The N-terminal domain of Brassica diacylglycerol acyltransferase1 binds CoA and acyl-CoA, contributing to feedback inhibition and activation, respectively, of triacylglycerol biosynthesis.
Abstract
Diacylglycerol acyltransferase 1 (DGAT1) is an integral membrane enzyme catalyzing the final and committed step in the acyl-coenzyme A (CoA)-dependent biosynthesis of triacylglycerol (TAG). The biochemical regulation of TAG assembly remains one of the least understood areas of primary metabolism to date. Here, we report that the hydrophilic N-terminal domain of Brassica napus DGAT1 (BnaDGAT11-113) regulates activity based on acyl-CoA/CoA levels. The N-terminal domain is not necessary for acyltransferase activity and is composed of an intrinsically disordered region and a folded segment. We show that the disordered region has an autoinhibitory function and a dimerization interface, which appears to mediate positive cooperativity, whereas the folded segment of the cytosolic region was found to have an allosteric site for acyl-CoA/CoA. Under increasing acyl-CoA levels, the binding of acyl-CoA with this noncatalytic site facilitates homotropic allosteric activation. Enzyme activation, on the other hand, is prevented under limiting acyl-CoA conditions (low acyl-CoA-to-CoA ratio), whereby CoA acts as a noncompetitive feedback inhibitor through interaction with the same folded segment. The three-dimensional NMR solution structure of the allosteric site revealed an α-helix with a loop connecting a coil fragment. The conserved amino acid residues in the loop interacting with CoA were identified, revealing details of this important regulatory element for allosteric regulation. Based on these results, a model is proposed illustrating the role of the N-terminal domain of BnaDGAT1 as a positive and negative modulator of TAG biosynthesis.
Triacylglycerol (TAG) is the predominant component of the seed oil of oleaginous plants. Not only does TAG serve as an energy reserve to fuel germination and early seedling growth, but this highly reduced form of carbon is a nutritional source of dietary oil for humans and animals. Plant TAG also can serve as a feedstock for petrochemical alternatives such as biolubricants, biopolymers, and biodiesel (Metzger and Bornscheuer, 2006). Diacylglycerol acyltransferase (DGAT; EC 2.3.1.20) catalyzes the acyl-CoA-dependent acylation of sn-1,2-diacylglycerol to generate TAG, and in some species, such as Brassica napus, the level of enzyme activity appeared to have a substantial effect on the flow of carbon into TAG (Weselake et al., 2008; Liu et al., 2012). In developing seeds, two families of integral nonhomologous membrane enzymes, DGAT1 and DGAT2, are known to contribute to catalyzing TAG formation. Because of the importance of the enzyme in carbon flow, overexpression of various plant DGAT cDNAs has been used to increase seed oil content in Arabidopsis (Arabidopsis thaliana; Jako et al., 2001) and oil crops such as canola-type B. napus (Weselake et al., 2008; Taylor et al., 2009) and soybean (Glycine max; Lardizabal et al., 2008; Roesler et al., 2016). Increased DGAT production also has been used as a molecular strategy to boost the TAG content of algal species (Iwai et al., 2014). Mammalian DGAT1, on the other hand, is relevant in medicine, as it has been investigated as a drug target for a variety of lipid-related metabolic diseases, including obesity and diabetes (Zammit et al., 2008; Birch et al., 2010). Many DGAT1 inhibitors have already been designed, synthesized, and tested (DeVita and Pinto, 2013). Bovine (Bos taurus) DGAT1 also is important in the cattle industry, as it is used as a polymorphic genetic marker for the fat content of milk (Winter et al., 2002; Grisart et al., 2004). Despite the enzyme’s key role in primary metabolism and its biotechnological and clinical relevance, little is known about the catalytic mechanism and regulation of DGAT1.
DGAT1 belongs to a family of enzymes named membrane-bound O-acyltransferases (MBOATs). Due to their hydrophobic nature, structural investigation to date of MBOATs has been limited to topological analysis. MBOAT members have highly conserved Asn/Asp and His residues that have been hypothesized to be involved in acyltransferase activity. There is little detailed structure-function information on any member of the MBOAT family, and furthermore, no 3D structure has been solved for any MBOAT. The DGAT1 family is highly conserved. DGAT1 possesses a very hydrophilic N-terminal region corresponding to about the first 100 residues, which is followed by eight to ten predicted transmembrane segments (Liu et al., 2012). The putative active site His residue of murine (Mus musculus) DGAT1 is predicted to be near the C terminus of the enzyme (McFie et al., 2010). That study also demonstrated the importance of the N-terminal region in forming dimers and tetramers and hypothesized the role of this region in enzyme regulation. The active site region in bovine DGAT1 was characterized through secondary structure analysis and binding studies of short peptide fragments spanning the predicted substrate binding sites (Lopes et al., 2014). The N and C termini of tung tree (Vernicia fordii) DGAT1 were both localized in the cytosol, and a C-terminal endoplasmic reticulum (ER) retrieval motif was shown to target the plant enzyme to the ER (Shockey et al., 2006). Recently, four highly homologous isoforms of B. napus DGAT1, recombinantly produced in Saccharomyces cerevisiae H1246, were partially characterized (Aznar-Moreno et al., 2015; Greer et al., 2015, 2016), and recombinant isoform BnaC.DGAT1.a was highly purified in active form (Caldo et al., 2015). A recombinant hydrophilic N-terminal fragment of isoform BnaA.DGAT1.b was shown previously to self-associate and interact with acyl-CoA through positive cooperativity based on the Lipidex-1000 assay (Weselake et al., 2006). The recombinant hydrophilic N-terminal fragment of mouse DGAT1 also was shown to interact with acyl-CoA through positive cooperativity (Siloto et al., 2008). Recently, it was reported that Corylus americana DGAT1, several recombinant enzyme variants, and Zea mays DGAT1 exhibited positive cooperativity through kinetic studies (Roesler et al., 2016). Thus, various lines of biochemical evidence suggest that DGAT1 enzymes may be regulated through allosteric interactions. The self-association properties of DGAT1 enzymes are consistent with the fact that most allosteric enzymes exhibit quaternary structure.
In this study, the structure and function of the hydrophilic N-terminal domain of B. napus DGAT1 (isoform BnaC.DGAT1.a) were examined. This domain was found to have an intrinsically disordered region (IDR) and a folded section. IDRs are recognized as important regions in proteins due to their roles in cellular signaling and regulation (Wright and Dyson, 2015). The highly disordered segment was found to be involved in the down-regulation of DGAT1 activity, suggesting the presence of an autoinhibitory motif. BnaC.DGAT1.a also was found to exhibit positive cooperativity, the extent of which diminished as more of the N terminus was removed. Therefore, the involvement of the N-terminal domain in self-association may mediate positive cooperativity. The folded section, on the other hand, is important to maintain high acyl-CoA affinity at the active site and activity. In addition, CoA noncompetitively inhibited BnaC.DGAT1.a, further confirming the presence of an allosteric site for CoA. The 3D NMR solution structure of the folded segment containing the allosteric site was determined. The CoA/acyl-CoA binding site in the hydrophilic N-terminal domain and specific interactions involved in CoA recognition also were identified.
RESULTS
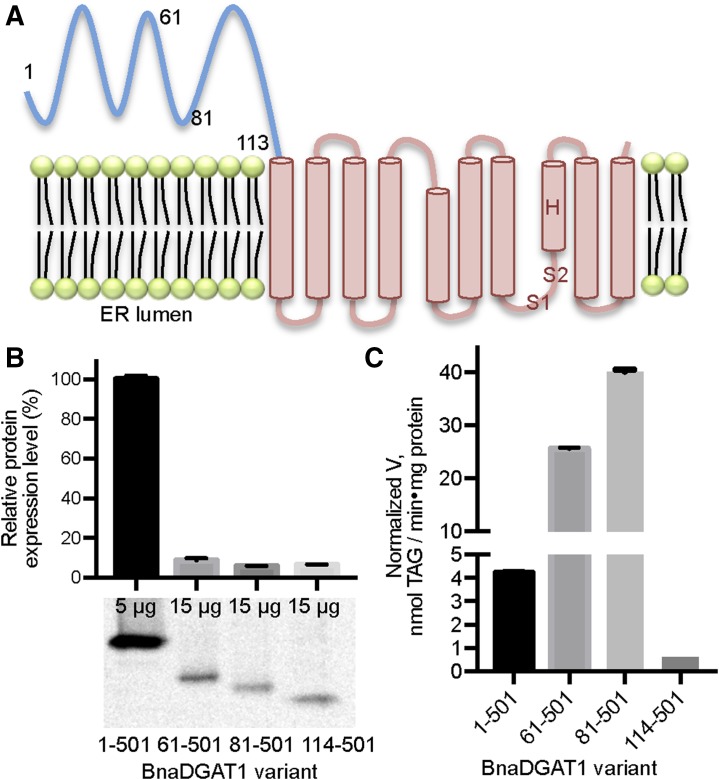
The BnaDGAT1 N-Terminal Domain Is Not Necessary for Catalysis But Contributes to Modulating Activity
The BnaC.DGAT1.a isoform (hereafter referred to as BnaDGAT1) is predicted to have several membrane-spanning segments preceded by a relatively large hydrophilic N-terminal domain that comprises more than 20% of the polypeptide (Fig. 1A). These features are conserved in all DGAT1s from various species. The BnaDGAT1 schematic shown in Figure 1A is based on a topology prediction of ten transmembrane domains, but other predictions indicate eight transmembrane domains. To probe the importance of the N-terminal domain in catalysis, the full-length enzyme and various truncated forms were recombinantly produced in S. cerevisiae H1246, which is devoid of the ability to synthesize TAG (Sandager et al., 2002). The specific activities were determined and normalized by the production level of each enzyme form relative to full-length BnaDGAT1 (Fig. 1B). Interestingly, BnaDGAT161-501 and BnaDGAT181-501 exhibited higher normalized specific activity compared with the full-length enzyme (Fig. 1C). These results suggest that the presence of amino acid residues 1 to 80 down-regulates enzymatic activity. Furthermore, despite the lower production level of BnaDGAT161-501 and BnaDGAT181-501, these enzyme forms were able to generate TAG amounting to about 60% of the total TAG produced by the full-length enzyme in situ (Supplemental Fig. S1). On the other hand, BnaDGAT1114-501, which is devoid of the entire N-terminal domain, was about 10-fold less active than the full-length enzyme (Fig. 1C). These results indicate that the N-terminal domain is not necessary to form an active enzyme but that distinct segments of this domain contribute to modulating activity.
Figure 1.
Truncation analysis of the BnaDGAT1 N-terminal domain. A, BnaDGAT1 topology as predicted by TMHMM. BnaDGAT1 has a relatively large hydrophilic N-terminal domain (shown in blue) in the cytosolic face of the ER and 10 predicted transmembrane segments, housing the catalytic site, within the remainder of the enzyme. Numerals indicate the locations of the different enzyme truncation points. S1 and S2 correspond to the acyl-CoA and diacylglycerol binding regions in the active site, respectively, which were identified in bovine DGAT1 (Lopes et al., 2014). The putative catalytic His (H), identified in murine DGAT1, also is shown in the topology (McFie et al., 2010). B, Protein expression level of BnaDGAT1 proteins with various truncations. The amount of microsomal protein used for blotting is indicated for each BnaDGAT1 construct. The amount of enzyme was semiquantified using densitometric analysis using blots performed in duplicate. C, Normalized DGAT activity of BnaDGAT11-501, BnaDGAT161-501, BnaDGAT181-501, or BnaDGAT1114-501. Specific activity was obtained at 2 μm oleoyl-CoA, and the values were normalized by the protein expression level of each form. Reported values are means ± sd; n = 3.
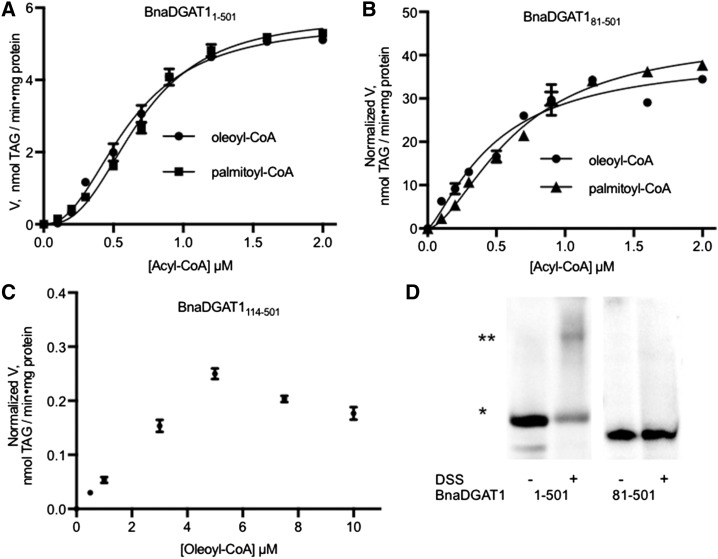
The activities of BnaDGAT11-501, BnaDGAT181-501, and BnaDGAT1114-501 were assayed at increasing concentrations of acyl-CoA (Fig. 2, A, B, and C, respectively). Normalized DGAT1 activities are depicted on the y axis of each part. Maximum enzyme activity was observed at 1 to 2 µm acyl-CoA for BnaDGAT11-501 or BnaDGAT181-501. In contrast, maximum activity with BnaDGAT1114-501 was not observed until about 5 µm acyl-CoA (Fig. 2C). This result suggests that the affinity of BnaDGAT1114-501 for acyl-CoA was much lower than that of the full-length BnaDGAT1 or BnaDGAT181-501 and that residues 81 to 113 are important in maintaining high activity and affinity for the acyl donor at the active site.
Figure 2.
The BnaDGAT1 N-terminal domain partially mediates positive cooperativity. A to C, Substrate saturation curves of full-length BnaDGAT1 (A), BnaDGAT181-501 (B), and BnaDGAT1114-501 (C). The data were fitted with the Hill equation using GraphPad Prism 7.0a. The kinetic parameters are listed in Table I. D, Cross-linking studies of BnaDGAT1 and truncated versions of the enzyme using disuccinimidyl suberate (DSS). The monomeric species is indicated by one asterisk, while the dimer is indicated by two asterisks. The BnaDGAT1 N-terminal region is required for interactions leading to the dimeric enzyme form, which may allow it to partially mediate positive cooperativity through intermolecular interaction. Reported values are means ± sd; n = 3.
The substrate saturation plots for BnaDGAT1 and the N-terminally truncated enzyme variants were found to be a better fit to the Hill equation over the Michaelis-Menten model based on R2 values and residual analysis (Table I). In the case of BnaDGAT1114-501, the plot did not reach the asymptote due to substrate inhibition observed at higher concentrations of acyl-CoA (Fig. 2C). This plot did not fit any specific enzyme kinetic model. The Hill coefficients of BnaDGAT1 are 2.28 ± 0.13 and 2.65 ± 0.19 for oleoyl-CoA and palmitoyl-CoA, respectively, and are indicative of positive cooperativity. The Hill coefficient for N-terminally truncated enzyme BnaDGAT181-501 decreased to 1.26 ± 0.19 and 1.64 ± 0.11 for oleoyl-CoA and palmitoyl-CoA, respectively, suggesting that the N-terminal domain partially mediates positive cooperativity.
Table I. Kinetic parameters of full-length and truncated BnaDGAT1 using palmitoyl-CoA and oleoyl-CoA as acyl donors.
The data for the substrate saturation assays were fitted with the Hill equation using GraphPad Prism 7.0a. The apparent Vmax values were not normalized for DGAT abundance. Reported values are means ± sd; n = 3.
| Parameter | BnaDGAT11-501 |
BnaDGAT181-501 |
||
|---|---|---|---|---|
| Oleoyl-CoA | Palmitoyl-CoA | Oleoyl-CoA | Palmitoyl-CoA | |
| Apparent Vmax (nmol TAG min−1 mg−1 protein) | 5.58 ± 0.14 | 5.75 ± 0.16 | 2.27 ± 0.24 | 2.18 ± 0.09 |
| Hill coefficient | 2.28 ± 0.13 | 2.65 ± 0.19 | 1.26 ± 0.19 | 1.64 ± 0.11 |
| Apparent S0.5 (μm) | 0.61 ± 0.02 | 0.69 ± 0.02 | 0.55 ± 0.11 | 0.67 ± 0.05 |
| Goodness of fit/R2 | 0.994 | 0.992 | 0.961 | 0.992 |
In positive cooperativity, the binding of substrate at one site increases the affinity of substrate at another site. This effect is commonly achieved through intersubunit communication in a quaternary structure situation (Laskowski et al., 2009). Taking this possibility into account, we investigated the ability of the N-terminal domain to facilitate the formation of a quaternary structure. The oligomeric states of BnaDGAT1 were determined using cross-linking experiments. Yeast microsomes containing recombinant variants of BnaDGAT1 were incubated with the membrane-permeable cross-linker, disuccinimidyl suberate. Treated or untreated protein samples were then subjected to SDS-PAGE and western-blot analysis (Fig. 2D). Untreated BnaDGAT1 showed one band with a molecular mass of about 50 kD. For treated BnaDGAT1, two polypeptides were detected: the 50-kD species and a second species with molecular mass twice the size of the 50-kD species. This result suggested that the full-length enzyme is capable of forming a dimer. In contrast, treatment of BnaDGAT181-501 with cross-linking agent did not result in any detectable polypeptide oligomerization (Fig. 2D), which suggested that dimer formation capability was associated with the first 80 amino acid residues of the enzyme. In addition, BnaDGAT181-501 still exhibited a certain degree of cooperativity, based on the Hill coefficients, shown in Table I, in the absence of dimerization. This observation suggests that the binding of acyl-CoA to an allosteric site in the hydrophilic N-terminal domain increases the affinity of the active site for acyl-CoA, which is associated with the remainder of the same polypeptide.
CoA Modulates the Activity of the Active Site Acyltransferase
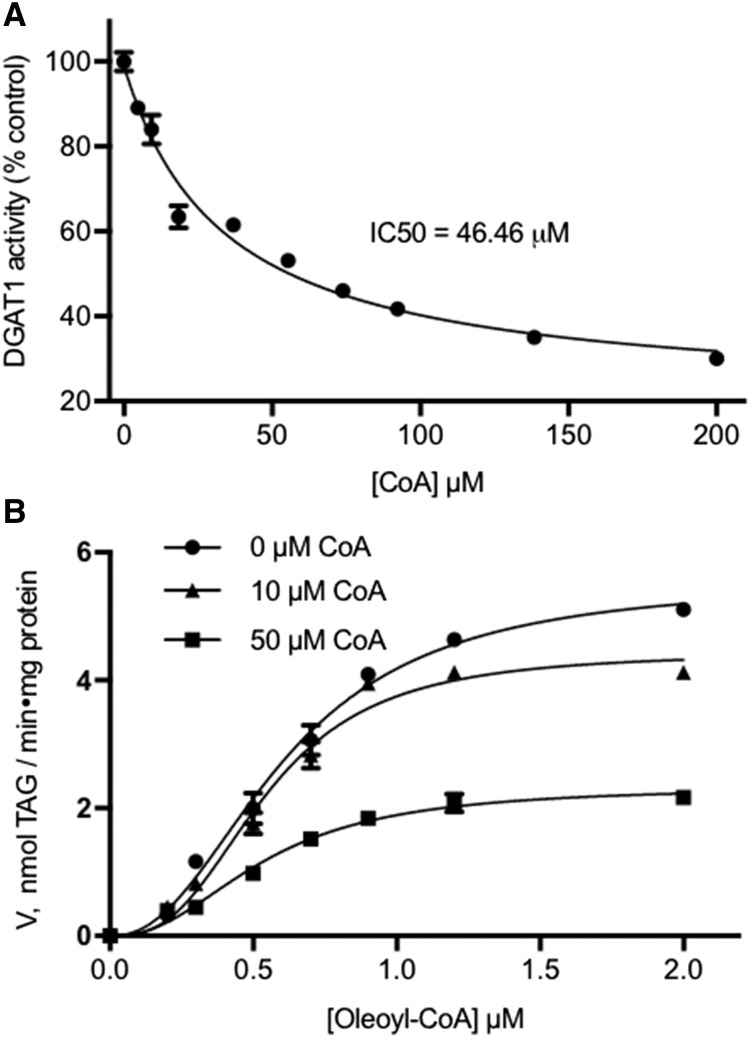
Previous Lipidex-1000 binding experiments showed that the N-terminal domain of isoform BnaA.DGAT1.b can interact with acyl-CoA at a possible allosteric site and that CoA could displace the thioester (Weselake et al., 2006). In this study, the effect of CoA on BnaDGAT1 activity was investigated. The presence of increasing concentrations of CoA in the reaction mixture led to a corresponding decrease in acyltransferase activity (Fig. 3A). The activity was reduced by about 10% in the presence of 5 μm CoA and by as much as 70% when the CoA concentration was increased to 200 μm. The IC50 for CoA was found to be about 47 μm. To determine the mode of inhibition, the effect of increasing CoA concentration on the oleoyl-CoA saturation plot was evaluated (Fig. 3B; Table II). In the presence of 10 μm CoA, the apparent Vmax of the enzyme decreased from 5.55 ± 0.17 to 4.56 ± 0.25 nmol TAG min−1 mg−1 protein. The apparent Vmax decreased further to 2.46 ± 0.16 nmol TAG min−1 mg−1 protein upon the addition of 50 μm CoA. On the other hand, the apparent S0.5 of the enzyme remained essentially the same under the above conditions. These results indicate that CoA is a noncompetitive BnaDGAT1 inhibitor that binds to an allosteric site, which may be within the N-terminal domain of the enzyme.
Figure 3.
Activity assay of BnaDGAT1 in the presence of CoA. A, BnaDGAT1 activity in the presence of increasing concentrations of CoA. CoA was found to inhibit BnaDGAT1. B, Oleoyl-CoA saturation curve in the presence of different concentrations of CoA. The apparent Vmax decreased progressively upon the addition of increasing amounts of CoA, while the apparent S0.5 remained the same. These results indicate that CoA is a noncompetitive inhibitor of the enzyme and, thereby, inhibits BnaDGAT1 by binding to an allosteric site. Reported values are means ± sd; n = 3.
Table II. Kinetic parameters of BnaDGAT1 using oleoyl-CoA as an acyl donor in the presence of varying amounts of CoA.
The data for the substrate saturation assays were fitted with the Hill equation using GraphPad Prism 7.0a. Reported values are means ± sd; n = 3. CoA was found to be a noncompetitive inhibitor of BnaDGAT1.
| [CoA] | Apparent Vmax | Apparent S0.5 |
|---|---|---|
| nmol TAG min−1 mg−1 protein | μm | |
| Control | 5.55 ± 0.17 | 0.61 ± 0.02 |
| 10 μm | 4.56 ± 0.25 | 0.60 ± 0.04 |
| 50 μm | 2.46 ± 0.16 | 0.62 ± 0.05 |
The BnaDGAT1 N-Terminal Domain Is Structurally Flexible
The BnaDGAT1 N-terminal domain (amino acid residues 1–113) is not homologous to any structurally characterized protein domain. We first analyzed the amino acid sequence of BnaDGAT11-113 to obtain insights on its structure-function characteristics. The N-terminal domain is depleted of hydrophobic amino acid residues, which are important for promoting protein folding, and is composed predominantly of charged and polar residues. This combination has been correlated with intrinsic disorder in proteins (Uversky et al., 2000). This observation agrees with secondary structure analysis using DISOPRED (Ward et al., 2004), which showed that the majority of the N-terminal hydrophilic region is likely in a disordered state, a characteristic that appears to be conserved among plant and animal DGAT1s (Supplemental Fig. S2). The BnaDGAT1 N-terminal domain exhibits high sequence variability with a prevalence of insertions/deletions in the primary structure, both of which are characteristics of IDRs (Brown et al., 2002; Light et al., 2013). In contrast, the remainder of BnaDGAT1 is relatively highly conserved, enriched in hydrophobic amino acid residues, and expected to be well folded based on DISOPRED analysis.
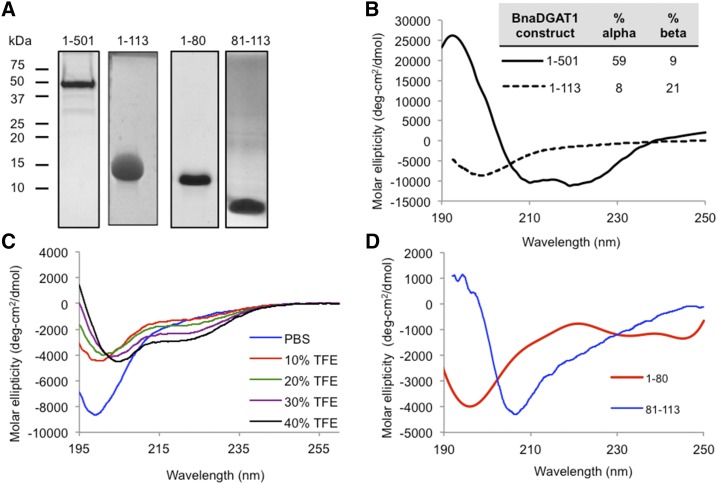
For structural investigation, full-length recombinant BnaDGAT1 was produced in S. cerevisiae H1246, solubilized in n-dodecyl-β-d-maltopyranoside (DDM), and purified as described previously (Fig. 4A; Caldo et al., 2015). In addition, BnaDGAT11-113 was recombinantly produced in Escherichia coli and purified (Fig. 4A; Supplemental Fig. S3A). Far-UV circular dichroism (CD) spectroscopy was then used to analyze the secondary structure of purified BnaDGAT1 or BnaDGAT11-113 (Fig. 4B). The CD spectrum of full-length BnaDGAT1 in DDM micelles reveals a well-folded protein that is predominantly α-helical. In contrast, the CD spectrum of BnaDGAT11-113 was indicative of a random coil polypeptide. This result confirmed that this region of the BnaDGAT1 polypeptide is mainly intrinsically disordered. Analysis of the CD spectra using K2D3 software (Louis-Jeune et al., 2012) showed that the full-length polypeptide is predominantly well folded (about 68% α-helices/β-sheets), while the N-terminal domain only has about 29% α-helices/β-sheets.
Figure 4.
The BnaDGAT1 N-terminal domain is composed of an IDR and a folded segment, while full-length BnaDGAT1 is well folded. A, SDS-PAGE of purified BnaDGAT1 in DDM micelles, BnaDGAT11-113, BnaDGAT11-80, and BnaDGAT181-113. B, Far-UV CD spectra of BnaDGAT1 and BnaDGAT11-113 in DDM micelles and phosphate-buffered saline, respectively. The percentage of secondary structure based on K2D3 analysis of CD spectra is indicated. Full-length BnaDGAT1 is predominantly α-helical, while BnaDGAT11-113 is mainly random coil. C, The BnaDGAT1 N-terminal domain has a propensity to form secondary structure upon titration with increasing amounts of TFE. Some IDRs tend to form a transient structure upon interaction with protein-binding partners. D, CD spectra of BnaDGAT11-80 and BnaDGAT181-113. Residues 1 to 80 are highly disordered, while residues 81to 113 appear to form defined secondary structures.
Some IDRs have the ability to form specific stable conformations upon interacting with proteins/ligands (Wright and Dyson, 2015). The presence of trifluoroethanol (TFE) simulates the hydrophobic condition that can arise upon forming protein-protein interaction and has been used to assess the propensity of IDRs to undergo induced folding (Sun et al., 2010; Marín et al., 2012). The N-terminal domain of BnaDGAT1 was titrated with increasing amounts of TFE followed by CD analysis (Fig. 4C). Increasing amounts of TFE resulted in the appearance of distinct minima at 208 and 220 nm. This suggests that, given the presence of the right binding partner or condition, specific segments of BnaDGAT1 N-terminal IDR can potentially form a defined structure. DISOPRED analysis identified the presence of possible protein-ligand binding sites within the disordered region (Supplemental Fig. S2).
The BnaDGAT1 N-Terminal Domain Is Composed of an IDR and a Folded Portion
Truncation analysis showed that the removal of the first 80 amino acid residues did not decrease the responsiveness of the enzyme to increasing concentrations of acyl-CoA, while the opposite was observed upon deletion of amino acid residues 81 to 113 (Fig. 2). Our results here along with a previous study on the N-terminal domain of DGAT1 (Weselake et al., 2006) suggest that this binding site is localized along amino acid residues 81 to 113. DISOPRED analysis predicted that segment 1–80 of BnaDGAT1 is disordered, whereas segment 81–113 has a secondary structure (Supplemental Fig. S2). Initial NMR structure elucidation of the entire N-terminal domain was not plausible due to the lack of a defined structure. We then recombinantly produced and purified BnaDGAT11-80 and BnaDGAT181-113 (Fig. 4A; Supplemental Fig. S3, B and C). Both polypeptide segments were subjected to far-UV CD analysis (Fig. 4D). The CD spectrum of BnaDGAT11-80 is similar to that of BnaDGAT11-113, indicating a random coil structure. The CD spectrum of BnaDGAT181-113, however, was indicative of a well-folded structure. These results suggested that BnaDGAT181-113, which may include an acyl-CoA/CoA binding site, would be suitable for structural elucidation using solution NMR spectroscopy. In turn, natural abundance 15N-HSQC, 1H-1H-TOCSY, NOESY, and COSY data sets were acquired for BnaDGAT181-113 in 30 mm sodium phosphate buffer (pH 6.3) with 25 mm NaCl. The 15N-HSQC spectrum (Supplemental Fig. S4) showed a relatively good dispersion of the amide proton signals, confirming that BnaDGAT181-113 exhibits a defined structure. Peak lists of the chemical shift assignments and NOE constraints were then inputted into CYANA 2.1 for the structural calculations. The final calculation utilized 356 NOE constraints with 300 short-range, 49 medium-range, and seven long-range constraints (Supplemental Table S2). The Ramachandran plot shows that no φ or ψ backbone angles are in the disallowed regions (Supplemental Table S2).
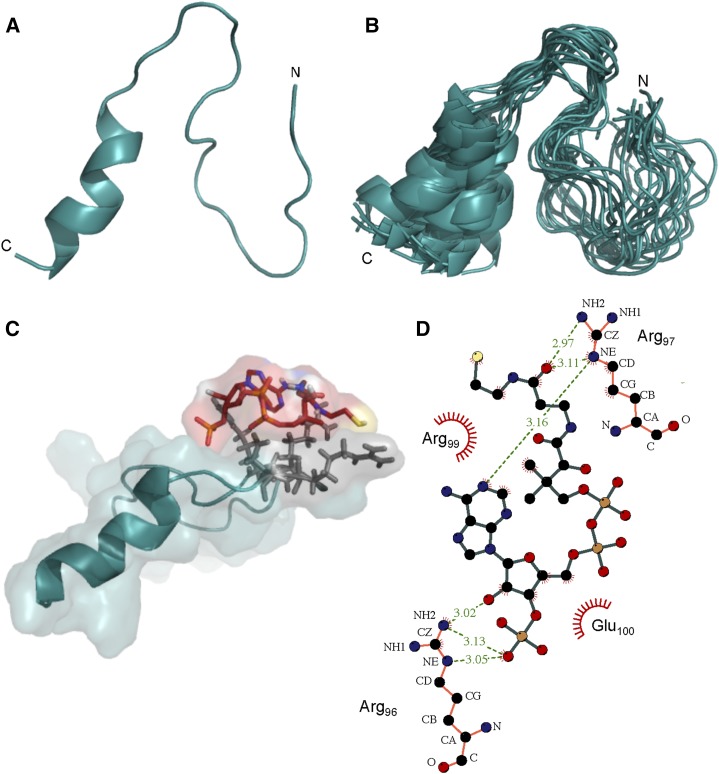
The calculated structure of BnaDGAT181-113 showed that it is composed of an α-helix at the C terminus spanning Leu-103 to Ser-112, a defined loop from Ala-94 to Pro-102, and an unstructured N terminus (Fig. 5A). The root-mean-square deviation (RMSD) values for the structured regions were calculated to be 0.51 and 0.49 Å for the α-helix and loop backbone atoms, respectively. The low RMSD values indicate that the observed conformations are consistent throughout the 20 calculated structures (Fig. 5B). On the other hand, a higher RMSD of 1.58 Å for the backbone atoms was calculated for Asn-81 to Pro-93, which forms a random coil. This coil is connected to the segment comprising amino acid residues 1 to 80 of the N-terminal domain, which is an IDR based on DISOPRED, CD, and NMR analyses.
Figure 5.
3D structure of the allosteric site in the BnaDGAT1 N-terminal domain and binding studies with CoA. A, NMR solution structure of BnaDGAT181-113 in 30 mm sodium phosphate buffer (pH 6.3) and 25 mm NaCl exhibiting an α-helix at the C terminus. B, Overlay of the 20 lowest energy conformers. C, Docking of the allosteric site with CoA using AutoDock Vina (Trott and Olson, 2010). The interacting residues were identified using NMR titration experiments (Supplemental Fig. S5). D, Layout of interacting residues obtained using LigPlot software (Wallace et al., 1995). Hydrogen bonds are shown by green dashed lines, while hydrophobic interactions are shown by red rays arranged in a circular fashion.
The Folded Portion Has the Allosteric Site for CoA
A previous study demonstrated that CoA alone could displace bound oleoyl-CoA from the N-terminal domain of BnaDGAT1, suggesting that the CoA moiety of acyl-CoA is critical to effective binding (Weselake et al., 2006). NMR titration of BnaDGAT181-113 with CoA was performed and binding was monitored through 15N-HSQC (Supplemental Fig. S5A). The results showed that, while most of the signals remained at exactly the same positions, movement of four amide protons was observed, signifying a change in chemical environment upon the addition of CoA. No further chemical shift changes were observed beyond a 1:1 BnaDGAT181-113:CoA molar ratio, indicating saturation of binding and a stoichiometry of 1:1. The cross peaks that moved were found to correspond to four polar amino acids (Arg-96, Arg-97, Arg-99, and Glu-100) that are located in the loop region (Supplemental Fig. S5A). A closer examination of the structure of BnaDGAT181-113 revealed that all four residues are located on the same side of the loop (Fig. 5C). To examine the part of CoA that interacts with BnaDGAT181-113, 15N-HSQC and 31P-NMR were performed with CoA in the absence or presence of the peptide segment. 15N-HSQC analysis of CoA, before and after binding, showed slight movement of the amide hydrogens near the diphosphate group (Supplemental Fig. S5B). 31P-NMR showed three major peaks corresponding to the phosphorus of the 3′-phosphate of ribose and two phosphorus atoms in the diphosphate moiety (Supplemental Fig. S5C). Upon addition of the peptide, 31P-HSQC revealed that the signal for 31P of the phosphate group attached to ribose moved upfield from about 1 to 0 ppm. On the other hand, the 31P signals of the diphosphate moiety moved slightly downfield. These results suggest that the phosphate groups are involved in binding to the loop region of BnaDGAT181-113. Taking into account these experimental results, a molecular model of the complex was derived by docking the calculated NMR solution structure of BnaDGAT181-113 with CoA using AutoDock Vina (Trott and Olson, 2010). The model suggests the formation of hydrogen bonds between the side chain of Arg-96 and the 3′-phosphate and 2′-hydroxyl groups of CoA (Fig. 5D). Hydrogen bonds also are observed between CoA and Arg-97, while hydrophobic interactions are present between CoA and all four amino acid residues (Arg-96, Arg-97, Arg-99, and Glu-100) that were identified earlier to be involved in binding through NMR titration experiments.
The Allosteric Site Also Is Needed for Acyl-CoA-Mediated Homotropic Allosteric Activation
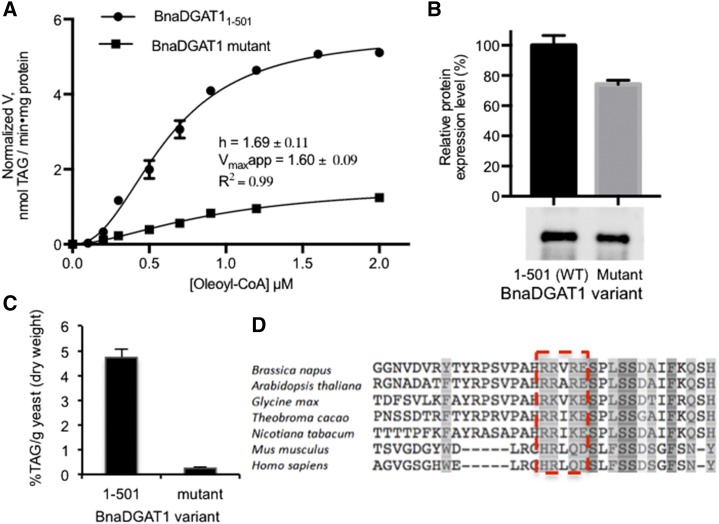
Mutational analysis of the loop amino acid residues implicated in CoA recognition was then performed to investigate their role in acyltransferase activity. A BnaDGAT1 variant having amino acid residue substitutions R96A, R97A, R99A, and E100A was recombinantly produced in S. cerevisiae H1246, and both in vivo DGAT activity and in vitro microsomal DGAT activity were assessed (Fig. 6, A and B). The BnaDGAT1 mutant was found to exhibit low normalized in vitro microsomal activity, having a normalized apparent Vmax value of 1.6 ± 0.09 nmol TAG min−1 mg−1 protein, which is only about 29% of that of the wild-type enzyme (Fig. 6A). The BnaDGAT1 mutant also exhibited low activity in vivo compared with the wild-type enzyme, as shown by the results of TAG quantification using gas chromatography-mass spectrometry (GC-MS) analysis (Fig. 6C). These results demonstrate that the same allosteric site also is important for binding acyl-CoA, which allows the enzyme to form the more active state through homotropic allosteric activation. Furthermore, sequence alignment of DGAT1 from several plant and animal species indicated that the nature of the four amino acid residues implicated in CoA binding and homotropic activation is highly conserved (Fig. 6D). NMR analysis of the interaction of BnaDGAT181-113 with various species of acyl-CoA also was explored, but this led to precipitation of the complex.
Figure 6.
Mutational studies of the CoA binding site and its role for binding acyl-CoA for homotropic allosteric activation. A, Mutational analysis of the CoA binding site through Ala mutation. Normalized activity of the BnaDGAT1 mutant with R96A, R97A, R99A, and E100A mutations exhibited low activity in vitro compared with the wild-type enzyme, suggesting that these residues are needed for intramolecular homotropic allosteric activation with respect to acyl-CoA. B, Western blot and relative production level of a recombinant BnaDGAT1 variant compared with the wild-type (WT; 1–501) enzyme. C, The TAG produced by yeast H1246 producing recombinant BnaDGAT1 variants (R96A, R97A, R99A, E100A) compared with the wild-type enzyme analyzed by GC-MS. The low in vivo activity in H1246 of the BnaDGAT1 mutant compared with the wild-type enzyme also supports the importance of the identified residues for homotropic activation. D, Multiple sequence alignment of DGAT1 from plant and animal species showing the highly conserved loop region (boxed) implicated in CoA/acyl-CoA binding. Reported values are means ± sd; n = 3.
DISCUSSION
Previous studies have demonstrated that N-terminally truncated forms of plant DGAT1 are capable of catalyzing TAG synthesis (Nykiforuk et al., 2002; Siloto et al., 2009; Ahmad et al., 2015). Although the hydrophilic N-terminal domain of BnaDGAT1 does not participate in catalysis directly, our work demonstrates that this domain plays a key regulatory role in enzymatic function.
In this study, CD and NMR experiments revealed that the regulatory N-terminal domain is composed of an IDR and a folded section. The presence of IDRs is common in higher organisms. Indeed, a recent study found that IDRs are found in 50% of membrane proteins and usually are localized at the cytoplasmic face of the lipid bilayer (Bürgi et al., 2016). Computational studies also showed that multipass membrane proteins tend to have longer (∼70 residues) IDRs facing the cytosol (Bürgi et al., 2016). This agrees with previous studies of plant DGAT1 indicating that the N-terminal region resides on the cytosolic face of the ER (Shockey et al., 2006; Weselake et al., 2006). Sequence analysis showed that the intrinsically disordered nature of the N-terminal domain is conserved in other plant DGAT1s as well as in nonplant DGAT1s from human (Homo sapiens), murine, and bovine (Supplemental Fig. S2). This suggests that the properties rendered by the N-terminal domain on DGAT1 might be conserved across different species.
IDRs are recognized as important regions of amino acid residues, as they have been found to perform a variety of roles in protein regulation and cellular signaling (Wright and Dyson, 2015). The IDR in the N-terminal domain of BnaDGAT1 has a down-regulatory effect on enzyme activity, which is due to the presence of an autoinhibitory sequence. Similarly, the increase in activity upon truncation of the N-terminal domain also was observed in murine DGAT1 (McFie et al., 2010). A study of autoinhibited proteins revealed that inhibitory motifs are localized predominantly in disordered regions (Trudeau et al., 2013). In addition, that study suggested that disordered regions usually contain multiple phosphorylation sites and exhibit flexible structural conformations, which can operate combinatorially to enable the tight regulation of activity. Murine DGAT1 was found to have 12 putative phosphorylation sites within the N-terminal domain, six of which were confirmed through mass spectrometry analysis (Yu et al., 2015). BnaDGAT1 has 14 putative phosphorylation sites based on the NetPhos 3.1 server (Blom et al., 1999). Interestingly, CTP:phosphocholine cytidyltransferase (CCT), a key regulatory enzyme involved in the biosynthesis of another major lipid group (phosphatidylcholine), also was found to have an autoinhibitory motif within a structurally flexible regulatory tail (Ding et al., 2012). The CCT regulatory tail has a number of putative phosphorylation sites similar to the DGAT1 N-terminal domain. The elucidated 3D structure of CCT demonstrated that the autoinhibitory motif within the regulatory tail interacts with the catalytic domain (Lee et al., 2014). This interaction blocks access into the active site and hinders the dynamics of the key catalytic residue. The autoinhibitory motif was found to form an induced amphipathic helix upon binding, while the rest of the regulatory tail remained disordered (Lee et al., 2014). Autoinhibitory motifs are known to inhibit the activity of other domains through intramolecular interactions (Pufall and Graves, 2002). DISOPRED analysis showed that the BnaDGAT1 IDR has a propensity to form protein-protein interactions (Supplemental Fig. S2). The BnaDGAT1 autoinhibitory sequence may interact with cytosolic loops associated with the catalytic region of the enzyme, thereby down-regulating the activity of the enzyme. As mentioned above, the full-length BnaDGAT1 is well folded and predominantly α-helical in DDM micelles, while the IDR with an autoinhibitory motif has a propensity to form a structure (Fig. 4, B and C). It remains to be determined whether the IDR of BnaDGAT1 forms a 3D structure with the cytoplasmic loops of segment 114–501, which contains the catalytic site and numerous transmembrane domains (Fig. 1A).
The highly disordered region of BnaDGAT1, residues 1 to 80, was found to facilitate dimerization, as evident from cross-linking studies (Fig. 7). This agrees with our studies of purified full-length BnaDGAT1 in DDM micelles, which showed that the enzyme, when subjected to gel filtration, predominantly elutes with a retention time indicative of a dimer (Caldo et al., 2015). Previous studies on murine DGAT1 and the BnaA.DGAT1.b N-terminal fragment also showed that the N-terminal domain plays a role in self-association (Weselake et al., 2006; McFie et al., 2010). The formation of a quaternary structure could possibly facilitate intermolecular communication between subunits.
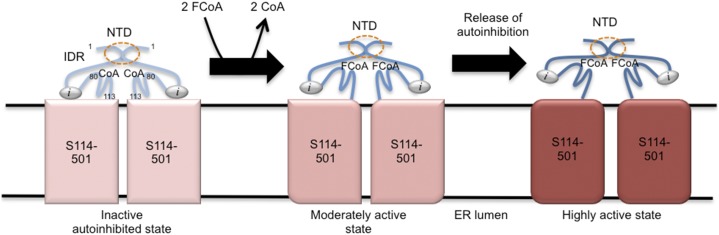
Figure 7.
Proposed model for BnaDGAT1 regulation involving the hydrophilic N-terminal domain (NTD). The NTD (amino acid residues 1–113) and the remainder of the enzyme (S114–501) are shown in shades of blue and red, respectively. The enzyme initially exists in the inactive state with an autoinhibitor motif (i) in the NTD bound to S114–501, the latter of which houses the transmembrane segments and catalytic domain. High levels of CoA stabilize the inactive state by binding to the allosteric site in the NTD. CoA can be displaced through increased availability of acyl-CoA (FCoA), which activates the enzyme by binding to the allosteric site. The binding of FCoA activates the catalytic domain in S114–501 via a conformational change in the same polypeptide, and the signal also is transmitted to the other subunit through the dimerization segment (circled) in the IDR in the NTD. The release of the autoinhibitory region is hypothesized to lead to further conformational changes resulting in the highly active state.
Our kinetic analysis of BnaDGAT1 showed that it exhibits positive cooperativity. This behavior for plant DGAT1 also was reported recently for both C. americana DGAT1 and Z. mays DGAT1 (Roesler et al., 2016). That study found that plant DGAT1s exhibited a sigmoidal response to increasing concentrations of acyl-CoA, consistent with the findings on BnaDGAT1 in this study. Furthermore, in this study, deletion of the N-terminal domain led to a decrease in the Hill coefficient, suggesting that this region partially mediates positive cooperativity through intermolecular interactions. This observation agrees with the acyl-CoA binding characteristics of recombinant forms of the N-terminal region of B. napus DGAT1 or murine DGAT1 (Weselake et al., 2006; Siloto et al., 2008). Lipidex-1000 assays showed that the binding of the BnaA.DGAT1.b N-terminal fragment (amino acid residues 1–113) with various types of acyl-CoA followed a sigmoidal behavior, suggesting that the N-terminal domain can facilitate regulation through positive cooperativity. In addition, free CoA was shown to displace acyl-CoA from the N-terminal fragment, suggesting that this noncatalytic site could interact with either thioester or CoA (Weselake et al., 2006). In this study, in vitro enzyme assays showed that CoA is a noncompetitive inhibitor of BnaDGAT1, which in turn, suggested that CoA might be a physiological negative effector of the enzyme.
Kinetic analysis also showed that the first 80 amino acid residues of BnaDGAT1 do not play a significant role in binding acyl-CoA, while removal of amino acid residues 81 to 113 led to substantially reduced acyl-CoA affinity and enzyme activity. This finding showed that the acyl-CoA binding property, identified previously in BnaA.DGAT1.b (Weselake et al., 2006), lies specifically along amino acid residues 81 to 113 in the BnaC.DGAT1.a isoform, under investigation in this study. The 3D structure of the acyl-CoA/CoA binding site involving amino acid residues 81 to 113 was characterized. It is important to note that no 3D structure has been experimentally elucidated previously for any important motif of MBOATs. In contrast to the IDR in the BnaDGAT1 N-terminal domain, the putative allosteric acyl-CoA binding site is a folded segment composed of loops and an α-helix. Subsequent structural and ligand-binding analyses identified the specific amino acid residues involved in recognition of the CoA moiety. The amino acid residue substitutions R96A, R97A, R99A, and E100A resulted in a substantial reduction in DGAT activity, which clearly demonstrated that this segment of the enzyme is involved in regulating enzyme activity. Binding of acyl-CoA at the allosteric site in the N terminus may enhance DGAT activity, whereas binding of CoA may decrease enzyme activity.
Loops in proteins are common ligand-binding sites. Importantly, the amino acid residues implicated in CoA binding are highly conserved among DGAT1 homologs, including human and murine DGAT1s, suggesting a common mechanism of binding (Fig. 5C). The motif identified is composed of [HR][RK]X[KRQ][DE]. The 3′-phosphate of the adenosine moiety of CoA was found to be a major point for interaction in this study. Previously, CoA was found to be a feedback inhibitor of phosphopantetheine adenylyltransferase, which is a regulatory enzyme catalyzing the second last reaction involved in CoA biosynthesis (Rubio et al., 2008). The IC50 of CoA for phosphopantetheine adenylyltransferase is about 39 μm, which is close to the IC50 of about 47 μm for the CoA-BnaDGAT1 interaction based on our kinetic analysis.
An enzyme model is proposed wherein BnaDGAT1 activity is modulated by acyl-CoA and CoA (Fig. 7). Under conditions of high acyl-CoA levels, the level of free CoA is expected to decrease, and vice versa. The presence of at least two acyl-CoA binding sites (allosteric and catalytic sites) and positive cooperativity in BnaDGAT1 indicate that homotropic allosteric activation operates in BnaDGAT1, enabling its activity to adjust based on acyl-CoA levels. As observed in autoinhibited proteins, it is proposed that BnaDGAT1 initially exists in an inactive state with an autoinhibitor motif (i) bound to segment 114–501 (S114–501), which contains the numerous transmembrane domains and catalytic site. High levels of CoA stabilize the inactive state. CoA can be displaced by increasing the concentration of acyl-CoA, which activates the enzyme due to a conformational change induced by the binding of thioester to the allosteric site. The binding of acyl-CoA in the N-terminal domain activates the catalytic domain within the remainder of the polypeptide (S114–501), and the activation signal also is transmitted to the other subunit through the dimerization segment (circled) in the IDR (Fig. 7). The process of regulation also may include other unidentified biochemical effectors, which may be involved in the release of the autoinhibitory region from S114–501. The proposed model, however, does not account for the apparent substrate inhibition observed for BnaDGAT1114-501 at the two concentrations of acyl-CoA above 5 μm (Fig. 2C), where the enzyme might have a second lower affinity noncatalytic acyl-CoA binding site in S114–501 that results in down-regulation of the enzyme. Homotropic allosteric regulation in membrane proteins is not uncommon. Recently, another intramembrane enzyme, rhomboid protease, was shown to be regulated through homotropic allosterism (Arutyunova et al., 2014).
It is interesting to consider the above model for BnaDGAT1 regulation in a physiological context. Acyl-CoAs are used in many other biochemical reactions (Ohlrogge and Jaworski, 1997), and the tight regulation of BnaDGAT1 activity may be needed to ensure that the enzyme is only highly active when there is a surplus of substrates for TAG biosynthesis. Long-chain acyl-CoAs have been shown to have a regulatory role in mammalian lipid metabolism (Hu et al., 2005; Nagy et al., 2014). An early study implicated CoA and oleoyl-CoA as possible inhibitors of neutral lipase activity in the endosperm of 4-d-old castor (Ricinus communis) seedlings (Hills et al., 1989). Acyl-CoAs also were shown to inhibit Glc-6-P transporter and adenylate transporter in isolated plastids prepared from developing seeds of B. napus (Fox et al., 2000). The inhibition of these transport proteins reduced the flux of carbon from Glc-6-P or from acetate into long-chain fatty acids. The rate of plant fatty acid biosynthesis, which determines the level of acyl-CoA, can vary greatly depending on various factors, such as plant developmental stage, time of day, and growth rate (Ohlrogge and Jaworski, 1997). The concentration of long-chain acyl-CoA in developing B. napus seeds has been determined to be in the range of 3 to 6 μm (Larson and Graham, 2001). This is somewhat lower than the Kd of 17 μm determined for the binding of oleoyl-CoA to the recombinant N-terminal fragment of BnaA.DGAT1.b (Weselake et al., 2006). The availability of acyl-CoA under physiological conditions, however, has to be considered in terms of the interaction of these thioesters with membranes and acyl-CoA binding proteins (Yurchenko and Weselake, 2011; Lung and Chye, 2016). Thus, it may be worthwhile to further explore the activation of BnaDGAT1 in the presence of acyl-CoA binding proteins or other acyl lipids. Given the absence of the acyl chain, free CoA concentrations are probably less affected by membranes and acyl-CoA binding proteins. In lyophilized developing Arabidopsis seeds, acyl-CoA content was determined to be over 10-fold greater than free CoA content (Gibon et al., 2002; Tumaney et al., 2004).
Physiologically, the situation may be more intricate in vivo due to different combinations of B. napus DGAT1 isoform monomers. Recently, it was reported that four homologous B. napus DGAT1 genes encode four isoforms of the enzyme (Aznar-Moreno et al., 2015; Greer et al., 2015), referred to as BnaA.DGAT1.a, BnaA.DGAT1.b, BnaC.DGAT1.a, and BnaC.DGAT1.b (Greer et al., 2015), with the main differences in amino acid sequence occurring in the IDR. The A and C genomes of B. napus each encode two isoforms of DGAT1 (Greer et al., 2015). Based on sequence identity, BnaA.DGAT1.a and BnaC.DGAT1.a belong to clade I, whereas BnaA.DGAT1.b and BnaC.DGAT1.b belong to clade II (Greer et al., 2015, 2016). In vitro assays of DGAT activity with microsomes from yeast producing different recombinant isoforms of B. napus DGAT1 have shown that clade II enzymes exhibit a preference for linoleoyl-CoA as an acyl donor (Greer et al., 2016). Within the developing embryo of B. napus, the possibility exists for the formation of dimers consisting of various combinations of the four B. napus DGAT1 isoforms. Analysis of B. napus DGAT1 transcripts has indicated that BnaA.DGAT1.a and BnaC.DGAT1.a are expressed at substantially higher levels than the two other transcripts (BnaA.DGAT1.b and BnaC.DGAT1.b) at all stages of seed development up to 35 d after flowering (Aznar-Moreno et al., 2015). If it is assumed that the transcript levels are generally reflective of relative enzyme polypeptide levels, then the majority of B. napus DGAT1 dimers would be based on different combinations involving monomers, BnaA.DGAT1.a and BnaC.DGAT1.a, which are clade I enzymes that do not show a preference for linoleoyl-CoA.
CONCLUSION
This study sheds light on the regulatory function of the hydrophilic N-terminal domain of BnaDGAT1. This domain consists of an IDR and a folded allosteric site for the binding of acyl-CoA and/or CoA. The regulatory roles of distinct portions of the N-terminal region were inferred through enzyme truncation and kinetic analysis. IDRs have been found recently to perform many more functions than thought previously, particularly in their role in cell signaling and regulation, and our results in the N-terminal domain agree with the developing body of knowledge on IDRs. The 3D structure of the acyl-CoA/CoA binding site also was elucidated through solution NMR, and the specific interactions with CoA were determined. BnaDGAT1 activity may be modulated based on the availability of acyl-CoA and CoA and the ratio of the two metabolites.
MATERIALS AND METHODS
Production of Recombinant Full-Length and Truncated BnaDGAT1 in Saccharomyces cerevisiae H1246 and in Vivo Assay for Neutral Lipid Content
BnaC.DGAT1.a cDNA (AAF64065.1) was codon optimized and purchased from BioBasic. The regions corresponding to amino acid residues 1 to 501 (full length), 61 to 501, 81 to 501, and 114 to 501 were cloned into pYES2.1 vector (Invitrogen) and produced in S. cerevisiae H1246, which is devoid of TAG biosynthesis (Sandager et al., 2002). The clones were grown on solid synthetic medium lacking uracil with 2% (w/v) Glc. For recombinant protein production, a colony was picked and inoculated into 3 mL of synthetic medium lacking uracil with 2% raffinose for overnight incubation at 30°C. The seed culture was transferred into 50 mL of synthetic medium lacking uracil with 1% raffinose and 2% Gal and grown again overnight at 30°C. The cultures were then analyzed after 24 h through the Nile Red assay according to previously described methods (Siloto et al., 2009). Briefly, 100 μL of yeast culture was added with 5 μL of 0.1 mg mL−1 dye solution (Nile Red dissolved in methanol). Fluorescence emission was measured before and after the addition of dye solution, and the change in fluorescence units over optical density was used as an indicator of neutral lipid content (Chen et al., 2017). Three technical replicates were performed for each BnaDGAT1 construct.
Yeast Fatty Acid Analysis Using GC-MS
Fifty-milliliter cultures of induction medium were harvested after 24 h, flash frozen, and freeze dried. Lipids were then extracted using the Folch method with minor modifications (Christie and Han, 2010). The lipid extracts were resolved on thin-layer chromatography plates (silica G25) using an 80:20:1 hexane:diethyl ether:acetic acid solvent system as a mobile phase. The plates were sprayed with 0.5% primulin in 80:20 (v/v) acetone:water solution to visualize and identify the TAG spots under UV. The TAG isolates were derivatized by incubation in 2 mL of methanolic HCl for 1 h at 80°C. Reactions were quenched with the addition of 2 mL of saline, and methyl esters were isolated with two hexane extractions. Isolated fatty acid methyl esters were separated along a 30-m DB23 column (Agilent Technologies) using a 6896 N network gas chromatography system (Agilent Technologies) and quantified using a 5975 inert XL mass selection detector (Agilent Technologies). Triheptadecanoin and heneicosanoic methyl ester (Nu-Chek Prep) were used as external and internal controls, respectively. Three biological replicates were analyzed for each construct.
Yeast Microsome Isolation, DGAT Activity Assay, and Western-Blot Analysis
Yeast cells grown using the above conditions were harvested at an OD600 of 6. The cell pellet was resuspended in lysis buffer (50 mm Tris-HCl buffer, pH 8, containing 300 mm NaCl, 10% [v/v] glycerol, 10 mm imidazole, 2 μg mL−1 pepstatin, 2 μg mL−1 leupeptin, and 2 mm phenylmethylsulfonyl fluoride) and lysed through bead beating. The cell lysate was recovered after spinning at 10,000g for 30 min and then subjected to ultracentrifugation at 105,000g for 1 h. The 10,000g to 105,000g microsomal pellet was recovered, resuspended in lysis buffer, and stored at −80°C. The protein content of microsomes was determined using the Bradford assay using BSA as a standard (Bradford, 1976). For the DGAT assay, the reaction was performed at 30°C for 30 s to 1 min (10 min for BnaDGAT1114-501) in a 60-μL reaction mixture containing 100 μm sn-1,2-diolein (predispersed in 0.2% [v/v] Tween 20), 0.1 to 15 μm [14C]oleoyl-CoA (60 mCi mmol−1) or [14C]palmitoyl-CoA (60 mCi mmol−1), 200 mm HEPES-NaOH, pH 7.4, 4.6 mm MgCl2, and 1 to 10 μg of protein. The enzyme reaction was initiated by the addition of acyl-CoA and quenched with 10 μL of 10% (w/v) SDS. For competition assays with CoA, CoA trilithium salt was dissolved in water and then added into the reaction mixture at the desired concentration. The resulting mixture was applied onto a preparative thin-layer chromatography plate (silica G25; DC-Fertigplatten) with a separate lane for [14C]triolein standard. The plate was developed with hexane:diethyl ether:acetic acid (80:20:1, v/v/v). The products were visualized by phosphorimaging (Typhoon Trio Variable Mode Imager; GE Healthcare). Spots corresponding to TAG were scraped and analyzed for radioactivity using Beckman-Coulter LS6500. Using GraphPad Prism 7.0a, the data were fitted with the Michaelis-Menten equation or the Hill equation.
For quantification of recombinant protein production level, microsomes (containing 5–15 μg of protein as indicated in “Results”) were incubated with 5× SDS loading buffer at room temperature for 15 min, and the proteins were resolved through SDS-PAGE followed by electroblotting onto polyvinylidene fluoride membranes. The recombinant enzyme was probed with anti-V5-HRP conjugated antibody (Invitrogen) and detected through incubation with the ECL Advance Western Blotting Detection Kit (Amersham) for 5 min. The blot was scanned using a variable mode imager (Typhoon Trio Variable Mode Imager; GE Healthcare), and the protein bands were semiquantified using ImageJ software (Schneider et al., 2012).
Production and Purification of Recombinant BnaDGAT11-113
BnaDGAT11-113 was cloned into pET-16b vector between the BamHI and XhoI restriction sites. The plasmid was transformed into BL21 DE3 (New England Biolabs) and grown overnight in 200 mL of Luria broth with 100 μg mL−1 ampicillin. The seed culture was inoculated in 2 L of Luria broth with 100 μg mL−1 ampicillin and grown to an OD600 of 0.8. After which, the production of recombinant proteins was induced through the addition of 1 mm isopropyl-β-d-thiogalactopyranoside and subsequent incubation at 30°C for 6 h. The cells were harvested and suspended in 100 mL of lysis buffer, consisting of 50 mm Tris-HCl, pH 8, and 150 mm NaCl. The cells were lysed by sonication six times at 60% power with a 10-s on and 10-s off cycle. The cell lysate was recovered after spinning at 20,000g for 30 min, and the resulting supernatant was incubated with 2 mL of nickel-NTA (McLab). After transferring the resin to a small disposable column, the resin was washed with several volumes of lysis buffer. The fusion protein was eluted from the resin with 5 mL of lysis buffer containing 500 mm imidazole. For the final purification step, the eluted proteins were subjected to fast protein liquid chromatography-size exclusion chromatography (Superdex 200 16/40).
Production and Purification of Recombinant BnaDGAT11-80 and BnaDGAT181-113
BnaDGAT11-80 was cloned into pET-16b, expressed, and purified using nickel-NTA as described above. BnaDGAT181-113 was cloned into the pET-SUMO vector (Invitrogen) according to the manufacturer’s instructions. The plasmid was transformed into BL21 DE3 (New England Biolabs) and grown overnight in 50 mL of Luria broth with 50 μg mL−1 kanamycin. The seed culture was inoculated in 2 L of Luria broth-kanamycin and grown to an OD600 of 0.8. After which, the expression of the SUMO- BnaDGAT181-113 was induced through the addition of 1 mm isopropyl-β-d-thiogalactopyranoside and incubated subsequently at 30°C for 6 h. The cells were harvested, suspended in 100 mL of lysis buffer consisting of 50 mm Tris-HCl, pH 8, and 100 mm NaCl, and lysed through sonication. The cell lysate was recovered after spinning at 20,000g for 30 min, and the resulting supernatant was incubated with 2 mL of nickel-NTA resin (Thermo Scientific) for 2 h and transferred into a small column. The fusion protein was eluted from the resin with 5 mL of lysis buffer containing 500 mm imidazole. The SUMO tag was cleaved from the fusion protein using His-tagged SUMO protease (McLab) at 1 unit of protease per 20 μg of fusion protein. The cleavage cocktail was composed of 50 mm Tris-HCl, pH 8, 0.2% IGEPAL CA-360 (Sigma), 1 mm DTT, and 150 mm NaCl. Cleavage was complete after 20 h at 25°C. SDS-PAGE was run to confirm the completion of the cleavage. Partial purification of the recombinant truncated BnaDGAT1 in the cleavage mixture was done with the use of 1 mL of nickel-NTA agarose resin (Qiagen), which removed the His-tagged SUMO and His-tagged SUMO protease. Further purification of BnaDGAT181-113 was accomplished by reverse-phase HPLC using a Phenomenex Luna C18(2) semipreparative column (5 μm particle size, 100 Å pore size, 10 mm × 250 mm). The detector was set at 220 nm, while the flow rate was at 3 mL min−1. Solvent A (water with 0.1% trifluoroacetic acid) and solvent B (acetonitrile with 0.1% trifluoroacetic acid) were used. Solvent B was set at 10% for 10 min, slowly increased to 20% for 10 min, to 50% for another 15 min, and ramped up to 95% for 5 min. BnaDGAT181-113 eluted at 27 min and was concentrated under vacuum, lyophilized, and stored at −20°C.
MALDI Time-of-Flight Mass Spectrometry
MALDI time-of-flight spectra were acquired using the Perspective Biosystems Voyager Elite MALDI-TOF mass spectrometer in reflectron, delayed-extraction, positive ion mode. Sinapinic acid was used as the sample matrix.
CD Spectroscopy
CD spectra of samples at 0.2 mg mL−1 were recorded on a Jasco J-810 spectropolarimeter. For far-UV measurement (190–260 nm), a 0.1-cm quartz cell was used in a nitrogen atmosphere. Ellipticity was recorded at a scan speed of 50 nm min−1, 0.5-nm resolution, 1-nm bandwidth, and five accumulations. The proteins were dissolved in 50 mm phosphate buffer, pH 6.3, and 50 mm NaCl (phosphate-buffered saline) for most experiments. For determination of the propensity of the protein to form regular secondary structure, increasing amounts of TFE in water (0%–40%) were used. The solvent spectrum also was recorded as background, which was subtracted from the sample spectra.
NMR Spectroscopy
BnaDGAT181-113 was dissolved in 600 μL of 9:1H2O:D2O that contained 30 mm sodium phosphate buffer (pH 6.3) and 25 mm NaCl. The sample was added with 4,4-dimethyl-4-silapentane-1-sulfonic acid (0.01%, w/v) for referencing and loaded into a standard 5-mm NMR tube. One-dimensional 1H-NMR and two-dimensional homonuclear 1H-1H-TOCSY, 1H-1H-gDQF-COSY, 1H-1H-NOESY, and heteronuclear natural abundance 1H-15N-HSQC spectra were acquired at 27°C on a triple resonance HCN cryoprobe-equipped Varian VNMRS 700-MHz spectrometer with z-axis pulsed-field gradients and VNMRJ 4.2A as a host control. The water signal was suppressed by either presaturation during the relaxation delay or water gradient-tailored excitation. The experimental details are summarized in Supplemental Table S1. NMRPipe and NMRView were used to process and analyze the data sets (Delaglio et al., 1995; Johnson, 2004). Chemical shifts were assigned manually based on a precedent literature procedure (Wider et al., 1984; Wüthrich, 1986). The assignments are shown in Supplemental Table S3.
Structure Calculations
The structure of BnaDGAT181-113 was calculated using CYANA 2.1 (Güntert et al., 1997). Automatically and manually assigned NOE cross peaks combined with angle restraints obtained from TALOS (Cornilescu et al., 1999) were utilized for the structural calculations that involved seven cycles with 10,000 steps per cycle. Simulated annealing calculated 100 conformers. The 20 conformers with the lowest energy were used for further analysis. The output data from CYANA included the RMSD and Ramachandran plot. PyMOL was used to generate figures of the calculated structure. Coordinates of the structure were deposited in the Protein Data Bank (5UZL), while the chemical shift assignments were deposited in the Biological Magnetic Resonance Data Bank (30256).
HSQC Binding Experiments, Phosphorus NMR, and Docking Studies
The binding of BnaDGAT181-113 (1.5 mm) to CoA in phosphate-buffered saline (pH 6.3) was monitored using 15N-HSQC with increasing amounts of CoA (0.75, 1.5, and 3 mm). Octanoyl-CoA or palmitoyl-CoA also was tested, but drastic precipitation was encountered upon addition of the acyl-CoA. 31P-NMR and 31P-HSQC were performed to monitor chemical shift changes of phosphorus in CoA upon addition of the peptide. For the docking studies, AutoDockTools (version 1.5.6) was used to convert the files of BnaDGAT181-113 and palmitoyl-CoA from .pdb to .pdbqt extension. After which, AutoDock Vina (Trott and Olson, 2010) was used to dock CoA to BnaDGAT181-113. Figures were generated using PyMOL and LigPlot (Wallace et al., 1995).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. In vivo and in vitro activity of BnaDGAT1 variants.
Supplemental Figure S2. Intrinsic disorder profile of DGAT1 from different species and prediction for likelihood for protein-binding sites.
Supplemental Figure S3. MALDI-TOF spectra of purified BnaDGAT1 N-terminal hydrophilic domain and the two distinct segments.
Supplemental Figure S4. 15N-HSQC spectrum of BnaDGAT181-113.
Supplemental Figure S5. Ligand-binding studies of BnaDGAT181-113 with CoA.
Supplemental Table S1. NMR experimental parameters used for BnaDGAT181-113.
Supplemental Table S2. Structure calculation statistics for BnaDGAT181-113.
Supplemental Table S3. BnaDGAT181-113 chemical shift assignments.
Acknowledgments
We thank Mark Miskolzie for assistance on NMR data acquisition and Dr. Sten Stymne of the Swedish University of Agricultural Sciences for providing S. cerevisiae H1246.
Glossary
- TAG
triacylglycerol
- ER
endoplasmic reticulum
- IDR
intrinsically disordered region
- DDM
n-dodecyl-β-d-maltopyranoside
- CD
circular dichroism
- TFE
trifluoroethanol
- RMSD
root-mean-square deviation
- GC-MS
gas chromatography-mass spectrometry
Footnotes
This work was supported by the Canada Research Chairs (R.J.W.), Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grants (RGPIN-2015-05163 to J.C.V., RGPIN-2016-06478 to M.J.L., and RGPIN-2014-04585 to R.J.W.), Alberta Innovates Bio Solutions (R.J.W.), Deutsche Forschungsgemeinschaft of the International Research Training Group 1830 (M.J.L.), Alberta Innovates Health Solutions Scholar Program (M.J.L.) and Graduate Scholarship (J.Z.A.), Alberta Innovates Technology Futures Graduate Scholarship (K.M.P.C.), Alberta Canola Producers Commission Graduate Award (K.M.P.C.), and NSERC-International Research Training Group in Membrane Biology Postdoctoral Fellowship (R.P.). Infrastructure used in this work was funded by the Canadian Foundation for Innovation.
Articles can be viewed without a subscription.
References
- Ahmad I, Sharma AK, Daniell H, Kumar S (2015) Altered lipid composition and enhanced lipid production in green microalga by introduction of brassica diacylglycerol acyltransferase 2. Plant Biotechnol J 13: 540–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arutyunova E, Panwar P, Skiba PM, Gale N, Mak MW, Lemieux MJ (2014) Allosteric regulation of rhomboid intramembrane proteolysis. EMBO J 33: 1869–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar-Moreno J, Denolf P, Van Audenhove K, De Bodt S, Engelen S, Fahy D, Wallis JG, Browse J (2015) Type 1 diacylglycerol acyltransferases of Brassica napus preferentially incorporate oleic acid into triacylglycerol. J Exp Bot 66: 6497–6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birch AM, Buckett LK, Turnbull AV (2010) DGAT1 inhibitors as anti-obesity and anti-diabetic agents. Curr Opin Drug Discov Devel 13: 489–496 [PubMed] [Google Scholar]
- Blom N, Gammeltoft S, Brunak S (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294: 1351–1362 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Brown CJ, Takayama S, Campen AM, Vise P, Marshall TW, Oldfield CJ, Williams CJ, Dunker AK (2002) Evolutionary rate heterogeneity in proteins with long disordered regions. J Mol Evol 55: 104–110 [DOI] [PubMed] [Google Scholar]
- Bürgi J, Xue B, Uversky VN, van der Goot FG (2016) Intrinsic disorder in transmembrane proteins: roles in signaling and topology prediction. PLoS ONE 11: e0158594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldo KM, Greer MS, Chen G, Lemieux MJ, Weselake RJ (2015) Purification and properties of recombinant Brassica napus diacylglycerol acyltransferase 1. FEBS Lett 589: 773–778 [DOI] [PubMed] [Google Scholar]
- Chen G, Xu Y, Siloto RMP, Caldo KMP, Vanhercke T, El Tahchy A, Niesner N, Chen Y, Mietkiewska E, Weselake RJ (2017) High performance variants of plant diacylglycerol acyltransferase 1 generated by directed evolution provide insights into structure-function. Plant J http://dx.doi.org/10.1111/tpj.13652 [DOI] [PubMed]
- Christie WW, Han X (2010) Lipid Analysis: Isolation, Separation, Identification and Lipidomic Analysis, Ed 4 Oily Press, Bridgwater, UK [Google Scholar]
- Cornilescu G, Delaglio F, Bax A (1999) Protein backbone angle restraints from searching a database for chemical shift and sequence homology. J Biomol NMR 13: 289–302 [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A (1995) NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR 6: 277–293 [DOI] [PubMed] [Google Scholar]
- DeVita RJ, Pinto S (2013) Current status of the research and development of diacylglycerol O-acyltransferase 1 (DGAT1) inhibitors. J Med Chem 56: 9820–9825 [DOI] [PubMed] [Google Scholar]
- Ding Z, Taneva SG, Huang HK, Campbell SA, Semenec L, Chen N, Cornell RB (2012) A 22-mer segment in the structurally pliable regulatory domain of metazoan CTP:phosphocholine cytidylyltransferase facilitates both silencing and activating functions. J Biol Chem 287: 38980–38991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox SR, Rawsthorne S, Hills MJ (2000) Role of acyl-CoAs and acyl-CoA-binding protein in regulation of carbon supply for fatty acid biosynthesis. Biochem Soc Trans 28: 672–674 [PubMed] [Google Scholar]
- Gibon Y, Vigeolas H, Tiessen A, Geigenberger P, Stitt M (2002) Sensitive and high throughput metabolite assays for inorganic pyrophosphate, ADPGlc, nucleotide phosphates, and glycolytic intermediates based on a novel enzymic cycling system. Plant J 30: 221–235 [DOI] [PubMed] [Google Scholar]
- Greer MS, Pan X, Weselake RJ (2016) Two clades of type-1 Brassica napus diacylglycerol acyltransferase exhibit differences in acyl-CoA preference. Lipids 51: 781–786 [DOI] [PubMed] [Google Scholar]
- Greer MS, Truksa M, Deng W, Lung SC, Chen G, Weselake RJ (2015) Engineering increased triacylglycerol accumulation in Saccharomyces cerevisiae using a modified type 1 plant diacylglycerol acyltransferase. Appl Microbiol Biotechnol 99: 2243–2253 [DOI] [PubMed] [Google Scholar]
- Grisart B, Farnir F, Karim L, Cambisano N, Kim JJ, Kvasz A, Mni M, Simon P, Frère JM, Coppieters W, et al. (2004) Genetic and functional confirmation of the causality of the DGAT1 K232A quantitative trait nucleotide in affecting milk yield and composition. Proc Natl Acad Sci USA 101: 2398–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntert P, Mumenthaler C, Wüthrich K (1997) Torsion angle dynamics for NMR structure calculation with the new program DYANA. J Mol Biol 273: 283–298 [DOI] [PubMed] [Google Scholar]
- Hills MJ, Murphy DJ, Beevers H (1989) Inhibition of neutral lipase from castor bean lipid bodies by coenzyme A (CoA) and oleoyl-CoA. Plant Physiol 89: 1006–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Deeney JT, Nolan CJ, Peyot ML, Ao A, Richard AM, Luc E, Faergeman NJ, Knudsen J, Guo W, et al. (2005) Regulation of lipolytic activity by long-chain acyl-coenzyme A in islets and adipocytes. Am J Physiol Endocrinol Metab 289: E1085–E1092 [DOI] [PubMed] [Google Scholar]
- Iwai M, Ikeda K, Shimojima M, Ohta H (2014) Enhancement of extraplastidic oil synthesis in Chlamydomonas reinhardtii using a type-2 diacylglycerol acyltransferase with a phosphorus starvation-inducible promoter. Plant Biotechnol J 12: 808–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jako C, Kumar A, Wei Y, Zou J, Barton DL, Giblin EM, Covello PS, Taylor DC (2001) Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiol 126: 861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. (2004) Using NMRView to visualize and analyze the NMR spectra of macromolecules. Methods Mol Biol 278: 313–352 [DOI] [PubMed] [Google Scholar]
- Lardizabal K, Effertz R, Levering C, Mai J, Pedroso MC, Jury T, Aasen E, Gruys K, Bennett K (2008) Expression of Umbelopsis ramanniana DGAT2A in seed increases oil in soybean. Plant Physiol 148: 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson TR, Graham IA (2001) A novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J 25: 115–125 [DOI] [PubMed] [Google Scholar]
- Laskowski RA, Gerick F, Thornton JM (2009) The structural basis of allosteric regulation in proteins. FEBS Lett 583: 1692–1698 [DOI] [PubMed] [Google Scholar]
- Lee J, Taneva SG, Holland BW, Tieleman DP, Cornell RB (2014) Structural basis for autoinhibition of CTP:phosphocholine cytidylyltransferase (CCT), the regulatory enzyme in phosphatidylcholine synthesis, by its membrane-binding amphipathic helix. J Biol Chem 289: 1742–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light S, Sagit R, Ekman D, Elofsson A (2013) Long indels are disordered: a study of disorder and indels in homologous eukaryotic proteins. Biochim Biophys Acta 1834: 890–897 [DOI] [PubMed] [Google Scholar]
- Liu Q, Siloto RM, Lehner R, Stone SJ, Weselake RJ (2012) Acyl-CoA:diacylglycerol acyltransferase: molecular biology, biochemistry and biotechnology. Prog Lipid Res 51: 350–377 [DOI] [PubMed] [Google Scholar]
- Lopes JL, Nobre TM, Cilli EM, Beltramini LM, Araújo AP, Wallace BA (2014) Deconstructing the DGAT1 enzyme: binding sites and substrate interactions. Biochim Biophys Acta 1838: 3145–3152 [DOI] [PubMed] [Google Scholar]
- Louis-Jeune C, Andrade-Navarro MA, Perez-Iratxeta C (2012) Prediction of protein secondary structure from circular dichroism using theoretically derived spectra. Proteins 80: 374–381 [DOI] [PubMed] [Google Scholar]
- Lung SC, Chye ML (2016) Deciphering the roles of acyl-CoA-binding proteins in plant cells. Protoplasma 253: 1177–1195 [DOI] [PubMed] [Google Scholar]
- Marín M, Thallmair V, Ott T (2012) The intrinsically disordered N-terminal region of AtREM1.3 remorin protein mediates protein-protein interactions. J Biol Chem 287: 39982–39991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFie PJ, Stone SL, Banman SL, Stone SJ (2010) Topological orientation of acyl-CoA:diacylglycerol acyltransferase-1 (DGAT1) and identification of a putative active site histidine and the role of the N terminus in dimer/tetramer formation. J Biol Chem 285: 37377–37387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger JO, Bornscheuer U (2006) Lipids as renewable resources: current state of chemical and biotechnological conversion and diversification. Appl Microbiol Biotechnol 71: 13–22 [DOI] [PubMed] [Google Scholar]
- Nagy HM, Paar M, Heier C, Moustafa T, Hofer P, Haemmerle G, Lass A, Zechner R, Oberer M, Zimmermann R (2014) Adipose triglyceride lipase activity is inhibited by long-chain acyl-coenzyme A. Biochim Biophys Acta 1841: 588–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykiforuk CL, Furukawa-Stoffer TL, Huff PW, Sarna M, Laroche A, Moloney MM, Weselake RJ (2002) Characterization of cDNAs encoding diacylglycerol acyltransferase from cultures of Brassica napus and sucrose-mediated induction of enzyme biosynthesis. Biochim Biophys Acta 1580: 95–109 [DOI] [PubMed] [Google Scholar]
- Ohlrogge JB, Jaworski JG (1997) Regulation of fatty acid synthesis. Annu Rev Plant Physiol Plant Mol Biol 48: 109–136 [DOI] [PubMed] [Google Scholar]
- Pufall MA, Graves BJ (2002) Autoinhibitory domains: modular effectors of cellular regulation. Annu Rev Cell Dev Biol 18: 421–462 [DOI] [PubMed] [Google Scholar]
- Roesler K, Shen B, Bermudez E, Li C, Hunt J, Damude HG, Ripp KG, Everard JD, Booth JR, Castaneda L, et al. (2016) An improved variant of soybean type 1 diacylglycerol acyltransferase increases the oil content and decreases the soluble carbohydrate content of soybeans. Plant Physiol 171: 878–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio S, Whitehead L, Larson TR, Graham IA, Rodriguez PL (2008) The coenzyme A biosynthetic enzyme phosphopantetheine adenylyltransferase plays a crucial role in plant growth, salt/osmotic stress resistance, and seed lipid storage. Plant Physiol 148: 546–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandager L, Gustavsson MH, Ståhl U, Dahlqvist A, Wiberg E, Banas A, Lenman M, Ronne H, Stymne S (2002) Storage lipid synthesis is non-essential in yeast. J Biol Chem 277: 6478–6482 [DOI] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockey JM, Gidda SK, Chapital DC, Kuan JC, Dhanoa PK, Bland JM, Rothstein SJ, Mullen RT, Dyer JM (2006) Tung tree DGAT1 and DGAT2 have nonredundant functions in triacylglycerol biosynthesis and are localized to different subdomains of the endoplasmic reticulum. Plant Cell 18: 2294–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siloto RM, Madhavji M, Wiehler WB, Burton TL, Boora PS, Laroche A, Weselake RJ (2008) An N-terminal fragment of mouse DGAT1 binds different acyl-CoAs with varying affinity. Biochem Biophys Res Commun 373: 350–354 [DOI] [PubMed] [Google Scholar]
- Siloto RMP, Truksa M, He X, McKeon T, Weselake RJ (2009) Simple methods to detect triacylglycerol biosynthesis in a yeast-based recombinant system. Lipids 44: 963–973 [DOI] [PubMed] [Google Scholar]
- Sun X, Jones WT, Harvey D, Edwards PJ, Pascal SM, Kirk C, Considine T, Sheerin DJ, Rakonjac J, Oldfield CJ, et al. (2010) N-terminal domains of DELLA proteins are intrinsically unstructured in the absence of interaction with GID1/gibberellic acid receptors. J Biol Chem 285: 11557–11571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DC, Zhang Y, Kumar A, Francis T, Giblin EM, Barton DL, Ferrie JR, Laroche A, Shah S, Zhu W, et al. (2009) Molecular modification of triacylglycerol accumulation by over-expression of DGAT1 to produce canola with increased seed oil content under field conditions. Botany 87: 533–543 [Google Scholar]
- Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31: 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau T, Nassar R, Cumberworth A, Wong ET, Woollard G, Gsponer J (2013) Structure and intrinsic disorder in protein autoinhibition. Structure 21: 332–341 [DOI] [PubMed] [Google Scholar]
- Tumaney AW, Ohlrogge JB, Pollard M (2004) Acetyl coenzyme A concentrations in plant tissues. J Plant Physiol 161: 485–488 [DOI] [PubMed] [Google Scholar]
- Uversky VN, Gillespie JR, Fink AL (2000) Why are “natively unfolded” proteins unstructured under physiologic conditions? Proteins 41: 415–427 [DOI] [PubMed] [Google Scholar]
- Wallace AC, Laskowski RA, Thornton JM (1995) LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein Eng 8: 127–134 [DOI] [PubMed] [Google Scholar]
- Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT (2004) Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol 337: 635–645 [DOI] [PubMed] [Google Scholar]
- Weselake RJ, Madhavji M, Szarka SJ, Patterson NA, Wiehler WB, Nykiforuk CL, Burton TL, Boora PS, Mosimann SC, Foroud NA, et al. (2006) Acyl-CoA-binding and self-associating properties of a recombinant 13.3 kDa N-terminal fragment of diacylglycerol acyltransferase-1 from oilseed rape. BMC Biochem 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weselake RJ, Shah S, Tang M, Quant PA, Snyder CL, Furukawa-Stoffer TL, Zhu W, Taylor DC, Zou J, Kumar A, et al. (2008) Metabolic control analysis is helpful for informed genetic manipulation of oilseed rape (Brassica napus) to increase seed oil content. J Exp Bot 59: 3543–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wider G, Macura S, Kumar A, Ernst RR, Wüthrich K (1984) Homonuclear two-dimensional 1H NMR of proteins: experimental procedures. J Magn Reson 56: 207–234 [Google Scholar]
- Winter A, Krämer W, Werner FAO, Kollers S, Kata S, Durstewitz G, Buitkamp J, Womack JE, Thaller G, Fries R (2002) Association of a lysine-232/alanine polymorphism in a bovine gene encoding acyl-CoA:diacylglycerol acyltransferase (DGAT1) with variation at a quantitative trait locus for milk fat content. Proc Natl Acad Sci USA 99: 9300–9305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PE, Dyson HJ (2015) Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol 16: 18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüthrich K. (1986) NMR of Proteins and Nucleic Acids. Wiley-Interscience, New York [Google Scholar]
- Yu J, Li Y, Zou F, Xu S, Liu P (2015) Phosphorylation and function of DGAT1 in skeletal muscle cells. Biophys Rev 1: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurchenko OP, Weselake RJ (2011) Involvement of low molecular mass soluble acyl-CoA-binding protein in seed oil biosynthesis. N Biotechnol 28: 97–109 [DOI] [PubMed] [Google Scholar]
- Zammit VA, Buckett LK, Turnbull AV, Wure H, Proven A (2008) Diacylglycerol acyltransferases: potential roles as pharmacological targets. Pharmacol Ther 118: 295–302 [DOI] [PubMed] [Google Scholar]