Abstract
Mice lacking distal tubular expression of CLDN10, the gene encoding the tight junction protein Claudin-10, show enhanced paracellular magnesium and calcium permeability and reduced sodium permeability in the thick ascending limb (TAL), leading to a urine concentrating defect. However, the function of renal Claudin-10 in humans remains undetermined. We identified and characterized CLDN10 mutations in two patients with a hypokalemic-alkalotic salt-losing nephropathy. The first patient was diagnosed with Bartter syndrome (BS) >30 years ago. At re-evaluation, we observed hypocalciuria and hypercalcemia, suggesting Gitelman syndrome (GS). However, serum magnesium was in the upper normal to hypermagnesemic range, thiazide responsiveness was not blunted, and genetic analyses did not show mutations in genes associated with GS or BS. Whole-exome sequencing revealed compound heterozygous CLDN10 sequence variants [c.446C>G (p.Pro149Arg) and c.465–1G>A (p.Glu157_Tyr192del)]. The patient had reduced urinary concentrating ability, with a preserved aquaporin-2 response to desmopressin and an intact response to furosemide. These findings were not in line with any other known salt-losing nephropathy. Subsequently, we identified a second unrelated patient showing a similar phenotype, in whom we detected compound heterozygous CLDN10 sequence variants [c.446C>G (p.(Pro149Arg) and c.217G>A (p.Asp73Asn)]. Cell surface biotinylation and immunofluorescence experiments in cells expressing the encoded mutants showed that only one mutation caused significant differences in Claudin-10 membrane localization and tight junction strand formation, indicating that these alterations do not fully explain the phenotype. These data suggest that pathogenic CLDN10 mutations affect TAL paracellular ion transport and cause a novel tight junction disease characterized by a non-BS, non-GS autosomal recessive hypokalemic-alkalotic salt-losing phenotype.
Keywords: familial nephropathy, kidney tubule, hypokalemia, Cell & Transport Physiology, ion transport, salt-losing nephropathy
Hypokalemic alkalosis is often due to increased water and sodium delivery to the collecting duct with concomitant enhanced aldosterone action due to volume contraction. Both increase sodium reabsorption, which creates a lumen-negative potential that eventually promotes excretion of potassium and hydrogen ions. After the exclusion of acquired and/or drug-induced causes of hypokalemic alkalosis, the differential diagnosis includes several rare (genetic) renal tubular disorders. The most important molecular player in the pathogenesis of hypokalemic alkalosis is the apically located epithelial sodium channel (ENaC). Gain of function mutations in the genes encoding ENaC β- and γ-subunits cause Liddle syndrome, presenting with a hypokalemic alkalosis and hypertension at young age.1,2 More proximal defects in sodium reabsorption, so-called salt-losing nephropathies, can also induce a hypokalemic alkalosis by increasing distal tubular flow and sodium delivery. Several molecular players in these reabsorption processes were identified by studying the rare genetic tubular salt-losing disorders Bartter syndrome (BS) and Gitelman syndrome (GS). In BS, transcellular sodium-chloride reabsorption in the thick ascending limb of Henle loop (TAL) is disrupted due to mutations in transporters and channels involved in this process.3–7 GS is caused by mutations affecting distal convoluted tubular (DCT) transcellular sodium-chloride reabsorption.8
Salt-losing disorders distal from the TAL (e.g., GS) generally lead to hypocalciuria due to either increased proximal tubular paracellular transport or increased transcellular calcium reabsorption in the DCT.9–11 In contrast, salt-losing disorders in the TAL (e.g., BS) are frequently accompanied by hypercalciuria due to reduced passive paracellular reabsorption of calcium (and magnesium) in the TAL. In this tubular segment, about 60%–70% of filtered magnesium and 10%–20% of calcium are reabsorbed, driven by the lumen-positive transepithelial gradient that is maintained by transcellular sodium, chloride, and potassium transport processes.12 The lumen-positive gradient permits the passive paracellular transport of cations like calcium and magnesium but also, sodium from the prourine through tight junctions and back to the interstitium and vascular compartment.12,13 The main constituent of the size- and charge-selective tight junction is the family of claudins, encompassing at least 24 members in mammals.14 Claudin proteins consist of four transmembrane segments, intracellular N and C termini, and two extracellular loops that extend into the intercellular cleft between adjacent cells. They interact with other claudins within the same cell membrane (in cis) and claudins in the membrane of the adjacent cell (in trans). Trans-interaction via the extracellular loops bridges the cleft between neighboring cells and results in the formation of selective paracellular pores. The sum of multiple claudin interactions leads to the establishment of a complex tight junction strand meshwork.
The paracellular calcium and magnesium reabsorption in the TAL closely depends on the expression of Claudin-16 and -19, and mutations in the corresponding genes CLDN16 and CLDN19 cause familial hypomagnesemia with hypercalciuria and nephrocalcinosis, in which renal calcium and magnesium wasting occurs.15–18 Claudin-14 was shown to be a negative regulator of paracellular calcium reabsorption, probably by modifying the permeability properties of Claudin-16.19,20 Claudin-10 exists in two major isoforms, Claudin-10a and -10b, due to alternative splicing.21 Claudin-10a and -10b differ only in their first transmembrane segment and first extracellular loop. Claudin-10a is present in the proximal renal tubule (PT) and thought to form an anion-selective paracellular pore. In contrast, Claudin-10b is a component of the paracellular pathway in the TAL and confers permeability to small cations, like sodium, when overexpressed in cell culture.21–23 Mice with a conditional knockout of Cldn10 in nephron segments distal from PT show enhanced TAL paracellular magnesium and calcium permeability and reduced paracellular sodium permeability, leading to a urine concentrating defect. The functional significance of renal Claudin-10 expression in humans has remained elusive.
In this study, we report the molecular identification of a novel non-Bartter, non-Gitelman hereditary salt-losing nephropathy and describe the clinical phenotype in two unrelated patients, in whom we identified compound heterozygous variants in the CLDN10 gene encoding Claudin-10. This is the first description of a hypokalemic-alkalotic salt-losing nephropathy putatively on the basis of a primary defect of paracellular ion transport in TAL.
Results
Patient 1
This woman was referred to the endocrinologist in 1980 at the age of 21 years old because of hypokalemia detected at cardiologic evaluation for atypical chest pains. Detailed examination revealed a hypokalemic alkalosis with mild renal insufficiency, a polyuria of 3–5 L/d, and a reduced urine concentrating ability (Tables 1 and 2). Her BP was in the lower normal range without orthostatic hypotension. Serum magnesium was reported once, which was in the normal range (0.98 mmol/L). At that time, in the absence of genetic screening tests, a presumptive diagnosis of BS was made.
Table 1.
Clinical data of patients and family members
| Subject | Sex | Age,a yr | Serum | eGFR/ECC, >90 ml/min | TTKG, % | FE K, % | FE Mg, % | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K, 3.4–4.7 mM | Mg, 0.7–1.1 mM | Ca, 2.20–2.65 mM | HCO3, 22.0–28.0 mM | pH, 7.31–7.41 | Creatinine, μM | |||||||
| Patient 1 | ||||||||||||
| II:2 | W | 21 | 2.1 | 0.98 | 2.66 | 31.7 | 109 | 49 | ||||
| II.2 | 53 | 4.1b | 1.19 | 2.61 | 29.8 | 7.43 | 144 | 33/37 | 19.9 | 90 | 3.7 | |
| Family members | ||||||||||||
| II:3 | W | 58 | 3.6 | 0.86 | 2.44 | 30.7 | 7.39 | 83 | 62 | 7.5 | 14 | 6.4 |
| II:4 | W | 45 | 3.6 | 0.83 | 2.22 | 25.8 | 7.34 | 70 | 78 | 7.5 | 10 | 3.9 |
| III:1 | W | 26 | 4.0 | 0.98 | 2.47 | 26.0 | 69 | >90 | 8.1 | 17 | 2.3 | |
| III:2 | M | 24 | 3.8 | 0.96 | 2.38 | 32.6 | 86 | >90 | 7.8 | 20 | 4.1 | |
| Patient 2 | ||||||||||||
| II.2 | M | 15 | 2.8 | 1.03 | 2.59 | 30.1 | 7.57 | 90 | >90/149 | |||
| II.2 | 18 | 2.7 | 0.90 | 2.59 | 28.7 | 7.42 | 88 | >90 | 14.1 | 36 | 5.1 | |
| Family members | ||||||||||||
| I:1 | M | 48 | 3.6 | 0.80 | 2.29 | 27.0 | 7.38 | 89 | 88 | 4.8 | 7 | 3.6 |
| I:2 | W | 46 | 3.8 | 0.90 | 2.34 | 25.2 | 7.41 | 85 | 71 | 5.6 | 7 | 3.8 |
| II:1 | M | 21 | 3.9 | 0.90 | 2.37 | 24.1 | 7.38 | 90 | >90 | 4.7 | 9 | 3.9 |
| II:3 | W | 14 | 3.8 | 0.90 | 2.45 | 24.5 | 7.39 | 66 | >90 | 5.7 | 7 | 6.4 |
Individual subject numbers correspond with Figure 1. K, potassium; Mg, magnesium; Ca, calcium; HCO3, bicarbonate; ECC, endogenous creatinine clearance rate; TTKG, transtubular potassium gradient; FE, fractional excretion; W, woman; M, man.
Age at examination.
Serum potassium while on potassium supplementation.
Table 2.
Clinical data of patients and family members
| Subject | Urine | Thiazide Test; FE Cl,a 2.3%–4.9% | DDAVP Test | Furosemide Test; ΔFE Cl,a 8%–15% | Genetic Analysis CLDN10 Gene | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24-h Ca Excretion, mM | Ca/Creatinine, mM/mM | 24 h Mg Excretion, mM | Mg/Creatinine, mM/mM | ΔUrine Osmolality, mosmol/kg | Maximum Urine Osmolality, >800 mosmol/kg | Nucleotide Change | Consequence | |||
| Patient 1 | ||||||||||
| II:2 | 0.6–1.4 | |||||||||
| II:2 | < | < | 2.0 | 0.21 | 12.9 | 34/39 | 546/603 | 26.0 | c.446C>G, c.465–1G>T | p.Pro149Arg, p.Glu157-Tyr192del |
| Family members | ||||||||||
| II:3 | 0.43 | 0.46 | 294 | 825 | c.446C>G | p.Pro149Arg | ||||
| II:4 | 0.58 | 0.32 | 136 | 1179 | c.446C>G | p.Pro149Arg | ||||
| III:1 | 0.21 | 0.23 | c.446C>G | p.Pro149Arg | ||||||
| III:2 | 0.28 | 0.32 | 210 | 1048 | c.465–1G>T | p.Glu157-T yr192del | ||||
| Patient 2 | ||||||||||
| II.2 | < | < | ||||||||
| II.2 | 0.03 | 0.26 | 5.6 | c.446C>G, c.217G>A | p.Pro149Arg, p.Asp73Asn | |||||
| Family members | ||||||||||
| I:1 | 0.15 | 0.23 | c.446C>G | p.Pro149Arg | ||||||
| I:2 | 0.05 | 0.28 | c.217G>A | p.Asp73Asn | ||||||
| II:1 | 0.21 | 0.27 | None | |||||||
| II:3 | 0.06 | 0.61 | c.446C>G | p.Pro149Arg | ||||||
Individual subject numbers correspond with Figure 1. DDAVP was the synthetic arganine vasopressin analog. Ca, calcium; Mg, magnesium; FE, fractional excretion; Cl, chloride; <, below detection limit (urine calcium <0.1 mmol/L).
Maximum change of FE Cl as the main outcome measure in thiazide and furosemide responsiveness testing, with a ΔFE Cl <2.3% in thiazide responsiveness testing suggested to be abnormal and suggestive of GS.
In 2012, she was re-evaluated at our nephrology outpatient clinic. Her renal insufficiency had only mildly progressed, but a persistent hypocalciuria with serum calcium levels in the upper normal to high range was noted, which in retrospect, had been present since diagnosis in 1980 (Tables 1 and 2). A hypokalemic alkalosis with hypocalciuria is more in line with GS, which is classically accompanied by hypomagnesemia. However, serum magnesium levels were persistently normal to even increased (up to 1.22 mmol/L) at several occasions, and a challenge with hydrochlorothiazide showed an exaggerated rather than the generally blunted response expected in GS (Tables 1 and 2). Moreover, a challenge with furosemide, which can be blunted in patients with BS, also resulted in an exaggerated response. Mutations in SLC12A3 and CLCNKB were not detected by Sanger sequencing. Recently, we showed that patients with ADTKD-HNF1β can also show a hypo- or normomagnesemic Gitelman-like phenotype with either preserved or blunted response to thiazides.24,25 Because of a small right kidney with a single cyst, the presence of an HNF1B mutation or deletion was also excluded by Sanger sequencing and Multiplex Ligation–Dependent Probe Amplification (MLPA). Renal ultrasound and abdominal CT scanning did not show any other structural renal abnormalities apart from the small right kidney and the normal-sized left kidney or signs of nephrocalcinosis.
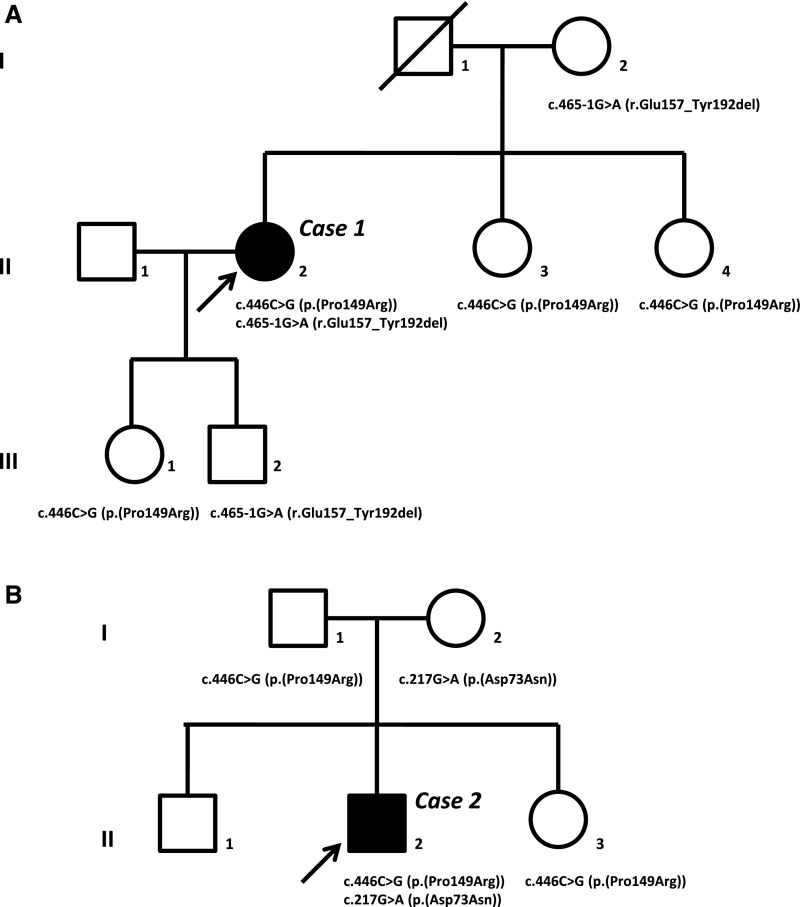
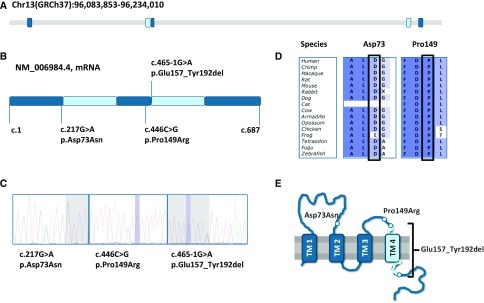
Subsequently, she was referred to our multidisciplinary nephrogenetic outpatient clinic, and whole-exome sequencing was performed, in which the data analysis was initially confined to a set of 177 genes confirmed to be associated with isolated or complex kidney diseases in humans. Open exome analysis thereafter identified two heterozygous sequence variants in the CLDN10 gene [c.446C>G (p.(Pro149Arg)) and c.465–1G>A (p.(Glu157_Tyr192del))]. The family pedigree is depicted in Figure 1A. The presence of one CLDN10 variant in her mother (I:2), the other CLDN10 variant detected in two sisters (II:3 and II:4), and both her children (III:1 and III:2) inheriting different variants are compatible with compound heterozygosity in the patient. One CLDN10 variant results in a single-amino acid substitution [p.(Pro149Arg)] in an evolutionary conserved amino acid in the second extracellular loop, whereas the other was predicted to disrupt the consensus splice site [p.(Glu157_Tyr192del)], leading to loss of exon 4 and deletion of the fourth transmembrane segment in the Claudin-10 protein (Figure 2). According to their localization, the mutations found in patient 1 affect both major Claudin-10 isoforms, Claudin-10a and -10b.
Figure 1.
Pedigrees of the families of patients 1 and 2 show that the clinical phenotype co-segregates with compound heterozygous CLDN10 variants. Arrows indicate index patients in (A) family 1 (subject II:2 or patient 1) and (B) family 2 (subject II:2 or patient 2). Affected individuals are indicated in black. The specific CLDN10 sequence variants found in the index patients and their family members and the predicted effects on protein level are depicted.
Figure 2.
Sequence analyses and evolutionary conservation of the CLDN10 gene and the identified CLDN10 variants. (A) Genomic structure of the human CLDN10 gene located at chromosome 13. (B) Schematic representation of the CLDN10 cDNA on the basis of the longest protein coding sequence (NM_006984.4). CLDN10 variants are depicted by vertical lines indicating the cDNA position and describing the effect on protein level. (C) Chromatograms for the different mutations confirmed by Sanger sequencing. (D) Evolutionary conservation of Asp73 and Pro149. The third CLDN10 variant is predicted to induce an alternative splice site leading to loss of glutamic acid at position 157 to tyrosine at position 192 and therefore, not depicted. (E) Predicted topology of the Claudin-10 protein with location of the different mutations and deletion indicated. TM, transmembrane region.
Patient 2
After the identification of CLDN10 sequence variants as a possible cause of the hypokalemic alkalosis with hypocalciuria and the tendency toward hypermagnesemia in patient 1, we recognized a similar phenotype in a second unrelated patient. He presented to the emergency department after a (near) collapse at age 15 years old in 2013, and a hypokalemia (2.6 mmol/L) and metabolic alkalosis were shown (Tables 1 and 2). In retrospect, hypokalemia was already present at a previous emergency department visit at age 8 years old. There had been recurrent episodes of collapse or falls with minor injuries without specific cause. The BP was normal, with no orthostatic hypertension tested. At adult age, his BP was rather low, with a mean of 105/55 mm Hg. There was a hypocalciuria with serum calcium ranging from 2.54 to 2.59 mmol/L. Hypokalemic alkalosis with hypocalciuria had prompted the suspicion of GS, but hypomagnesemia was absent with a serum magnesium in the normal range (0.98–1.03 mmol/L), and no sequence variants in SLC12A3 and CLCNKB were detected by Sanger sequencing. After genetic counseling, the now adult patient opted for whole-exome sequencing, in which data analyses for known kidney disease–associated genes excluded pathogenic mutations. Because the phenotype resembled that of patient 1, we also tested the CLDN10 gene in this analysis, and two CLDN10 variants were identified. These included the same c.446C>G (p.(Pro149Arg)) variant as detected in patient 1 as well as a novel c.217G>A (p.(Asp73Asn)) variant (Figure 2). The latter mutation affects exon 1b, which is only present in Claudin-10b but not present in Claudin-10a, and affects the first extracellular loop of the protein. Segregation analysis by Sanger sequencing in the parents of patient 2 confirmed compound heterozygosity for the CLDN10 variants in this patient. The pedigree of this family is depicted in Figure 1B. Thiazide responsiveness testing showed an exaggerated response to thiazide administration, consistent with patient 1. Renal imaging showed kidneys without structural abnormalities or nephrocalcinosis.
Family members of both patients were screened for electrolyte disturbances. The full hypokalemic-alkalotic, hypomagnesuric, hypocalciuric salt-losing phenotype was clearly restricted to the compound heterozygous patients. Interestingly, family members heterozygous for one CLDN10 variant showed serum potassium levels in the lower one half of the reference interval (Tables 1 and 2), and an occasional mild hypokalemia was reported in the daughter (III:1) of patient 1 and the father (I:1) and sister (II:3) of patient 2. Some heterozygous family members also showed high serum bicarbonate levels (Tables 1 and 2).
The identified CLDN10 sequence variants were not reported in the Exome Aggregation Consortium database (Cambridge, MA), a large database collecting next generation sequencing variants in over 60,000 exomes as proxy for variant allele frequencies in the general population. In addition, these sequence variants were not reported in other databases, like the Genome of the Netherlands Consortium and the Human Genetic Variation Database (Kyoto, Japan).
Urine Concentrating Defect
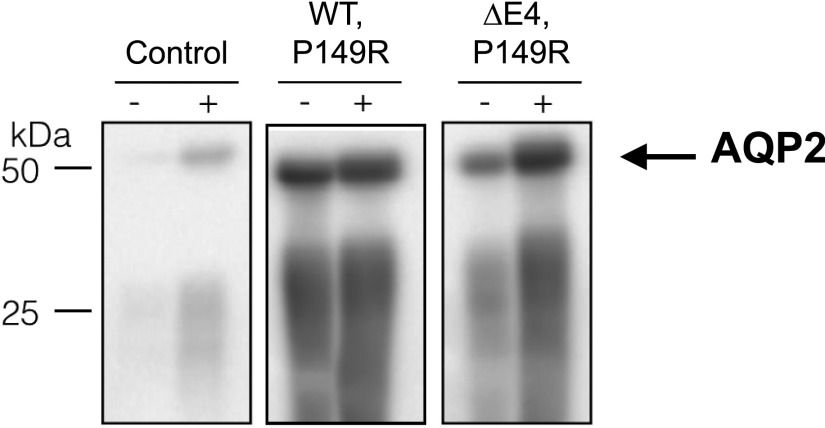
Because the conditional knockout of Cldn10 in nephron segments distal from PT in mice reduced calcium and magnesium excretion but increased sodium loss with a urine concentrating defect,26 we tested urinary concentrating ability in patient 1. This showed a blunted urine concentrating response to thirsting and desmopressin acetate (DDAVP) on two separate occasions. Heterozygous family members with only one of either CLDN10 variants showed normal responses to thirsting and/or DDAVP (Tables 1 and 2). A reduced response to DDAVP can result from both the inability of the collecting duct principal cells to react to the synthetic vasopressin analog and insufficient buildup of the interstitial tonicity (e.g., by dysfunction of sodium reabsorption in the TAL). Determination of aquaporin-2 expression in urinary exosomes before and after DDAVP administration showed the expected increase in aquaporin-2 expression in the family members as well as the patient, consistent with an appropriate collecting duct response to DDAVP (Figure 3). Chronic hypokalemia is also suggested to affect urine concentrating ability directly. However, urine concentration was determined during potassium supplementation therapy, with a serum potassium in the range of 3.8–4.0 mmol/L (normal range of 3.5–4.7 mmol/L).
Figure 3.
Both patient 1 and her family members showed a normal physiological AQP2 response to DDAVP. Immunoblotting for AQP2 was performed on urinary exosomes derived from urine collected before (−) and after (+) administration of DDAVP. Murine immortalized cortical collecting duct cells cultured in the presence or absence of DDAVP served as controls. Representative blots showing AQP2 response to DDAVP administration in a heterozygous family member as well as patient 1 are shown. WT, wild type.
Subcellular Localization of Claudin-10b Variants
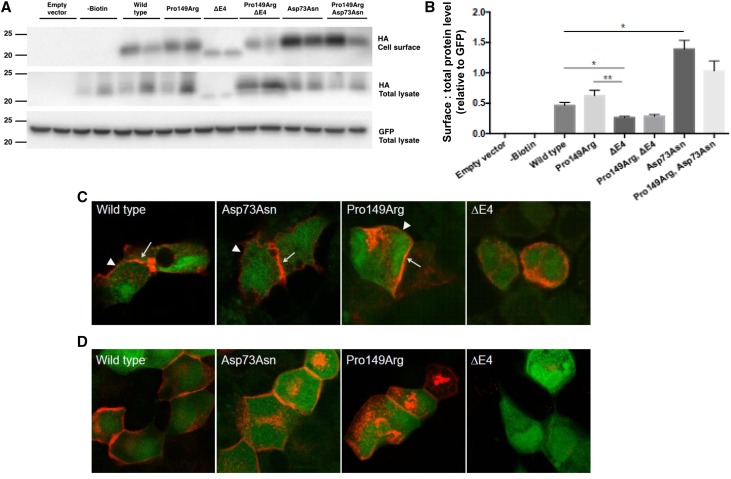
Because the renal phenotype of our patients resembled that of mice with a distal tubular Claudin-10 deletion (Claudin-10b), we analyzed the subcellular localization of the three Claudin-10b variants in cell culture models. To study whether the phenotype in these patients might be due to a reduction or lack of Claudin-10b expression at the cell membrane, cell surface biotinylation experiments were performed with the Claudin-10b wild-type and mutated proteins expressed in HEK293 cells (Figure 4A). The wild-type Claudin-10b protein was detected at the cell surface. The Pro149Arg Claudin-10b immunoreactive bands were detected at a similar density at the cell surface compared with the wild-type protein, with green fluorescent protein immunoblotting serving to show equal transfection efficiency and loading, indicating correct expression and cellular trafficking of this mutant protein. However, total as well as cell surface expression of the smaller Glu157_Tyr192del Claudin-10b variant predicted to lead to loss of exon 4 (ΔE4) were reduced (Figure 4B). In contrast, cell surface expression of the Asp73Asn Claudin-10b variant was significantly increased. Cotransfection of the respective variants, reconstituting the patient genotypes, also showed correct cellular trafficking with reduced overall Claudin-10 cell surface expression in the presence of the ΔE4 variant and increased Claudin-10 cell surface expression in the presence of the Asp73Asn variant.
Figure 4.
Variable response of Claudin-10b variants on cell surface expression and tight junction strand formation when expressed in HEK293 and MDCK-C7 cells. (A) Representative immunoblots of Claudin-10b variant–transfected HEK293 cell surface and total lysates probed for the hemagglutinin (HA) tag or green fluorescent protein (GFP) expressed with the Claudin-10b variant. (B) Densitometry of immunoreactive bands quantified from three independent experiments expressed relative to GFP as an indication of successful transfection. Statistical analysis was performed with an unpaired t test. *P≤0.05; **P≤0.01. (C) Immunohistochemistry of HEK293 cells lacking endogenous claudin expression and transiently transfected with Claudin-10b wild type, Asp73Asn, and Pro149Arg (red) enriched at cell-cell contacts (arrows) compared with cell membranes without contact to a transfected cell (arrowheads), indicating autonomous tight junction formation. In contrast, Claudin-10b ΔE4 was not localized within the cell membrane and thus, could not form tight junction strands. GFP is shown in green. (D) Immunohistochemistry of MDCK subclone C7 cells with endogenous claudin expression and tight junction formation transiently transfected with Claudin-10b variants, Claudin-10b wild type, Asp73Asn, and Pro149Arg (red) integrated in the cell membrane. Claudin-10b ΔE4 was only weakly expressed and did not localize to the cell membrane. GFP is shown in green.
To investigate whether the Claudin-10b variants were capable of forming tight junction strands in HEK293 cells, expression and localization of Claudin-10b wild type or variants were further studied by immunostaining and confocal laser-scanning microscopy. Claudin-10b wild type, Asp73Asn, and Pro149Arg localized to the cell membrane (Figure 4C). Moreover, they were enriched at cell-cell contacts between transfected cells compared with cell membranes without contact to a transfected cell. Claudin contact enrichment in HEK293 cells is a clear indicator for tight junction strand formation27,28 on the basis of both cis- and trans-interaction of heterologous claudins. In sharp contrast to wild-type Claudin-10b and the other two variants, Claudin-10b ΔE4 was not able to localize to the cell membrane and form tight junction strands but was retained in the cytosol.
To study whether Claudin-10b Asp73Asn and Pro149Arg integrate into already existing cell junctions as previously shown for the wild-type protein,22 both variants were expressed in the MDCK-C7 epithelial kidney cell line. This cell line differentiates into an epithelial monolayer with well equipped tight junctions, comparatively high transepithelial resistance, low permeability to ions, and no endogenous expression of Claudin-10.22,29,30 Both the Claudin-10b Asp73Asn and Pro149Arg variants localized to the cell junctions, thus forming tight junction strands in a differentiated epithelial monolayer (Figure 4D). Claudin-10b ΔE4 was only weakly expressed after several days of culture and never localized to cell membranes in MDCK-C7 cells.
Discussion
We identified a new hypokalemic-alkalotic salt-losing nephropathy in two unrelated patients with compound heterozygous mutations in CLDN10. Using whole-exome sequencing, we detected three different CLDN10 variants in these two patients. Given their absence in large exome sequencing databases, these are very rare variants virtually absent in the normal population. This is the first report on putatively pathogenic CLDN10 variants in humans. The patients’ phenotype consisted of a hypokalemic alkalosis together with reduced renal calcium and magnesium excretion, which resulted in occasional hypercalcemia and hypermagnesemia. In addition, we report a urine concentrating defect with a preserved aquaporin-2 response to DDAVP, thus likely caused by a reduced medullary osmolality. The renal tubular role of Claudin-10 was previously studied in mice with conditional knockout of Cldn10 in nephron segments distal from the PT, resulting in absence of Claudin-10b from the TAL.26 Similar to our patients, these mice showed reduced paracellular sodium permeability resulting in a urine concentrating defect accompanied by an enhanced TAL paracellular magnesium and calcium reabsorptive capacity.26 Therefore, we propose that CLDN10 is a candidate gene in patients with a non-Bartter, non-Gitelman hypokalemic-alkalotic salt-losing nephropathy with presumed autosomal recessive inheritance and hypothesize that this phenotype results from a primary defect of TAL paracellular ion transport.
Importantly, distal tubular Cldn10 knockout mice showed a reduced TAL sodium permeability. The exaggerated response of both patients to hydrochlorothiazide in the context of a hypokalemic alkalosis and enhanced tubular aldosterone activity is consistent with reduced sodium reabsorption proximal to the DCT, leading to compensatory enhanced thiazide-sensitive sodium reabsorption in DCT as well as increased ENaC-mediated sodium reabsorption more distally leading to the hypokalemic alkalosis. Together with the response of patient 1 to furosemide, suggesting intact or even enhanced transcellular sodium reabsorption in TAL, this would be consistent with a defect in paracellular sodium reabsorption. Cldn10 knockout mice also showed a urine concentration defect with reduced urine osmolality and increased urine volumes as well as a blunted response to water deprivation compared with wild-type littermates. In patient 1, there was polyuria and reduced renal concentrating response to thirsting at presentation. Recent DDAVP response testing showed a blunted response to thirsting and DDAVP but a sustained AQP2 response, in line with a disturbed concentrating ability due to insufficient buildup of the interstitial osmolality because of TAL dysfunction. Claudin-10 expression in rodent nephron segments distal from the TAL was reported in some studies21,22,31 but was not reported in others.32–34 Therefore, we cannot exclude that direct effects of Claudin-10(b) loss in, for example, collecting duct mediate the disturbed concentrating ability. Apart from similarities between distal tubular Cldn10 knockouts and our patients, supporting that the CLDN10 sequence variants are involved in these patients’ tubulopathy, there are also differences. Most strikingly, the hypokalemia, which was the presenting feature in both patients, is absent in the mouse model.26 This is remarkable, because increased distal tubular sodium delivery expected to accompany the reduced TAL sodium reabsorption should enhance potassium excretion in the collecting duct. This discrepancy could be due to species differences e.g., Slc12a3 knockout mice as a model for GS also lack the characteristic hypokalemia when maintained on normal chow.35 Only when put on a stringent low-potassium diet do Slc12a3 knockouts show significantly lower serum potassium levels compared with their wild-type littermates.
Conditional Cldn10 knockout mice showed hypermagnesemia combined with hypomagnesuria and increased TAL magnesium reabsorptive capacity. Patients only occasionally showed increased serum magnesium levels, such as was the case for calcium. Mice showed hypocalciuria and increased TAL calcium reabsorption, but serum calcium was not elevated, possibly due to effects mediated by the hypermagnesemia on calcium-sensing receptor signaling and ensuing lower parathyroid hormone levels. Cldn10 knockout mice showed nephrocalcinosis, whereas the patients did not. Apart from species differences and different (tubular) compensatory mechanisms in mice and humans, the renal tubular Claudin-10 expression pattern should be considered. Claudin-10 is expressed along the renal tubule, with the anion-selective Claudin-10a isoform predominantly expressed in the PT and the relatively cation-selective Claudin-10b isoform predominantly in the TAL.13,22,23 Two of the CLDN10 mutations (Pro149Arg and ΔE4) found in our patients affect both Claudin-10 isoforms, and only one of them (Asp73Asn) is specific for Claudin-10b. According to its effects in cell culture models, Claudin-10a is suggested to facilitate paracellular chloride reabsorption in the PT; however, data on its specific function are not available. Possible defects in PT ion transport could be compensated in downstream nephron segments, and thus, the patients’ phenotype may be primarily on the basis of Claudin-10b defects. However, the influence of Claudin-10a mutations on tubular function remains elusive. Which Claudin-10 isoform is expressed in the thin limb and its tubular transport role there are also unresolved.
In the TAL, Claudin-10b exhibits a characteristic expression pattern along the corticomedullary axis. In the TAL of the inner stripe of outer medulla, Claudin-10b is the only claudin present and allows paracellular reabsorption of sodium along the electrical gradient.26,36 In the outer stripe of outer medulla and cortical TAL, claudins are arranged in a mosaic expression pattern, with Claudin-10b alone and Claudin-3, -16, and -19 in a complex.37 Although Claudin-10b paracellular channels have a preference for monovalents and primarily conduct sodium, tight junctions dominated by Claudin-16/19 complexes prefer magnesium over sodium. The conditional knockout of Cldn10 led to not only an increase in Claudin-14, -16, and -19 expression but also, a broad expansion of the other TAL claudins over all tight junctions, tentatively in line with the reported increased TAL calcium and magnesium reabsorptive capacity.26 In contrast to mere absence of Claudin-10b in these mice, the patients express different mutant variants of Claudin-10b, of which the ΔE4 variant failed to insert into the cell membrane, in line with former studies showing that lack of the fourth transmembrane segment prevents membrane localization and leads to complete loss of function.22 Both patients carry the Pro149Arg variant, affecting a residue that is conserved in the majority of claudins. Pro149 is suggested to stabilize the conformation of the second extracellular loop and play a crucial role in correct tight junction strand arrangement.28 A substitution of the respective residue in murine Claudin-5 (Pro150Ala) sustained trans- and cis-interaction capability but showed a reduced surface biotinylation and increased ER localization.28 Whereas the capability of Claudin-10b Pro149Arg to form autonomous tight junction strands is in accordance with these findings, a reduced membrane localization of Claudin-10b Pro149Arg was not observed in biotinylation assays. Claudin-10b Asp73Asn affects the first extracellular loop, responsible for pore selectivity.21,22,31 Asp73 is not conserved among claudins, and the significance of this residue for pore selectivity has not been determined.14 Exchange of negatively charged residues by a hydrophilic residue in the first extracellular loop of a cation-selective claudin is considered important, but charged residues in similar regions of Claudin-2 or -10a showed only small or no effect on charge selectivity.21,38 Even when Asp73 is not directly involved in cation pore lining, an indirect effect on pore formation is possible. Pore-specific mutations of the Claudin-16 protein have been shown to affect ion selectivity,17 with Claudin-16 proteins reaching the cell surface exhibiting either partial or complete loss of function. Whereas defects of all three Claudin-10b variants in forming a functional cation pore in the TAL could explain the salt-losing phenotype with urine concentrating defect and secondary hypokalemia, a certain residual protein function of Asp73Asn and Pro149Arg thus has to be considered. The basal capability for tight junction formation of variants Asp73Asn and Pro149Arg could be an explanation for the relatively mild calcium and magnesium disturbances in patients compared with Cldn10 knockout mice. Because these two variants form autonomous Claudin-10b tight junctions, Claudin-16/19 is possibly prevented from entering those junctions and thus, greatly enhancing calcium and magnesium reabsorption as observed in the mouse model. This could tentatively explain why one patient (patient 1) seems to have developed a more severe phenotype, because she carries the ΔE4 mutation with a complete loss of protein function, whereas patient 2 expresses Asp73Asn with a putative residual protein function.
A limitation of our study is that we identified only one family member with biallelic CLDN10 variants in each family, which limits the value of cosegregation analysis. Furthermore, it is difficult to ascertain which clinical signs and symptoms are directly caused by the CLDN10 mutations (e.g., the renal insufficiency in patient 1 does not seem to be present in patient 2 as yet), and because Claudin-10 has a broad (extrarenal) tissue expression, there might also be additional extrarenal effects.
In conclusion, the salt-losing hypokalemic-alkalotic phenotype in our two unrelated patients seems to be due to the presence of compound heterozygous CLDN10 mutations. None of these variants were found in large exome sequencing databases as proxy for variant allele frequencies in the general population, the particular variants are theoretically in line with functionally relevant alterations in a protein suggested to be involved in relevant renal tubular electrolyte transport processes, and the phenotype resembles mice with renal tubular loss of the candidate protein. However, more research is needed to detail the exact mechanism of disease, and identification of more patients with CLDN10 mutations and supportive data from generating knock-in animals and/or diet studies in Cldn10 knockout mice should further confirm causality and pathogenesis. On the basis of our patients’ phenotype, CLDN10 mutations should be considered in patients with a hypokalemic non-Bartter, non-Gitelman salt-losing nephropathy and a renal concentration defect with low urine calcium excretion and unexpectedly normal to high serum magnesium levels. Because this phenotypical description is on the basis of only two patients, it seems justified to sequence CLDN10 in all incident and prevalent patients with a hypokalemic-alkalotic salt-losing nephropathy, in whom mutations in the currently known causative genes have been excluded.
Concise Methods
Genetic Analyses
Initial genetic testing to detect or exclude specific mutations in the known salt-losing nephropathy genes SLC12A3, CLCKNB, or HNF1β was done by Sanger sequencing and MLPA on genomic DNA derived from peripheral blood cells.
Whole-Exome Sequencing
Whole-exome sequencing was essentially performed as previously described.39 Capture of exons was done using an Agilent SureSelectXT Human All Exon 50-Mb Kit. Sequencing was performed using a Life Technologies 5500XL machine or an Illumina HisEquation 2000. Read mapping and variant calling were done using LifeScope TM for the 5500XL data or BWA (mapping) and GATK (calling) for the Illumina data. A filter for a renal disorders gene panel was applied, consisting of approximately 200 genes implicated in hereditary kidney disorders. Genes and coverage statistics can be found at www.genomediagnosticsnijmegen.nl/exome. Mutations were prioritized on the basis of the following criteria: frequency, nucleotide and amino acid conservation, relation of the gene to disease (per family), and inheritance pattern. Reported variants were confirmed by Sanger sequencing. Mutation nomenclature is according to HGVS guidelines (www.hgvs.org). As reference sequence, the longest protein coding transcript was used on the basis of NM_006984.4.
Thiazide and Furosemide Responsiveness Testing
The thiazide and furosemide tests were performed on the basis of the protocols described by Colussi et al.40 and Nozu et al.41 A washout period of 7 days for therapy that could interfere with the test (i.e., diuretics, NSAIDs) was required, but potassium salts were allowed. Patients visited the clinic in the morning after an overnight fast. They were instructed to drink 10 ml/kg tap water (t=0). At t=30 and t=90 minutes, patients were asked to void, but urine was discarded. At t=120 and t=150 minutes, basal urine samples were taken. At t=150 minutes, basal blood samples were taken, and subsequently, 50 mg hydrochlorothiazide or 40 mg furosemide was administered orally. Urine samples were taken every 30 minutes until t=330 minutes. Another blood sample was drawn at t=270 minutes. As reference and cutoff values, the data derived from the papers from Colussi et al.40 and Nozu et al.41 were used.
DDAVP Responsiveness Testing
The DDAVP test was performed after an overnight thirst starting at 10:00 p.m. the day before the test. At t=0 (baseline) as well as at t=30, t=60, t=90, t=120, t=150, and t=180 minutes after the subcutaneous administration of 4 μg DDAVP, urine samples were taken to determine urine osmolality. In addition, serum samples were taken at t=60, t=120, and t=180 minutes after DDAVP administration to determine serum osmolality.
Urinary Exosome AQP2 Analyses
Patient urine was collected before and after administration of DDAVP and treated with protease inhibitors before urinary exosome isolation, which was performed as described previously.42 For immunoblotting, exosome samples were loaded according to urinary creatinine and separated by SDS-PAGE as previously described.42 Murine immortalized cortical collecting duct cells cultured in the presence or absence of DDAVP served as controls. AQP2 antibody (KP9201; Merck Milipore, Amsterdam, The Netherlands) was used at a concentration of 1:5000.
In Vitro CLDN10 Expression Analyses
Constructs were generated using human influenza hemagglutinin-tagged human Claudin-10b, and mutant Pro149Arg, p.Glu157_Tyr192del, and p.Asp73Asn were inserted into a pcNeo IRES vector and transiently expressed in HEK293 cells. Subsequently, cell surface biotinylation was performed to determine expression and trafficking of wild-type Claudin-10 and -10 sequence variants.43 Resultant HEK293 lysate immunoreactive band volumes were quantified using ImageStudio Lite (Licor) by normalizing hemagglutinin tag (Antibody 6E2; 1:5000; Bioké, Leiden, The Netherlands) expression to green fluorescent protein (1:5000; G1544; Sigma-Aldrich, St. Louis, MO) and depicted as the mean±SEM.
To further analyze subcellular localization and tight junction strand formation of Claudin-10b wild type or variants, HEK293 cells were grown on coverslips and transfected with the respective constructs. One day post-transfection, cells were fixed with 4% PFA, blocked with 5% BSA/0.5% Triton X, and exposed to anti–Claudin-10 mAb (Thermo Fisher Scientific) followed by incubation with a secondary antibody (Anti-Mouse DyLight 633; Thermo Fisher Scientific). Images were taken using a confocal laser-scanning microscope 510 (Zeiss). MDCK cells, subclone C7, were obtained from Albrecht Schwab (University of Münster, Muenster, Germany). Transfection and immunostaining procedures were carried out as described for HEK293 cells, except that MDCK-C7 cells were fixed 4 days post-transfection to allow differentiation into a functional epithelial monolayer.
Disclosures
None.
Acknowledgments
We thank the patients and their family members for participating in additional studies and clinical characterization. We thank Albrecht Schwab (University of Münster, Muenster, Germany) for the gift of MDCK-C7 cells.
This research was partly supported by the EURenOmics project from European Union Seventh Framework Programme FP7/2007–2013 agreement 305608 (to L.M.S. and J.G.J.H.), grants from the Innovation Fund of the Dutch health insurance companies (to A.P.B. and T.N.), and The Netherlands Organization for Scientific Research grant VICI 016.130.668 (to J.G.J.H.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Hansson JH, Schild L, Lu Y, Wilson TA, Gautschi I, Shimkets R, Nelson-Williams C, Rossier BC, Lifton RP: A de novo missense mutation of the beta subunit of the epithelial sodium channel causes hypertension and Liddle syndrome, identifying a proline-rich segment critical for regulation of channel activity. Proc Natl Acad Sci USA 92: 11495–11499, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansson JH, Nelson-Williams C, Suzuki H, Schild L, Shimkets R, Lu Y, Canessa C, Iwasaki T, Rossier B, Lifton RP: Hypertension caused by a truncated epithelial sodium channel gamma subunit: Genetic heterogeneity of Liddle syndrome. Nat Genet 11: 76–82, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T: Association between activating mutations of calcium-sensing receptor and Bartter’s syndrome. Lancet 360: 692–694, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Vargas-Poussou R, Huang C, Hulin P, Houillier P, Jeunemaître X, Paillard M, Planelles G, Déchaux M, Miller RT, Antignac C: Functional characterization of a calcium-sensing receptor mutation in severe autosomal dominant hypocalcemia with a Bartter-like syndrome. J Am Soc Nephrol 13: 2259–2266, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Simon DB, Karet FE, Rodriguez-Soriano J, Hamdan JH, DiPietro A, Trachtman H, Sanjad SA, Lifton RP: Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet 14: 152–156, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP: Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13: 183–188, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, John E, Lifton RP: Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat Genet 17: 171–178, 1997 [DOI] [PubMed] [Google Scholar]
- 8.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP: Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ: Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest 115: 1651–1658, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nijenhuis T, Hoenderop JG, Loffing J, van der Kemp AW, van Os CH, Bindels RJ: Thiazide-induced hypocalciuria is accompanied by a decreased expression of Ca2+ transport proteins in kidney. Kidney Int 64: 555–564, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Loffing J, Vallon V, Loffing-Cueni D, Aregger F, Richter K, Pietri L, Bloch-Faure M, Hoenderop JG, Shull GE, Meneton P, Kaissling B: Altered renal distal tubule structure and renal Na(+) and Ca(2+) handling in a mouse model for Gitelman’s syndrome. J Am Soc Nephrol 15: 2276–2288, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Alexander RT, Hoenderop JG, Bindels RJ: Molecular determinants of magnesium homeostasis: Insights from human disease. J Am Soc Nephrol 19: 1451–1458, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu AS: Claudins and the kidney. J Am Soc Nephrol 26: 11–19, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milatz S, Breiderhoff T: One gene, two paracellular ion channels-claudin-10 in the kidney. Pflugers Arch 469: 115–121, 2017 [DOI] [PubMed] [Google Scholar]
- 15.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S: Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79: 949–957, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kausalya PJ, Amasheh S, Günzel D, Wurps H, Müller D, Fromm M, Hunziker W: Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16. J Clin Invest 116: 878–891, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou J, Renigunta A, Konrad M, Gomes AS, Schneeberger EE, Paul DL, Waldegger S, Goodenough DA: Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest 118: 619–628, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon DB, Lu Y, Choate KA, Velazquez H, Al-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriguez-Soriano J, McCredie D, Milford D, Sanjad S, Lifton RP: Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285: 103–106, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Gong Y, Renigunta V, Himmerkus N, Zhang J, Renigunta A, Bleich M, Hou J: Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J 31: 1999–2012, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimke H, Desai P, Borovac J, Lau A, Pan W, Alexander RT: Activation of the Ca(2+)-sensing receptor increases renal claudin-14 expression and urinary Ca(2+) excretion. Am J Physiol Renal Physiol 304: F761–F769, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Itallie CM, Rogan S, Yu A, Vidal LS, Holmes J, Anderson JM: Two splice variants of claudin-10 in the kidney create paracellular pores with different ion selectivities. Am J Physiol Renal Physiol 291: F1288–F1299, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Günzel D, Stuiver M, Kausalya PJ, Haisch L, Krug SM, Rosenthal R, Meij IC, Hunziker W, Fromm M, Müller D: Claudin-10 exists in six alternatively spliced isoforms that exhibit distinct localization and function. J Cell Sci 122: 1507–1517, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Günzel D, Yu AS: Function and regulation of claudins in the thick ascending limb of Henle. Pflugers Arch 458: 77–88, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bech AP, Wetzels JF, Bongers EM, Nijenhuis T: Thiazide responsiveness testing in patients with renal magnesium wasting and correlation with genetic analysis: A diagnostic test study. Am J Kidney Dis 68: 168–170, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Verhave JC, Bech AP, Wetzels JF, Nijenhuis T: Hepatocyte nuclear factor 1β-associated kidney disease: More than renal cysts and diabetes. J Am Soc Nephrol 27: 345–353, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Breiderhoff T, Himmerkus N, Stuiver M, Mutig K, Will C, Meij IC, Bachmann S, Bleich M, Willnow TE, Müller D: Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci U S A 109: 14241–14246, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piontek J, Fritzsche S, Cording J, Richter S, Hartwig J, Walter M, Yu D, Turner JR, Gehring C, Rahn HP, Wolburg H, Blasig IE: Elucidating the principles of the molecular organization of heteropolymeric tight junction strands. Cell Mol Life Sci 68: 3903–3918, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piontek J, Winkler L, Wolburg H, Müller SL, Zuleger N, Piehl C, Wiesner B, Krause G, Blasig IE: Formation of tight junction: Determinants of homophilic interaction between classic claudins. FASEB J 22: 146–158, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Wünsch S, Gekle M, Kersting U, Schuricht B, Oberleithner H: Phenotypically and karyotypically distinct Madin-Darby canine kidney cell clones respond differently to alkaline stress. J Cell Physiol 164: 164–171, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Gekle M, Wünsch S, Oberleithner H, Silbernagl S: Characterization of two MDCK-cell subtypes as a model system to study principal cell and intercalated cell properties. Pflugers Arch 428: 157–162, 1994 [DOI] [PubMed] [Google Scholar]
- 31.Inai T, Sengoku A, Guan X, Hirose E, Iida H, Shibata Y: Heterogeneity in expression and subcellular localization of tight junction proteins, claudin-10 and -15, examined by RT-PCR and immunofluorescence microscopy. Arch Histol Cytol 68: 349–360, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Kirk A, Campbell S, Bass P, Mason J, Collins J: Differential expression of claudin tight junction proteins in the human cortical nephron. Nephrol Dial Transplant 25: 2107–2119, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S: Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol 13: 875–886, 2002 [DOI] [PubMed] [Google Scholar]
- 34.Lee JW, Chou CL, Knepper MA: Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 26: 2669–2677, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultheis PJ, Lorenz JN, Meneton P, Nieman ML, Riddle TM, Flagella M, Duffy JJ, Doetschman T, Miller ML, Shull GE: Phenotype resembling Gitelman’s syndrome in mice lacking the apical Na+-Cl- cotransporter of the distal convoluted tubule. J Biol Chem 273: 29150–29155, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Plain A, Wulfmeyer VC, Milatz S, Klietz A, Hou J, Bleich M, Himmerkus N: Corticomedullary difference in the effects of dietary Ca2+ on tight junction properties in thick ascending limbs of Henle’s loop. Pflugers Arch 468: 293–303, 2016 [DOI] [PubMed] [Google Scholar]
- 37.Milatz S, Himmerkus N, Wulfmeyer VC, Drewell H, Mutig K, Hou J, Breiderhoff T, Müller D, Fromm M, Bleich M, Günzel D: Mosaic expression of claudins in thick ascending limbs of Henle results in spatial separation of paracellular Na+ and Mg2+ transport. Proc Natl Acad Sci USA 114: E219–E227, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu AS, Cheng MH, Angelow S, Günzel D, Kanzawa SA, Schneeberger EE, Fromm M, Coalson RD: Molecular basis for cation selectivity in claudin-2-based paracellular pores: Identification of an electrostatic interaction site. J Gen Physiol 133: 111–127, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neveling K, Feenstra I, Gilissen C, Hoefsloot LH, Kamsteeg EJ, Mensenkamp AR, Rodenburg RJ, Yntema HG, Spruijt L, Vermeer S, Rinne T, van Gassen KL, Bodmer D, Lugtenberg D, de Reuver R, Buijsman W, Derks RC, Wieskamp N, van den Heuvel B, Ligtenberg MJ, Kremer H, Koolen DA, van de Warrenburg BP, Cremers FP, Marcelis CL, Smeitink JA, Wortmann SB, van Zelst-Stams WA, Veltman JA, Brunner HG, Scheffer H, Nelen MR: A post-hoc comparison of the utility of sanger sequencing and exome sequencing for the diagnosis of heterogeneous diseases. Hum Mutat 34: 1721–1726, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Colussi G, Bettinelli A, Tedeschi S, De Ferrari ME, Syrén ML, Borsa N, Mattiello C, Casari G, Bianchetti MG: A thiazide test for the diagnosis of renal tubular hypokalemic disorders. Clin J Am Soc Nephrol 2: 454–460, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Nozu K, Iijima K, Kanda K, Nakanishi K, Yoshikawa N, Satomura K, Kaito H, Hashimura Y, Ninchoji T, Komatsu H, Kamei K, Miyashita R, Kugo M, Ohashi H, Yamazaki H, Mabe H, Otsubo A, Igarashi T, Matsuo M: The pharmacological characteristics of molecular-based inherited salt-losing tubulopathies. J Clin Endocrinol Metab 95: E511–E518, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Tutakhel OA, Jeleń S, Valdez-Flores M, Dimke H, Piersma SR, Jimenez CR, Deinum J, Lenders JW, Hoenderop JG, Bindels RJ: Alternative splice variant of the thiazide-sensitive NaCl cotransporter: A novel player in renal salt handling. Am J Physiol Renal Physiol 310: F204–F216, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Blanchard MG, Kittikulsuth W, Nair AV, de Baaij JH, Latta F, Genzen JR, Kohan DE, Bindels RJ, Hoenderop JG: Regulation of Mg2+ reabsorption and transient receptor potential melastatin type 6 activity by cAMP signaling. J Am Soc Nephrol 27: 804–813, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]