Abstract
We investigated the value of genetic, histopathologic, and early treatment response information in prognosing long-term renal outcome in children with primary steroid-resistant nephrotic syndrome. From the PodoNet Registry, we obtained longitudinal clinical information for 1354 patients (disease onset at >3 months and <20 years of age): 612 had documented responsiveness to intensified immunosuppression (IIS), 1155 had kidney biopsy results, and 212 had an established genetic diagnosis. We assessed risk factors for ESRD using multivariate Cox regression models. Complete and partial remission of proteinuria within 12 months of disease onset occurred in 24.5% and 16.5% of children, respectively, with the highest remission rates achieved with calcineurin inhibitor–based protocols. Ten-year ESRD-free survival rates were 43%, 94%, and 72% in children with IIS resistance, complete remission, and partial remission, respectively; 27% in children with a genetic diagnosis; and 79% and 52% in children with histopathologic findings of minimal change glomerulopathy and FSGS, respectively. Five-year ESRD-free survival rate was 21% for diffuse mesangial sclerosis. IIS responsiveness, presence of a genetic diagnosis, and FSGS or diffuse mesangial sclerosis on initial biopsy as well as age, serum albumin concentration, and CKD stage at onset affected ESRD risk. Our findings suggest that responsiveness to initial IIS and detection of a hereditary podocytopathy are prognostic indicators of favorable and poor long-term outcome, respectively, in children with steroid-resistant nephrotic syndrome. Children with multidrug-resistant sporadic disease show better renal survival than those with genetic disease. Furthermore, histopathologic findings may retain prognostic relevance when a genetic diagnosis is established.
Keywords: nephrotic syndrome, children, podocytopathies, immunosuppression, steroid resistance, outcomes
Although most children with idiopathic nephrotic syndrome readily respond to glucocorticoid therapy, approximately 10% of patients turn out to be steroid resistant (steroid-resistant nephrotic syndrome [SRNS]). The predominant histopathologic finding associated with steroid resistance is FSGS. SRNS/FSGS is associated with an increased risk of developing ESRD. Patients with SRNS/FSGS account for 15% of all children with CKD requiring RRT.1 However, disease courses are highly variable, indicating etiologic heterogeneity of the disorder. Although a considerable proportion of patients respond to intensified immunosuppression (IIS) protocols, others show multidrug resistance. IIS-responsive forms of SRNS may show better long-term outcomes than IIS-resistant forms.2 In addition, in recent years, abnormalities in a growing number of genes specifically expressed in podocytes have been identified as underlying causes of SRNS. Comprehensive genetic screening currently identifies hereditary podocytopathies in up to 30% of children with SRNS.3
Historically, the prognosis of SRNS was largely staged according to histopathologic findings, with limited predictability of medium- to long-term disease outcomes.4–9 The recent insights should allow reclassification of SRNS taking into account information about genetic disease causes and IIS responsiveness. However, most SRNS cohorts assessing long-term outcomes on the basis of genetic information and IIS responsiveness were limited in size, follow-up time, and/or completeness of information. Important open questions concern the prognostic effect of partial versus complete proteinuria remission in response to IIS, the relative roles of IIS responsiveness, genetic disease, histopathologic findings and other potential risk modifying factors, such as age and disease severity at onset, and the frequency and relevance of the anecdotally reported responsiveness of genetic SRNS forms to IIS.
In the work presented here, we sought to address these open issues by interrogating the PodoNet Registry database. In this international patient registry comprehensive clinical, biochemical, treatment-related, genetic, and histopathologic information is collected from pediatric patients with primary steroid resistance with up to 15 years of follow-up.10 The average duration of follow-up from first disease manifestation was 3.6 years in this cohort.
Results
Patient Characteristics
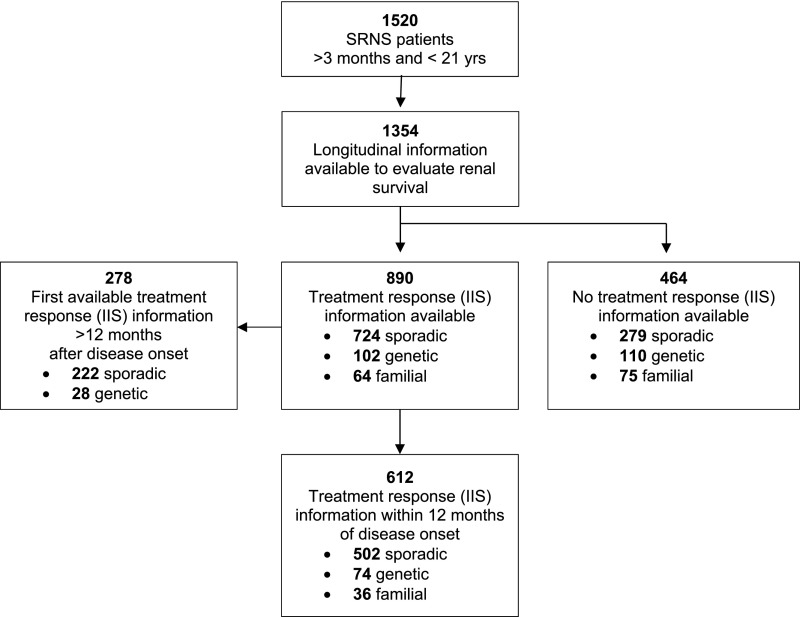
In total, 1354 patients with 10,409 clinical updates were included in the analysis (Figure 1). These included 713 patients with sporadic disease and negative genetic testing, 212 patients in whom a genetic cause was ascertained (Supplemental Table 1), 139 patients with familial disease without established genetic diagnosis, and 290 patients with sporadic disease occurrence in whom no testing was performed. Information on the type of and response to immunosuppressive therapies during the first year after disease onset was available in 612 children (Figure 1). Detailed patient characteristics of the overall cohort and each subgroup are given in Table 1.
Figure 1.
Distribution of the selected PodoNet Registry cohort. Selection of patients for IIS response and renal survival analyses from the total PodoNet Registry cohort.
Table 1.
Patient characteristics by response to immunosuppressive therapy, familial occurrence, and genetic findings
| Patient Characteristics | First-Year Treatment Response Information Available (%) | First-Year Treatment Response Information Not Available (%) | All, N=1354 (%) | |||
|---|---|---|---|---|---|---|
| Complete Remission, N=150 | Partial Remission, N=101 | No Remission, N=361 | Genetic/Familial, N=241 | Sporadic, N=501 | ||
| Cause of disease | ||||||
| Sporadic, no mutation identified | 92 (61.3) | 67 (66.3) | 228 (63.2) | — | 326 (65.1) | 713 (52.7) |
| Sporadic, not tested | 45 (30.0) | 20 (19.8) | 50 (13.9) | — | 175 (34.9) | 290 (21.4) |
| Genetic | 2 (1.3) | 8 (7.9) | 64 (17.7) | 138 (57.3) | — | 212 (15.7) |
| Familial, no mutation identified | 11 (7.3) | 6 (5.9) | 19 (5.3) | 103 (42.7) | — | 139 (10.3) |
| Histopathologic diagnosis | ||||||
| MCGN | 37 (28.0) | 24 (25.0) | 70 (21.0) | 16 (8.4) | 102 (25.3) | 249 (21.6) |
| MesPGN | 15 (11.4) | 12 (12.5) | 44 (13.2) | 29 (15.2) | 44 (10.9) | 144 (12.5) |
| FSGS | 74 (56.1) | 55 (57.3) | 202 (60.7) | 114 (59.7) | 220 (54.6) | 666 (57.6) |
| DMS | 0 | 0 | 7 (2.1) | 12 (6.3) | 7 (1.7) | 26 (2.3) |
| Other | 6 (4.5) | 5 (5.2) | 10 (3.0) | 20 (10.5) | 30 (7.4) | 72 (6.1) |
| Unknown/no biopsy | 18 | 5 | 28 | 50 | 98 | 199 |
| Characteristics at disease onset | ||||||
| Age | ||||||
| >3 mo and <1 yr | 11 (7.3) | 1 (1.0) | 17 (4.7) | 40 (16.6) | 44 (8.8) | 113 (8.3) |
| ≥1 and <6 yr | 90 (60.0) | 55 (54.5) | 194 (53.7) | 122 (50.6) | 265 (52.9) | 726 (53.6) |
| ≥6 and <12 yr | 30 (20.0) | 25 (24.8) | 92 (25.5) | 44 (18.3) | 134 (26.7) | 325 (24.0) |
| ≥12 yr | 19 (12.7) | 20 (19.8) | 58 (16.1) | 35 (14.5) | 58 (11.6) | 190 (14.0) |
| Serum albumin, g/L | 18.9 (14.0–23.0) | 20.0 (15.1–29.0) | 19.5 (15.0–25.0) | 24.0 (17.5–35.0) | 20.0 (16.0–29.0) | 20.0 (16.0–28.0) |
| Ninfo | 120 | 89 | 273 | 171 | 355 | 1008 |
| Proteinuria | ||||||
| Nephrotic range | 124 (93.2) | 86 (92.5) | 315 (96.9) | 167 (86.5) | 336 (90.1) | 1028 (92.0) |
| Non-nephrotic range | 9 (6.8) | 7 (7.5) | 10 (3.1) | 26 (13.5) | 37 (9.9) | 89 (8.0) |
| Ninfo | 133 | 93 | 325 | 193 | 373 | 1117 |
| Renal function | ||||||
| CKD stage 1 | 83 (69.7) | 61 (76.3) | 206 (69.6) | 78 (51.7) | 164 (57.1) | 592 (63.5) |
| CKD stage 2 | 23 (19.3) | 14 (17.5) | 62 (20.9) | 35 (23.2) | 85 (29.6) | 219 (23.5) |
| CKD stage 3 | 11 (9.2) | 5 (6.3) | 21 (7.1) | 29 (19.2) | 27 (9.4) | 93 (10.0) |
| CKD stage 4 | 2 (1.7) | 0 | 7 (2.4) | 9 (6.0) | 11 (3.8) | 29 (3.1) |
| Ninfo | 119 | 80 | 296 | 151 | 287 | 933 |
| Follow-up information | ||||||
| Duration of follow-up, yr | 3.9 (1.5–6.3) | 3.2 (1.6–5.6) | 3.0 (1.2–5.7) | 3.5 (1.0–7.2) | 4.0 (1.8–7.8) | 3.6 (1.5–6.8) |
| ESRD at last observation | 7 (4.7) | 12 (11.9) | 115 (31.9) | 116 (47.9) | 113 (22.6) | 363 (26.8) |
| Time to ESRD, yr | 2.4 (0.8–4.8) | 3.3 (1.1–4.6) | 3.0 (1.6–5.3) | 3.0 (0.8–6.4) | 2.6 (0.8–5.8) | 2.8 (1.1–5.6) |
| Renal survival, yr | ||||||
| ≤1 | 2 (1.5) | 2 (2.3) | 20 (6.7) | 34 (15.7) | 30 (6.6) | 88 (7.4) |
| >1 | 130 (98.5) | 86 (97.7) | 279 (93.3) | 180 (84.1) | 422 (93.4) | 1097 (92.6) |
| Ninfo | 132 | 88 | 299 | 214 | 452 | 1185 |
Data are given as N (percentage) per analysis group (column) or median (interquartile range). MCGN, minimal-change glomerulopathy; MesPGN, mesangioproliferative glomerulonephritis; DMS, diffuse mesangial sclerosis.
Efficacy of IIS Protocols
In total, 906 individual treatment periods were recorded in 612 patients during the first year from disease onset; 380 patients were treated with one immunosuppressive medication, 173 patients were treated with two different immunosuppressive medications, and 59 patients were treated with three or more different immunosuppressive medications. The treatment results are provided in Tables 1 and 2. Altogether, complete remission of proteinuria was observed with 18.5% of therapies and in 24.5% of patients. The highest rates of complete or partial remission were achieved with calcineurin inhibitor (CNI)–based protocols, whereas steroid pulses, cyclophosphamide (CPH), and mycophenolate mofetil (MMF) showed lacking efficacy in >80% of patients.
Table 2.
Response to IIS treatment episodes during the first year after disease onset in 612 patients with SRNS
| Treatment Episodes (±Oral Steroid, ±RAS) | Complete Remission | Partial Remission | No Remission | Total |
|---|---|---|---|---|
| Oral | ||||
| CNI | 129 (29.8) | 82 (18.9) | 222 (51.3) | 433 |
| CPH | 9 (9.2) | 8 (8.2) | 81 (82.7) | 98 |
| MMF | 2 (8.3) | 2 (8.3) | 20 (83.3) | 24 |
| CNI + MMF | 4 (11.8) | 10 (29.4) | 20 (58.8) | 34 |
| iv Pulse | ||||
| Steroid pulse | 16 (6.8) | 25 (10.6) | 195 (82.6) | 236 |
| iv + Oral | ||||
| Steroid pulse + CNI | 4 (8.2) | 5 (10.2) | 40 (81.6) | 49 |
| Steroid pulse + other | 1 (5.9) | 1 (5.9) | 15 (88.2) | 17 |
| CPH pulse ± other | 1 (12.5) | 1 (12.5) | 6 (75.0) | 8 |
| Rituximab ± other | 2 (28.6) | 0 | 5 (71.4) | 7 |
| All first-year treatment episodes | 168 (18.5) | 134 (14.8) | 604 (66.7) | 906 |
| Best response in treated patients | 150 (24.5) | 101 (16.5) | 361 (59.0) | 612 |
In total, 232 (38%) patients were treated with more than one treatment protocol during the first year after disease onset. Most efficacious treatment was used to classify patients. Data are given as number (percentage). RAS, renin-angiotensin system; CPH, cyclophosphamide.
Among the 502 patients with sporadic disease without a genetic diagnosis, 139 (27.3%) achieved complete remission, and another 87 (17.3%) achieved partial remission. Similar response rates were observed among 36 patients with familial disease but without established genetic diagnosis, with 11 (31%) patients achieving complete remission and six (17%) patients achieving partial remission. In the subgroup of patients with familial genetically unexplained disease, none of 17 children with IIS responsiveness but four of 19 IIS-unresponsive children progressed to ESRD. One of three kidney transplant recipients developed post-transplant proteinuria recurrence.
Among 74 children with a documented genetic diagnosis, transient complete remission was documented in two (2.7%) children, and partial remission was documented in eight (11%) children. The detailed genetic information is given in Supplemental Table 2. One patient with a WT1 mutation achieved complete remission on cyclosporin A (CsA) and methylprednisolone pulses for 2 weeks followed by mild subnephrotic-range proteinuria. The partial remission status was maintained for >11 years. At last follow-up, 12 years after disease onset, the patient was still in CKD stage 2. The other patient, compound heterozygous in NPHS2, also showed transient complete remission for 4–6 weeks before a relapse was documented. He progressed to ESRD within 4 years. Partial remission with reduction of proteinuria to the non-nephrotic range was observed in eight patients with genetic disease while receiving CsA and four patients receiving CsA combined with RAAS antagonists. Five of these eight patients were nephrotic, and four were in CKD stages 3–5 at last observation.
Long-Term Renal Survival
According to Kaplan–Meier analysis, the proportion of patients with SRNS and preserved renal function was 74% (95% confidence interval [95% CI], 71% to 77%) at 5 years, 58% (95% CI, 53% to 61%) at 10 years, and 48% (95% CI, 43% to 53%) at 15 years.
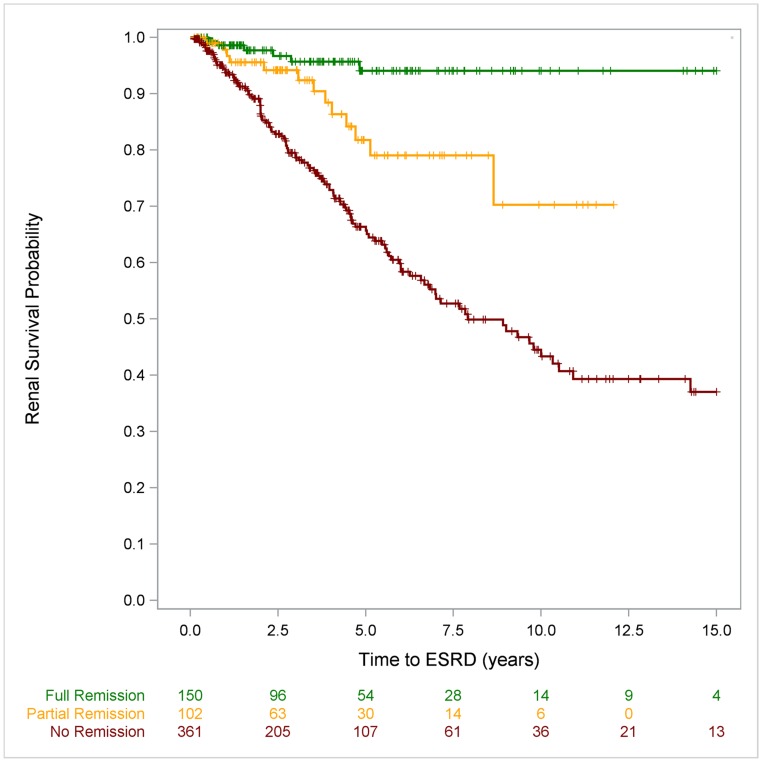
Ten-year renal survival was 94% (95% CI, 87% to 97%) among patients who achieved complete remission in the first disease year, 72% (95% CI, 47% to 86%) in those who achieved partial remission, and 43% (95% CI, 35% to 51%) in the patients with multidrug resistance (log rank: P<0.001). Fifteen-year survival was 94% (95% CI, 87% to 97%) in complete responders to initial IIS compared with 37% (95% CI, 28% to 46%) in the multidrug-resistant cohort (log rank: P<0.001) (Figure 2 and Supplemental Figure 1).
Figure 2.
Renal survival, analysed by response to IIS, is excellent in children with SRNS achieving full remission following IIS compared to patients being resistant to IIS. Ten-year ESRD-free survival rates were 94% (95% CI, 87% to 97%) in patients achieving full remission, 72% (95% CI, 48% to 86%) in patients with partial remission, and 43% (95% CI, 35% to 51%) in patients resistant to IIS.
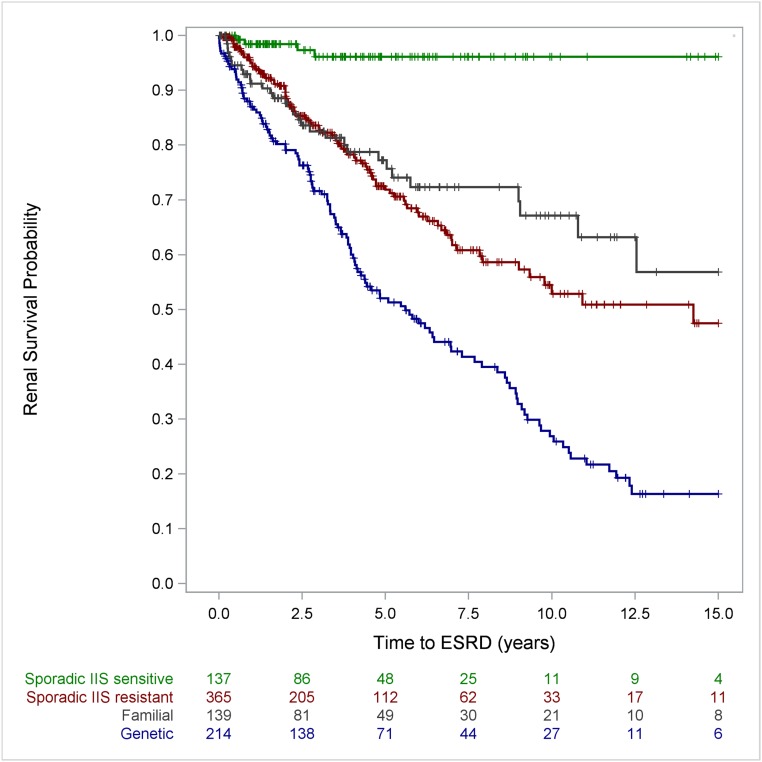
Analysis of the data including hereditary and genetic information showed excellent long-term outcomes in IIS-sensitive patients with SRNS and sporadic disease occurrence (96%; 95% CI, 90% to 99%, 10- and 15-year renal survival rates). The diagnosis of a genetic disease markedly affected ESRD risk: 10- and 15-year ESRD-free survival rates were 27% (95% CI, 20% to 35%) and 17% (95% CI, 10% to 25%) in patients with a genetic diagnosis compared with 53% (95% CI, 44% to 61%) and 48% (95% CI, 37% to 58%) in patients with sporadic multidrug-resistant disease without a genetic diagnosis (log rank: P<0.001) (Figure 3 and Supplemental Figure 2).
Figure 3.
The analysis of renal survival by disease category showed an excellent long-term outcome in IIS sensitive SRNS patients with sporadic disease occurrence and poor long-term outcome in patients with genetic disease. IIS resistant patients with sporadic disease had a better renal survival compared to patients with genetic disease (patients with partial IIS responsiveness were classified IIS resistant for this analysis).
Further breakdown by genetic diagnosis showed largely uniform renal survival times of the major genetic entities, with estimated 10-year ESRD-free survival rates of 28% (95% CI, 16% to 42%) for NPHS2-associated nephropathy, 23% (95% CI, 10% to 39%) for WT1-associated disease, and 29% (95% CI, 19% to 42%) for the less common podocytopathies (Supplemental Figure 3).
Notably, patients with familial disease but without established genetic diagnosis showed better 10-year renal survival (67%; 95% CI, 55% to 77%) than patients with a genetic diagnosis (log rank: P<0.001) (Figure 3 and Supplemental Figure 2).
The 501 patients with sporadic disease occurrence in the cohort in whom no IIS response information (n=279) at all and no response information during the first 12 months (n=222) were documented suffered a 10-year ESRD risk of 32% (95% CI, 26% to 38%), likely representing a mixture of patients with and without IIS responsiveness.
The histopathologic findings at the time of diagnosis were strongly associated with long-term renal survival (Supplemental Figure 4). ESRD-free survival rates in children with minimal change glomerulopathy (MCGN) MCGN were 92% (95% CI, 86% to 95%) at 5 years and 79% (95% CI, 69% to 86%) at 10 and 15 years compared with 69% (95% CI, 65% to 73%) 5-year, 52% (95% CI, 46% to 57%) 10-year, and 37% (95% CI, 30% to 44%) 15-year renal survival rates in children diagnosed with FSGS. The most unfavorable outcome was observed in patients with diffuse mesangial sclerosis (DMS) who have an 80% (95% CI, 60% to 93%) ESRD risk at 5 years after initial manifestation.
Cox regression analysis was performed to identify predictors of renal survival in unadjusted, hereditary disease–adjusted, and multivariate models (Table 3). An age of 1–5 years at disease onset was associated with a lower ESRD risk, whereas advanced CKD at initial presentation and nephrotic-range proteinuria increased the likelihood of progressing to ESRD both in the univariate models and when adjusting for hereditary disease. Age 1–5 years old and advanced CKD at first presentation but not nephrotic-range proteinuria remained significant risk factors for ESRD in the fully adjusted multivariate model. The histopathologic diagnosis was clearly predictive of ESRD. Even adjusted for age, proteinuria level, CKD, genetic status, and IIS responsiveness, DMS (hazard ratio, 12.3; 95% CI, 6.3 to 24.0) or FSGS (hazard ratio, 2.9; 95% CI, 1.9 to 4.5) on biopsy implied an increased risk of progressing to ESRD. Moreover, the independent effect of genetic diagnosis and IIS responsiveness suggested in the Kaplan–Meier survival analysis was confirmed by multivariate Cox regression modeling. The ESRD risk was increased by 150% in patients in whom a genetic diagnosis was shown and reduced by 87% in patients who achieved complete remission and by 50% in those with partial remission in response to IIS during the first year. These associations were still present after adjustment for the characteristics at disease onset and histologic diagnosis.
Table 3.
Risk factors for ESRD according to unadjusted Cox regression analysis, a model adjusting for hereditary disease, and a multivariate model
| Variable | Unadjusted | Adjusted for Hereditary Disease | Multivariate Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Characteristics at disease onset | |||||||||
| Age (reference ≥12 yr) | |||||||||
| >3 mo and <1 yr | 1.21 | 0.81 to 1.85 | 0.34 | 0.99 | 0.62 to 1.44 | 0.79 | 0.88 | 0.55 to 1.39 | 0.60 |
| ≥1 and <6 yr | 0.59 | 0.43 to 0.80 | 0.001 | 0.57 | 0.41 to 0.78 | 0.001 | 0.68 | 0.48 to 0.97 | 0.04 |
| ≥6 and <12 yr | 0.91 | 0.65 to 1.28 | 0.59 | 0.93 | 0.66 to 1.30 | 0.66 | 1.16 | 0.80 to 1.68 | 0.44 |
| Proteinuria (reference subnephrotic range) | |||||||||
| Nephrotic range | 1.67 | 0.94 to 2.96 | 0.08 | 1.73 | 1.00 to 3.00 | 0.05 | 1.33 | 0.74 to 2.41 | 0.34 |
| Serum albumin per 10-g/L increase | 1.01 | 0.88 to 1.16 | 0.90 | 0.93 | 0.81 to 1.08 | 0.36 | 0.86 | 0.72 to 1.02 | 0.09 |
| CKD (reference stage 1) | |||||||||
| Stage 2 | 1.34 | 1.00 to 1.79 | 0.05 | 1.25 | 0.93 to 1.68 | 0.14 | 1.17 | 0.88 to 1.57 | 0.28 |
| Stage 3 | 2.29 | 1.58 to 3.31 | <0.001 | 2.19 | 1.48 to 3.25 | 0.001 | 2.03 | 1.37 to 3.00 | 0.001 |
| Stage 4 | 6.21 | 3.92 to 9.82 | <0.001 | 5.77 | 3.70 to 8.98 | <0.001 | 5.10 | 3.12 to 8.34 | <0.001 |
| Histopathology (reference MCGN) | |||||||||
| FSGS | 3.96 | 2.57 to 6.08 | <0.001 | 3.61 | 2.34 to 5.56 | <0.001 | 2.90 | 1.85 to 4.53 | <0.001 |
| DMS | 19.9 | 10.7 to 37.0 | <0.001 | 13.2 | 7.01 to 25.0 | <0.001 | 12.3 | 6.29 to 24.0 | <0.001 |
| MesPGN | 1.69 | 0.93 to 3.07 | 0.09 | 1.49 | 0.82 to 2.72 | 0.19 | 1.26 | 0.68 to 2.35 | 0.46 |
| Other | 3.59 | 1.87 to 6.87 | 0.001 | 3.76 | 1.96 to 7.23 | <0.001 | 3.42 | 1.75 to 6.67 | 0.003 |
| Unknown | 4.81 | 2.91 to 7.95 | <0.001 | 3.88 | 2.34 to 6.44 | <0.001 | 3.59 | 2.13 to 6.04 | <0.001 |
| Cause of disease (reference sporadic) | |||||||||
| Familial | 1.18 | 0.79 to 1.75 | 0.42 | 1.10 | 0.73 to 1.66 | 0.66 | |||
| Genetic | 3.11 | 2.45 to 3.94 | <0.001 | 2.39 | 1.83 to 3.13 | <0.001 | |||
| Response to IIS (reference no remission) | |||||||||
| Partial remission | 0.34 | 0.18 to 0.63 | 0.001 | 0.42 | 0.23 to 0.79 | <0.01 | 0.49 | 0.26 to 0.92 | 0.03 |
| Complete remission | 0.11 | 0.05 to 0.24 | <0.001 | 0.14 | 0.06 to 0.31 | <0.001 | 0.13 | 0.06 to 0.30 | <0.001 |
| Unknown | 0.82 | 0.63 to 1.07 | 0.14 | 0.82 | 0.63 to 1.08 | 0.16 | 0.76 | 0.57 to 1.01 | 0.06 |
Only covariates with significant contribution to the model are shown. HR, hazard ratio; MCGN, minimal-change glomerulopathy; DMS, diffuse mesangial sclerosis; MesPGN, mesangio-proliferative glomerulonephritis.
Discussion
This integrative analysis of the largest global cohort of pediatric SRNS provides unequivocal evidence for the independent prognostic effect of an established genetic diagnosis, the histopathologic findings at disease onset, and the early responsiveness of proteinuria to IIS therapy.
The average overall ESRD-free survival rates in this unselected cohort of pediatric patients with primary SRNS were 74% at 5 years and 48% at 15 years after diagnosis, well in line with previous cohort studies reporting 65%–92% renal survival at 5 years and 34%–76% at 15 years.5,6,9,11,12 We used the extensive information collected in the PodoNet Registry to delineate key factors helping to predict long-term renal outcome.
A strong predictive value of the responsiveness to initial CNI therapy in SRNS for long-term renal survival has recently been reported in a multicenter study of 169 children with primary SRNS.2 Our analysis confirms and extends this observation to IIS therapies in general, with 10- and 15-year kidney survival rates differing by as much as 50% between patients who achieved complete proteinuria remission in the year after diagnosis and those who were found to be multidrug resistant. The predictive value of IIS responsiveness remained highly significant when the genetic diagnosis was taken into account and was also independent of the histopathologic diagnosis as well as age, renal function, and clinical presentation at disease onset. Calcineurin inhibition was found to be the most efficacious immunosuppressive therapy, yielding complete remission in 30% of all patients and partial remission in another 19% of all patients. This finding is in keeping with previous reports, in which CNI response rates ranged from 31% to 89% for complete remission and from 19% to 38% for partial remission depending on the selection criteria chosen.13–17 By contrast, we observed full remission in <10% of patients exposed to steroid pulses, CPH, or MMF, in keeping with previous studies.18–23 Our findings provide some evidence against the use of these therapeutic protocols. In accordance with our results, a much lower proteinuria response rate and poorer long-term renal survival with CPH were observed in a recent prospective study comparing this agent with CsA in children with SRNS.18 Our findings lend further support to the current consensus that CPH should not be used in SRNS due to its poor risk-benefit profile.18–25 Likewise, we provide further evidence that MMF is of very limited efficacy in inducing proteinuria remission in pediatric SRNS as suggested by two small-scale pediatric studies.26,27
In a sizable fraction of patients exposed to IIS, protein excretion was not completely normalized but was reduced to the subnephrotic range. It is often difficult to causally attribute this partial remission pattern to the administered immunosuppressive therapies due to the frequent coadministration of RAAS antagonists, which reliably lower proteinuria by 40%–50% in patients with SRNS.28,29 Notwithstanding this potential source of confounding, it is noteworthy that partial reduction of proteinuria in the first year after diagnosis in patients receiving IIS was associated with significantly improved long-term renal survival relative to that in patients with multidrug-resistant proteinuria. Even when adjusting for genetic and histopathologic findings, age, initial disease severity, and renal function, partial responsiveness to initial IIS was associated with a reduction of the ESRD risk by >50%.
In 20.2% of the included patients (age at disease onset >3 months old but <20 years old), a genetic podocytopathy was identified. Although NPHS2 and WT1 mutations accounted for two thirds of patients, the other abnormalities were scattered over 17 different podocyte genes (Supplemental Table 1). Children in whom a genetic diagnosis was established carried a highly unfavorable long-term prognosis, with 85% progressing to ESRD within 15 years. Notably, this outcome was significantly poorer than that of children with sporadic multidrug-resistant disease in whom no genetic abnormality was established, highlighting the added prognostic value obtained from genetic screening.
The observed difference in outcome between the genetic and the multidrug-resistant cases without a genetic diagnosis may even be underestimated, because a fraction of multidrug-resistant children was not screened comprehensively in the more recently identified genes or did not undergo genetic screening at all. Among the 43% of patients in our cohort who underwent next generation gene panel sequencing (NGS), a genetic diagnosis was established in 23%. A recent large study using NGS panel screening identified genetic causes in 29% of an SRNS cohort including patients with congenital disease.3
Our study contributes important information to the ongoing controversy of whether some patients diagnosed with genetic disease may still respond to immunosuppressive treatment. Specifically, a nonimmunologic antiproteinuric action of CNI mediated by stabilization of the actin cytoskeleton has been suggested.30 Our 74 patients with a genetic disease and documented IIS represent the largest published cohort of patients with hereditary SRNS treated with IIS. Only two of these, one diagnosed with WT1 and one diagnosed with NPHS2 glomerulopathy, transiently achieved complete remission in the first year of disease while on CsA treatment. The patient with WT1 disease (previously published31) still has stable renal function after 12 years of follow-up; the other patient progressed to ESRD within <5 years. Another eight patients achieved the criteria for partial remission for some time while receiving CsA. However, remission persisted in none of the children followed for >30 months, and four of five children with long-term follow-up were in CKD stages 3–5 at last observation. Also, 50% of the children were cotreated with RAAS antagonists, which may explain the observed reduction of proteinuria. Our data confirm observations of previous case series and small studies generally suggesting nonresponsiveness to IIS in hereditary podocytopathies.32–36 Complete proteinuria remission on calcineurin inhibition has been reported in only four subjects, and partial remission on calcineurin inhibition has been reported in 17 subjects to date, almost all of whom were simultaneously receiving RAAS antagonists.2,31,32,37,38 Nearly all reported patients had a poor long-term renal outcome. Hence, the current state of evidence allows concluding that, in hereditary forms of SRNS, calcineurin inhibition does not offer a therapeutic benefit over RAAS blockade alone, and hence, patients should be spared the side effects of immunosuppressive therapy.
Another interesting subgroup is made up of the 139 patients with familial SRNS in whom no known genetic disease could be identified by NGS gene panel screening. Some 31% of the children with documented first-year IIS achieved complete remission, a rate similar to that observed in children with sporadic disease without a genetic diagnosis. None of the IIS responders with familial disease progressed to ESRD. One of three transplant recipients with familial disease developed post-transplant recurrence. The long-term renal survival rate of the patients with genetically unexplained familial cases (67% at 10 years) was 15% better than that of the patients with sporadic cases with multidrug resistance and almost 40% better than that of the patients with an established genetic diagnosis. It is interesting to speculate about as-yet undiscovered genetic entities in these families, which might involve variants in genes regulating the immune system rather than podocyte structure and function and may, in some patients, show sensitivity to pharmacologic modulation. The favorable response to IIS in general and the observed case of post-transplant recurrence are suspicious for an immunologic pathogenesis in these patients with familial cases and provide a rationale for a trial of IIS therapy in patients with familial cases in whom no genetic diagnosis can be established.
Traditionally, the diagnostic categorization and prognostic judgment in SRNS relied on the histopathologic assessment of kidney tissue. In this cohort, we expectedly found that the diagnosis of FSGS associated with a fourfold increase of ESRD risk relative to MCGN and that the diagnosis of DMS associated with a 20-fold increase of ESRD risk relative to MCGN. Notably, FSGS and DMS largely retained their independent prognostic value when adjusting for CKD stage at first manifestation, responsiveness to IIS, and the presence of a genetic disease. Hence, a patients who is multidrug resistant with a genetic diagnosis and a given GFR will still have a nearly threefold higher ESRD risk when diagnosed with FSGS compared with MCGN, suggesting that histopathologic assessment may be still relevant in the genetic era. Because the prognostic value of renal biopsy is still unproven in genetic SRNS, further detailed studies will be required to address which histopathologic features are most predictive in patients with IIS-resistant SRNS with or without an established genetic disease.
Although the very large size of the cohort and the comprehensive and long-term data collection are major strengths of this international study, it is, at the same time, limited by the incompleteness of reporting. We attempted to maximize genetic information by NGS gene panel sequencing of all patients who did not achieve complete remission by IIS but were able to retrieve DNA samples in only 85% of these individuals. Finally, the common use of polypragmatic therapeutic approaches with frequent coadministration of RAAS blockers was a major source of confounding to the analysis of treatment responses.
Nonetheless, the PodoNet cohort proved to be a unique source of information on short- and long-term outcomes in children with primary SRNS. We found clear evidence that the response to initial immunosuppressive therapy and the diagnosis of an underlying genetic disease are important independent prognostic indicators in addition to the histopathologic diagnosis, age, and renal function at first presentation.
Concise Methods
Patient Cohort and Analytic Approach
The PodoNet Registry is an international web-based clinical registry (www.podonet.org) for primary SRNS and congenital nephrotic syndrome (CNS). The PodoNet Registry accepts patients with childhood-onset (age ≤20 years old) primary SRNS, CNS, or persistent subnephrotic proteinuria with likely genetic disease. Patients with secondary SRNS are not included in this cohort. The registry study protocol, description, and characterization of the PodoNet cohort were recently published.10
For these analyses, patients with CNS , patients with adult disease onset, and patients without clinical outcome information were excluded. Hence, of 1840 patients registered in the PodoNet Registry, 1354 unrelated children ages 3 months old to 20 years old at disease onset with available longitudinal clinical information were selected (Figure 1). In case of several registered family members, one representative family member was randomly selected and included into the analyses. The included patients were treated at 62 centers in 21 countries. In 612 patients, sufficient information was available to evaluate the response to different immunosuppressive treatment strategies within 12 months after disease onset (Figure 1, Table 1).
The prevalence of genetic disease was 20.2% in the analysis cohort, from which CNS was excluded. Disease-causing gene variants were primarily identified by Sanger sequencing of individual genes in 107 of 607 patients and NGS of 30 podocytopathy-associated genes in 105 of 457 patients.
IIS therapies used for first-, second-, and third-line treatment after confirming steroid resistance (persistent nephrotic-range proteinuria after 4-week treatment with oral prednisone at 60 mg/m2 per day) included intravenous steroid pulses, CNIs, MMF, CNI combined with MMF, oral or intravenous CPH, and rituximab.
The diagnosis of steroid resistance and the response to IIS were evaluated according to a standardized set of criteria taking into account changes in proteinuria and serum albumin.
As previously defined,10 complete remission after IIS was diagnosed in case of proteinuria reduction to <100 mg/m2 24-hour protein excretion, <0.2 mg/mg protein-to-creatinine ratio in spot urine (if age <2 years old: <0.5 mg/mg), a negative dipstick reading, or serum albumin >30 g/L combined with dipstick trace (+).
Partial remission was defined as persistent non-nephrotic–range proteinuria with a 24-hour protein excretion >100 mg/m2 per day but <1 g/m2 per day, urine protein-to-creatinine ratio of 0.2–2 mg/mg (if age <2 years old: 0.5–2 mg/mg), dipstick 1+ in combination with serum albumin >30 g/L, or dipstick trace (+) in combination with serum albumin <30 g/L.
Lack of remission was defined as persistent nephrotic-range proteinuria as defined by 24-hour protein excretion ≥1 g/m2 per day, urine protein-to-creatinine ratio of >2 mg/mg, dipstick 2+ or greater, or dipstick 1+ with serum albumin ≤30 g/L.
Because the propensity of achieving a favorable response to IIS treatment may depend on the duration of IIS treatment and disease, the evaluation of IIS responsiveness was limited to the first year after disease onset to minimize potential bias. The evaluation time window to assess responsiveness to an IIS protocol included the exposure time plus the first 6 weeks after drug discontinuation if no medications other than oral steroids and/or RAAS antagonists were applied during that period.
In patients who received more than one immunosuppressive treatment in the first year, the most efficacious treatment and the best antiproteinuric response were considered to classify IIS responsiveness. ESRD was defined by attainment of CKD stage 5 and/or start of RRT.
Statistical Analyses
Throughout the manuscript, data are given as medians (interquartile ranges) or percentages relative to all patients with available information regarding the item of interest. Kaplan–Meier analysis and log-rank tests were used to analyze time to ESRD according to IIS responsiveness, hereditary disease, and histopathologic diagnosis. Confidence limits for proportions without ESRD are on the basis of normal approximation of log-log–transformed survival estimates.
Cox regression analyses were performed to identify risk factors for ESRD. The analyses were stratified by treatment center to account for potential center effects. Small units contributing <20 patients were considered as one center. Analyses were performed in an unadjusted model, a model adjusting only for hereditary disease, and a multivariate model accounting for genetic diagnosis, histopathologic diagnosis, degree of proteinuria, serum albumin, age, CKD at disease onset, and IIS responsiveness. Additional covariates tested (but without significance in any of the models) were sex and ethnicity.
Missing values for the variables CKD, proteinuria, and serum albumin at disease onset were imputed on the basis of missing at random assumption using fully conditional specification methods.39,40 Ten imputations were performed, and analysis results were pooled using rules by Rubin.41
Fully conditional specification (FCS) discriminant function, FCS logistic regression, and FCS regression method were used for imputation of CKD, proteinuria, and serum albumin, respectively. Imputation was on the basis of information on age, CKD, proteinuria, serum albumin, and closest available information as well as time to next available information. Information on cause of disease was also used for imputation of CKD and proteinuria.
P values were not adjusted for multiple comparisons due to the exploratory character of the study. SAS, version 9.4 was used for all statistical analyses.
Disclosures
None.
Supplementary Material
Acknowledgments
The PodoNet project has been made possible by support received from E-Rare (German Ministry of Education and Research), European Union (EU) Seventh Framework Programme (EURenOmics) grant 2012-305608, Polish Ministry of Science and Education grant N402631840, German Research Foundation grant Scha 477/11-1, and Scientific and Technological Research Council of Turkey grant 108S417.
The PodoNet collaborators were M.A., Lily Quiroz (Roberto del Rio Children's Hospital, Santiago de Chile, Chile), L.M.S.H., Jiří Dušek (University Hospital Motol, Prague, Czech Republic), B.R., Michel Fischbach (University Children's Hospital, Strasbourg), Tinatin Davitaia (M.Iashvili Children Central Hospital, Tbilisi, Georgia), J.G., J.O., A.M., F.S., Marianne Wigger (University Children's Hospital, Rostock, Germany), N.P., Peter Sallay (Semmelweis University, Budapest, Hungary), Alaleh Gheissari (Isfahan University of Medical Science, St. Al Zahra Hospital, Isfahan, Iran), Marina Noris (IRCCS - Istituto di Richerche Farmacologiche “Mario Negri” and Azienda Ospedaliera Papa Giovanni XXXI, Bergamo, Italy), Andrea Pasini (S.Orsola-Malpighi Hospital, Bologna, Italy), Gian Marco Ghiggeri (Istituto Giannina Gaslini, Genoa, and University of Parma, Italy), Gianluigi Ardissino (IRCCS Ca'Granda, Osepdale Maggiore Policlinico, Milano, Italy), Elisa Benetti (Hospital of Padua, Italy), F.E., Bilal Aoun (Rafic Hariri University Hospital, Beirut, Lebanon), Pauline Abou-Jaoudé (Notre Dame de Secours University Hospital, Byblos, Lebanon), A.J., A.W., Ewa Gacka (Pediatrics and Oncology Center, Chorzow, Poland), A.Z., D.D., M.T., Małgorzata Stańczyk (Polish Mothers Memorial Hospital Research Institute, Lodz, Poland), H.B., Magdalena Silska (Poznan University of Medical Sciences, Poznan, Poland), Tomasz Urasinski (Pomeranian Academy of Medicine, Szczecin, Poland), A.F.-A., Joanna Ksiazek (Centrum Zdrowia Dziecka, Warsaw, Poland), E.K.-M., Anna Medynska (Medical University, Wroclaw, Poland), M.S., Alberto Caldas Afonso (Hospital S. Joao Pediatrics, Porto, Portugal), H.J., Amira Peco-Antic (University Children's Hospital, Belgrade, Serbia), R.B., Rafael T. Krmar (Karolinska University Hospital, Stockholm, Sweden), Giacomo D. Simonetti (University Children's Hospital, Bern, Switzerland), B.S., A.A., A.B., E.B., Nilgun Cakar (Diskapi Children's Hospital, Ankara, Turkey), O.E., Birsin Özcakar (Ankara University Medical School, Ankara, Turkey), F.O., Onur Sakallioglu (Gulhane Military Academy of Medicine, Ankara, Turkey), Oguz Soylemezoglu (Gazi University Hospital, Ankara, Turkey), Sema Akman (Akdeniz University, Antalya, Turkey), Faysal Gok (Gulhane Military Academy of Medicine, Gulhane, Turkey), S.C., Cengiz Candan (Gaztepe Hospital, Istanbul, Turkey), A.Y., S.M., Ipek Akil (Celal Bayar University, Manisa, Turkey), Pelin Ertan (Celal Bayar University, Manisa, Turkey), Ozan Özkaya (Ondokuz Mayis University, Samsun, Turkey), Mukaddes Kalyoncu (Karadeniz University, Trabzon, Turkey), E.S., Entesar Alhammadi (Al Qassimi Hospital, Sharjah, United Arab Emirates), and Roman Sobko (Western Ukrainian Specialized Medical Center, Lviv, Ukraine).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016101121/-/DCSupplemental.
Contributor Information
Collaborators: M Azocar, Lily Quiroz, LM Serna Higuita, Jiří Dušek, B Ranchin, Michel Fischbach, Tinatin Davitaia, J Gellerman, J Oh, A Melk, F Schaefer, Marianne Wigger, N Printza, Peter Sallay, Alaleh Gheissari, Marina Noris, Andrea Pasini, Gian Marco Ghiggeri, Gianluigi Ardissino, Elisa Benetti, F Emma, Bilal Aoun, Pauline Abou-Jaoudé, A Jankauskiene, A Wasilewska, Ewa Gacka, A Zurowska, D Drozdz, M Tkaczyk, Małgorzata Stańczyk Lodz, H Borzecka, Magdalena Silska, Tomasz Jarmolinski, A Firszt-Adamczyk, Joanna Ksiazek, E Kuzma-Mroczkowska, Anna Medynska, M Szczepanska, Alberto Caldas Afonso, H Jardim, Amira Peco-Antic Belgrade, R Bogdanovic, Rafael T Krmar, Giacomo D Simonetti, B Saeed, A Anarat, A Balat, E Baskin, Nilgun Cakar, O Erdogan, Birsin Özcakar, F Ozaltin, Onur Sakallioglu, Oguz Soylemezoglu, Sema Akman, Faysal Gok, S Caliskan, Cengiz Candan, A Yilmaz, S Mir, Ipek Akil, Pelin Ertan, Ozan Özkaya, Mukaddes Kalyoncu, E Simkova, Entesar Alhammadi, and Roman Sobko
References
- 1.NAPRTCS : NAPRTCS 2008 Annual Report (Renal Transplantation, Dialysis, Chronic Renal Insufficiency), Rockville, MD, The Emmes Corporation, 2008 [Google Scholar]
- 2.Büscher AK, Beck BB, Melk A, Hoefele J, Kranz B, Bamborschke D, Baig S, Lange-Sperandio B, Jungraithmayr T, Weber LT, Kemper MJ, Tönshoff B, Hoyer PF, Konrad M, Weber S; German Pediatric Nephrology Association (GPN) : Rapid response to cyclosporin A and favorable renal outcome in nongenetic versus genetic steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 11: 245–253, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadowski CE, Lovric S, Ashraf S, Pabst WL, Gee HY, Kohl S, Engelmann S, Vega-Warner V, Fang H, Halbritter J, Somers MJ, Tan W, Shril S, Fessi I, Lifton RP, Bockenhauer D, El-Desoky S, Kari JA, Zenker M, Kemper MJ, Mueller D, Fathy HM, Soliman NA, Hildebrandt F; SRNS Study Group : A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol 26: 1279–1289, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cattran DC, Rao P: Long-term outcome in children and adults with classic focal segmental glomerulosclerosis. Am J Kidney Dis 32: 72–79, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Gipson DS, Chin H, Presler TP, Jennette C, Ferris ME, Massengill S, Gibson K, Thomas DB: Differential risk of remission and ESRD in childhood FSGS. Pediatr Nephrol 21: 344–349, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Paik KH, Lee BH, Cho HY, Kang HG, Ha IS, Cheong HI, Jin DK, Moon KC, Choi Y: Primary focal segmental glomerular sclerosis in children: Clinical course and prognosis. Pediatr Nephrol 22: 389–395, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Abrantes MM, Cardoso LS, Lima EM, Penido Silva JM, Diniz JS, Bambirra EA, Oliveira EA: Predictive factors of chronic kidney disease in primary focal segmental glomerulosclerosis. Pediatr Nephrol 21: 1003–1012, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Abeyagunawardena AS, Sebire NJ, Risdon RA, Dillon MJ, Rees L, Van’t Hoff W, Kumarasiri PV, Trompeter RS: Predictors of long-term outcome of children with idiopathic focal segmental glomerulosclerosis. Pediatr Nephrol 22: 215–221, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Martinelli R, Okumura AS, Pereira LJ, Rocha H: Primary focal segmental glomerulosclerosis in children: Prognostic factors. Pediatr Nephrol 16: 658–661, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Trautmann A, Bodria M, Ozaltin F, Gheisari A, Melk A, Azocar M, Anarat A, Caliskan S, Emma F, Gellermann J, Oh J, Baskin E, Ksiazek J, Remuzzi G, Erdogan O, Akman S, Dusek J, Davitaia T, Özkaya O, Papachristou F, Firszt-Adamczyk A, Urasinski T, Testa S, Krmar RT, Hyla-Klekot L, Pasini A, Özcakar ZB, Sallay P, Cakar N, Galanti M, Terzic J, Aoun B, Caldas Afonso A, Szymanik-Grzelak H, Lipska BS, Schnaidt S, Schaefer F; PodoNet Consortium : Spectrum of steroid-resistant and congenital nephrotic syndrome in children: The PodoNet registry cohort. Clin J Am Soc Nephrol 10: 592–600, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mekahli D, Liutkus A, Ranchin B, Yu A, Bessenay L, Girardin E, Van Damme-Lombaerts R, Palcoux JB, Cachat F, Lavocat MP, Bourdat-Michel G, Nobili F, Cochat P: Long-term outcome of idiopathic steroid-resistant nephrotic syndrome: A multicenter study. Pediatr Nephrol 24: 1525–1532, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Niaudet P: Pediatric Nephrology, 5th Ed, Philadelphia, Lippincott Williams and Wilkins, 2004 [Google Scholar]
- 13.Niaudet P; French Society of Pediatric Nephrology : Treatment of childhood steroid-resistant idiopathic nephrosis with a combination of cyclosporine and prednisone. J Pediatr 125: 981–986, 1994 [DOI] [PubMed] [Google Scholar]
- 14.Cattran DC, Appel GB, Hebert LA, Hunsicker LG, Pohl MA, Hoy WE, Maxwell DR, Kunis CL; North America Nephrotic Syndrome Study Group : A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. Kidney Int 56: 2220–2226, 1999 [DOI] [PubMed] [Google Scholar]
- 15.El-Husseini A, El-Basuony F, Mahmoud I, Sheashaa H, Sabry A, Hassan R, Taha N, Hassan N, Sayed-Ahmad N, Sobh M: Long-term effects of cyclosporine in children with idiopathic nephrotic syndrome: A single-centre experience. Nephrol Dial Transplant 20: 2433–2438, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Ghiggeri GM, Catarsi P, Scolari F, Caridi G, Bertelli R, Carrea A, Sanna-Cherchi S, Emma F, Allegri L, Cancarini G, Rizzoni GF, Perfumo F: Cyclosporine in patients with steroid-resistant nephrotic syndrome: An open-label, nonrandomized, retrospective study. Clin Ther 26: 1411–1418, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Hino S, Takemura T, Okada M, Murakami K, Yagi K, Fukushima K, Yoshioka K: Follow-up study of children with nephrotic syndrome treated with a long-term moderate dose of cyclosporine. Am J Kidney Dis 31: 932–939, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Inaba A, Hamasaki Y, Ishikura K, Hamada R, Sakai T, Hataya H, Komaki F, Kaneko T, Mori M, Honda M: Long-term outcome of idiopathic steroid-resistant nephrotic syndrome in children. Pediatr Nephrol 31: 425–434, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Plank C, Kalb V, Hinkes B, Hildebrandt F, Gefeller O, Rascher W; Arbeitsgemeinschaft für Pädiatrische Nephrologie : Cyclosporin A is superior to cyclophosphamide in children with steroid-resistant nephrotic syndrome-a randomized controlled multicentre trial by the Arbeitsgemeinschaft für Pädiatrische Nephrologie. Pediatr Nephrol 23: 1483–1493, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tarshish P, Tobin JN, Bernstein J, Edelmann CM Jr.: Cyclophosphamide does not benefit patients with focal segmental glomerulosclerosis. A report of the International Study of Kidney Disease in Children. Pediatr Nephrol 10: 590–593, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Bajpai A, Bagga A, Hari P, Dinda A, Srivastava RN: Intravenous cyclophosphamide in steroid-resistant nephrotic syndrome. Pediatr Nephrol 18: 351–356, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Hinkes B, Vlangos C, Heeringa S, Mucha B, Gbadegesin R, Liu J, Hasselbacher K, Ozaltin F, Hildebrandt F; APN Study Group : Specific podocin mutations correlate with age of onset in steroid-resistant nephrotic syndrome. J Am Soc Nephrol 19: 365–371, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latta K, von Schnakenburg C, Ehrich JH: A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol 16: 271–282, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Hodson EM, Willis NS, Craig JC: Interventions for idiopathic steroid-resistant nephrotic syndrome in children. Cochrane Database Syst Rev 11: CD003594, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Anonymous: Prospective, controlled trial of cyclophosphamide therapy in children with nephrotic syndrome. Report of the International study of Kidney Disease in Children. Lancet 2: 423–427, 1974 [PubMed] [Google Scholar]
- 26.Gargah TT, Lakhoua MR: Mycophenolate mofetil in treatment of childhood steroid-resistant nephrotic syndrome. J Nephrol 24: 203–207, 2011 [DOI] [PubMed] [Google Scholar]
- 27.de Mello VR, Rodrigues MT, Mastrocinque TH, Martins SP, de Andrade OV, Guidoni EB, Scheffer DK, Martini Filho D, Toporovski J, Benini V: Mycophenolate mofetil in children with steroid/cyclophosphamide-resistant nephrotic syndrome. Pediatr Nephrol 25: 453–460, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Bagga A, Mudigoudar BD, Hari P, Vasudev V: Enalapril dosage in steroid-resistant nephrotic syndrome. Pediatr Nephrol 19: 45–50, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Duan C, He J, Wu T, Xun M, Zhang Y, Yin Y: Mycophenolate mofetil therapy for children with steroid-resistant nephrotic syndrome. Pediatr Nephrol 25: 883–888, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gellermann J, Stefanidis CJ, Mitsioni A, Querfeld U: Successful treatment of steroid-resistant nephrotic syndrome associated with WT1 mutations. Pediatr Nephrol 25: 1285–1289, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, Zalewski I, Imm A, Ruf EM, Mucha B, Bagga A, Neuhaus T, Fuchshuber A, Bakkaloglu A, Hildebrandt F; Arbeitsgemeinschaft Für Pädiatrische Nephrologie Study Group : Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol 15: 722–732, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Caridi G, Bertelli R, Di Duca M, Dagnino M, Emma F, Onetti Muda A, Scolari F, Miglietti N, Mazzucco G, Murer L, Carrea A, Massella L, Rizzoni G, Perfumo F, Ghiggeri GM: Broadening the spectrum of diseases related to podocin mutations. J Am Soc Nephrol 14: 1278–1286, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Frishberg Y, Rinat C, Megged O, Shapira E, Feinstein S, Raas-Rothschild A: Mutations in NPHS2 encoding podocin are a prevalent cause of steroid-resistant nephrotic syndrome among Israeli-Arab children. J Am Soc Nephrol 13: 400–405, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Weber S, Gribouval O, Esquivel EL, Morinière V, Tête MJ, Legendre C, Niaudet P, Antignac C: NPHS2 mutation analysis shows genetic heterogeneity of steroid-resistant nephrotic syndrome and low post-transplant recurrence. Kidney Int 66: 571–579, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Lipska BS, Ranchin B, Iatropoulos P, Gellermann J, Melk A, Ozaltin F, Caridi G, Seeman T, Tory K, Jankauskiene A, Zurowska A, Szczepanska M, Wasilewska A, Harambat J, Trautmann A, Peco-Antic A, Borzecka H, Moczulska A, Saeed B, Bogdanovic R, Kalyoncu M, Simkova E, Erdogan O, Vrljicak K, Teixeira A, Azocar M, Schaefer F; PodoNet Consortium : Genotype-phenotype associations in WT1 glomerulopathy. Kidney Int 85: 1169–1178, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Malina M, Cinek O, Janda J, Seeman T: Partial remission with cyclosporine A in a patient with nephrotic syndrome due to NPHS2 mutation. Pediatr Nephrol 24: 2051–2053, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Choudhry S, Bagga A, Hari P, Sharma S, Kalaivani M, Dinda A: Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: A randomized controlled trial. Am J Kidney Dis 53: 760–769, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Brand JPL: Development, Implementation, and Evaluation of Multiple Imputation Strategies for the Statistical Analysis of Incomplete Data Sets. PhD Thesis, Erasmus University Rotterdam, Netherlands, 1999 [Google Scholar]
- 40.van Buuren S: Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 16: 219–242, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Rubin D: Multiple Imputation for Nonresponse Surveys, New York, John Wiley & Sons, Inc., 1987 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.