Abstract
The demand for establishment of high-throughput biodosimetric methods is increasing. Our aim in this study was to identify low-molecular-weight urinary radiation-responsive molecules using electrospray ionization Fourier transform mass spectrometry (ESI-FT MS), and our final goal was to develop a sensitive biodosimetry technique that can be applied in the early triage of a radiation emergency medical system. We identified nine metabolites by statistical comparison of mouse urine before and 8 h after irradiation. Time-course analysis showed that, of these metabolites, thymidine and either thymine or imidazoleacetic acid were significantly increased dose-dependently 8 h after radiation exposure; these molecules have already been reported as potential radiation biomarkers. Phenyl glucuronide was significantly decreased 8 h after radiation exposure, irrespective of the dose. Histamine and 1-methylhistamine were newly identified by MS/MS and showed significant, dose-dependent increases 72 h after irradiation. Quantification of 1-methylhistamine by enzyme-linked immunosorbent assay (ELISA) analysis also showed a significant increase 72 h after 4 Gy irradiation. These results suggest that urinary metabolomics screening using ESI-FT MS can be a powerful tool for identifying promising radiation-responsive molecules, and that urinary 1-methylhistamine is a potential radiation-responsive molecule for acute, high-dose exposure.
Keywords: biodosimetry, metabolomics analysis, urine, 1-methylhistamine
INTRODUCTION
Human beings have already experienced some serious radiation accidents. Among these, the JCO criticality accident, in which three workers at a conversion test facility in Japan were heavily exposed to radiation (up to 20 GyEq) in 1999, is relatively fresh in our mind [1]. These workers showed varying symptoms of acute radiation syndrome. On the other hand, fortunately, after the Fukushima Daiichi nuclear power plant disaster in Japan, which occurred in March 2011, none of the workers showed acute radiation syndrome [2]. As radiation usage is increasing, not only in nuclear power plants, but also in medical and industrial applications, another radiation disaster could occur. Dose estimation using biological materials may save a person who has potentially been exposed to ionizing radiation, because it is well known that the health effects induced by radiation exposure are completely dependent on the total radiation dose, as indicated by the JCO accident [1]. To date, counting chromosome aberrations, including dicentric and ring chromosomes, has been the most accurate method for estimating exposure dose; however, it is time-consuming and requires expertise. Therefore, the development of a simple high-throughput method to evaluate the existence of radiation exposure is desirable.
Using matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry, we have previously shown that hepcidin-2 in mouse urine is a radiation-responsive candidate molecule [3]. The upregulation pattern of hepcidin-2 was shown to differ between high- and low-dose radiation exposure and was induced, at least in part, by the expression of the hepcidin-2 gene in the liver. These results indicated that newly developed, high-sensitivity mass spectrometry techniques can enable us to identify new radiation biomarkers for dose estimation.
Diverse materials, including organic acids, amino acids, purines, pyrimidines and monosaccharides, exist not only in blood but also in urine, and measurements of these low-molecular-weight metabolites in blood or urine have provided much useful information for disease diagnosis. Urine normally contains a low protein level, a small number of cells, and many metabolites, and has a metabolic signature derived from many biochemical pathways [4]. Measurement of specific metabolite levels in urine has become an important method for detecting disease in its early stages [5, 6]. The individual metabolic state is regarded as normally stable [7], which suggests that the damage induced by radiation exposure might alter the amount of certain metabolites in urine, ideally in a dose-dependent manner.
In this study, urine specimens obtained from mice before and after radiation exposure, or after sham-irradiation, were analyzed by electrospray ionization Fourier transform mass spectrometry (ESI-FT MS) to identify radiation-responsive molecules, particularly low-molecular-weight metabolites. The statistical comparison of mouse urine samples before and 8 h after irradiation allowed us to identify nine candidate metabolites. Time-course analysis showed that thymidine and either thymine or imidazoleacetic acid, which have already been reported as potential radiation biomarkers, were significantly increased dose-dependently 8 h after radiation exposure [8, 9]. Levels of phenyl glucuronide were significantly decreased 8 h after radiation exposure, irrespective of the dose. Histamine and 1-methylhistamine were newly identified by MS/MS and showed significant, dose-dependent increases 72 h after irradiation. Quantification of 1-methylhistamine by enzyme-linked immunosorbent assay (ELISA) showed a significant increase 72 h after 4 Gy irradiation.
MATERIALS AND METHODS
Ethics
The Animal Experimentation Committee of Hiroshima University, Hiroshima, Japan, approved all the animal experiments.
Animal experiments and gamma irradiation
The animal experiments and gamma irradiation technique have been previously described in detail [3]. Eight-week-old male B6C3F1/Crlj mice were purchased from Charles River Laboratories Japan Inc. (Yokohama, Japan). At nine weeks of age, the mice were exposed to whole-body gamma radiation from a 137Cs source (Gammacell; Best Theratronics, Ottawa, Canada) at a dose rate of 1.0 Gy/min, or sham-irradiated. The urine samples of each mouse were collected directly into tubes before and after gamma irradiation. We could not collect urine from all mice at each time point; irradiated mice appeared to have smaller urine volumes. For this reason, we analyzed the urine of only a subset of mice. Collected urine samples were kept at −80°C until analysis. Each animal experiment (Table 1; n = 6, Figs 3 and 4; n = 5) was performed independently.
Table 1.
Nine metabolites satisfying the statistical comparison between mouse urine before and 8 h after 4 Gy irradiation
| m/z | Before irradiation | 8 h after 4 Gy irradiation | P valuea | Log fold changeb | Predicted metabolitesc | ||
|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | ||||
| 265.080 | 0.0048 | 0.0032 | 0.13 | 0.043 | 0.0009 | 1.4 | Thymidine |
| 112.051 | 0.0070 | 0.0038 | 0.024 | 0.0064 | 0.0005 | 0.54 | Cytosine |
| 200.035 | 0.015 | 0.0051 | 0.045 | 0.0087 | 0.0001 | 0.47 | N-Formylmethionine |
| 103.039 | 0.0088 | 0.0057 | 0.022 | 0.0076 | 0.007 | 0.40 | either Succinic acid semialdehyde, Acetoacetic acid or 3-methyl pyruvic acid |
| 112.087 | 0.13 | 0.030 | 0.30 | 0.061 | 0.0005 | 0.36 | Histamine |
| 126.103 | 0.11 | 0.027 | 0.24 | 0.022 | 0.000005 | 0.34 | 1-Methylhistamine |
| 127.050 | 0.014 | 0.0025 | 0.029 | 0.0064 | 0.001 | 0.33 | Thymine or Imidazoleacetic acid |
| 250.039 | 0.0049 | 0.0013 | 0.0020 | 0.00082 | 0.002 | −0.39 | Norepinephrine sulfate |
| 293.064 | 0.039 | 0.019 | 0.0067 | 0.0041 | 0.007 | −0.77 | Phenyl glucuronide |
aWelch's t-test.
bLog ratio of the average intensity value of ‘8 h after 4 Gy irradiation’ to that of ‘before irradiation’.
cPredicted formulae of each molecular mass were determined by METLIN, and were searched against the Human Metabolome Database to match previously identified metabolites in normal human urine.
Fig. 3.
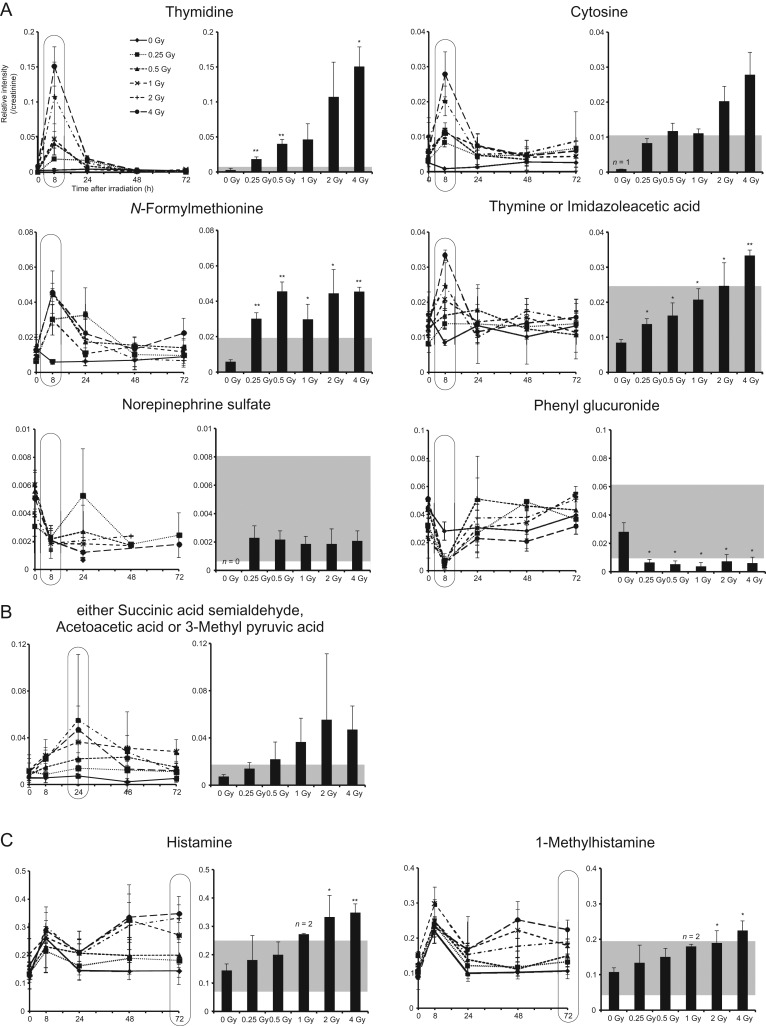
Urinary excretion of the candidate metabolites. (Left) Relative intensity shown as the ratio of intensity of (A) thymidine, cytosine, N-formylmethionine, thymine or imidazoleacetic acid, norepinephrine sulfate and phenyl glucuronide, (B) either succinic acid semialdehyde, acetoacetic acid or 3-methyl pyruvic acid and (C) histamine and 1-methylhistamine, to that of creatinine. (Right) Dose-dependent changes were observed. The time point selected is indicated with circles in the corresponding left panel. The results are presented as mean ± SD. **P < 0.01, *P < 0.05, compared with sham-irradiated samples. Shaded portion shows mean ± 2 SD of each measurement of all sham-irradiated and unirradiated (0 h time point) mice urine. Due to the limit of the measurements and the number of mice available (n = 5), some dose/time points were missed, or are represented by one single, or the average of two, intensity values.
Fig. 4.
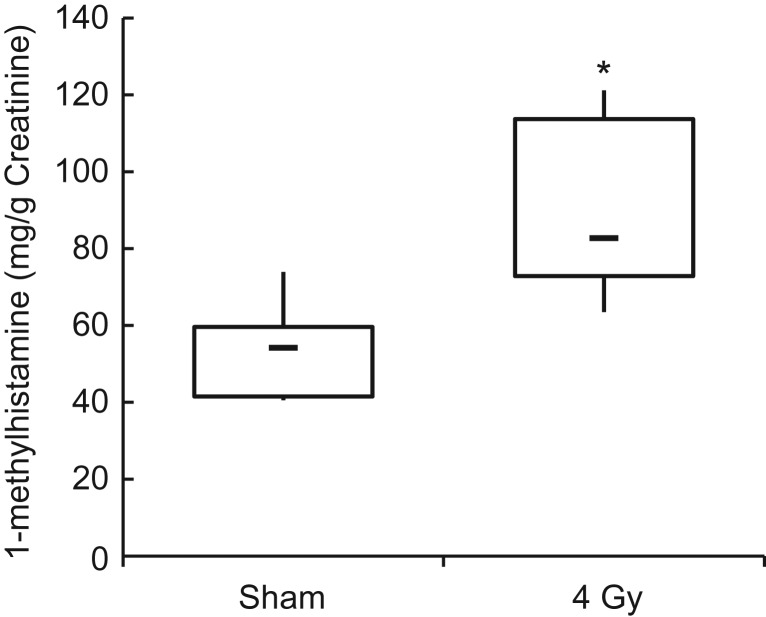
Box-and-whisker plots showing the amount of 1-methylhistamine in mouse urine (n = 5), as measured by ELISA. 1-methylhistamine levels were normalized with creatinine (mg/g creatinine). Vertical bars extend from the minimum to the maximum value; box, 25 and 75 percentiles; horizontal bar, median. *P < 0.05, compared with sham-irradiated samples.
Electrospray ionization Fourier transform mass spectrometry analysis
Defrosted urine samples (10 μl) were mixed with 990 μl of ice-cold methanol/0.1% formic acid (v/v) and deproteinized by centrifugation. Alpha-cyano-4-hydroxycinnamic acid was added to each deproteinized sample at 40 μM final concentration. ESI-FT MS was performed on an LTQ Orbitrap XL (Thermo Fisher Scientific, San Jose, CA) equipped with an autosampler (Accela, Thermo Fisher Scientific). Samples (5 μl) were injected at a flow rate of 200 μl/min using methanol as the mobile phase. Positive ion mode was set as follows: ion source voltage 5 kV, capillary voltage 32 V, capillary temperature 330°C, sheath gas flow 35 l/h, aux gas flow 10 l/h. Data within the range m/z 100 to 1000 were collected for 1 min, and maximum injection time was 500 ms. The MS resolution was set at 60 000 and mass calibration was performed using the Polytyrosine 1, 3, 6 standard (CS Bio Corp., Menlo Park, CA). The obtained spectra were processed with Xcalibur Qual Browser software (Thermo Fisher Scientific), and after normalization with creatinine, these values were statistically analyzed.
To identify putative urinary radiation-responsive molecules, MS/MS measurements were performed using an LTQ Orbitrap XL equipped with a fine-precision XYZ stage (NTMS, Nikkyo Technos Co. Ltd, Tokyo, Japan). Urine samples (5 µl) were loaded into a nanospray tip (HUMANIX, Hiroshima, Japan) using GELoader tips (Eppendorf, Hamburg, Germany). Positive ion mode was set as follows: ion source voltage 1.0–1.3 kV, capillary voltage 32 V, capillary temperature 120°C. The MS resolution was set at 60 000. Data within the range m/z 100 to 1000 were collected; maximum injection time was 500 ms. After selection of the precursor ion, data within the range m/z 50 to 500 were collected under a collision energy of 20–30 eV. Mass calibration was performed using the Polytyrosine 1, 3, 6 standard. The obtained spectra were processed using Xcalibur Qual Browser software.
ELISA analysis of 1-methylhistamine
ELISA analysis of 1-methylhistamine in the urine samples was performed using a 1-methylhistamine ELISA kit (DLD Diagnostika GmbH, Hamburg, Germany) according to the manufacturer's instructions. ELISA analysis of creatinine was also performed according to the manufacturer's instructions (R&D systems, Minneapolis, MN), to normalize the obtained data.
Data analysis
To identify candidate metabolites, each molecular mass was searched against the METLIN database (Metabolite and Tandem MS Database; http://metlin.scripps.edu/) at an accuracy of 5 ppm with either hydrogen, sodium or potassium adducts, and then each possible formula was searched against the Human Metabolome Database (http://www.urinemetabolome.ca/) for matches with known metabolites in normal human urine. The results are presented as mean ± SD. Statistically significant differences between groups were tested with Welch's t-test, and P < 0.05 was accepted as statistically significant.
RESULTS
Identification of radiation-responsive, low-molecular-weight molecules in mouse urine
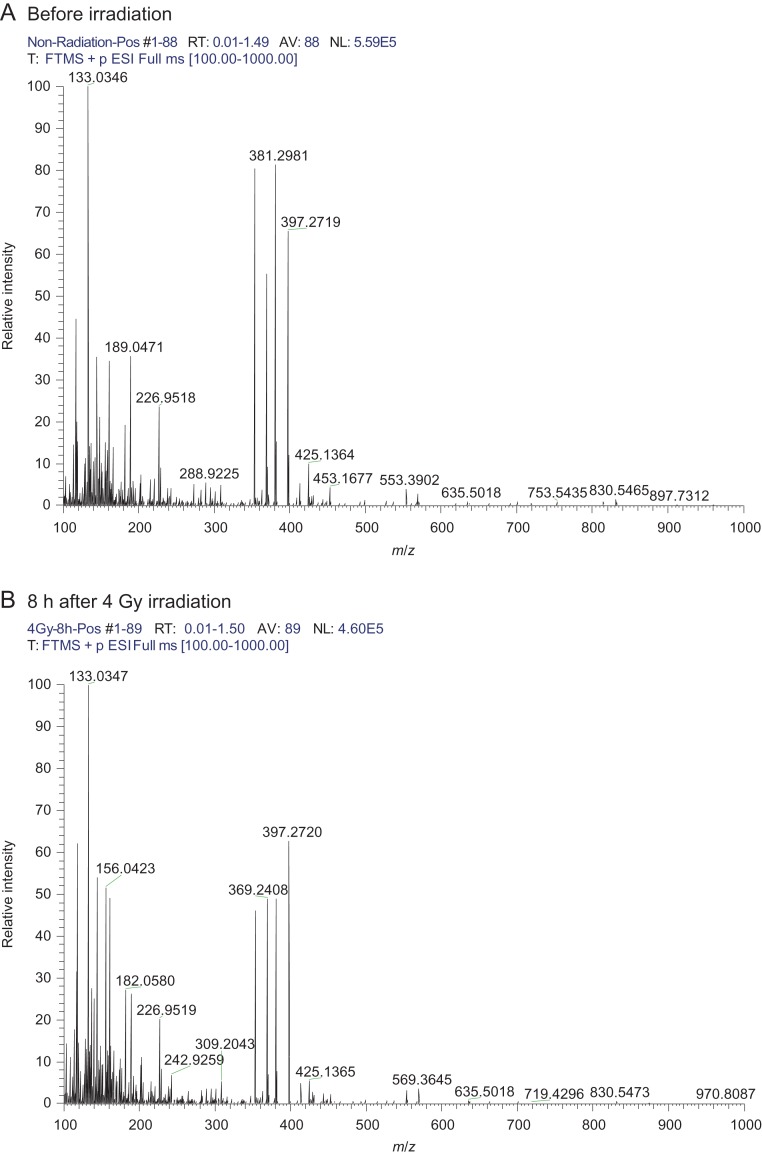
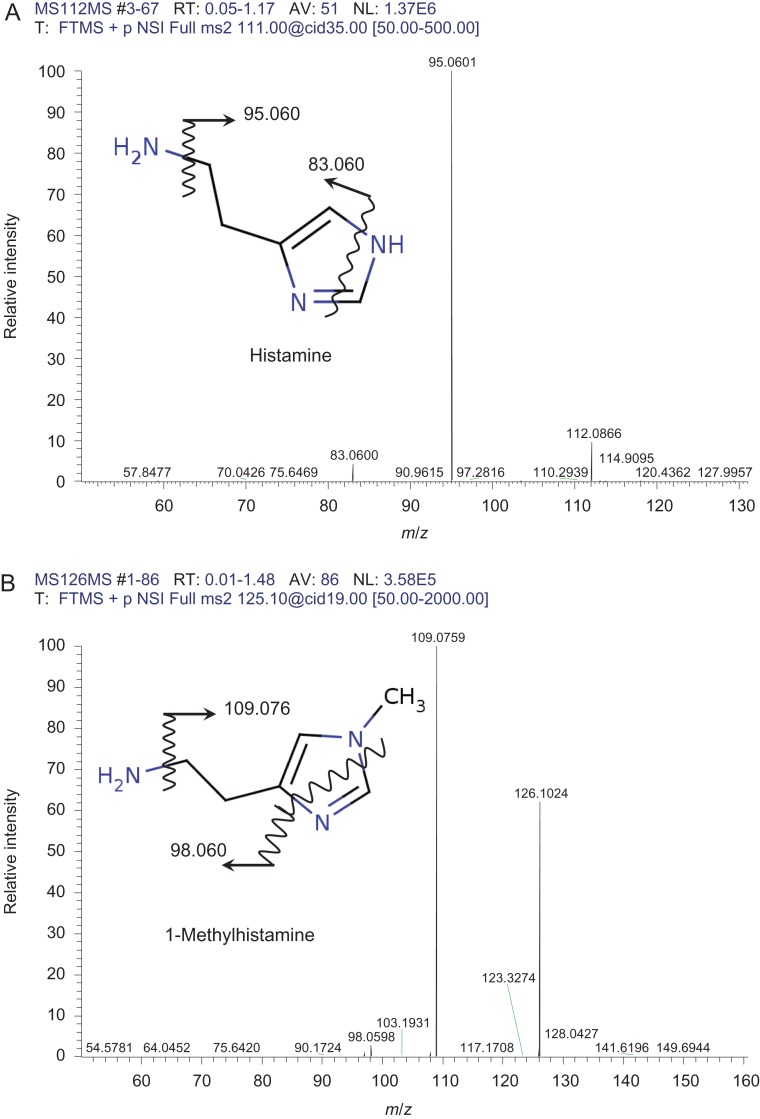
To identify urinary radiation-responsive, low-molecular-weight molecules, ESI-FT MS analysis was performed. Figure 1 shows representative ESI-FT MS spectra from mouse urine. The average peak intensity of mouse urine before, and 8 h after 4 Gy irradiation was compared statistically (Welch's t-test, P < 0.01), and an absolute log fold change >0.30 was set as the significance cut-off, identifying 39 candidate changes in peak intensity. Of these metabolites, thymidine (m/z 265.080), cytosine (m/z 112.051), N-formylmethionine (m/z 200.035), either succinic acid semialdehyde, acetoacetic acid or 3-methyl pyruvic acid (m/z 103.039), histamine (m/z 112.087), 1-methylhistamine (m/z 126.103), thymine or imidazoleacetic acid (m/z 127.050), norepinephrine sulfate (m/z 250.039) and phenyl glucuronide (m/z 293.064) were identified by a database search, summarized in Table 1 (a detailed summary of all metabolites is shown in Supplementary Table 1). Histamine and 1-methylhistamine were identified and verified through the database search and MS/MS analysis (Fig. 2A and B).
Fig. 1.
Representative electrospray ionization Fourier transform mass spectrometry spectra of mouse urine. Vertical and horizontal axes represent relative intensity and mass-to-charge ratio (m/z), respectively. Urine samples were collected before (A) and 8 h after 4 Gy irradiation (B).
Fig. 2.
Representative MS/MS fragmentation spectra of (A) histamine and (B) 1-methylhistamine. Vertical and horizontal axes represent relative intensity and mass-to-charge ratio (m/z), respectively. Proposed fragmentation patterns are shown in the molecular diagram. These fragmentation spectra were identical to those shown in the database (Wiley Subscription Services, Inc.)
Time-course analysis of these candidate metabolites after radiation exposure
To evaluate the possibility of using the candidate metabolites (as shown in Table 1) as radiation-responsive molecules, time-course analysis was performed using ESI-FT MS (Fig. 3, Supplementary Fig. 1 and Supplementary Table 2). Time-course analysis showed that the kinetics after radiation exposure were roughly divided into three patterns (i.e. the peak changes that were induced at 8 h, 24 h and 72 h after irradiation). It was shown that levels of thymidine, cytosine, N-formylmethionine and either thymine or imidazoleacetic acid were increased 8 h after irradiation, and then decreased to basal levels 48 h after irradiation (Fig. 3A, left panel). Of these, levels of thymidine and either thymine or imidazoleacetic acid were significantly increased dose-dependently 8 h after irradiation (Fig. 3A, right panel). Levels of N-formylmethionine and phenyl glucuronide were significantly increased and decreased, respectively, 8 h after irradiation, irrespective of the dose (Fig. 3A, right panel). The change in norepinephrine sulfate level was shown not to be radiation responsive, partly due to the variety of individual differences, the limit of the measurements and the number of mice available (n = 5). Levels of either succinic acid semialdehyde, acetoacetic acid or 3-methyl pyruvic acid were also shown to have increased dose-dependently 24 h after irradiation (Fig. 3B, right panel). In contrast, 8 h after irradiation, the histamine and 1-methylhistamine levels increased irrespective of the dose, suggesting a possible stress response effect induced by the experimental procedure for these mice (Fig. 3C, left panel). Levels of both metabolites were significantly increased dose-dependently 72 h after irradiation (Fig. 3C, left panel). Finally, we quantified 1-methylhistamine levels. ELISA analysis showed a statistically significant increase of 1-methylhistamine 72 h after 4 Gy irradiation (Fig. 4).
DISCUSSION
In this study, we identified 39 radiation-responsive candidate metabolites through changes in peak intensity by using ESI-FT MS. A database search identified those peaks showing changes as nine metabolites (Table 1); histamine and 1-methylhistamine were also verified by MS/MS analysis (Fig. 2). Time-course experiments showed that levels of all of these metabolites, with the exception of N-formylmethionine, norepinephrine sulfate and phenyl glucuronide, were increased dose-dependently after radiation exposure, but the kinetics were not identical. Five metabolites showed significant increases either 8 h (thymidine, N-formylmethionine and either thymine or imidazoleacetic acid) or 72 h (histamine and 1-methylhistamine) after radiation exposure (Fig. 3). On the other hand, the level of phenyl glucuronide was significantly decreased 8 h after irradiation. ELISA analysis showed a statistically significant increase in 1-methylhistamine at 72 h after 4 Gy irradiation (Fig. 4).
Thymidine, thymine and cytosine have already been identified as biomarkers of radiation exposure [8, 9], and our data (Fig. 3A) showed the same tendency, suggesting the possibility of identical metabolites.
Histamine is known to be involved in immediate-type allergy reactions, and is produced by macrophages and neutrophils during inflammation [10, 11]. Exposure to ionizing radiation has been reported to increase blood levels of histamine in humans undergoing radiation therapy [12], as well as in irradiated rats [13]. Recent findings demonstrated that histamine treatment reduced mucosal injury of the small intestine after radiation exposure [14], and that histamine reduced ionizing radiation toxicity in bone marrow cells in mice [15]. These observations suggest that histamine release has an important physiological role in counteracting the acute effects of radiation exposure.
1-Methylhistamine is formed from histamine by the enzyme histamine methyltransferase [16]. Endogenous 1-methylhistamine has been found in human body fluids and in tissues. It is well known that the intake of histamine-rich food may influence the urinary excretion levels of histamine and 1-methylhistamine [17]. Evaluations of 1-methylhistamine have shown it to be a good surrogate marker for histamine, as 1-methylhistamine is more stable than histamine in urine [18]. The increase in urinary 1-methylhistamine was also shown to be an indicator of histamine release in immediate allergic reactions [19]. Our observation indicated that 1-methylhistamine was a potential radiation-responsive molecule. However, the use of this molecule as a radiation biomarker might be limited only to high-dose exposure because the release of histamine is also induced by either inflammation or the stress response (Fig. 3C). Imidazoleacetic acid is another histamine metabolite, converted by diamine oxidase [16]. We could not identify m/z 127.050 by MS/MS analysis; however, the time-course analysis result suggested that it was mainly thymine, as the kinetics look similar to those of thymidine. Biological significance of the changes in the other radiation-responsive candidate metabolites (N-formylmethionine, either succinic acid semialdehyde, acetoacetic acid or 3-methyl pyruvic acid, and phenyl glucuronide) has not yet been demonstrated, and further investigation will be needed.
Increased DNA degradation and cell turnover are events that take place after the initial responses to radiation, and are key processes for the urinary excretion of metabolites including thymidine, cytosine and thymine [8, 9]. The excretion of thymidine in urine has been reported to be undetectable by 48 h after irradiation [20], consistent with our observation (Fig. 3 and Supplementary Fig. 1). On the other hand, histamine and its derivative, 1-methylhistamine, are related to inflammation, and we observed them to increase gradually at least up to 72 h after irradiation. Thus, the mechanism of induction of these metabolites, at least in part, explains their different time-dependent changes in level (Supplementary Fig. 1). This would be the advantage of using 1-methylhistamine as a biomarker of high-dose-radiation exposure, because many of the affected people in a radiation accident could be examined only after a few days. Development of radiation biomarkers for a longer time (1 week) after radiation exposure is thus warranted. Indeed, some metabolites associated with perturbation of important biological processes (fatty acid beta oxidation, steroid hormone biosynthesis, etc.) have been identified as longer-term radiation biomarkers [21]. Combinations of biomarkers measured at different time points will improve the accuracy of radiation biodosimetry.
In the present study, we describe an analysis of candidate metabolites of radiation exposure using positive ion mode ESI-FT MS. In general, MS coupled with reversed-phase ultra-performance liquid chromatography has been predominantly applied in radiation-metabolomics analysis [9, 22–24]. This reversed-phase liquid chromatographic separation is a very popular and reliable method; however, the ability to analyze polarized, ionic species (e.g. amino acids) is limited due to poor retention of these molecules [25]. Without applying chromatographic separation, we could identify histamine (known to have a high polarity) using ESI-FT MS analysis. ESI positive ion mode is known to be more sensitive than negative ion mode, due to the ease of cationization of molecules. Analysis in negative ion mode will be necessary for the establishment of radiation biodosimetric methods.
In conclusion, our observations have demonstrated the possibility of using metabolites, including 1-methylhistamine, as radiation-responsive molecules, but further investigation is warranted for the development of new biodosimetry methods for radiation exposure.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank M. Yano and J. Takemoto for technical assistance, and the staff at the Radiation Research Center for Frontier Science Facilities, Hiroshima University, for managing the radiation sources. This study was presented at the meeting of the Young Radiation Biologists’ Association of Japan (28 March 2013).
SUPPLEMENTARY DATA
Supplementary data are available at the Journal of Radiation Research online.
FUNDING
This work was supported in part by Grants-in-Aid for both Challenging Exploratory Research and Young Scientists (B) from the Ministry of Education, Culture, Sports, Science and Technology, Japan [No. 25550031 to K.K., 23710071 to D.I.].
CONFLICT OF INTEREST
The authors declare that no conflict of interest is associated with this manuscript.
REFERENCES
- 1. Akashi M, Hirama T, Tanosaki S et al. . Initial symptoms of acute radiation syndrome in the JCO criticality accident in Tokai-mura. J Radiat Res 2001;42:S157–66. [DOI] [PubMed] [Google Scholar]
- 2. Suto Y, Hirai M, Akiyama M et al. . Biodosimetry of restoration workers for the Tokyo Electric Power Company (TEPCO) Fukushima Daiichi nuclear power station accident. Health Phys 2013;105:366–73. [DOI] [PubMed] [Google Scholar]
- 3. Iizuka D, Yoshioka S, Kawai H et al. . Hepcidin-2 in mouse urine as a candidate radiation-responsive molecule. J Radiat Res 2016;57:142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang X, Zhang A, Han Y et al. . Urine metabolomics analysis for biomarker discovery and detection of jaundice syndrome in patients with liver disease. Mol Cell Proteomics 2012;11:370–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Won AJ, Kim S, Kim YG et al. . Discovery of urinary metabolomic biomarkers for early detection of acute kidney injury. Mol Biosyst 2016;12:133–44. [DOI] [PubMed] [Google Scholar]
- 6. Liang Q, Liu H, Wang C et al. . Phenotypic characterization analysis of human hepatocarcinoma by urine metabolomics approach. Sci Rep 2016;6:19763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bernini P, Bertini I, Luchinat C et al. . Individual human phenotypes in metabolic space and time. J Proteome Res 2009;8:4264–71. [DOI] [PubMed] [Google Scholar]
- 8. Lanz C, Patterson AD, Slavik J et al. . Radiation metabolomics. 3. Biomarker discovery in the urine of gamma-irradiated rats using a simplified metabolomics protocol of gas chromatography–mass spectrometry combined with random forests machine learning algorithm. Radiat Res 2009;172:198–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tyburski JB, Patterson AD, Krausz KW et al. . Radiation metabolomics. 1. Identification of minimally invasive urine biomarkers for gamma-radiation exposure in mice. Radiat Res 2008;170:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shiraishi M, Hirasawa N, Oikawa S et al. . Analysis of histamine-producing cells at the late phase of allergic inflammation in rats. Immunology 2000;99:600–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shiraishi M, Hirasawa N, Kobayashi Y et al. . Participation of mitogen-activated protein kinase in thapsigargin- and TPA-induced histamine production in murine macrophage RAW 264.7 cells. Br J Pharmacol 2000;129:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lasser EC, Stenstrom KW. Elevation of circulating blood histamine in patients undergoing deep roentgen therapy. Am J Roentgenol Radium Ther Nucl Med 1954;72:985–8. [PubMed] [Google Scholar]
- 13. Weber RP, Steggerda FR. Histamine in rat plasma; correlation with blood pressure changes following X-irradiation. Proc Soc Exp Biol Med 1949;70:261–3. [DOI] [PubMed] [Google Scholar]
- 14. Medina VA, Croci M, Mohamad NA et al. . Mechanisms underlying the radioprotective effect of histamine on small intestine. Int J Radiat Biol 2007;83:653–63. [DOI] [PubMed] [Google Scholar]
- 15. Medina VA, Croci M, Carabajal E et al. . Histamine protects bone marrow against cellular damage induced by ionising radiation. Int J Radiat Biol 2010;86:283–90. [DOI] [PubMed] [Google Scholar]
- 16. Green JP, Prell GD, Khandelwal JK et al. . Aspects of histamine metabolism. Agents Actions 1987;22:1–15. [DOI] [PubMed] [Google Scholar]
- 17. Imamura I, Watanabe T, Maeyama K et al. . Effect of food intake on urinary excretions of histamine, N tau-methylhistamine, imidazole acetic acid and its conjugate(s) in humans and mice. J Biochem 1984;96:1931–7. [DOI] [PubMed] [Google Scholar]
- 18. Keyzer JJ, Breukelman H, Wolthers BG et al. . Measurement of N tau-methylhistamine concentrations in plasma and urine as a parameter for histamine release during anaphylactoid reactions. Agents Actions 1985;16:76–9. [DOI] [PubMed] [Google Scholar]
- 19. Stephan V, Zimmermann A, Kühr J et al. . Determination of N-methylhistamine in urine as an indicator of histamine release in immediate allergic reactions. J Allergy Clin Immunol 1990;86:862–8. [DOI] [PubMed] [Google Scholar]
- 20. Mak TD, Tyburski JB, Krausz KW et al. . Exposure to ionizing radiation reveals global dose- and time-dependent changes in the urinary metabolome of rat. Metabolomics 2015;11:1082–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pannkuk EL, Laiakis EC, Authier S et al. . Global metabolomic identification of long-term dose-dependent urinary biomarkers in nonhuman primates exposed to ionizing radiation. Radiat Res 2015;184:121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson CH, Patterson AD, Krausz KW et al. . Radiation metabolomics. 5. Identification of urinary biomarkers of ionizing radiation exposure in nonhuman primates by mass spectrometry-based metabolomics. Radiat Res 2012;178:328–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson CH, Patterson AD, Krausz KW et al. . Radiation metabolomics. 4. UPLC-ESI-QTOFMS-based metabolomics for urinary biomarker discovery in gamma-irradiated rats. Radiat Res 2011;175:473–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tyburski JB, Patterson AD, Krausz KW et al. . Radiation metabolomics. 2. Dose- and time-dependent urinary excretion of deaminated purines and pyrimidines after sublethal gamma-radiation exposure in mice. Radiat Res 2009;172:42–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gika HG, Theodoridis GA, Plumb RS et al. . Current practice of liquid chromatography–mass spectrometry in metabolomics and metabonomics. J Pharm Biomed Anal 2014;87:12–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.