Abstract
Biopsy findings at the time of procurement of deceased donor kidneys remain the most common reason cited for kidney discard. To determine the value of renal allograft histology in predicting outcomes, we evaluated the significance of histologic findings, read by experienced renal pathologists, in 975 postreperfusion biopsy specimens collected from 2005 to 2009 after living donor (n=427) or deceased donor (n=548) renal transplant. We evaluated specimens for the degree of glomerulosclerosis, interstitial fibrosis and tubular atrophy, and vascular disease; specimens with a score of 0 or 1 (scale, 0–3) for each parameter were considered optimal. Overall, 66.3% of living donor kidneys and 50.7% of deceased donor kidneys received an optimal histology score (P<0.001). Irrespective of donor status, suboptimal kidneys came from older donors with a higher incidence of diabetes mellitus, hypertension, and obesity and a higher mean kidney donor risk index (all P<0.001). Death-censored outcomes after transplant differed significantly between optimal and suboptimal kidneys only in the deceased donor transplants (P=0.02). Regardless of histologic classification, outcomes with deceased donor kidneys were inferior to outcomes with living donor kidneys. However, 73.2% of deceased donor kidneys with suboptimal histology remained functional at 5 years. Our findings suggest that histologic findings on postreperfusion biopsy associate with outcomes after deceased donor but not living donor renal transplants, thus donor death and organ preservation–related factors may be of greater prognostic importance. Discarding donated kidneys on the basis of histologic factors may be inappropriate and merits further study.
Keywords: transplant pathology, transplant outcomes, renal transplantation, kidney histology, post-reperfusion biopsy
Discard rates for deceased donor kidneys in the United States are at an all-time high, and the most common reason cited for discard is biopsy findings at the time of procurement.1–4 As a result, there is considerable interest in the true value of renal histologic findings in predicting long-term outcomes after renal transplantation.5 Most of the studies that have analyzed the value of histologic findings in predicting long-term post-transplant outcomes have been relatively small cohort studies or have been limited in the biopsy sample features examined.6,7 Additionally, few studies have attempted to link histologic findings with subsequent transplant function.7,8 Although small studies have suggested that the value of histologic findings noted on frozen section before implantation is predictive of outcomes, a recent systematic review of preimplantation biopsies underscored both the poor quality of the data in the literature and the poor association of findings with outcomes.6 The majority of analyses have been confounded by the use of frozen tissue, wedge biopsies versus needle biopsies, and by pathologists with limited expertise in the evaluation of renal tissue, all of which have contributed to the inability to draw definitive conclusions.6,9–11 We attempt to determine the ability of postreperfusion needle core renal allograft biopsies, optimally processed with formalin-fixed, paraffin embedded sections and read/classified (Table 1) by experienced renal pathologists, to predict post-transplant renal function as well as early and long-term outcomes after living and deceased donor transplantation (LDRT and DDRT).
Table 1.
Histologic classification and scoringa
| Assigned Score | GS, % | IFTA, % | Vascular Disease |
|---|---|---|---|
| 0 | <5 | <5 | None |
| 1 | 5–10 | 5–10 | Mild |
| 2 | 11–25 | 11–25 | Moderate |
| 3 | >25 | >25 | Severe |
Optimal histology is defined by a score of 0–1 for each of the three histologic parameters. Suboptimal histology is defined by a score of 2 or more for at least one of the three histologic parameters. Unfavorable histology is defined by a score of 2 or more for each of the three histologic parameters.
GS and IFTA were graded on the basis of percentage involvement. The degree of vascular disease was a composite assessment of arteries and arterioles, focused on blood vessels with the most severe changes, and was on the basis of the degree of intimal expansion as compared with the thickness of the media. When the degree of intimal expansion was less than the thickness of the media, a grade of mild was assigned. Moderate vascular disease was defined as intimal thickening roughly equivalent to medial thickness, whereas a grade of severe was given to cases in which the intimal expansion exceeded the thickness of the media.
Results
Our final cohort of 975 patients included 548 (56.2%) DDRT and 427 (43.8%) LDRT kidneys (Table 2). Compared with the DDRT group, the LDRT donors were older (43.4 versus 40.8 years; P<0.01), less likely to be male (P<0.001) or black (P=0.001), and had a significantly lower serum creatinine (0.88 versus 1.52 mg/dl; P<0.001) at the time of organ donation or procurement (Table 2). Consistent with our selection criteria at Columbia University Medical Center (CUMC), living donors did not have a history of diabetes and only a small minority (2.3%) had a history of well controlled hypertension using a single antihypertensive agent. At the time of organ donation there was no significant difference in body mass index (BMI) among living and deceased donors (27.5 versus 26.8 kg/m2; P=0.12); however, a higher proportion of deceased donors were obese (30.8% versus 19.4%; P<0.001). In the DDRT group, recipients were more likely to be older (53.4 versus 47.5 years; P<0.001), black (22.7% versus 9.8%; P<0.001), and diabetic (35.1% versus 25.5%; P=0.002) when compared with living donor transplant recipients. Deceased donor kidneys had a significantly higher prevalence of moderate-to-severe vascular disease (35.2% versus 20.6%), glomerulosclerosis (GS) (9.2% versus 5.1%), acute tubular injury (ATI) (46.7% versus 10.3%), and interstitial fibrosis and tubular atrophy (IFTA) ≥5% (15.7% versus 3.7%) than LDRT kidneys (all P<0.001; Table 2).
Table 2.
Comparison of donor and recipient characteristics among kidneys from living or deceased donors and with optimal or suboptimal histology at CUMC, 2005–2009 (n=975)
| Characteristic | Total | Donor Type | P Value | Donor Histology | P Value | ||
|---|---|---|---|---|---|---|---|
| Deceased | Living | Suboptimal | Optimal | ||||
| N (%) | 975 (100.0) | 548 (56.2) | 427 (43.8) | 414 (42.5) | 561 (57.5) | ||
| Donor characteristics | |||||||
| Age, yr | 42.0±15.9 | 40.8±18.3 | 43.4±11.8 | <0.01 | 50.4±11.4 | 35.7±15.8 | <0.001 |
| Female | 46.4 | 39.6 | 55.0 | <0.001 | 50.7 | 43.1 | 0.02 |
| Black | 15.0 | 18.4 | 10.5 | 0.001 | 16.9 | 13.5 | 0.15 |
| Final creatininea, mg/dl | 1.23±0.91 | 1.52±1.12 | 0.88±0.19 | <0.001 | 1.30±0.83 | 1.19±0.96 | 0.09 |
| BMI≥30 kg/m2 | 25.9 | 30.8 | 19.4 | <0.001 | 33.7 | 20.1 | <0.001 |
| Hypertension | 22.8 | 38.7 | 2.3 | <0.001 | 40.8 | 9.4 | <0.001 |
| Diabetes | 5.0 | 8.9 | 0.0 | <0.001 | 9.1 | 2.0 | <0.001 |
| ECDb | 27.7 | 27.7 | NA | NA | 48.9 | 7.2 | <0.001 |
| KDRI | 1.45±0.43 | 1.45±0.43 | NA | NA | 1.65±0.40 | 1.25±0.35 | <0.001 |
| Recipient characteristics | |||||||
| Age at transplant, yr | 50.8±14.6 | 53.4±13.8 | 47.5±15.0 | <0.001 | 53.2±14.5 | 49.0±14.5 | <0.001 |
| Female | 39.5 | 37.6 | 41.9 | 0.17 | 37.2 | 41.2 | 0.21 |
| Black | 17.1 | 22.7 | 9.8 | <0.001 | 18.7 | 15.9 | 0.25 |
| BMI≥30 kg/m2 | 24.3 | 25.9 | 22.2 | 0.17 | 26.3 | 22.9 | 0.22 |
| Hypertension | 86.1 | 86.5 | 85.5 | 0.67 | 88.2 | 84.5 | 0.10 |
| Diabetes | 30.9 | 35.1 | 25.5 | 0.002 | 34.7 | 28.1 | 0.03 |
| Transplant characteristics | |||||||
| HLA mismatches, no. | 4.0±1.6 | 4.5±1.2 | 3.3±1.7 | <0.001 | 4.1±1.5 | 3.8±1.6 | 0.002 |
| CIT, hb | 30.9±10.6 | 30.9±10.6 | NA | NA | 32.3±9.5 | 29.6±11.5 | 0.004 |
| Previous kidney transplant | 19.3 | 23.2 | 14.3 | 0.001 | 17.1 | 20.9 | 0.15 |
| Biopsy findings | |||||||
| Number of glomeruli | 20.0±12.2 | 20.9±13.2 | 18.9±10.6 | 0.01 | 18.5±10.8 | 21.1±13.0 | <0.001 |
| Glomeruli sclerosed, % | 7.4±10.6 | 9.2±12.4 | 5.1±7.1 | <0.001 | 14.8±12.6 | 1.9±3.0 | <0.001 |
| ATI presence | 30.8 | 46.7 | 10.3 | <0.001 | 37.2 | 26.0 | <0.001 |
| Vascular disease moderate/severe | 28.8 | 35.2 | 20.6 | <0.001 | 67.9 | 0.0 | <0.001 |
| IFTA with ≥5% presence | 10.5 | 15.7 | 3.7 | <0.001 | 23.9 | 0.5 | <0.001 |
| Transplant outcomes | |||||||
| Median follow-up, mo (IQR) | 79.9 (39.1) | 77.1 (57.1) | 84.8 (33.4) | <0.001 | 75.7 (25.4) | 83.1 (34.9) | <0.001 |
| Graft status | |||||||
| Functioning, % | 67.1 | 57.3 | 79.3 | <0.001 | 60.3 | 72.0 | <0.001 |
| Dead, % | 11.2 | 14.1 | 7.5 | 14.2 | 9.0 | ||
| Fail, % | 21.8 | 28.6 | 13.2 | 25.5 | 19.0 | ||
| 5-yr graft survival, % | 83.8 | 77.6 | 91.4 | <0.001 | 79.7 | 86.7 | 0.004 |
| DGF | 25.0 | 40.7 | 4.9 | <0.001 | 32.6 | 19.4 | <0.001 |
| Most recent creatininec, mg/dl | 1.61±0.86 | 1.70±0.94 | 1.51±0.61 | 0.003 | 1.55±0.58 | 1.53±0.85 | 0.78 |
Continuous variables are shown as mean ± standard deviation; categorical variables are reported as column percentages; median follow up is reported as median (interquartile range). ECD, expanded criteria donor; KDRI, kidney donor risk index; HLA, histocompatibility leukocyte antigen; CIT, cold ischemia time; IQR, interquartile range; DGF, delayed graft function.
Most recent creatinine before donation for living donors or terminal creatinine for deceased donors.
Represented for deceased donor transplants only (n=548).
For functioning allografts only.
Utilizing the criteria in Table 1, 57.5% of kidneys in our cohort met the definition for optimal histology (Table 2), including 66.3% of LDRT kidneys versus 50.7% of the DDRT kidneys (Table 3; P<0.001). A direct comparison of optimal versus suboptimal kidneys, irrespective of donor status, demonstrated that suboptimal kidneys come from older donors with a higher incidence of diabetes mellitus, hypertension, and obesity and a higher Kidney Donor Risk Index (KDRI) (all P<0.001; Table 2). Consistent with the definitions employed and irrespective of donor status, suboptimal kidneys had significantly more GS, IFTA, ATI, and vascular disease (all P<0.001; Table 2). The difference was particularly large for vascular disease, which was moderate-to-severe in 67.9% of suboptimal kidneys but, by definition, absent or mild in all optimal kidneys. As such, vascular disease was the most common basis for classifying a kidney as suboptimal. Not surprisingly, optimal histology was associated with lower rates of delayed graft function (P<0.001; Table 2) and better long-term allograft outcomes (P<0.001; Table 2).
Table 3.
Donor, recipient, transplant, and histologic characteristics of the cohort stratified by histology status and donor type, 2005–2009 (n=975)
| Characteristic | Total | Suboptimal | Optimal | P Value | ||
|---|---|---|---|---|---|---|
| Deceased Donor Histology | Living Donor Histology | Deceased Donor Histology | Living Donor Histology | |||
| N (%) | 975 (100.0) | 270 (27.7) | 144 (14.8) | 278 (28.5) | 283 (29.0) | |
| Donor characteristics | ||||||
| Age, yr | 42.0±15.9 | 51.4±11.6a | 48.6±10.7b | 30.5±17.9 | 40.8±11.5 | <0.001 |
| Female, % | 46.4 | 46.3a | 59.0 | 33.1 | 53.0 | <0.001 |
| Black, % | 15.0 | 20.4 | 10.4 | 16.5 | 10.6 | 0.004 |
| Final creatininec, mg/dl | 1.23±0.91 | 1.52±0.94 | 0.86±0.19 | 1.51±1.28 | 0.88±0.19 | <0.001 |
| BMI≥30 kg/m2, % | 25.9 | 40.4a | 20.7 | 21.6 | 18.7 | <0.001 |
| Hypertension, % | 22.8 | 60.4a | 4.2 | 17.6 | 1.4 | <0.001 |
| Diabetes, % | 5.0 | 14.0a | 0.0 | 4.0 | 0.0 | <0.001 |
| ECDd, % | 27.7 | 48.9a | NA | 7.2 | NA | <0.001 |
| KDRI | 1.45±0.43 | 1.65±0.40a | NA | 1.25±0.35 | NA | <0.001 |
| Recipient characteristics | ||||||
| Age at transplant, yr | 50.8±14.6 | 55.5±13.2a | 49.1±15.9 | 51.3±14.1 | 46.7±14.4 | <0.001 |
| Female, % | 39.5 | 37.4 | 36.8 | 37.8 | 44.5 | 0.23 |
| Black, % | 17.1 | 24.6 | 7.6 | 20.9 | 11.0 | <0.001 |
| BMI≥30 kg/m2, % | 24.3 | 28.1 | 22.9 | 23.8 | 21.9 | 0.37 |
| Hypertension, % | 86.1 | 87.2 | 90.1 | 85.8 | 83.2 | 0.25 |
| Diabetes, % | 30.9 | 36.8 | 30.7 | 33.3 | 22.9 | 0.004 |
| Transplant characteristics | ||||||
| HLA mismatches, no. | 4.0±1.6 | 4.6±1.1 | 3.4±1.7 | 4.4±1.3 | 3.2±1.7 | <0.001 |
| CIT, hd | 30.9±10.6 | 32.3±9.5a | NA | 29.6±11.5 | NA | NA |
| Previous kidney transplant, % | 19.3 | 20.7 | 10.4 | 25.5 | 16.3 | 0.001 |
| Biopsy findings | ||||||
| Number of glomeruli | 20.0±12.2 | 18.7±11.0a | 18.0±10.4 | 23.0±14.7 | 19.3±10.7 | 0.003 |
| Glomeruli sclerosed, % | 7.4±10.6 | 16.7±13.9a | 11.1±8.7b | 1.8±2.9 | 2.1±3.0 | <0.001 |
| ATI presence, % | 30.8 | 51.1e | 11.1 | 42.4 | 9.9 | <0.001 |
| Vascular disease present as moderate or severe, % | 28.8 | 71.5a | 61.1b | 0.0 | 0.0 | <0.001 |
| IFTA with ≥5% presence, % | 10.5 | 30.7a | 11.1b | 1.1 | 0.0 | <0.001 |
| Transplant outcomes | ||||||
| Median follow-up, mo (IQR) | 79.9 (39.1) | 71.3 (60.0)a | 84.9 (34.5) | 80.5 (46.4) | 84.5 (33.1) | <0.001 |
| Graft status | ||||||
| Functioning, % | 67.1 | 52.8 | 74.1 | 61.7 | 82.0 | <0.001 |
| Dead, % | 11.2 | 15.1 | 12.6 | 16.1 | 5.0 | |
| Fail, % | 21.8 | 32.1 | 13.3 | 25.2 | 13.1 | |
| 5-yr graft survival, % | 83.8 | 73.2e | 91.2 | 81.7 | 91.4 | <0.001 |
| DGF | 25.0 | 47.8a | 4.2 | 33.8 | 5.3 | <0.001 |
| Most recent creatininef, mg/dl | 1.61±0.86 | 2.00±1.13a | 1.55±0.58 | 1.43±0.64 | 1.53±0.85 | <0.001 |
Continuous variables are shown as mean ± standard deviation; categorical variables are reported as column percentages; median follow up is reported as median (interquartile range). ECD, expanded criteria donor; NA, Not applicable; KDRI, kidney donor risk index; HLA, histocompatibility leukocyte antigen; CIT, cold ischemia time; IQR, interquartile range; DGF, delayed graft function.
Comparison of optimal to suboptimal DDRT significantly different, P<0.001.
Comparison of optimal to suboptimal LDRT significantly different, P<0.001.
Most recent creatinine before donation for living donors or terminal creatinine for deceased donors.
Represented for deceased donor transplants only (n=548).
Comparison of optimal to suboptimal DDRT significantly different, P<0.05.
For functioning allografts only.
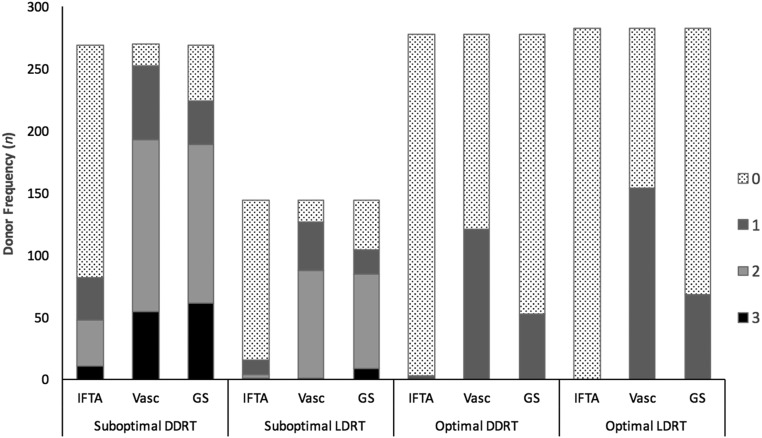
Donor kidneys subsequently were classified into four groups on the basis of donor type and histology: suboptimal deceased donor; suboptimal living donor; optimal deceased donor; or optimal living donor (Figure 1, Table 3).
Figure 1.
Suboptimal DDRT and LDRT kidneys had significantly more IFTA, vascular disease, and GS than optimal DDRT and LDRT kidneys, respectively. For suboptimal DDRT, n=270; for suboptimal LDRT, n=144; for optimal DDRT, n=278; and for optimal LDRT, n=283. IFTA, interstitial fibrosis and tubular atrophy; Vasc, vascular disease; GS, glomerulosclerosis.
Deceased Donor Transplants
Consistent with our definitions, suboptimal DDRT kidneys had significantly more GS, vascular disease, IFTA, and ATI (Figure 1, Table 3) than DDRT kidneys with optimal histology. Suboptimal deceased donor kidneys tended to come from older, more often female, donors who were more likely to have diabetes, hypertension, and be obese (all P values <0.001; Table 3). Additionally, suboptimal deceased donor kidneys were more likely to come from extended criteria donors and had higher KDRI scores and longer cold ischemia times than deceased donor kidneys with optimal histology (P=0.004; Table 3). Recipients of suboptimal deceased donor kidneys were similar to those who received optimal kidneys with respect to sex, race, prior transplants, the prevalence of diabetes, hypertension, and obesity; however, recipients of suboptimal deceased donor kidneys were significantly older (P<0.001).
For deceased donor renal transplants, the degree of GS noted on biopsies was associated with donor age, BMI, history of hypertension, and diabetes on univariate analysis (all P values ≤0.001); however, on multivariable analysis the association between GS and donor diabetes was no longer significant (P=0.35) (Supplemental Table 1A). On univariate analysis, increased odds of ATI were only significantly seen in black donors (P=0.03) and donors with higher terminal creatinine (P<0.001) (Supplemental Table 1B). On multivariable analysis, only terminal creatinine was predictive of ATI (adjusted odds ratio [aOR], 1.68; 95% CI 1.39 to 2.02; aOR, 1.68 95% confidence interval [95% CI], 1.39 - 2.02; P<0.001). IFTA was significantly associated with donor age, donor BMI, a history of diabetes or hypertension (P values <0.001), and longer cold ischemia time (P=0.03) on univariate analysis; however, on multivariable analysis only the association with donor age (aOR, 1.05; 95% CI, 1.03 to 1.08; P<0.001) and hypertension (aOR, 2.43; 95% CI, 1.35 to 4.38; P=0.003) persisted (Supplemental Table 1C). The presence of moderate-to-severe vascular disease was significantly associated with donor age, male sex, and BMI, as well as a history of diabetes and hypertension (P values <0.001). On multivariable analysis, the association with donor age, BMI, and hypertension persisted (Supplemental Table 1D).
The ability of pathologic parameters at the time of transplantation to predict long-term outcome (as reflected by serum creatinine) is provided in Table 4. Among DDRT, only GS was predictive of final serum creatinine at a statistically significant level. The presence of moderate-to-severe vascular disease, IFTA>5%, or ATI was associated with a higher serum creatinine level at last follow-up, but none reached the level of statistical significance.
Table 4.
Most recent serum creatinine among kidney transplant recipients with functioning allografts at Columbia University Medical Center, 2005–2009
| Pathologic Finding at Time of Transplantation | Deceased Donor Serum Creatinine (mg/dl) | P Value | Living Donor Serum Creatinine (mg/dl) | P Value |
|---|---|---|---|---|
| ATI absent | 2.51±2.36 (98.5±16.5) | 0.45 | 1.87±1.69 (91.6±17.9) | 0.61 |
| ATI present | 2.67±2.34 (89±16.1)a | 1.73±1.01 (85.9±21.2) | ||
| Vascular disease absent/mild | 2.55±2.43 (96±16.8) | 0.63 | 1.84±1.67 (91.2±17.7) | 0.69 |
| Vascular disease moderate/severe | 2.65±2.21 (90.1±16.1)a | 1.92±1.45 (89.7±21.2) | ||
| IFTA<5% | 2.50±2.35 (94.4±17) | 0.05 | 1.85±1.64 (90.9±17.9) | 0.60 |
| IFTA>5% | 3.07±2.32 (91.7±16.3) | 2.09±1.23 (91.7±28.4) | ||
| GS<5% | 2.27±2.29 (95.5±16.9) | 0.003 | 1.87±1.84 (91.2±18.3) | 0.87 |
| GS>5% | 2.90±2.39 (92.4±17) | 1.84±1.25 (90.6±18.4) | ||
| GS<20% | 2.43±2.31 (94.2±16.9) | 0.001 | 1.87±1.68 (91.2±18.5) | 0.49 |
| GS>20% | 3.42±2.47 (92.7±17.3) | 1.63±0.43 (88±15.8) |
Time to follow-up in months is listed in parentheses for each group as mean±SD.
Durations of follow-up that are statistically significant at a P<0.05.
After adjusting for the KDRI, allograft survival using deceased donor optimal histology kidneys was not significantly better than that with suboptimal histology (hazard ratio [HR], 0.91; 95% CI, 0.63 to 1.31; P=0.62). Similarly, the presence of vascular disease, >5% IFTA, and ATI were not associated with worse long-term outcomes after adjusting for KDRI. However, the percent GS remained significantly associated with shorter graft survival after adjustment for KDRI (HR, 1.14 for each 10% rise in GS; 95% CI, 1.00 to 1.29; P=0.02).
Living Donor Transplants
As expected, suboptimal living donor kidneys had significantly greater GS, IFTA, ATI, and vascular disease than optimal LDRT kidneys; however, the observed difference in the presence of ATI between both groups was not clinically meaningful (11.1% versus 9.9%; Figure 1, Table 3). Donors of suboptimal LDRT kidneys were older but otherwise similar to optimal donors. The overall prevalence of pathologic findings on postimplantation biopsies of kidneys from LDRT was low, although moderate-to-severe vascular disease was present in 20.6%, including 61.1% with suboptimal histology and, by definition, none of the patients with optimal histology (Table 3). There were no significant differences between the key clinical characteristics of recipients of optimal and suboptimal LDRT kidneys (Table 3). Among LDRT kidneys, increasing donor age was associated with increasing GS (odds ratio [OR], 1.05; 95% CI, 1.01 to 1.09; P=0.01; Supplemental Table 2A), IFTA (aOR, 1.07; 95% CI, 1.02 to 1.13; P=0.004; Supplemental Table 2C), and moderate-to-severe vascular disease (aOR, 1.07; 95% CI, 1.04 to 1.09; P<0.001; Supplemental Table 2D), although only the latter two associations persisted on multivariate analysis. On both univariate and multivariable analysis, there was increased odds of IFTA among black donors compared with donors of other races (OR, 4.22; 95% CI, 1.40 to 12.75; P=0.01; and aOR, 5.32; 95% CI, 1.42 to 19.96; P=0.01). IFTA was also more likely to be observed among donors with higher BMIs (OR, 1.10; 95% CI, 1.03 to 1.16; P=0.002; and aOR, 1.10; 95% CI, 1.02 to 1.17; P=0.01; Supplemental Table 2C). Pathologic parameters in the postreperfusion biopsy samples were not predictive of serum creatinine in the long-term among LDRT recipients (Table 4).
Allograft Outcome
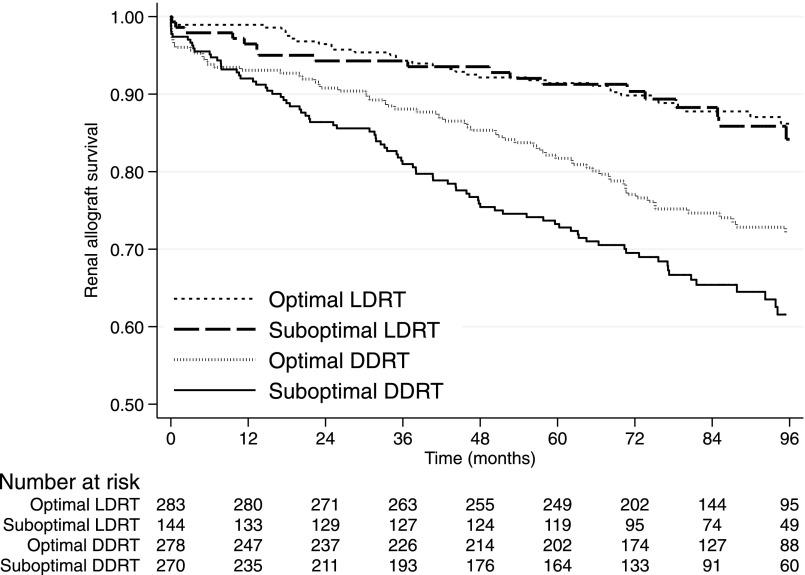
For LDRT, optimal and suboptimal histology had similar death-censored allograft outcomes (log rank=0.04, P=0.84; Figure 2). In contrast, there was a significant difference in outcome for optimal versus suboptimal kidneys after DDRT (log rank=5.06, P=0.02), both of which were inferior to outcomes with LDRT kidneys. Specifically and notably, outcomes were better for LDRT kidneys with suboptimal histology than for DDRT kidneys with optimal histology. The differences in outcomes among donor type and histologic status persisted on multivariable analysis after adjustment for race (donor and recipient), donor hypertension, recipient diabetes, and HLA mismatch (Supplemental Table 3, Table 5). Among kidneys that were still functioning at the end of follow-up, optimal DDRT had a significantly lower serum creatinine than their suboptimal counterparts (P<0.001) but there was no significant difference between the groups of LDRT (P=0.10).
Figure 2.
Kaplan–Meier curves for death-censored graft survival showing the similarity between suboptimal and optimal LDRT and the difference between optimal and suboptimal DDRT.
Table 5.
Univariate and multivariable analysis for death-censored graft survival
| Parameter | Univariate | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | SD Error | P Value | 95% CI | HR | SD Error | P Value | 95% CI | |||
| Donor age | 1.00 | 0.004 | 0.81 | 0.99 | 1.01 | |||||
| Donor sex (male) | 1.12 | 0.155 | 0.43 | 0.85 | 1.47 | |||||
| Donor race (black) | 1.59 | 0.269 | 0.01 | 1.14 | 2.21 | 1.35 | 0.237 | 0.10 | 0.95 | 1.90 |
| Obese donor | 1.27 | 0.194 | 0.12 | 0.94 | 1.71 | |||||
| Donor diabetes | 1.66 | 0.444 | 0.06 | 0.98 | 2.80 | |||||
| Donor hypertension | 1.75 | 0.262 | <0.001 | 1.30 | 2.34 | 0.99 | 0.178 | 0.97 | 0.70 | 1.41 |
| KDRI | 2.01 | 0.343 | <0.001 | 1.44 | 2.81 | |||||
| Recipient age | 1.00 | 0.005 | 0.62 | 0.99 | 1.01 | |||||
| Recipient sex (male) | 0.90 | 0.126 | 0.47 | 0.69 | 1.19 | |||||
| Recipient race (black) | 1.43 | 0.239 | 0.03 | 1.03 | 1.99 | 1.04 | 0.185 | 0.81 | 0.74 | 1.48 |
| Obese recipient | 1.17 | 0.183 | 0.33 | 0.86 | 1.59 | |||||
| Recipient diabetes | 0.97 | 0.150 | 0.87 | 0.72 | 1.32 | 0.89 | 0.138 | 0.44 | 0.65 | 1.20 |
| Recipient hypertension | 0.87 | 0.169 | 0.46 | 0.59 | 1.27 | |||||
| HLA mismatch | 1.19 | 0.057 | <0.001 | 1.09 | 1.31 | 1.06 | 0.056 | 0.25 | 0.96 | 1.18 |
| CIT | 1.01 | 0.006 | 0.19 | 1.00 | 1.02 | |||||
| Optimal LDRT | Ref | |||||||||
| Suboptimal LDRT | 1.06 | 0.298 | 0.85 | 0.61 | 1.84 | 1.11 | 0.314 | 0.72 | 0.63 | 1.93 |
| Optimal DDRT | 2.13 | 0.434 | 0.000 | 1.43 | 3.18 | 1.94 | 0.422 | 0.002 | 1.26 | 2.97 |
| Suboptimal DDRT | 3.08 | 0.608 | 0.000 | 2.10 | 4.54 | 2.86 | 0.673 | <0.001 | 1.80 | 4.54 |
95% CI, 95% confidence interval; HLA, histocompatibility leukocyte antigen; CIT, cold ischemia time; Ref, reference; LRT, living donor recipient transplant.
Unfavorable Histology
We assessed the effect of unfavorable histologic changes, defined by the presence of ≥11% GS, ≥11% IFTA, and moderate or severe vascular disease. Unfavorable histology was identified in 3.4% of our cohort, including 31 deceased donor kidneys and three living donor kidneys, and was associated with 1- and 5-year graft survivals of 79.4% (95% CI, 61.6% to 81.6%) and 50% (95% CI, 32.4% to 65.2%), respectively.
Discussion
The true value of pre- and postimplantation biopsies has been the focus of intense debate, especially given the rising number of potentially transplantable kidneys that are discarded—apparently on account of adverse histologic findings on a preimplantation biopsy and in the absence of an association between preimplantation histologic features and outcomes.4–6,12,13 Understanding the relative value of histologic abnormalities on renal biopsy and their effect on long-term outcomes is essential to being able to use this information appropriately to guide clinical decision-making; particularly given conflicting results on the value of GS, IFTA, and vascular changes.6,13–18 Our analysis uses postimplantation needle biopsies and formalin-fixed, paraffin-embedded tissue sections reviewed by experienced renal pathologists, a process that is different from how preimplantation biopsy samples are currently evaluated.9,11 In contrast, preimplantation biopsies are typically wedge biopsies that disproportionately sample the immediate subcapsular cortex, an area in which more extensive GS and tubulointerstitial scarring are seen, frequently resulting in an inadvertent overestimation of the degree of renal parenchymal scarring and thus an underestimation of the true quality of the kidney.10,17,19,20 Therefore, the evaluation process used in this analysis represents a “best case scenario” to determine the true value of histology in determining whether a kidney should or should not be utilized for transplantation.6,9
In our cohort, DDRT kidneys were significantly more likely to have suboptimal histologic features as compared with LDRT kidneys, consistent with the strict criteria that are employed in the selection of living donors by transplant centers (49.3% versus 33.7%; P<0.001). Despite thorough screening before transplantation, moderate-to-severe vascular disease was surprisingly common in LDRT kidneys, with a prevalence of 20.6%. Nonetheless, outcomes after LDRT were independent of the observed histology at the time of transplantation, suggesting that factors other than histology were the primary drivers of outcomes. As expected, LDRT kidneys were associated with better graft survival. A more interesting finding was the observation that LDRT kidneys with suboptimal histology had better outcomes than DDRT kidneys with optimal histology (Figure 2). This suggests that differences in outcome between LDRT and DDRT are primarily the result of factors unrelated to donor histology and, instead, related to the negative consequences of factors associated with donor death and ischemic organ preservation. In contrast to outcomes after LDRT, outcomes after DDRT were affected adversely by renal histology, with optimal kidneys significantly outperforming kidneys with suboptimal histology. Not surprisingly, deceased donor kidneys with suboptimal histologic findings accrued more cold ischemia time, reflecting the fact that they were harder to place, and the differences in outcomes persisted even after adjusting for the differences in cold ischemia time (HR, 1.40; 95% CI, 1.00 to 1.95; P<0.05; versus unadjusted HR, 1.43; 95% CI, 1.05 to 1.98; P=0.03). Of note, even with suboptimal histology and greater cold ischemia time experienced by these organs, 73.2% of DDRT were still functioning after 5 years (compared with 81.7% of DDRT with optimal histology). This suggests that reasonable outcomes can be achieved even with DDRT kidneys exhibiting suboptimal histology, supporting the notion that organ discards on the basis of histologic factors are likely to be inappropriate and perhaps driven by factors other than organ quality.21 Currently in the United States, over a third of kidneys available for DDRT that are discarded are purportedly discarded due to unfavorable histologic findings despite only a small fraction of kidneys appearing to have histologic changes severe enough that the risk may outweigh the benefits.3,21 These decisions are on the basis of the aforementioned suboptimal circumstances that appear to carry far less prognostic value.6,7,9
In summary, our results demonstrate the limited value of renal histologic findings on postreperfusion biopsies as a prognostic marker for allograft outcomes after LDRT but demonstrate their significant prognostic value after DDRT. Optimal DDRT kidneys appear to have better long-term outcomes than those with suboptimal histologic features even after adjusting for key donor, recipient, and transplant characteristics. However, even suboptimal histologic findings, identified on optimally processed biopsies are associated with reasonable intermediate to long-term outcomes, further supporting their use. Histologic information available from a postimplantation biopsy may help ascertain the optimal level of function that one might reasonably expect from a given allograft. Our findings also suggest that although postimplantation biopsies may provide prognostic information for DDRT, their utility for allograft outcomes in the setting of LDRT is limited.
Concise Methods
Postimplantation biopsies have been performed 1 hour postreperfusion as standard of care at CUMC’s renal transplant program since 2004. Over a 5-year period from January 1, 2005 through December 31, 2009, 1015 patients underwent DDRT or LDRT at our center, followed by a postreperfusion biopsy. After excluding ABO incompatible transplants (n=40), our final cohort of 975 transplant recipients included 427 biopsies from LDRT and 548 biopsies from DDRT. Renal biopsies were performed using an 18-gauge needle to obtain two tissue cores that were formalin-fixed, paraffin-embedded, and processed according to standard techniques, which included the use of the hematoxylin and eosin, periodic acid Schiff, trichrome, and Jones methenamine silver stains.
Biopsy Variables
Renal pathologists at CUMC reviewed the postreperfusion renal allograft biopsies and determined the degree of GS, IFTA, and vascular disease. Kidneys were classified as “optimal” if they had a score of 0 or 1 for each of the three histologic parameters (Table 1). In contrast, a score of 2 or 3 for any of the three parameters resulted in a designation of “suboptimal.” Sensitivity analyses were performed by limiting analyses to biopsy samples with ≥10 glomeruli, consistent with the Banff recommendations.22,23 The presence or absence of ATI also was documented.
Clinical Variables
We obtained recipient and donor demographics (age, sex, race), anthropometrics (height, weight, BMI), comorbidities (hypertension, diabetes), and whether recipients had received a previous renal transplant, as well as the donor terminal creatinine or creatinine at the time of donation for living donors. Recipients and donors were defined as obese if their BMI at the time of organ donation or transplantation was >30 kg/m2. Recipient status at the time of last follow-up was defined as alive with a functioning allograft, alive with a failed allograft, or dead with a functioning allograft. Analyses that used most recent creatinine as an outcome were restricted to those patients who still had an allograft available at the end of the follow-up period. Transplant-specific characteristics including total number of HLA mismatches and cold ischemia time were obtained.
We used the KDRI, which is currently part of the new Organ Procurement Transplant Network allocation policy for kidneys in the United States, as a composite measure of deceased donor organ quality.24 The KDRI is calculated using ten donor-specific clinical characteristics: age, height, weight, ethnicity, history of hypertension, history of diabetes, cause of death, serum creatinine, hepatitis C virus status, and donation after cardiac death status.24,25 KDRI is only calculated for kidneys from deceased donors.
Statistical Analyses
Categoric variables were compared using the chi-squared test and continuous variables were compared using Kruskal–Wallis across the four groups. Time-to-event analyses were performed using univariate Kaplan–Meier methods to compare the outcomes of optimal and suboptimal kidneys from both LDRT and DDRT. Cox proportional hazards regression was used for the multivariable models. All analyses were performed using Stata 13.1 (Stata Corp., College Station, TX). A P value <0.05 was deemed to be significant. The CUMC Institutional Review Board approved the study.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported in part by the Laura and John Arnold Foundation, American Society of Transplant Surgeons and American Society of Transplantation’s Transplantation and Immunology Research Network, as well as the National Institute of Minority Health and Health Disparities (R01010290).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016121330/-/DCSupplemental.
References
- 1.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, Stewart DE, Cherikh WS, Wainright JL, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2012 Annual Data Report: Kidney. Am J Transplant 14[Suppl 1]: 11–44, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Matas AJ, Smith JM, Skeans MA, Lamb KE, Gustafson SK, Samana CJ, Stewart DE, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2011 Annual Data Report: Kidney. Am J Transplant 13[Suppl 1]: 11–46, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Mohan S, Foley K, Patzer R, Cohen D, Pastan S: Characteristics of discarded kidneys from deceased donors in the United States. Am J Transplant 14: 587, 2014 [Google Scholar]
- 4.De Vusser K, Lerut E, Kuypers D, Vanrenterghem Y, Jochmans I, Monbaliu D, Pirenne J, Naesens M: The predictive value of kidney allograft baseline biopsies for long-term graft survival. J Am Soc Nephrol 24: 1913–1923, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2013 Annual Data Report: Kidney. Am J Transplant 15[Suppl 2]: 1–34, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Wang CJ, Wetmore JB, Crary GS, Kasiske BL: The donor kidney biopsy and its implications in predicting graft outcomes: A systematic review. Am J Transplant 15: 1903–1914, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Hall IE, Reese PP, Weng FL, Schröppel B, Doshi MD, Hasz RD, Reitsma W, Goldstein MJ, Hong K, Parikh CR: Preimplant histologic acute tubular necrosis and allograft outcomes. Clin J Am Soc Nephrol 9: 573–582, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Randhawa PS, Minervini MI, Lombardero M, Duquesnoy R, Fung J, Shapiro R, Jordan M, Vivas C, Scantlebury V, Demetris A: Biopsy of marginal donor kidneys: Correlation of histologic findings with graft dysfunction. Transplantation 69: 1352–1357, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Azancot MA, Moreso F, Salcedo M, Cantarell C, Perello M, Torres IB, Montero A, Trilla E, Sellarés J, Morote J, Seron D: The reproducibility and predictive value on outcome of renal biopsies from expanded criteria donors. Kidney Int 85: 1161–1168, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Kasiske BL, Stewart DE, Bista BR, Salkowski N, Snyder JJ, Israni AK, Crary GS, Rosendale JD, Matas AJ, Delmonico FL: The role of procurement biopsies in acceptance decisions for kidneys retrieved for transplant. Clin J Am Soc Nephrol 9: 562–571, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagasta A, Sánchez-Escuredo A, Oppenheimer F, Paredes D, Musquera M, Campistol JM, Solé M: Pre-implantation analysis of kidney biopsies from expanded criteria donors: Testing the accuracy of frozen section technique and the adequacy of their assessment by on-call pathologists. Transpl Int 29: 234–240, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Rudge CJ: Should retrieved kidneys ever be discarded? Transplantation 81: 974–975, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Sung RS, Christensen LL, Leichtman AB, Greenstein SM, Distant DA, Wynn JJ, Stegall MD, Delmonico FL, Port FK: Determinants of discard of expanded criteria donor kidneys: Impact of biopsy and machine perfusion. Am J Transplant 8: 783–792, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Cockfield SM, Moore RB, Todd G, Solez K, Gourishankar S: The prognostic utility of deceased donor implantation biopsy in determining function and graft survival after kidney transplantation. Transplantation 89: 559–566, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Kayler LK, Mohanka R, Basu A, Shapiro R, Randhawa PS: Correlation of histologic findings on preimplant biopsy with kidney graft survival. Transpl Int 21: 892–898, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Munivenkatappa RB, Schweitzer EJ, Papadimitriou JC, Drachenberg CB, Thom KA, Perencevich EN, Haririan A, Rasetto F, Cooper M, Campos L, Barth RN, Bartlett ST, Philosophe B: The Maryland aggregate pathology index: A deceased donor kidney biopsy scoring system for predicting graft failure. Am J Transplant 8: 2316–2324, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Pokorná E, Vítko S, Chadimová M, Schück O, Ekberg H: Proportion of glomerulosclerosis in procurement wedge renal biopsy cannot alone discriminate for acceptance of marginal donors. Transplantation 69: 36–43, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Malek SK: Procurement biopsies in kidneys retrieved for transplantation. Clin J Am Soc Nephrol 9: 443–444, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas M: Donor kidney biopsies: Pathology matters, and so does the pathologist. Kidney Int 85: 1016–1019, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Muruve NA, Steinbecker KM, Luger AM: Are wedge biopsies of cadaveric kidneys obtained at procurement reliable? Transplantation 69: 2384–2388, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Mohan S, Foley K, Chiles MC, Dube GK, Patzer RE, Pastan SO, Crew RJ, Cohen DJ, Ratner LE: The weekend effect alters the procurement and discard rates of deceased donor kidneys in the United States. Kidney Int 90: 157–163, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Marcussen N, Mihatsch MJ, Nadasdy T, Nickerson P, Olsen TS, Papadimitriou JC, Randhawa PS, Rayner DC, Roberts I, Rose S, Rush D, Salinas-Madrigal L, Salomon DR, Sund S, Taskinen E, Trpkov K, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 23.Wang HJ, Kjellstrand CM, Cockfield SM, Solez K: On the influence of sample size on the prognostic accuracy and reproducibility of renal transplant biopsy. Nephrol Dial Transplant 13: 165–172, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Revision to the deceased donor kidney allocation policy by UNOS/OPTN Board. Available at: https://optn.transplant.hrsa.gov/news/board-approves-significant-revisions-to-deceased-donor-kidney-allocation-policy/. Accessed July 21, 2016
- 25.Guide to calculating and interpreting KDPI. Available at: https://optn.transplant.hrsa.gov/media/1512/guide_to_calculating_interpreting_kdpi.pdf. Accessed July 31, 2016
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.