Abstract
Multilineage-differentiating stress-enduring (Muse) cells are nontumorigenic endogenous pluripotent-like stem cells that can be collected from various organs. Intravenously administered Muse cells have been shown to spontaneously migrate to damaged tissue and replenish lost cells, but the effect in FSGS is unknown. We systemically administered human bone marrow–derived Muse cells without concurrent administration of immunosuppressants to severe combined immune-deficient (SCID) and BALB/c mouse models with adriamycin-induced FSGS (FSGS-SCID and FSGS-BALB/c, respectively). In FSGS-SCID mice, human Muse cells preferentially integrated into the damaged glomeruli and spontaneously differentiated into cells expressing markers of podocytes (podocin; 31%), mesangial cells (megsin; 13%), and endothelial cells (CD31; 41%) without fusing to the host cells; attenuated glomerular sclerosis and interstitial fibrosis; and induced the recovery of creatinine clearance at 7 weeks. Human Muse cells induced similar effects in FSGS-BALB/c mice at 5 weeks, despite xenotransplant without concurrent immunosuppressant administration, and led to improvement in urine protein, creatinine clearance, and plasma creatinine levels more impressive than that in the FSGS-SCID mice at 5 weeks. However, functional recovery in FSGS-BALB/c mice was impaired at 7 weeks due to immunorejection, suggesting the importance of Muse cell survival as glomerular cells in the FSGS kidney for tissue repair and functional recovery. In conclusion, Muse cells are unique reparative stem cells that preferentially home to damaged glomeruli and spontaneously differentiate into glomerular cells after systemic administration. Introduction of genes to induce differentiation is not required before Muse cell administration; thus, Muse cells may be a feasible therapeutic strategy in FSGS.
Keywords: chronic kidney disease, adult stem cells, cell survival, glomerulosclerosis, podocyte
Stem/progenitor cell therapy to replenish damaged glomerular cells is anticipated to be an effective strategy for CKD.1,2 Multilineage-differentiating stress-enduring (Muse) cells are stress-tolerant nontumorigenic endogenous stem cells collectable as pluripotent surface marker stage-specific embryonic antigen-3 (SSEA-3)–positive cells from the peripheral blood, connective tissue of various organs as well as bone marrow (BM) as approximately 0.03% of the mononucleated fraction.3–5 Muse cells express the pluripotency markers Sox2, Oct3/4, and Nanog; are able to differentiate into cells representative of all three germ layers from a single cell; and show self-renewability of triploblastic differentiation over several generations in vitro, suggesting their pluripotent-like properties.3,6–8 These properties are reproducible in Muse cells directly collected from BM aspirates, indicating that their properties are not newly acquired by in vitro manipulation or they modified under culture conditions.3 Approximately 50 ml fresh human BM aspirate directly yields 1 million Muse cells within approximately 2 days of culture, suggesting their therapeutic feasibility.5
Differentiation of Muse cells into triploblastic lineages is also shown by their in vivo reparative effects; intravenously or topically administered naïve Muse cells migrate to and integrate into damaged tissue with high selectivity and replenish lost cells by spontaneous in vivo differentiation into tissue-compatible cells, leading to tissue repair in models of stroke, liver cirrhosis, muscle degeneration, and skin ulcers of diabetes mellitus.3,9–11 Muse cells circulate in the peripheral blood of healthy donors, and endogenous circulating Muse cells are increased in patients after stroke in the acute phase, possibly by mobilizing from the BM in response to severe damage.12 The use of naturally existing reparative stem cells that do not require the introduction of genes or induction into purposive cells before transplantation is suggested to provide a clinically relevant therapeutic strategy for kidney diseases.13
Adriamycin (doxorubicin hydrochloride [DOX]) nephropathy is a well established rodent model of chronic proteinuric kidney disease resembling human FSGS with podocyte loss, focal segmental and global sclerosis, tubulointerstitial inflammation, and fibrosis.14 In severe combined immune-deficient (SCID) and BALB/c mouse models of FSGS, we intravenously injected human xenograft BM-derived Muse cells and evaluated structural and functional recovery. Human cell xenotransplantation has several advantages compared with allogeneic and autogenic transplantation; the efficiency of human cells can be estimated in vivo, and human-specific markers can be used to confirm integration of the transplanted cells in mouse tissue.
Although SCID mice are beneficial for tracing the integration and differentiation of human cells, they may not accurately reflect the pathology of human FSGS, because FSGS progresses in combination with inflammatory cytokines and immune responses,15 which are deficient in SCID mice. Furthermore, migration and engraftment of Muse cells might be affected, because inflammatory cytokines and immune responses are reported to affect the migration activity of stem cells, such as mesenchymal stem cells (MSCs).16 Therefore, to address these concerns, we performed the same xenograft experiment with BALB/c mice without immunosuppression.
This study showed that systemically administered human Muse cells led to structural and functional regeneration in mouse FSGS models, suggesting the potential feasibility of Muse cells for the treatment of FSGS.
Results
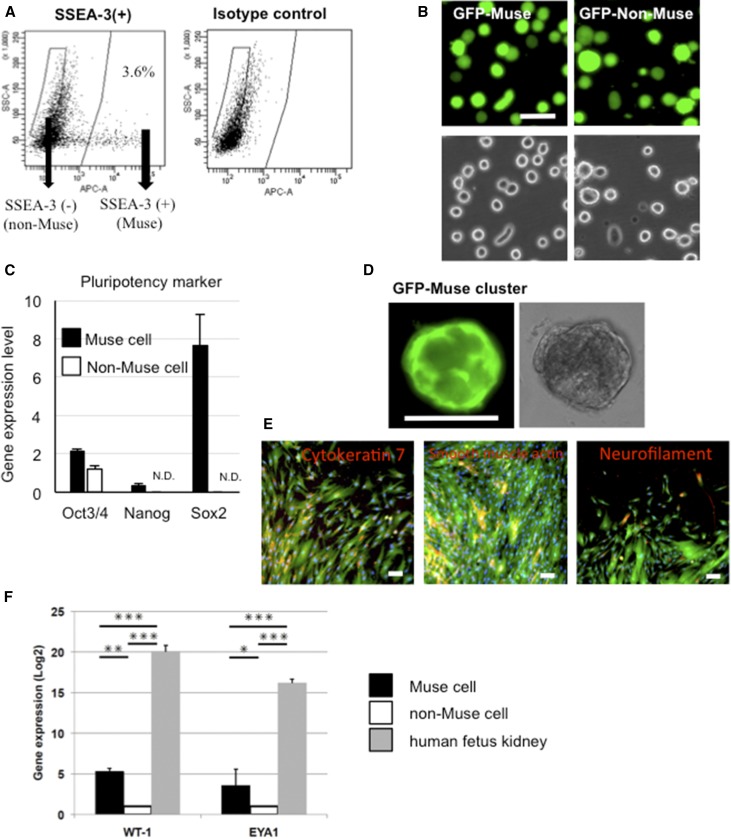
Characteristics of Human BM-Muse Cells
Muse and non-Muse cells were obtained as SSEA-3(+) and SSEA-3(−) cells, respectively, from human BM-MSCs labeled with lentivirus-green fluorescent protein (GFP) by FACS as described previously (Figure 1, A and B, Supplemental Result 1).3,4 Muse cells expressed Oct3/4, Nanog, and Sox2, whereas non-Muse cells exhibited minimal expression of these markers, with levels of Nanog and Sox2 falling beneath the detection threshold (Figure 1C). Muse cells formed characteristic clusters similar to embryonic stem cell–derived embryoid bodies in single-cell suspension (Figure 1D), whereas none of the non-Muse cells generated such clusters. These clusters spontaneously generated ectodermal (neurofilament), mesodermal (α-smooth muscle actin), and endodermal (cytokeratin 7) lineage cells in the adherent expanded cells after transferring the cluster onto a gelatin-coated dish (Figure 1E). These results are consistent with previous reports.3,6 To investigate the ability to differentiate into renal lineage cells in vitro, Muse and non-Muse cells were subjected to cytokine induction with all trans retinoic acid, activin A, and bone morphologic protein-7, a well known cocktail for renal cell induction.17 Three weeks later, Muse cells expressed higher levels of the developmental renal markers WT1 and EYA1 compared with non-Muse cells, although to a lesser extent compared with human fetus kidney (Figure 1F). Before cytokine induction, neither naive Muse nor non-Muse cells expressed either marker (data not shown), suggesting that the expression was newly induced by the cytokines.
Figure 1.
Muse cells exhibit triploblastic differentiation ability. (A) An example of the separation of Muse and non-Muse cells from human GFP(+) BM-MSCs by FACS. (B) GFP(+) Muse and GFP(+) non-Muse cells after isolation by FACS. (C) Gene expression of the pluripotency markers Oct3/4, Nanog, and Sox2 normalized by β-actin. N.D., not detectable. (D) A GFP(+) Muse cell–derived cluster formed from a single cell in suspension culture for 10 days. (E) Spontaneous differentiation of cells expanded from GFP(+) Muse cell–derived clusters on gelatin-coated dishes into cytokeratin 7 (endodermal), α-smooth muscle actin (mesodermal), and neurofilament (ectodermal; red). (F) Gene expression of the renal lineage markers WT1 and Eya1 3 weeks after induction. The expression level was normalized to glyceraldehyde-3-phosphate dehydrogenase. Scale bars, 50 μm in B; 100 μm in D and E. *P<0.05; **P<0.01; ***P<0.001.
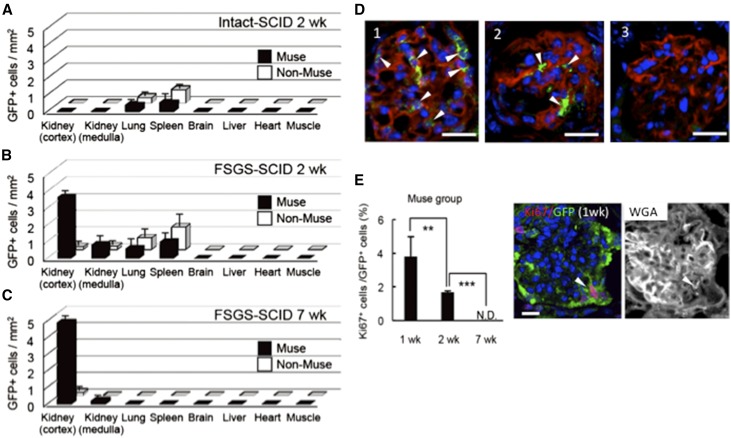
Organ Distribution of Muse Cells in FSGS-SCID Mice
One week after DOX administration, 2×104 human Muse cells (Muse group), non-Muse cells (non-Muse group), or an equivalent volume of sterile saline (vehicle group) were infused into FSGS-SCID mice via the tail vein. The in vivo dynamics of systemically administered cells were evaluated. When the cells were injected into intact normal SCID mice, small numbers of GFP(+) Muse and non-Muse cells were detected in the lung and spleen at 2 weeks but scarcely detected in the kidney (Figure 2A). In the FSGS-SCID mice, however, GFP(+) human Muse cells were detected in the damaged kidney, both the cortex and medulla, and the lung and spleen at 2 weeks but were not detected in other organs. The highest accumulation of Muse cells was detected in the cortex of the kidney. In contrast, GFP(+) human non-Muse cells accumulated mainly in the spleen and lung but only scarcely in the damaged kidney (Figure 2B, Supplemental Result 2). At 7 weeks, GFP(+) Muse cells were detected mainly in the cortex, particularly in the glomerulus (Supplemental Result 2), and only scarcely in the medulla of the damaged kidney but not in the other organs. A very small number of GFP(+) non-Muse cells were detected in the cortex of the kidney but were not detected in other organs (Figure 2C, Supplemental Results 2 and 3). Quantitative PCR for the human-specific Alu sequence at 2 and 7 weeks also revealed the highest engraftment of Muse cells in the cortex of the damaged kidney (Supplemental Result 2). The extent of integration of GFP(+) Muse cells at 7 weeks, however, varied among glomeruli (Figure 2D, Supplemental Results 4 and 5).
Figure 2.
Preferential spontaneous homing of Muse cells into damaged glomeruli in FSGS-SCID mice. Distribution of GFP(+) cells per square millimeter in the kidney (cortex and medulla), lung, spleen brain, liver, heart, and muscle of the Muse and non-Muse groups. (A) The intact SCID (2 weeks), (B) FSGS-SCID (2 weeks), and (C) FSGS-SCID (7 weeks). (D) GFP(+) Muse cells in the FSGS-SCID glomerulus costained with wheat germ agglutinin (WGA; red) at 7 weeks: (1) many, (2) several, and (3) lesser/no integration of GFP(+) cells. (E) Percentage of Ki67(+) cells in GFP(+) Muse cells at 1, 2, and 7 weeks. An example of Ki67(+)/GFP(+) cell with WGA staining on the same section is shown. The single-channel images used to produce D and E are shown in Supplemental Results 5 and 6, respectively. N.D., not detectable. **P<0.01; ***P<0.001. Scale bars, 20 μm.
The Ki67(+) ratio in GFP(+) Muse cells was 3.8%±1.2% at 1 week and 1.7%±0.1% at 2 weeks, and it became undetectable at 7 weeks (Figure 2E, Supplemental Result 6), indicating that Muse cells did not show tumorigenic proliferative activity after integration.
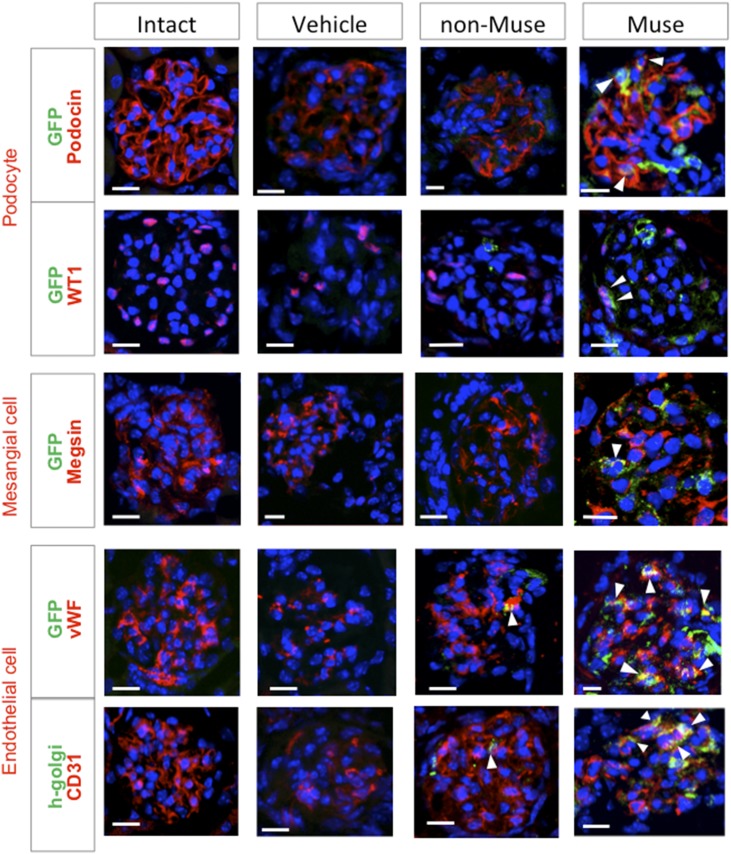
Glomerular Marker Expression in Muse Cells in FSGS-SCID Mice
Histologic analysis was performed at 7 weeks (Figure 3, Supplemental Result 7). Integration of human Muse cells was confirmed using the anti-human Golgi complex in addition to GFP. Glomerular cells were positive for GFP-expressing podocin, which localizes at the podocyte cytoplasm, and WT1, a nuclear marker for podocytes. They also expressed megsin, a cytoplasmic marker for mesangial cells. As for endothelial cell markers, vWF, a cytoplasmic marker, and CD31, which localizes at the cell membrane, were expressed in GFP(+) or human Golgi complex(+) glomerular cells. The specificity of each antibody was confirmed with the use of negative controls (Supplemental Result 8). Of the GFP(+) cells in the glomerulus, 31.1%±1.7% expressed podocin, 12.6%±1.7% expressed megsin, and 41.0%±4.3% expressed CD31.
Figure 3.
Muse cells spontaneously differentiate into glomerular cells in FSGS-SCID mice (7 weeks). Expression of the podocyte markers podocin and WT1, the mesangial cell marker megsin, and the endothelial cell markers vWF and CD31 in the glomerulus of intact SCID and FSGS-SCID of the vehicle, non-Muse, and Muse groups. Human Muse and non-Muse cells were detected as GFP- or human Golgi complex–positive cells. The single-channel images used to produce these figures are shown in Supplemental Result 7. Scale bars, 20 μm.
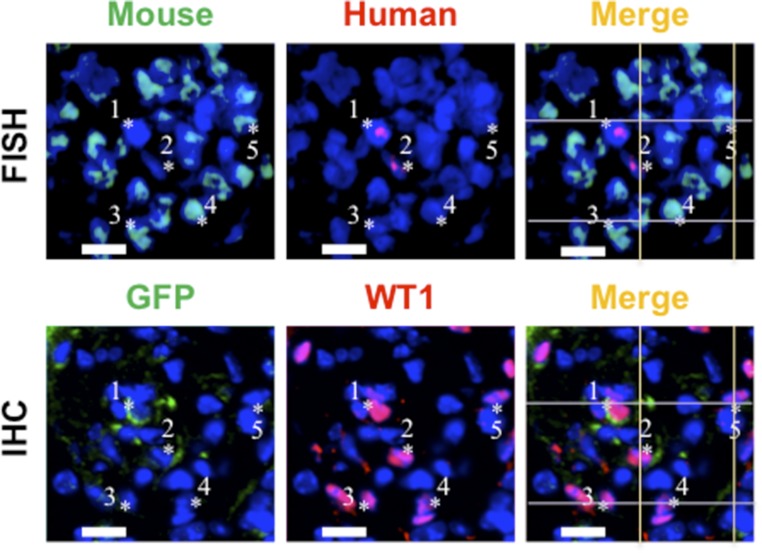
We further performed fluorescence in situ hybridization (FISH) analysis for murine and human genomic DNA (Figure 4). Adjacent sections were simultaneously subjected to immunohistochemistry to determine the differentiation state of each cell in the FISH. GFP(+) human Muse cells that expressed WT1 appeared to be positive for the human DNA probe but not the mouse probe (Figure 4). No human/mouse probe double-positive cells in the GFP(+)/WT1(+) cells were detected. Although incomplete matching of cell positions in neighboring sections may limit the findings, the result suggested that fusion between Muse cells and host glomerular cells is not likely a major mechanism that explains the differentiation of Muse cells in the glomerulus.
Figure 4.
Muse cells differentiate into glomerular cells without fusing with the host cells. Mouse and human chromosomes are indicated by light blue and red signals, respectively, in FISH; *1 to approximately *2 cells were GFP(+)/WT1(+) (in immunohistochemistry of the neighboring section [IHC]) and possessed only human chromosomes (red signal in FISH), whereas*3 to approximately *5 cells were GFP(−)/WT1(+) and possessed only mouse chromosomes (light blue signal in FISH). Because the tissue section thickness was approximately 8 μm, the nuclear location and morphology of the cytoplasm by immunohistochemistry are not exactly the same as those assessed by FISH. Scale bars, 20 μm.
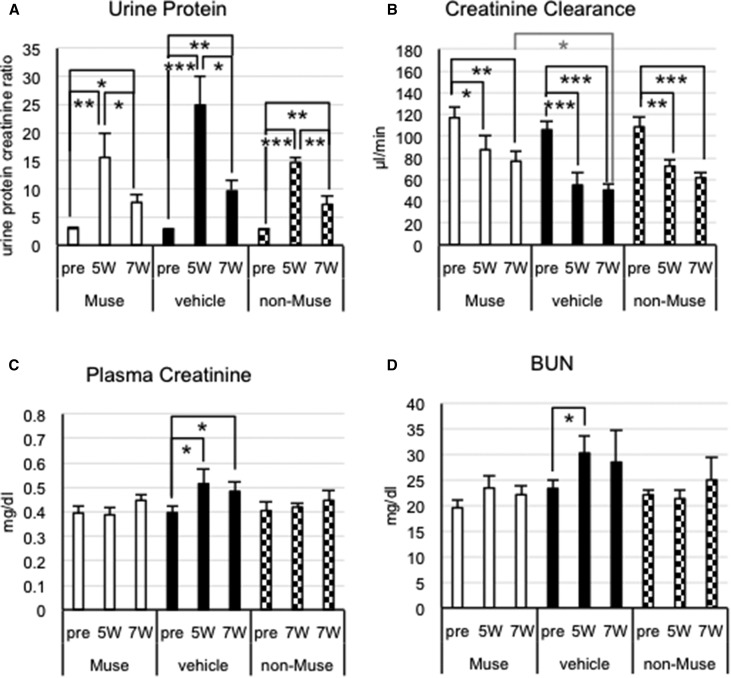
Renal Function in FSGS-SCID Mice
The urine protein-to-creatinine ratio at 5 and 7 weeks was significantly higher than that before DOX injection (pre-DOX) in all three groups (P<0.01) (Figure 5A). The actual values at 7 weeks were smaller in the Muse and non-Muse groups than in the vehicle group, although the differences among the three groups were not significant at either time point (Figure 5A). At 7 weeks, creatinine clearance recovered significantly in the Muse group (76.9±8.7 μl/min) compared with the vehicle group (50.4±6.1 μl/min; P<0.05), representing approximately 45% recovery in the Muse group (Figure 5B). The non-Muse group showed no significant recovery compared with the vehicle group (Figure 5B). Plasma creatinine (Figure 5C) and BUN (Figure 5D) levels did not change significantly among pre-DOX (5 and 7 weeks) in either group, although plasma creatinine was significantly increased at 5 and 7 weeks and BUN was significantly increased at 5 weeks compared with pre-DOX in the vehicle group (P<0.05) (Figure 5C). Notably, the non-Muse and vehicle groups did not differ significantly with regard to any of the four parameters at 5 and 7 weeks (Figure 5). Methods for measuring creatinine clearance and interpretation of renal functions are provided in Supplemental Result 9.
Figure 5.
Muse cells improve renal functions in FSGS-SCID mice. (A) Urine protein-to-creatinine ratio (urine protein), (B) creatinine clearance, (C) plasma creatinine, and (D) BUN. (B) Creatinine clearance was significantly higher in the Muse group at 7 weeks compared with in the vehicle group. Urine protein-to-creatinine ratio, plasma creatinine, and BUN did not significantly differ among the three groups at any time point. *P<0.05; **P<0.01; ***P<0.001.
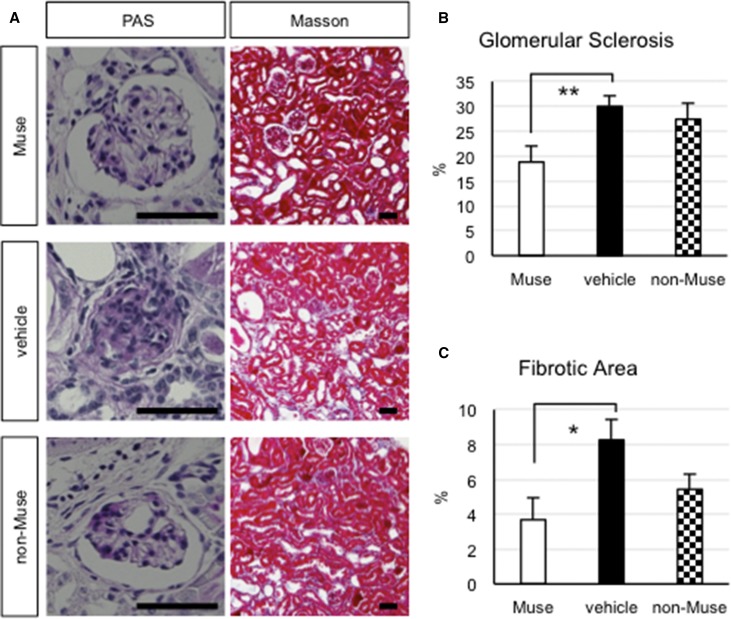
We next evaluated the glomerular sclerosis (periodic acid–Schiff-positive area per total glomerular area in the glomeruli) and interstitial fibrosis (Masson trichrome per area of one optical field) at 7 weeks. The vehicle group exhibited the most glomerular sclerosis (30.0%±2.1%) and interstitial fibrosis (8.2%±1.1%), whereas the Muse group exhibited the least glomerular sclerosis (18.8%±3.2%; approximately 40% improvement; P<0.01 versus vehicle group) and interstitial fibrosis (3.6%±1.2%; approximately 56% improvement; P<0.05 versus the vehicle group), indicating greater tissue repair effects in the Muse group (Figure 6). The pathologic findings in the non-Muse group were slightly better than those of the vehicle group, but the differences were not statistically significant between the groups (Figure 6, B and C).
Figure 6.
Muse cells attenuate glomerular sclerosis and interstitial fibrosis in the FSGS-SCID kidney. (A) Paraffin sections were stained with periodic acid–Schiff (PAS) to evaluate glomerular sclerosis (left panel) and Masson trichrome to evaluate interstitial fibrosis (right panel). (B) Glomerular sclerosis and (C) interstitial fibrosis were significantly attenuated in the Muse group compared with in the vehicle group. Scale bars, 50 μm. *P<0.05; **P<0.01.
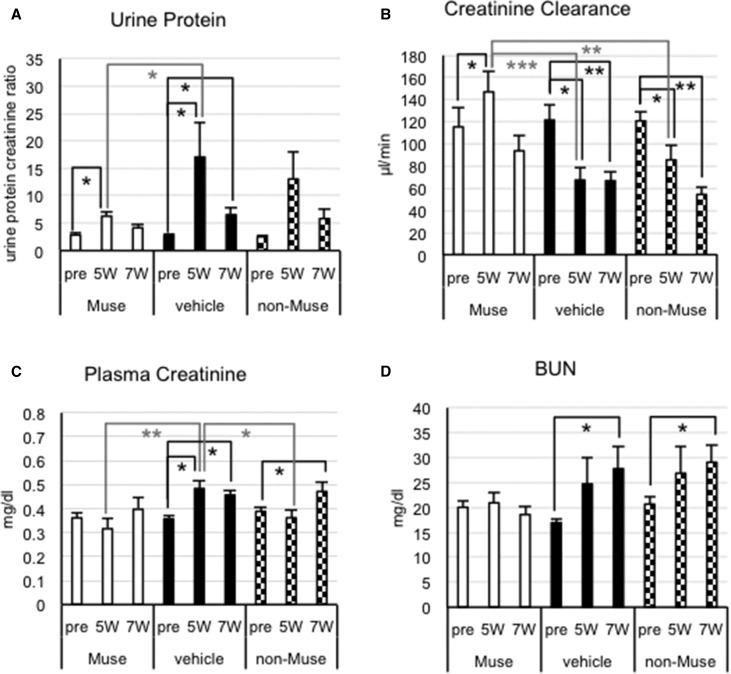
Renal Function in FSGS-BALB/c Mice after Muse Cell Infusion
Cells were infused without immunosuppression in FSGS-BALB/c mice. The urine protein-to-creatinine ratio in each group exhibited the same tendency; the actual value substantially increased after 5 weeks compared with pre-DOX and decreased at 7 weeks, although not to basal levels (Figure 7A). At 5 weeks, the urine protein-to-creatinine ratio in the Muse group was dramatically reduced (approximately 65%) compared with that in the vehicle group (P<0.05), whereas the non-Muse group exhibited no significant change compared with the vehicle group (Figure 7A). No significant differences, however, were detected among the three groups at 7 weeks.
Figure 7.
Recovery of renal functions in FSGS-BALB/c mice at 5 weeks after Muse cell infusion. (A) Urine protein-to-creatinine ratio (urine protein), (B) creatinine clearance, (C) plasma creatinine, and (D) BUN. (A) Urine protein levels were significantly attenuated in the Muse group compared with in the vehicle group at 5 weeks (P<0.05). (B) The Muse group showed significantly higher creatinine clearance compared with the vehicle (P<0.001) and non-Muse (P<0.01) groups, indicating substantial recovery of renal function in the Muse group at 5 weeks. (C) Plasma creatinine was significantly lower in the Muse (P<0.01) and non-Muse (P<0.05) groups than in the vehicle group at 5 weeks. (D) BUN was stable in the Muse group, whereas it was significantly increased in the vehicle and non-Muse groups at 7 weeks. *P<0.05; **P<0.01; ***P<0.001.
The vehicle group showed reduced creatinine clearance at 5 weeks compared with pre-DOX (approximately 52% of pre-DOX). In contrast, the creatinine clearance values were twofold higher in the Muse group than in the vehicle group at 5 weeks (P<0.001), and there was also a significant difference between the Muse and non-Muse groups (P<0.01) (Figure 7B). In contrast, creatinine clearance did not differ between the non-Muse and vehicle groups at any time point (Figure 7B). The significant recovery observed in the Muse group at 5 weeks, however, disappeared at 7 weeks, and significant differences were no longer detected among the three groups (Figure 7B).
Plasma creatinine continuously increased in the vehicle group compared with pre-DOX (Figure 7C). Plasma creatinine values were significantly lower in the Muse group than in the vehicle group at 5 weeks (P<0.01), but they were slightly elevated at 7 weeks. The non-Muse group had a lower plasma creatinine level than the vehicle group at 5 weeks (P<0.05), and it was increased at 7 weeks (pre-DOX versus 7 weeks; P<0.05). At 7 weeks, there were no significant differences among the three groups. BUN levels were increased in the vehicle and non-Muse groups at 7 weeks compared with pre-DOX, whereas the Muse group exhibited stable BUN levels (Figure 7D).
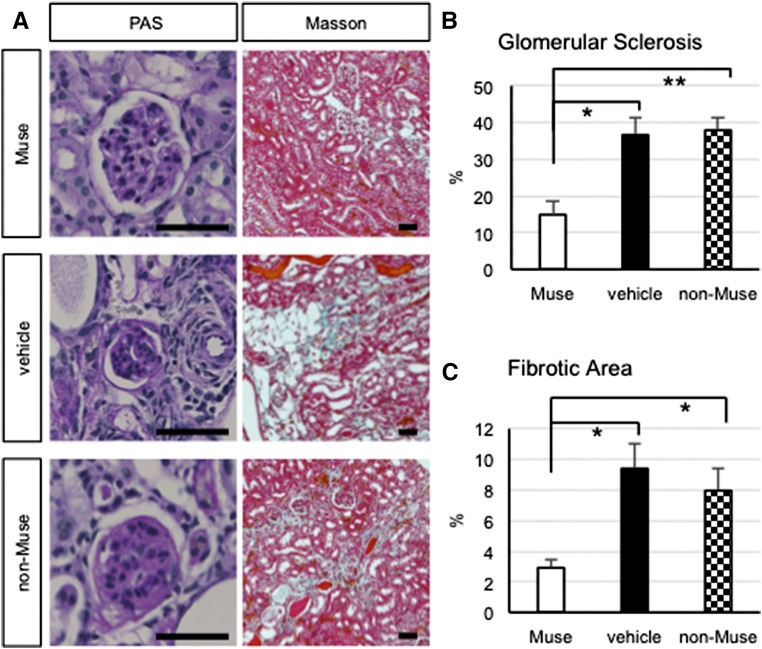
In general, the Muse group exhibited significant recovery in urine protein, creatinine clearance, and plasma creatinine compared with the vehicle and non-Muse (creatinine clearance) groups at 5 weeks, although such effects were not maintained at 7 weeks. Glomerular sclerosis and interstitial fibrosis were analyzed at 7 weeks. The Muse group showed statistically significant improvement in both parameters compared with the vehicle and non-Muse groups, whereas the non-Muse group was not significantly different from the vehicle group (Figure 8). The area of glomerular sclerosis was reduced in the Muse group (14.9%±3.8%) compared with both the vehicle group (36.6%±4.9%; representing an approximately 60% improvement in the sclerotic glomeruli; P<0.05) and the non-Muse group (37.9%±3.2%; P<0.01) (Figure 8B). The interstitial fibrotic area was also diminished in the Muse group (2.9%±0.5%) compared with both the vehicle group (9.4%±1.7%; representing an approximately 68% reduction in the fibrotic area; P<0.05) and the non-Muse group (8.0%±1.5%; P<0.05) (Figure 8C). The non-Muse group showed no changes in the degree of fibrosis compared with the vehicle group (Figure 8C).
Figure 8.
Muse cells attenuate glomerular sclerosis and interstitial fibrosis in the FSGS-BALB/c kidney. Paraffin sections were stained with periodic acid–Schiff (PAS) to evaluate glomerular sclerosis (left panel) and Masson trichrome to evaluate interstitial fibrosis (right panel). (B) Glomerular sclerosis and (C) interstitial fibrosis were significantly attenuated in the Muse group compared with in the vehicle and non-Muse groups. Scale bars, 50 μm. *P<0.05; **P<0.01.
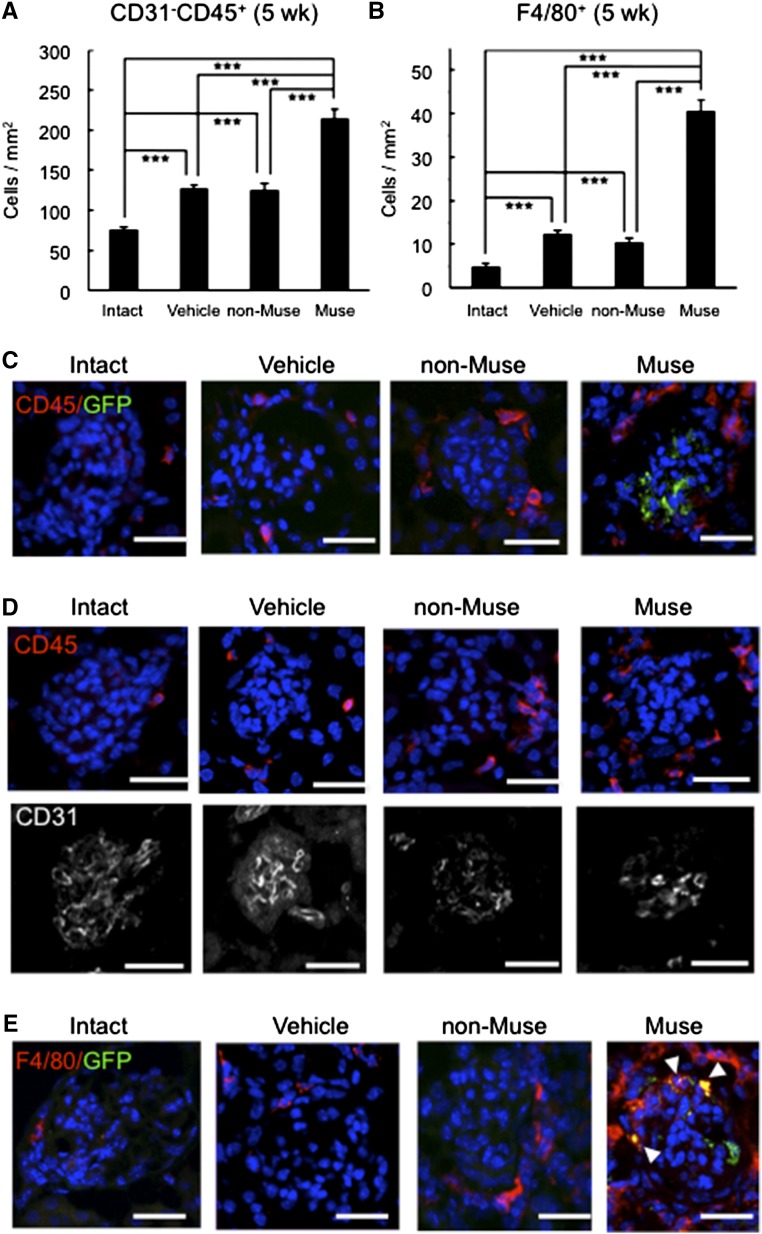
Integration and Differentiation of Muse Cells in FSGS-BALB/c Mice
Almost no GFP(+) xenograft cells were observed in FSGS-BALB/c mouse glomeruli in either the Muse or non-Muse groups at 7 weeks (data not shown), probably due to the lack of immunosuppression. In fact, invasion of CD31(−)/CD45(+) cells (leukocytes) (Figure 9, A–D) and F4/80(+) cells (macrophages) (Figure 9, B and E) was substantially higher in the Muse group at 5 weeks compared with in the non-Muse group (P values <0.001) (Figure 9, A and B), in which the injected cells basically did not remain in the kidney even at 2 weeks (Figure 2B). The distribution of F4/80(+) cells in most of the glomeruli was similar to that of CD45(+) cells (Figure 9, C–E) in the Muse group. The number of glomeruli that exhibited phagocytosis of GFP(+) cells by F4/80(+) cells was very small at 5 weeks, probably because immunorejection had just started at this time point (Figure 9E). The fact that Muse cells remained until 5 weeks may be partly explained by the immunomodulatory effect of Muse cells as assessed by a mixed lymphocyte proliferation assay and IFN-γ–induced expression of indoleamine-2,3 dioxygenase, a mediator of immunosuppression (Supplemental Result 10).18,19 These results are consistent with the optimal functional recovery exhibited by the Muse group at 5 weeks but not at 7 weeks (Figure 7, A–C).
Figure 9.
Immunologic cells invade within the FSGS-BALB/c glomeruli at 5 weeks. (A) Because CD45 reacts with not only leukocytes but also, endothelial cells, we counted only CD31(−)/CD45(+) cells to exclude endothelial cells. The number of CD31(−)/CD45(+) cells was highest in the Muse group among the four groups. (B) The number of F4/80(+) macrophages was also highest in the Muse group. ***P<0.001. (C–E) Localization of (C) CD45 (leukocytes)/GFP (Muse or non-Muse cells), (D) CD45 (leukocytes)/CD31 (endothelial) double staining on the same section, and (E) F4/80 (macrophages)/GFP (Muse or non-Muse cells). In D, most of CD45 did not merge with CD31. In the Muse group, the distribution of F4/80(+) cells in most of the glomeruli was similar to that of CD45 shown in C and D. As shown in E, however, a very small number of glomeruli showed invasion of F4/80(+) cells. Note that arrowheads in E show the GFP(+) fragments/particles in F4/80 cytoplasm, suggesting phagocytosis of GFP(+) fragments/particles by F4/80(+) macrophages. Scale bars, 20 μm.
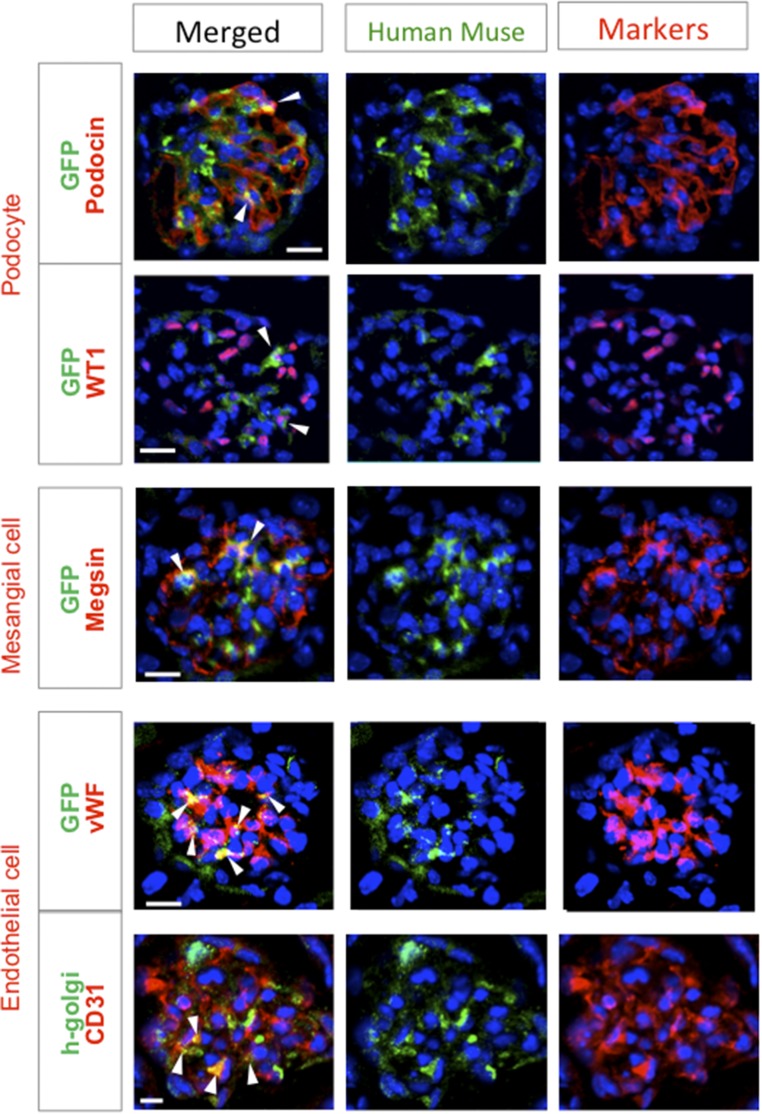
On the basis of the above observations, histologic assessment was performed at 5 weeks. Marker expression levels in the intact, vehicle, and non-Muse groups tended to be similar to those in FSGS-SCID mice (Supplemental Result 11). GFP(+) or human Golgi complex(+) glomerular cells that expressed podocin, WT1, megsin, vWF, or CD31 were only present in the Muse group (Figure 10). Of the GFP(+) cells in the glomerulus, 31.0%±2.2% expressed podocin, 11.7%±1.8% expressed megsin, and 46.6%±3.7% expressed CD31.
Figure 10.
Muse cells spontaneously differentiate into glomerular cells in FSGS-BALB/c mice (5 weeks). GFP- or human Golgi complex–positive cells coexpressed the podocyte markers podocin and WT1, the mesangial marker megsin, and the endothelial markers vWF and CD31 (arrowheads). Scale bars, 20 μm.
Capacity of Muse Cells for Migration and Renoprotection
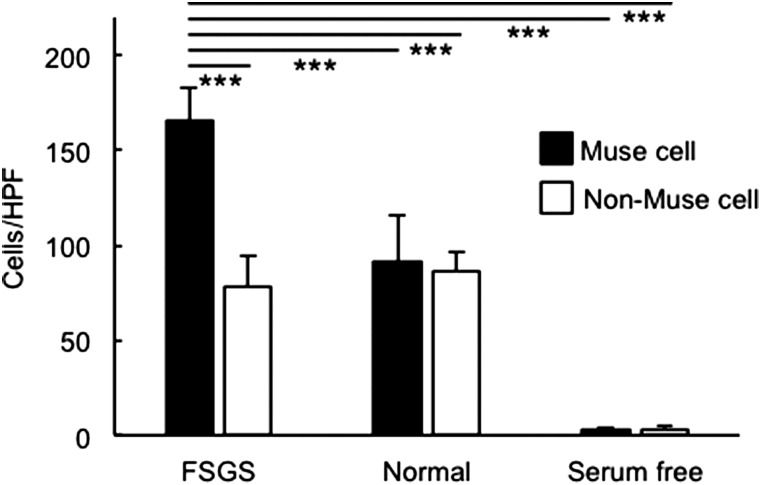
The migration capacity of human Muse and non-Muse cells toward the serum from both normal and FSGS-SCID mice (1 week after DOX administration) was assessed using a matrigel invasion chamber. Compared with the serum of intact normal mice, a significantly greater number of Muse cells migrated to FSGS mouse serum compared with non-Muse cells (P<0.001) (Figure 11).
Figure 11.
Muse cells have migration capacity toward the serum from FSGS-SCID mice. The number of migrated human Muse and non-Muse cells was counted after 22 hours of incubation in a matrigel invasion chamber with serum from either normal SCID or FSGS-SCID mice. Serum free was set as the control. ***P<0.001.
The ratios of apoptotic podocin(+) and CD31(+) cells at 3 days and apoptotic podocin (+) at 10 days tended to be lower in the Muse group than in the vehicle group (Supplemental Result 12). The ratio of PCNA(+) cells among WT1(+) cells and that of Ki67(+) cells among CD31(+) cells at 10 days tended to be higher in the Muse group compared with the other two groups (Supplemental Result 12). The renal tissue of the Muse group showed significantly higher gene expression levels of IGF-1, VEGF-A, VEGF-B, VEGF-C, VEGF-D, EGF, and TGF-α, which are suggested to be involved in renoprotection,20 compared with the vehicle group (Supplemental Result 13).
Discussion
Muse cells are unique stem cells that (1) preferentially migrate to the damaged kidney as suggested by an in vitro migration assay using FSGS-SCID serum, which allowed intravenously administered Muse cells to efficiently home to and integrate into damaged glomeruli; (2) are stress-tolerant3,5 and thus, able to survive the harsh microenvironment of damaged glomeruli; (3) spontaneously differentiate after integration; and (4) attenuate glomerular sclerosis and interstitial fibrosis. These properties are not exhibited by other stem cells in CKD models.21,22 Renal function recovery was more clearly recognized in FSGS-BALB/c than in FSGS-SCID, probably because functional impairment, which usually progresses with inflammatory responses that are deficient in SCID mice, was incomplete in FSGS-SCID.
A critical issue regarding the use of stem cell/progenitors cells for FSGS is the low survival rate of the cells after administration. The harsh environment in the damaged tissue due to proapoptotic and proinflammatory factors and reactive oxygen and nitrogen species makes it very difficult for the cells to survive after transplantation.23–25 Because such a hostile environment is difficult to overcome, a potential solution is to use stem cells that are able to adapt to a stressful environment, which could significantly improve the homing and survival rates. Muse cells are stress tolerant and adaptable to severe conditions.3,5,7 This study also showed the intrinsic property of Muse cells to remain in the damaged tissue; homing and survival of Muse cells were observed in the kidneys of FSGS-SCID (up to 7 weeks) and FSGS-BALB/c (up to 5 weeks) in contrast to non-Muse cells, which were mainly trapped in the lung and spleen and did not remain in the damaged kidney.
The in vivo dynamics showed that Muse cells survived only in the kidney and did not survive in other organs at 7 weeks, whereas non-Muse cells, corresponding to 98% to approximately 99% of MSCs, were mainly detected in the lung and spleen and did not remain in the kidney, even at 2 weeks. Integration of Muse cells into the kidney was also confirmed by multiple approaches: detection of the human-specific Alu sequence in quantitative PCR, GFP- and human Golgi complex–positive signals in immunohistochemistry and the human DNA probe in FISH. These findings suggest that homing and integration of Muse cells into the damaged glomeruli were driven by a positive mechanism, including migration of Muse cells toward signals that were produced by the damaged kidney and released into the blood in FSGS mice. The results of the in vitro migration assay shown in Figure 11 may support this hypothesis.
In the Muse group of FSGS-BALB/c mice, functional recovery of the urine protein-to-creatinine ratio, creatinine clearance, and plasma creatinine was clearly observed at 5 weeks compared with in the vehicle and non-Muse (only for creatinine clearance) groups but was significantly impaired at 7 weeks, and there were no significant differences among the three groups at 7 weeks. Functional recovery was observed during the period when Muse cells survived in the host kidney environment until 5 weeks even without immunosuppressant but was later abrogated—most likely when the Muse cells differentiated into glomerular cells, which may have allowed the host immune cells to reject them as suggested by the invasion of immunologic cells. A similar phenomenon was reported in a study of MSCs in a rat myocardial infarction model, in which the MHC profile changed, and the immunomodulatory function of allogeneic MSCs associated with differentiation into myocardial cells was lost.26 Muse cells have immunomodulatory effects,27 similar to MSCs. MSCs from mice and humans have different mechanisms of modulating the immune system (iNOS and indoleamine-2,3 dioxygenase, respectively)18,19; however, it is unclear how effectively the immunomodulatory effect of human Muse cells functions in FSGS-BALB/c mice. Nevertheless, it is plausible that the immunomodulatory effect of Muse cells protected the Muse cells from immunologic attack in the earlier period; however, this protective effect was lost after differentiation, and the cells were eventually immunorejected. Furthermore, Muse cells were inferred to maintain a tissue repair effect, even with immunologic cell invasion, partially through regulating the balance of macrophages toward M2-type macrophages—anti-inflammatory cells that promote tissue repair, similar to MSCs.28,29 Other than immunomodulation, Muse cells were suggested to have a renoprotective effect (Supplemental Results 12 and 13). On the basis of the timing of immunorejection and impairment of functional recovery and the multiple actions of Muse cells, the survival of the integrated Muse cells is considered important for maintaining functional recovery of the damaged kidney.
In contrast to mesangial and endothelial cells, podocytes do not normally regenerate.30,31 A possible explanation for podocyte differentiation in Muse cells might be that systemically administered Muse cells might have passed through the basement membrane of impaired/disrupted vessels into the glomerular portion followed by differentiation into podocytes (Supplemental Result 14). DOX has vascular toxicity and causes vessel impairment.32,33 Nearly one half (approximately 41% for SCID and approximately 47% for BALB/c) of the GFP(+) Muse cells that integrated into the glomeruli expressed endothelial marker CD31(+). Turnover and replacement of endothelial cells are major mechanisms that underlie maintenance of the vascular integrity within the kidney.34,35 It is possible that replacement of endothelial cells by Muse cells induces the formation of new blood vessels with consequent improvement of cell-cell contact and paracrine interactions in the glomerulus, leading to tissue repair and functional recovery of the kidney.34,35 If so, differentiation of Muse cells into endothelial cells might have contributed to the improvement of glomerular sclerosis and fibrosis. In fact, Kinoshita et al.9 reported the ability of human Muse cells to produce cytokines and trophic factors relevant to tissue repair. Because no direct proof of replacement of damaged glomerular cells through the differentiation of Muse cells is provided in our study, differentiation is not likely a primary mechanism of renoprotection. Rather, Muse cells could have multiple effects to deliver functional recovery by synergistic influences on cell differentiation, paracrine effects, immunomodulation, and suppression of fibrosis.
Despite the disappearance of Muse cells after 5 weeks, the Muse FSGS-BALB/c group sustained efficient reduction of glomerular sclerosis and interstitial fibrosis of the renal tissue compared with the vehicle and non-Muse groups at 7 weeks. MSCs have the ability to attenuate sclerosis/interstitial fibrosis through their trophic effects,36–39 and such effects are most potent in the early phase after transplantation and sustained for a certain period, even after the cells disappear.40,41 Considering that Muse cells are a small subpopulation of MSCs and may have similar effects, the efficient reduction of glomerular sclerosis and interstitial fibrosis of the renal tissue may be explained by the same mechanism as that of MSCs.
Because intravenous injection of naïve Muse cells led to functional recovery in the mouse FSGS model, this simple, cost-effective strategy—namely collection of Muse cells from the BM, large-scale expansion, and intravenous injection—is a feasible treatment for FSGS. Induction of Muse cells into glomerular cells by gene introduction or cytokine treatment in a cell processing center before transplantation is not necessary in this procedure. Finally, the findings in this model may be most relevant to human kidney disease characterized by progressive glomerulosclerosis and may not be applicable to other forms of CKD.
Concise Methods
All animal experiments were approved by Tohoku University’s Committee of Bioethics. FSGS was induced by tail vein injection of DOX (Wako, Tokyo, Japan) at a concentration of 5.3 mg/kg body wt for SCID mice (11–14 weeks old) and 11.5 mg/kg body wt for BALB/c mice (11–14 weeks old). One week later, the SCID and BALB/c mice were divided into three groups: Muse and non-Muse (n=9 for SCID, n=5 for BALB/c) as well as vehicle (n=10 for SCID, n=5 for BALB/c) groups, and 2×104 cells suspended in sterile saline were injected into the tail vein. Data are shown as means±SEM. Detailed protocols for the other experiments are provided in Supplemental Material.
Disclosures
N.U., Y. Kushida, S.W., and M.D. are affiliated with the Department of Stem Cell Biology and Histology at Tohoku University Graduate School of Medicine (Sendai, Japan), which is party to a codevelopment agreement with Life Science Institute, Inc. (LSII; Tokyo, Japan). S.W. and M.D. have a patent for Muse cells and the isolation method thereof licensed to LSII. Y.H. is an employee of LSII. G.C. is a consultant for ClusterXStem Inc.
Supplementary Material
Acknowledgments
This study was supported by a Grant-in-Aid for Scientific Research (B) of the Japan Society for the Promotion of Science, The Eunice Kennedy Shriver National Institute of Child Health & Human Development, and the National Institutes of Health through cooperative agreement P50 HD071836.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016070775/-/DCSupplemental.
References
- 1.Aggarwal S, Moggio A, Bussolati B: Concise review: Stem/progenitor cells for renal tissue repair: Current knowledge and perspectives. Stem Cells Transl Med 2: 1011–1019, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hickson LJ, Eirin A, Lerman LO: Challenges and opportunities for stem cell therapy in patients with chronic kidney disease. Kidney Int 89: 767–778, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, Goda M, Akashi H, Inutsuka A, Niwa A, Shigemoto T, Nabeshima Y, Nakahata T, Nabeshima Y, Fujiyoshi Y, Dezawa M: Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci USA 107: 8639–8643, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M: Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc 8: 1391–1415, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Alessio N, Özcan S, Tatsumi K, Murat A, Peluso G, Dezawa M, Galderisi U: The secretome of MUSE cells contains factors that may play a role in regulation of stemness, apoptosis and immunomodulation. Cell Cycle 16: 33–44, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wakao S, Kitada M, Kuroda Y, Shigemoto T, Matsuse D, Akashi H, Tanimura Y, Tsuchiyama K, Kikuchi T, Goda M, Nakahata T, Fujiyoshi Y, Dezawa M: Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci USA 108: 9875–9880, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heneidi S, Simerman AA, Keller E, Singh P, Li X, Dumesic DA, Chazenbalk G: Awakened by cellular stress: Isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PLoS One 8: e64752, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogura F, Wakao S, Kuroda Y, Tsuchiyama K, Bagheri M, Heneidi S, Chazenbalk G, Aiba S, Dezawa M: Human adipose tissue possesses a unique population of pluripotent stem cells with nontumorigenic and low telomerase activities: Potential implications in regenerative medicine. Stem Cells Dev 23: 717–728, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Kinoshita K, Kuno S, Ishimine H, Aoi N, Mineda K, Kato H, Doi K, Kanayama K, Feng J, Mashiko T, Kurisaki A, Yoshimura K: Therapeutic potential of adipose-derived SSEA-3-positive Muse cells for treating diabetic skin ulcers. Stem Cells Transl Med 4: 146–155, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uchida H, Morita T, Niizuma K, Kushida Y, Kuroda Y, Wakao S, Sakata H, Matsuzaka Y, Mushiake H, Tominaga T, Borlongan CV, Dezawa M: Transplantation of unique subpopulation of fibroblasts, Muse cells, ameliorates experimental stroke possibly via robust neuronal differentiation. Stem Cells 34: 160–173, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Iseki M, Kushida Y, Wakao S, Akimoto T, Mizuma M, Motoi F, Asada R, Shimizu S, Unno M, Chazenbalk G, Dezawa M: Muse cells, nontumorigenic pluripotent-like stem cells, have liver regeneration capacity through specific homing and cell replacement in a mouse model of liver fibrosis. Cell Transplant 26: 821–840, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hori E, Hayakawa Y, Hayashi T, Hori S, Okamoto S, Shibata T, Kubo M, Horie Y, Sasahara M, Kuroda S: Mobilization of pluripotent multilineage-differentiating stress-Enduring cells in ischemic stroke. J Stroke Cerebrovasc Dis 25: 1473–1481, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Dezawa M: Muse cells provide the pluripotency of mesenchymal stem cells: Direct contribution of Muse cells to tissue regeneration. Cell Transplant 25: 849–861, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Lee VW, Harris DC: Adriamycin nephropathy: A model of focal segmental glomerulosclerosis. Nephrology (Carlton) 16: 30–38, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Zoja C, Garcia PB, Remuzzi G: The role of chemokines in progressive renal disease. Front Biosci (Landmark Ed) 14: 1815–1822, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Eggenhofer E, Luk F, Dahlke MH, Hoogduijn MJ: The life and fate of mesenchymal stem cells. Front Immunol 5: 148, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batchelder CA, Lee CC, Matsell DG, Yoder MC, Tarantal AF: Renal ontogeny in the rhesus monkey (Macaca mulatta) and directed differentiation of human embryonic stem cells towards kidney precursors. Differentiation 78: 45–56, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML: Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant 19: 667–679, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling W, Zhang J, Yuan Z, Ren G, Zhang L, Chen X, Rabson AB, Roberts AI, Wang Y, Shi Y: Mesenchymal stem cells use IDO to regulate immunity in tumor microenvironment. Cancer Res 74: 1576–1587, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ezquer ME, Ezquer FE, Arango-Rodríguez ML, Conget PA: MSC transplantation: A promising therapeutic strategy to manage the onset and progression of diabetic nephropathy. Biol Res 45: 289–296, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Villanueva S, Ewertz E, Carrión F, Tapia A, Vergara C, Céspedes C, Sáez PJ, Luz P, Irarrázabal C, Carreño JE, Figueroa F, Vio CP: Mesenchymal stem cell injection ameliorates chronic renal failure in a rat model. Clin Sci (Lond) 121: 489–499, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Ninichuk V, Gross O, Segerer S, Hoffmann R, Radomska E, Buchstaller A, Huss R, Akis N, Schlöndorff D, Anders HJ: Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int 70: 121–129, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Arumugam TV, Phillips TM, Cheng A, Morrell CH, Mattson MP, Wan R: Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol 67: 41–52, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song J, Finnerty CC, Herndon DN, Boehning D, Jeschke MG: Severe burn-induced endoplasmic reticulum stress and hepatic damage in mice. Mol Med 15: 316–320, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fulda S, Gorman AM, Hori O, Samali A: Cellular stress responses: Cell survival and cell death. Int J Cell Biol 2010: 214074, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang XP, Sun Z, Miyagi Y, McDonald Kinkaid H, Zhang L, Weisel RD, Li RK: Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation 122: 2419–2429, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Gimeno ML, Fuertes F, Barcala Tabarrozzi AE, Attorressi AI, Cucchiani R, Corrales L, Oliveira TC, Sogayar MC, Labriola L, Dewey RA, Perone MJ: Pluripotent nontumorigenic adipose tissue-derived Muse cells have immunomodulatory capacity mediated by transforming frowth factor-beta1. Stem Cells Transl Med 6: 161–173, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Novak ML, Koh TJ: Macrophage phenotypes during tissue repair. J Leukoc Biol 93: 875–881, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y: Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med 46: e70, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin SV, Petermann AT, Durvasula RV, Shankland SJ: Podocyte proliferation and differentiation in glomerular disease: Role of cell-cycle regulatory proteins. Nephrol Dial Transplant 18[Suppl 6]: vi8–vi13, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Guo JK, Cantley LG: Cellular maintenance and repair of the kidney. Annu Rev Physiol 72: 357–376, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Sun YB, Qu X, Zhang X, Caruana G, Bertram JF, Li J: Glomerular endothelial cell injury and damage precedes that of podocytes in adriamycin-induced nephropathy. PLoS One 8: e55027, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson EL, Sidaway JE, Cross MJ: Cardiotoxic drugs Herceptin and doxorubicin inhibit cardiac microvascular endothelial cell barrier formation resulting in increased drug permeability. Biol Open 5: 1362–1370, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guerrot D, Dussaule JC, Kavvadas P, Boffa JJ, Chadjichristos CE, Chatziantoniou C: Progression of renal fibrosis: The underestimated role of endothelial alterations. Fibrogenesis Tissue Repair 5[Suppl 1]: S15, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, Ding BS, Schachterle W, Liu Y, Rosenwaks Z, Butler JM, Xiang J, Rafii A, Shido K, Rabbany SY, Elemento O, Rafii S: Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 26: 204–219, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magnasco A, Corselli M, Bertelli R, Ibatici A, Peresi M, Gaggero G, Cappiello V, Chiavarina B, Mattioli G, Gusmano R, Ravetti JL, Frassoni F, Ghiggeri GM: Mesenchymal stem cells protective effect in adriamycin model of nephropathy. Cell Transplant 17: 1157–1167, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Zoja C, Garcia PB, Rota C, Conti S, Gagliardini E, Corna D, Zanchi C, Bigini P, Benigni A, Remuzzi G, Morigi M: Mesenchymal stem cell therapy promotes renal repair by limiting glomerular podocyte and progenitor cell dysfunction in adriamycin-induced nephropathy. Am J Physiol Renal Physiol 303: F1370–F1381, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Baulier E, Favreau F, Le Corf A, Jayle C, Schneider F, Goujon JM, Feraud O, Bennaceur-Griscelli A, Hauet T, Turhan AG: Amniotic fluid-derived mesenchymal stem cells prevent fibrosis and preserve renal function in a preclinical porcine model of kidney transplantation. Stem Cells Transl Med 3: 809–820, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franquesa M, Herrero E, Torras J, Ripoll E, Flaquer M, Gomà M, Lloberas N, Anegon I, Cruzado JM, Grinyó JM, Herrero-Fresneda I: Mesenchymal stem cell therapy prevents interstitial fibrosis and tubular atrophy in a rat kidney allograft model. Stem Cells Dev 21: 3125–3135, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunter U, Rong S, Djuric Z, Boor P, Müller-Newen G, Yu D, Floege J: Transplanted mesenchymal stem cells accelerate glomerular healing in experimental glomerulonephritis. J Am Soc Nephrol 17: 2202–2212, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J: Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol 18: 1754–1764, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.