Abstract
The presence of sex disparity in living donor kidney transplantation (LDKT) remains controversial. To determine if women fall behind men in LDKT evaluation, we performed an intention to treat study of 2587 candidates listed for kidney transplant at a single transplant center over 7 years. We found that women and men kidney transplant candidates engaged an equivalent type and number of prospective living donors. However, sex-specific differences in sensitization history and histocompatibility reduced the rate of LDKT for women by 30%. Pregnancy-induced incompatibility with spouse donors was limiting given that spouses were among the individuals most likely to complete donation. Notably, participation in a kidney paired exchange program eliminated sex-based differences in LDKT. Collectively, these data suggest that pregnancy is a formidable biologic barrier for women and contributes uniquely to sex disparity in LDKT. Targeted efforts to improve transplant center participation in paired kidney exchanges may increase sex equity in LDKT.
Keywords: gender difference, kidney donation, kidney transplantation, pregnancy, disparity
Living donor kidney transplantation (LDKT) represents the ideal therapy for transplant candidates with ESRD.1–3 However, LDKT access is limited for thousands of patients, including low-income groups, ethnic minorities, and women.4–16 Factors contributing to LDKT disparities for disadvantaged populations are being explored and addressed,4,17–19 including differences in social networks,20 attitudes toward transplantation,21,22 and biologic and economic barriers.4,6,8–11,13,16 However, no studies have identified drivers of the lower rates of LDKT among women. Although marked differences in LDKT rates in men and women are not obvious in national datasets,23 these data do not capture the number of women versus men who present with a living donor but fail to achieve transplantation. It is, therefore, unknown whether rates of living donor volunteerism, progression through evaluation, and completion of donation are equivalent for men versus women LDKT candidates.
To address this knowledge gap, we, therefore, performed an intention to treat analysis of candidates listed for kidney transplantation at a single high-volume center. We hypothesized that the trajectories to successful LDKT diverge for the two sexes. We expected that we would uncover differences at all steps toward LDKT, including donor referral, donor-recipient evaluation, and conversion to successful living donor transplantation. Our primary objective was to identify sex-specific points of attrition where women failed to progress toward successful LDKT. A secondary objective was to identify programmatic interventions that improve sex equity in LDKT.
Results
Emergence and Effect of Sex Disparity in LDKT
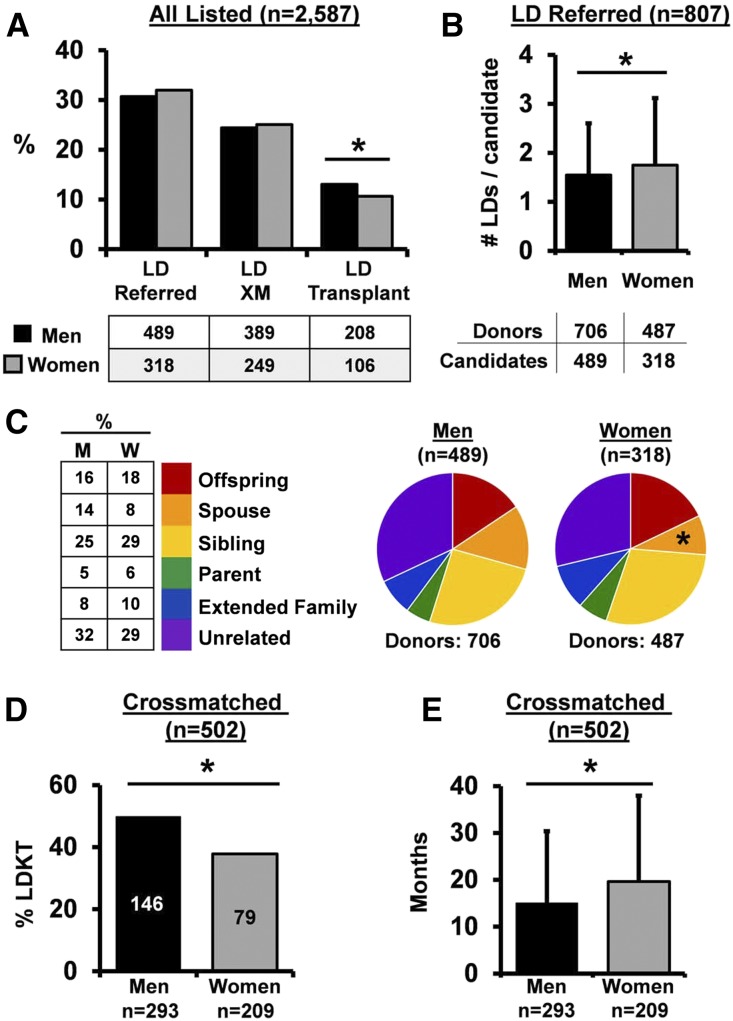
We identified 2587 candidates listed for renal transplantation between January 1, 2007 and December 31, 2013 at our transplant center (men=62%; women=38%). Approximately one third of all listed candidates had a living donor referred (men=31%; women=32%) (Figure 1A). The number and type of referrals per candidate were largely similar between men and women, although women received slightly more living donor referrals overall (Figure 1B) with fewer referrals from spouses (Figure 1C). The pattern of donor referral at our center mirrored national transplantation trends,23 with the majority of referrals from unrelated individuals and siblings. Men and women progressed equally to the next stage of evaluation and were crossmatched at similar rates (80% of transplant candidates with a living donor referral) (Figure 1A). Despite similar patterns of living donor referral and crossmatching, successful LDKT occurred less frequently among listed women candidates (Figure 1A).
Figure 1.
Sex differences in LDKT evaluation arise during histocompatibility testing. (A) Frequency of LD referral, crossmatch (XM), and transplantation for all patients listed for kidney transplantation (men=1593; women=994). The table indicates the number of listed patients in each group. *P=0.01. (B) Average number of LD referrals per candidate (among candidates with at least one LD referred; n=807). Error bars represent SD. *P=0.03. (C) Distribution of relationship of referred LDs. The table indicates percentage of donors in each group. It excludes donors with an undocumented relationship to the candidate (n=12 referred donors [men]; n=20 referred donors [women]). M, men; W, women. *P<0.01 (spouses, men versus women). (D) Rate of LDKT among candidates with complete data who were XM with an LD (n=502; men =293, women =209). *P<0.01. (E) Wait times for patients who were transplanted by sex (men =171, women =118). Error bars represent SD. *P=0.02.
To compare candidate progression through histocompatibility testing, we focused our subsequent analysis on the cohort of crossmatched candidates with a complete history of sensitizing events (pregnancy, blood transfusion, or prior transplant; n=502 [293 men, 209 women]). The clinical and demographic characteristics of these candidates and their prospective living donors were largely similar (Tables 1 and 2, respectively). Among these crossmatched candidates, 50% of men versus 35% of women achieved LDKT (P=0.01) (Figure 1D). This difference in LDKT rate did not translate into less frequent transplantation of women at our center, because women were able to fill this transplant deficit with deceased donor kidney transplants (DDKTs) (overall rate of transplant: 59% [men] versus 57% [women] of all listed patients [P=0.71; men: 174=146 LDKT+28 DDKT; women: 120=79 LDKT+41 DDKT]). However, there was a significant prolongation of waitlist time for women compared with men (men: 15±15 versus women: 20±18 months; P=0.02) (Figure 1E). The increased rate of LDKT in men was additionally shown in a secondary survival analysis, in which DDKT was treated as a competing risk (subdistribution hazard ratio, 1.48; 95% confidence interval, 1.13 to 1.95; P<0.01).
Table 1.
Patient characteristics (n=502)
| Candidate Characteristic | Men, n=293 | Women, n=209 | P Value |
|---|---|---|---|
| Age at listing, yr, mean (SD) | 46.5 (14) | 46.1 (13) | 0.72 |
| Length of follow-up, mo, mean (SD) | 20 (18) | 23 (19.6) | 0.07 |
| Race, % | 0.46 | ||
| Black | 22.5 | 26.3 | |
| Asian | 3.4 | 5.3 | |
| Hispanic | 2.7 | 6.9 | |
| Other | 3.8 | 2.9 | |
| White | 67.6 | 61.2 | |
| Blood type, % | 0.23 | ||
| A | 38.6 | 31.6 | |
| AB | 4.8 | 7.7 | |
| B | 14.7 | 18.2 | |
| O | 42 | 42.6 | |
| ESRD etiology, % | 0.001 | ||
| Diabetes | 25.6 | 35.9 | |
| Hypertension | 10.6 | 8.1 | |
| FSGS | 11.9 | 13.4 | |
| PCKD | 9.6 | 10 | |
| SLE | 2 | 9.6 | |
| IgA nephropathy | 10.6 | 3.3 | |
| Alport | 3.7 | 0.5 | |
| Othera | 27 | 30.6 | |
| Renal replacement | |||
| On dialysis when listed, % | 43 | 43 | 0.81 |
| On dialysis when transplanted, % | 69 | 70 | 0.86 |
| Time on dialysis, d, mean (SD) | 857 (762) | 899 (674) | 0.60 |
| Among patients who were transplanted | 713 (795) | 765 (638) | 0.47 |
| Among still listed at study end | 1014 (707) | 1089 (687) | 0.64 |
| GFR at listing (if not on dialysis), ml/min, mean (SD) | 15.4 (4.5) | 15 (4.3) | 0.44 |
| GFR at transplant (if not on dialysis), ml/min, mean (SD) | 13.1 (6.5) | 10.5 (3.6) | 0.01 |
PCKD, polycystic kidney disease.
Includes Goodpasture syndrome, GN of unclear etiology, reflux disease, other congenital abnormality, or unknown etiology.
Table 2.
Characteristics of prospective donors (n=502 candidates)
| Donor Characteristic | Men, n=293 (475 Donors) | Women, n=209 (351 Donors) | P Value |
|---|---|---|---|
| Prospective donor age, yr, mean (SD) | 41.7 (12) | 41.4 (12) | 0.70 |
| Prospective donor race, % | 0.14 | ||
| Black | 12.0 | 17.7 | |
| Asian | 2.1 | 2.0 | |
| Hispanic | 1.9 | 2.3 | |
| White | 84.0 | 78.1 | |
| Prospective donor sex, % | 0.72 | ||
| Women | 56 | 57 |
Pregnancy Sensitization Contributes Uniquely to Sex Disparity
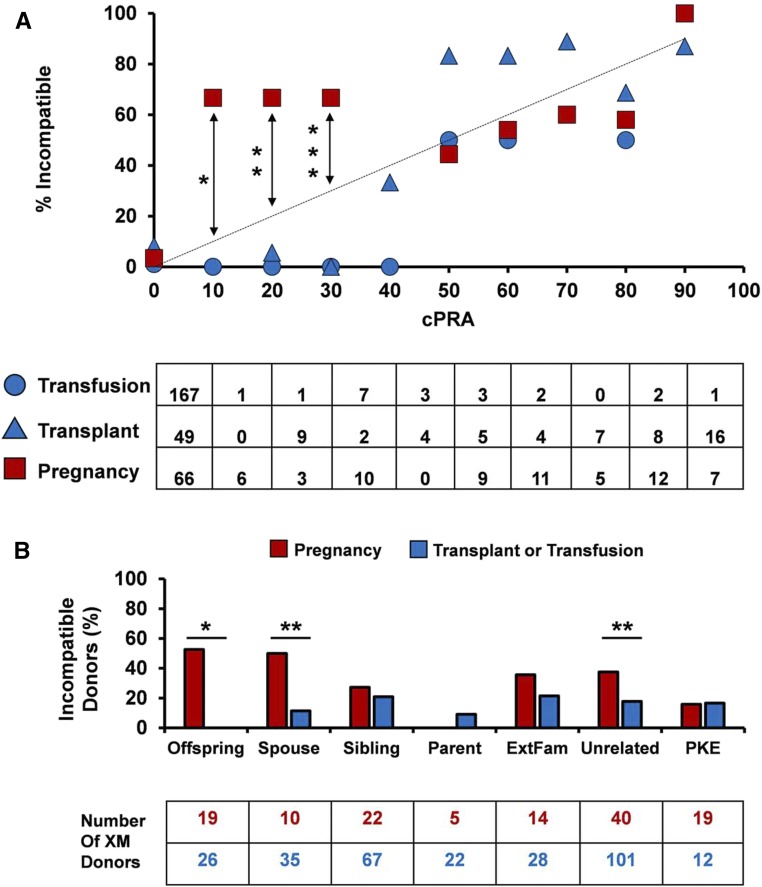
Given that living donor access diverged most significantly between men and women at the point of histocompatibility testing, we next compared living donor compatibility and degree of sensitization between men and women. Women were incompatible with at least one living donor three times more frequently than men (Figure 2A). Notably, 21% of women versus 5% of men were incompatible with all of their crossmatched living donors (P<0.001), which resulted in a loss of 31% of potential donors for women versus 9% for men (P<0.001). As expected, women were significantly more sensitized than men as evidenced by a greater frequency of sensitizing events (men =51% versus women =90%; P<0.001) and higher calculated panel reactive antibody (cPRA; men =7%±22% versus women =24%±35%; P<0.001) (Figure 2B). However, women were incompatible with their crossmatched living donors at a higher rate than predicted by the cPRA (women: cPRA, 23% versus living donor incompatibility, 29%; P=0.02; men: cPRA, 7% versus living donor incompatibility, 9%; P=0.17) (Figure 2C). Taken together, these data suggest that differences in both the prevalence and effect of sensitization underlie sex disparity in LDKT.
Figure 2.
The greater burden of sensitization in female LDKT candidates is underestimated by cPRA. (A) Frequency of living donor incompatibility in women versus men LDKT candidates. *P<0.001. (B) Frequency and magnitude of sensitization between men and women LDKT candidates. The bar graph represents the percentage of men or women with each sensitizing event. The table indicates the number of candidates in each group. X indicates mean cPRA for men and women in each group. Preg, pregnancy; Tfn, transfusion; Txp, prior transplant; Uns, unsensitized. (C) Frequency of living donor incompatibility as a function of cPRA. The dotted line represents a 1:1 correlation between living donor incompatibility and cPRA. *P=0.02.
In light of the frequency of pregnancy in our study group (77% of all women), we asked to what degree an individual sensitizing event would affect living donor incompatibility for men versus women. We found that incompatibility with a potential living donor was equivalent between men and women who were sensitized by either a prior blood transfusion or a transplant (transfusion: 2% men versus 9% women [P=0.07]; transplant: 42% men versus 21% women [P=0.12]). Living donor incompatibility for these candidates was also concordant with cPRA (Figure 3A) and equally distributed across donor types (data not shown). In contrast, living donor incompatibility was significantly higher than predicted by cPRA among weakly sensitized candidates with a history of pregnancy (cPRA<50%) (Figure 3A). In this analysis, we avoided potential confounding between pregnancy and other sensitizing events by including women with a history of pregnancy alone (n=72). Nevertheless, identical results were obtained when living donor compatibility was plotted against cPRA for women sensitized by both pregnancy and transfusion or women sensitized by all three events (data not shown). Not surprisingly, the distribution of donor-specific incompatibility was skewed for candidates sensitized by pregnancy, with significantly higher rates of offspring and spouse incompatibility than candidates sensitized by other events (Figure 3B). Collectively, these data show that pregnancy restricts living donor compatibility more significantly than blood transfusion or transplantation, particularly in weakly sensitized women crossmatched with a spouse or offspring.
Figure 3.
Pregnancy promotes living donor incompatibility to a greater degree than prior transplantation or blood transfusion. (A) Frequency of living donor incompatibility as a function of cPRA for candidates with a history of a sensitizing event. The dotted line represents a 1:1 correlation between living donor incompatibility and cPRA. Transfusion and transplantation groups include both men and women. Number of crossmatched donors is shown in the table for each group. *P<0.001; **P=0.04; ***P=0.01. (B) Frequency of incompatible donor type among candidates grouped by sensitizing event. Number of crossmatched donors is shown in the table for each group. Candidates sensitized by prior transplant or transfusion include men and women, except when crossmatched with an offspring living donor (men only). XM, crossmatched. *P<0.001; **P=0.02.
Attrition and Utilization of Compatible Living Donors Is Independent of Sex
We next examined the final phase of LDKT evaluation when compatible prospective living donors proceeded to complete donation. Variables that may affect donor transition through this final stage include donor race, sex, and medical suitability as well as overall commitment to donate. We, therefore, considered these variables both directly and indirectly to determine whether these factors affected donor utilization by men or women LDKT candidates.
We first evaluated whether donor sex or race affected utilization of compatible donors in men versus women. Although most compatible prospective donors were women (58%), we did not find any significant differences between men and women donors with respect to overall donation frequency (29% of men completed donation versus 33% of women; P=0.28). Although most compatible donors of either sex donated to men (63% of men donors and 67% of women donors donated to men recipients), the majority of both men and women recipients had women kidney donors (58% of women LDKT recipients versus 63% of men LDKT recipients; P=0.56). These trends were largely consistent across ethnicities, although black women donors donated more frequently to women than women donors of other ethnicities (67% of black women donors to women versus 29% [white] and 20% [other]; P=0.02). Donor race did not otherwise affect frequency of donation (percentage of compatible donors who completed donation: 27% [Asian], 24% [black], 35% [Hispanic], 33% [white]; P=0.44).
Attrition of compatible living donors was comparable between men and women LDKT candidates (66% versus 68%, respectively; P=0.61). Attrition was due primarily to donor medical contraindications (men =34% versus women =39%; P=0.47), donor withdrawal (men =26% versus women =26%; P>0.99), and recipient contraindications (men =22% versus women =13%; P=0.11). Medical contraindications to donation included high body mass index (BMI; in excess of self-reported BMI on screening questionnaire), impaired glucose tolerance testing, significant cardiovascular disease, low creatinine clearance, kidney stones, and complex renal arterial anatomy; these subcategories did not differ between men and women (data not shown). Less common reasons for attrition of compatible donors also did not differ significantly between men and women LDKT candidates; these included incompletely treated psychiatric disease or poor social support (8%), adjudication as a backup donor (5%), and strong family history of renal or cardiovascular disease (3%).
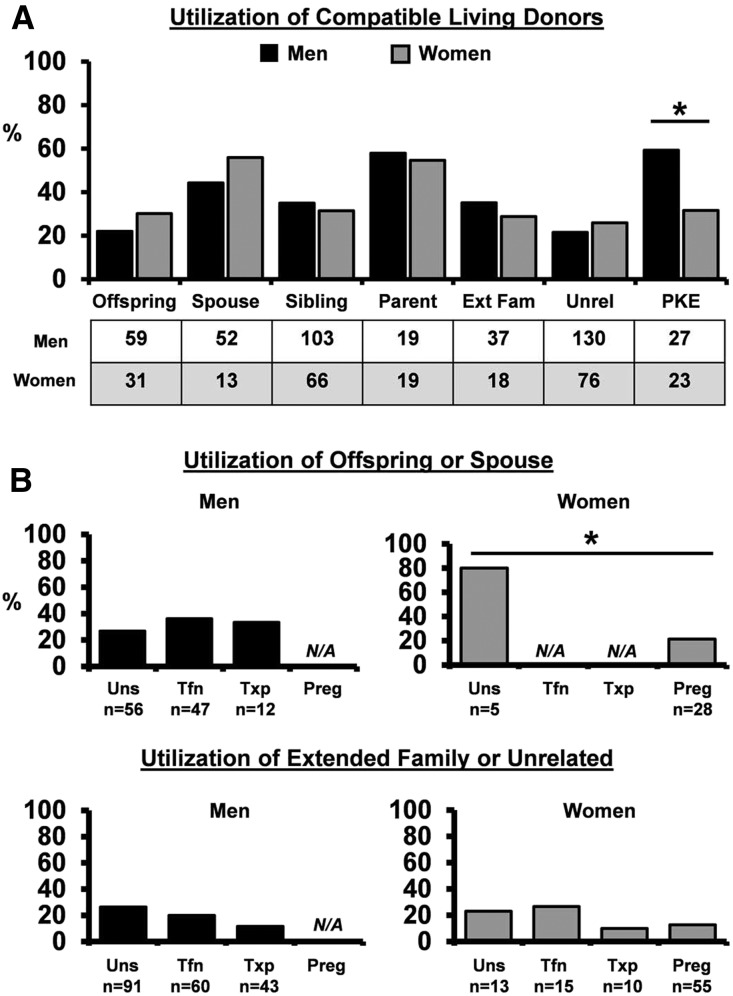
With respect to the relationship between donor and recipient, we found that men and women recipients ultimately used similar types of compatible living donors (Figure 4A). However, not all donor types were equally likely to complete donation (Figure 4A). Parents and spouses represented the prospective donors most likely to actualize donation for both men and women. Given the importance of spouses for all LDKT candidates, we next determined the effect of pregnancy-induced histoincompatibility on living donor utilization for women (Figure 4B). We found that sensitizing event did not substantially alter utilization of parents, siblings, extended family, or unrelated living donors (Figure 4B, lower panel) (data not shown). However, offspring and spouse living donor utilization was reduced by threefold among women with a history of pregnancy (Figure 4B, upper panel). These data confirm that histoincompatibility due specifically to pregnancy restricts utilization of a critical group of potential living donors for women.
Figure 4.
Utilization of compatible living donors reveals the impact of pregnancy-induced incompatibility on women. (A) Living donor utilization among compatible donor types. Men are shown in black, and women are shown in gray. The number of compatible prospective donors per group is shown. Ext Fam, extended family; Unrel, unrelated. *P=0.05. (B) Effect of sensitizing event on utilization of offspring or spouse living donors (upper panel) and extended family or unrelated living donors (lower panel). Number of compatible prospective donors per group is shown. Not applicable (N/A; women) indicates that, by definition, offspring were not available for crossmatch among women sensitized only by transfusion or transplantation. No spouses were used among women sensitized by transfusion or transplantation alone. Preg, pregnancy; Tfn, transfusion; Txp, prior transplant; Uns, unsensitized. *P=0.02.
Paired Exchange as a Strategy to Restore Sex Parity in LDKT
Having identified the contribution of histoincompatibility to LDKT sex disparity in our study population, we considered whether the inclusion of living donor–incompatible women in paired kidney exchange (PKE) would restore sex parity in LDKT. We found that sensitized men and women who were incompatible with at least one living donor were crossmatched with a PKE donor with similar frequency (men: three of 25 [12%] versus women: eight of 62 [13%]; P>0.99). Moreover, men and women who were registered in the PKE for HLA incompatibility were transplanted at comparable rates through the PKE (one of three men [all sensitized by prior transplant] versus five of eight women [seven sensitized by pregnancy; one sensitized by prior transplant]; P=0.54). Although our initial analysis of PKE utilization was confounded by differences in the number of men and women enrolled because of blood type incompatibility (Figure 4A), utilization of compatible PKE donors equalized between men and women when we limited the analysis to candidates with a positive crossmatch (data not shown). Altogether, these results suggest that PKE presents an effective programmatic strategy to maintain the access of women to LDKT.
Discussion
The resolution of LDKT disparities will improve patient longevity and decrease demand on the pool of deceased donor kidneys. Although ethnic and economic disparities within LDKT are well appreciated, the effect of sex on access and utilization of living donors has not been as well studied. The goals of this intention to treat study were, therefore, to (1) define the magnitude of sex disparity in contemporary LDKT, (2) identify when potential living donors become inaccessible to women kidney recipients, and (3) identify opportunities for intervention by the transplant provider. To achieve these objectives, we directly compared the ability of men and women to successfully navigate three phases of LDKT evaluation and transplantation at a high-volume transplant center. These phases included (1) living donor referral, (2) donor-specific histocompatibility testing, and (3) conversion to actual living donation. Given that living donor referral and selection patterns are influenced by numerous factors, we were surprised to find that the age, race, sex, number, and relationship of prospective donors were largely similar for men and women candidates at our center. We thus conclude that men and women have comparable initial donor access (phase 1) and equivalent utilization of compatible living donors (phase 3). It is during histocompatibility testing (phase 2) that women LDKT candidates fall behind their men counterparts.
The most significant finding of our study is that a convergence of biologic and sociologic phenomena limits LDKT in women. Although it is well appreciated that pregnancy induces alloantibodies that restrict transplantation for women,24–30 our study reveals that the cost of pregnancy is compounded for LDKT candidates given that spouses are among the most likely prospective donors to complete donation. Although our study does not reveal why certain donor types are more likely to complete donation, this observation adds a novel dimension to our understanding of the effect of donor incompatibility.
A second important finding of our study is that pregnancy contributes disproportionately to the incompatibility of women with their living donors, beyond what would be expected from cPRA. We hypothesize that this occurs because of the over-representation of uncommon HLA alleles in an individual candidate’s living donor pool. It is also possible that positive crossmatches result from non-HLA antibodies or a combination of weakly reactive antibodies that individually do not meet median fluorescence intensity criteria for the assignment of unacceptable antigens. Although kidney transplant recipients with low-level pretransplant donor-specific antibody and a negative crossmatch have excellent graft survival,31 it is unknown how frequently weakly reactive antibody in women sensitized by pregnancy affects the crossmatch interpretation of candidates tested against donors to whom they were directly sensitized. An increased number of failed crossmatches may also explain, in part, why offspring and spouse donor utilization has decreased more in women than in men since the wide adoption of sensitive solid-phase histocompatibility testing (approximately 2007) (Supplemental Figure 1).23,32,33 Future improvements in the molecular characterization of immunogenic epitopes that provoke alloantibody production during pregnancy34–35 may improve our ability to accurately designate unacceptable antigens and predict histocompatibility between women and their prospective living donors.
What strategies can transplant centers use to surmount this immunologic barrier and achieve sex parity in LDKT? Desensitization and subsequent incompatible LDKT are an option for some centers but may not be broadly applicable due to concerns over efficacy, long-term outcomes, and cost.36,37 Given that our center does not perform incompatible LDKT, we aimed to identify alternative interventions that would increase the rate of compatible LDKT for women. Several potential strategies emerged from our dataset. First, we found an important role for liberal evaluation and crossmatching policies that support diversification of a women candidate’s portfolio of potential living donors. An appreciation of the key role of parents, spouses, and siblings in maintaining the access of women to LDKT may also influence transplant programs to preferentially crossmatch and/or approve the selection of these donors whenever possible. Second, increased engagement in PKE may also improve the rate of LDKT for women; in our study, HLA-incompatible women were enrolled and transplanted as frequently as men through paired exchange efforts. However, the observation that only 10%–15% of living donor–incompatible candidates at our center were crossmatched with a PKE donor reflects the numerous logistic and financial barriers to PKE that are well appreciated within the transplant community.38–40 Although it remains unknown whether the designation of a national organization to administer kidney paired donation will help foster transplant center participation in PKE or reduce costs,39,41 our study makes a compelling argument to pursue these efforts to improve sex equity in LDKT. Higher rates of LDKT in women at transplant centers with aggressive PKE programs will further validate this approach.
Finally, our results should prompt all transplant centers to reconsider the necessity of the histocompatibility requirements that are currently used for women transplant candidates. In light of the superlative outcomes expected of LDKT recipients, we expect that transplant centers may be reluctant to transplant patients with low levels of donor-specific anti-HLA antibody. Consequently, some transplant candidates may be labeled incompatible at one transplant center but compatible at another, even in the absence of desensitization. We, therefore, speculate that the present regulatory climate encourages the application of stringent histocompatibility criteria for LDKT recipients. Is such stringency necessary, and is it potentially harmful? Our data suggest that the only cost of living donor incompatibility at our center is the prolongation of wait time for women patients, because we did not observe any differences in overall transplant rates and post-transplant outcomes between men and women (data not shown). However, we suspect that the cost of increasing wait time is underestimated in light of the recent demonstration that recipients of HLA-incompatible living donor transplants have increased survival over patients on the waitlist.42 Additional work in this arena is critical to determine whether relaxation of traditionally held immunologic barriers will harm or benefit women transplant recipients.
In summary, we present the first intention to treat analysis of sex disparity in contemporary LDKT. Our center-specific data identify an important contribution of pregnancy-induced sensitization to the decreased rate of LDKT among women. Although relaxation of stringent histocompatibility criteria or increased participation in PKE may decrease the sex gap in LDKT, our data suggest that diversification of the donor pool represents an immediate strategy to attenuate sex disparity in LDKT.
Concise Methods
Given that the Scientific Registry of Transplant Recipients does not capture data on living donor referral and evaluation for candidates listed for a kidney transplant, we elected to perform a retrospective intention to treat analysis of all listed kidney transplant candidates at our high-volume center. After approval by the Institutional Review Board (819422), we collected demographic, clinical, and histocompatibility data on 2587 men and women listed for renal transplantation between January 1, 2007 and December 31, 2013 at the University of Pennsylvania. We selected this particular timeframe to maximize follow-up of candidates listed after solid-phase histocompatibility testing became routine at our center. All listed patients during this time period were considered equally likely to have a living donor referred and thus, included in the initial cohort. Follow-up data were collected through March 31, 2014 and included listing outcome (transplantation [deceased or living donor], list removal, or patient death) and patient and graft survival after transplantation. Additional data for patients who were transplanted included time to transplant and time to graft failure. Graft failure was defined as initiation of dialysis, retransplantation, or death after transplant.
We compared the ability of men and women candidates to navigate all phases of the LDKT evaluation process. These phases include (1) initial contact by referred donors with the transplant center, (2) histocompatibility testing with prospective donors, and (3) conversion from compatible prospective LDKT candidate to living donor recipient. For the purposes of this study, any listed candidate transformed into an LDKT candidate when a prospective living donor filled out an initial screening questionnaire. We defined submission of this questionnaire as the point of living donor referral and the start of the first phase of the evaluation.
At the University of Pennsylvania, prospective LDKT pairs are selected to move into phase 2 of evaluation after physician review of the donor screening questionnaire. Prospective donors are deferred if they report any number of medical or psychosocial contraindications to live donation at our center. A complete list of the selection criteria for living kidney donation at our center can be found in the Penn Transplant Institute Policy Manual and is available on request. In brief, absolute contraindications to donation at our center include (1) active substance abuse, (2) diabetes, (3) uncontrolled hypertension or hypertension with evidence of end organ damage, (4) a history of kidney disease, (5) BMI>35, (6) 24-hour urine protein >300 mg, (6) active or incompletely treated malignancy, (7) evidence of coercion, and/or (8) inability to understand the risks associated with donation. Relative contraindications include (1) age <21 or >70 years old; (2) psychiatric history requiring inpatient treatment; (3) multiagent hypertension; (4) kidney stones >4 mm; (5) extensive family history of kidney disease, diabetes, and/or hypertension; (6) smoking; (7) lack of medical insurance; and/or (8) a history of gestational diabetes. However, donors with relative contraindications are considered on a patient by patient basis and are not deferred at the initial screening questionnaire. Importantly, these specific practices may vary between transplant centers. At the commencement of phase 2 of evaluation, prospective donors are scheduled for a clinic visit with a nephrologist. Laboratory tests drawn at the time of this visit include ABO typing, HLA typing, and a sample for crossmatching with the intended recipient. Crossmatching with the intended recipient is performed concurrently with the donor’s HLA typing; in this regard, crossmatches are liberally performed without consideration of the potential recipient’s antibody burden against the prospective donor. Reporting of compatibility results to potential donors advances the pair into the final phase of evaluation, when all compatible donors are discussed in a multidisciplinary selection meeting before scheduling of surgery. Blood type– or HLA-incompatible donors willing to participate in paired exchange are also promoted into phase 3.
Because our center does not perform desensitization, we rely on paired exchange matches built by the National Kidney Registry (NKR) to transplant blood type– or HLA-incompatible candidates. Since its inception in 2008, the NKR has facilitated over 2000 kidney transplants.43 Our center has performed 38 transplants through the NKR and currently ranks 14 of 75 participating centers on the basis of NKR transplant volume.44 In 2016, our program evaluated 81 potential swaps, which included transplant candidates enrolled at our center. Proposed swaps usually include two to eight individual transplant centers, and a proposed swap will fail if any individual center declines the specific donor offered to their enrolled recipient or a center cannot accommodate the proposed surgical date(s). Hence, multiple swaps may be proposed before any given enrolled pair is ultimately transplanted. To manage this administrative burden, one of our two dedicated living donor coordinators devotes approximately 50% of her time to the NKR at our center. Over the course of 2016, we ultimately transplanted seven recipients and enrolled 14 new pairs through the NKR, which is consistent with reported NKR transplant rates and wait times.43
Histocompatibility testing between transplant candidates and potential living donors was prospectively performed using a T cell and B cell flow cytometry crossmatch. Crossmatch reactivity is expressed by using a relative ratio of the molecules of equivalent soluble fluorescence of the tested sample over a negative control. Reactivity is subsequently determined as follows: negative (ratio <1.5), weakly reactive (1.5–2.5), or positive (>2.5). Crossmatches were performed prospectively at the time of living donor evaluation and repeated within 2 weeks of LDKT. HLA typing of both recipient and donor was performed using DNA-based methods. Anti-HLA antibody screening was also performed on all recipients using a combination of LABScreen mixed and single-antigen beads (One Lambda) on a Luminex platform. Median fluorescence intensity over 3000 is used to define unacceptable antigens, which are subsequently entered into UNet to form the basis of the cPRA for each recipient.
Living donor utilization for each donor type was calculated by dividing the number of transplants derived from that donor type by the number of crossmatched donors in the available pool. For example, if ten spouses were in the pool and three were used, the spouse utilization rate would be 0.3. For living donor utilization from compatible donors, the number of transplants was divided by the number of crossmatched and compatible donors.
Continuous variables between groups of patients were analyzed with either a paired t test or ANOVA where appropriate. Categorical variables across groups were analyzed for significance using the chi-squared or Fisher exact test. Kaplan–Meier survival curves were generated, and differences between groups were compared using the log rank test. Cox regression was used to compare the LDKT rate between men and women given the competing risk of DDKT. A P value of <0.05 was deemed significant in all statistical analyses. Statistical analyses were performed using SPSS software (version 23.0; IBM, Armonk, NY) and STATA (version 14.1; Statacorp LP, College Station, TX).
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the living donor kidney transplant team, with special thanks to Donna Collins, Maral Palanjian, and Mary Houston. We also thank Nancy Durnin for administrative support.
We thank the Alpha Omega Alpha Carolyn Kuckein Student Research Fellowship and the Harrison Research Scholarship for providing financial support for this work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Sex and Kidney Transplantation: Why Can’t a Woman Be More Like a Man?,” on pages 2829–2831.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016101059/-/DCSupplemental.
References
- 1.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, Stewart DE, Cherikh WS, Wainright JL, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2012 annual data report: Kidney. Am J Transplant 14[Suppl 1]: 11–44, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Roodnat JI, van Riemsdijk IC, Mulder PG, Doxiadis I, Claas FH, IJzermans JN, van Gelder T, Weimar W: The superior results of living-donor renal transplantation are not completely caused by selection or short cold ischemia time: A single-center, multivariate analysis. Transplantation 75: 2014–2018, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Chkhotua AB, Klein T, Shabtai E, Yussim A, Bar-Nathan N, Shaharabani E, Lustig S, Mor E: Kidney transplantation from living-unrelated donors: Comparison of outcome with living-related and cadaveric transplants under current immunosuppressive protocols. Urology 62: 1002–1006, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Rodrigue JR, Kazley AS, Mandelbrot DA, Hays R, LaPointe Rudow D, Baliga P; American Society of Transplantation : Living donor kidney transplantation: Overcoming disparities in live kidney donation in the US-recommendations from a consensus conference. Clin J Am Soc Nephrol 10: 1687–1695, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gore JL, Danovitch GM, Litwin MS, Pham PT, Singer JS: Disparities in the utilization of live donor renal transplantation. Am J Transplant 9: 1124–1133, 2009 [DOI] [PubMed] [Google Scholar]
- 6.Roodnat JI, Laging M, Massey EK, Kho M, Kal-van Gestel JA, Ijzermans JN, van de Wetering J, Weimar W: Accumulation of unfavorable clinical and socioeconomic factors precludes living donor kidney transplantation. Transplantation 93: 518–523, 2012 [DOI] [PubMed] [Google Scholar]
- 7.Hall EC, James NT, Garonzik Wang JM, Berger JC, Montgomery RA, Dagher NN, Desai NM, Segev DL: Center-level factors and racial disparities in living donor kidney transplantation. Am J Kidney Dis 59: 849–857, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Bloembergen WE, Port FK, Mauger EA, Briggs JP, Leichtman AB: Gender discrepancies in living related renal transplant donors and recipients. J Am Soc Nephrol 7: 1139–1144, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Kayler LK, Rasmussen CS, Dykstra DM, Ojo AO, Port FK, Wolfe RA, Merion RM: Gender imbalance and outcomes in living donor renal transplantation in the United States. Am J Transplant 3: 452–458, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Segev DL, Kucirka LM, Oberai PC, Parekh RS, Boulware LE, Powe NR, Montgomery RA: Age and comorbidities are effect modifiers of gender disparities in renal transplantation. J Am Soc Nephrol 20: 621–628, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peracha J, Hayer MK, Sharif A: Gender disparity in living-donor kidney transplant among minority ethnic groups. Exp Clin Transplant 14: 139–145, 2016 [PubMed] [Google Scholar]
- 12.Tuohy KA, Johnson S, Khwaja K, Pavlakis M: Gender disparities in the live kidney donor evaluation process. Transplantation 82: 1402–1407, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Kayler LK, Meier-Kriesche HU, Punch JD, Campbell DA Jr ., Leichtman AB, Magee JC, Rudich SM, Arenas JD, Merion RM: Gender imbalance in living donor renal transplantation. Transplantation 73: 248–252, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Khajehdehi P: Living non-related versus related renal transplantation--Its relationship to the social status, age and gender of recipients and donors. Nephrol Dial Transplant 14: 2621–2624, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Schaubel DE, Stewart DE, Morrison HI, Zimmerman DL, Cameron JI, Jeffery JJ, Fenton SS: Sex inequality in kidney transplantation rates. Arch Intern Med 160: 2349–2354, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Gill J, Dong J, Gill J: Population income and longitudinal trends in living kidney donation in the United States. J Am Soc Nephrol 26: 201–207, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigue JR, Cornell DL, Lin JK, Kaplan B, Howard RJ: Increasing live donor kidney transplantation: A randomized controlled trial of a home-based educational intervention. Am J Transplant 7: 394–401, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Boulware LE, Hill-Briggs F, Kraus ES, Melancon JK, Falcone B, Ephraim PL, Jaar BG, Gimenez L, Choi M, Senga M, Kolotos M, Lewis-Boyer L, Cook C, Light L, DePasquale N, Noletto T, Powe NR: Effectiveness of educational and social worker interventions to activate patients’ discussion and pursuit of preemptive living donor kidney transplantation: A randomized controlled trial. Am J Kidney Dis 61: 476–486, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sickand M, Cuerden MS, Klarenbach SW, Ojo AO, Parikh CR, Boudville N, Garg AX; Donor Nephrectomy Outcomes Research Network : Reimbursing live organ donors for incurred non-medical expenses: A global perspective on policies and programs. Am J Transplant 9: 2825–2836, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladin K, Hanto DW: Understanding disparities in transplantation: Do social networks provide the missing clue? Am J Transplant 10: 472–476, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Salter ML, Gupta N, King E, Bandeen-Roche K, Law AH, McAdams-DeMarco MA, Meoni LA, Jaar BG, Sozio SM, Kao WH, Parekh RS, Segev DL: Health-related and psychosocial concerns about transplantation among patients initiating dialysis. Clin J Am Soc Nephrol 9: 1940–1948, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gillespie A, Hammer H, Kolenikov S, Polychronopoulou A, Ouzienko V, Obradovic Z, Urbanski MA, Browne T, Silva P: Sex differences and attitudes toward living donor kidney transplantation among urban black patients on hemodialysis. Clin J Am Soc Nephrol 9: 1764–1772, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Available at: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data. Accessed January 19, 2017
- 24.Weng FL, Reese PP, Mulgaonkar S, Patel AM: Barriers to living donor kidney transplantation among black or older transplant candidates. Clin J Am Soc Nephrol 5: 2338–2347, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Rood JJ, Eernisse JG, Van Leeuwen A: Leucocyte antibodies in sera from pregnant women. Nature 181: 1735–1736, 1958 [DOI] [PubMed] [Google Scholar]
- 26.Densmore TL, Goodnough LT, Ali S, Dynis M, Chaplin H: Prevalence of HLA sensitization in female apheresis donors. Transfusion 39: 103–106, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Regan L, Braude PR, Hill DP: A prospective study of the incidence, time of appearance and significance of anti-paternal lymphocytotoxic antibodies in human pregnancy. Hum Reprod 6: 294–298, 1991 [DOI] [PubMed] [Google Scholar]
- 28.Hönger G, Fornaro I, Granado C, Tiercy JM, Hösli I, Schaub S: Frequency and determinants of pregnancy-induced child-specific sensitization. Am J Transplant 13: 746–753, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Hyun J, Park KD, Yoo Y, Lee B, Han BY, Song EY, Park MH: Effects of different sensitization events on HLA alloimmunization in solid organ transplantation patients. Transplant Proc 44: 222–225, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Lopes D, Barra T, Malheiro J, Tafulo S, Martins L, Almeida M, Pedroso S, Dias L, Castro Henriques A, Cabrita A: Effect of different sensitization events on HLA alloimmunization in kidney transplantation candidates. Transplant Proc 47: 894–897, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Yoo PS, Bonnel A, Kamoun M, Levine MH: Clinical outcomes among renal transplant recipients with pre-transplant weakly reactive donor-specific antibodies. Clin Transplant 28: 127–133, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gibney EM, Cagle LR, Freed B, Warnell SE, Chan L, Wiseman AC: Detection of donor-specific antibodies using HLA-coated microspheres: Another tool for kidney transplant risk stratification. Nephrol Dial Transplant 21: 2625–2629, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Vaidya S, Partlow D, Susskind B, Noor M, Barnes T, Gugliuzza K: Prediction of crossmatch outcome of highly sensitized patients by single and/or multiple antigen bead luminex assay. Transplantation 82: 1524–1528, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Duquesnoy RJ, Marrari M, Mulder A: Usefulness of the nonself-self algorithm of HLA epitope immunogenicity in the specificity analysis of monospecific antibodies induced during pregnancy. Front Immunol 6: 180, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duquesnoy RJ, Hönger G, Hösli I, Marrari M, Schaub S: Identification of epitopes on HLA-DRB alleles reacting with antibodies in sera from women sensitized during pregnancy. Hum Immunol 77: 214–222, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Orandi BJ, Garonzik-Wang JM, Massie AB, Zachary AA, Montgomery JR, Van Arendonk KJ, Stegall MD, Jordan SC, Oberholzer J, Dunn TB, Ratner LE, Kapur S, Pelletier RP, Roberts JP, Melcher ML, Singh P, Sudan DL, Posner MP, El-Amm JM, Shapiro R, Cooper M, Lipkowitz GS, Rees MA, Marsh CL, Sankari BR, Gerber DA, Nelson PW, Wellen J, Bozorgzadeh A, Gaber AO, Montgomery RA, Segev DL: Quantifying the risk of incompatible kidney transplantation: A multicenter study. Am J Transplant 14: 1573–1580, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Higgins RM, Daga S, Mitchell DA: Antibody-incompatible kidney transplantation in 2015 and beyond. Nephrol Dial Transplant 30: 1972–1978, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massie AB, Gentry SE, Montgomery RA, Bingaman AA, Segev DL: Center-level utilization of kidney paired donation. Am J Transplant 13: 1317–1322, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melcher ML, Blosser CD, Baxter-Lowe LA, Delmonico FL, Gentry SE, Leishman R, Knoll GA, Leffell MS, Leichtman AB, Mast DA, Nickerson PW, Reed EF, Rees MA, Rodrigue JR, Segev DL, Serur D, Tullius SG, Zavala EY, Feng S: Dynamic challenges inhibiting optimal adoption of kidney paired donation: Findings of a consensus conference. Am J Transplant 13: 851–860, 2013 [DOI] [PubMed] [Google Scholar]
- 40.Rodrigue JR, Leishman R, Vishnevsky T, Evenson A, Mandelbrot DA: Concerns of ABO incompatible and crossmatch-positive potential donors and recipients about participating in kidney exchanges. Clin Transplant 29: 233–241, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Irwin FD, Bonagura AF, Crawford SW, Foote M: Kidney paired donation: A payer perspective. Am J Transplant 12: 1388–1391, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Orandi BJ, Luo X, Massie AB, Garonzik-Wang JM, Lonze BE, Ahmed R, Van Arendonk KJ, Stegall MD, Jordan SC, Oberholzer J, Dunn TB, Ratner LE, Kapur S, Pelletier RP, Roberts JP, Melcher ML, Singh P, Sudan DL, Posner MP, El-Amm JM, Shapiro R, Cooper M, Lipkowitz GS, Rees MA, Marsh CL, Sankari BR, Gerber DA, Nelson PW, Wellen J, Bozorgzadeh A, Gaber AO, Montgomery RA, Segev DL: Survival benefit with kidney transplant from HLA-incompatible Live Donors. N Engl J Med 374: 940–950, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Kidney Registry: Paired Exchange Results Quarterly Report. Available at http://www.kidneyregistry.org/pages/p399/NKR_Quarterly_Report_Q4_2016.php. Accessed March 1, 2017
- 44.Available at: http://www.kidneytransplantcenters.org/center/index. Accessed March 14, 2017
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.