Fine-scale characterization of light absorption and photosynthesis across plant tissue sections shows that quantum yields of PSII are highly affected by tissue light gradients.
Abstract
In vivo variable chlorophyll fluorescence measurements of photosystem II (PSII) quantum yields in optically dense systems are complicated by steep tissue light gradients due to scattering and absorption. Consequently, externally measured effective PSII quantum yields may be composed of signals derived from cells differentially exposed to actinic light, where cells located deeper inside tissues receive lower irradiance than cells closer to the surface and can display distinct photophysiological status. We demonstrate how measured distributions of PSII quantum yields in plant tissue change under natural tissue light gradients as compared with conventionally measured quantum yields with even exposure to actinic light. This was achieved by applying actinic irradiance perpendicular to one side of thallus cross sections of the aquatic macrophyte Fucus vesiculosus with laser light sheets of defined spectral composition, while imaging variable chlorophyll fluorescence from cross sections with a microscope-mounted pulse amplitude-modulated imaging system. We show that quantum yields are highly affected by light gradients and that traditional surface-based variable chlorophyll fluorescence measurements result in substantial underestimations and/or overestimations, depending on incident actinic irradiance. We present a method for using chlorophyll fluorescence profiles in combination with integrating sphere measurements of reflectance and transmittance to calculate depth-resolved photon absorption profiles, which can be used to correct apparent PSII electron transport rates to photons absorbed by PSII. Absorption profiles of the investigated aquatic macrophyte were different in shape from what is typically observed in terrestrial leaves, and based on this finding, we discuss strategies for optimizing photon absorption via modulation of the structural organization of phytoelements according to in situ light environments.
Estimating photosynthetic parameters using variable chlorophyll fluorescence techniques has become increasingly popular due to its ease of use and noninvasive nature. The basic fluorescence signals of open and closed reaction centers change according to actinic irradiance and are powerful monitors of the status and activity of the photosynthetic apparatus (Baker, 2008). Most measurements of variable chlorophyll fluorescence in complex plant tissues, and in other surface-associated cell assemblages like biofilms and sediments, rely on external measurements with fiber-optic or imaging fluorimeters under the assumptions that (1) different cells are subjected to the same amount of measuring light and actinic irradiance, (2) saturating pulses are indeed saturating all cells, and (3) the fluorescence detected is emitted equally from all sampled cells (Serodio, 2004). These assumptions are influenced by the optical density of the sample, where optical dilute refers to a negligible or only moderate light attenuation through a sample (e.g. a dilute algal suspension or plant tissue with only a few cell layers), while optically dense samples such as algal biofilms and thicker plant tissues absorb all, or most, of the incident light. As a result, the assumptions are usually valid in optically dilute samples (Klughammer and Schreiber, 2015), whereas steep light gradients in densely pigmented tissues or algal biofilms will distort the measurements of maximal and effective PSII quantum yields. Cells located deeper inside tissues will receive less actinic irradiance than cells close to the surface. Thus, externally integrated measurements of variable chlorophyll fluorescence contain a complex mixture of signals originating from different layers in the structure exposed to different levels of measuring and actinic light, and the actual operational depth of such measurements remains unknown. This inherent limitation of such measurements can lead to light-dependent overestimations of effective PSII quantum yields of up to 40% (e.g. in microphytobenthic assemblages; Serodio, 2004).
Previous efforts to describe the internal gradients of photosynthetic efficiencies have used microfiber-based pulse amplitude modulation (PAM) techniques (Schreiber et al., 1996), revealing distinct differences between such internal and external variable chlorophyll fluorescence measurements (Oguchi et al., 2011). Another challenge is to quantify the internal light gradients to estimate the total actinic light exposure in different tissue layers (i.e. the scalar irradiance). The scalar component becomes increasingly important in deeper tissue layers as light becomes progressively more diffuse due to multiple scattering (Kühl and Jørgensen, 1994). This can be measured with fiber-optic scalar irradiance microprobes (Kühl, 2005; Rickelt et al., 2016), which collect light isotropically via a small (30–150 µm wide) spherical tip cast on the end of a tapered optical fiber. Such measurements enabled estimates of internal rates of PSII electron transport corrected for the specific tissue light gradients in corals and plants (Lichtenberg and Kühl, 2015; Lichtenberg et al., 2016). However, to obtain absolute electron transport rates (ETRs) through PSII, it is necessary to know the absorption factor, which describes the PSII absorption cross section and the balance between PSI and PSII photochemistry, and these parameters cannot be calculated from measurements of light availability. In addition, due to the small tip size of fiber-optic radiance microprobes (usually less than 50 µm) used to detect the fluorescence, microfiber-based measurements of variable chlorophyll fluorescence also are prone to reflect the natural heterogeneity of such systems (Lichtenberg and Kühl, 2015; Lichtenberg et al., 2016). A method was recently proposed for calculating absolute electron turnover rates of PSII, but the approach was limited to surface measurements or optically thin systems (Szabó et al., 2014). It is thus of great importance to further explore how steep gradients of light influence photosynthetic efficiencies in complex photosynthetic tissues and surface-associated phototrophic communities.
Internal gradients of light absorption have been quantified from fluorescence profiles in terrestrial leaves (Takahashi et al., 1994; Vogelmann and Han, 2000; Slattery et al., 2016), and this technique has been combined with fine-scale measurements of CO2 fixation to investigate the relationship between chlorophyll concentration, light absorption, and photosynthesis at high spatial resolution (Vogelmann and Evans, 2002; Evans and Vogelmann, 2003). These studies generally found a good correlation between the light absorption of different spectral ranges and the associated CO2 fixation profiles. However, the CO2 fixation rates relied on freeze clamping 14CO2-preincubated leaf samples with concomitant paradermal sectioning and measurements by scintillation counting, which is a laborious process that is limited in the spatial resolution by the sectioning process to ∼40 µm (Vogelmann and Evans, 2002). Here, we present a novel experimental approach and show its application for mapping gradients of light absorption and photosynthesis in aquatic plant tissue.
The lower community photosynthesis often observed in aquatic systems as compared with terrestrial systems (Sand-Jensen and Krause-Jensen, 1997) can be largely explained by the inability of aquatic macrophytes to obtain an optimal 3D structural organization in relation to the incident irradiance (Binzer and Sand-Jensen, 2002a, 2002b), unlike their terrestrial counterparts, which can regulate leaf inclination to increase canopy light utilization (McMillen and McClendon, 1979; Myers et al., 1997). In addition, specialized cell/tissue structures in terrestrial plants can increase photon absorption such as in sun-adapted leaves with well-developed palisade cells that can act as light funnels directing light into the photosynthetically active mesophyll layer (Vogelmann and Martin, 1993), while some shade-adapted understory plants can alleviate light limitation by focusing light in the mesophyll layer via planoconvex epidermal cells and intercellular air spaces (Vogelmann et al., 1996; Brodersen and Vogelmann, 2007). In contrast, most macroalgae are not recognized to have specialized tissue structures to facilitate the penetration of light, although there have been reports of light guides in some green algae (Ramus, 1978).
Macroalgal members of the Fucales have morphologically differentiated tissues such as the basal thallus, the growing sterile frond, and fertile receptacles, while cells are differentiated into meristoderm, cortex, and medullary layers on the tissue scale (Garbary and Kim, 2005). While all cell types contain plastids (Moss, 1983), the outer meristoderm and cortex cells contain more chloroplasts and thylakoids than the medullary filaments. It has been suggested that the medullary filaments could play a role in the longitudinal translocation of materials (Moss, 1983; Raven, 2003) and, further, that they may play a structural role in providing elasticity in terms of a cushion-like effect protecting against wave action (Moss, 1983). The medulla layer is surrounded on both sides by anatomically similar layers of cortex, meristoderm, and epidermis cells (henceforth referred to as the cortex), in contrast to bifacial terrestrial plant leaves that display morphologically and physiologically differentiated abaxial and adaxial domains. In Fucus spp., steep gradients of light and photosynthesis have been measured using fiber-optic microprobes and microelectrodes, although this approach is rather challenging in such cohesive tissues (Spilling et al., 2010; Lichtenberg and Kühl, 2015).
In this study, we aimed to resolve how photosynthetic efficiencies are affected by steep light gradients in different spectral regions. This was accomplished by the use of a novel multicolor laser light sheet microscopy setup to image the distribution of light absorption and photosynthetic activity over transverse sections of an aquatic macrophyte to resolve how photosynthetic efficiencies are affected by steep light gradients in different spectral regions. We applied laser light sheets of defined spectral composition perpendicular to one side of thallus cross sections while imaging the distribution of chlorophyll fluorescence and variable chlorophyll fluorescence from the cut surface. We compared such data with measurements obtained with equal illumination of the cross section to describe, to our knowledge for the first time, how PSII quantum yields are affected by natural light gradients in optically dense tissues. This novel method can resolve such gradients routinely and with higher resolution as compared with other microscale approaches such as mapping with fiber-optic probes (Kühl and Jørgensen, 1994; Lichtenberg et al., 2016).
RESULTS
Cross-Thallus Chlorophyll Fluorescence Profiles
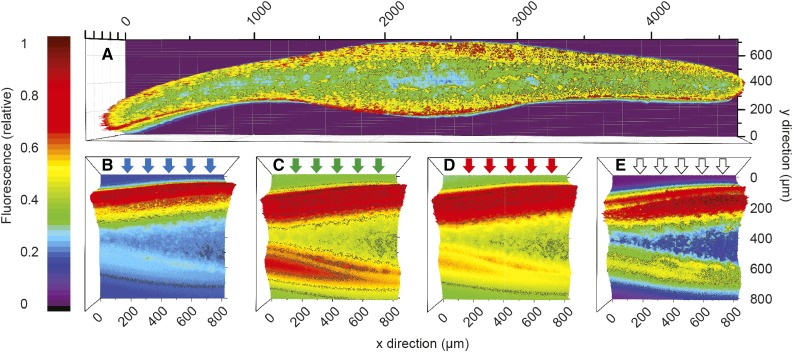
Using a novel microscopic setup (Fig. 1; for details, see “Materials and Methods”), we used both even illumination of plant tissue cross sections and illumination with laser sheets of defined spectral composition incident perpendicularly on tissue cross sections. When illuminated homogenously across the algal thallus cross section, both cortex layers of Fucus vesiculosus displayed equally high amounts of chlorophyll that were 2.5- to 5-fold higher than in the central medulla (Figs. 2 and 3), assuming that relative chlorophyll content can be estimated from fluorescence using epiillumination (Vogelmann and Evans, 2002). The fluorescence profiles under light sheet illumination perpendicular to one side of the cross section showed that blue light (425–475 nm) was attenuated most strongly in an exponential manner with depth and decreased to less than 21% of the maximum fluorescence (Fmax) ∼250 µm inside the thallus (Fig. 3). Fluorescence profiles over the thallus cross section using green (525–575 nm) and red (615–665 nm) light showed similar attenuation but decreased to a minimum fluorescence more than 2 times higher than was found for blue light at a similar depth in the thallus. Blue, green, and red light-induced fluorescence profiles all displayed Fmax values close to the thallus surface. When using broadband white light illumination, a peak was located at the same position as the Fmax of the blue, green, and red profiles followed by an intermittent decrease before reaching Fmax ∼100 µm inside the thallus (Fig. 3). Common for all profiles was that the fluorescence showed a peak close to the illuminated cortex followed by a decrease toward the center of the medulla before increasing again toward the shaded cortex. The relative largest increase toward the shaded thallus side was in the order blue < red < < green < white. The width of the peaks was of similar size and extended 150 to 200 µm from the surfaces toward the center of the thallus (Fig. 3).
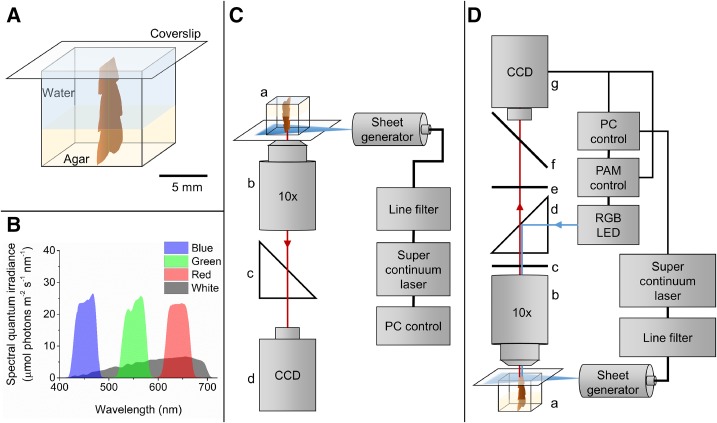
Figure 1.
Experimental setup for light sheet microscopy in combination with variable chlorophyll fluorescence imaging. A, The sample holder consisted of a cuvette cut down to 11-mm height (internal dimensions of 10 × 10 × 10 mm). The sample was mounted in agar in the bottom of the sample holder, which was filled with seawater and then closed with a coverslip. B, Spectral composition of the laser light used for measurements of chlorophyll fluorescence profiles and the actinic light for measurements of variable chlorophyll fluorescence. The laser was adjusted to have the same absolute photon irradiance independent of spectral composition. C, Schematic drawing of the experimental setup for measuring chlorophyll fluorescence profiles. a, The algal thallus sample positioned in the cuvette; b, microscope objective; c, filter cube with long-pass filter; and d, CCD camera. Illumination of the sample was done with a personal computer (PC)-controlled supercontinuum laser connected to a spectral line filter unit and a laser sheet generator. D, Schematic drawing of the experimental setup for variable chlorophyll fluorescence microscopy. a, Sample fixed in the cuvette; b, microscope objective; c, emission filter; d, dichroic beam splitter cube; e, dichroic filter; f, mirror (to ocular); and g, CCD camera. Weak nonactinic modulated measuring light was provided by a software-controlled red-green-blue (RGB) LED unit. Actinic light was provided perpendicular to one side of the thallus surface by a personal computer-controlled supercontinuum laser connected to a spectral line filter unit and a laser sheet generator.
Figure 2.
Color-coded maps showing the distribution of chlorophyll a fluorescence (normalized to maximum fluorescence) of an F. vesiculosus thallus cross section illuminated evenly over the cut side with 430-nm light from a xenon lamp (the plot is composed of multiple images taken through a 10× objective and stitched together using Adobe Photoshop; A) and thallus cross sections illuminated perpendicular to one side of the thallus surface with a supercontinuum laser sheet of different spectral compositions of blue light (425–475 nm; B), green light (525–575 nm; C), red light (615–665 nm; D), and white light (400–700 nm; E).
Figure 3.
A and B, Epifluorescence microscopy image (false colors) of an F. vesiculosus thallus cross section illuminated evenly with blue light (430 nm) from a xenon lamp (A) and the associated fluorescence profile (normalized to maximum fluorescence; B). C and D, Example of a fluorescence image (false colors) of a thallus fragment irradiated perpendicularly to one side of the thallus surface (arrows) with a laser sheet of red light (615–665 nm) from a supercontinuum laser (C) and chlorophyll fluorescence profiles of cross sections of apical thallus fragments of F. vesiculosus irradiated perpendicular to the thallus surface with different spectral bands of blue light (425–475 nm), green light (525–575 nm), red light (615–665 nm), and white light (400–700 nm; D). Data were normalized to maximum fluorescence, and actual data points are spaced 0.8 µm apart. Error bars are not shown for clarity, but mean relative sd was ±7.7% (n = 5).
Absorption Profiles
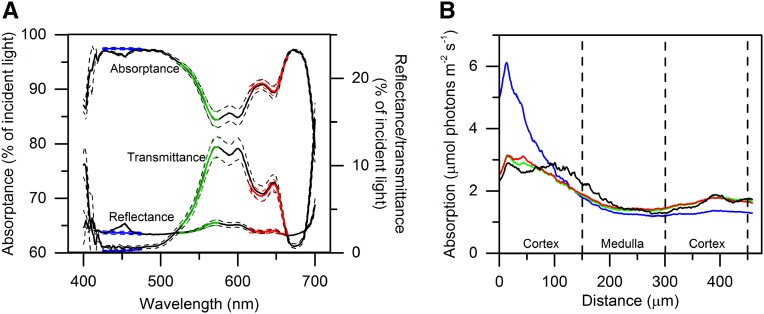
Integrating sphere measurements of thallus reflectance, transmittance, and absorptance displayed typical characteristics for densely pigmented opaque plant tissues (Fig. 4). Reflectance was relatively uniform at ∼3% of the incident irradiance, although slightly higher in the green/yellow part of the spectrum (around 570 nm). Absorptance spectra (Fig. 4; Supplemental Fig. S1) showed in vivo absorption peaks from major photopigments present in brown macroalgae, such as chlorophyll a (440 and 675 nm; Johnsen et al., 1994) and chlorophyll c (460, 590, and 635 nm; Shibata and Haxo, 1969; Kühl et al., 1995), fucoxanthin (in vitro absorption peaks in hexane at 425, 450, and 475 nm and extending to 580 nm in vivo; Govindjee and Braun, 1974), and other carotenoids (400–540 nm; Govindjee and Braun, 1974). The mean absorptance averaged over photosynthetically active radiation (PAR; 400–700 nm) using broadband white light was 92% of the incident irradiance. Transmittance was highest (10%–13%) in the green/yellow part (around 570 nm) of the spectrum and was close to zero in the blue and red spectral regions, while the mean transmittance was 5% of the incident irradiance (Fig. 4).
Figure 4.
A, Spectral measurements of reflectance, transmittance, and absorptance of an F. vesiculosus thallus fragment using an integrating sphere (Supplemental Fig. S1). Data were recorded using either incident blue laser light (425–475 nm; blue lines), green laser light (525–575 nm; green lines), red laser light (615–665 nm; red lines), or white laser light (400–700 nm; black lines). Dashed lines indicate ±1 sd (n = 3). B, Calculated absorption profiles of cross sections of apical thallus fragments of F. vesiculosus irradiated perpendicular to one side of the thallus surface with different spectral bands of blue laser light (425–475 nm), green laser light (525–575 nm), red laser light (615–665 nm), and white laser light (400–700 nm). Absorption was calculated from the measured chlorophyll fluorescence profiles (Fig. 3D) and was normalized to the bulk absorption measured with an integrating sphere (A). Dashed lines indicate the borders of the cortex and medulla tissue layers. Data points are spaced 0.8 µm apart (n = 5).
By normalizing the chlorophyll fluorescence profiles (Fig. 3) to the total absorption measured for blue, green, red, and white light with an integrating sphere (Fig. 4), we could calculate the depth of specific photon absorption inside the thallus (Fig. 4; Supplemental Fig. S3). The different thallus regions (cortex/medulla) were estimated to be, on average, 150 µm in thickness (Fig. 3). When illuminating the thallus with the laser sheet, the apparent absorption of photons was always highest in the upper and lower cortex as compared with the medulla, where the fractional absorption was lowest (Fig. 4; Table I).
Table I. Photosynthetic ETR from PSII versus photon absorption curve parameters and the fractional photon absorption (Abs; in percentage of total absorption) calculated for the upper cortex, medulla, and lower cortex under blue (425–475 nm), green (525–575 nm), red (615–665 nm), and white (400–700 nm) irradiance applied perpendicular to one side of the thallus surface of F. vesiculosus.
Slopes on the subsaturated part of the ETR versus light curve, maximum ETR values (ETRmax), and the light acclimation index Ek were estimated from curve fitting of the ETR versus photon absorption curves with an exponential function (Webb et al., 1974) using a nonlinear Levenberg-Marquardt fitting algorithm. The RGB values were calculated as average curves of blue, green, and red. Pairwise superscript letters indicate statistically significant differences (one-way ANOVA, P < 0.01; n = 5 for white and n = 4 for RGB).
| Light | Upper Cortex |
Medulla |
Lower Cortex |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | ETRmax | Ek | Abs | Slope | ETRmax | Ek | Abs | Slope | ETRmax | Ek | Abs | |
| % | % | % | ||||||||||
| Red | 0.60 | 197.09 | 327.23 | 44.0 | 0.59 | 80.43 | 135.82 | 26.0 | 0.60 | 192.68 | 319.05 | 30.0 |
| Green | 0.47 | 225.31 | 482.46 | 44.0 | 0.45 | 81.33 | 180.71 | 26.0 | 0.47 | 228.90 | 489.60 | 30.0 |
| Blue | 0.54 | 878.52 | 1,620.05 | 57.0 | 0.54 | 254.76 | 469.67 | 21.0 | 0.55 | 599.82 | 1,082.76 | 22.0 |
| White | 0.60a | 378.40 | 629.79 | 45.0 | 0.58b | 125.48 | 216.22 | 26.0 | 0.60c | 349.31 | 586.39 | 29.0 |
| RGB | 0.54a | – | – | – | 0.53b | – | – | – | 0.54c | – | – | – |
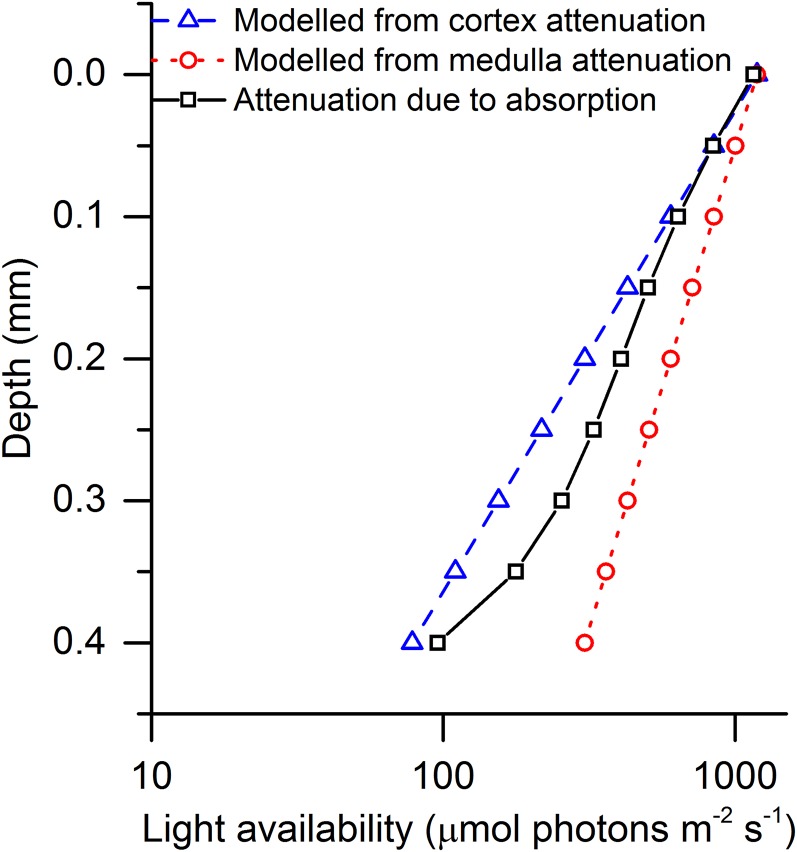
We modeled the light availability in the F. vesiculosus thallus by using measured scalar irradiance attenuation coefficients of cortex and medulla layers from the closely related brown alga Fucus serratus (Lichtenberg and Kühl, 2015), assuming monoexponential attenuation of light in the thallus (Fig. 5; see “Materials and Methods”). These modeled curves of light attenuation were compared with the curves of attenuation due to absorption found in this study (Fig. 5) to test if the found absorption profiles were in the same order as the attenuation profiles, as would be expected for such densely pigmented systems. We found an average light attenuation coefficient of 5.64 mm−1 (R2 = 0.97) over the entire thallus, with higher attenuation coefficients in the cortex layers (upper cortex = 6 mm−1 [R2 = 0.99] and lower cortex = 9.8 mm−1 [R2 = 0.96]) than in the medulla layer (4.3 mm−1 [R2 = 0.99]; Fig. 5). These values were in the same order as the light attenuation coefficients of cortex and medulla layers in F. serratus (6.8 and 3.4 mm−1 for cortex and medulla, respectively; Lichtenberg and Kühl, 2015), suggesting that the distribution of photon absorption can be found by a combination of chlorophyll fluorescence profiles and measurements of total absorption.
Figure 5.
Plots of scalar irradiance attenuation profiles of blue light (420–520 nm) calculated using attenuation coefficients from the cortex (blue triangles) and medulla (red circles) layers (Lichtenberg and Kühl, 2015), and a profile of the attenuation due to the absorption of blue light (425–475 nm) estimated from the observed fluorescence profile (black squares).
PSII Quantum Yields and Photosynthetic Electron Transport
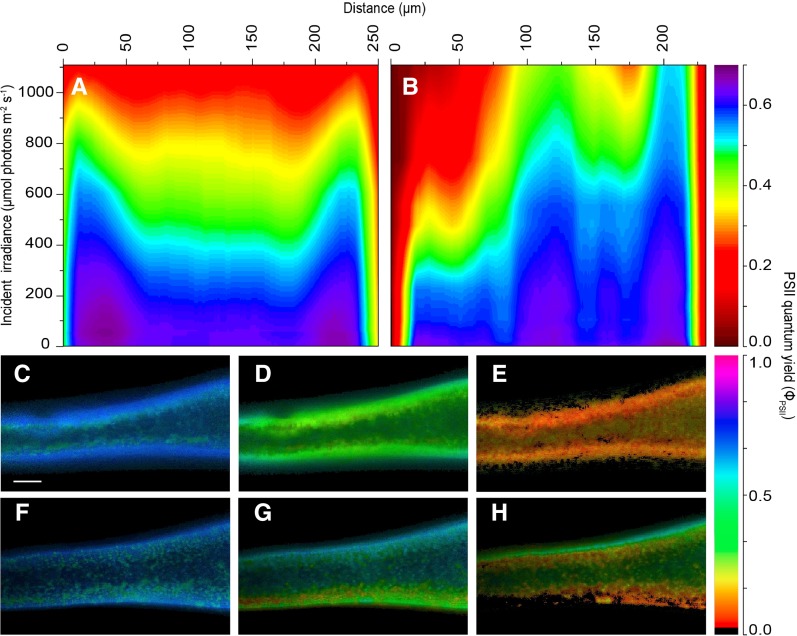
In the dark-acclimated state, all thallus layers displayed a maximal PSII quantum yield of greater than 0.6, indicating no major stress factor on photosynthetic performance due to cutting or sample handling (Supplemental Fig. S4). When applying actinic irradiance homogenously over the cross sections via the built-in LEDs of the microscope PAM system (Fig. 1), the effective PSII quantum yield decreased in all thallus layers but more so in the medulla as compared with the cortex layers. The highest decrease was found under high incident irradiance, where the effective PSII quantum yield in the medulla decreased to less than 0.3 (Fig. 6). This pattern changed under actinic laser sheet illumination of the cross section from one side. While the PSII quantum yield distribution was apparently unaffected by the changed actinic light geometry in the dark-acclimated state and under very low irradiance, the PSII quantum yield decreased rapidly over the illuminated cortex and reached levels of less than 0.1 under the highest irradiance. Due to the strong light attenuation across the thallus, the PSII quantum yields in the medulla and the shaded cortex layers decreased less than when illuminated homogenously via the imaging PAM actinic light source, and the effective PSII quantum yield in the shaded cortex remained at levels similar to those in dark-acclimated states (greater than 0.6) even at the highest irradiance (Fig. 6).
Figure 6.
Isopleths (A and B) and images (C–H) of effective PSII quantum yield in apical thallus fragments of F. vesiculosus illuminated evenly on a cross section or perpendicular on one side of the thallus surface. Images were acquired under red light using either direct light from the built-in LEDs of the variable chlorophyll fluorescence imaging system (590–650 nm) or light perpendicular to the thallus surface provided by a supercontinuum laser (615–665 nm) connected to a tunable single-line filter and delivered via a laser sheet generator. The isopleths (A and B) show the influence of actinic irradiance on the effective PSII quantum yield (in µmol electrons m−2 s−1 [µmol photons m−2 s−1]−1) as a function of the depth in the tissue when illuminated either directly on the cross section (A) or perpendicular to the surface of the thallus (B). Illumination in B was given from left to right. Line profiles (line width = 15 pixels) were taken on thallus parts with similar thickness (∼250 µm), with cortex layers also displaying similar thicknesses (∼50–75 µm). C to H show images of effective PSII quantum yield in darkness, moderate irradiance (567 ± 18 µmol photons m−2 s−1), and saturating irradiance (1,087 ± 30 µmol photons m−2 s−1) under direct even illumination of the cross section (C–E) and with laser light sheet illumination perpendicular to the thallus surface (F–H). Illumination in F to H was given from the bottom to the top. Bar = 0.2 mm.
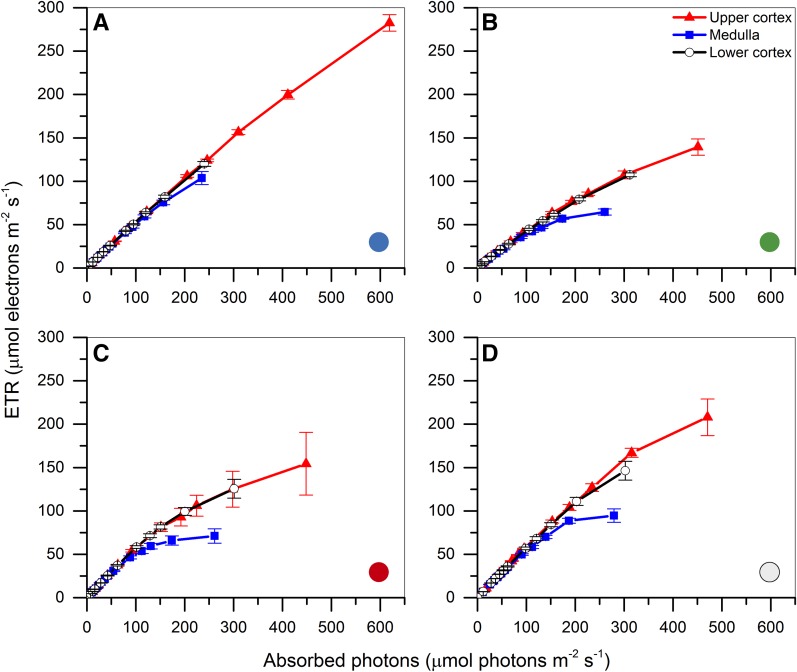
Apparent ETRs through PSII were calculated for the illuminated (upper) cortex, the medulla, and the shaded (lower) cortex, and the rates were corrected for the amount of absorbed photons in the respective tissue layers. In all cases, the ETRs in the upper and lower cortex layers were very similar when corrected for absorbed light. The medulla ETRs were similar to the cortex activity on the subsaturated part of the ETR versus irradiance curve but saturated at higher irradiance (Fig. 7).
Figure 7.
Apparent ETRs through PSII corrected for absorbed photons (Fig. 4). Measurements were performed with 20-s acclimation to each increasing actinic irradiance level of blue light (425–475 nm; A), green light (525–575 nm; B), red light (615–665 nm; C), and white light (400–700 nm; D) as provided by a laser sheet illuminating a thallus fragment perpendicular to one side of the thallus surface. Data points represent means ± se (n = 5, except A, where n = 4).
The slope of the ETR versus absorbed light curve under blue light was lower than for red and white light but reached higher ETRs (Fig. 7; Table I). The curves appeared similar under green and red light, although green light yielded a lower slope on the subsaturated part of the ETR versus absorption curve. The ETR curves measured under broadband white light appeared qualitatively as a combination of the curves measured under blue, green, and red light, where saturation occurred at higher irradiance, similar to the green and red curves, but reaching higher ETR values, probably caused by the blue light component. However, the slopes on the subsaturated part of the ETR versus irradiance curves were significantly different between white light and the average of the blue, green, and red curves in all thallus layers (Table I). The green and red ETR versus absorbed light curves were similar in appearance, in correspondence with their associated absorption profiles, which also were similar (Figs. 4 and 7). Surprisingly, the ETR curves under blue light did not reach saturation, and ETRs in the cortex and medulla layers were very similar except at the highest irradiance, where a decrease in the medulla layer was observed (Fig. 7). Even at the highest irradiance, PSII quantum yields in the cortex layers remained high, and only a small increase in the nonphotochemical quenching was observed (Supplemental Fig. S4), which could point to better light-processing properties of blue light compared with green and red light.
DISCUSSION
A novel combination of multicolor light sheet microscopy with variable chlorophyll fluorescence imaging enabled the mapping of light absorption and photosynthetic efficiencies in densely pigmented tissues. By combining well-tested methods of integrating sphere measurements and the chlorophyll fluorescence profile technique (Takahashi et al., 1994; Vogelmann and Han, 2000; Slattery et al., 2016), we propose a method for calculating profiles of photon absorption across plant tissue sections, which can be combined with variable chlorophyll fluorescence imaging of photosynthetic efficiency across tissue light gradients.
The conversion of PSII quantum yields measured by variable chlorophyll fluorescence to absolute rates of photosynthetic electron transport activity requires precise measurements of (1) mean effective PAR, (2) the PSII absorption cross section, and (3) knowledge about the partitioning between PSI and PSII photochemistry (Klughammer and Schreiber, 2015). While such information can be obtained in dilute suspensions of chloroplasts and microalgae (Klughammer and Schreiber, 2015), to measure these parameters in dense algal solutions, plant tissue, and algal biofilms is not trivial (Szabó et al., 2014; Klughammer and Schreiber, 2015). In optically dense systems, light gradients are affected by both multiple scattering and absorption, and it is important to take diffuse light into account when quantifying actinic light levels (i.e. by measuring the incident photon flux from all directions with scalar irradiance microprobes; Kühl, 2005). While such sensors have tip diameters down to 30 µm (Rickelt et al., 2016), it is difficult to perform scalar irradiance measurements in thin, cohesive plant tissues, where measurements can be biased by tissue compression due to the physical impact of the microprobe (Spilling et al., 2010; Lichtenberg and Kühl, 2015). The mean effective PAR also can be calculated from complex measurements of the angular radiance distribution with field radiance microprobes (Vogelmann and Björn, 1984; Vogelmann et al., 1989; Kühl and Jørgensen, 1994). Alternatively, information on the cell size distribution and the inherent optical properties (i.e. the scattering phase function and the scattering and absorption coefficients) allows calculations of PAR gradients, but these parameters are difficult to determine in optically dense media (Privoznik et al., 1978; Berberoglu et al., 2009; Klughammer and Schreiber, 2015), albeit recent experimental and theoretical advances in biomedical optics have allowed detailed characterization of tissue optics using combinations of optical reflection spectroscopy, optical coherence tomography, and Monte Carlo simulations (Wang et al., 1995; Wangpraseurt et al., 2016a, 2017).
An experimental solution to the above-mentioned complications relies on measuring the chlorophyll fluorescence profile, which represents the net outcome of photon absorption along the actinic light gradient in the tissue (Takahashi et al., 1994; Vogelmann and Han, 2000). By correlating fluorescence profiles to total absorption, we could measure the direct result of absorption, and the values obtained are thus only affected by the quantum yield of fluorescence and energy transfer between antenna pigment molecules and PSII and PSI. However, due to the invasive nature of the method, some actinic light will be lost from the cut surface, causing some underestimation of the tissue absorption in situ (Ichiro et al., 2016). Furthermore, this method allows estimates of the distribution of photon absorption, but it does not enable a separation of possible changes in the absorption cross section or balance between PSI and PSII absorption in different tissue layers.
We found total absorption values from integrating sphere measurements that were similar to those of terrestrial leaves (Gorton et al., 2010), although the absorption of green/yellow light was higher in F. vesiculosus due to the presence of accessory brown algal pigments such as fucoxanthin, which displays a high efficiency of energy transfer to chlorophyll a (∼95%; Yukihira et al., 2017). Due to the low reflection and transmission in the thallus, the absorption will be in the same order as the light attenuation. Comparing the calculated attenuation of light due to absorption with the light attenuation calculated using scalar irradiance microprofile measurements from a previous study (Lichtenberg and Kühl, 2015; see “Materials and Methods”), we found a whole-thallus absorption coefficient that was lower than cortex attenuation coefficients and higher than medulla attenuation coefficients (Fig. 5). We predicted identical absorption coefficients in the cortex layers, since these layers are not anatomically different and, in addition, displayed similar levels of chlorophyll fluorescence under uniform epi-illumination. Surprisingly, the absorption coefficient of the shaded cortex was larger than the one found in the illuminated cortex (Fig. 5). We speculate that the light field angularity could have caused this difference. Previously, it was shown that the absorptance of plant tissue can be different under collimated versus diffuse light (Brodersen and Vogelmann, 2010; Gorton et al., 2010), and here, the incident light on the illuminated cortex was collimated while light reaching the shaded cortex had a higher diffuse component due to internal scattering. Furthermore, we note that our calculations were based on profiles of blue light, which was almost completely absorbed, making the calculations of absorption in the shaded cortex more prone to errors due to the lower signal-to-noise ratio. Absorption profiles are further complicated due to the self-absorption of red fluorescence by chlorophyll. Thus, red fluorescence profiles are likely to better represent absorption profiles than profiles of far-red fluorescence (Vogelmann and Han, 2000). Here, we used a long-pass filter (greater than 670 nm) to detect fluorescence; therefore, the resulting profiles comprised both red and far-red chlorophyll fluorescence, and future studies should divide the detected fluorescence signals into red and far-red fluorescence. We also note that our laser sheet had a Gaussian beam profile, which makes positioning close to the edge of the cut thallus surface difficult and may create a potential spillover of photons onto the cut side. This limitation could be resolved by shaping the beam (e.g. by the generalized phase-contrast method; Bañas et al., 2014) to transform the Gaussian beam profile to a sharp rectangular shape, and such work is now under way.
The shape of the white fluorescence profile across the thallus was slightly different in appearance from the profiles for blue, green, and red light. Previously, it was shown that profiles of carbon assimilation and chlorophyll fluorescence profiles followed each other closely depending on the spectral quality (Sun et al., 1996; Vogelmann and Han, 2000), and it has been proposed that profiles of carbon fixation under white light can be described as the mean when using blue, green, and red light (Sun et al., 1998; Vogelmann and Han, 2000). Here, we show that, for plant tissue harboring a range of accessory pigments such as fucoxanthin, the absorptive properties are more complex, resulting in a different response to white light than what can be expected from the combination of measurements made under monochromatic light. Furthermore, it was shown that green light drives photosynthesis more efficiently deeper in terrestrial plant tissue than blue and red light, due to a larger penetration depth of green light in leaf tissues (Sun et al., 1998; Terashima et al., 2009). In contrast, the presence of fucoxanthin and carotenes in Fucus spp. caused the green light (525–575 nm) to be absorbed equally effectively as red light. However, not all wavelengths were absorbed equally effectively, and the spectral region around 570 to 605 nm displayed reduced absorption as compared with the other spectral regions of PAR. Since only the white light treatment covered this part of the spectrum, it is possible that illumination with broadband white light caused the differently shaped absorption profile. This might be confirmed by measuring additional chlorophyll fluorescence profiles using yellow light (e.g. 570–605 nm) to validate if this would result in fluorescence profiles with Fmax located deeper in the thallus, similar to fluorescence profiles in terrestrial leaves illuminated with green light (Vogelmann and Han, 2000).
Photosynthesis
We demonstrate that PSII quantum yields and derived apparent ETR across the thallus cross sections strongly differed between homogenous actinic light illumination of the cross section and unidirectional actinic light illumination on one side of the thallus with a laser light sheet. Under low incident irradiance, the yields were very similar in all layers and between measurements. However, as incident light directly on the cut side increased, the yields decreased across the tissue, with the highest decreases found in the medulla (Fig. 6). This was not the case under laser light sheet illumination perpendicular to one side of the thallus surface, where we found strongly reduced yields in the illuminated cortex, while yields in the shaded cortex were unaffected (Fig. 6), even at the highest incident photon irradiance (1,108 µmol photons m−2 s−1). Thus, when applying actinic light directly on a cross section, our data show that it is possible to both underestimate and overestimate PSII quantum yields as compared with yields found under natural light gradients. Using a multilayer leaf model, Evans (2009) found that, as irradiance increased on the adaxial side, the quantum yields were reduced progressively deeper into the mesophyll. However, quantum yields at the abaxial side were unaltered even under high blue irradiance, which is in good agreement with the findings of this study. Evans (2009) showed that surface-based measurements of ϕPSII to estimate ETR were only valid in some cases but overestimated ETR when the leaf was inverted. Similarly, mesophyll conductance measurements could be influenced when estimated using surface-based ϕPSII values (Evans, 2009). With our new method, ϕPSII values can now be measured under natural tissue light gradients and can be corrected for photon absorption, thus making it possible to get more detailed insights into mesophyll conductance heterogeneities (e.g. by using depth-resolved ϕPSII data in combination with gas-exchange measurements; Evans, 2009; Pons et al., 2009).
The differences in PSII quantum yield measured under illumination directly on the cross section or perpendicular to the side of the thallus surface indicate the difference between the photosynthetic potential under equal illumination and the realized photosynthesis under tissue light gradients. We show here that, even under high incident irradiance, photosynthetic electron transport in the lower cortex was not saturated; therefore, an even illumination of tissue cross sections will underestimate the PSII quantum yield as compared with shaded parts during high unidirectional illumination. Apparent ETRs in the cortex layers were very similar when corrected for absorption, while the medulla layer displayed saturation and lower ETRs at increasing irradiance, indicating a lower photosynthetic capacity, probably due to lower pigment content (Figs. 3 and 7). The slope of the initial part of the ETR curves, which is a measure of the light utilization efficiency at subsaturating photon flux, was similar in all thallus layers, albeit consistently slightly lower in the medulla (Table I). In a recent study, absolute ETRs of thin-tissued corals were calculated (Szabó et al., 2014), and the rates found in this study were of similar magnitude. Szabó et al. (2014) also found initial slopes of the subsaturated part of the ETR versus irradiance curves that were slightly higher, probably reflecting differences in photochemical acclimatization that have been shown to be tightly linked to the optical properties of coral tissues (Lichtenberg et al., 2016; Wangpraseurt et al., 2016a, 2016b).
Fucus spp. are often found in the intertidal zone and, thus, on a daily basis, experiences a variable light environment as a function of water depth and concentration of organic and inorganic particles as well as dissolved organic matter attenuating solar irradiance. Therefore, air-exposed plant parts will be subjected to the full solar spectrum, while the incident light field on algae situated in deeper oligotrophic waters will be blue shifted due to the absorption of red light by water. In contrast, the incident light field on algae in shallow eutrophic waters will be green shifted due to the absorption of blue and red wavelengths by suspended phytoplankton. On a tissue scale, the medulla layer of Fucus spp. will, on average, experience the lowest photon irradiance compared with the cortex layers (Lichtenberg et al., 2016) and, therefore, could be thought of as a shade-adapted compartment in the algal thallus. Shade-acclimatized phytoelements will normally display higher light use efficiencies (i.e. steeper initial slope on the photosynthesis-irradiance curve) but lower photosynthetic capacity (Pmax) due to increased pigment content and biochemical regulations in the photosynthetic machinery and/or ultrastructural changes in the chloroplasts (Lichtenthaler et al., 1981, 2007; Lichtenthaler and Babani, 2004; Sarijeva et al., 2007). However, as the initial slopes of the ETR versus absorbed light curves in the medulla were both lower and displayed saturation at lower irradiances, medulla layers cannot be described as photosynthetically shade adapted in conventional terms. Conversely, it appears that the structural organization of the thallus layers could be adapted to maximize photon absorption in the outer cortex layers while having a relatively translucent central medulla with low absorptive properties. This is in contrast to terrestrial leaves, where the even illumination of tissue layers is achieved by increased internal scattering due to intercellular airspaces, with the concomitant absorption profiles following an exponential attenuation with depth (Vogelmann and Han, 2000). This fundamental difference is in good agreement when considering their respective positions in terrestrial and aquatic habitats, as terrestrial leaves can organize their positions according to the angle of solar radiation, whereas aquatic macrophytes are limited in their structural organization by strong drag and shearing forces imposed by waves and currents, randomly exposing both sides of the thallus to direct light. By maximizing absorption in the outer layers and having a translucent central layer, F. vesiculosus can maximize light harvesting by allowing photons not absorbed in the illuminated thallus to propagate to tissue layers with unused photosynthetic potential, thereby ensuring a more efficient resource distribution.
The ETR versus absorbed light curves measured in different tissue layers under laser light sheet illumination were similar to what was found previously in Fucus spp. (Lichtenberg and Kühl, 2015), although these microfiber PAM-based measurements were associated with high sd values due to the small measurement volume of the fiber-optic microprobe, which makes such measurements prone to microscale tissue heterogeneity effects. With the experimental approach presented in this study, it is now possible to integrate photosynthetic responses from specific tissue layers much more precisely and, in addition, to correct them for the amount of photons absorbed by that given tissue layer. Here, we used a 10× microscope objective, but in principle, such measurements could be performed at even higher magnification (e.g. to investigate single cell gradients of light) and photosynthetic efficiencies (e.g. in large algal cells). The combination of multicolor light sheet microscopy with variable chlorophyll fluorescence imaging on plant tissue cross sections provides an alternative to more destructive methods such as constructing profiles of CO2 fixation from paradermal sectioning (Evans and Vogelmann, 2003) or nanoscale secondary ion mass spectroscopy (Kilburn et al., 2010; Wangpraseurt et al., 2016b) and allows sequential measurements on the same sample (e.g. under different levels of actinic irradiance) or comparisons of diffuse versus collimated light fields (Brodersen et al., 2008). Here, we demonstrated the application on aquatic macrophyte tissue, but the technique is readily applicable to many other types of plant tissues, including terrestrial leaves as well as photosynthetic biofilms and symbioses.
CONCLUSION
The combination of multicolor light sheet microscopy and variable chlorophyll fluorescence imaging is a powerful technique that enables fine-scale characterization of light absorption and PSII quantum yields across plant tissue sections. Furthermore, quantification of photon absorption from light sheet-induced cross-tissue fluorescence profiles can be used in concert with variable chlorophyll fluorescence imaging, enabling calculations of ETRs that otherwise require knowledge of the absorption cross section and the mean effective PAR.
The spectral flexibility of a white supercontinuum laser source allows this method to be used in other photosynthetic systems with different anatomical structures and pigmentation. In this manner, the role of specific accessory pigments in light propagation and photosynthesis can be investigated further.
MATERIALS AND METHODS
Sample Collection and Preparation
Stands of the brown macroalga Fucus vesiculosus were collected in the littoral zone at various locations around Helsingør, Denmark, during late summer and were maintained in 10-L buckets continuously flushed with 0.2 µm of filtered aerated seawater (temperature = 16°C, salinity = 32) for up to 1 week prior to experiments. Samples were kept under a 12:12-h light:dark cycle under a photon irradiance of ∼50 µmol photons m−2 s−1 (PAR, 400–700 nm) as provided by a fluorescent tube (Philips Master TL-D90, 18 W; Philips).
Prior to measurements, an apical thallus fragment was cut ∼1 cm from the thallus tip with a razor blade, and the cut side was rinsed in seawater with a transfer pipette to wash away pigments leaking from cut chloroplasts. The sample holder (Fig. 1) consisted of a standard plastic cuvette, cut down to a height of 11 mm to allow insertion on the microscope (internal size = 10 × 10 × 10 mm). To fix the sample in the cuvette, ∼300 µL of 20 g L−1 seawater agar (Sigma-Aldrich) was transferred to the cuvette and allowed to cool to 20°C, after which a slit was cut parallel to the cuvette window to allow insertion of the thallus fragment. The thallus was inserted flush with the edge of the cuvette, filled with seawater (16°C, salinity = 32), and closed with a coverslip.
Chlorophyll Fluorescence Profiles
Profiles of absorbed light as estimated from chlorophyll fluorescence profiles across tissue sections were imaged with a customized microscope setup (Fig. 1). The sample cuvette (see above) was mounted on an inverted microscope (IX81; Olympus) with a 10× objective (UPlanSApo 10×/0.40; Olympus). The sample was illuminated perpendicular to one side of the thallus surface by a supercontinuum laser (SuperK Extreme, EX-B; NKT Photonics). The laser was connected to a tunable single-line filter module (SuperK Varia; NKT Photonics) via a single-mode fiber with a collimated output. The tunable single-line filter could be tuned from 400 to 840 nm to produce bandwidths from 1 to 400 nm. Light from the single-line filter module was delivered, via an alignment tool (SuperK Connect; NKT Photonics), to an endlessly single-mode large-mode-area photonic crystal fiber (FD7; NKT Photonics) with a collimated output. The collimated output was connected to a cylindrical laser sheet generator (NKT Photonics) with a 14° light sheet half angle, yielding a 5-cm longitudinal line at 10-cm distance. The generated laser sheet had a Gaussian beam profile of ∼1 mm on the latitudinal axis. The output laser optics was mounted on a manual micromanipulator (MM33; Märzhäuser) that allowed easy positioning of the laser sheet in the focal plane of the microscope.
The sample was positioned with the cut side facing the microscope objective, and the laser sheet was adjusted to hit as close to the edge of the cut as possible without illumination spillover to the cut side. After positioning, the sample was allowed to dark adapt for 15 min.
Images of chlorophyll fluorescence from the cross section were taken with a sensitive CCD camera (iXon) using a fixed exposure time of 70 ms. Chlorophyll fluorescence was detected by placing an ultra-steep long-pass edge filter (BLP01-664R; Semrock) in the light path between the objective and the camera with a transmission close to 100% at wavelengths greater than 670 nm and close to 0% at wavelengths less than 670 nm. Illumination in different spectral bands was achieved by control of the laser and the spectral filtering module with the manufacturer’s software (NKTP Control; NKT Photonics). Four different spectral illumination bands were composed (Fig. 1) and adjusted to the same photon irradiance (1,190.3 ± 1.9 µmol photons m−2 s−1) as measured with a microquantum sensor (MC-MQS; Walz) connected to a calibrated quantum irradiance meter (ULM-500; Walz). We used broadband illumination (50 nm or greater) to ensure the excitation of both PSII and PSI, thus avoiding eventual red-drop effects.
Illumination of the sample during individual image acquisitions was limited to less than 2 s per image, and the spectral sequence was randomized between replicates. The effect of irradiance on the emitted fluorescence was tested by treatment with 3-(3,4-dichlorophenyl)-1,1-dimethyl-urea (Supplemental Fig. S2). Images were analyzed in ImageJ (version 1.50B), where gray values were extracted either by the line profile tool or by extracting all gray values. The images had a spatial resolution of 0.8 × 0.8 µm pixel−1. Maximum chlorophyll fluorescence was normalized to 1 in all images to allow comparison between images. Plots were made in OriginPro (version 9.3; OriginLab). The thickness of the thallus varied, both between samples and depending on the location in the cross section (Fig. 2). Therefore, profiles of fluorescence were taken at thallus thicknesses of ∼450 µm to allow comparison of thallus light gradients over the same tissue thickness.
Integrating Sphere Measurements of Reflectance and Transmittance
Thallus reflectance and transmittance were measured using an integrating sphere (diameter = 10 cm, port diameters = 2.5 cm; Labsphere Instruments). The sphere had three port openings: two located opposite each other and one orthogonal to the two other openings (Supplemental Fig. S1). The incident light from the supercontinuum laser was tuned to different spectral ranges each with the same photon irradiance (see above and Fig. 1). Light was measured with a calibrated spectral irradiance meter (MSC15; Gigahertz Optik) connected to the orthogonally located port on the integrating sphere. For transmittance measurements, a thallus fragment was mounted in front of the entrance port between the light source and the integrating sphere, and the port opposite to the entrance was covered with a white reflecting plate. For reflectance measurements, a thallus fragment was placed in the port opening opposite the incident laser beam at an angle of 5° to 10° to capture both the specular and diffuse reflections. Total absorptance (A) by the thallus was estimated as:
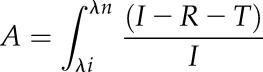 |
(1) |
where I is the incident photon irradiance, R is the reflectance, and T is the transmittance, all integrated over the spectral region of interest (λi − λn).
Modeling of Light Attenuation
Light attenuation profiles were modeled using attenuation coefficients, α, of blue scalar irradiance (420–520 nm) in the cortex and medulla layers measured in a previous study (Lichtenberg and Kühl, 2015). The model assumed monoexponential attenuation of incident irradiance, I0, over tissue depth intervals Δz, and the light availability in different tissue depths was then calculated as:
| (2) |
These data were compared with the estimated attenuation due to absorption quantified as induced fluorescence and corrected for total absorption (Supplemental Fig. S3) across the thallus under blue irradiance (425–475 nm) in this study. The attenuation due to absorption was calculated by subtracting the cumulative absorption (Fig. 4), integrated in 50-µm increments, from the incident irradiance.
Variable Chlorophyll Fluorescence Imaging
PAM variable chlorophyll fluorescence imaging (Imaging-PAM) with the saturation pulse method (Schreiber et al., 1995; Schreiber, 2004; Kühl and Polerecky, 2008) was used to assess the photosynthetic performance over cross sections of the algal thallus mounted in cuvettes as described above. Measurements were performed with a microscope Imaging-PAM system (Fig. 1) fitted with a RGB-LED excitation lamp (IMAG-RGB; Heinz Walz) as described in detail elsewhere (Trampe et al., 2011). The microscope was fitted with a high numerical aperture objective (10×/NA0.8, Plan-Apochromat; Carl Zeiss). Fast measurements of the effective PSII quantum yield under increasing photon irradiance (each irradiance step was applied for 20 s) were used to measure so-called rapid light curves (White and Critchley, 1999) of relative PSII electron transport versus irradiance curves. Two different approaches were applied: (1) using increasing actinic irradiances of red light (590–650 nm) as provided by the system-default internal RGB-LED lamp for equal excitation of the exposed cross-section of the thallus from above, in combination with the customizable automated light curve function of the software provided by the system software (ImagingWin; Heinz Walz); and (2) using the supercontinuum laser setup as described above as an external actinic light source, illuminating the thallus with a light sheet perpendicular to the thallus surface. The actinic light sheet was controlled manually in stepwise increments in sync with the custom-defined automated light curve function of the ImagingWin software, facilitating a semiautomated acquisition of rapid light curves, while the system-default internal actinic RGB-LED light source was disconnected. Rapid light curves with the laser light sheet were obtained with increasing actinic irradiance of blue (425–475 nm), green (525–575 nm), red (615–665 nm), or white (400–700 nm) light. Nonactinic modulated blue measuring light was provided by the built-in LEDs of the Imaging-PAM system during both approaches. The two setups were calibrated using a photon irradiance meter connected to a cosine-corrected mini quantum PAR irradiance sensor (ULM-500, MQS-B; Walz). All samples were allowed to dark adapt for 15 min before the measurements started. When in the dark-acclimated state, all reactions centers of PSII were open, enabling imaging of the minimal fluorescence yield (F0). Upon exposure to a high-intensity saturation pulse, all PSII reaction centers closed, permitting imaging of the maximal fluorescence yield (Fm) over the thallus cross section. From these images, the maximum PSII quantum yield could be calculated as described by Schreiber (2004):
| (3) |
From imaging of the fluorescence yield, F, while the sample was illuminated with a predefined level of actinic light (PAR, in μmol photons m−2 s−1), and the maximum fluorescence yield during a saturation pulse, Fm′, images of the effective PSII quantum yield could be calculated as:
| (4) |
From these values, the relative photosynthetic electron transport rate (rETR) is usually derived using the equation:
| (5) |
where PAR is the incident photon irradiance and AF (the absorption factor) is a constant set to 0.42, assuming that 84% of the incident light is absorbed (Björkman and Demmig, 1987) and an even distribution of absorbed photons between PSII and PSI (Schreiber et al., 2012). In our study, we estimated internal ETR rates (in units of µmol electrons m−2 s−1) in different regions of the thallus (cortex/medulla) by multiplying average ϕPSII values (in units of µmol electrons m−2 s−1 [µmol photons m−2 s−1]−1) for a given area with the total amount of absorbed photons (in units of µmol photons m−2 s−1) in that area. The wavelength-dependent absorption was calculated by normalizing the chlorophyll fluorescence profiles to the total absorption (Supplemental Fig. S3). This essentially gives an estimate of the wavelength-dependent photon absorption at any given depth in the thallus, assuming that 1 unit of fluorescence was the direct result of 1 unit of absorption. In reality, however, this is affected by factors such as the quantum yield of fluorescence and energy transfer between antenna pigment molecules and PSII/PSI. Therefore, the true photon absorption will vary slightly, and the calculated photon absorptions are only estimates.
The light saturation coefficient (i.e. the photon scalar irradiance at the onset of light saturation of photosynthesis, Ek) was calculated as 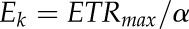 , where ETRmax is the maximum activity and α is the initial slope of the ETR versus photon absorption curve; both parameters were obtained by curve fitting of ETR measurements using an exponential function (Webb et al., 1974) by means of a nonlinear Levenberg-Marquardt fitting algorithm (OriginPro 2015; OriginLab).
, where ETRmax is the maximum activity and α is the initial slope of the ETR versus photon absorption curve; both parameters were obtained by curve fitting of ETR measurements using an exponential function (Webb et al., 1974) by means of a nonlinear Levenberg-Marquardt fitting algorithm (OriginPro 2015; OriginLab).
Statistics
One-way ANOVAs were applied to test differences in the slopes of the ETR curves between different light treatments. Data were first tested for normality (Shapiro-Wilk) and for equal variance. Statistical analysis was performed in SigmaPlot (version 12.5), and the significance level was set to P < 0.01.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Schematic drawing of integrating sphere measurements of transmittance and reflectance.
Supplemental Figure S2. Chlorophyll fluorescence profiles over cross sections of apical thallus fragments of F. vesiculosus.
Supplemental Figure S3. Concept of the calculations of photon absorption profiles across plant tissue cross sections.
Supplemental Figure S4. PSII quantum yield and nonphotochemical quenching as a function of absorbed photons in the upper cortex, medulla, and lower cortex of F. vesiculosus.
Acknowledgments
We thank NKT Photonics for generous loan of the supercontinuum laser and for excellent technical support.
Glossary
- PAM
pulse amplitude modulation
- ETR
electron transport rate
- PAR
photosynthetically active radiation
- RGB
red-green-blue
Footnotes
This study was supported by a Sapere-Aude Advanced grant from the Danish Council for Independent Research/Natural Sciences (M.K.) and grants from the Carlsberg Foundation (M.K.).
References
- Baker NR. (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59: 89–113 [DOI] [PubMed] [Google Scholar]
- Bañas A, Palima D, Villangca M, Aabo T, Glückstad J (2014) GPC light shaper for speckle-free one- and two-photon contiguous pattern excitation. Opt Express 22: 5299–5311 [DOI] [PubMed] [Google Scholar]
- Berberoglu H, Gomez PS, Pilon L (2009) Radiation characteristics of Botryococcus braunii, Chlorococcum littorale, and Chlorella sp. used for CO2 fixation and biofuel production. J Quant Spectrosc Radiat Transf 110: 1879–1893 [Google Scholar]
- Binzer T, Sand-Jensen K (2002a) Importance of structure and density of macroalgae communities (Fucus serratus) for photosynthetic production and light utilisation. Mar Ecol Prog Ser 235: 53–62 [Google Scholar]
- Binzer T, Sand-Jensen K (2002b) Production in aquatic macrophyte communities: a theoretical and empirical study of the influence of spatial light distribution. Limnol Oceanogr 47: 1742–1750 [Google Scholar]
- Björkman O, Demmig B (1987) Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170: 489–504 [DOI] [PubMed] [Google Scholar]
- Brodersen CR, Vogelmann TC (2007) Do epidermal lens cells facilitate the absorptance of diffuse light? Am J Bot 94: 1061–1066 [DOI] [PubMed] [Google Scholar]
- Brodersen CR, Vogelmann TC (2010) Do changes in light direction affect absorption profiles in leaves? Funct Plant Biol 37: 403–412 [Google Scholar]
- Brodersen CR, Vogelmann TC, Williams WE, Gorton HL (2008) A new paradigm in leaf-level photosynthesis: direct and diffuse lights are not equal. Plant Cell Environ 31: 159–164 [DOI] [PubMed] [Google Scholar]
- Evans JR. (2009) Potential errors in electron transport rates calculated from chlorophyll fluorescence as revealed by a multilayer leaf model. Plant Cell Physiol 50: 698–706 [DOI] [PubMed] [Google Scholar]
- Evans JR, Vogelmann TC (2003) Profiles of C-14 fixation through spinach leaves in relation to light absorption and photosynthetic capacity. Plant Cell Environ 26: 547–560 [Google Scholar]
- Garbary DJ, Kim KY (2005) Anatomical differentiation and photosynthetic adaptation in brown algae. Algae 20: 233–238 [Google Scholar]
- Gorton HL, Brodersen CR, Williams WE, Vogelmann TC (2010) Measurement of the optical properties of leaves under diffuse light. Photochem Photobiol 86: 1076–1083 [DOI] [PubMed] [Google Scholar]
- Govindjee, Braun BZ (1974) Light absorption emission and photosynthesis. In Stewart WDP, ed, Botanical Monographs: Algal Physiology and Biochemistry, Vol 10 University of California Press, Berkeley, CA, pp 346–390 [Google Scholar]
- Ichiro T, Hiroki O, Takashi F, Riichi O (2016) Light environment within a leaf. II. Progress in the past one-third century. J Plant Res 129: 353–363 [DOI] [PubMed] [Google Scholar]
- Johnsen G, Samset O, Granskog L, Sakshaug E (1994) In vivo absorption characteristics in 10 classes of bloom-forming phytoplankton: taxonomic characteristics and responses to photoadaptation by means of discriminant and HPLC analysis. Mar Ecol Prog Ser 105: 149–157 [Google Scholar]
- Kilburn MR, Jones DL, Clode PL, Cliff JB, Stockdale EA, Herrmann AM, Murphy DV (2010) Application of nanoscale secondary ion mass spectrometry to plant cell research. Plant Signal Behav 5: 760–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U (2015) Apparent PS II absorption cross-section and estimation of mean PAR in optically thin and dense suspensions of Chlorella. Photosynth Res 123: 77–92 [DOI] [PubMed] [Google Scholar]
- Kühl M. (2005) Optical microsensors for analysis of microbial communities. Methods Enzymol 397: 166–199 [DOI] [PubMed] [Google Scholar]
- Kühl M, Cohen Y, Dalsgaard T, Jørgensen BB, Revsbech NP (1995) Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studied with microsensors for O2, pH and light. Mar Ecol Prog Ser 117: 159–172 [Google Scholar]
- Kühl M, Jørgensen BB (1994) The light-field of microbenthic communities: radiance distribution and microscale optics of sandy coastal sediments. Limnol Oceanogr 39: 1368–1398 [Google Scholar]
- Kühl M, Polerecky L (2008) Functional and structural imaging of phototrophic microbial communities and symbioses. Aquat Microb Ecol 53: 99–118 [Google Scholar]
- Lichtenberg M, Kühl M (2015) Pronounced gradients of light, photosynthesis and O2 consumption in the tissue of the brown alga Fucus serratus. New Phytol 207: 559–569 [DOI] [PubMed] [Google Scholar]
- Lichtenberg M, Larkum AWD, Kühl M (2016) Photosynthetic acclimation of Symbiodinium in hospite depends on vertical position in the tissue of the scleractinian coral Montastrea curta. Front Microbiol 7: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK, Ac A, Marek MV, Kalina J, Urban O (2007) Differences in pigment composition, photosynthetic rates and chlorophyll fluorescence images of sun and shade leaves of four tree species. Plant Physiol Biochem 45: 577–588 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK, Babani F (2004) Light adaptation and senescence of the photosynthetic apparatus: changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity. In GC Papageorgiou, Govindjee, eds, Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer, Dordrecht, The Netherlands, pp 713–736 [Google Scholar]
- Lichtenthaler HK, Buschmann C, Döll M, Fietz HJ, Bach T, Kozel U, Meier D, Rahmsdorf U (1981) Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth Res 2: 115–141 [DOI] [PubMed] [Google Scholar]
- McMillen GG, McClendon JH (1979) Leaf angle: an adaptive feature of sun and shade leaves. Bot Gaz 140: 437–442 [Google Scholar]
- Moss BL. (1983) Sieve elements in the Fucales. New Phytol 93: 433–437 [Google Scholar]
- Myers DA, Jordan DN, Vogelmann TC (1997) Inclination of sun and shade leaves influences chloroplast light harvesting and utilization. Physiol Plant 99: 395–404 [Google Scholar]
- Oguchi R, Douwstra P, Fujita T, Chow WS, Terashima I (2011) Intra-leaf gradients of photoinhibition induced by different color lights: implications for the dual mechanisms of photoinhibition and for the application of conventional chlorophyll fluorometers. New Phytol 191: 146–159 [DOI] [PubMed] [Google Scholar]
- Pons TL, Flexas J, von Caemmerer S, Evans JR, Genty B, Ribas-Carbo M, Brugnoli E (2009) Estimating mesophyll conductance to CO2: methodology, potential errors, and recommendations. J Exp Bot 60: 2217–2234 [DOI] [PubMed] [Google Scholar]
- Privoznik KG, Daniel KJ, Incropera FP (1978) Absorption, extinction and phase function measurements for algal suspensions of Chlorella pyrenoidosa. J Quant Spectrosc Radiat Transf 20: 345–352 [Google Scholar]
- Ramus J. (1978) Seaweed anatomy and photosynthetic performance: ecological significance of light guides, heterogeneous absorption and multiple scatter. J Phycol 14: 352–362 [Google Scholar]
- Raven JA. (2003) Long-distance transport in non-vascular plants. Plant Cell Environ 26: 73–85 [Google Scholar]
- Rickelt LF, Lichtenberg M, Trampe ECL, Kühl M (2016) Fiber-optic probes for small-scale measurements of scalar irradiance. Photochem Photobiol 92: 331–342 [DOI] [PubMed] [Google Scholar]
- Sand-Jensen K, Krause-Jensen D (1997) Broad-scale comparison of photosynthesis in terrestrial and aquatic plant communities. Oikos 80: 203–208 [Google Scholar]
- Sarijeva G, Knapp M, Lichtenthaler HK (2007) Differences in photosynthetic activity, chlorophyll and carotenoid levels, and in chlorophyll fluorescence parameters in green sun and shade leaves of Ginkgo and Fagus. J Plant Physiol 164: 950–955 [DOI] [PubMed] [Google Scholar]
- Schreiber U. (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In GC Papageorgiou, Govindjee, eds, Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer, Dordrecht, The Netherlands, pp 279–319 [Google Scholar]
- Schreiber U, Bilger W, Neubauer C (1995) Chlorophyll fluorescence as a nonintrusive indicator for rapid assessment of in vivo photosynthesis. In Schulze ED, Caldwell MM, eds, Ecophysiology of Photosynthesis. Springer, Berlin, pp 49–70 [Google Scholar]
- Schreiber U, Klughammer C, Kolbowski J (2012) Assessment of wavelength-dependent parameters of photosynthetic electron transport with a new type of multi-color PAM chlorophyll fluorometer. Photosynth Res 113: 127–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber U, Kühl M, Klimant I, Reising H (1996) Measurement of chlorophyll fluorescence within leaves using a modified PAM fluorometer with a fiber-optic microprobe. Photosynth Res 47: 103–109 [DOI] [PubMed] [Google Scholar]
- Serodio J. (2004) Analysis of variable chlorophyll fluorescence in microphytobenthos assemblages: implications of the use of depth-integrated measurements. Aquat Microb Ecol 36: 137–152 [Google Scholar]
- Shibata K, Haxo FT (1969) Light transmission and spectral distribution through epi- and endozoic algal layers in the brain coral, Favia. Biol Bull 136: 461–468 [Google Scholar]
- Slattery RA, Grennan AK, Sivaguru M, Sozzani R, Ort DR (2016) Light sheet microscopy reveals more gradual light attenuation in light-green versus dark-green soybean leaves. J Exp Bot 67: 4697–4709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilling K, Titelman J, Greve TM, Kühl M (2010) Microsensor measurements of the external and internal microenvironment of Fucus vesiculosus (Phaeophyceae). J Phycol 46: 1350–1355 [Google Scholar]
- Sun J, Nishio JN, Vogelmann TC (1996) High light alters photosynthetic carbon fixation gradients across sun and shade leaves. Plant Cell Environ 19: 1261–1271 [Google Scholar]
- Sun J, Nishio JN, Vogelmann TC (1998) Green light drives CO2 fixation deep within leaves. Plant Cell Physiol 39: 1020–1026 [Google Scholar]
- Szabó M, Wangpraseurt D, Tamburic B, Larkum AW, Schreiber U, Suggett DJ, Kühl M, Ralph PJ (2014) Effective light absorption and absolute electron transport rates in the coral Pocillopora damicornis. Plant Physiol Biochem 83: 159–167 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Mineuchi K, Nakamura T, Koizumi M, Kano H (1994) A system for imaging transverse-distribution of scattered-light and chlorophyll fluorescence in intact rice leaves. Plant Cell Environ 17: 105–110 [Google Scholar]
- Terashima I, Fujita T, Inoue T, Chow WS, Oguchi R (2009) Green light drives leaf photosynthesis more efficiently than red light in strong white light: revisiting the enigmatic question of why leaves are green. Plant Cell Physiol 50: 684–697 [DOI] [PubMed] [Google Scholar]
- Trampe E, Kolbowski J, Schreiber U, Kühl M (2011) Rapid assessment of different oxygenic phototrophs and single-cell photosynthesis with multicolour variable chlorophyll fluorescence imaging. Mar Biol 158: 1667–1675 [Google Scholar]
- Vogelmann TC, Björn LO (1984) Measurement of light gradients and spectral regime in plant-tissue with a fiber optic probe. Physiol Plant 60: 361–368 [Google Scholar]
- Vogelmann TC, Bornman JF, Josserand S (1989) Photosynthetic light gradients and spectral regime within leaves of Medicago sativa. Philos Trans R Soc Lond B Biol Sci 323: 411–421 [Google Scholar]
- Vogelmann TC, Bornman JF, Yates DJ (1996) Focusing of light by leaf epidermal cells. Physiol Plant 98: 43–56 [Google Scholar]
- Vogelmann TC, Evans JR (2002) Profiles of light absorption and chlorophyll within spinach leaves from chlorophyll fluorescence. Plant Cell Environ 25: 1313–1323 [Google Scholar]
- Vogelmann TC, Han T (2000) Measurement of gradients of absorbed light in spinach leaves from chlorophyll fluorescence profiles. Plant Cell Environ 23: 1303–1311 [Google Scholar]
- Vogelmann TC, Martin G (1993) The functional-significance of palisade tissue: penetration of directional versus diffuse light. Plant Cell Environ 16: 65–72 [Google Scholar]
- Wang L, Jacques SL, Zheng L (1995) MCML: Monte Carlo modeling of light transport in multi-layered tissues. Comput Methods Programs Biomed 47: 131–146 [DOI] [PubMed] [Google Scholar]
- Wangpraseurt D, Jacques SL, Petrie T, Kühl M (2016a) Monte Carlo modeling of photon propagation reveals highly scattering coral tissue. Front Plant Sci 7: 1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangpraseurt D, Pernice M, Guagliardo P, Kilburn MR, Clode PL, Polerecky L, Kühl M (2016b) Light microenvironment and single-cell gradients of carbon fixation in tissues of symbiont-bearing corals. ISME J 10: 788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangpraseurt D, Wentzel C, Jacques SL, Wagner M, Kühl M (2017) In vivo imaging of coral tissue and skeleton with optical coherence tomography. J R Soc Interface 14: 20161003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb WL, Newton M, Starr D (1974) Carbon dioxide exchange of Alnus rubra: a mathematical model. Oecologia 17: 281–291 [DOI] [PubMed] [Google Scholar]
- White AJ, Critchley C (1999) Rapid light curves: a new fluorescence method to assess the state of the photosynthetic apparatus. Photosynth Res 59: 63–72 [Google Scholar]
- Yukihira N, Sugai Y, Fujiwara M, Kosumi D, Iha M, Sakaguchi K, Katsumura S, Gardiner AT, Cogdell RJ, Hashimoto H (2017) Strategies to enhance the excitation energy-transfer efficiency in a light-harvesting system using the intra-molecular charge transfer character of carotenoids. Faraday Discuss 198: 59–71 [DOI] [PubMed] [Google Scholar]