Abstract
Stenosis from venous neointimal hyperplasia is common in native arteriovenous fistulas (AVFs). However, the preexisting histologic characteristics of veins at fistula creation, and associations thereof with baseline patient factors, have not been well characterized. In this study, we conducted histologic analysis of a segment of the vein used for anastomosis creation, obtained during AVF creation from 554 of the 602 participants in the multicenter Hemodialysis Fistula Maturation Cohort Study. We quantified intimal and medial areas and lengths of the internal and external elastic lamina by morphometry and assessed venous wall cells by immunohistochemistry, extracellular matrix with Movat stain, and calcium deposition by alizarin red stain. We also studied a representative subset of veins for markers of monocyte/macrophage content, cell proliferation, apoptosis, and neoangiogenesis. Neointima occupied >20% of the lumen in 57% of fully circumferential vein samples, and neointimal hyperplasia associated positively with age and inversely with black race. The neointima was usually irregularly thickened, sometimes concentric, and contained α–smooth muscle actin–expressing cells of smooth muscle or myofibroblast origin. Proteoglycans admixed with lesser amounts of collagen constituted the predominant matrix in the neointima. In 82% of vein samples, the media of vessel walls contained large aggregates of collagen. A minority of veins expressed markers of inflammation, cell proliferation, cell death, calcification, or neoangiogenesis. In conclusion, we observed preexisting abnormalities, including neointimal hyperplasia and prominent accumulation of extracellular matrix, in veins used for AVF creation from a substantial proportion of this cohort.
Keywords: arteriovenous access, arteriovenous fistula, pathology, dialysis access
The arteriovenous fistula (AVF) is the preferred means of vascular access enabling maintenance hemodialysis. However, many AVFs (50%–60%) fail to adequately mature to support the hemodynamic requirements of the most common method of treatment of ESRD in the United States.1,2 Several previous studies have shown that venous neointimal hyperplasia may be present within segments of veins adjacent to the segments used for fistula creation surgery. Preexisting venous neointimal hyperplasia could reduce luminal area for blood flow and/or reduce vein distensibility, and lead to failure of the AVF to mature adequately to support hemodialysis. However, available studies examining this question have shortcomings, including lack of morphometric histologic analysis, confinement to single institutions, and small sample sizes, that may contribute to the wide range reported for prevalence of preexisting venous neointima.3–9 Furthermore, little is known about alterations in other structural compartments of the vein or their potential pathophysiologic significance.
The Hemodialysis Fistula Maturation (HFM) Study was a prospective, multicenter, cohort study conducted in the United States to identify predictors of fistula maturation failure and elucidate underlying mechanisms of fistula dysfunction among patients with advanced kidney disease and already undergoing or likely to soon need RRT.10 Pre-, peri-, and postoperative measurements and assessments were performed, including standardized collection of vein samples immediately adjacent to the portion of vein used for fistula creation. We describe selected histologic features, including neointima formation, composition of the media, and calcification of vein samples obtained from HFM Study participants at the time of fistula creation. We also describe their associations with baseline clinical and demographic factors.
Results
A total of 602 participants about to undergo AVF construction were recruited between March of 2010 and September of 2013. Nearly all participants provided written consent to permit vein tissue collection, and vein segments were obtained from 554 (92.03%). Initial histologic sections revealed that 371 (67.0%) of the veins demonstrated a complete circumferential structure. The remaining vein samples were estimated from their shapes to contain 10%–90% of a fully intact structure. The lack of complete circumferential structure was likely due to difficulties in handling and subdividing, for multiple uses, the small vein samples obtained (5 mm or less in length). Baseline demographic and clinical characteristics of participants were similar in the subgroup with full cross-section vein samples (Table 1) compared with the overall cohort.
Table 1.
Baseline characteristics of the HFM Study Cohort and subgroups with full cross-sections, partial cross-sections, and no vein specimen available
| Factor | All Study Participants (n=602) | Participants with Full Cross-Section (n=371) | Participants with Partial Cross-Section (n=183) | Participants without Vein Specimen (n=48) |
|---|---|---|---|---|
| Age at time of surgery, years | 55.2±13.4 | 55.6±13.8 | 54.7±13.1 | 53.1±11.2 |
| Female | 179 (29.7) | 112 (30.2) | 53 (29.0) | 14 (29.2) |
| Blacka | 264 (44.4) | 155 (42.5) | 85 (47.0) | 24 (50.0) |
| Years of prior dialysis (hemo or peritoneal) | 0.96±2.20 | 0.94±2.23 | 1.13±2.34 | 0.46±1.17 |
| Diabetes prevalence | 353 (58.6) | 223 (60.1) | 105 (57.4) | 25 (52.1) |
| Cardiovascular diseaseb | 315 (52.3) | 202 (54.5) | 93 (50.8) | 20 (41.7) |
| Coronary artery diseasec | 156 (25.9) | 106 (28.6) | 43 (23.5) | 7 (14.6) |
| Cerebrovascular diseased | 88 (14.6) | 54 (14.6) | 24 (13.1) | 10 (20.8) |
| Venous thromboembolic diseasee | 37 (6.1) | 23 (6.2) | 11 (6.0) | 3 (6.3) |
| Peripheral arterial diseasef | 91 (15.1) | 54 (14.6) | 32 (17.5) | 5 (10.4) |
| Body mass index, kg/m2 | 30.38±7.57 | 30.44±7.84 | 30.24±7.13 | 30.51±7.09 |
| Tobacco use (current, former)a | 325 (54.4) | 212 (57.5) | 92 (50.5) | 21 (45.7) |
| On dialysis (HD or PD) at time of surgery | 387 (64.3) | 235 (63.3) | 125 (68.3) | 27 (56.3) |
| Fistula location: upper arm | 459 (76.2) | 281 (75.7) | 146 (79.8) | 32 (66.7) |
| Draining vein | ||||
| Basilic vein | 172 (28.6) | 130 (35.0) | 32 (17.5) | 10 (20.8) |
| Cephalic vein | 418 (69.4) | 236 (63.6) | 145 (79.2) | 37 (77.1) |
| Brachial vein | 12 (2.0) | 5 (1.3) | 6 (3.3) | 1 (2.1) |
Data are displayed as count (%) or, for quantitative variables, mean ± SD. HD, hemodialysis; PD, peritoneal dialysis.
Race is not reported for eight participants. Tobacco use is not reported for five participants. Percent is calculated out of available data.
History of any of the following: myocardial infarction, angina, coronary artery bypass surgery or angioplasty, congestive heart failure, cardiac arrhythmias or conduction problems, stroke, transient ischemic attack, carotid endarterectomy, carotid artery angioplasty, claudication, lower extremity arterial bypass surgery or angioplasty, and nontraumatic amputation.
History of any of the following: angina, coronary artery angioplasty or bypass, or myocardial infarction.
History of any of the following: stroke, transient ischemic attack, carotid endarterectomy, or carotid angioplasty.
History of any of the following: deep venous thrombosis, or pulmonary embolism.
History of any of the following: claudication, lower extremity angioplasty or bypass surgery, or nontraumatic amputation.
Neointima Formation
We performed histologic analysis on all vein samples. Figures 1 and 2 illustrate selected circumferential vein samples with and without a neointima. However, because many of the expanded neointimas were eccentric in distribution (Figure 3), we limited morphometric analysis of the extent of luminal narrowing by neointima formation to the 371 samples with a complete circumferential structure, but further excluding six cases in which accurate measurement of the neointimal area was precluded by valves in the plane of histologic sectioning.
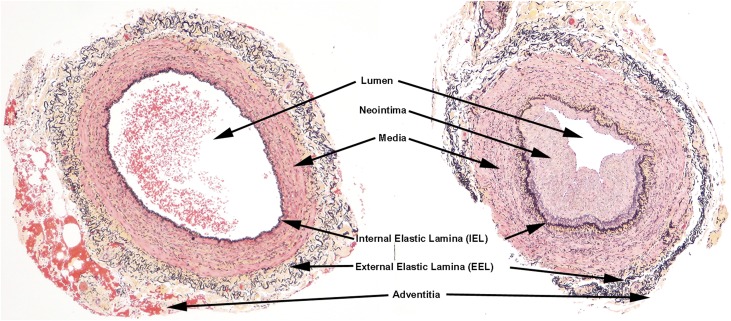
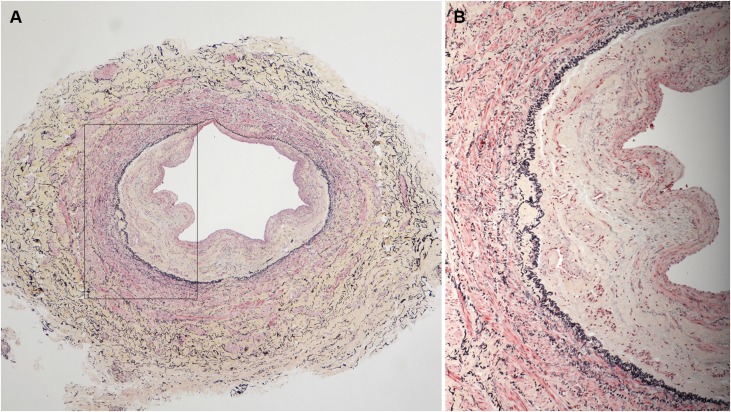
Figure 1.
Illustration of characteristic landmarks of both normal veins and abnormal veins. There is a neointima and accumulation of medial collagen (yellow staining) adjacent to the IEL in the abnormal vein (right). In the abnormal veins, the indicated landmarks were measured morphometrically to determine the extent of luminal compromise by neointima (Movat pentachrome stain).
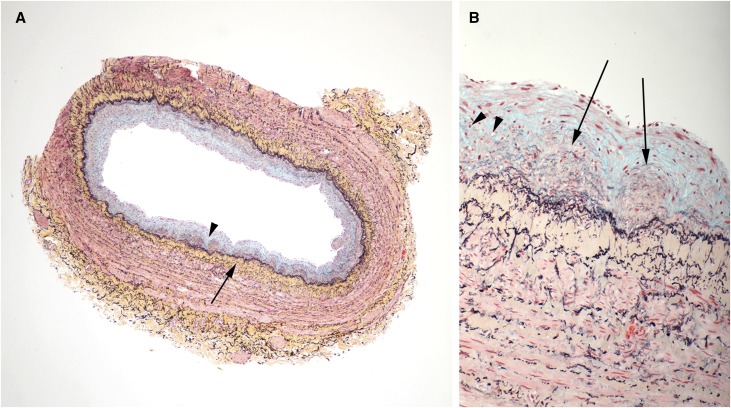
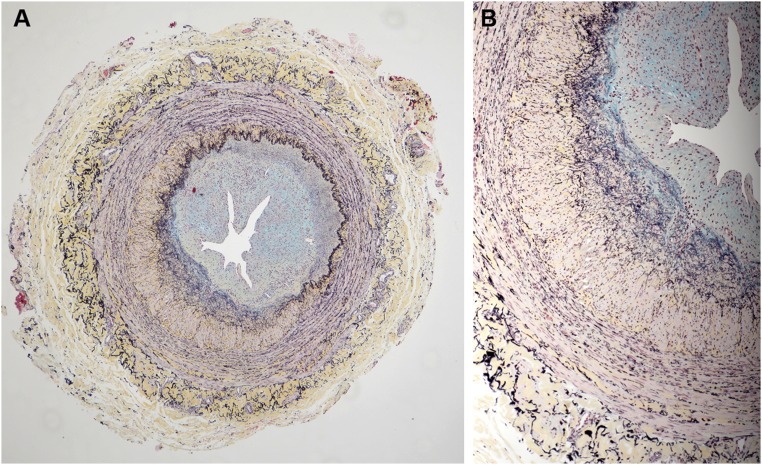
Figure 2.
Vein with concentric neointima formation. A lower power image is on the left (A) and a higher power image of a section of the same vein is shown on the right (B). (A) The neointima contains large amounts of blue staining material (proteoglycans, arrowhead), whereas there is prominent concentric accumulation of collagen (yellow stain, long arrow) in the media immediately adjacent to the IEL. No significant inflammatory infiltrates are identified. (B) Discrete microdomains of admixed collagen (yellow) and proteoglycans (blue), indicated by arrows, were observed within the neointima. Some microdomains appeared to have a component of neoangiogenesis (arrowheads). (Movat pentachrome stain)
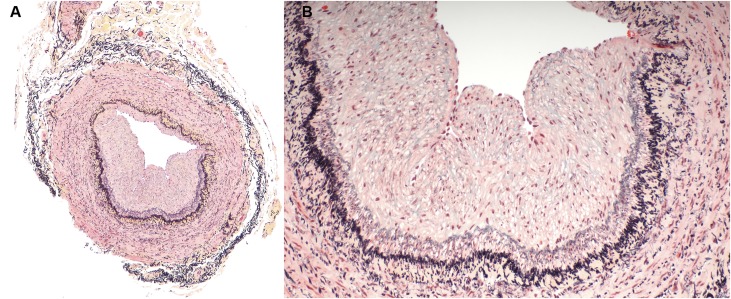
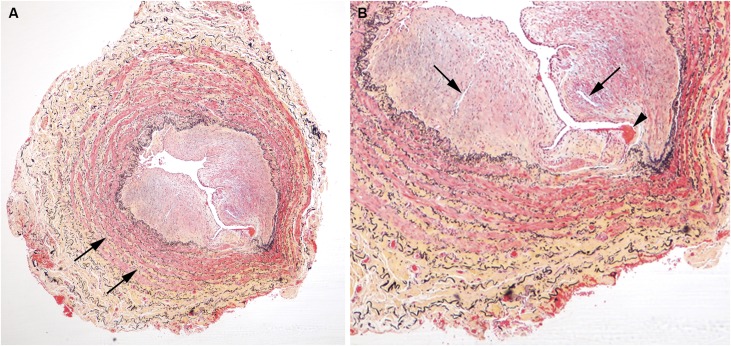
Figure 3.
Abnormal vein with eccentric neointima formation, stained with Movat pentachrome. (A) is a low power image and (B) is a higher power image of the vein section shown on the right in Figure 1. Less proteoglycans (blue staining) are noted in the neointima of this sample and less collagen (yellow staining) is noted in the vessel wall. Inflammatory infiltrates are not present in any of the layers of the vessel wall.
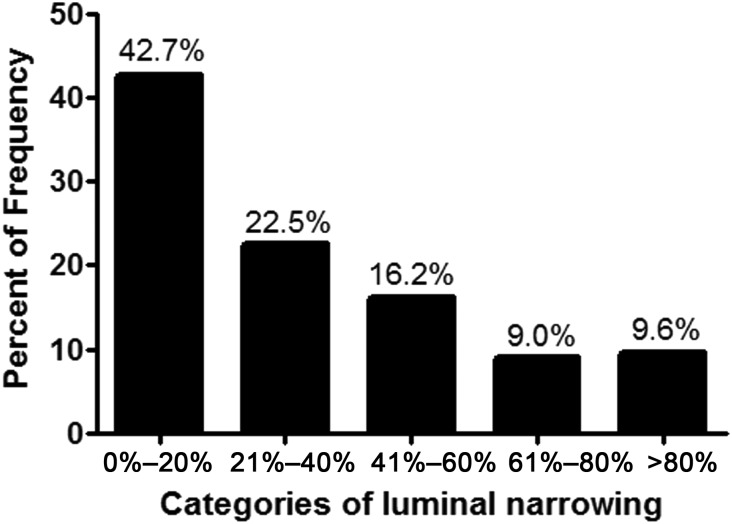
Most (88%) of the remaining 365 veins on which morphometric analysis was performed showed some degree of expansion of the intimal layer (neointima formation). Luminal narrowing in full cross-section samples varied from minimal or mild (20% or less of the total luminal area occupied by neointima) to severe (>80% of the total luminal area occupied by neointima) (Figure 4). The majority of cases (57%) had substantial neointimal occupation of >20% of the total luminal area.
Figure 4.
Graphic depiction of the extent of luminal narrowing by formation of a neointima in the 365 veins undergoing morphometric analysis. The majority of veins (57%) show 20% or greater encroachment of the lumen area by neointima. Nearly 10% of veins showed severe luminal narrowing of 80% or more.
Table 2 relates luminal narrowing to characteristics of the patient and to AVF location. The mean percent lumen area occupied by neointimal hyperplasia increased by 0.24%/yr of age (P=0.03) and by 2.13% (P=0.002) per year spent on dialysis. Results were substantively similar when age and dialysis duration were adjusted for each other and/or when Tweedie regression, an approach oriented specifically to skewed responses with clumping at zero, was employed. Luminal narrowing was lower in blacks compared with other races. Neointimas occupied higher fractions of lumen area in smokers than nonsmokers, in participants with histories of cardiovascular and peripheral arterial diseases than those without, and in upper-arm versus forearm veins. Overall, narrowing was greatest in basilic (mean [SD] %, 39.5 [30.4]) veins used for fistula creation and lowest in brachial veins (21.2 [23.4]), with cephalic veins (28.1 [27.2]) intermediate (P<0.001), and when restricted to upper-arm AVFs, lower in cephalic than other veins (30.6 [27.4] versus 40.3 [30.2]; P=0.01). Sex, histories of diabetes, coronary artery, cerebrovascular and venous thromboembolic diseases, body mass index, and current maintenance dialysis status were not significantly associated with luminal narrowing (Table 2).
Table 2.
Percent luminal narrowing of vein samples with complete cross-sections (n=365a) by baseline attributes
| Factorb | Factor Present | Factor Absent | P Value | ||
|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | ||
| Female | 111 | 36.3 (31.7) | 254 | 30.2 (27.4) | 0.08 |
| Black | 154 | 28.8 (29.2) | 205 | 34.8 (28.7) | 0.05 |
| Diabetes prevalence | 219 | 33.6 (28.3) | 146 | 29.7 (29.6) | 0.22 |
| Cardiovascular disease | 198 | 35.3 (29.3) | 167 | 28.1 (27.9) | 0.02 |
| Peripheral arterial disease | 51 | 42.2 (33.7) | 314 | 30.4 (27.7) | 0.02 |
| Coronary artery disease | 104 | 36.5 (29.0) | 261 | 30.2 (28.7) | 0.06 |
| Cerebrovascular disease | 53 | 36.0 (31.5) | 312 | 31.4 (28.4) | 0.32 |
| Venous thromboembolic disease | 22 | 38.8 (32.2) | 343 | 31.6 (28.6) | 0.32 |
| Tobacco use | 208 | 35.1 (28.1) | 155 | 28.1 (29.5) | 0.02 |
| On dialysis at time of surgery | 232 | 33.6 (29.4) | 133 | 29.3 (27.7) | 0.16 |
| Forearm fistula | 90 | 22.9 (26.2) | 275 | 35.0 (29.1) | <0.001 |
| Upper-arm fistula: basilic or brachial vein | 126 | 40.3 (30.2) | |||
| Upper-arm fistula: cephalic vein | 149 | 30.6 (27.4) | |||
| P<0.01c | |||||
Six of the 371 vein samples with complete cross-section were obscured by valves.
See Table 1 footnotes for specific factor definitions.
For comparison of cephalic vein upper-arm fistulas to pooled basilic and brachial vein upper-arm fistulas.
Composition of the Neointimas
Neointimas in these veins were composed predominantly of extracellular matrix with admixed mesenchymal cells that largely expressed α–smooth muscle actin (Supplemental Figures 1–4), and did not display the large accumulations of foam cells, monocytes/macrophages, and lipid cores characteristic of atherosclerosis (Figures 2, 3, 5, and 6). Immunohistochemistry performed on a subset of vein samples revealed that the great majority of neointimal cells express α–smooth muscle actin, thereby identifying these cells as either smooth muscle cells, myofibroblasts, or “myofibroblast-like” cells (Supplemental Figure 3). Staining of replicate tissue sections with markers of endothelial cells (CD31) and pericytes (PDGF β) showed colocalization of pericytes with penetrating capillaries within the neointima, but that the great preponderance of smooth muscle actin–expressing cells were not pericytes and hence either smooth muscle cells or myofibroblasts (Supplemental Figure 4).
Figure 5.
Example of vein, stained with Movat pentachrome, showing highest degree of luminal occlusion by a neointima. (A) This vein shows accumulations of collagen (yellow-stained matrix, arrows) dispersed throughout the media. (B) High-power view of vein in (A) shows a small thrombus (arrowhead) at the neointimal surface. Several small vessels within a more cellular region of the neointima are present (arrows). The IEL (black stain) is disrupted in the portion of the vein near the thrombus.
Figure 6.
A vein sample, stained with Movat pentachrome, with a very eccentric neointimal layer. A lower power image is on the left (A) and a higher power image (B) of a section of the same vein is shown on the right that was obtained from the vessel area indicated by the black outline in (A). This sample had a large component of collagen (yellow staining) in the neointimal and adventitial layers with very little proteoglycan content (blue staining).
Movat-stained sections showed that the extracellular matrix is composed of elastic fibers, collagen, and proteoglycans. Although these components often occupied discrete foci within the expanded intimas, in some samples we also identified discrete microdomains of admixed collagen and proteoglycans that were generally immediately adjacent to the internal elastic lamina (IEL) and distinct from the remaining portions of the neointimas (Figures 2 and 6).
The presence of inflammation, characterized by the presence of monocytes/macrophages within the intima, was studied in a subset of 48 veins. Approximately one-half (25/48=52.1%) of the vein samples utilized for immunohistochemistry contained at least one CD68-expressing monocyte/macrophage within the intima (Supplemental Figure 2B). However, the mean number of CD68+ cells present in any involved neointima was small (7.7 cells per involved neointima), and only rarely were these cells observed as a cluster. When CD68+ cells appeared as a cluster, they were associated with foci of neoangiogenesis, characterized by clusters of microvessels lined by CD31+-expressing endothelial cells. These microvessels were also present in 25 of 48 (52.1%) of the neointimas examined; among neointimas with microvessels, we observed a mean of 20.4 microvessels per neointima (Figure 7). Immunohistochemistry showed limited evidence of cell turnover. Almost half (22/48=45.8%) of the neointimas contained at least one Ki-67+-proliferating cell, with a mean of 4.2 cells in involved neointimas (Supplemental Figure 2A). Only rarely did we identify apoptotic cells by their expression of cleaved caspase 3 (4/48=12.5% of neointimas, mean 2.0 cells/involved neointima, Supplemental Figure 2C).
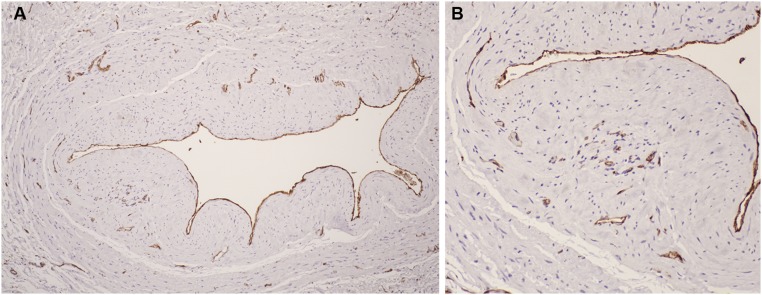
Figure 7.
Immunostaining with anti-CD31 antibody to detect endothelial cells. Rust color indicates positive immunostaining. A lower power image is on the left (A) and a higher power image of a section of the same vein is shown on the right (B). Endothelial cells lining the vein lumen are highlighted. Positive immunostaining of microvessels in the neointima and adventitia is visible in (B), suggesting neoangiogenesis in these regions.
Composition of the Media
All vein samples (n=554), regardless of their circumferential structure on histologic sections, were utilized for this analysis. We found prominent collagen aggregates within the medial portion of the vein (Figures 2 and 8). Most veins (79.2%) showed collagen aggregates localized to the portion of the media adjacent to the IEL. In about one-third of the veins (33.6%), collagen was observed to be localized within the media near the IEL, but did not form a continuous ring throughout the sampled media. In another 35.7%, such collagen formed a circumferential, continuous ring, at times consisting of broad collagen bands, whereas in an additional 9.9% of the veins such collagen rings were sufficiently large as to increase media thickness relative to the normal vein structure. The remaining 115 veins (20.8%) showed no collagen aggregates in the sub-IEL portion of the media. Table 3 shows the prevalence of IEL-adjacent collagen in all samples, in relation to several patient characteristics. IEL-adjacent medial collagen aggregates were statistically significantly more prevalent in upper-arm than in forearm veins; less evident in brachial than other veins, although the number of brachial vein samples was small (brachial, 5/11=45.4%; basilic, 137/162=84.6%; cephalic, 297/381=77.9%; exact test P<0.01); and more common among tobacco users than nonusers. Age was also associated with increasing collagen prevalence (prevalence odds ratio [OR], 1.02/decade, 95% confidence interval [95% CI], 1.004 to 1.034; P=0.01). Table 4 relates continuous and apparently fully circumferential IEL-adjacent collagen to patient factors, but is restricted to the subset of 426 samples either fully circumferential (n=371) or otherwise assessed to include ≥75% of a full cross-section (n=55), for whom extensive continuity could be most reliably observed. In this subset, fully circumferential collagen was also more common in older patients (OR, 1.02; 95% CI, 1.002 to 1.031; P=0.03) and upper-arm versus forearm veins; least common in brachial veins (brachial, 0/7=0.0%; basilic, 68/143=47.6%; cephalic, 151/276=54.7%; exact test P<0.01) although, in upper-arm samples, it was less common in cephalic than basilic or brachial veins (Table 4). Fully circumferential collagen was more common for tobacco users versus nonusers, and among patients with diabetes or cardiovascular disease (Table 4).
Figure 8.
A vein sample, stained with Movat pentachrome, with a very eccentric neointimal layer. A lower power image is on the left (A) and a higher power image of a section of the same vein is shown on the right (B). This sample had large aggregates of collagen (yellow staining) in the media adjacent to the IEL and in the adventitia, with large proteoglycan content (blue staining) in the neointima.
Table 3.
Prevalence of IEL-adjacent collagen (all samples, n=554) by baseline attributes
| Factora | Factor Present | Prevalence of Collagen | Factor Absent | Prevalence of Collagen | P Value |
|---|---|---|---|---|---|
| N | N (%) | N | N (%) | ||
| Female | 165 | 133 (80.6) | 389 | 306 (78.7) | 0.60 |
| Black | 240 | 184 (76.7) | 306 | 247 (80.7) | 0.25 |
| Diabetes prevalence | 328 | 265 (80.8) | 226 | 174 (77.0) | 0.28 |
| Cardiovascular disease | 295 | 237 (80.3) | 259 | 202 (78.0) | 0.50 |
| Coronary artery disease | 149 | 121 (81.2) | 405 | 318 (78.5) | 0.49 |
| Cerebrovascular disease | 78 | 61 (78.2) | 476 | 378 (79.4) | 0.81 |
| Venous thromboembolic disease | 34 | 26 (76.5) | 520 | 413 (79.4) | 0.68 |
| Peripheral arterial disease | 86 | 71 (82.6) | 468 | 368 (78.6) | 0.41 |
| Tobacco use | 304 | 252 (82.9) | 247 | 184 (74.5) | 0.02 |
| On dialysis at time of surgery | 360 | 291 (80.8) | 194 | 148 (76.3) | 0.21 |
| Forearm fistula | 127 | 87 (68.5) | 427 | 352 (82.4) | <0.001 |
| Upper-arm fistula: basilic or brachial vein | 163 | 134 (82.2) | |||
| Upper-arm fistula: cephalic vein | 264 | 218 (82.6) |
See Table 1 footnotes for specific factor definitions.
Table 4.
Prevalence of IEL-adjacent collagen with continuous circumferential ring in samples with ≥75% circumferential cross-sections (n=426) by baseline attributes
| Factora | Present | Absent | P Value | ||
|---|---|---|---|---|---|
| N | N (%) | N | N (%) | ||
| Female | 129 | 66 (51.2) | 297 | 153 (51.5) | 0.95 |
| Black | 183 | 89 (48.6) | 237 | 128 (54.0) | 0.27 |
| Diabetes prevalence | 252 | 141 (56.0) | 174 | 78 (44.8) | 0.02 |
| Cardiovascular disease | 230 | 130 (56.5) | 196 | 89 (45.4) | 0.02 |
| Coronary artery disease | 118 | 68 (57.6) | 308 | 151 (49.0) | 0.11 |
| Cerebrovascular disease | 64 | 38 (59.4) | 362 | 181 (50.0) | 0.17 |
| Venous thromboembolic disease | 29 | 18 (62.1) | 397 | 201 (50.6) | 0.23 |
| Peripheral arterial disease | 64 | 37 (57.8) | 362 | 182 (50.3) | 0.27 |
| Tobacco use | 244 | 137 (56.2) | 180 | 81 (45.0) | 0.02 |
| On dialysis at time of surgery | 274 | 137 (50.0) | 152 | 82 (54.0) | 0.43 |
| Forearm fistula | 99 | 33 (38.4) | 327 | 181 (55.4) | <0.001 |
| Upper-arm fistula: basilic or brachial vein | 141 | 65 (46.1) | |||
| Upper-arm fistula: cephalic vein | 186 | 116 (62.4) | |||
| P=0.003b | |||||
See Table 1 footnotes for specific factor definitions.
For comparison of cephalic vein upper-arm fistulas to pooled basilic and brachial vein upper-arm fistulas.
In addition to collagen aggregates adjacent to the IEL, collagen could be identified in the remaining portions of the medial smooth muscle layer, although distributed more diffusely and not concentrated in discrete aggregates. Only rarely (2.7%) were collagen accumulations within the medial smooth muscle layer not observed; in these cases, the small amount of collagen normally present in the media was not detected with the Movat stain. The extent of medial area occupied by collagen was estimated semiquantitatively from the photographed histologic specimens. In 238 veins (43.0%), up to 25% of the medial area was considered to be so occupied; in 260 veins (46.9%), 26%–50%; and in 41 veins (7.4%), 51%–75%. Table 5 shows ORs from ordinal proportional odds models of the semiquantitative medial area categories in relation to selected clinical and demographic characteristics of study participants and the fistula; these are also restricted to the 426 samples with ≥75 cross-sections. Although similarly prevalent in males and females, collagen was somewhat more extensive in males (proportional odds model OR, 1.98 per increasing category of extent of the media [0, ≤25%, 26%–50%, 51%–75%]; 95% CI, 1.32 to 2.96; P=0.001) and upper-arm fistulas (proportional odds model OR, 2.03; 95% CI, 1.30 to 3.17; P=0.002).
Table 5.
Summary ORs, with 95% confidence limits and P values, from proportional (cumulative) odds models for extent of collagen in medial smooth muscle layer (none, ≤25%, 25%–50%, 50%–75%, 75%–100%; among samples with ≥75% full cross-sections; n=426) by baseline attributes
| Factora | OR | 95% Wald Confidence Limits | P Value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age per decade | 1.10 | 0.97 | 1.26 | 0.15 |
| Female | 0.51 | 0.34 | 0.76 | <0.001 |
| Black | 0.86 | 0.59 | 1.24 | 0.41 |
| Diabetes prevalence | 0.87 | 0.60 | 1.26 | 0.45 |
| Cardiovascular disease | 1.05 | 0.73 | 1.51 | 0.81 |
| Coronary artery disease | 0.73 | 0.49 | 1.01 | 0.14 |
| Cerebrovascular disease | 0.85 | 0.51 | 1.42 | 0.54 |
| Venous thromboembolic disease | 0.49 | 0.23 | 1.02 | 0.06 |
| Peripheral arterial disease | 0.98 | 0.59 | 1.63 | 0.93 |
| Body mass index, per kg/m2 | 1.00 | 0.98 | 1.02 | 0.88 |
| Tobacco use | 0.80 | 0.56 | 1.17 | 0.25 |
| On dialysis at time of surgery | 1.23 | 0.87 | 1.87 | 0.21 |
| Forearm fistula | 0.49 | 0.31 | 0.77 | 0.002 |
See Table 1 footnotes for specific factor definitions.
Calcification
Presence and pattern of calcification were determined in each of the principal vein layers of all 554 samples. When present, calcification was most commonly identified within the adventitia (Table 6). Within the adventitia, one distinctive location of calcium deposition was in adventitial microvessels, a pattern similar to calciphylaxis. Calcification was also often identified as discrete aggregates within the media and adventitia (Figure 9). In only two samples, calcification was present as discrete aggregates within the neointima (Figure 9). A very few cases showed a more finely stippled distribution of calcium deposits in each of the three tissue layers (Table 6).
Table 6.
Calcification (all samples, n=554)
| Tissue Layer | Calcium Deposition Pattern, n (%) | Total, n (%) | |
|---|---|---|---|
| Distinct Aggregate | Stippled | ||
| Intima | 10 (1.8) | 2 (0.4) | 12 (2.2) |
| Media | 54 (9.7) | 3 (0.5) | 57 (10.3) |
| Adventitia | 80 (14.4) | 6 (1.1) | 86 (15.5) |
| Adventitial microvessels | 20 (3.6) | 3 (0.5) | 23 (4.1) |
| Distributed calcium, unattached to tissue | 6 (1.1) | 0 (0.0) | 6 (1.1) |
Figure 9.
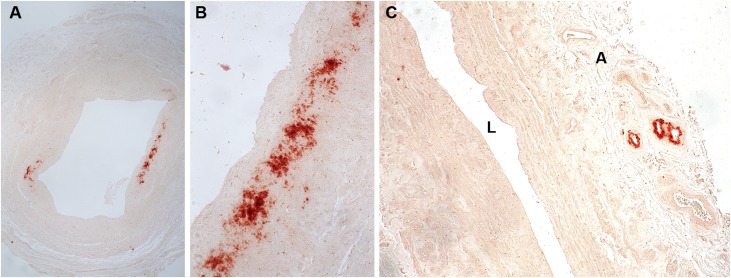
Examples of calcification in vein detected by alizarin red S reagent. A lower power image (A) shows discrete areas of calcium accumulation (dark red). A higher power image of the calcium deposits in (A) is shown in (B). In (C), another vein sample had calcium deposits confined to neovessels in the adventitia (A). Calcification was typically detected in the medial and adventitial layers of veins with little accumulation of calcium within neointimas. A, adventitia; L, vein lumen.
Table 7 relates calcification prevalence to patient and sample characteristics. Black race was associated with lower prevalence of vein calcification in either intima or media. Vein calcification prevalence was higher in patients with upper-arm fistulas and histories of diabetes and coronary artery disease, and increased with age (OR, 1.05/decade; 95% CI, 1.03 to 1.07; P<0.001).
Table 7.
Vein calcification prevalence in intima or media (all samples, n=554) by baseline attributes
| Factora | Present | Absent | P Value | ||
|---|---|---|---|---|---|
| N | N (%) | N | N (%) | ||
| Female | 165 | 18 (10.9) | 389 | 46 (11.8) | 0.76 |
| Black | 240 | 16 (6.7) | 306 | 47 (15.4) | 0.002 |
| Diabetes prevalence | 328 | 46 (14.0) | 226 | 18 (8.0) | 0.03 |
| Cardiovascular disease | 295 | 41 (13.9) | 259 | 23 (9.2) | 0.07 |
| Coronary artery disease | 149 | 29 (19.5) | 405 | 35 (8.6) | <0.001 |
| Cerebrovascular disease | 78 | 13 (16.7) | 476 | 51 (10.7) | 0.13 |
| Venous thromboembolic disease | 34 | 6 (17.6) | 520 | 58 (11.1) | 0.25 |
| Peripheral arterial disease | 86 | 13 (15.1) | 468 | 51 (10.9) | 0.26 |
| Tobacco use | 304 | 35 (11.4) | 247 | 29 (11.7) | 0.93 |
| On dialysis at time of surgery | 360 | 43 (11.9) | 194 | 21 (10.8) | 0.69 |
| Forearm fistula | 127 | 5 (3.9) | 427 | 59 (13.8) | <0.01 |
| Upper-arm fistula: basilic or brachial vein | 163 | 21 (12.9) | |||
| Upper-arm fistula: cephalic vein | 264 | 38 (14.4) | |||
See Table 1 footnotes for specific factor definitions.
Discussion
The HFM Study is the largest to date correlating histologic characteristics of prospectively collected vein samples with demographic and functional patient characteristics at the time of AVF creation. Each sample was evaluated for presence of a neointima and accumulations of collagen, proteoglycans, or other components of extracellular matrix, and presence of calcium. The limited data available in humans when this study commenced suggested that preexisting intimal hyperplasia is present in at least some veins used for fistula creation, and might be a determining factor in subsequent fistula failure. These data further suggested that cellular proliferation and/or inflammation, if identified as key mechanistic processes leading to venous intimal hyperplasia, could serve as targets for therapeutic interventions to improve fistula outcome. Our data demonstrate that neointimal hyperplasia is present in the great majority of veins utilized for AVF; that neointimal hyperplasia may be concentric or eccentric in distribution; that matrix accumulations in abnormal veins are composed of both collagens and proteoglycans; and that calcification is uncommon. Accumulated matrix proteins are not always distributed homogeneously in the neointima or throughout the vessel wall layers, but can appear localized to microdomains (i.e., proteoglycans in the intima; collagen abundantly in regions immediately below the IEL in addition to regions within the neointimas). This study also demonstrates that neointimal hyperplasia, at least at the time of fistula creation, is unaccompanied by prominent inflammatory cell infiltrates, apoptosis, or proliferative activity.
Although prior studies had indicated that preexisting neointima formation was a feature of some veins utilized for AVF, this large, multicenter study documents the high prevalence of this process. Eighty-eight percent of 365 veins in which the full circumference was present in histologic sections had some degree of neointima formation; in 57% of the 365 veins the neointimas occupied 20% or more of the luminal area of the vein. Neointima formation was present in a similar proportion of veins from which circumferential sections were not obtained, and for which the degree of neointima formation could not be quantitated on the basis of the calculation method of this manuscript.
Our studies also provide new insight into neointimal composition. The preoperative fistula vein neointimas are predominantly extracellular matrix with admixed smooth muscle or myofibroblasts or “myofibroblast-like” cells, and histologically resemble the neointimas that form in arteries after angioplasty or similar trauma.11–13 The extracellular matrix is composed both of collagen and proteoglycans either as discrete foci within the expanded intimas or sometimes as distinct from the remaining portions of the neointimas. Whether these microdomains, previously undescribed in neointimas of abnormal veins and arteries in other settings, might have a role in the evolution of a neointima, is unknown. Our histologic characterization of the composition of the neointimas does not in itself allow us to distinguish between two alternative lesion etiologies: neointima formation in response to remote injury such as venipuncture, or as a consequence of abnormal metabolism or exposure to uremic toxins in the course of CKD. However, there was no excess of neointimal lesions in the more superficial upper-arm cephalic AVFs as compared with upper-arm basilic or brachial AVFs, which tend to be deeper and generally less accessible to standard venipuncture. This suggests a primary role for uremia-driven oxidative stress, endothelial dysfunction, or inflammation in the pathogenesis of these lesions, rather than injury from repeated venipuncture, to which the more accessible superficially located veins would be most vulnerable (Table 2).
Histologic evaluation of the entire vein cohort showed little evidence of foci of neointimal or medial inflammation, except in rare cases with a small thrombus present or with inflammation present in neoangiogenesis sites. The subset of veins stained for CD68-expressing monocytes/macrophages confirmed this histologic assessment. The absence of prominent numbers of monocytes/macrophages in the vein samples, or of histologically identifiable foci of inflammation, strongly argues against a hypothesis that the extent of inflammation in veins at the time of fistula creation can be predictive of successful vein maturation, and indicates that, at least at the time of AVF creation, the uremic state has not led to an active, ongoing process of inflammation driving neointimal expansion.
Cellular activity could influence venous capacity to remodel and mature in settings of increased pressures and flows after fistula creation. Although there is evidence that cell proliferation is greatly increased in neointimas of stenotic lesions surgically removed from human AVFs,14 whether this is influenced by the preoperative status of the vein is unknown. To address these issues, we utilized well established immunohistochemical markers of cell proliferation (Ki-67) and apoptotic cell death (cleaved caspase 3) in a subsample to define the degree of cellular activity in the abnormal veins. We found little evidence of prominent cell turnover in any vein wall layer at the time of fistula creation. This suggests that the neointimal fibroplasia identified in our study represents established and generally quiescent lesions at the time of fistula creation; although how hemodynamic stressors post–AVF creation would influence preexisting lesions is unknown.
Neoangiogenesis is commonly present in vasculopathies characterized by the formation of a neointima, including atherosclerosis, vein graft stenosis after coronary artery bypass grafting, and restenosis after atherectomy or angioplasty.15 The close correlation of their presence in pathologically abnormal vessels with neointimas has generated theories that these processes are linked and that neoangiogenesis enables neointimal growth, much as tumor angiogenesis enables the growth of cancers. Neovessels could enable neointimal expansion by providing nutrient and oxygen delivery to the neointima, by serving as a site of egress for monocytes/macrophages and other inflammatory cells, and as a site for local propagation of chemokines and other signaling molecules. Yet our studies yielded equivocal results. Using the CD31 immunohistochemical marker of vascular endothelium to detect microvessels in all vein wall layers, we found that foci of neoangiogenesis involved only a minority of vein samples in the subset utilized for immunohistochemistry studies.
Calcification was present in only a minority of the vein samples, and when present was most commonly identified within the adventitia. The calcifications most often identified in the adventitia were localized to adventitial microvessels, a pattern typical of uremic calciphylaxis and not a pattern of dystrophic calcification secondary to trauma, which would most likely occur as aggregates within the soft connective tissue comprising the adventitia.
Our data also clearly identify differences at a histologic level between forearm and upper-arm veins. There are significant differences in the pattern and timeline of vascular remodeling of forearm AVFs as compared with upper-arm AVFs. The excesses in our data of collagen in samples from males versus females, and of neointima, collagen, and calcification in upper-arm as compared with forearm AVFs, seem surprising because maturation failure has often appeared to be more common in females and forearm fistulas.16–18 However, either the advantages of relatively larger vessels in males and in upper-arm AVFs, or of postoperative changes in vein biology induced by hemodynamic stress, may outweigh the effects of differentially compromised histologic features.
An important limitation of our findings is that we analyzed veins at one time point, and so cannot adequately characterize the evolution and even possibly the regression of the lesions observed. However, the preoperative pathologic findings can help distinguish between features that are more versus less likely to be predictive of AVF maturation, and thereby be indirectly useful for identifying appropriate therapeutic targets to modify these veins to improve future rates of AVF maturation and survival. An additional caveat is that we are unable to determine whether the preexisting neointimal hyperplasia in the veins has a similar underlying mechanism as neointimal hyperplasia that forms after AVF creation.
These data also afford a rare glimpse into the baseline effect of uremia per se (with its attendant inflammation, oxidative stress, and endothelial dysfunction) on venous biology before the effect of hemodynamic stressors. Lastly, although our main focus has been on associations within the realm of dialysis vascular access, we note that significant or suggestive associations of (1) neointimal hyperplasia with coronary artery (P=0.06) and peripheral vascular diseases (P=0.02), (2) venous calcification with coronary artery disease, and (3) tobacco use with several histologic parameters suggest further avenues of investigation, although caution is warranted due to the multiple comparisons from which they arise.
In summary, preexisting neointimal hyperplasia and prominent accumulation of extracellular matrix (specifically, collagen and proteoglycans) were highly prevalent in veins used for AVF creation among HFM Study participants. Conversely, there was little evidence of active cellular remodeling, inflammation, neoangiogenesis, or calcification as potential mediators of neointimal hyperplasia, suggesting that either these biologic processes were quiescent at the time point examined, or that other factors are more responsible for neointimal hyperplasia in this patient population. The next step to the studies presented here has been to assess the clinical significance of the histologic findings to the clinical development of AVF stenosis assessed by ultrasound and to clinical AVF maturation. These are reported in a subsequent manuscript.
Concise Methods
Study Participants
Patients either currently undergoing dialysis, or <80 years old and anticipated to require dialysis within 3 months, were recruited from seven academic medical centers before planned single-stage fistula creation surgery.10 The Institutional Review Boards of each of these institutions and the Data Coordinating Center approved the study. Each participant provided written informed consent.
Histology
A 5–10 mm segment of vein immediately adjacent to that portion of the vein used for fistula creation was surgically removed at the time of fistula creation, and within 15 minutes subdivided into four discrete samples with one portion placed in 10% neutral buffered formalin, as previously described,10 and utilized for all of the histologic studies described in this manuscript. After 24–72 hours of fixation, the formalin-fixed tissue was placed in 70% ethanol for short-term storage and shipped from each of the participating centers to the central histology core laboratory. Upon receipt, the tissue was processed and embedded in paraffin following standard protocols. Four-micrometer sections were placed onto slides and stained with the Movat pentachrome procedure for histology and with alizarin red S for calcium assessment.19 Five-hundred and fifty-four cases met study criteria and were accessioned for pathology assessment. Two patients refused consent for specimen collection, and no vein specimen was obtained during surgery from 46 others.
Movat Pentachrome Stain
The Movat pentachrome procedure stains collagen yellow, proteoglycans and mucin blue, nuclei and elastic fibers black, muscle red, and fibrin intense red, thereby allowing sharp definition of the various elements composing vein structures. This stain allows in a single histologic section distinction between the three layers composing a vein wall (Supplemental Figure 1). The intima is the area closest to the lumen, separated from the remaining portion of the vein by an IEL. The media is composed of a uniform population of smooth muscle cells with finely distributed elastic tissue around individual cells, located between the black-staining IEL and the external elastic lamina (EEL). The adventitia is composed of loose connective tissue and microvessels and is located external to the EEL. These features are illustrated in Supplemental Figure 1. These staining features allowed definition of the vein lumen and space occupied by any neointimal hyperplasia, morphometric analysis of the amount of luminal area occupied by an expanded neointima (Supplemental Figure 1), and localization of pathologic alterations such as accumulations of leukocytes and/or proliferation of small blood vessels.
Morphometric analysis was performed on the Movat pentachrome–stained slides of all cases in which a full circumferential histologic section of the vein was obtained (n=371), excluding six of these samples with internal structure obscured by valves, leaving n=365. Photographs of each slide were taken at magnification ×40 and imported into ImagePro Plus software. Calibrated measurements of IEL length, EEL length, luminal area, maximal luminal area (open luminal area plus luminal area occupied by neointima), and total vein area were obtained, as illustrated in Supplemental Figure 1. The presence of collagen, identified by its staining qualities with the Movat stain, was recorded when it could be localized to the region within the medial portion of the vessel wall immediately adjacent to the IEL. Such IEL-adjacent medial collagen was further characterized as being circumferential in distribution or not, and occurring in large aggregates or not. The extent of collagen accumulations in the medial layer of the vessel walls, but not adjacent to the IEL, was scored as none (amount too little to be detected by Movat staining), or occupation of up to 25%, 26%–50%, and 51%–75% of the medial area by collagen.
Calcium deposits were identified in histologic slides stained with the alizarin red S reagent. The presence of calcium was further characterized as present in neointima, media, adventitial microvessels, and/or extravascular adventitia. In addition, the pattern of staining either as aggregates of calcium or as a finely stippled distribution was recorded.
A subset of 50 cases, representative of the entire cohort, was utilized for additional immunohistochemical analysis. This subset was drawn from vein specimens with at least 75% estimated cross-section available, selected proportionately from strata formed by combining clinical site, full or partial cross-section, and significant (≥20%) versus nonsignificant (<20%) luminal narrowing. Of these 50 cases, two were subsequently eliminated from this portion of the study because they were collected during a postsurgery study fistula intervention and had been included in the sample pool in error. Immunohistochemistry, along with appropriate controls, was performed using general procedures previously described.20,21 These cases were stained with antibodies to CD68 (clone PGM1; Dako, Carpinteria, CA) to assess monocyte content within the tissue as a general measure of inflammation; CD31 (Clone JC70A; Dako), a marker of endothelium as a general marker of neoangiogenesis; Ki67 (clone Sp6; ThermoFisher Scientific, Waltham, MA) as a marker of cell proliferation; cleaved caspase 3 (rabbit polyclonal, #9661; Cell Signaling, Danvers, MA) as a marker of apoptosis; α–smooth muscle actin (Clone 1A4; Dako) as a marker of mesenchymal cells with a smooth muscle cell or myofibroblast or myofibroblast-like phenotype; and platelet-derived growth factor β as a marker of pericytes (Epitomics [catalog # ab32570, rabbit monoclonal clone Y92]; Abcam). Deparaffinized sections were subjected to heat-mediated antigen retrieval in citrate buffer, pH 6 (HIER-L; ThermoFisher Scientific), blocked with 2.5% normal horse serum, incubated with primary antibody overnight at 4°C followed by incubation with host matched ImmPress horse radish peroxidase reagent (Vector Laboratory, Burlingame, CA), developed with QuantoDAB substrate (ThermoFisher Scientific), counterstained with hematoxylin, dehydrated, and coverslipped. Positive staining was quantitated (total number of positive cells) in the neointima and media for each antibody, except for CD31 and α–smooth muscle actin. When present, CD68-expressing cells were localized to specific anatomic compartments of the vein (intima, neointima, media, adventitia). Within each compartment, CD68+ cells were quantitated per total area of that compartment. Qualitative assessments of unique patterns of CD68+ cell localization (e.g., at sites of preferential collagen accumulation, sites of pronounced cellular proliferation, or sites of neoangiogenesis) were noted. Immunohistochemical staining for CD31 allowed the number of microvessels within each compartment (neointima or media) to be quantitated by staining of the endothelial lining of each microvessel for CD31. α–smooth muscle actin–expressing cells were too numerous to count and were assessed qualitatively for overall distribution patterns within the vein.
Statistical Analyses
Demographic and baseline clinical characteristics were summarized and compared between the complete HFM Study population and the groups of participants with no vein specimen, or respectively with partial and full cross-section vein samples available, using means and SDs for continuous variables and percentages of participants for categoric variables. The relationships of categoric baseline characteristics to luminal narrowing were examined using paired t test. Continuous predictors were examined through ordinary linear regression and Tweedie regression to assure robustness against skewing. ORs and Pearson chi-squared test were used to examine the relationships to categorical factors of vein calcification in either intima or media, total collagen in the media, and presence of a circumferential collagen band in full cross-section samples. Logistic regression was used to relate these histologic features to continuous patient characteristics, and proportional odds models to study associations of patient and vein factors with ordinal categories of collagen extent among samples judged to include at least 75% vein cross-sections. Computations were performed using SAS 9.3.
Disclosures
Dr. Allon serves as a consultant to CorMedix. Dr. Cheung was a member of the Data and Safety Monitoring Board for a trial on vascular grafts co-sponsored by Humacyte, Inc. and the National Heart, Lung, and Blood Institute, and a member of the Clinical Events Committee and Data Safety and Monitoring Board for the Novel Endovascular Access Trial sponsored by TVA Medical, Inc. Dr. Roy-Chaudhury serves as a consultant to WL Gore, Bard Peripheral Vascular, Medtronic, and TVA.
Supplementary Material
Acknowledgments
The Hemodialysis Fistula Maturation Study was funded by grants U01DK066597, U01DK082179, U01DK082189, U01DK082218, UO1DK082232, U01DK082236, and U01DK082240 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Portions of this work were presented at the American Society of Nephrology Kidney Week in San Diego, California, 30 October to 4 November, 2012.
Members of the Hemodialysis Fistula Maturation Study Group: Chair, Steering Committee—University of Pennsylvania: H. Feldman. Clinical Centers—Boston University: L. Dember (PI), A. Farber, J. Kaufman, L. Stern, P. LeSage, C. Kivork, D. Soares, M. Malikova; University of Alabama: M. Allon (PI), C. Young, M. Taylor, L. Woodard, K. Mangadi. University of Cincinnati: P. Roy‐Chaudhury (PI), R. Munda, T. Lee, R. Alloway, M. El‐Khatib, T. Canaan, A. Pflum, L. Thieken, B. Campos-Naciff; University of Florida: T. Huber (PI), S. Berceli, M. Jansen, G. McCaslin, Y. Trahan; University of Texas Southwestern: M. Vazquez (PI), W. Vongpatanasin, I. Davidson, C. Hwang, T. Lightfoot, C. Livingston, A. Valencia, B. Dolmatch, A. Fenves, N. Hawkins; University of Utah: A. Cheung (PI), L. Kraiss, D. Kinikini, G. Treiman, D. Ihnat, M. Sarfati, I. Lavasani, M. Maloney, L. Schlotfeldt, C. Terry, Y-T Shiu; University of Washington: J. Himmelfarb (PI), C. Buchanan, C. Clark, C. Crawford, J. Hamlett, J. Kundzins, L. Manahan, J. Wise. Data Coordinating Center—Cleveland Clinic: G. Beck (PI), J. Gassman, T. Greene, P. Imrey, L. Li, J. Alster, M. Li, J. MacKrell, M. Radeva, B. Weiss, K. Wiggins. Cores: Histology Core—University of Washington: C. Alpers (PI), K. Hudkins, T. Wietecha. Ultrasound Core—University of Alabama at Birmingham: M. Robbin (PI), H. Umphrey, L. Alexander, C. Abts, L. Belt. Vascular Function Core—Boston University: J. Vita (PI, deceased), M. Duess, N. Hamburg, A. Levit. Repositories: NIDDK Biosample Repository—Fisher BioServices: H. Higgins, S. Ke, O. Mandaci, C. Snell. NIDDK DNA Repository—Fred Hutchinson Cancer Research Center: J. Gravley, S. Behnken, R. Mortensen. External Expert Panel: G. Chertow (Chair), A. Besarab, K. Brayman, M. Diener-West, D. Harrison, L. Inker, T. Louis, W. McClellan, J. Rubin. NIDDK: J. Kusek, R. Star.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Moving Beyond the Assumed: Improving Fistula Success Rates,” on pages 2827–2829.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016050598/-/DCSupplemental.
Contributor Information
Collaborators: LM Dember, PB Imrey, GJ Beck, AK Cheung, J Himmelfarb, TS Huber, JW Kusek, P Roy-Chaudhury, MA Vazquez, CE Alpers, ML Robbin, JA Vita, T Greene, JJ Gassman, and HI Feldman
References
- 1.Schinstock CA, Albright RC, Williams AW, Dillon JJ, Bergstralh EJ, Jenson BM, McCarthy JT, Nath KA: Outcomes of arteriovenous fistula creation after the fistula first initiative. Clin J Am Soc Nephrol 6: 1996–2002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon BS: Why don’t fistulas mature? Kidney Int 70: 1413–1422, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Allon M, Litovsky S, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Robbin ML: Medial fibrosis, vascular calcification, intimal hyperplasia, and arteriovenous fistula maturation. Am J Kidney Dis 58: 437–443, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allon M, Litovsky S, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Robbin ML: Correlation of pre-existing vascular pathology with arteriovenous graft outcomes in hemodialysis patients. Am J Kidney Dis 62: 1122–1129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allon M, Robbin ML, Young CJ, Deierhoi MH, Goodman J, Hanaway M, Lockhart ME, Litovsky S: Preoperative venous intimal hyperplasia, postoperative arteriovenous fistula stenosis, and clinical fistula outcomes. Clin J Am Soc Nephrol 8: 1750–1755, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee T, Chauhan V, Krishnamoorthy M, Wang Y, Arend L, Mistry MJ, El-Khatib M, Banerjee R, Munda R, Roy-Chaudhury P: Severe venous neointimal hyperplasia prior to dialysis access surgery. Nephrol Dial Transplant 26: 2264–2270, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wali MA, Eid RA, Dewan M, Al-Homrany MA: Pre-existing histopathological changes in the cephalic vein of renal failure patients before arterio-venous fistula (AVF) construction. Ann Thorac Cardiovasc Surg 12: 341–348, 2006. [PubMed] [Google Scholar]
- 8.Wasse H, Huang R, Naqvi N, Smith E, Wang D, Husain A: Inflammation, oxidation and venous neointimal hyperplasia precede vascular injury from AVF creation in CKD patients. J Vasc Access 13: 168–174, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lazich I, Chang A, Watson S, Dhar P, Madhurapantula RS, Hammes M: Morphometric and histological parameters in veins of diabetic patients undergoing brachiocephalic fistula placement. Hemodial Int 19: 490–498, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dember LM, Imrey PB, Beck GJ, Cheung AK, Himmelfarb J, Huber TS, Kusek JW, Roy-Chaudhury P, Vazquez MA, Alpers CE, Robbin ML, Vita JA, Greene T, Gassman JJ, Feldman HI; Hemodialysis Fistula Maturation Study Group : Objectives and design of the hemodialysis fistula maturation study. Am J Kidney Dis 63: 104–112, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabas I, García-Cardeña G, Owens GK: Recent insights into the cellular biology of atherosclerosis. J Cell Biol 209: 13–22, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hao H, Gabbiani G, Bochaton-Piallat ML: Arterial smooth muscle cell heterogeneity: implications for atherosclerosis and restenosis development. Arterioscler Thromb Vasc Biol 23: 1510–1520, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz SM, deBlois D, O’Brien ER: The intima. Soil for atherosclerosis and restenosis. Circ Res 77: 445–465, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Lee T, Wang Y, Arend L, Cornea V, Campos B, Munda R, Roy-Chaudhury P: Comparative analysis of cellular phenotypes within the neointima from vein segments collected prior to vascular access surgery and stenotic arteriovenous dialysis accesses. Semin Dial 27: 303–309, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khurana R, Simons M, Martin JF, Zachary IC: Role of angiogenesis in cardiovascular disease: A critical appraisal. Circulation 112: 1813–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Masengu A, Maxwell AP, Hanko JB: Investigating clinical predictors of arteriovenous fistula functional patency in a European Cohort. Clin Kidney J 9: 142–147, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGrogan DG, Maxwell AP, Khawaja AZ, Inston NG: Current tools for prediction of arteriovenous fistula outcomes. Clin Kidney J 8: 2820–2829, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Jaishi AA, Oliver MJ, Thomas SM, Lok CD, Zhang JC, Garg AX, Kosa SD: Patency rates of the arteriovenous fistula for hemodialysis: A systematic review and meta-analysis. Am J Kid Dis 63: 464–478, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Garvey W, Fathi A, Bigelow F, Carpenter B, Jimenez C: Improved Movat pentachrome stain. Stain Technol 61: 60–62, 1986 [DOI] [PubMed] [Google Scholar]
- 20.Hudkins KL, Pichaiwong W, Wietecha T, Kowalewska J, Banas MC, Spencer MW, Muhlfeld A, Koelling M, Pippin JW, Shankland SJ, Askari B, Rabaglia ME, Keller MP, Attie AD, Alpers CE: BTBR Ob/Ob mutant mice model progressive diabetic nephropathy. J Amer Soc Nephrol 21: 1533–1542, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shankland SJ, Eitner F, Hudkins KL, Goodpaster T, D'Agati V, Alpers CE: Differential expression of cyclin-dependent kinase inhibitors in human glomerular disease: Role in podocyte proliferation and maturation. Kidney international 58: 674–683, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.