Abstract
Prior studies of sex differences in kidney graft survival showed conflicting results. We hypothesized that the association between recipient sex and kidney graft failure risk differs by recipient age and donor sex. We evaluated 159,417 patients recorded in the Scientific Registry of Transplant Recipients database who received a first deceased-donor kidney transplant (1995–2013). We used time-varying Cox models to estimate the association between recipient sex and death-censored graft failure. Models, stratified on donor sex and adjusted for potential confounders, included a recipient sex by current age interaction term. Among recipients of male donors, females of all ages had significantly higher graft failure risks than males (adjusted hazard ratios 0–14 years: 1.51 [95% confidence intervals 1.19 to 1.90]; 15–24 years: 1.37 [1.18 to 1.59]; 25–44 years: 1.14 [1.03 to 1.26]; 45 years: 1.05 [1.01 to 1.09]). Among recipients of female-donor grafts, only female recipients aged 15–24 years had a significantly higher graft failure risk than their male counterparts had (1.28 [1.06 to 1.53]). Indeed, female recipients aged ≥45 years had a significantly lower graft failure risk than their male counterparts had (0.95 [0.91 to 0.99]). These observations might be explained by the combined influence of several factors, including recognition of sex-determined minor histocompatibility antigens, influence of sex hormones on immune activation, sex- and age-related differences in medication adherence, and sex-related differences in body size. Additional studies should determine whether sex- and age-specific immunosuppression strategies are warranted for kidney graft recipients.
Keywords: transplant outcomes, Epidemiology and outcomes, kidney transplantation
Prior studies comparing kidney graft outcomes between males and females showed conflicting results. Whereas studies including pediatric recipients, or restricted to children and younger adults, found poorer outcomes among females compared with males,1–3 studies focusing exclusively on adult recipients found no differences by sex4,5 or slightly better outcomes among females.6 These discrepancies suggest that sex differences in kidney graft outcomes may differ by age.
Several factors may contribute to differences in graft survival by recipient sex. First, there may be sex-related differences in the level of immune activation. The higher prevalence of autoimmune diseases in females, and the higher risk of infection in males, prompted study of sex differences in immune activation outside the field of transplantation.7 In general, females produce more vigorous cellular and humoral immune reactions than males,8 driven in part by immune-enhancing effects of estrogens and suppressive effects of androgens.9,10 Any effects of sex hormones on immune activation likely vary by age, particularly among women, in whom sex hormone levels decline sharply after menopause. Second, medication adherence may differ by sex.11–13 Third, the smaller body size of women (on average), and resulting lower metabolic demand on the graft, may provide a graft survival advantage for females compared with males.14,15 Finally, the potential for immune recognition of sexually determined minor histocompatibility antigens (H-Y antigens)16,17 among female recipients of male-donor kidneys may contribute to higher graft failure risk among female recipients. The relationship between recipient sex and graft survival is complex, and likely to be modified by both age and donor sex. The combined effects of interacting factors of different relative strengths will determine overall differences in graft survival between female and male recipients.
We hypothesized that females of premenopausal age have poorer graft survival than males of the same age, whereas females of postmenopausal age have graft survival similar to males of the same age, and that differences in graft survival between female and male recipients also differ by donor sex. We aimed to determine whether death-censored graft survival differs between male and female recipients and whether the association between recipient sex and graft survival differs by age and by donor sex. This is the first study to assess differences in graft outcomes between female and male recipients across the entire age spectrum.
Results
We identified 159,417 individuals recorded in the Scientific Registry of Transplant Recipients (SRTR) who had a first deceased-donor kidney transplant between January 1, 1995 and December 31, 2013. Patients were followed for a median of 4.9 years (interquartile range, 2.0–9.1 years) after transplantation, with a total of 965,100 person-years of observation. There were 66,562 graft failures and 37,564 deaths. Living-donor kidney recipients were not included because body size information, an important potential confounder, was missing in >20% of patients.
Recipient and Transplant Characteristics
In order to examine how the effect of recipient sex may change with age, while matching on time since transplant, we used Cox models with time-varying recipient age, categorized in four intervals: 0–14, 15–24, 25–44, and ≥45 years. Recipients started observation in the age interval in which they received their transplant; recipient age was updated as age changed over time. Individuals could contribute observation to more than one age interval. Table 1 (recipient characteristics) and Table 2 (donor and transplant characteristics) summarize the composition of the observed recipient experience for males and females within each age interval. There were few differences in characteristics by sex. A greater proportion of the observation time of females than males was contributed by patients with GN; the magnitude of these differences diminished with increasing age. Among those <25 years, a greater proportion of the observation time of males than females was contributed by patients with congenital anomalies of the kidneys and urinary tract. Among those ≥15 years, females were shorter and lighter than males. Females 0–14 years were taller and heavier, and also slightly older than males 0–14 years. Males tended to have larger donors than females, but only among those ≥25 years. Panel reactive antibody was substantially higher in females than males ≥25 years.
Table 1.
Composition of the contrasted experience by recipient sex and age: recipient characteristics
| Characteristics | 0–14 yr | 15–24 yr | 25–44 yr | ≥45 yr | ||||
|---|---|---|---|---|---|---|---|---|
| Females (n=1773) | Males (n=2483) | Females (n=3869) | Males (n=4987) | Females (n=17,869) | Males (n=25,443) | Females (n=47,930) | Males (n=76,750) | |
| Person-years of observation | 10,468 | 16,063 | 18,206 | 24,655 | 116,854 | 170,142 | 237,683 | 371,029 |
| Graft failures | 659 | 728 | 1383 | 1538 | 6539 | 9476 | 16,772 | 29,467 |
| Deaths | 92 | 100 | 206 | 206 | 2091 | 3149 | 11,266 | 20,454 |
| Age at transplant (mean±SD), yr | 7.9±3.7 | 7.0±3.7 | 16.7±3.8 | 16.5±4.1 | 33.3±6.0 | 33.4±6.1 | 55.0±8.7 | 55.1±8.8 |
| Race, % | ||||||||
| White | 45 | 46 | 42 | 43 | 42 | 46 | 53 | 55 |
| Black | 19 | 23 | 24 | 27 | 33 | 32 | 26 | 25 |
| Other | 36 | 31 | 34 | 30 | 25 | 22 | 20 | 19 |
| Primary disease, % | ||||||||
| Diabetes | 0 | 0 | 1 | 0 | 13 | 12 | 24 | 28 |
| Hypertension | 1 | 1 | 5 | 5 | 17 | 27 | 22 | 27 |
| GN | 32 | 19 | 46 | 35 | 44 | 34 | 25 | 21 |
| CAKUT | 28 | 47 | 17 | 32 | 7 | 11 | 16 | 12 |
| Other | 39 | 32 | 31 | 26 | 16 | 15 | 12 | 11 |
| Missing | 0 | 0 | 1 | 1 | 2 | 2 | 1 | 1 |
| Insurer, % | ||||||||
| Private | 29 | 31 | 31 | 34 | 28 | 27 | 34 | 34 |
| Public | 70 | 69 | 68 | 65 | 72 | 72 | 65 | 65 |
| No coverage | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Missing | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Recipient height (mean±SD), cm | 114.8±23.8 | 110.0±24.3 | 151.4±15.0 | 159.0±20.5 | 161.5±8.2 | 175.2±9.7 | 162.0±7.3 | 176.0±8.3 |
| Missing, % | 9.8 | 9.7 | 9.7 | 9.7 | 9.9 | 10.1 | 8.0 | 8.2 |
| Recipient weight (mean±SD), kg | 25.9±13.7 | 23.8±12.7 | 52.6±17.9 | 58.0±21.4 | 68.2±17.8 | 80.6±19.2 | 71.8±15.7 | 84.7±16.5 |
| Missing, % | 8.3 | 8.3 | 10.8 | 10.7 | 11.7 | 11.5 | 8.9 | 8.9 |
| Era, % | ||||||||
| 1995–1999 | 26 | 28 | 32 | 34 | 41 | 42 | 35 | 35 |
| 2000–2004 | 27 | 27 | 30 | 29 | 31 | 31 | 32 | 31 |
| 2005–2009 | 35 | 33 | 30 | 30 | 22 | 22 | 26 | 26 |
| 2010–2013 | 12 | 12 | 8 | 8 | 6 | 6 | 8 | 8 |
| Duration of dialysis before transplant (mean±SD), yr | 1.7±1.4 | 1.8±1.4 | 2.2±1.9 | 2.1±1.9 | 3.8±3.1 | 3.6±3.0 | 3.5±3.0 | 3.4±2.7 |
| Missing, % | 19.4 | 24.3 | 12.7 | 14.2 | 10.1 | 8.1 | 13.0 | 10.5 |
The number of individuals contributing observation to each age interval is indicated. However, because age was treated as a time-varying variable, individuals could contribute observation to more than one age interval. Because the unit of analysis was person-time, rather than person, the characteristics presented are weighted by a factor derived from the number of person-years of observation and number of events, and presented as weighted mean±SD, weighted median (interquartile range [IQR]), or percent (%). For example, 45% of the person-years contributed by females between 0 and 14 years were by white recipients. CAKUT, congenital anomalies of the kidneys or urinary tract.
Table 2.
Composition of the contrasted experience by recipient sex and age: transplant-related characteristics
| Characteristics | 0–14 yr | 15–24 yr | 25–44 yr | ≥45 yr | ||||
|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | Females | Males | |
| Donor weight | ||||||||
| Median (IQR), kg | 69.0 (54.9, 81.6) | 69.0 (54.9, 81.6) | 71.0 (58.0, 84.4) | 72.5 (59.0, 85.0) | 73.9 (61.0, 87.2) | 75.0 (63.0, 88.5) | 75.3 (63.5, 90.0) | 77.1 (65.0, 90.7) |
| Missing, % | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Donor height | ||||||||
| Median (IQR), cm | 170 (158, 178) | 170 (160, 178) | 170 (160, 178) | 170 (163, 178) | 170 (163, 178) | 173 (163, 180) | 170 (163, 178) | 173 (163, 180) |
| Missing, % | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 |
| Donor age | ||||||||
| Median (IQR), yr | 21.0 (16.0, 28.0) | 20.0 (15.0, 28.0) | 24.0 (17.0, 37.0) | 25.0 (18.0, 37.0) | 34.0 (20.0, 47.0) | 35.0 (21.0, 47.0) | 41.0 (24.0, 53.0) | 43.0 (26, 53) |
| Missing, % | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Male donor sex, % | 65.8 | 63.8 | 63.2 | 62.7 | 59.8 | 61.6 | 57.3 | 59.6 |
| HLA mismatch | ||||||||
| Mean | 4.3 | 4.3 | 4.0 | 4.0 | 3.4 | 3.5 | 3.4 | 3.5 |
| SD | 1.3 | 1.3 | 1.5 | 1.5 | 1.8 | 1.7 | 1.8 | 1.7 |
| Missing, % | 0.2 | 0.2 | 0.4 | 0.3 | 0.4 | 0.4 | 0.7 | 0.9 |
| Panel reactive antibody at transplantation | ||||||||
| Mean | 2.5 | 2.3 | 4.0 | 3.2 | 12.1 | 3.9 | 12.4 | 3.0 |
| SD | 9.7 | 9.1 | 13.5 | 12.2 | 24.4 | 13.6 | 24.3 | 11.4 |
| Missing, % | 6.4 | 5.6 | 4.9 | 4.9 | 4.3 | 3.9 | 5.6 | 5.3 |
| Cold ischemia time | ||||||||
| Median (IQR), h | 13.0 (9.0, 19.0) | 13.7 (9.4, 19.0) | 15.5 (11.4, 22.0) | 16.0 (11.0, 22.0) | 18.0 (13.0, 24.0) | 18.0 (12.7, 24.0) | 18.0 (12.0, 24.0) | 17.5 (12.0, 24.0) |
| Missing, % | 11.4 | 10.8 | 8.8 | 7.5 | 7.4 | 7.0 | 6.6 | 7.0 |
Because the unit of analysis was person-time, rather than person, the characteristics presented are weighted by a factor derived from the number of person-years of observation and number of events, and presented as weighted mean±SD, weighted median (interquartile range [IQR]), or percent (%).
Comparison of Death-Censored Graft Survival by Recipient Sex and Current Age, Stratified on Donor Sex
We considered the possibility that the association between recipient sex and graft survival differed not only by recipient age (by including a recipient sex by current age interaction), but also by donor sex, by including a three-way recipient sex by current age by donor sex interaction term. Both interactions were statistically significant (sex by age interaction, P=0.003, and sex by age by donor sex interaction, P=0.03). Therefore, all subsequent models were stratified by donor sex.
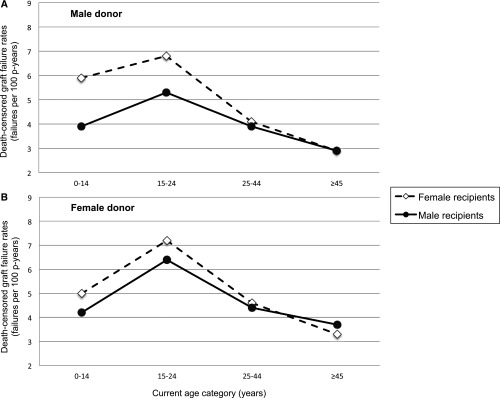
Figure 1 shows crude sex-specific death-censored graft failure rates for recipients of (A) male and (B) female donors, in the four current-age intervals. Graft failure rates were consistently higher in female than male recipients <45 years, regardless of donor sex. Among recipients ≥45 years, crude failure rates did not differ by sex when the donor was male, but were lower in females than males when the donor was female. The crude age- and sex-specific death-censored graft failure rates (with 95% confidence intervals [95% CI]) are shown in Supplemental Table 1.
Figure 1.
Crude death-censored graft failure rates are higher in young female than young male recipients. When the donor is male (A), sex differences are prominent among recipients <25 years old. When the donor is female (B), failure rates are slightly higher in females than males <25 years and slightly lower in females than males ≥45 years. Because age was treated as a time-varying variable, individuals could contribute person-time to multiple current-age intervals. Rates are provided in tabular form in the Supplemental Material. p-years, person years.
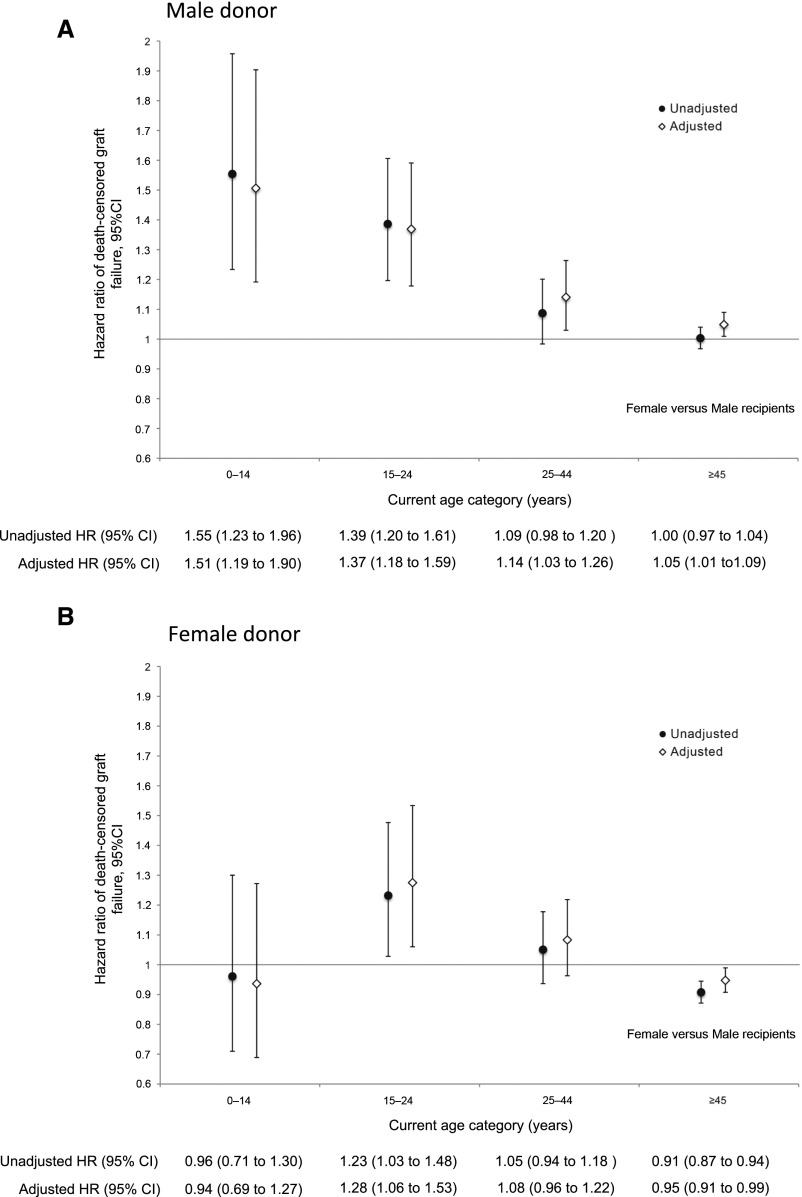
As illustrated in Figure 2A, when the donor was male, female recipients of all ages had significantly higher death-censored graft failure rates than male recipients of the same age: the adjusted hazard ratio (aHR) for female recipients (versus male) was 1.51 (95% CI, 1.19 to 1.90) among those 0–14 years, 1.37 (95% CI, 1.18 to 1.59) among those 15–24 years, 1.14 (95% CI, 1.03 to 1.26) among those 25–44 years, and 1.05 (95% CI, 1.01 to 1.09) among those ≥45 years.
Figure 2.
Relative hazards of death censored graft failure in female versus male recipients differ by age and by donor sex. Adjusted hazards of death-censored graft failure are significantly higher in females relative to males of all ages when the donor is male (A). When the donor is female (B) females 15–24 years have significantly higher graft failure risks than males the same age, whereas risks are lower in females than males ≥45 years. Hazard ratios are shown with 95% CIs. Final models were adjusted for race, primary cause of ESRD, duration of dialysis pretransplant, donor age, donor weight, recipient weight, and panel reactive antibody. HR, hazard ratio.
When the donor was female (Figure 2B), females 15–24 years had significantly higher death-censored graft failure rates compared with male recipients of the same age (aHR, 1.28; 95% CI, 1.06 to 1.53), whereas females ≥45 years had significantly lower death-censored graft failure rates (aHR, 0.95; 95% CI, 0.91 to 0.99) than males ≥45 years. There was no significant difference between female and male recipients among those 0–14 years (aHR, 0.94; 95% CI, 0.69 to 1.27) and those 25–44 years (aHR, 1.08; 95% CI, 0.96 to 1.22).
We examined the same relationships with respect to all-cause graft failure, defined as death or return to dialysis. Results were similar and are shown in Supplemental Figure 1).
Sensitivity Analyses
Models were refit including recipient and donor height, and including recipient and donor body surface area rather than weight as the measures of body size; results were essentially unchanged. Models were also refit including a single donor weight/recipient weight ratio to capture mismatch in body size, rather than including donor and recipient weight as separate variables; results were unchanged. Analyses were also repeated splitting the 0–14-years age interval into two: 0–10 years (representing prepubertal) and 11–14 years (representing early puberty). Results were essentially unchanged, although power was limited due to small numbers in the 11–14-years interval. The results of all sensitivity analyses are presented in the Supplemental Material.
Discussion
Although prior studies examined associations between graft survival and each of recipient sex,5,18,19 recipient age,1 and donor sex,4,6,20 none considered the possibility that these three factors may interact. However, it is reasonable to hypothesize that both immune biology and behavior may change with development and aging in different ways for females compared with males, resulting in differences in the immunologic risk by sex at different ages. In addition, sex differences in graft survival may also differ by donor sex.
Among >159,000 American deceased-donor kidney transplant recipients, we found important differences in graft outcomes between female and male recipients. The relationship between recipient sex and graft survival was modified by both recipient age and donor sex. Among recipients 15–24 years, females had significantly poorer graft outcomes than males, regardless of donor sex. In contrast, among recipients 0–14 years, 25–44 years, and ≥45 years, graft outcomes were poorer for females than males only when the donor was male. When the donor was female, there were no significant differences in graft failure rates by sex for 0–14 and 25–44 year-olds. Interestingly, among recipients ≥45 years, females had significantly better graft outcomes than males when the donor was female.
The pattern of sex differences in graft outcomes that we observed is likely due to a complex interplay of several factors, including age-related differences in immune potency,7 the effects of sex hormones on immune activation,9,21 sex differences in adherence to immunosuppressive medications,11–13 differing metabolic demands on the basis of sex-related differences in body size,14,15 donor sex-related differences in nephron mass,22,23 and immune reactivity to sexually determined minor histocompatibility antigens (H-Y effect).16,17 Changes in the potency of the immune response with increasing age may also modify the effects of other factors affecting graft survival. Table 3 provides a schematic illustration of the way the putative factors contributing to sex differences in graft outcomes may combine within each age interval to affect overall graft failure risk.
Table 3.
Hypothesized effects of different factors potentially influencing graft failure risk in female recipients (versus male recipients)
| Variable | Putative Contributing Factors | Total Combined Effect | |||
|---|---|---|---|---|---|
| Sex Hormones | H-Y Antigen | Adherence | Recipient Body Size | ||
| Male donor: recipient age, yr | |||||
| 0–14 | — | ↑ | — | —/↓ | ↑ |
| 15–24 | ↑↑ | ↑ | ↓ | ↓ | ↑ |
| 25–44 | ↑ | ↑ | ↓ | ↓ | ↑ |
| ≥45 | — | ↑ | ↓ | ↓ | — |
| Female donor: recipient age, yr | |||||
| 0–14 | — | — | — | —/↓ | —/↓ |
| 15–24 | ↑↑ | — | ↓ | ↓ | ↑ |
| 25–44 | ↑ | — | ↓ | ↓ | — |
| ≥45 | — | — | ↓ | ↓ | ↓ |
Arrows indicate the proposed effect of each factor on graft failure risk among female recipients (versus males). ↑ indicates that the factor leads to an increased risk of graft failure; ↓ indicates that the factor leads to a decreased risk of graft failure; — indicates that the factor has no substantial effect on the risk of graft failure. Smaller arrows represent a weaker proposed effect. For example, because adult females tend to have a smaller body size than adult males, the effect of recipient body size would be to give females a lower risk of graft failure than males. The column labeled “Total Combined Effect” indicates the proposed overall risk for graft failure in female recipients (versus males) within each age interval; total combined effect may not be strictly additive, but will depend on the relative strengths of the effects of each individual factor.
Among the youngest recipients, poorer graft outcomes in females are likely driven primarily by the H-Y effect—present only in the setting of a male donor; any effect of sex hormones is likely small in a group that is largely prepubertal. There is no evidence of sex differences in medication adherence in pediatric transplant recipients, possibly because parents take primary responsibility for adherence in children.24–26 Differences in metabolic demand due to sex-related differences in body size are likely very small or absent among children. The poorer outcomes observed among 15–24 year-old females compared with males of the same age, despite evidence that objectively measured adherence may be better in adolescent and adult females than males,27–29 suggest an important role for sex hormones. That this sex difference is preserved even in the setting of a female donor (when there is no H-Y effect), points to the immune-enhancing properties of estrogens as a primary driver of poorer outcomes in females in this age category. The relatively small sex differences in graft outcomes observed for 25–44 year-old recipients of male donors, and lack of significant sex differences for 25–44 year-old recipients of female donors were surprising, but likely reflect the interaction of competing factors with influences in different directions. It is possible that an overall waning of immune potency with increasing age dampens the effects of other factors on graft failure risk7,21; lower levels of sex hormones among women at the upper end of the age range may also reduce the magnitude of sex differences in this age interval. Among those ≥45 years, any effect of sex hormones on immune activation is likely sharply diminished due to menopause.7 In the setting of a female donor (no H-Y effect), the lower death-censored graft failure rates observed among women ≥45 years compared with males of the same age highlight the likely dominant roles in this relation of better adherence among adult women than men,11,27,28 and/or lower metabolic demands by women due to smaller body size.14
The potential role for age-related changes in immune activation in the known association between recipient age and graft failure risk deserves consideration. Among those whose grafts survive the immediate post-transplant period, graft failure rates are lowest in the youngest children, then increase during adolescence, peaking at 17–24 years, and declining through adulthood.1,30 Although age-related changes in medication adherence may play an important role in this relationship, differences in immune activation by age may also be important. Infants have a less robust immune response than older children, both to vaccines and to infections.31,32 The innate and adaptive immune systems mature throughout infancy and childhood, and are fully developed by early adulthood.31 Changes in immune activation in the interval between infancy and young adulthood are not well characterized. However, reductions in immune activation with increasing age—termed immune senescence—have been demonstrated, beginning from young adulthood.33,34 Immune senescence has been linked to involution of the thymus, with decreasing output of naïve T cells into the periphery as the thymus regresses with age.7 This results in an accumulation of antigen-experienced T cells relative to naïve T cells and therefore a dramatic reduction in diversity of the antigen recognition repertoire with age. It is estimated that young adults have a repertoire of 108 antigens that are recognized versus only 106 in the elderly.34
The dramatic changes in sex hormone levels that accompany puberty and menopause likely also contribute to age-associated changes in immune activation, and may play an important role in the observed sex differences in graft survival. Sex hormone receptors are present on many immune cell types. Differences in T lymphocyte, B lymphocyte, and regulatory T cell numbers and functions have been described both between men and women and between pre- versus postmenopausal women.7–10,35 Overall, estrogens enhance, and androgens suppress, immune activation.8–10 However, factors other than sex hormones may also be at play, including differential expression of immune function–influencing genes in males and females36: sex-based differences in immune activation have been observed from the first years of life.37 Sex differences in immune activation have been proposed as an explanation for the higher prevalence of autoimmune diseases in females, and the higher risk of infection in males,7 and may also contribute to sex differences in graft survival.
Several prior studies suggested that female recipients may have a graft survival advantage over males due to generally smaller body size, and therefore lower metabolic demand on the transplanted organ.6,20 Male-donor kidneys are believed to offer superior graft survival due to a greater nephron mass than female-donor kidneys.22,23,38–41 Indeed, prior studies concluded that male recipients of female-donor kidneys have poorer graft survival than all other recipient-donor sex combinations.38–41 We do not dispute the validity of this conclusion in populations dominated by recipients >45 years old. However, in younger recipients other competing factors appear to counterbalance these effects.
The observed modifying effect of donor sex on the association between recipient sex and graft outcomes may be explained by the effect of H-Y antigens encoded by the Y-chromosome.16,17,42 H-Y antigens are expressed only on tissues from male donors, and are therefore at risk of being recognized as foreign by female—but not male—recipients. Prior studies considering the possible effect of donor-recipient sex mismatch on graft outcomes were restricted to recipients >18 years old, and none considered interactions by recipient age.4,6,20,43 Two studies4,6 drew different conclusions using the same methodology. Whereas a European study found a significantly higher risk of graft failure among female recipients of male donors compared with all other donor-recipient sex combinations, an American study found no significant differences. However, by comparing female recipients of male donors to all other combinations, both these studies assumed that outcomes for male-to-male, female-to-male, and female-to-female grafts are equivalent—which is likely not true. Furthermore, neither study adjusted for donor or recipient body size. A more recent study examined the associations between donor sex and graft survival, stratified on recipient sex, adjusting for donor and recipient body size (among other covariates). The authors concluded that there was evidence of an H-Y effect, because female recipients did not benefit from the presumably greater nephron “dose” provided by a male donor, whereas male recipients of a male donor showed better graft survival than male recipients of a female donor.43 Our study also supports the contribution of an H-Y effect on graft outcomes among females.
Our study has several limitations. First, we acknowledge that we can only speculate as to the reasons for the observed sex differences in graft outcomes. These speculations are on the basis of the results of prior studies in transplant recipients and on existing knowledge from nontransplant populations regarding sex and age differences in immunity and associations between age and sex hormone levels. However, the database did not include any measures of immune activation, sex hormone levels, or medication adherence. Therefore, we cannot draw firm conclusions regarding the causes of the observed associations. It would be of interest to assess the pattern of differences in acute rejection risk between female and male recipients of different ages, by donor sex; such differences would lend support to the hypothesis that immune activation differs by sex. Unfortunately, acute rejections are poorly captured into the SRTR database: in the first two reporting periods after transplant, a response to the question on acute rejections during the period is missing in >40% patients. A recent validation study found that only 43% of acute rejections were captured into the database, and concluded that acute rejection data are unreliable.44 An additional limitation is the fact that our analyses were restricted to deceased-donor transplant recipients. This was done because donor body size information was missing for >20% of living-donor recipients, and this was considered a critical potential confounder. Consequently, we cannot determine whether these results also apply to living-donor recipients. Missing data may also have had an effect on our results. Although multiple imputation is the best way to minimize bias due to missing data, the possibility remains that missing values influenced the results if data were not missing at random. Finally, it is important to note that we cannot draw conclusions about the relative strengths of the associations between each of recipient sex, recipient age, and donor sex on graft outcomes. Our analyses only allow comparisons of outcomes between female and male recipients, accounting for the modifying effects of recipient age and donor sex.
This study has potentially important clinical implications. The recipient age– and donor sex–dependent nature of the differences in graft outcomes that we observed between female and male recipients suggests that immunologic factors may be driving these sex differences. Although additional studies are needed to clarify the role of age- and sex-related differences in immune activation in the kidney transplant population, our findings suggest that sex- and age-specific immunosuppression and monitoring strategies may be warranted.
Concise Methods
Data Source and Population
This was a retrospective cohort study of individuals recorded in the SRTR database who received a first renal transplant from a deceased donor in the United States between January 1, 1995 and December 31, 2013. Recipients were followed until September 2, 2015. The SRTR database includes data on all donors, wait-listed candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
Exposure and Outcome Definitions
Primary Exposure
Recipient sex was the primary exposure. We considered the possibility that sex differences in graft outcomes may differ by recipient current age by including a recipient sex by current age interaction term. Current age, a time-varying variable, was categorized as 0–14, 15–24, 25–44, or ≥45 years.1,45,46 We also considered the possibility that sex differences in graft outcomes may differ not only by recipient age but by donor sex by including a recipient sex by recipient time-varying current age by donor sex interaction.
Primary Outcome
The primary outcome was death-censored graft failure, defined as return to dialysis or retransplantation, with observation censored at death.
Statistical Analyses
Crude Death-Censored Graft Failure Rates by Current Age Stratified on Recipient Sex and Donor Sex
For each current-age interval, death-censored graft failure rates were calculated (failures per 100 person-years of observation within that interval) for male and female recipients of male and female donors separately. Individuals could contribute person-time to multiple current-age intervals.
Association Between Recipient Sex and Graft Survival
Cox models with time-varying covariates were used to assess the association between recipient sex and death-censored graft failure. Time zero was the date of transplant. The first model included a recipient sex by current age interaction term and a three-way recipient sex by current age by donor sex interaction term, to examine the possibility that sex differences in graft outcomes differed by recipient age and by donor sex. Both interactions were statistically significant. Therefore, subsequent models (which included recipient sex by current age interaction terms) were stratified on donor sex. These models allowed estimation of hazards ratios associated with female recipient sex (versus male) within each current-age category for recipients of male and female donors, separately.
Unadjusted analyses were followed by multivariable analysis including the following potential confounders: primary renal disease (categorized as diabetes, hypertension, GN, congenital anomalies of the kidneys and urinary tract, or other), race (white, black, other), insurer (public, private, none), duration of dialysis before transplant, transplant era (1995–1999, 2000–2004, 2005–2009, 2010–2013), donor age, donor weight, recipient weight, panel reactive antibody, HLA mismatch, and cold ischemia time. Initial models included all of the above covariates. We then reran the models excluding one covariate at a time. Covariates whose singular exclusion resulted in a <2% change in the point estimates associated with recipient sex (the primary exposure) were not included in the final models.47 Insurer, transplant era, HLA mismatch, and cold ischemia time were excluded on this basis.
The proportionality of hazards was assessed by examining Kaplan–Meier plots comparing graft survival in female versus male recipients; hazards appeared proportional. In addition, proportionality was assessed by refitting the models, censoring all observation at 5 years, and again censoring at 10 years. Results were unchanged, indicating that hazards were proportional.48
We conducted several sensitivity analyses. First, we fit the same models, replacing donor and recipient weight with donor and recipient height and with donor and recipient body surface area as the body size variables. In addition, we considered models including a donor weight/recipient weight ratio variable (to capture size mismatch between donor and recipient). Finally, we refit the models, splitting the 0–14 years current-age interval into two smaller intervals: 0–10 years, representing the prepubertal period, and 11–14 years, representing early puberty; the other age categories remained the same.
Missing covariate values were imputed using multiple imputation methods, on the basis of the joint distributions of all other variables in the model. Multiple imputation is the recommended method of dealing with missing data, and avoids the bias that may result if patients with missing data are simply excluded.49
Data analyses were performed using Statistical Analysis Software 9.4 (SAS Institute, Cary, NC) and S-plus (version 6.1); a two-sided P value <0.05 was considered statistically significant. The study was approved by the McGill University Health Center Research Ethics Board.
Supplemental Analyses
We used the same approach to assess the association between recipient sex and all-cause graft failure (defined as return to dialysis, retransplantation, or death) instead of death-censored graft failure.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was presented at the meeting of the American Society of Nephrology in Chicago, Illinois (November 15–20, 2016).
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Sex and Kidney Transplantation: Why Can’t a Woman Be More Like a Man?,” on pages 2829–2831.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016121380/-/DCSupplemental.
References
- 1.Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA: Association between age and graft failure rates in young kidney transplant recipients. Transplantation 92: 1237–1243, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Keith DS, Cantarovich M, Paraskevas S, Tchervenkov J: Recipient age and risk of chronic allograft nephropathy in primary deceased donor kidney transplant. Transpl Int 19: 649–656, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Kaboré R, Couchoud C, Macher M-A, Salomon R, Ranchin B, Lahoche A, Roussey-Kesler G, Garaix F, Decramer S, Piètrement C, Lassalle M, Baudouin V, Cochat P, Niaudet P, Joly P, Leffondré K, Harambat J: Age dependent risk of graft failure in young kidney transplant recipients [published online ahead of print August 1, 2016]. Transplantation 10.1097/TP.0000000000001372 [DOI] [PubMed] [Google Scholar]
- 4.Kim SJ, Gill JS: H-Y incompatibility predicts short-term outcomes for kidney transplant recipients. J Am Soc Nephrol 20: 2025–2033, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier-Kriesche HU, Ojo AO, Leavey SF, Hanson JA, Leichtman AB, Magee JC, Cibrik DM, Kaplan B: Gender differences in the risk for chronic renal allograft failure. Transplantation 71: 429–432, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Gratwohl A, Döhler B, Stern M, Opelz G: H-Y as a minor histocompatibility antigen in kidney transplantation: A retrospective cohort study. Lancet 372: 49–53, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Giefing-Kröll C, Berger P, Lepperdinger G, Grubeck-Loebenstein B: How sex and age affect immune responses, susceptibility to infections, and response to vaccination. Aging Cell 14: 309–321, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein SL, Marriott I, Fish EN: Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg 109: 9–15, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouman A, Heineman MJ, Faas MM: Sex hormones and the immune response in humans. Hum Reprod Update 11: 411–423, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Trigunaite A, Dimo J, Jørgensen TN: Suppressive effects of androgens on the immune system. Cell Immunol 294: 87–94, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Denhaerynck K, Steiger J, Bock A, Schäfer-Keller P, Köfer S, Thannberger N, De Geest S: Prevalence and risk factors of non-adherence with immunosuppressive medication in kidney transplant patients. Am J Transplant 7: 108–116, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Kiley DJ, Lam CS, Pollak R: A study of treatment compliance following kidney transplantation. Transplantation 55: 51–56, 1993 [DOI] [PubMed] [Google Scholar]
- 13.Frazier PA, Davis-Ali SH, Dahl KE: Correlates of noncompliance among renal transplant recipients. Clin Transplant 8: 550–557, 1994 [PubMed] [Google Scholar]
- 14.Oh C-K, Lee BM, Jeon KO, Kim HJ, Pelletier SJ, Kim SI, Kim YS: Gender-related differences of renal mass supply and metabolic demand after living donor kidney transplantation. Clin Transplant 20: 163–170, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Feldman HI, Fazio I, Roth D, Berlin JA, Brayman K, Burns JE, Grossman RA: Recipient body size and cadaveric renal allograft survival. J Am Soc Nephrol 7: 151–157, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Tan JC, Wadia PP, Coram M, Grumet FC, Kambham N, Miller K, Pereira S, Vayntrub T, Miklos DB: H-Y antibody development associates with acute rejection in female patients with male kidney transplants. Transplantation 86: 75–81, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeffer PF, Thorsby E: HLA-restricted cytotoxicity against male-specific (H-Y) antigen after acute rejection of an HLA-identical sibling kidney: Clonal distribution of the cytotoxic cells. Transplantation 33: 52–56, 1982 [DOI] [PubMed] [Google Scholar]
- 18.Yuge J, Cecka JM: Sex and age effects in renal transplantation. In: Clinical Transplants, edited by Terasaki P, Los Angeles, UCLA Tissue Typing Laboratory, 1991, pp 257–267 [PubMed] [Google Scholar]
- 19.Chen P-D, Tsai M-K, Lee C-Y, Yang C-Y, Hu R-H, Lee P-H, Lai H-S: Gender differences in renal transplant graft survival. J Formos Med Assoc 112: 783–788, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Zeier M, Döhler B, Opelz G, Ritz E: The effect of donor gender on graft survival. J Am Soc Nephrol 13: 2570–2576, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Fish EN: The X-files in immunity: Sex-based differences predispose immune responses. Nat Rev Immunol 8: 737–744, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan JC, Paik J, Chertow GM, Grumet FC, Busque S, Lapasia J, Desai M: Validity of surrogate measures for functional nephron mass. Transplantation 92: 1335–1341, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF: A stereological study of glomerular number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int 63 [Suppl 83]: S31–S37, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Chisholm-Burns MA, Spivey CA, Rehfeld R, Zawaideh M, Roe DJ, Gruessner R: Immunosuppressant therapy adherence and graft failure among pediatric renal transplant recipients. Am J Transplant 9: 2497–2504, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Dew MA, Dabbs AD, Myaskovsky L, Shyu S, Shellmer DA, DiMartini AF, Steel J, Unruh M, Switzer GE, Shapiro R, Greenhouse JB: Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation 88: 736–746, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN: Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: A systematic review. Pediatr Transplant 14: 603–613, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Spivey CA, Chisholm-Burns MA, Damadzadeh B, Billheimer D: Determining the effect of immunosuppressant adherence on graft failure risk among renal transplant recipients. Clin Transplant 28: 96–104, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Chisholm-Burns MA, Spivey CA, Tolley EA, Kaplan EK: Medication therapy management and adherence among US renal transplant recipients. Patient Prefer Adherence 10: 703–709, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boucquemont J, Foster BJ; The TAKE-IT investigators: Sex and Age Differences in Medication Adherence in Adolescent and Young Adult Kidney Transplant Recipients [Abstract]. J Am Soc Nephrol 27: 721A, 2016
- 30.Sapir-Pichhadze R, Young A, Joseph Kim S: Living donor age and kidney transplant outcomes: An assessment of risk across the age continuum. Transpl Int 26: 493–501, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Simon AK, Hollander GA, McMichael A: Evolution of the immune system in humans from infancy to old age. Proc Biol Sci 282: 20143085, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.PrabhuDas M, Adkins B, Gans H, King C, Levy O, Ramilo O, Siegrist CA: Challenges in infant immunity: Implications for responses to infection and vaccines. Nat Immunol 12: 189–194, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Wikby A, Månsson IA, Johansson B, Strindhall J, Nilsson SE: The immune risk profile is associated with age and gender: Findings from three Swedish population studies of individuals 20-100 years of age. Biogerontology 9: 299–308, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Weng NP: Aging of the immune system: How much can the adaptive immune system adapt? Immunity 24: 495–499, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nie J, Li YY, Zheng SG, Tsun A, Li B: FOXP3(+) treg cells and gender bias in autoimmune diseases. Front Immunol 6: 493, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oertelt-Prigione S: The influence of sex and gender on the immune response. Autoimmun Rev 11: A479–A485, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Uekert SJ, Akan G, Evans MD, Li Z, Roberg K, Tisler C, Dasilva D, Anderson E, Gangnon R, Allen DB, Gern JE, Lemanske RF Jr: Sex-related differences in immune development and the expression of atopy in early childhood. J Allergy Clin Immunol 118: 1375–1381, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Kolonko A, Chudek J, Wiecek A: Nephron underdosing as a risk factor for impaired early kidney graft function and increased graft loss during the long-term follow-up period. Transplant Proc 45: 1639–1643, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Shaheen MF, Shaheen FAM, Attar B, Elamin K, Al Hayyan H, Al Sayyari A: Impact of recipient and donor nonimmunologic factors on the outcome of deceased donor kidney transplantation. Transplant Proc 42: 273–276, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Santiago EV, Silveira MR, Araújo VE, Farah Kde P, Acurcio Fde A, Ceccato Md: Gender in the allocation of organs in kidney transplants: Meta-analysis. Rev. Saude Publica 49: 68, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J-Y, Cheng J, Huang H-F, Shen Y, Jiang Y, Chen J-H: The effect of donor-recipient gender mismatch on short- and long-term graft survival in kidney transplantation: A systematic review and meta-analysis. Clin Transplant 27: 764–771, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Wagner S: H-Y antigen in kidney transplant: Does gender matter? Gend Med 9: 387–388, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Tan JC, Kim JP, Chertow GM, Grumet FC, Desai M: Donor-recipient sex mismatch in kidney transplantation. Gend Med 9: 335–347.e2, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Potluri VS, Parikh CR, Hall IE, Ficek J, Doshi MD, Butrymowicz I, Weng FL, Schröppel B, Thiessen-Philbrook H, Reese PP: Validating early post-transplant outcomes reported for recipients of deceased donor kidney transplants. Clin J Am Soc Nephrol 11: 324–331, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Foster BJ, Dahhou M, Zhang X, Dharnidharka VR, Conway J, Ng VL: High risk of liver allograft failure during late adolescence and young adulthood. Transplantation 100: 577–584, 2016 [DOI] [PubMed] [Google Scholar]
- 46.Foster BJ, Dahhou M, Zhang X, Dharnidharka V, Ng V, Conway J: High risk of graft failure in emerging adult heart transplant recipients. Am J Transplant 15: 3185–3193, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Rothman KJ, Greenland S: Introduction to stratified analysis. In: Modern Epidemiology, 2nd Ed., edited by Rothman KJ, Greenland S, Philadelphia, Lippincott-Raven, 1998, p 256 [Google Scholar]
- 48.Allison PD: Survival Analysis Using SAS: A Practical Guide, 2nd ed., edited by Press SAS , North Carolina, SAS Institute, 2010 [Google Scholar]
- 49.Schafer JL: Multiple imputation: A primer. Stat Methods Med Res 8: 3–15, 1999 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.