Dear Editor,
Pan et al. (2016) presented evidence that the plant ubiquitin E3 ligase SP1 directs proteasome-mediated regulation of the peroxisomal protein import machinery. This was surprising, as SP1 was originally identified as a chloroplast outer envelope-localized regulator of the chloroplast protein import machinery (Ling et al., 2012; Ling and Jarvis, 2015), and there was nothing in the original datasets to suggest functions elsewhere in the cell. It was also difficult to understand how a key regulator of chloroplast protein import, which mediates nuanced and selective effects during development and under stress (Ling et al., 2012; Ling and Jarvis, 2015), could additionally operate in a second organelle with a very different protein import system (Léon et al., 2006). We sought to address these issues by conducting additional experimental analyses.
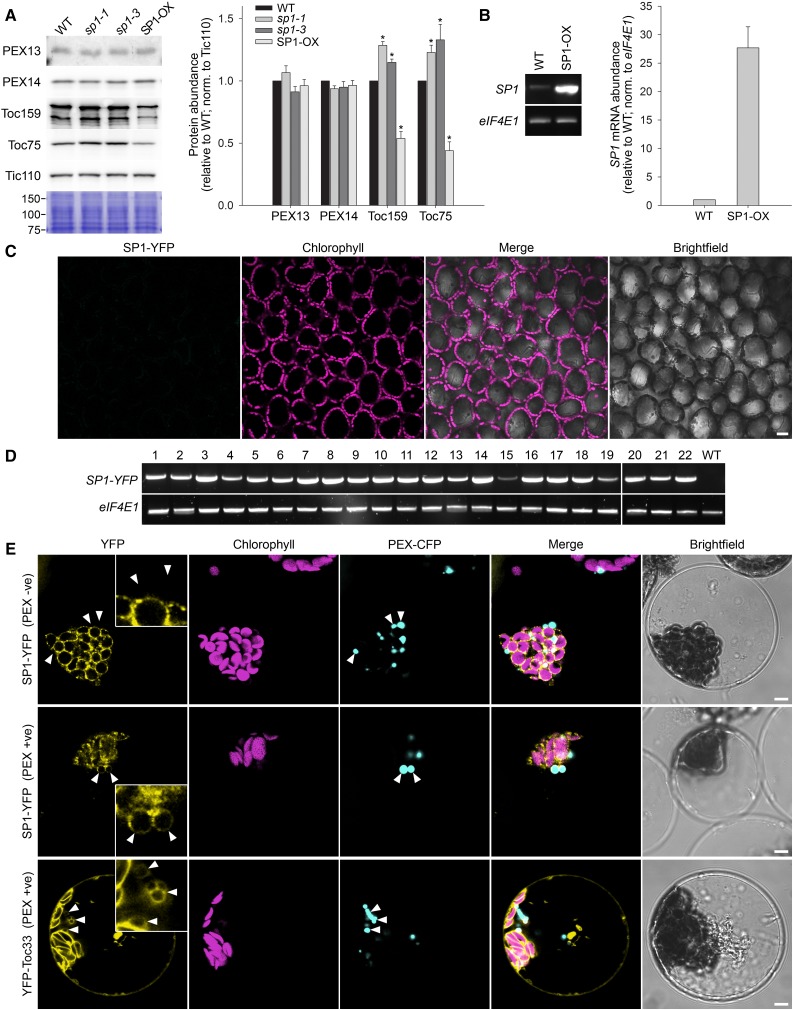
The veracity of the model proposed by Pan et al. (2016) depended heavily on qualitative immunoblot data showing opposing effects on the abundance of peroxisomal protein import apparatus components (PEX13 and PEX14) in sp1 mutant and SP1 overexpressor plants. This was because much of the other data presented in support of the model were either physiological measurements of a general nature that cannot be conclusively linked to peroxisomes (i.e. hypocotyl and root growth, which are both known to be influenced by chloroplast protein import; Huang et al., 2011) or analyses of fusion proteins with large tags transiently overexpressed in a heterologous system for which physiological relevance cannot be assured (Dixit et al., 2006; Tanz et al., 2013). Owing to the critical importance of the PEX protein abundance data, we sought to reproduce them in quantitative fashion using plants equivalent to those analyzed previously (Pan et al., 2016). However, in repeated experiments, we observed no significant changes in the levels of either PEX protein in response to SP1 manipulation, relative to the wild type (Fig. 1, A and B). In contrast, the levels of chloroplast TOC proteins, which are well-established targets of SP1, were significantly changed in both genotypes (Fig. 1, A and B), in line with previous observations (Ling et al., 2012; Ling and Jarvis, 2015); this clearly demonstrated the sensitivity of our assay conditions.
Figure 1.
Analysis of the proposed role of SP1 in peroxisomes. A and B, Analysis of the abundance of peroxisomal and chloroplast proteins in sp1 mutant and SP1 overexpressor (SP1-OX) plants. A, Total protein extracts from 9-d-old Arabidopsis (Arabidopsis thaliana) seedlings were analyzed by immunoblotting using previously described antibodies (Ling et al., 2012; Pan et al., 2016). A section of a Coomassie-stained gel covering several midintensity bands (75–150 kD) is shown below the blots to illustrate sample normalization. Specific immunoreactive bands derived from three or four biological replicates were quantified as described previously (Ling et al., 2012). Error bars indicate se (n = 3–4). Asterisks indicate statistically significant differences from the wild type, as determined using a Student’s t test (P < 0.05). B, Analysis of SP1 mRNA expression in the SP1-OX line used in A. Total RNA samples from 9-d-old seedlings were analyzed by reverse transcription PCR. Amplifications used a limited number of cycles (24 in each case) to avoid saturation, and products were analyzed by agarose gel electrophoresis. Bands were quantified as in A. Error bars indicate se (n = 3). The data confirmed that the SP1-OX line expresses SP1 at least as highly as the line employed by Pan et al. (2016). C and D, Analysis of transgenic plants expressing an SP1pro:SP1-YFP construct. The SP1 native promoter-driven SP1-YFP construct (SP1pro:SP1-YFP) employed by Pan et al. (2016) was recreated precisely and used to stably transform Arabidopsis plants. Leaves of more than 60 independent SP1pro:SP1-YFP transformants were screened by confocal microscopy, and none of them displayed detectable YFP fluorescence (data for a representative line are shown in C). Scale bar indicates 20 μm. All of the more than 60 lines were verified by genotyping as carrying the relevant construct (data not shown), while 22 lines selected at random were shown to express the corresponding mRNA by reverse transcription PCR (D); PCR amplification in D was nonsaturating and employed 27 (SP1-YFP) or 24 (eIF4E1) cycles. Transgenic plants expressing a constitutive 35S promoter-driven SP1-YFP construct also did not yield detectable fluorescence, nor did leaves that had received SP1pro:SP1-YFP construct by agrobacterial infiltration (Sparkes et al., 2006; Li et al., 2009; data not shown). E, Analysis of cells transiently overexpressing SP1 or Toc33 YFP fusions. Arabidopsis protoplasts from a transgenic line expressing peroxisome-targeted CFP (PEX-CFP; Arabidopsis Biological Resource Center stock no. CS16259) were transfected with 35S promoter-driven constructs encoding SP1-YFP or YFP-Toc33. Over three independent experiments, 75 cells showing clear SP1-YFP fluorescence (with PEX-CFP fluorescence that could be spatially differentiated from the chloroplast compartment) were analyzed by confocal microscopy, and of these most displayed YFP fluorescence that was clearly restricted to the chloroplast envelope (top row; PEX -ve). Nonetheless, six of the YFP-positive cells (8% of those analyzed) did show some apparently peroxisome-associated fluorescence (middle row; PEX +ve). Similarly, 90 cells showing YFP-Toc33 fluorescence were analyzed, and again most them displayed only chloroplast envelope fluorescence. However, as with SP1-YFP, a small proportion of cells (12 of those analyzed; approximately 13%) did display peroxisome-associated fluorescence (bottom row; PEX +ve). The positions of particularly informative peroxisomes are indicated with white arrowheads, and these are also shown in the insets in the YFP panels that show 3-fold higher magnification. Scale bars indicate 5 μm in the main panels. WT, Wild type.
Next, we reassessed the subcellular localization of SP1. We began by analyzing an SP1-YFP fusion by confocal microscopy in stably transformed Arabidopsis plants and in leaves transiently transformed by agrobacterial infiltration. However, YFP signals were not detectable in these experiments (Fig. 1, C and D; data not shown), so we concluded that SP1(-YFP) protein abundance is suppressed by stringent (auto)regulation, as is typical for ubiquitin E3 ligases. Our inability to detect YFP signals akin to those described by Pan et al. (2016) suggested that the cells in the previous study may have overaccumulated SP1-YFP to unusually high levels for some reason.
To aid detection of SP1-YFP, we next used transient protoplast transfection, which achieves overexpression by greatly increasing gene copy-number per cell. Over three independent experiments, we analyzed 75 cells displaying clear SP1-YFP fluorescence. Of these, the majority displayed fluorescence that was obviously restricted to the chloroplast envelope (Fig. 1E, top row). Nonetheless, approximately 8% of cells (six in total) did show a degree of extrachloroplastic fluorescence, some of which appeared to be associated with the peroxisomes (Fig. 1E, middle row). It is well documented that the overaccumulation of a protein can force its mislocalization (Dixit et al., 2006; Tanz et al., 2013). Moreover, cells possess quality control systems to eliminate mistargeted proteins, suggesting that minor mislocalization may not be an uncommon event even under native conditions (Chen et al., 2014). Thus, while the possibility that more extensive SP1-peroxisome association occurs in other cell types could not be excluded, we suspected that the rare peroxisomal signals detected in mesophyll protoplasts were the result of mislocalization caused by overexpression.
To assess whether the tendency of SP1-YFP to occasionally associate with peroxisomes in transfected protoplasts is a unique feature of SP1, or a more general phenomenon linked to overexpression, we similarly analyzed protoplasts expressing a YFP-Toc33 fusion. Over more than 2 decades of research, Toc33 has been clearly established as a core component of the chloroplast protein import machinery with no suggested functions elsewhere in the cell (Paila et al., 2015). Most YFP-Toc33-expressing cells showed only chloroplast envelope fluorescence, but, as with SP1-YFP, a small proportion of cells (approximately 13% of those analyzed) unexpectedly displayed peroxisomal signals (Fig. 1E, bottom row). Thus, we concluded that the microscopy data linking SP1 to peroxisomes (Pan et al., 2016) are of questionable physiological relevance. Pan et al. (2016) also used subfractionation to support their contention that SP1 is in peroxisomes, but these data too are equivocal owing to their reliance on an overexpressed, tagged protein for detection, and the fact the protocol used is described by its authors as being unable to yield pure peroxisome fractions devoid of chloroplast contamination (Reumann and Singhal, 2014; Reumann and Lisik, 2017).
In summary, in view of the uncertainties concerning the proposed localization of SP1 in peroxisomes, the significant caveats associated with physiological measurements of a general nature and with analyses of transiently overexpressed proteins with large tags, and, most importantly, the irreproducibility of the critically important PEX protein abundance data, we conclude that the proposal by Pan et al. (2016) that SP1 additionally acts in peroxisomal protein import regulation is unproven.
ACKNOWLEDGMENT
We thank Professor Bonnie Bartel for the gift of the PEX13 antibody.
Footnotes
This work was supported by the Biotechnology and Biological Sciences Research Council (grant no. BB/N006372/1).
[CC-BY]: Article free via Creative Commons CC-BY 4.0 license.
References
- Chen YC, Umanah GK, Dephoure N, Andrabi SA, Gygi SP, Dawson TM, Dawson VL, Rutter J (2014) Msp1/ATAD1 maintains mitochondrial function by facilitating the degradation of mislocalized tail-anchored proteins. EMBO J 33: 1548–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit R, Cyr R, Gilroy S (2006) Using intrinsically fluorescent proteins for plant cell imaging. Plant J 45: 599–615 [DOI] [PubMed] [Google Scholar]
- Huang W, Ling Q, Bédard J, Lilley K, Jarvis P (2011) In vivo analyses of the roles of essential Omp85-related proteins in the chloroplast outer envelope membrane. Plant Physiol 157: 147–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon S, Goodman JM, Subramani S (2006) Uniqueness of the mechanism of protein import into the peroxisome matrix: transport of folded, co-factor-bound and oligomeric proteins by shuttling receptors. Biochim Biophys Acta 1763: 1552–1564 [DOI] [PubMed] [Google Scholar]
- Li JF, Park E, von Arnim AG, Nebenführ A (2009) The FAST technique: a simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Methods 5: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Q, Huang W, Baldwin A, Jarvis P (2012) Chloroplast biogenesis is regulated by direct action of the ubiquitin-proteasome system. Science 338: 655–659 [DOI] [PubMed] [Google Scholar]
- Ling Q, Jarvis P (2015) Regulation of chloroplast protein import by the ubiquitin E3 ligase SP1 is important for stress tolerance in plants. Curr Biol 25: 2527–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paila YD, Richardson LGL, Schnell DJ (2015) New insights into the mechanism of chloroplast protein import and its integration with protein quality control, organelle biogenesis and development. J Mol Biol 427: 1038–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan R, Satkovich J, Hu J (2016) E3 ubiquitin ligase SP1 regulates peroxisome biogenesis in Arabidopsis. Proc Natl Acad Sci USA 113: E7307–E7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S, Lisik P (2017) Isolation of Arabidopsis leaf peroxisomes and the peroxisomal membrane. Methods Mol Biol 1511: 97–112 [DOI] [PubMed] [Google Scholar]
- Reumann S, Singhal R (2014) Isolation of leaf peroxisomes from Arabidopsis for organelle proteome analyses. Methods Mol Biol 1072: 541–552 [DOI] [PubMed] [Google Scholar]
- Sparkes IA, Runions J, Kearns A, Hawes C (2006) Rapid, transient expression of fluorescent fusion proteins in tobacco plants and generation of stably transformed plants. Nat Protoc 1: 2019–2025 [DOI] [PubMed] [Google Scholar]
- Tanz SK, Castleden I, Small ID, Millar AH (2013) Fluorescent protein tagging as a tool to define the subcellular distribution of proteins in plants. Front Plant Sci 4: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]