 | .053 | .807 | 93 | .051 | .808 | |||||||
| 0.6, 0.7 | 118 | .054 | .806 | 102 | .054 | .806 | 79 | .053 | .808 | |||||||
| 0.7, 0.8 | 95 | .054 | .808 | 81 | .055 | .808 | 60 | .054 | .809 | |||||||
|
| ||||||||||||||||
| (3,2,1) | 0.2, 0.3 | 80 | .048 | .808 | 75 | .048 | .807 | 73 | .049 | .803 | ||||||
| 0.3, 0.4 | 97 | .050 | .806 | 86 | .048 | .806 | 83 | .049 | .805 | |||||||
| 0.4, 0.5 | 104 | .051 | .811 | 86 | .051 | .806 | 80 | .048 | .806 | |||||||
| 0.5, 0.6 | 100 | .052 | .807 | 78 | .051 | .809 | 67 | .048 | .802 | |||||||
| 0.6, 0.7 | 88 | .053 | .807 | 63 | .052 | .807 | 49 | .048 | .803 | |||||||
| 0.7, 0.8 | 69 | .054 | .813 | 46 | .053 | .814 | 31 | .048 | .812 | |||||||
|
| ||||||||||||||||
| (3,2,2) | 0.2, 0.3 | 88 | .051 | .805 | 82 | .050 | .807 | 77 | .049 | .805 | ||||||
| 0.3, 0.4 | 112 | .052 | .809 | 101 | .051 | .808 | 91 | .050 | .807 | |||||||
| 0.4, 0.5 | 123 | .052 | .808 | 108 | .051 | .806 | 92 | .050 | .806 | |||||||
| 0.5, 0.6 | 123 | .052 | .807 | 105 | .052 | .809 | 84 | .050 | .806 | |||||||
| 0.6, 0.7 | 112 | .054 | .809 | 92 | .054 | .807 | 69 | .050 | .808 | |||||||
| 0.7, 0.8 | 90 | .054 | .809 | 72 | .055 | .809 | 50 | .053 | .810 | |||||||
|
| ||||||||||||||||
| (3,3,2) | 0.2, 0.3 | 84 | .050 | .807 | 78 | .051 | .808 | 74 | .048 | .804 | ||||||
| 0.3, 0.4 | 105 | .051 | .804 | 93 | .049 | .808 | 84 | .049 | .804 | |||||||
| 0.4, 0.5 | 115 | .052 | .809 | 97 | .051 | .808 | 83 | .049 | .807 | |||||||
| 0.5, 0.6 | 113 | .052 | .807 | 91 | .052 | .807 | 72 | .050 | .807 | |||||||
| 0.6, 0.7 | 101 | .054 | .806 | 78 | .054 | .809 | 56 | .050 | .808 | |||||||
| 0.7, 0.8 | 81 | .055 | .808 | 60 | .054 | .809 | 38 | .051 | .810 | |||||||
Table 4.
Sample sizes (n) were calculated from formula (4) under the contiguous alternative for the Weibull, gamma, log-logistic, log-normal, and Gompertz distributions with nominal type I error of 0.05 and power of 80%. The corresponding empirical type I errors and powers were estimated based on 100,000 simulation runs.
| Distribution | Design | n |
|
|
n |
|
|
n |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WB(λ, κ) | S0(2) vs S1(2) | κ = 0.5 | κ = 1 | κ = 2 | ||||||||||||
|
| ||||||||||||||||
| 0.2 vs 0.35 | 45 | .052 | .810 | 44 | .051 | .808 | 43 | .050 | .806 | |||||||
| 0.2 vs 0.4 | 27 | .052 | .815 | 26 | .052 | .812 | 26 | .050 | .815 | |||||||
| 0.3 vs 0.45 | 56 | .053 | .809 | 54 | .053 | .808 | 51 | .051 | .805 | |||||||
| 0.5 vs 0.65 | 60 | .054 | .806 | 57 | .054 | .812 | 50 | .052 | .812 | |||||||
| 0.6 vs 0.75 | 54 | .055 | .811 | 50 | .056 | .808 | 42 | .053 | .810 | |||||||
| 0.7 vs 0.8 | 104 | .055 | .805 | 95 | .054 | .807 | 77 | .055 | .807 | |||||||
|
| ||||||||||||||||
| GM(γ, k) | k = 0.5 | k = 1 | k = 2 | |||||||||||||
|
| ||||||||||||||||
| 0.2 vs 0.35 | 45 | .052 | .813 | 44 | .050 | .811 | 44 | .050 | .810 | |||||||
| 0.2 vs 0.4 | 27 | .053 | .819 | 26 | .052 | .811 | 26 | .050 | .813 | |||||||
| 0.3 vs 0.45 | 55 | .052 | .806 | 54 | .052 | .808 | 53 | .050 | .809 | |||||||
| 0.5 vs 0.65 | 59 | .055 | .806 | 57 | .055 | .813 | 53 | .054 | .807 | |||||||
| 0.6 vs 0.75 | 53 | .056 | .809 | 50 | .056 | .808 | 46 | .054 | .812 | |||||||
| 0.7 vs 0.8 | 103 | .056 | .807 | 95 | .056 | .806 | 85 | .055 | .810 | |||||||
|
| ||||||||||||||||
| LG(λ, p) | p = 0.5 | p = 1 | p = 2 | |||||||||||||
|
| ||||||||||||||||
| 0.2 vs 0.35 | 46 | .050 | .812 | 45 | .051 | .807 | 45 | .051 | .811 | |||||||
| 0.2 vs 0.4 | 27 | .052 | .809 | 27 | .052 | .815 | 27 | .051 | .818 | |||||||
| 0.3 vs 0.45 | 57 | .052 | .807 | 56 | .053 | .806 | 55 | .052 | .811 | |||||||
| 0.5 vs 0.65 | 62 | .054 | .809 | 59 | .056 | .808 | 55 | .053 | .809 | |||||||
| 0.6 vs 0.75 | 55 | .056 | .810 | 52 | .056 | .807 | 47 | .055 | .811 | |||||||
| 0.7 vs 0.8 | 106 | .054 | .809 | 99 | .055 | .806 | 86 | .055 | .809 | |||||||
|
| ||||||||||||||||
| LN(μ, σ) | σ = 2 | σ = 1 | σ = 0.5 | |||||||||||||
|
| ||||||||||||||||
| 0.2 vs 0.35 | 45 | .051 | .808 | 45 | .052 | .811 | 44 | .050 | .803 | |||||||
| 0.2 vs 0.4 | 27 | .052 | .815 | 27 | .051 | .818 | 26 | .050 | .807 | |||||||
| 0.3 vs 0.45 | 56 | .053 | .811 | 55 | .052 | .809 | 53 | .052 | .809 | |||||||
| 0.5 vs 0.65 | 60 | .054 | .807 | 57 | .054 | .811 | 51 | .051 | .808 | |||||||
| 0.6 vs 0.75 | 53 | .056 | .807 | 49 | .057 | .809 | 42 | .054 | .812 | |||||||
| 0.7 vs 0.8 | 102 | .057 | .807 | 91 | .054 | .809 | 73 | .055 | .807 | |||||||
|
| ||||||||||||||||
| GZ(θ, γ) | γ = 0.5 | γ = 1 | γ = 2 | |||||||||||||
|
| ||||||||||||||||
| 0.2 vs 0.35 | 43 | .050 | .807 | 43 | .048 | .809 | 44 | .049 | .808 | |||||||
| 0.2 vs 0.4 | 25 | .050 | .808 | 25 | .050 | .807 | 25 | .049 | .800 | |||||||
| 0.3 vs 0.45 | 51 | .050 | .806 | 50 | .051 | .805 | 50 | .049 | .806 | |||||||
| 0.5 vs 0.65 | 50 | .054 | .807 | 46 | .051 | .810 | 42 | .048 | .804 | |||||||
| 0.6 vs 0.75 | 43 | .054 | .812 | 37 | .053 | .809 | 32 | .049 | .809 | |||||||
| 0.7 vs 0.8 | 80 | .054 | .809 | 65 | .055 | .808 | 51 | .051 | .800 | |||||||
Footnote: abbreviation WB: Weibull; GM: gamma; LG: log-logistic; LN: log-normal; GZ: Gompetz
6 Example
Between January 1974 and May 1984, the Mayo Clinic conducted a double-blind randomized trial on treating primary biliary cirrhosis of the liver (PBC), comparing the drug D-penicillamine (DPCA) with a placebo (Fleming and Harrington, 1991). PBC is a rare but fatal chronic liver disease of unknown cause, with a prevalence of approximately 50 cases per million in the population. The primary pathologic event appears to be the destruction of the interlobular bile ducts, which may be mediated by immunologic mechanisms. Of 158 patients treated with DPCA, 65 died. The median survival time was 9 years. Suppose an experimental treatment is now available and investigators wish to design a new trial using the Mayo Clinic patients treated with DPCA as the historical data with which to formulate the hypothesis. The survival distribution of the DPCA data is estimated by a Kaplan-Meier curve, a spline version of the survival distribution, which is fitted by using the R function oldlogspline, and the Weibull distribution, which is fitted by using the R function survreg with the estimated shape parameter κ = 1.22 (Figure 1). Both the spline and Weibull distributions are fitted well and are close to the Kaplan-Meier curve. The 5-year survival probability estimate from the Kaplan-Meier curve is 71%. Thus, for the trial design, S0(5) = 71% is the 5-year survival probability at which investigators are no longer interested in the experimental treatment, and S1(5) = 82% is the 5-year survival probability at which investigators consider the experimental treatment to be promising. Then, the hazard ratio is δ = log(0.82) / log(0.71) = 0.58. To calculate the sample size, we assume a uniform accrual with an accrual period ta = 8 years and a follow-up period tf = 3 years, with no patient being lost to follow-up. Thus, given a type I error of α = 5% and power of 1−β = 80%, the required sample sizes calculated using the R function SIZE (Appendix 2) are 63, 63, and 63 under the Weibull, spline and Kaplan-Meier curve, respectively. With a power of 90%, the required sample sizes are 88, 87 and 88 under the Weibull, spline and Kaplan-Meier curve, respectively. Sample size calculations under the spline distribution and Kaplan-Meier curve make no assumption regarding the underlying survival distribution. Thus, this approach takes advantage of possible misspecification by using a parametric survival distribution for the trial design.
Figure 1.
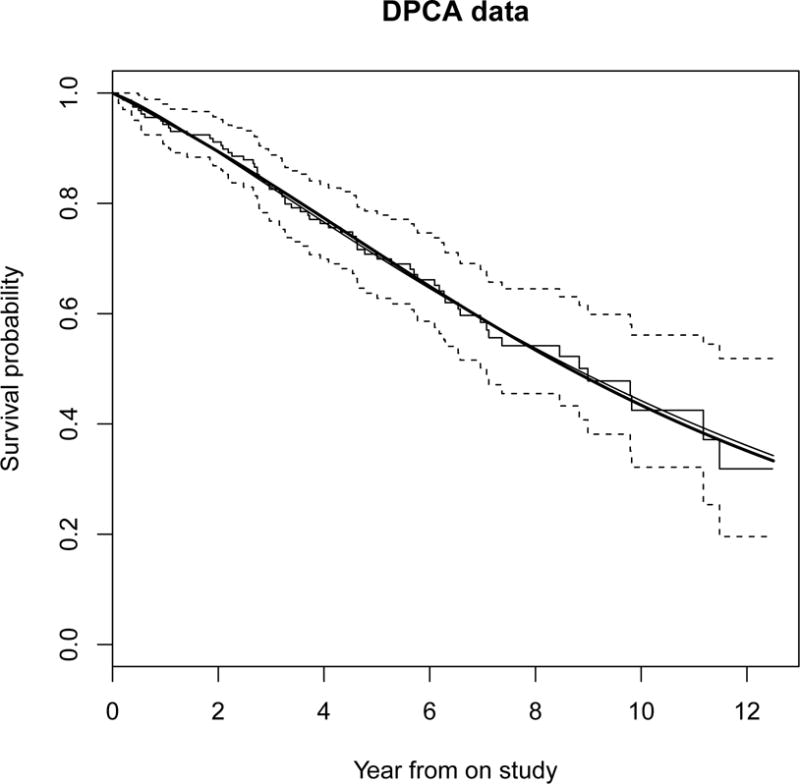
Step functions are the Kaplan-Meier survival curve and its 95% confidence boundaries. Solid and dark solid curves are the fitted Weibull and spline survival distributions, respectively.
7 Conclusion
In this paper, formulae for the number of events and sample size for a single-arm phase II survival trial are derived for the MOSLRT under the proportional hazards model. The new sample size formula is simple and easy to compute. The simulation results show that the proposed formula provides an accurate estimation of sample size for trial design. The sample size calculation using the new formula is extended to a class of flexible survival distributions, including a Kaplan-Meier curve or a spline version of the survival distribution, and has been implemented in the R function SIZE for trial design.
Table 5.
Sample sizes (n) were calculated from formula (5) under the fixed alternative for the Weibull, gamma, log-logistic, log-normal, and Gompertz distributions with nominal type I error of 0.05 and power of 80%. The empirical type I errors and powers were estimated based on 100,000 simulation runs.
| Distribution | Design | n |
|
|
n |
|
|
n |
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WB(λ, κ) | S0(2) vs S1(2) | κ = 0.5 | κ = 1 | κ = 2 | ||||||||||||
|
| ||||||||||||||||
| 0.2 vs 0.35 | 44 | .051 | .801 | 44 | .051 | .811 | 44 | .049 | .812 | |||||||
| 0.2 vs 0.4 | 26 | .051 | .802 | 26 | .051 | .811 | 26 | .052 | .818 | |||||||
| 0.3 vs 0.45 | 55 | .053 | .803 | 53 | .051 | .803 | 51 | .050 | .807 | |||||||
| 0.5 vs 0.65 | 58 | .054 | .795 | 55 | .052 | .799 | 49 | .053 | .805 | |||||||
| 0.6 vs 0.75 | 52 | .056 | .796 | 48 | .056 | .798 | 41 | .055 | .804 | |||||||
| 0.7 vs 0.8 | 100 | .055 | .793 | 91 | .055 | .794 | 75 | .055 | .798 | |||||||
|
| ||||||||||||||||
| GM(γ, k) | k = 0.5 | k = 1 | k = 2 | |||||||||||||
|
| ||||||||||||||||
| 0.2 vs 0.35 | 44 | .051 | .806 | 44 | .051 | .810 | 44 | .051 | .812 | |||||||
| 0.2 vs 0.4 | 26 | .051 | .808 | 26 | .051 | .812 | 26 | .051 | .813 | |||||||
| 0.3 vs 0.45 | 54 | .053 | .803 | 53 | .053 | .804 | 52 | .051 | .802 | |||||||
| 0.5 vs 0.65 | 57 | .054 | .795 | 55 | .054 | .798 | 52 | .054 | .800 | |||||||
| 0.6 vs 0.75 | 51 | .056 | .796 | 48 | .055 | .797 | 44 | .055 | .796 | |||||||
| 0.7 vs 0.8 | 98 | .055 | .792 | 91 | .056 | .790 | 82 | .054 | .795 | |||||||
|
| ||||||||||||||||
| LG(λ, p) | p = 0.5 | p = 1 | p = 2 | |||||||||||||
|
| ||||||||||||||||
| 0.2 vs 0.35 | 45 | .053 | .804 | 45 | .052 | .807 | 44 | .051 | .803 | |||||||
| 0.2 vs 0.4 | 27 | .053 | .808 | 27 | .053 | .813 | 26 | .052 | .805 | |||||||
| 0.3 vs 0.45 | 56 | .053 | .804 | 55 | .053 | .801 | 54 | .052 | .804 | |||||||
| 0.5 vs 0.65 | 60 | .056 | .799 | 57 | .054 | .795 | 54 | .054 | .804 | |||||||
| 0.6 vs 0.75 | 53 | .056 | .798 | 50 | .056 | .796 | 45 | .055 | .797 | |||||||
| 0.7 vs 0.8 | 101 | .055 | .791 | 95 | .055 | .795 | 83 | .054 | .797 | |||||||
|
| ||||||||||||||||
| LN(μ, σ) | σ = 2 | σ = 1 | σ = 0.5 | |||||||||||||
|
| ||||||||||||||||
| 0.2 vs 0.35 | 45 | .052 | .805 | 44 | .051 | .808 | 44 | .050 | .805 | |||||||
| 0.2 vs 0.4 | 27 | .053 | .813 | 26 | .051 | .807 | 26 | .051 | .809 | |||||||
| 0.3 vs 0.45 | 55 | .052 | .801 | 54 | .054 | .806 | 53 | .051 | .807 | |||||||
| 0.5 vs 0.65 | 58 | .055 | .798 | 55 | .053 | .800 | 50 | .052 | .803 | |||||||
| 0.6 vs 0.75 | 51 | .056 | .794 | 47 | .056 | .796 | 41 | .052 | .803 | |||||||
| 0.7 vs 0.8 | 98 | .057 | .793 | 88 | .055 | .798 | 72 | .054 | .803 | |||||||
|
| ||||||||||||||||
| GZ(θ, γ) | γ = 0.5 | γ = 1 | γ = 2 | |||||||||||||
|
| ||||||||||||||||
| 0.2 vs 0.35 | 43 | ,049 | .810 | 43 | .049 | .809 | 45 | .050 | .816 | |||||||
| 0.2 vs 0.4 | 26 | .049 | .818 | 26 | .050 | .821 | 27 | .049 | .824 | |||||||
| 0.3 vs 0.45 | 51 | .052 | .804 | 50 | .050 | .810 | 51 | .050 | .811 | |||||||
| 0.5 vs 0.65 | 50 | .052 | .808 | 46 | .052 | .811 | 44 | .049 | .820 | |||||||
| 0.6 vs 0.75 | 42 | .055 | .805 | 37 | .053 | .811 | 33 | .051 | .819 | |||||||
| 0.7 vs 0.8 | 77 | .054 | .797 | 64 | .053 | .802 | 53 | .050 | .814 | |||||||
Footnote: abbreviation WB: Weibull; GM: gamma; LG: log-logistic; LN: log-normal; GZ: Gompetz
Acknowledgments
The author acknowledges two anonymous reviewers and an editor for their valuable comments that improved an earlier version of the paper. The work was supported in part by the National Cancer Institute support grant P30CA021765 and ALSAC.
Appendix 1: Derivation of the asymptotic distribution for the MOSLRT
The methods used to derive the asymptotic distribution of the MOSLRT under the contiguous alternative are similar to the derivation of the two-sample log-rank test (Fleming and Harrington, 1991).
Consider a sequence of contiguous alternatives H1n : λ1n(t) = e−γ1nλ0(t), where γ1n is a sequence of positive constants satisfying n1/2γ1n = b < ∞. Then, the weighted one-sample log-rank score W is given by
where wn(t) is a weight function convergence to w(t) as n → ∞. If we further define a sequence of martingale by
and let
then we have
It is easy to show that
and
As
where π(t) = P(X ≥ t), we have where
where
Therefore,
By the martingale central limit theorem (Fleming and Harrington, 1991), W is approximate normal with mean −bV and variance V. When w(t) = 1, the variance V reduces to
Hence,
By the dominated convergence theorem, . Finally, by Slutsky’s theorem, it follows that
Appendix 2: R code for the sample size calculation under the Weibull distribution, spline distribution, and Kaplan-Meier curve
| library (survival) | ||||||||||||
| library (polspline) | ||||||||||||
| time=c | (1.10, | 12.33, | 2.77, | 5.27, | 6.58, | 9.82, | 0.36, | 11.59, | 1.84, | 11.18, | 10.78, | 0.61, |
| 6.29, | 12.24, | 3.70, | 12.48, | 6.18, | 7.12, | 6.54, | 2.74, | 3.93, | 3.73, | 8.99, | 12.22, | |
| 6.09, | 11.96, | 10.94, | 11.48, | 11.07, | 3.21, | 9.47, | 5.01, | 3.26, | 0.19, | 4.63, | 10.16, | |
| 6.96, | 9.79, | 11.10, | 4.54, | 0.54, | 4.77, | 7.37, | 1.06, | 10.72, | 2.05, | 10.55, | 10.47, | |
| 8.49, | 8.45, | 8.83, | 7.08, | 5.77, | 6.44, | 2.68, | 9.14, | 2.97, | 6.27, | 1.41, | 5.57, | |
| 9.03, | 2.66, | 8.41, | 2.26, | 2.84, | 8.87, | 8.07, | 8.63, | 8.49, | 8.19, | 3.55, | 8.38, | |
| 8.36, | 8.21, | 2.09, | 7.86, | 3.16, | 7.84, | 0.38, | 6.78, | 5.63, | 2.95, | 4.61, | 7.38, | |
| 7.05, | 7.28, | 7.24, | 4.09, | 7.07, | 7.00, | 7.00, | 6.92, | 4.32, | 6.39, | 6.86, | 6.69, | |
| 6.71, | 6.38, | 6.47, | 6.48, | 4.36, | 6.22, | 5.70, | 6.18, | 5.95, | 2.48, | 5.97, | 5.94, | |
| 5.95, | 3.38, | 0.92, | 5.33, | 5.54, | 2.74, | 0.95, | 5.35, | 5.29, | 5.23, | 5.16, | 1.90, | |
| 5.02, | 4.96, | 4.63, | 3.93, | 2.01, | 4.88, | 3.99, | 4.85, | 4.84, | 2.02, | 4.66, | 4.42, | |
| 4.66, | 4.42, | 0.49, | 3.26, | 4.30, | 4.18, | 3.96, | 3.70, | 4.06, | 3.87, | 0.11, | 3.84, | |
| 3.86, | 3.38, | 2.19, | 3.73, | 2.47, | 3.57, | 2.40, | 1.46, | 3.54, | 3.48, | 3.37, | 3.16, | |
| 2.57, | 2.30) | |||||||||||
| status=c | (1, | 0, | 1, | 1, | 1, | 1, | 1 | 0, | 1, | 1, | 0, | 1, | 1, | 0, | 1, | 0, | 1, | 1, | 1, | 1, | 1, | 1, | 1, | 0, | 1, | 0, | 0, | 1, |
| 0, | 1, | 0, | 1, | 1, | 1, | 1 | 0, | 1, | 1, | 0, | 1, | 1, | 1, | 1, | 1, | 0, | 1, | 0, | 0, | 0, | 1, | 1, | 1, | 1, | 0, | 1, | 0, | |
| 1, | 1, | 1, | 0, | 0, | 1, | 0 | 1, | 1, | 0, | 0, | 0, | 0, | 0, | 1, | 0, | 0, | 0, | 1, | 0, | 1, | 0, | 1, | 0, | 1, | 1, | 1, | 0, | |
| 0, | 0, | 0, | 1, | 0, | 0, | 0 | 0, | 1, | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 1, | 0, | 0, | 1, | 0, | 0, | 0, | 1, | 1, | 0, | |
| 0, | 1, | 1, | 0, | 0, | 0, | 0 | 1, | 0, | 0, | 1, | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 1, | 1, | 0, | 0, | 0, | 0, | |
| 0, | 0, | 1, | 0, | 0, | 0, | 1 | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 0, | 0) |
| dat=data.frame(time=time, status=status) | ||
| SIZE=function(delta, ta, tf, alpha, beta, data) | ||
| { | ||
| tau=tf+ta | ||
| z0=qnorm(1-alpha) | ||
| z1=qnorm(1-beta) | ||
| ######### fit KM curve ############# | ||
| surv=Surv(time, status) | ||
| fitKM<- survfit(surv ~ 1, data = dat) | ||
| p0<-c(1, summary(fitKM)$surv) | # KM survival probability ### | |
| t0<-c(0, summary(fitKM)$time) | # ordered failure times ### | |
| outKM<-data.frame(t0=t0,p0=p0) | ||
| KM<-function(t){ | ||
| t0=outKM$t0; p0=outKM$p0; k=length(t0) | ||
| if (t>=t0[k] || t<0) {ans<-0} | ||
| for (i in 1:(k-1)){ | ||
| if (t>=t0[i] & t<t0[i+1]) {S0=p0[i]}} | ||
| return(S0)} | ||
| ######### fit Weibull curve ########### | ||
| fitWB=survreg(formula=surv~1, dist=“weibull”) | ||
| scale=as.numeric(exp(fitWB$coeff)) | ||
| shape=1/fitWB$scale | ||
| WB=function(t){ | ||
| kappa=shape; lambda0=1/scaleˆkappa | ||
| S0 = exp(-lambda0*tˆkappa); return(S0)} | ||
| ######## fit spline curve ########## | ||
| fitSP=oldlogspline(time[status == 1], time[status == 0], lbound = 0) | ||
| SP=function(t) {S0=1-poldlogspline(t, fitSP); return(S0)} | ||
| ####### sample size calculation ##### | ||
| S0=function(t){WB(t)} | ||
| S1=function(t){WB(t)ˆdelta} | ||
| p0=1-integrate(S0, tf, tau)$value/ta | ||
| p1=1-integrate(S1, tf, tau)$value/ta | ||
| PWB=(p0+p1)/2 | ||
| S0=function(t){SP(t)} | ||
| S1=function(t){SP(t)ˆdelta} | ||
| p0=1-integrate(S0, tf, tau)$value/ta | ||
| p1=1-integrate(S1, tf, tau)$value/ta | ||
| PSP=(p0+p1)/2 | ||
| S0=function(t){KM(t)} | ||
| S1=function(t){KM(t)ˆdelta} | ||
| p0=1-(S0(tf)+4*S0(0.5*ta+tf)+S0(ta+tf))/6 | ||
| p1=1-(S1(tf)+4*S1(0.5*ta+tf)+S1(ta+tf))/6 | ||
| PKM=(p0+p1)/2 | ||
| d0=(z0+z1)ˆ2/log(delta)ˆ2 | # number of events formula (3) | |
| nWB=ceiling(d0/PWB) | # sample size formula (4) under Weibull model | |
| nSP=ceiling(d0/PSP) | # sample size formula (4) under spine curve | |
| nKM=ceiling(d0/PKM) | # sample size formula (4) under KM curve | |
| d=ceiling(d0) | ||
| ans=list(c(d=d, nWB=nWB, nSP=nSP, nKM=nKM)) | ||
| return(ans) | ||
| } | ||
| #### 80% power #### | ||
| SIZE(delta=0.58, ta=8, tf=3, alpha=0.05, beta=0.2, data=dat) | ||
| d nWB nSP nKM | ||
| 21 63 63 63 | ||
| #### 90% power #### | ||
| SIZE(delta=0.58, ta=8, tf=3, alpha=0.05, beta=0.1, data=dat) | ||
| d nWB nSP nKM | ||
| 29 88 87 88 | ||
References
- Anderson TML, Dickman PW, Eloranta S, Lambe M, Lambert PC. Estimating the loss in expectation of life due to cancer using flexible parametric survival models. Statistics in Medicine. 2013;32:5286–5300. doi: 10.1002/sim.5943. [DOI] [PubMed] [Google Scholar]
- Bantis LE, Tsimikas JV, Georgiou SD. Survival estimation through the cumulative hazard function with monotone natural cubic splines. Lifetime Data Analysis. 2012;18:364–396. doi: 10.1007/s10985-012-9218-4. [DOI] [PubMed] [Google Scholar]
- Barthel FMS, Babiker A, Royston P, Parmar MKB. Evaluation of sample size and power for multi-arm survival trials allowing for nonproportional hazards, loss to follow-up and crossover. Statistics in Medicine. 2006;25:2521–2542. doi: 10.1002/sim.2517. [DOI] [PubMed] [Google Scholar]
- Breslow NE. Analysis of survival data under the proportional hazards model. International Statistical Review. 1975;43:44–58. [Google Scholar]
- Finkelstein DM, Muzikansky A, Schoenfeld DA. Comparing survival of a sample to that of a standard population. Journal of National Cancer Institute. 2003;95:1434–1439. doi: 10.1093/jnci/djg052. [DOI] [PubMed] [Google Scholar]
- Fleming TR, Harrington DP. Counting processes and survival analysis. New York: John Wiley and Sons; 1991. [Google Scholar]
- George SL, Desu MM. Planning the size and duration of a clinical trial studying the time to some critical event. Journal of Chronic Diseases. 1977;27:15–24. doi: 10.1016/0021-9681(74)90004-6. (1977) [DOI] [PubMed] [Google Scholar]
- Kooperberg C, Stone CJ. Logspline density estimation for censored data. Journal of Computational and Graphical Statistics. 1992;1:301–328. [Google Scholar]
- Kwak M, Jung SH. Phase II clinical trials with time-to-event endpoints: Optimal two-stage designs with one-sample log-rank test. Statistics in Medicine. 2014;33:2004–2016. doi: 10.1002/sim.6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin DY, Yao Q, Ying ZL. A general theory on stochastic curtailment for censored survival data. Journal of the American Statistical Association. 1999;94:510–521. [Google Scholar]
- Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Controlled Clinical Trials. 1981;2:93–114. doi: 10.1016/0197-2456(81)90001-5. [DOI] [PubMed] [Google Scholar]
- Lakatos E. Sample size based on the log-rank statistic in complex clinical trials. Biometrics. 1988;44:229–241. [PubMed] [Google Scholar]
- Owzar K, Jung SH. Designing phase II trials in cancer with time-to-event endpoints (with discussion) Clinical Trials. 2008;5:209–221. doi: 10.1177/1740774508091748. [DOI] [PubMed] [Google Scholar]
- Rubenstein LV, Gail MH, Santner TJ. Planning the duration of a comparative clinical trial with loss to follow-up and a period of continued observation. Journal of Chronic Diseases. 1981;34:469–479. doi: 10.1016/0021-9681(81)90007-2. [DOI] [PubMed] [Google Scholar]
- Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics. 1983;39:499–503. [PubMed] [Google Scholar]
- Sun XQ, Peng P, Tu DS. Phase II cancer clinical trial with a one-sample log-rank test and its corrections based on the Edgeworth expansion. Contemporary Clinical Trials. 2011;32:108–113. doi: 10.1016/j.cct.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Woolson RF. Rank-tests and a one-sample log-rank test for comparing observed survival-data to a standard population. Biometrics. 1981;37:687–696. [Google Scholar]
- Wu J. Single-arm phase II cancer survival trial designs. Journal of Biopharmaceutical Statistics. 2015 doi: 10.1080/10543406.2015.1052494. [DOI] [PMC free article] [PubMed] [Google Scholar]


