Abstract
The recent discovery of mutations in the gene encoding diacylglycerol kinase ε (DGKE) identified a novel pathophysiologic mechanism leading to HUS and/or MPGN. We report ten new patients from eight unrelated kindreds with DGKE nephropathy. We combined these cases with all previously published cases to characterize the phenotypic spectrum and outcomes of this new disease entity. Most patients presented with HUS accompanied by proteinuria, whereas a subset of patients exhibited clinical and histologic patterns of MPGN without TMA. We also report the first two patients with clinical and histologic HUS/MPGN overlap. DGKE-HUS typically manifested in the first year of life but was not exclusively limited to infancy, and viral triggers frequently preceded HUS episodes. We observed signs of complement activation in some patients with DGKE-HUS, but the role of complement activation remains unclear. Most patients developed a slowly progressive proteinuric nephropathy: 80% of patients did not have ESRD within 10 years of diagnosis. Many patients experienced HUS remission without specific treatment, and a few patients experienced HUS recurrence despite complete suppression of the complement pathway. Five patients received renal allografts, with no post-transplant recurrence reported. In conclusion, we did not observe a clear genotype-phenotype correlation in patients with DGKE nephropathy, suggesting additional factors mediating phenotypic heterogeneity. Furthermore, the benefits of anti-complement therapy are questionable but renal transplant may be a feasible option in the treatment of patients with this condition.
Keywords: DGKE, diacylglycerol kinase epsilon, atypical hemolytic uremic syndrome, membranoproliferative glomerulonephritis, thrombotic microangiopathy
Atypical HUS is a TMA characterized by microangiopathic hemolytic anemia, thrombocytopenia, and renal function abnormalities. Since the discovery of complement involvement in the physiopathology of aHUS, abnormalities have been detected in several genes encoding proteins implicated in the alternative complement pathway (AP).1 Excessive complement activation may be caused by gain-of-function in complement factors (genetic mutations) or loss-of-function in complement regulatory proteins (genetic mutations or autoantibodies). Aberrant complement activation affects primarily the renal microcirculation, resulting in glomerular TMA. Approximately 60% of patients with aHUS are diagnosed with a molecular AP abnormality.2–4
Lemaire et al. recently reported that recessive mutations in the gene encoding for diacylglycerol kinase ε (DGKE) cause HUS.5 DGKE is an intracellular lipid kinase that phosphorylates diacylglycerol to phosphatidic acid. Current evidence suggests this novel form of HUS does not require activation of the AP. The mechanism by which DGKE deficiency in glomerular cells (endothelia, podocytes) leads to HUS is poorly understood.6 A total of 34 patients with DGKE nephropathy are described in the literature. Although most patients presented with a clinical and histologic picture consistent with HUS,7–10 a subgroup showed a membranoproliferative pattern of glomerular changes without signs of HUS.11 Here we report ten new patients with DGKE nephropathy from eight unrelated kindreds, including three novel pathogenic genotypes. We also provide a comprehensive analysis of the phenotypic spectrum of DGKE nephropathy on the basis of all cases reported to date.
Results
Genetic Findings
Recessive DGKE mutations were identified in ten new patients from eight kindreds with a clinical diagnosis of aHUS (Table 1). The complement genes were free of mutations in all patients, and no anti-CFH autoantibodies or evidence of Shiga-toxin producing Escherichia coli were uncovered when tested. Consanguinity was only reported for kindred 1. Seven patients were homozygous and three were compound heterozygous for a total of six individual genotypes (Table 2). Novel disease-causing mutations were identified in three kindreds: one originating from Asia (p.IVS11+2), one from the Arabian Peninsula (p.K109E) and one from Europe (p.I187Ffs*6). Patient 4.1 is included in this report even though his genotype (p.T204Nfs*4/p.C167W) was previously reported because the original publication did not provide a detailed description of his phenotype.12 In silico analysis13–18 revealed that the two missense mutations are predicted to be deleterious (Supplemental Table 1) and both loci also display evolutionary conservation at the amino acid levels (Supplemental Figure 1).
Table 1.
Summary of findings of all DGKE-mediated kidney disease cases reported to date
| Patient Characteristics | All Patients (n=44) | HUS (n=35) | MPGN-Like (n=9) |
|---|---|---|---|
| Genetic status, n (%) | |||
| Parental consanguinity | 21/44 (48) | 12/35 (34) | 9/9 (100) |
| Homozygous DGKE mutation | 30/44 (68) | 21/35 (60) | 9/9 (100) |
| Clear loss-of-function DGKE genotypea | 33/44 (75) | 24/35 (69) | 9/9 (100) |
| Concurrent complement mutations | 3/44 (7) | 3/35 (86) | 0/9 (0) |
| First disease manifestation/diagnosis | |||
| Median age in yr (range) | 0.7 (0.2–17) | 0.6 (0.2–4.9)b | 2 (0.8–17)b |
| Documented viral trigger, n (%)c | 13/44 (30) | 13/35 (37) | — |
| Proteinuria at onset, n (%) | 26/26d (100) | 17/17 (100) | 9/9 (100) |
| Nephrotic-range proteinuria, n (%) | 21/26 (81) | 13/17 (76) | 8/9 (89) |
| Median sCr in mg/dl (range) | 2.1 (0.2–11.4) | 2.8 (0.2–11.4) | 0.6 (0.3–3) |
| Need for dialysis, n (%) | 22/42 (52) | 22/33 (67) | 0/9 |
| HUS relapses, n (%) | |||
| No. of HUS relapses | |||
| None | 10/35 (31) | 10/35 (31) | — |
| 1–2 | 13/35 (34) | 13/35 (34) | — |
| ≥3 | 12/35 (34) | 12/35 (34) | — |
| Viral trigger documented for at least one HUS relapse | 14/25 (56) | 14/25 (56) | — |
| Renal status at last documented clinic visit | |||
| Age in yr at last follow-up (range) | 10 (1–30) | 9 (1–25) | 19 (2–30) |
| Any proteinuria, n (%) | 36/42 (86) | 28/34 (82) | 8/8 (100) |
| Nephrotic-range proteinuria, n (%) | 13/42 (31) | 8/34 (24) | 5/8 (63) |
| Hematuria, n (%) | 24/31 (77) | 25/31 (81) | N/A |
| Progression to ESRD, n (%) | 10/44 (23) | 7/35 (20) | 3/9 (33) |
| Age at ESRD, in yr (range) | 12 (0.3–23) | 11 (0.3–18) | 19 (8–23) |
| Evidence of systemic complement activation documented at any time, n (%) | 10/44 (23) | 10/35 (29) | 0/9 (0) |
—, not relevant to patients without HUS episodes; sCr, serum creatinine; N/A, not available.
“Clear loss-of-function DGKE genotypes” are defined as any combination of two nonsense, frameshift, or splice-site DGKE mutations.
P<0.05 for difference between subgroups.
The denominator for this parameter is imprecise because for most patients without documentation of a viral trigger, few clinicians formally documented the absence of infection.
Denominators that are discordant with the total number of patients represent the number of patients with available data.
Table 2.
Demographic and clinical characteristics of newly described patients with DGKE mutations
| ID | Kindred | Age at Diagnosis, yr | Sex | Origin | Parental Consanguinity | DGKE Mutations | C Activation | Histologic Diagnosis |
|---|---|---|---|---|---|---|---|---|
| 1.1 | 1 | 0.7 | M | United Arab Emirates | Yes | p.K109E/p.K109E | No | Not done |
| 1.2 | 1 | 2.0 | M | United Arab Emirates | Yes | p.K109E/p.K109E | No | Not done |
| 2.1 | 2 | 0.3 | M | Europe | No | p.W322*/p.W322* | No | TMA/MPGN |
| 2.2 | 2 | 0.2 | F | Europe | No | p.W322*/p.W322* | No | TMA |
| 3.1 | 3 | 4.9 | M | Europe | No | p.IVS5+40/p.W322* | No | TMA |
| 4.1 | 4 | 0.5 | M | Asia | No | p.T204Nfs*4/p.C167W | No | Not done |
| 5.1 | 5 | 0.6 | F | Asia | No | p.IVS11+2/p.IVS11+2 | Yes | Not done |
| 6.1 | 6 | 2.0 | F | Europe | No | p.I187Ffs*6/p.W322* | Yes | TMA |
| 7.1 | 7 | 0.4 | F | Europe | No | p.W322*/p.W322* | No | Not done |
| 8.1 | 8 | 2.5 | M | Europe | No | p.W322*/p.W322* | Yes | TMA/MPGN |
M, male; F, female; C, complement.
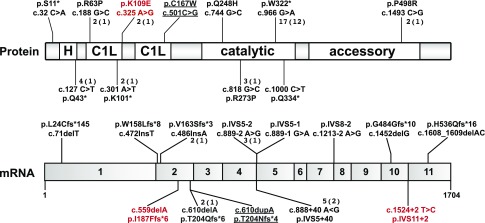
Including the patients presented here, 44 patients from 27 unrelated families with DGKE nephropathy have been documented to date. Parental consanguinity was documented in ten families. Homozygosity was found in 17, and compound heterozygosity in ten kindreds, accounting for a total of 23 distinct mutations (Figure 1). Three patients also had mutations in genes associated with aHUS (thrombomodulin or C3).9
Figure 1.
Schematic representations of DGKE protein and mRNA illustrating the relative positions of all pathogenic mutations reported to date. Novel mutations are highlighted in red. The two mutations of patient 4.1 are underlined because they were recently reported in a report that did not include detailed clinical information, which is part of this report. The numbers next to many mutations indicate how many individual patients have this genotype (number of kindreds). Mutations without an associated number were observed in one patient. C1L, C1 domains (presumed to bind diacylglycerol); H, hydrophobic domain.
Prevalence of DGKE Mutations in Large aHUS Cohorts
We sought to estimate the prevalence of DGKE mutations in two large pediatric aHUS cohorts that contained kindreds 1, 2, and 4 (Supplemental Table 2). The analysis yielded an overall prevalence of 3.1% and 2.0% in the cohorts from Heidelberg (n=96) and Seoul (n=51), respectively. In both cohorts, genetic abnormalities in any of the genes encoding members of the AP were about ten-fold more prevalent (30.2% and 19.8%, respectively).
Clinical Characteristics of DGKE-HUS
Eighty percent (35 of 44) of patients with DGKE nephropathy had clinical presentation and laboratory investigations that were consistent with HUS (Supplemental Tables 3 and 4, Table 3). Most patients with DGKE-HUS (30 of 35) had disease onset in the first year of life (median, 7 months; (95% confidence interval [95% CI], 5 to 8 months; range, 2–59 months). All initial HUS episodes were accompanied by hypertension and proteinuria, typically in the nephrotic range (Table 3). Few prior reports included data for these parameters at disease onset (Supplemental Table 4). Although two thirds of patients with DGKE-HUS had AKI requiring dialysis, renal function returned to normal in all but one patient after the first episode (patient 8-3 reported by Lemaire et al.5 had global cortical ischemia). Renal biopsy specimens from 20 of 35 patients with HUS exhibited features consistent with TMA. Relapsing HUS was documented in 70% (25 of 35) of patients, almost half of which experienced more than three HUS relapses. Most relapse episodes were accompanied by nephrotic-range proteinuria. Viral infections were reported as possible triggers for the first HUS episode in nine out of ten newly identified patients, and nearly a third of all patients with HUS. Signs of complement activation (low C3 and/or increased sC5b-9 levels) at any time during the disease course were documented in ten out of 35 patients.
Table 3.
Clinical and laboratory data for the first episode of newly described patients with DGKE
| ID | Hemoglobin Nadir, g/dl | LDH, IU/L | Platelet Nadir, 103/μl | sCr Peak, mg/dl | Proteinuria | Hematuria | HTN | Triggers | Dialysis | Other Treatments | Recovery of Renal Function |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 5.5 | N/A | 214 | 4 | N/A | N/A | Yes | Yes (viral) | Yes | None | Yes |
| 1.2 | 6.0 | 3207 | 207 | 1.1 | Yes, NR | Yes | Yes | Unknown | No | PI | Yes |
| 2.1 | 4.7 | 1235 | 25 | 4.0 | Yes, NR | Yes | Yes | Yes (GE) | Yes | None | Yes |
| 2.2 | 6.8 | 694 | 33 | 2.33 | Yes, NR | Yes | Yes | Yes (viral) | Yes | PE, steroids | Yes |
| 3.1 | 9.6 | 496 | 649 | 0.3 | Yes, NR | Yes | Yes | Yes (viral) | No | ACEi, heparin | Yes |
| 4.1 | 5.7 | 2154 | 45 | 4.4 | Yes, NR | Yes | Yes | Yes (viral) | Yes | PI | Yes |
| 5.1 | 8.2 | 7337 | 25 | 2.2 | N/A | N/A | Yes | Yes (viral) | Yes | PE, eculizumab | Yes |
| 6.1 | 11.2 | 1843 | 135 | <0.2 | Yes, NR | Yes | Yes | Yes (viral) | Yes | Steroids, PI, PE, eculizumab | Yes |
| 7.1 | 6.1 | 11,994 | 20 | 3.8 | Yes, NR | Yes | Yes | Yes (GE) | Yes | PI | Yes |
| 8.1 | 6.0 | N/A | 60 | 1.3 | Yes, NR | N/A | Yes | Yes (GE) | No | Steroids, PE, cyclosporin A, cyclophosphamide, vincristine | Yes |
LDH, lactate dehydrogenase; sCr, serum creatinine; HTN, hypertension; N/A, not available; NR, nephrotic range; PI, plasma infusions; GE, gastroenteritis; PE, plasma exchange; ACEi, angiotensin-converting enzyme inhibitors.
Two of these 35 patients with DGKE-HUS (patients 2.1 and 8.1) were notable because their original pathologic diagnoses were MPGN. No patient or clinical characteristics easily distinguish these two patients from the others. Both had the most common pathogenic genotype (p.W322*/p.W322*). Of note, the two siblings from kindred 2, who have the same genotype, showed significant differences in both histologic features (MPGN/TMA versus TMA) and outcomes (preserved renal function versus rapid ESRD development; Tables 2–4). Representative renal histology images are presented in Figure 2 (patient 2.1) and Supplemental Figure 2 (patient 2.2). The clinical presentation of patient 8.1 is the most unusual. A diagnosis of nephrotic syndrome accompanied by hypertension was posed 1 week before clear findings of aHUS were observed. It is possible that the relatively late age at diagnosis of this patient (2.5 years) may not accurately reflect disease activity. Indeed, he was diagnosed with transient erythroblastopenia of childhood 1 year before because of chronic unexplained anemia.
Table 4.
Long-term follow-up data and outcomes for newly described patients with DGKE mutations
| ID | HUS Relapse(s) | Long-Term Outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trigger | Proteinuria | Hematuria | Acute Treatment | Maintenance Therapy | Age at Last Follow-Up, yr | Current CKD Stage | Age at ESRD, yr | Renal Tx | Proteinuria | Hematuria | ||
| 1.1 | 2 | Yes (viral) | Yes, NR | Yes | None | None | 25 | HD | 16 | No | Yes, NR | N/A |
| 1.2 | 5 | Unknown | Yes, NR | Yes | PI, dialysis, eculizumab | None | 14 | HD | 10 | No | Yes, NR | N/A |
| 2.1 | 3 | Yes (viral) | Yes, NR | Yes | Steroids | Steroids ACEi, ARB | 15 | CKD1 | — | No | Yes | Yes |
| 2.2 | 1 | Yes (viral) | Yes, NR | Yes | Dialysis, PE, steroids | ARB | 9 | Tx | 0.3 | Yes | No | No |
| 3.1 | 0 | — | — | — | — | ACEi, ARB | 5 | CKD1 | — | No | Yes | Yes |
| 4.1 | 6 | Yes (viral) | Yes, NR | Yes | PI | PI | 9 | CKD1 | — | No | Yes, NR | Yes |
| 5.1 | 1 | Yes (viral) | Yes, NR | Yes | HD, PI, eculizumab | eculizumab | 1.5 | CKD1 | — | No | No | No |
| 6.1 | 0 | — | — | — | — | ACEi | 2.5 | CKD1 | — | No | Yes | Yes |
| 7.1 | 1 | Yes (viral) | Yes, NR | Yes | PI | ACEi | 2 | CKD1 | — | No | No | Yes |
| 8.1 | 0 | — | — | — | — | ACEi | 20 | CKD2 | — | No | Yes | Yes |
Tx, kidney transplantation; NR, nephrotic range; HD, hemodialysis; N/A, not available; PI, plasma infusions; ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; PE, plasma exchange.
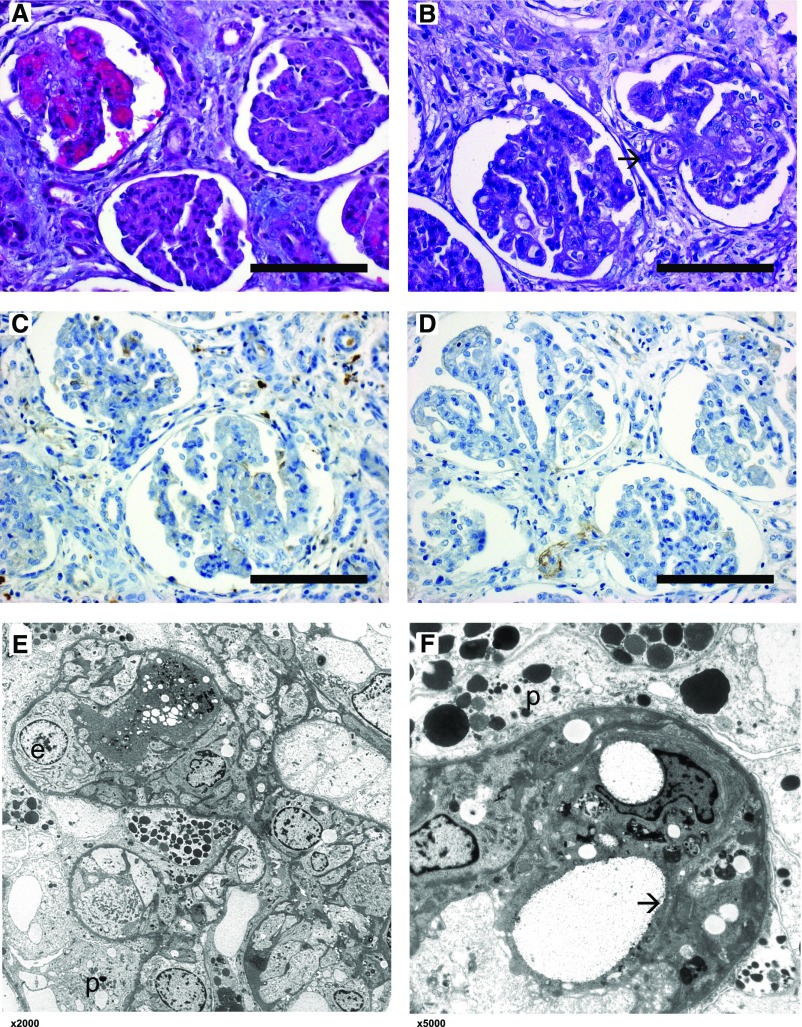
Figure 2.
Renal histology of patient 2.1 shows features of both MPGN and TMA. Light microscopical changes of affected glomeruli with lobulation of the glomerular tuft, thickening and double contours of glomerular basement membranes, collapse of the capillary tuft, and swelling of endothelial cells. One glomerulus shows active TMA (arrow). (A) Hematoxylin and eosin stain, original magnification, ×40; (B) periodic acid–Schiff stain, original magnification, ×40. (C and D) Immunohistochemistry using antibodies against IgG and C3c shows no specific glomerular staining. Original magnification, ×40. (E and F) Electron microscopy at various magnifications (×2000, ×5000) showing endothelial cell swelling (e) with subendothelial cleft formation (arrow) and capillary lumen obliteration. Glomerular basement membrane is intact. Podocytes (p) show enlarged cytoplasm and foot process effacement. No specific osmiophilic deposits and no fibrils.
Patient-level data of the new and previously identified patients with DGKE-HUS are provided in Supplemental Tables 3–6 and Tables 2–4, respectively. Detailed clinical histories for all new patients are available in Supplemental Appendix.
We compared various clinical characteristics between patients with DGKE nephropathy caused by two alleles predicted to result in complete loss-of-function (nonsense, frameshift, or splice-site mutations) to those with other genotypes. No differences were found (Supplemental Table 7).
Clinical Characteristics of DGKE-MPGN
The other nine patients, all reported by Ozaltin et al.11 showed no clinical signs of HUS. All harbored homozygous loss-of-function genotypes. Patients were from three unrelated consanguineous unions. Renal biopsies were done in all patients and specimens were read as “MPGN-like.” Patients with DGKE-MPGN were diagnosed later than those with DGKE-HUS (median, 2 years; 95% CI, 1.5 to 8 years; range, 0.8–17 years; P<0.001). All patients had nephrotic-range proteinuria at diagnosis that persisted long term. Complement investigations were done for seven out of nine patients and showed no abnormalities. Patient-level data of patients with DGKE-MPGN are provided in Supplemental Tables 3–6.
Assessment of Therapies Prescribed and Long-Term Outcomes
Management was restricted to supportive measures for 16 of 35 patients with DGKE-HUS at disease onset. Renal function returned to normal for all patients. Six of these patients (four with relapsing HUS episodes) never received any specific therapy (e.g., plasma therapy or eculizumab), and five out of six had normal renal function documented after a median follow-up of 5 years (95% CI, 4 to 13 years; range, 0.5–24 years). The other patient progressed to ESRD 15 years after the first HUS episode. All six had significant proteinuria at their last observation. Among the 19 patients with DGKE-HUS who received HUS-related therapy at disease onset (immunosuppression, plasma treatment, and/or eculizumab), one patient did not regain normal renal function (patient 8-3).
Out of the 29 of 35 patients with DGKE-HUS who did receive one of the HUS-specific therapies at any time during the disease course, detailed information was available for 16. Acute improvements were attributed to plasma therapy in ten out of 16 patients. Regular plasma infusions were continued for eight patients: four had no disease recurrences, and four developed HUS relapses after prolongation of intervals between plasma infusions that were reported as responsive to treatment intensification. An example of intermittent use of plasmapheresis for patient 4.1 for >8 years since diagnosis is provided in Supplemental Figure 3.
Three patients were treated with eculizumab at disease onset. Resolution of laboratory evidence of TMA was documented, along with recovery of renal function in two patients (patient reported by Miyata et al.8 and new patient 5.1). Regular eculizumab maintenance infusions were continued in both patients. The third patient (new patient 6.1) received only two eculizumab doses with no effect on laboratory signs of TMA or renal laboratory indices (details in Supplemental Figure 4).
Data on eculizumab therapy were available for three other patients treated for the first time after the initial HUS episode because of clinical worsening or persistent, mild HUS. In one patient (HUS272),9 it was associated with a transient increase in serum albumin whereas in the other (patient 1.2), no effects were documented. Patient 6-3 was already recovering from a HUS relapse when eculizumab was started.5
A history of biochemical signs of AP activations (typically, mildly depressed C3 levels) was documented in all patients who received eculizumab except patients 1.2 and 6-3.
The original report by Lemaire et al. described a patient who developed a HUS relapse while on eculizumab maintenance therapy (patient 6-3).5 We now describe a second patient with a similar clinical course (patient 5.1; details in Supplemental Figure 5). Evidence of adequate anti-complement therapy was documented for both patients.
More aggressive immunosuppressive therapy was administered to patients 2.1 and 8.1 because of histopathology consistent with MPGN. A course of steroids was associated with recovery of renal function in patient 2.1. The sister of patient 2.1 (patient 2.2) was also treated with steroids, with no apparent improvements: she rapidly progressed to ESRD after the first HUS relapse. Patient 8.1 received trials of steroids, cyclosporin A, and cyclophosphamide for persistent nephrotic-range proteinuria, with no effects. Subsequently, plasma exchange combined with vincristine, oral steroids, and angiotensin-converting enzyme inhibition were associated with attenuation of proteinuria.
All nine patients with MPGN-like phenotype received immunosuppressive therapies that were associated with partial remission in three patients, and complete remission in one patient.11 At last follow-up (median age, 10 years; median follow-up time, 8.9 years), all patients still exhibited proteinuria.
Four patients with HUS and one patient with MPGN-like disease received renal allografts; none of them developed post-transplant recurrence. One patient with DGKE-MPGN reported by Ozaltin et al. died at 4 years, from meningitis.11 Four siblings of reported patients (indicated in Supplemental Appendix) died in early childhood from HUS with no genetic testing or detailed history.
Cumulative Incidence and Renal Survival
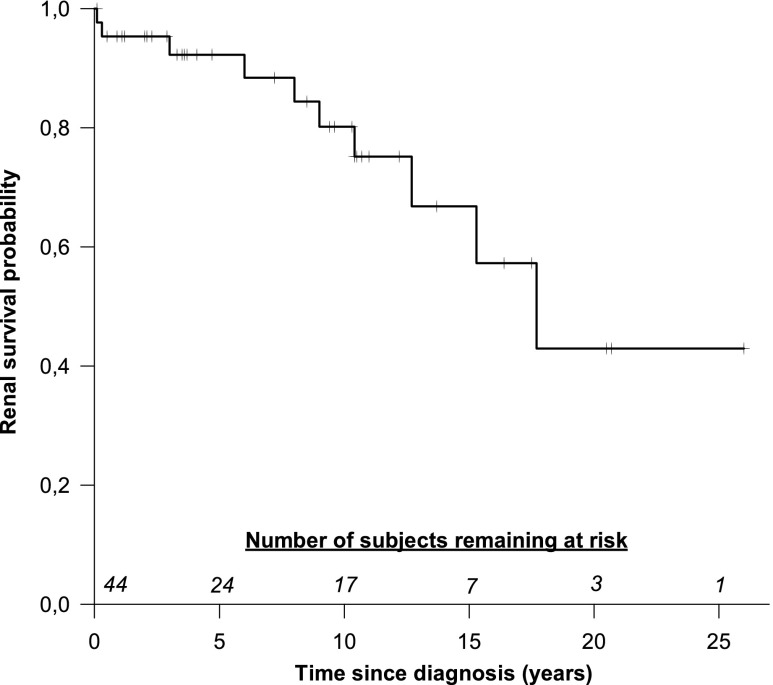
The cumulative incidence of all DGKE nephropathy cases is presented in Figure 3. The actuarial ESRD-free renal survival for the entire cohort is shown in Figure 4. A total of ten (22%) patients reached ESRD at a median age of 12 years (95% CI, 1 to 19 years). The 10-year renal survival rate was 80% in all patients with DGKE nephropathy, 89% in patients with DGKE-HUS, and 50% in patients with MPGN-like phenotype (HUS versus MPGN-like phenotype, P=0.37). Split actuarial survival for HUS and MPGN-like phenotype patients is shown in Supplemental Figure 6.
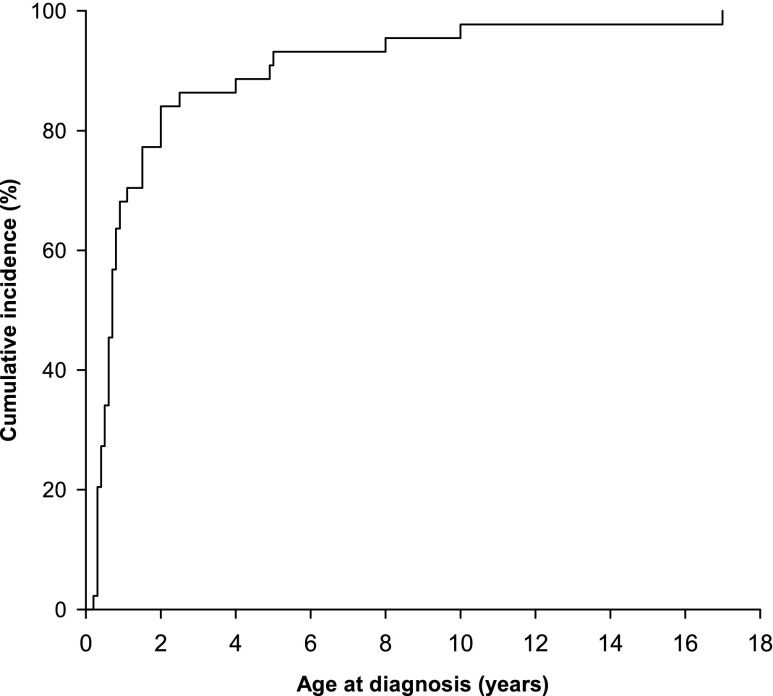
Figure 3.
Most patients with DGKE nephropathy are diagnosed before the age of two. Cumulative incidence of DGKE nephropathy diagnosis in the cohort of 44 patients (age at first HUS episode or at diagnosis of MPGN).
Figure 4.
Kaplan–Meier curve of renal survival, defined as progression to ESRD. The numbers above the x-axis indicate number of subjects remaining at risk at 5-year intervals.
Discussion
We describe the largest cohort of patients with DGKE nephropathy. The combined analysis of the ten newly identified cases with all previously published patients provides convincing evidence that this condition is a genetically and phenotypically distinct disease entity. However, the larger sample size has helped uncover findings that deviate from that of the original reports.
The predominant phenotype of DGKE nephropathy is early-onset relapsing HUS with chronic proteinuria and long-term progression to CKD. On the basis of the original report by Lemaire et al.5 and the reports that followed,7–10 DGKE-HUS appeared to be a disease with onset during early infancy. Aggregated data analysis show that indeed most patients with DGKE-HUS (86%) are diagnosed within the first year of life. However, there are now five patients diagnosed between the ages of 1 and 4 years. On that basis, it would be helpful to screen for DGKE mutations in all patients with aHUS, irrespective of age at diagnosis.
Chronic proteinuria is a distinctive feature of DGKE nephropathy. Proteinuria (mostly nephrotic range) was present in all patients at disease onset, regardless of clinical or histopathologic findings. Most patients displayed chronic proteinuria that was present even after resolution of HUS relapses. This degree of proteinuria could result from TMA-induced glomerular damages. The fact that chronic proteinuria is uncommon in patients with complement-mediated TMA lesions would suggest a distinct mechanism, such as primary endothelial and/or podocyte dysfunction.19 It is unclear if proteinuria contributed to the development of HUS lesions in these patients. The association between proteinuric glomerulopathies and HUS was recently reviewed.19
One of the most striking differences emerging from the two original reports5,11 is the clear clinical and histologic divide between patients who exhibit HUS or MPGN-like phenotypes. Patients with MPGN-like phenotypes were, on average, significantly older at diagnosis and were all noted to have a chronic proteinuria glomerulopathy devoid of TMA features.11 All cases reported since then have linked pathogenic DGKE genotypes with HUS.7–10 We identified two new subjects (patients 2.1 and 8.1) with histologic signs of MPGN that were diagnosed at age 0.6 and 2.5 years, respectively. In contrast to other patients with MPGN, these patients did exhibit clinical hallmarks of HUS. It is intriguing to consider that the transient erythroblastopenia of childhood documented in patient 8.1 at approximately 1.5 years may have reflected the recovery phase of an undiagnosed microangiopathic episode. It may be important to consider testing for renal dysfunction in young children with unexplained anemia to rule out the possibility of subclinical TMA. These new findings of phenotypic overlaps further expand the clinical and pathophysiologic spectrum of DGKE nephropathy.
HUS relapses are reported in two thirds of patients with DGKE-HUS. The relapse rates appear to be highly variable and do not seem to correlate with long-term outcomes (ESRD rates in two out of ten versus five out of 25 patients without and with relapses). The detailed analysis of the new patients reported here revealed a high incidence of viral infections preceding renal TMA development. Similar to complement-mediated aHUS,4 infectious triggers were previously documented in some patients with DGKE nephropathy.7–10 The apparently lower report rates in previous studies may be because of underreporting, variations in host inflammatory responses, or severity of infections.
DGKE-HUS was initially described as a complement-independent disorder because no patients had evidence of systemic complement activation, and one patient had a HUS relapse whilst on eculizumab therapy.5 Importantly, we now describe a second patient in which eculizumab could not prevent a HUS relapse (patient 5.1). This notion is supported by data from recent in vitro studies focusing on the role of DGKE in endothelial cells.6 Including our patients, there are now ten cases of DGKE nephropathy in which complement activation of various degrees was documented.7–10 In most patients, the reduction in plasma C3 levels was modest, and kidney biopsy specimens did not show C3 deposition. Concurrent heterozygous mutations in genes encoding AP components were found in only two of these patients.9 It is interesting to note that all of these patients were diagnosed with HUS, and none have developed ESRD. Also of note, most of the patients who showed signs of AP activation received complement targeting therapy, which could attenuate the effects of AP activation. It remains unclear if complement activation defines a subset of DGKE nephropathy or is a nonspecific secondary finding.
The optimal therapy for DGKE nephropathy is unclear because anti-complement and other immunosuppressive regimen do not appear to provide benefits. The presumptive diagnosis of aHUS or MPGN frequently led to the initiation of standard complement targeting or immunosuppressive therapies, respectively. It is important to note that 16 patients with DGKE-HUS who did not receive any specific therapy at disease onset showed spontaneous recovery from the initial HUS episode. Furthermore, only one out of six patients with no further therapy progressed to ESRD. Moreover, 11 out of 12 patients with DGKE-HUS who did progress to CKD stage 3 and beyond received specific treatments during their disease course, and two patients had HUS recurrence while on regular eculizumab infusions. The absence of differences in long-term outcomes and spontaneous disease evolution in patients with and without specific treatment put the value of complement targeting therapies in question. Disease recurrence after kidney transplantation is a well known issue for patients with complement-mediated aHUS, especially if not treated with eculizumab.20 None of the five patients with DGKE nephropathy that received a renal allograft developed post-transplant disease recurrence to date.
The genotypes of most patients with DGKE nephropathy are expected to result in a clear loss-of-function phenotype (nonsense, frameshift, or splice site). By extension, the missense DGKE mutations identified are predicted to also cause DGKE deficiency; this has yet to be demonstrated experimentally. The fact that these missense DGKE mutations span the entire coding region of the DGKE gene suggests that all functional modules are likely required for normal DGKE protein enzymatic activity.
No differences were found when comparing various clinical characteristics of patients with genotypes expected to result in complete loss-of-function with others. It is also remarkable that equally damaging recessive mutations were found in patients with either HUS or MPGN phenotype: complete DGKE functional deficiency is expected in all patients. Moreover, obvious intrafamilial phenotypic and outcome discordance is evident for the two affected siblings from kindred 2 (Tables 3 and 4). Overall, these data strongly suggest that patient-specific gene modifiers and/or exposure to environmental triggers may play a major role in defining the pattern of glomerular disease expression. DGKE nephropathy is thus another example of a complex Mendelian condition.21
The recognition of DGKE nephropathy has paved a new pathway in the understanding of a subset of patients with previously unexplained HUS and MPGN. It is a heterogenous glomerular disorder with histopathologic and clinical features ranging from HUS to MPGN, and displays poor genotype-phenotype correlations. Optimal treatment remains unclear and conventional therapies appear to be of questionable benefits. The disease evolves over time to a state of chronic proteinuria that slowly progresses to CKD. Renal transplantation is a safe option for patients with ESRD because there is no post-transplant HUS recurrence. The expanding phenotypic spectrum of DGKE nephropathy suggests a complex disease mechanism that remains to be elucidated. This expanded cohort is important to help refine the prognosis and the range of phenotypes associated with this condition.
Concise Methods
Identification of New Patients with DGKE Mutations
The patients with DGKE mutations were identified by either retrospective screening of known patients with aHUS or prospective diagnostic work-up. The affected patients were identified by pediatric nephrologists in Germany, Hungary, Poland, United States, United Arab Emirates, Canada, and South Korea, and referred to aHUS diagnostic reference laboratories. Detailed descriptions of each patient are provided in the online Supplemental Appendix.
All patients were screened for single nucleotide variants and short indel mutations in genes encoding for complement proteins and regulators of the alternative pathway (CFH, CFB, CFI, MCP, THBD, and C3) and DGKE using Sanger sequencing, next-generation sequencing multigene panel testing, or whole-exome sequencing. CFHR1–3 deletions were sought with multiplex ligation-dependent probe amplification and/or by high-coverage next-generation sequencing. ELISA was used to detect the presence of anti-CFH autoantibodies.
The pathogenic potential of the novel missense DGKE mutations was evaluated by assessing the degree of amino acid conservation across species and by integrating the results of various in silico predictions, such as PolyPhen-2, SIFT (Sorting Intolerant From Tolerant), Mutation Assessor, PROVEAN (Protein Variation Effect Analyzer), PANTHER (Protein Analysis Through Evolutionary Relationships), and CONDEL (Consensus Deleteriousness).12–17 A detailed description of the genetic and immunologic complement system analyses along with details about Shiga-toxin producing Escherichia coli testing performed on each family is presented in Supplemental Table 8.
Identification of Previously Published Cases of DGKE Nephropathy
Previously reported cases were identified by PubMed search (performed in July of 2016) using various combinations of relevant keywords: “DGKE,” “Diacylglycerol Kinase Epsilon,” “HUS,” “aHUS,” “TMA,” “Hemolytic Uremic Syndrome,” “Thrombotic microangiopathies,” “Membranoproliferative GN,” and “MPGN”. Genetic, clinical, and laboratory data of all previously reported cases were extracted from relevant articles, as available.
Statistical Analyses
Distribution-free 95% CIs were calculated for medians of non-normally distributed data. Group comparisons were performed using Mann–Whitney and chi-squared tests. Statistical analysis was performed with SigmaPlot software, version 13.0 (Systat Software, San Jose, CA) and SAS software, version 9.3 (SAS Institute Inc., Cary, NC).
Survival Analyses
Kaplan–Meier actuarial survival analysis and log-rank test was used to calculate and compare renal survival, defined as progression to ESRD. Time zero for the survival analysis was defined as age at first HUS episode or clinical MPGN diagnosis.
Disclosures
None.
Supplementary Material
Acknowledgments
Support for this work was received from the European Community’s Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 2012-305608 (EURenOmics) (to F.S. and V.F.B.), and from the Korean Health Technology R&D (Research and Development) Project, Ministry of Health and Welfare, Republic of Korea (grant no. HI12C0014) (to H.I.C.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017010031/-/DCSupplemental.
References
- 1.Loirat C, Frémeaux-Bacchi V: Atypical hemolytic uremic syndrome. Orphanet J Rare Dis 6: 60, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Noris M, Caprioli J, Bresin E, Mossali C, Pianetti G, Gamba S, Daina E, Fenili C, Castelletti F, Sorosina A, Piras R, Donadelli R, Maranta R, van der Meer I, Conway EM, Zipfel PF, Goodship TH, Remuzzi G: Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 5: 1844–1859, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noris M, Remuzzi G: Atypical hemolytic-uremic syndrome. N Engl J Med 361: 1676–1687, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Kavanagh D, Goodship TH: Atypical hemolytic uremic syndrome, genetic basis, and clinical manifestations. Hematology (Am Soc Hematol Educ Program) 2011: 15–20, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Lemaire M, Frémeaux-Bacchi V, Schaefer F, Choi M, Tang WH, Le Quintrec M, Fakhouri F, Taque S, Nobili F, Martinez F, Ji W, Overton JD, Mane SM, Nürnberg G, Altmüller J, Thiele H, Morin D, Deschenes G, Baudouin V, Llanas B, Collard L, Majid MA, Simkova E, Nürnberg P, Rioux-Leclerc N, Moeckel GW, Gubler MC, Hwa J, Loirat C, Lifton RP: Recessive mutations in DGKE cause atypical hemolytic-uremic syndrome. Nat Genet 45: 531–536, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruneau S, Néel M, Roumenina LT, Frimat M, Laurent L, Frémeaux-Bacchi V, Fakhouri F: Loss of DGKε induces endothelial cell activation and death independently of complement activation. Blood 125: 1038–1046, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Westland R, Bodria M, Carrea A, Lata S, Scolari F, Fremeaux-Bacchi V, D’Agati VD, Lifton RP, Gharavi AG, Ghiggeri GM, Sanna-Cherchi S: Phenotypic expansion of DGKE-associated diseases. J Am Soc Nephrol 25: 1408–1414, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyata T, Uchida Y, Ohta T, Urayama K, Yoshida Y, Fujimura Y: Atypical haemolytic uraemic syndrome in a Japanese patient with DGKE genetic mutations. Thromb Haemost 114: 862–863, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Sánchez Chinchilla D, Pinto S, Hoppe B, Adragna M, Lopez L, Justa Roldan ML, Peña A, Lopez Trascasa M, Sánchez-Corral P, Rodríguez de Córdoba S: Complement mutations in diacylglycerol kinase-ε-associated atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 9: 1611–1619, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mele C, Lemaire M, Iatropoulos P, Piras R, Bresin E, Bettoni S, Bick D, Helbling D, Veith R, Valoti E, Donadelli R, Murer L, Neunhäuserer M, Breno M, Frémeaux-Bacchi V, Lifton R, Remuzzi G, Noris M: Characterization of a new DGKE intronic mutation in genetically unsolved cases of familial atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol 10: 1011–1019, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozaltin F, Li B, Rauhauser A, An SW, Soylemezoglu O, Gonul II, Taskiran EZ, Ibsirlioglu T, Korkmaz E, Bilginer Y, Duzova A, Ozen S, Topaloglu R, Besbas N, Ashraf S, Du Y, Liang C, Chen P, Lu D, Vadnagara K, Arbuckle S, Lewis D, Wakeland B, Quigg RJ, Ransom RF, Wakeland EK, Topham MK, Bazan NG, Mohan C, Hildebrandt F, Bakkaloglu A, Huang CL, Attanasio M: DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN. J Am Soc Nephrol 24: 377–384, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JM, Park YS, Lee JH, Park SJ, Shin JI, Park YH, Yoo KH, Cho MH, Kim SY, Kim SH, Namgoong MK, Lee SJ, Lee JH, Cho HY, Han KH, Kang HG, Ha IS, Bae JS, Kim NK, Park WY, Cheong HI: Atypical hemolytic uremic syndrome: Korean pediatric series. Pediatr Int 57: 431–438, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Cooper GM, Stone EA, Asimenos G, Green ED, Batzoglou S, Sidow A; NISC Comparative Sequencing Program : Distribution and intensity of constraint in mammalian genomic sequence. Genome Res 15: 901–913, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garber M, Guttman M, Clamp M, Zody MC, Friedman N, Xie X: Identifying novel constrained elements by exploiting biased substitution patterns. Bioinformatics 25: i54–i62, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR: A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar P, Henikoff S, Ng PC: Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc 4: 1073–1081, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Schwarz JM, Rödelsperger C, Schuelke M, Seelow D: MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods 7: 575–576, 2010 [DOI] [PubMed] [Google Scholar]
- 18.Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S: Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLOS Comput Biol 6: e1001025, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noris M, Mele C, Remuzzi G: Podocyte dysfunction in atypical haemolytic uraemic syndrome. Nat Rev Nephrol 11: 245–252, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Noris M, Remuzzi G: Managing and preventing atypical hemolytic uremic syndrome recurrence after kidney transplantation. Curr Opin Nephrol Hypertens 22: 704–712, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Dipple KM, McCabe ER: Phenotypes of patients with “simple” Mendelian disorders are complex traits: Thresholds, modifiers, and systems dynamics. Am J Hum Genet 66: 1729–1735, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.