Abstract
Congenital anomalies of the kidney and urinary tract (CAKUT) occur in three to six of 1000 live births, represent about 20% of the prenatally detected anomalies, and constitute the main cause of CKD in children. These disorders are phenotypically and genetically heterogeneous. Monogenic causes of CAKUT in humans and mice have been identified. However, despite high-throughput sequencing studies, the cause of the disease remains unknown in most patients, and several studies support more complex inheritance and the role of environmental factors and/or epigenetics in the pathophysiology of CAKUT. Here, we report the targeted exome sequencing of 330 genes, including genes known to be involved in CAKUT and candidate genes, in a cohort of 204 unrelated patients with CAKUT; 45% of the patients were severe fetal cases. We identified pathogenic mutations in 36 of 204 (17.6%) patients. These mutations included five de novo heterozygous loss of function mutations/deletions in the PBX homeobox 1 gene (PBX1), a gene known to have a crucial role in kidney development. In contrast, the frequency of SOX17 and DSTYK variants recently reported as pathogenic in CAKUT did not indicate causality. These findings suggest that PBX1 is involved in monogenic CAKUT in humans and call into question the role of some gene variants recently reported as pathogenic in CAKUT. Targeted exome sequencing also proved to be an efficient and cost-effective strategy to identify pathogenic mutations and deletions in known CAKUT genes.
Keywords: kidney development, genetics and development, genetic renal disease
Congenital anomalies of the kidney and urinary tract (CAKUT) refer to a heterogeneous group of malformations of the kidney and the urinary tract due to defects in embryonic kidney development, a complex process that involves reciprocal interaction between the ureteric bud and the metanephric mesenchymal tissue. Prevalence of CAKUT has been estimated between three and six per 1000 live births. These kidney defects are frequently detected in utero by prenatal ultrasonography and account for 15%–20% of all of the prenatally detected congenital anomalies .1 Their severity is extremely variable, but CAKUT represent the leading cause of CKD in children.2 Genetic and environmental factors are proposed to be involved in CAKUT. Except for posterior urethral valves that usually appear as sporadic, familial clustering of CAKUT has been frequently described3; however, the heritability estimates remain unknown. In some families, CAKUT are inherited as a Mendelian monogenic disease, mostly transmitted as an autosomal dominant trait with variable expressivity. Although in these cases CAKUT are frequently associated with extrarenal anomalies (>100 syndromes including renal or urinary abnormalities, with a Mendelian transmission, have been described),4 they can also present with isolated kidney diseases, particularly at prenatal ultrasound screening. Identification of the molecular defect in these patients is important to provide genetic counseling to the families. A monogenic cause of CAKUT was reported to be identified in approximately 10%–15% of patients presenting with isolated CAKUT.5 Among the approximately 50 genes with reported mutations, PAX2 and HNF1B are the two genes most frequently mutated in syndromic or isolated CAKUT.6,7 Copy number variations (CNVs) have also been shown to be frequently associated with syndromic as well as isolated CAKUT; however, these CNVs are frequently attributable to known genomic disorders.8–10 Recently, the simultaneous analysis of multiple candidate genes in parallel by next generation sequencing (NGS) was reported not to lead to a substantial increase in the proportion of patients with CAKUT with identified mutations,11 suggesting that inheritance may frequently be more complex and/or involve noncoding regions, epigenetic modifications, or somatic mutations.
Here, we report targeted NGS of 330 genes known to be either involved in CAKUT or candidate genes in a large cohort of 204 unrelated patients with CAKUT, which led to the identification of a novel CAKUT gene, PBX1.
Results
Patient Cohort
Two hundred four unrelated patients with CAKUT were included (K1–K204 in Supplemental Table 1). Criteria for inclusion were CAKUT involving both kidneys or unilateral CAKUT associated with either extrarenal defects or familial history of CAKUT (Table 1). Patients with posterior urethral valves were not included in the study. Fifty of the 204 patients had been previously tested for mutations in HNF1B (involved in renal cysts and diabetes syndrome) and/or PAX2 (involved in papillorenal syndrome) and/or EYA1 (involved in branchio-oto-renal syndrome) by Sanger sequencing and were revealed to be negative, and nine fetuses had been tested for mutations in RET (involved in bilateral kidney agenesis12), with one identified as carrying a variant of unknown significance.13 In addition, we also tested 11 patients suspected to suffer from branchio-oto-renal syndrome because of branchial and/or ear abnormalities but without any renal phenotype (normal renal ultrasound) (BO1–BO11 in Supplemental Table 1). Sex ratio was 116 boys, 89 girls, and ten patients for which sex was unknown. Not including the 11 BO patients, 79 patients presented extrarenal anomalies. Fifty-five patients had familial history of renal disease affecting first-degree relative(s) (parents, children, or affected siblings), and eight additional patients had history of renal disease in more distant family. Moreover, seven patients had a parent with branchial defects and/or deafness (five), coloboma (one), or diabetes (one) without renal defect (Supplemental Table 1, Table 1). Ninety-three (45%) cases were fetuses affected with extremely severe renal anomalies at fetal ultrasound screening, which led to termination of pregnancy after parents’ request and case by case evaluation by a multidisciplinary prenatal committee in accordance with the French law. Autopsy was performed after informed consent in all cases.
Table 1.
Renal and extrarenal phenotypes of the cohort encompassing 204 patients with CAKUT and 11 patients with branchial and/or ear defect (215 patients)
| Phenotype | Patients | Extrarenal Phenotypea | Family History | |
|---|---|---|---|---|
| Renal Defect | Nonrenal Defect | |||
| Main CAKUT phenotype | ||||
| Bilateral kidney agenesis | 46 | 14 | 14 | |
| Bilateral multicystic dysplasia | 12 | 3 | 2 | |
| Unilateral kidney agenesis | 15 | 10 | 7 | 2b,c |
| Unilateral kidney agenesis plus secondary CAKUT phenotyped | 23 | 7 | 6 | 1b |
| Unilateral multicystic dysplasia | 5 | 3 | 2 | |
| Unilateral multicystic dysplasia plus secondary CAKUT phenotyped,e | 9 | 4 | 2 | 1f |
| Renal hypoplasia | 22 | 10 | 6 | 1b |
| Renal hypoplasia plus secondary CAKUT phenotyped,g | 34 | 12 | 9 | 2c,h |
| Renal dysplasiai | 21 | 8 | 7 | |
| Renal dysplasia plus secondary CAKUT phenotyped,j | 6 | 1 | 2 | |
| Otherk | 11 | 7 | 6 | |
| Total | 204 | 79 | 63 | 7 |
| Branchial signs and/or ear defect without CAKUT phenotype | 11 | 11 | 0 | |
Includes Mullerian anomalies, urogenital sinus, testis agenesis, anal atresia, cardiopathy, VATER (vertebrae, anus, trachea, esophagus, renal) syndrome, diaphragmatic hernia, cystic adenomatoid lung malformation, lung isomerism, bone defect (ribs, vertebra, or polydactyly), eye defect (coloboma or morning glory syndrome), external ear abnormalities, deafness, branchial defect, pancreatic hypoplasia, abnormal teeth, liver defect, microcephaly, dysmorphic features, central nervous system anomaly, intellectual disability, and mellitus diabetes.
Parent with BO without renal defect.
Parent with deafness without renal defect.
Includes all patients with two CAKUT phenotypes (e.g., unilateral kidney agenesis plus contralateral multicystic dysplastic kidney and renal hypoplasia plus pelviureteric junction obstruction).
Does not include unilateral multicystic dysplasia plus unilateral kidney agenesis, which is counted in the unilateral kidney agenesis plus secondary CAKUT diagnosis.
Parent with diabetes without renal defect.
Does not include renal hypoplasia plus unilateral kidney agenesis, which is counted in unilateral kidney agenesis plus secondary CAKUT diagnosis, or renal hypoplasia plus unilateral multicystic dysplasia, which is counted in multicystic kidney dysplasia plus secondary CAKUT.
Parent with coloboma without renal defect.
Renal dysplasia was defined by histology of the kidney and/or hyperechogenicity of the renal parenchyma at ultrasound with or without cysts.
Does not include renal dysplasia plus unilateral multicystic dysplasia, plus unilateral renal agenesis, or plus renal hypoplasia and/or plus renal ectopy, plus hydronephrosis, or plus vesicoureteral reflux.
Includes vesicoureteral reflux, pelviureteric junction obstruction, obstructive megaureter, hydronephrosis without further known diagnosis, and renal ectopy.
Screening the Candidate Genes and Identification of PBX1 as a Novel Gene Involved in Monogenic CAKUT
Among the 330 genes, 275 were candidate genes with no mutation reported in patients with CAKUT so far (Supplemental Table 2). Analysis of these genes in the 204 individuals with renal involvement led to the identification of 125 heterozygous variants never reported in the Exome Aggregation Consortium (ExAC) database in a total of 88 genes in 92 patients (Supplemental Tables 1 and 3). In one of these genes, PBX1, we identified five heterozygous loss of function mutations/deletions (Figure 1, Table 2). The first mutation (family 1) was a frameshift mutation (p.Asn143Thrfs*37) in a 21-year-old adult affected with renal hypoplasia and deafness. She has an eGFR of 40 ml/min per 1.73 m2. The second one (family 2) was a nonsense mutation (p.Arg184*) in a child presenting with small hyperechogenic kidneys with cysts, developmental delay, growth retardation, and long and narrow face. She had eGFR of 51 ml/min per 1.73 m2 at the age of 11 years old. The third one (family 3) was a splice mutation (c.511–2A>G) in a fetus with renal hypoplasia and oligoamnios. The autopsy showed extremely severe oligonephronia. RNA was not available to study the effect of that mutation on splicing, but the mutation is predicted to lead to total loss of the 3′ splice site and skipping of exon 4, resulting in a frameshift. The two last patients presented with a heterozygous deletion removing the whole PBX1 gene indicated by a reduced number of reads for the nine exons of the gene (Figure 1). The first one (family 4) was a 39-year-old adult woman followed for small and dysplastic horseshoe kidney also affected with profound deafness. She was the oldest patient of our series and had stage 3B renal failure (eGFR=40 ml/min per 1.73 m2) at last evaluation. The second one (family 5) was an infant with a single small hyperechogenic kidney also presenting developmental delay, microcephaly, and facial dysmorphism (with long and narrow face as well as abnormal ear lobes). He had normal renal function at the age of 18 months old. Comparative Genomic Hybrization (CGH) array analysis (Agilent 60 K) performed on DNA from patients’ lymphocytes confirmed presence of a 2.46-Mb deletion at 1q23.3q24.1 including PBX1 and seven other genes in a family 4′ patient and a 9.1-Mb deletion on the long arm of a chromosome 1 including PBX1 together with 130 other genes (Supplemental Figure 1) in a family 5′ patient. De novo occurrence of the deletion in patients K136 (family 5) was confirmed by fluorescence in situ hybridization on parents’ lymphocytes, which was normal (Supplemental Figure 2). The pelviureteric junction obstruction in the patient’s mother (shown in gray in Figure 1) is thus an incidental association. Sanger sequencing (families 1–3) or analysis of four microsatellite markers located in PBX1 (family 4) allowed us to show that the mutations/deletion in these families also occurred de novo (Figure 1, Supplemental Table 4). In all five families, misclassification of the parents was excluded by analysis of at least 12 unlinked microsatellite markers located on various chromosomes (Supplemental Table 4) and the identification of a paternally inherited CNV on 1p (family 5) (Supplemental Figure 2). Moreover, by retrospective analysis of our cohort of patients with CAKUT, we identified another individual not included in the 204 patients screened by targeted NGS, in whom a de novo 6.2-Mb deletion, including PBX1, had been identified by CGH array analysis. This patient presented with small hyperechogenic kidneys, developmental delay, and facial dysmorphism, similar to the patient from family 5. He already had severe proteinuria associated with a rapidly progressing renal failure (eGFR=60 ml/min per 1.73 m2) at the age of 10 years old.
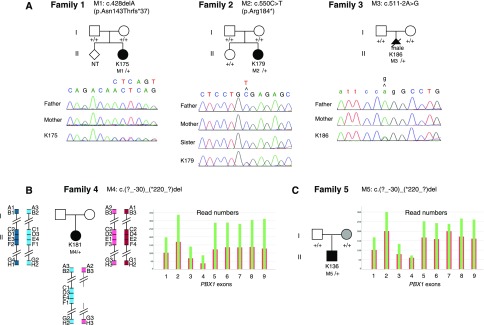
Figure 1.
Identification of de novo PBX1 mutations in three families and deletions in two families. (A) Pedigrees of three families with one affected individual (in black) carrying a de novo point mutations in PBX1. The affected individual in family 1 (K175) was a 21-year-old woman presenting with renal hypoplasia and deafness. The affected individual in family 2 (K179) was a 12-year-old girl presenting with small hyperechogenic kidneys with cysts, developmental delay, growth retardation, and long and narrow face. The affected individual in family 3 (K186) was a male fetus with extremely severe renal hypoplasia. Loss of function mutations (deletion of one base leading to a frameshift, nonsense mutation, or nucleotide change in the consensus acceptor splice site located in 3′ of intron 3) identified in patients K175, K179, and K186, respectively, were validated by Sanger sequencing. Absence of the mutation in the parents showed de novo occurrence in all patients. NT, not tested. (B) Pedigree of family 4 with a 40-year-old woman (K181) affected with small and dysplastic horseshoe kidney and deafness. NGS analysis of patient DNA revealed a reduced number of reads for all of the exons of PBX1 (in red) compared with the mean number of reads for the 37 other DNAs analyzed in the same run (in green), suggesting deletion of one PBX1 allele. Haplotypes of the affected woman and her parents were generated using four known microsatellite markers upstream and downstream of PBX1 (markers A, B, G, and H) as well as four intragenic PBX1 markers (markers C–F). Genomic positions of the microsatellite markers (on Human Genome version GRCh38.p7 from Ensembl) are A (D1S2675): chr1:162,240,203–162,240,364; B (D1S2844): chr1:162,979,036–162,979,218; C (in PBX1 intron 1): chr1:164,562,253–164,562,694; D (in PBX1 intron 2): chr1:164,651,731–164,652,170; E (in PBX1 intron 2): chr1:164,707,219–164,707,656; F (in PBX1 intron 8): chr1:164,833,830–164,834,270; G (D1S2762) chr1:166,986,900–166,987,137; and H (D1S196): chr1:167,635,063–167,635,195. Deletion of a <4-Mb region encompassing PBX1 located on the maternally inherited chromosome was shown by the lack of maternal contribution for markers C–F (genotypes are in Supplemental Table 3). (C) Pedigree of family 5 with a 2-year-old infant boy (K136) presenting with a single hyperechogenic kidney associated with developmental delay, microcephaly, and facial dysmorphism (with long and narrow face as well as abnormal ear lobes). The mother was presenting pyeloureteric junction obstruction. NGS analysis of K136 DNA revealed a reduced number of reads for all of the exons of PBX1 (in red) compared with the mean number of reads for the 38 other DNA analyzed in the same run (in green), suggesting deletion of one PBX1 allele. Validation of this deletion and identification of its extent and de novo occurrence were performed by CGH analysis (Supplemental Figure 1).
Table 2.
De novo loss of function variants in PBX1 (NM_002585)
| Patient | Kidney Phenotype | Renal Function | Extrarenal Phenotype | Nucleotide Change | Protein Change |
|---|---|---|---|---|---|
| K175 | Bilateral hypoplasia | eGFR=40 ml/min per 1.73 m2 (21 yr) | Deafness plus scoliosis | c.[428delA];[=] | p.[Asn143Thrfs*37];[=] |
| K179 | Bilateral cystic hypodysplasia | eGFR=51 ml/min per 1.73 m2 (11 yr) | Dysmorphic features plus developmental delay | c.[550C>T];[=] | p.[Arg184*];[=] |
| K186 | Bilateral hypoplasia with oligonephronia | Oligoamnios | No | c.[511–2A>G];[=] | |
| K181 | Hypoplasic horseshoe kidney, absence of corticomedullar differentiation | eGFR=40 ml/min per 1.73 m2 (39 yr) | Deafness | c.[(?_−30)_(*220_?)del];[=] | |
| K136 | Unilateral agenesis/small hyperechogenic kidney | Normal renal function (18 mo) | Dysmorphic features plus intellectual disability | c.[(?_−30)_(*220_?)del];[=] |
To precisely evaluate the strength of association between PBX1 mutations and CAKUT, we used a binomial probability test. The P value for identifying three de novo loss of function mutations in that gene among 204 patients was <0.001 (Supplemental Figure 3). The identification of three de novo loss of function mutations together with two de novo large deletions encompassing the gene among 204 patients with CAKUT strongly support the causal effect of the PBX1 mutations.
Screening the Known CAKUT Genes
In 31 of the 204 patients, we identified 24 pathogenic mutations in seven known genes associated with highly penetrant CAKUT, namely HNF1B, PAX2, EYA1, ANOS1, GATA3, CHD7, and KIF14 (Figure 2, Table 3). Three of the mutations/deletions, affecting GATA3 or KIF14, were identified in patients who had been previously screened for HNF1B, PAX2, and/or EYA1 by Sanger sequencing and remained unsolved. Renal phenotypes, extrarenal anomalies, and mutation inheritance are shown in Table 3 (Supplemental Table 1). As frequently observed for patients with mutations in these genes, 16 of the patients had extrarenal symptoms. Notably, the fetuses with biallelic KIF14 mutations presented severe microcephaly and agenesis of the corpus callosum as recently reported.14 Fourteen patients were sporadic, and de novo occurrence of the mutation was shown for the five families with available parental DNA. Somatic mosaicism in the mother was identified in one patient with a PAX2 mutation. Ten patients had a family history of CAKUT (Supplemental Table 1, Table 3), and presence of the mutation was shown in the affected related individual in the seven tested families. In addition, in six patients with a family history of branchial and/or ear defect (three patients), deafness (one patient), diabetes (one patient), or coloboma (one patient), mutations were inherited from a parent with branchial and/or ear defect (EYA1), diabetes (HNF1B), coloboma (PAX2), or deafness (GATA3) but no renal defect in agreement with incomplete penetrance of the renal defect associated with mutations in these genes.15–17 Altogether, 11 mutations were large deletions removing all exons of HNF1B (eight), GATA3 (two), or PAX2 (one); five were intragenic deletions removing few exons of PAX2 (two), EYA1 (one), ANOS1 (one), or KIF14 (one; homozygous); and 18 were point mutations (including small deletions up to 9 bp), all absent from the ExAC, except for one EYA1 mutation that was present with a frequency of one of 66,722 in the European population. The 15 heterozygous large deletions were confirmed by either CGH or multiplex ligation–dependent probe amplification, and the homozygous deletion of KIF14 was confirmed by sequencing of a cDNA synthesized from kidney RNA of the affected fetus (Supplemental Figure 4).
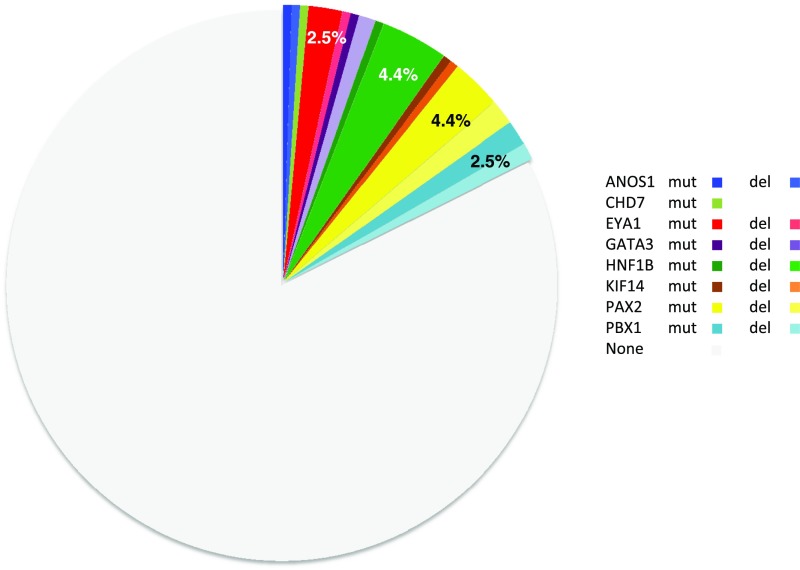
Figure 2.
Identification of causative mutations/deletions in 36 of the 204 CAKUT cases. For each gene (ANOS1, CHD7, EYA1, GATA3, HNF1B, KIF14, PAX2, and PBX1), the proportions of patients with mutations and deletions are shown in dark and light colors, respectively. All of the mutations/deletions are heterozygous, except for KIF4, for which they are biallelic.
Table 3.
Causative mutations in known genes associated with highly penetrant CAKUT phenotype
| Patient | Renal Phenotypea | Extrarenal Phenotype | Causal Gene | NM_ref | Nucleotide Change | Protein Change | PP2 | Sift | MT | Granthamb | GVDVc | No. of Missense Deleterious Scores | Splice Effect, % | ExAC | Inheritance | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rare variants in individuals with CAKUT | ||||||||||||||||
| K4 | RH | Bifid uterus | HNF1B | 000458 | c.[(?_−30)_(*220_?)del];[=] | 0 | NAd | 36 | ||||||||
| K50 | UMD+ | No | HNF1B | 000458 | c.[(?_−30)_(*220_?)del];[=] | 0 | Affected mothere | |||||||||
| K103 | RD+ | No | HNF1B | 000458 | c.[(?_−30)_(*220_?)del];[=] | 0 | Affected mothere | |||||||||
| K124 | RD+ | No | HNF1B | 000458 | c.[(?_−30)_(*220_?)del];[=] | 0 | De novod | |||||||||
| K143 | RD+ | No | HNF1B | 000458 | c.[(?_−30)_(*220_?)del];[=] | 0 | De novod | |||||||||
| K172 | UMD+ | Diabetes plus cryptorchidy | HNF1B | 000458 | c.[(?_−30)_(*220_?)del];[=] | 0 | Affected motherf | |||||||||
| K185 | RD | No | HNF1B | 000458 | c.[(?_−30)_(*220_?)del];[=] | 0 | NAd | |||||||||
| K203 | RD | No | HNF1B | 000458 | c.[(?_−30)_(*220_?)del];[=] | 0 | NAe | |||||||||
| K170 | RH+ | No | HNF1B | 000458 | c.[344+2_+5del];[=] | −100 | 0 | NAd | ||||||||
| K72 | RH | Papillary excavation | PAX2 | 003987 | c.[(?_−30)_(*220_?)del];[=] | 0 | Germinal mosaicism/two affected childrene | 37 | ||||||||
| K62 | RH | Eye coloboma | PAX2 | 003987 | c.[?−30_410+?del];[=] | 0 | NAd | |||||||||
| K79 | RD | Papillary coloboma | PAX2 | 003987 | c.[44−?_496+?del];[=] | 0 | De novod | |||||||||
| K117 | RH | No | PAX2 | 003987 | c.[183C>A];[=] | p.[Ser61Arg];[=] | 1 | 0 | dc | 110 | C0 | 4/5 | 0 | De novod | ||
| K133 | RH+ | Papillary coloboma | PAX2 | 003987 | c.[76del];[=] | p.[Val26Cysfs*3];[=] | 0 | Affected motherg | 38 | |||||||
| K146 | RH+ | No | PAX2 | 003987 | c.[446del];[=] | p.[Pro149Glnfs*10];[=] | 0 | NAd | ||||||||
| K148 | RH | No | PAX2 | 003987 | c.[76dup];[=] | p.[Val26Glyfs*28];[=] | 0 | Unaffected mother (somatic mosaicism)d | 39 | |||||||
| K176 | RH+ | No | PAX2 | 003987 | c.[331G>A];[=] | p.[Ala111Thr];[=] | 0.998 | 0 | dc | 58 | C0 | 4/5 | 0 | Affected mothere | 40 | |
| K199 | Oth | Papillary coloboma | PAX2 | 003987 | c.[212G>A];[=] | p.[Arg71Lys];[=] | 0.974 | 0 | dc | 26 | C0 | 3/5 | −49 | 0 | NAe,h | 41 |
| K80 | UKA+ | Preauricular pit plus ear tag | EYA1 | 000503 | c.[557−?_1597+?del];[=] | 0 | Affected motheri | |||||||||
| K78 | BKA | No | EYA1 | 000503 | c.[967–1G>C ]:[=] | −100 | 0 | Affected fathere | 42 | |||||||
| K119 | RH | Branchial defect plus deafness | EYA1 | 000503 | c.[1459T>C];[=] | p.[Ser487Pro];[=] | 0.132 | 0.07 | dc | 74 | C0 | 2/5 | 0.0015% NFE | Affected fatheri | 43 | |
| K135 | UKA | Preauricular pit | EYA1 | 000503 | c.[553C>T];[=] | p.[Gln185*];[=] | 0 | Affected motheri | 44 | |||||||
| K145 | RH | Branchial defect | EYA1 | 000503 | c.[1338_1346del];[=] | p.[Asn446_Tyr448del];[=] | 0 | Affected fathere | ||||||||
| K101 | UKA | Ear tag plus external ear canal stenosis | GATA3 | 001002295 | c.[(?_−30)_(*220_?)del];[=] | 0 | Affected fatherj | 45 | ||||||||
| K167 | UKA | Deafness plus intellectual disability | GATA3 | 001002295 | c.[(?_−30)_(*220_?)del];[=] | 0 | NAd | |||||||||
| K48 | RH+ | No | GATA3 | 001002295 | c.[829C>T];[=] | p.[Arg277*];[=] | 0 | De novod | 46 | |||||||
| K158 | BKA | No | ANOS1 | 000216 | c.[?−30_255+?del] | 0 | Unaffected mothere | 47 | ||||||||
| K26 | BKA | No | ANOS1 | 000216 | c.[769C>T];[=] | p.[Arg257*];[=] | 0 | NAd | 48 | |||||||
| K160 | UKA | Deafness plus branchial defect plus colobomatous microphtalmia | CHD7 | 017780 | c.[5050G>A];[=] | p.[Gly1684Ser];[=] | 0.929 | 0 | dc | 56 | C55 | 5/5 | −38 | 0 | Fatherk | 49 |
| K73 | RH+ | Lissencephaly plus agenesis of corpus callosum | KIF14 | 014875 | c.[3567−?_4072+?del];[(3567-?_4072+?del)] | 0 | NAd | |||||||||
| K195 | BKA | Craniostenosis plus microcephaly plus agenesis of corpus callosum | KIF14 | 014875 | c.[3910C>T];[1090C>T] | p.[Gln1304*];[Arg364Cys] | 1 | 0 | dc | 180 | C0 | 4/5 | 0 | One mutation from each parente | ||
| Rare variants in individuals with branchial signs and/or ear defect without CAKUT phenotype | ||||||||||||||||
| BO4 | No | Branchial defect plus preauricular pit plus deafness | EYA1 | 000503 | c.[1081C>T];[=] | p.[Arg361*];[=] | 0 | De novod | 50 | |||||||
| BO5 | No | Branchial defect plus deafness | SIX1 | 005982 | c.[273_274insC];[=] | p.[Tyr92Leufs*62];[=] | 0 | Affected motheri | ||||||||
PP2, Polyphen2; MT, MutationTaster; GVDV, Grantham variation score and Grantham difference score; RH, renal hypoplasia; NA, not available; UMD, unilateral multicystic dysplasia; RD, renal dysplasia; Oth, other; UKA, unilateral kidney agenesis; BKA, bilateral kidney agenesis; NFE, non-Finnish European population.
Plus indicates presence of (a) secondary CAKUT phenotype(s).
Considered as deleterious when ≥50.
Considered as deleterious when ≥C25.
Patient with sporadic case.
Patient with familial case.
Parent affected with diabetes without renal defect.
Parent affected with coloboma without renal defect.
Mutation transmitted to a son with coloboma without renal defect.
Parent affected with BO without renal defect.
Parent affected with deafness without renal defect.
Clinical information not available.
We also identified 21 rare heterozygous damaging variants in 12 genes reported as involved in dominant forms of CAKUT in a total of 17 individuals with CAKUT (Table 4). Extrarenal symptoms usually associated with mutations in some of these genes were never observed in these patients. All of the variants were missense predicted to be damaging by at least three of the five prediction programs used, and 13 of them, affecting a total of ten genes (BICC1, CDC5L, CHD1L, NOTCH2, RET, SALL1, SALL4, TBC1D1, TBX18, and TNXB), were absent from the ExAC. This suggests a causative effect for these variants, which remains to be shown. Of note, two of these variants were present in individuals with a pathogenic mutation identified (one variant in CDC5L in a child with a causative mutation in CHD7 and one in TBX18 in a fetus with a causative de novo mutation in GATA3), thus questioning the effect of these additional variants. Other patients were carrying variants in several genes, which may suggest oligogenic inheritance but also, highlights the arduousness of the interpretation of the variants (Table 4).
Table 4.
Variants in other genes reported in dominant forms of CAKUT, including previously reported variants and novel rare variants
| Patient | Renal Phenotypea | Extrarenal Phenotype | Gene | NM_ref | Nucleotide Change | Amino Acid Change | No. of Missense Deleterious Scoresb | ExACc | Inheritance | Ref. | Other Variant(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Novel rare variants in individuals with CAKUT | |||||||||||
| K94 | UKA+ | Didelphys uterus | BICC1 | 001080512 | c.[2264C>G];[=] | p.[Thr755Arg];[=] | 4/5 | 0 | NAd | ||
| K127 | UKA+ | No | BICC1 | 001080512 | c.[1052G>A];[=], | p.[Cys351Tyr];[=] | 3/5 | 0 | Unaffected mother plus affected brothere | DSTYK, SOX17f | |
| K160 | UKA | Deafness plus branchial defect plus colobomatous microphtalmia | CDC5L | 001253 | c.[2240T>G];[=] | p.[Leu747Trp];[=] | 4/5 | 0 | Fatherg | CHD7 causative | |
| K162 | RH+ | Palate cleft plus preauticular pit | CDC5L | 001253 | c.[251C>G];[=] | p.[Thr84Ser]; [=] | 5/5 | 0 | NAe | ||
| K70 | BMD | No | CHD1L | 004284 | c.[1557A>C];[=], | p.[Lys519Asn];[=] | 4/5 | 0 | NAd | NOTCH2, SALL4 | |
| K127 | UKA+ | No | DSTYK | 015375 | c.[2674G>A];[=] | p.[Asp892Asn];[=] | 3/5 | 0.0075% NFE | Unaffected mother plus affected brothere | BICC1, SOX17f | |
| K32 | RH | No | NOTCH2 | 024408 | c.[1063G>A];[=] | p.[Asp355Asn];[=] | 3/5 | 0.0015% NFE | NAd | RETf | |
| K70 | BMD | No | NOTCH2 | 024408 | c.[5156G>A];[=] | p.[Arg1719Gln];[=] | 3/5 | 0.012% EAS | NAd | CHD1L, SALL4 | |
| K190 | RH+ | No | NOTCH2 | 024408 | c.[854G>A];[5903C>T] | p.[Arg285His] | 4/5; 5/5 | 0.0087% AMR; 0 | One from each parenth | ||
| K83 | BMD | No | RET | 020975 | c.[2063C>T];[=] | p.[Ser688Phe];[=] | 5/5 | 0 | Unaffected fathere | ||
| K91 | UKA | No | RET | 020975 | c.[1903C>T];[=] | p.[Arg635Cys];[=] | 5/5 | 0.012% SAS | Unaffected mother plus affected brothere | ||
| K69 | BMD | No | SALL1 | 002968 | c.[3771C>G];[=] | p.[Asn1257Lys];[=] | 4/5 | 0 | NAd | ||
| K70 | BMD | No | SALL4 | 020436 | c.[2492G>A];[=] | p.[Arg831Gln];[=] | 3/5 | 0 | NAd | CHD1L, NOTCH2 | |
| K29 | BKA | Small testis | SIX5 | 175875 | c.[2189C>T];[=] | p.[Ser730Leu];[=] | 4/5 | 0.012% NFE | NAd | ||
| K56 | RD | Interventricular communication plus intrauterine growth retardation | TBC1D1 | 015173 | c.[2790G>A];[=] | p.[Met930Ile];[=] | 3/5 | 0 | NAd | ||
| K105 | RH+ | No | TBC1D1 | 015173 | c.[2152C>T];[=] | p.[Arg718Cys];[=] | 4/5 | 0.015% FIN | NAd | ||
| K21 | BMD | No | TBX18 | 001080508 | c.[610C>T]; [=] | p.[His204Tyr];[=] | 5/5 | 0.0015% NFE | NAd | ||
| K48 | RH+ | No | TBX18 | 001080508 | c.[1483C>A];[=] | p.[Gln495Leu];[=] | 5/5 | 0 | Unaffected fatherd | GATA3 causative | |
| K192 | BKA | No | TBX18 | 001080508 | c.[772A>G]; [=] | p.[Ile258Val];[=] | 3/5 | 0 | NAd | ||
| K169 | UKA+ | No | TNXB | 019105 | c.[563C>T]; [=] | p.[Pro188Leu];[=] | 3/5 | 0g | NAd | ||
| Previously reported variantsf in individuals with CAKUT | |||||||||||
| K3 | BKA | Right ventricular dilation | RET | 020975 | c.[1699G>A];[=] | p.[Asp567Asn];[=] | 3/5 | 0.11% OTH | Unaffected father (absent in affected sister)e | 13 | |
| K32 | RH | No | RET | 020975 | c.[2081G>A];[=] | p.[Arg694Gln];[=] | 2/5 | 0.049% SAS | NAd | 18 | NOTCH2 |
| K127 | UKA+ | No | SOX17 | 022454 | c.[775T>A]; [=] | p.[Tyr259Asn];[=] | 2/5 | 1.75% OTH | Unaffected father plus affected brothere | 19 | BICC1, DSTYK |
| Previously reported variantsf in individuals with BO | |||||||||||
| BO11 | No | Deafness | DSTYK | 015375 | c.[654+1G>A];[=] | 0.088% AMR | NAd | 20 |
UKA, unilateral kidney agenesis; NA, not available; RH, renal hypoplasia; BMD, bilateral multicystic dysplasia; NFE, non-Finnish European; EAS, East Asian; AMR, Latino; SAS, South Asian; BKA, bilateral kidney agenesis; RD, renal dysplasia; FIN, Finnish; OTH, Other populations.
Plus indicates presence of (a) secondary phenotype(s).
Number of programs that predicted the variant as damaging among Polyphen2 (probably and possibly damaging), Sift (deleterious), MutationTaster (deleterious), Grantham (considered as deleterious when ≥50), and Grantham variation score and Grantham difference score (considered as deleterious when ≥C25).
Only variant frequencies in the population with the higher level are indicated.
Sporadic.
Familial.
Variant reported in the literature with a frequency in the ExAC above the one to 5000 threshold.
Clinical information not available.
Mother affected with deafness without renal defect.
In addition, using less stringent criteria for variant filtering, we identified four previously reported mutations with frequency in the ExAC that was above the one to 5000 threshold (Table 4). The RET p.Asp567Gln variation in a fetus with bilateral kidney agenesis (that patient has already been reported13) was inherited from his unaffected father and not carried by his sister, who presented with a less severe renal phenotype. This variant was identified in 19 individuals without CAKUT (seven being unrelated) from our in-house exome database (>8000 exomes). The RET p.Arg694Gln variation detected in a fetus with bilateral hypoplasia has been reported in a patient with Hirschsprung disease without associated renal involvement.18 This mutation has low pathogenicity scores. The SOX17 p.Tyr259Asn variation, previously reported to be associated with vesicoureteral reflux,19 was identified in a patient also carrying missense variants in BICC1 and DSTYK. That SOX17 variant was inherited from his unaffected father. It is found with a frequency of one in 181 in the European population in the ExAC and in 82 individuals without CAKUT in our in-house exome database. Finally, the DSTYK splice variant (c.654+1), recently reported in a large family with CAKUT,20 was identified in a BO individual with normal renal ultrasound scan. That variant is found in the ExAC database with a frequency of one in 3563 in the European population and was identified in 11 individuals without CAKUT (ten being unrelated) in our in-house exome database.
Burden Analyses of Rare Variants in Patients with Unsolved Cases
Finally, for the 168 patients with no mutation identified in PBX1 or any of the highly penetrant CAKUT genes (HNF1B, PAX2, EYA1, ANOS1, GATA3, CHD7, or KIF14), we performed a burden test to look for genes with an enrichment in rare variants. When considering only loss of function variants, we did not identify any gene with nominal P value <0.05. When considering loss of function plus missense, we identified a few genes with nominal P value <0.05, but none of them reached significance with Bonferroni correction (P<1.5×10−4). These results are shown in Supplemental Table 5.
Discussion
Whole-exome sequencing is currently considered as the optimal approach for identifying new genes involved in human diseases. However, targeted exome sequencing, because it offers the advantage to analyze larger series of patients for the same cost, may prove more efficient for mutation identification in the context of highly genetically heterogeneous diseases, such as CAKUT, provided that the gene selection is relevant. Analysis of candidate genes allowed us to identify five de novo PBX1 mutations/deletions, including three loss of function point mutations, showing that this gene is a novel CAKUT gene. PBX1 encodes the Pre-B Cell Leukemia Transcription Factor 1, a TALE homeodomain transcription factor known to play a crucial role in several developmental processes and modify Hox gene activity.21 In the mouse, Pbx1 was notably shown to play a role during kidney development.22,23 It is expressed in the nephrogenic mesenchyme, weakly in the induced mesenchyme and strongly in the stroma after ureteric bud invasion. Its expression decreases when cells undergo epithelial differentiation, whereas it increases in glomerular cells at the stage of capillary loop extension. Except for Pdgfrb, which was recently reported as a direct target gene during vascular patterning,24 Pbx1 transcriptional targets in the kidney remain largely unknown. Although no defect was reported in Pbx1+/− kidneys, a majority of Pbx1−/− embryos exhibit hypoplastic kidneys (30% with unilateral kidney agenesis), poorly defined cortical and medullary regions (E13.0), and a reduced number of differentiating nephrons (E14.5). Mutant kidneys were also reported to be mispositioned caudally. Histologic analysis revealed defects in ureteric branching and abnormal expansion of induced mesenchyme, arising from a mesenchymal dysfunction that led to a reduced mesenchymal to epithelial differentiation.23 Pbx1−/− mouse kidney phenotypes are thus highly reminiscent of those of the patients with heterozygous PBX1 mutations (hypoplastic kidneys [all patients], unilateral agenesis [K136], oligonephronia [K186], and horseshoe kidney with absence of corticomedullar differentiation [K181]). A phenotype associated with haploinsufficiency in human but not in mouse has already been reported for other transcription factor–encoding genes involved in kidney development (e.g., HNF1B, SIX1, and SIX2).
In 2012, Sanna-Cherchi et al.9 reported a 0.5-Mb de novo deletion at 1q32, including PBX1, in a patient with renal hypoplasia. More recently, the study of overlapping deletions on the long arm of chromosome 1 in several patients presenting with renal malformation associated with other defects suggested that haploinsufficiency of PBX1 might be responsible for the renal phenotype.25 Our data confirm that hypothesis, because three PBX1 mutations were de novo point mutations leading to a null allele. As for CAKUT associated with heterozygous mutations in genes encoding other transcription factors (HNF1B, PAX2, EYA1, and SALL1), the severity of the renal disease associated with PBX1 defect seems variable: the oldest patient presents with stage 3 renal failure (eGFR of 40 ml/min per 1.73 m2) at the age of 39 years old, whereas the fetal case showed major renal hypoplasia leading to oligohydramnios. This could be due to variants in modifier genes. Associated deafness was present in two of the five patients with PBX1 mutation/deletion who were not carrying additional mutation or deletion of PAX2, GATA3, EYA1, or SIX1, four genes with mutations that may lead to deafness. In a mouse cochlear hair cell line, Pbx1 is coexpressed with Gata3,26 suggesting that PBX1 mutations might be responsible for deafness. However, the majority of patients with large deletions at 1q23.3q24.1 removing PBX1 do not have deafness.25 Microcephaly, facial dysmorphism, ear anomalies, and developmental delay in a family 5 patient were reported in other patients with large deletions at that locus25; however, developmental delay and facial dysmorphism were also present in the patient from family 2 carrying a PBX1 nonsense mutation. Screening of larger series of patients with CAKUT for mutations in PBX1 will be necessary to more precisely evaluate their association with extrarenal phenotypes.
Our strategy of targeted exome sequencing also allowed us to improve the efficiency of identification of causative mutations in patients with known CAKUT compared with our previous workflow (by Sanger sequencing and MLPA analysis), in which only HNF1B, PAX2, and/or EYA1 were screened. Altogether, causative mutations, including the five PBX1 mutations, were identified in 36 of the 204 (17.6%) patients with CAKUT. This rate is far above that reported recently by a similar NGS approach (six of 453),11 and this is likely due, at least in part, to the fact that the spectrum of phenotypes included was different. We only included CAKUT affecting both kidneys and/or familial and/or syndromic forms, excluding posterior urethral valves known to be most frequently sporadic, whereas Nicolaou et al.11 included unilateral forms and many posterior urethral valves. The rate of mutation that we observed here is also higher than that reported by Thomas et al.6 but close to that reported by Weber et al.5 or Madariaga et al.7 Of note, the proportion of patients with HNF1B, PAX2, or EYA1 mutations is probably slightly biased downward, because our cohort included 50 patients already known not to carry mutations in these genes. Interestingly, 40% of the identified mutations are deletions, removing either a few exons or the whole gene. Indeed, a major advantage of NGS is the identification of both point mutations and deletions (intragenic and whole-gene deletions) in a sole experiment. In addition to HNF1B deletions, heterozygous deletions, which would not have been diagnosed by our previous workflow, were identified in PAX2, EYA1, GATA3, and ANOS1. Also, one homozygous intragenic deletion of KIF14 was shown in a consanguineous family. No mutation was identified in ITGA8 or FGF20, two genes recently reported as responsible for autosomal recessive bilateral kidney agenesis.27,28
Among variants identified in this study, some are in other genes previously reported as responsible for dominant CAKUT (Table 4). However, although these variants met criteria supporting their pathogenicity (absence or low frequency in the ExAC and pathogenicity scores indicating deleterious effect), in the seven patients whose parents could be tested, the variants were inherited from an unaffected parent (Table 4), thus questioning their causality. In one patient with renal cystic hypodysplasia and deafness, compound heterozygous variants in NOTCH2 were shown in the index patient, the significance of which is unknown. Growing numbers of available data regarding the frequency of variants among large control populations can lead variants initially considered as pathogenic to be reclassified. The frequency of the SOX17 p.Tyr259Asn variation in the ExAC and our in-house database indicates that this variant is likely not causative, although it could be an at-risk allele as recently proposed.29 As for the DSTYK splice variant (c.654+1)20 that we identified in a BO individual without any renal involvement, the rate of this variant in the ExAC and our in-house database supports that, if involved in the development of a CAKUT phenotype, it has an incomplete penetrance. This highlights the fact that one must be cautious when interpreting variants reported as pathogenic in the literature. Indeed, some of them can reveal as low-penetrance alleles, modifiers, or even frequent polymorphisms instead of causative mutations.
Except for KIF14, no ballelic mutation was identified in any of the genes previously reported in recessive forms of CAKUT. As in the work by Nicolaou et al.,11 we did not identify any significant increase in rare variants in any of the tested genes, reflecting the complexity of genetic studies in CAKUT. Moreover, no pathogenic mutation or rare variant of unknown significance was identified in 40% of our series (Supplemental Table 1). Although mutations or CNVs in gene(s), noncoding regions, or microRNAs not targeted by our capture library could be involved in some of these patients, somatic events and/or environmental factors or epigenetic mechanisms likely explain at least part of this large fraction of patients. The familial aggregation of CAKUT as well as the wide spectrum of severity of the phenotypes suggest a complex genetic architecture as observed in neurodevelopmental disorders.30 This would fit with a model in which various combinations of variants as well as environmental factors will cause early kidney development insults and act together to alter renal and urinary tract formation. The timing, severity, location, and extent of the “deviation from an optimal renal developmental program” may determine the ultimate phenotype. Collaborative effort will be essential in the future to test a larger number of individuals by whole-exome sequencing, classify variants, and establish high-throughput functional assays to successfully decipher the complex genetic mechanisms of CAKUT and be able to propose appropriate genetic tests and counseling for the families.
During the review process of the paper, Le Tanno et al.31 reported a novel series of de novo 1q23.3-q24.1 microdeletions in patients with syndromic CAKUT. The patient carrying the smallest deletion, limited to PBX1, presented with mild developmental delay and hearing loss, which was observed in two patients of our series harboring PBX1 point mutations.
Concise Methods
Design of the Targeted SureSelect Library
The 330 genes included in our panel are shown in Supplemental Table 2 and include 55 genes with mutations reported in the literature in patients with isolated and/or syndromic CAKUT and 275 candidate genes (including 104 genes with knockout in mouse that led to kidney developmental defects, 84 genes involved in cellular processes/signaling pathways relevant for kidney development, 11 genes with a role in ureter/bladder development, 57 genes encoding reported targets of transcription factors WT1 or HNF1B, and 19 homologs of genes in the sublists above expressed during kidney/lower urinary tract development). The custom SureSelect gene panel was designed using the SureDesign software (Agilent). The target regions covered 1.38 Mb, including coding exons and splice junctions.
Targeted Exome Sequencing and Prioritization of the Variants
DNA was extracted from blood cells (living patients) or frozen tissues (fetuses). The study was approved by the Comité de Protection des Personnes pour la Recherche Biomédicale Ile de France 2, and informed consent was obtained from the patients or parents. Illumina-compatible precapture barcoded genomic DNA libraries were constructed, and a series of 16 or 36 barcoded libraries was pooled at equimolar concentrations. The capture process was performed according to the SureSelect protocol (Agilent) and sequencing on an Illumina HiSeq2500. Sequences were aligned to the reference human genome hg19 using the Burrows–Wheeler Aligner.
The mean depth of coverage ranged from 215 to 719, with ≥98% of the bases covered at least 30× (Supplemental Material). Variants were prioritized according to their frequency in the ExAC database32 and their predicted damaging effect. For known CAKUT genes, we selected previously published variants as well as nonsense, frameshift, splice variants, and missense predicted as damaging by at least three of five used prediction programs (Polyphen2, Sift, MutationTaster, Grantham score, and Align-GVGD) (Supplemental Material) with a minor allele frequency ≤1/5000 for genes involved in dominant forms and ≤1/1000 for those involved in recessive forms. For candidate genes, only variants that were absent from the ExAC were retained.
To evaluate duplication and large deletion events, for each individual, the relative read count for each targeted region was determined as the ratio of the read count for that region divided by the total absolute read counts of all targeted regions of the design. The ratio of the relative read count of a region in a given individual over the average relative read counts in other individuals of the run resulted in the estimated copy number for that region in that individual (method adapted from ref. 33).
Burden Test
To look for an excess of rare variants in the 330 genes in patients with CAKUT, we conducted a burden test using the Madsen and Browning34 Wilcoxon rank sum test and the SKAT-o test35 as implemented in EPACTS software (http://genome.sph.umich.edu/wiki/EPACTS). For the patients, we included the 168 patients with no mutation identified in PBX1 or any of the highly penetrant CAKUT genes (HNF1B, PAX2, EYA1, ANOS1, GATA3, CHD7, or KIF14). For the controls, we used 426 unrelated individuals who were unaffected parents of children presenting with immune deficiency that were sequenced by whole-exome sequencing on the same genomic platform. Variants in patients and controls were called together. Variants were filtered according to their frequency in public databases (the ExAC, dbSNP, 1000G, and EVS) using two different thresholds (<0.01% and <0.001%), and we then considered either loss of function variants (nonsense, frameshift, splice, start loss, and stop loss) or loss of function plus missense predicted as damaging by Polyphen2 (score >0.470) and Sift (score <0.05). Large deletions were not taken into account in this analysis.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank patients and families for their participation. We also thank the foetopathologists of the SOciété Française de FOETopatholgie for providing us with fetal tissue samples and the pediatric nephrologists from the French Society of Pediatric Nephrology for referring the patients. We thank Marie-Claire Gubler for her help with histology analysis of fetal kidneys. We thank Olivier Gribouval for help with microsatellite analysis.
The research leading to these results has received funding from the European Union’s Seventh Framework Programme (FP7/2007–2013) grant 305608 (EURenOmics). C. Humbert was funded by EURenOmics. This work was also supported by Programme Hospitalier de la Recherche Clinique Assistance Publique grant AOM07129 (to R.S.), Investments for the Future Program grant ANR-10-IAHY-01 (to S.S. and C.A.), and Fondation pour la Recherche Médicale grant DEQ20130326532 (to S.S.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017010043/-/DCSupplemental.
References
- 1.Loane M, Dolk H, Kelly A, Teljeur C, Greenlees R, Densem J; EUROCAT Working Group : Paper 4: EUROCAT statistical monitoring: Identification and investigation of ten year trends of congenital anomalies in Europe. Birth Defects Res A Clin Mol Teratol 91[Suppl 1]: S31–S43, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Ardissino G, Daccò V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, Marra G, Edefonti A, Sereni F; ItalKid Project : Epidemiology of chronic renal failure in children: Data from the ItalKid project. Pediatrics 111: e382–e387, 2003 [DOI] [PubMed] [Google Scholar]
- 3.Bulum B, Ozçakar ZB, Ustüner E, Düşünceli E, Kavaz A, Duman D, Walz K, Fitoz S, Tekin M, Yalçınkaya F: High frequency of kidney and urinary tract anomalies in asymptomatic first-degree relatives of patients with CAKUT. Pediatr Nephrol 28: 2143–2147, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Limwongse C: Syndromes and malformations of the urinary tract. In: Pediatric Nephrology, Vol. 1, 6th Ed., edited by Avner ED, Harmon WE, Niaudet P, Yoshikawa N, Berlin, Springer, 2009, pp 121–156 [Google Scholar]
- 5.Weber S, Moriniere V, Knüppel T, Charbit M, Dusek J, Ghiggeri GM, Jankauskiené A, Mir S, Montini G, Peco-Antic A, Wühl E, Zurowska AM, Mehls O, Antignac C, Schaefer F, Salomon R: Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: Results of the ESCAPE study. J Am Soc Nephrol 17: 2864–2870, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Thomas R, Sanna-Cherchi S, Warady BA, Furth SL, Kaskel FJ, Gharavi AG: HNF1B and PAX2 mutations are a common cause of renal hypodysplasia in the CKiD cohort. Pediatr Nephrol 26: 897–903, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madariaga L, Morinière V, Jeanpierre C, Bouvier R, Loget P, Martinovic J, Dechelotte P, Leporrier N, Thauvin-Robinet C, Jensen UB, Gaillard D, Mathieu M, Turlin B, Attie-Bitach T, Salomon R, Gübler MC, Antignac C, Heidet L: Severe prenatal renal anomalies associated with mutations in HNF1B or PAX2 genes. Clin J Am Soc Nephrol 8: 1179–1187, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez-Rivera E, Liu YP, Verbitsky M, Anderson BR, Capone VP, Otto EA, Yan Z, Mitrotti A, Martino J, Steers NJ, Fasel DA, Vukojevic K, Deng R, Racedo SE, Liu Q, Werth M, Westland R, Vivante A, Makar GS, Bodria M, Sampson MG, Gillies CE, Vega-Warner V, Maiorana M, Petrey DS, Honig B, Lozanovski VJ, Salomon R, Heidet L, Carpentier W, Gaillard D, Carrea A, Gesualdo L, Cusi D, Izzi C, Scolari F, van Wijk JA, Arapovic A, Saraga-Babic M, Saraga M, Kunac N, Samii A, McDonald-McGinn DM, Crowley TB, Zackai EH, Drozdz D, Miklaszewska M, Tkaczyk M, Sikora P, Szczepanska M, Mizerska-Wasiak M, Krzemien G, Szmigielska A, Zaniew M, Darlow JM, Puri P, Barton D, Casolari E, Furth SL, Warady BA, Gucev Z, Hakonarson H, Flogelova H, Tasic V, Latos-Bielenska A, Materna-Kiryluk A, Allegri L, Wong CS, Drummond IA, D’Agati V, Imamoto A, Barasch JM, Hildebrandt F, Kiryluk K, Lifton RP, Morrow BE, Jeanpierre C, Papaioannou VE, Ghiggeri GM, Gharavi AG, Katsanis N, Sanna-Cherchi S: Genetic drivers of kidney defects in the DiGeorge syndrome. N Engl J Med 376: 742–754, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanna-Cherchi S, Kiryluk K, Burgess KE, Bodria M, Sampson MG, Hadley D, Nees SN, Verbitsky M, Perry BJ, Sterken R, Lozanovski VJ, Materna-Kiryluk A, Barlassina C, Kini A, Corbani V, Carrea A, Somenzi D, Murtas C, Ristoska-Bojkovska N, Izzi C, Bianco B, Zaniew M, Flogelova H, Weng PL, Kacak N, Giberti S, Gigante M, Arapovic A, Drnasin K, Caridi G, Curioni S, Allegri F, Ammenti A, Ferretti S, Goj V, Bernardo L, Jobanputra V, Chung WK, Lifton RP, Sanders S, State M, Clark LN, Saraga M, Padmanabhan S, Dominiczak AF, Foroud T, Gesualdo L, Gucev Z, Allegri L, Latos-Bielenska A, Cusi D, Scolari F, Tasic V, Hakonarson H, Ghiggeri GM, Gharavi AG: Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet 91: 987–997, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Westland R, Verbitsky M, Vukojevic K, Perry BJ, Fasel DA, Zwijnenburg PJ, Bökenkamp A, Gille JJ, Saraga-Babic M, Ghiggeri GM, D’Agati VD, Schreuder MF, Gharavi AG, van Wijk JA, Sanna-Cherchi S: Copy number variation analysis identifies novel CAKUT candidate genes in children with a solitary functioning kidney. Kidney Int 88: 1402–1410, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolaou N, Pulit SL, Nijman IJ, Monroe GR, Feitz WF, Schreuder MF, van Eerde AM, de Jong TP, Giltay JC, van der Zwaag B, Havenith MR, Zwakenberg S, van der Zanden LF, Poelmans G, Cornelissen EA, Lilien MR, Franke B, Roeleveld N, van Rooij IA, Cuppen E, Bongers EM, Giles RH, Knoers NV, Renkema KY: Prioritization and burden analysis of rare variants in 208 candidate genes suggest they do not play a major role in CAKUT. Kidney Int 89: 476–486, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Skinner MA, Safford SD, Reeves JG, Jackson ME, Freemerman AJ: Renal aplasia in humans is associated with RET mutations. Am J Hum Genet 82: 344–351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeanpierre C, Macé G, Parisot M, Morinière V, Pawtowsky A, Benabou M, Martinovic J, Amiel J, Attié-Bitach T, Delezoide AL, Loget P, Blanchet P, Gaillard D, Gonzales M, Carpentier W, Nitschke P, Tores F, Heidet L, Antignac C, Salomon R; Société Française de Foetopathologie : RET and GDNF mutations are rare in fetuses with renal agenesis or other severe kidney development defects. J Med Genet 48: 497–504, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Filges I, Nosova E, Bruder E, Tercanli S, Townsend K, Gibson WT, Röthlisberger B, Heinimann K, Hall JG, Gregory-Evans CY, Wasserman WW, Miny P, Friedman JM: Exome sequencing identifies mutations in KIF14 as a novel cause of an autosomal recessive lethal fetal ciliopathy phenotype. Clin Genet 86: 220–228, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Bower M, Salomon R, Allanson J, Antignac C, Benedicenti F, Benetti E, Binenbaum G, Jensen UB, Cochat P, DeCramer S, Dixon J, Drouin R, Falk MJ, Feret H, Gise R, Hunter A, Johnson K, Kumar R, Lavocat MP, Martin L, Morinière V, Mowat D, Murer L, Nguyen HT, Peretz-Amit G, Pierce E, Place E, Rodig N, Salerno A, Sastry S, Sato T, Sayer JA, Schaafsma GC, Shoemaker L, Stockton DW, Tan WH, Tenconi R, Vanhille P, Vats A, Wang X, Warman B, Weleber RG, White SM, Wilson-Brackett C, Zand DJ, Eccles M, Schimmenti LA, Heidet L: Update of PAX2 mutations in renal coloboma syndrome and establishment of a locus-specific database. Hum Mutat 33: 457–466, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krug P, Morinière V, Marlin S, Koubi V, Gabriel HD, Colin E, Bonneau D, Salomon R, Antignac C, Heidet L: Mutation screening of the EYA1, SIX1, and SIX5 genes in a large cohort of patients harboring branchio-oto-renal syndrome calls into question the pathogenic role of SIX5 mutations. Hum Mutat 32: 183–190, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Lin YH, Wu CC, Hsu TY, Chiu WY, Hsu CJ, Chen PL: Identification of a novel GATA3 mutation in a deaf Taiwanese family by massively parallel sequencing. Mutat Res 771: 1–5, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Barceló M, Sham MH, Lee WS, Lui VC, Chen BL, Wong KK, Wong JS, Tam PK: Highly recurrent RET mutations and novel mutations in genes of the receptor tyrosine kinase and endothelin receptor B pathways in Chinese patients with sporadic Hirschsprung disease. Clin Chem 50: 93–100, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Gimelli S, Caridi G, Beri S, McCracken K, Bocciardi R, Zordan P, Dagnino M, Fiorio P, Murer L, Benetti E, Zuffardi O, Giorda R, Wells JM, Gimelli G, Ghiggeri GM: Mutations in SOX17 are associated with congenital anomalies of the kidney and the urinary tract. Hum Mutat 31: 1352–1359, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanna-Cherchi S, Sampogna RV, Papeta N, Burgess KE, Nees SN, Perry BJ, Choi M, Bodria M, Liu Y, Weng PL, Lozanovski VJ, Verbitsky M, Lugani F, Sterken R, Paragas N, Caridi G, Carrea A, Dagnino M, Materna-Kiryluk A, Santamaria G, Murtas C, Ristoska-Bojkovska N, Izzi C, Kacak N, Bianco B, Giberti S, Gigante M, Piaggio G, Gesualdo L, Kosuljandic Vukic D, Vukojevic K, Saraga-Babic M, Saraga M, Gucev Z, Allegri L, Latos-Bielenska A, Casu D, State M, Scolari F, Ravazzolo R, Kiryluk K, Al-Awqati Q, D’Agati VD, Drummond IA, Tasic V, Lifton RP, Ghiggeri GM, Gharavi AG: Mutations in DSTYK and dominant urinary tract malformations. N Engl J Med 369: 621–629, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moens CB, Selleri L: Hox cofactors in vertebrate development. Dev Biol 291: 193–206, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Yu J, McMahon AP, Valerius MT: Recent genetic studies of mouse kidney development. Curr Opin Genet Dev 14: 550–557, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Schnabel CA, Godin RE, Cleary ML: Pbx1 regulates nephrogenesis and ureteric branching in the developing kidney. Dev Biol 254: 262–276, 2003 [DOI] [PubMed] [Google Scholar]
- 24.Hurtado R, Zewdu R, Mtui J, Liang C, Aho R, Kurylo C, Selleri L, Herzlinger D: Pbx1-dependent control of VMC differentiation kinetics underlies gross renal vascular patterning. Development 142: 2653–2664, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackenroth L, Hackmann K, Klink B, Weber JS, Mayer B, Schröck E, Tzschach A: Interstitial 1q23.3q24.1 deletion in a patient with renal malformation, congenital heart disease, and mild intellectual disability. Am J Med Genet A 170: 2394–2399, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Rivolta MN, Halsall A, Johnson CM, Tones MA, Holley MC: Transcript profiling of functionally related groups of genes during conditional differentiation of a mammalian cochlear hair cell line. Genome Res 12: 1091–1099, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschké P, Salomon R, Antignac C, Ornitz DM, Kopan R: FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev Cell 22: 1191–1207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humbert C, Silbermann F, Morar B, Parisot M, Zarhrate M, Masson C, Tores F, Blanchet P, Perez MJ, Petrov Y, Khau Van Kien P, Roume J, Leroy B, Gribouval O, Kalaydjieva L, Heidet L, Salomon R, Antignac C, Benmerah A, Saunier S, Jeanpierre C: Integrin alpha 8 recessive mutations are responsible for bilateral renal agenesis in humans. Am J Hum Genet 94: 288–294, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Combes P, Planche V, Eymard-Pierre E, Sarret C, Rodriguez D, Boespflug-Tanguy O, Vaurs-Barriere C: Relevance of SOX17 variants for hypomyelinating leukodystrophies and congenital anomalies of the kidney and urinary tract (CAKUT). Ann Hum Genet 76: 261–267, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Tordjman S, Somogyi E, Coulon N, Kermarrec S, Cohen D, Bronsard G, Bonnot O, Weismann-Arcache C, Botbol M, Lauth B, Ginchat V, Roubertoux P, Barburoth M, Kovess V, Geoffray MM, Xavier J: Gene × Environment interactions in autism spectrum disorders: Role of epigenetic mechanisms. Front Psychiatry 5: 53, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Tanno P, Breton J, Bidart M, Satre V, Harbuz R, Ray PF, Bosson C, Dieterich K, Jaillard S, Odent S, Poke G, Beddow R, Digilio MC, Novelli A, Bernardini L, Pisanti MA, Mackenroth L, Hackmann K, Vogel I, Christensen R, Fokstuen S, Bena F, Amblard F, Devillard F, Vieville G, Apostolou A, Jouk PS, Guebre-Egziabher F, Sartelet H, Coutton C: PBX1 haploinsufficiency leads to syndromic congenital anomalies of the kidney and urinary tract (CAKUT) in humans [published online ahead of print March 7, 2017]. J Med Genet doi: 10.1136/jmedgenet-2016-104435 [DOI] [PubMed]
- 32.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG; Exome Aggregation Consortium : Analysis of protein-coding genetic variation in 60,706 humans. Nature 536: 285–291, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goossens D, Moens LN, Nelis E, Lenaerts AS, Glassee W, Kalbe A, Frey B, Kopal G, De Jonghe P, De Rijk P, Del-Favero J: Simultaneous mutation and copy number variation (CNV) detection by multiplex PCR-based GS-FLX sequencing. Hum Mutat 30: 472–476, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Madsen BE, Browning SR: A groupwise association test for rare mutations using a weighted sum statistic. PLoS Genet 5: e1000384, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S, Wu MC, Lin X: Optimal tests for rare variant effects in sequencing association studies. Biostatistics 13: 762–775, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellanné-Chantelot C, Clauin S, Chauveau D, Collin P, Daumont M, Douillard C, Dubois-Laforgue D, Dusselier L, Gautier JF, Jadoul M, Laloi-Michelin M, Jacquesson L, Larger E, Louis J, Nicolino M, Subra JF, Wilhem JM, Young J, Velho G, Timsit J: Large genomic rearrangements in the hepatocyte nuclear factor-1beta (TCF2) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes 54: 3126–3132, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Benetti E, Artifoni L, Salviati L, Pinello L, Perrotta S, Zuffardi O, Zacchello G, Murer L: Renal hypoplasia without optic coloboma associated with PAX2 gene deletion. Nephrol Dial Transplant 22: 2076–2078, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Schimmenti LA, Shim HH, Wirtschafter JD, Panzarino VA, Kashtan CE, Kirkpatrick SJ, Wargowski DS, France TD, Michel E, Dobyns WB: Homonucleotide expansion and contraction mutations of PAX2 and inclusion of Chiari 1 malformation as part of renal-coloboma syndrome. Hum Mutat 14: 369–376, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Sanyanusin P, McNoe LA, Sullivan MJ, Weaver RG, Eccles MR: Mutation of PAX2 in two siblings with renal-coloboma syndrome. Hum Mol Genet 4: 2183–2184, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Sellick GS, Longman C, Tolmie J, Newbury-Ecob R, Geenhalgh L, Hughes S, Whiteford M, Garrett C, Houlston RS: Genomewide linkage searches for Mendelian disease loci can be efficiently conducted using high-density SNP genotyping arrays. Nucleic Acids Res 32: e164, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higashide T, Wada T, Sakurai M, Yokoyama H, Sugiyama K: Macular abnormalities and optic disk anomaly associated with a new PAX2 missense mutation. Am J Ophthalmol 139: 203–205, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Rickard S, Boxer M, Trompeter R, Bitner-Glindzicz M: Importance of clinical evaluation and molecular testing in the branchio-oto-renal (BOR) syndrome and overlapping phenotypes. J Med Genet 37: 623–627, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Levi-Acobas F, Cruaud C, Le Merrer M, Mathieu M, König R, Vigneron J, Weissenbach J, Petit C, Weil D: Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYA1. Hum Mol Genet 6: 2247–2255, 1997 [DOI] [PubMed] [Google Scholar]
- 44.Orten DJ, Fischer SM, Sorensen JL, Radhakrishna U, Cremers CW, Marres HA, Van Camp G, Welch KO, Smith RJ, Kimberling WJ: Branchio-oto-renal syndrome (BOR): Novel mutations in the EYA1 gene, and a review of the mutational genetics of BOR. Hum Mutat 29: 537–544, 2008 [DOI] [PubMed] [Google Scholar]
- 45.Fukami M, Muroya K, Miyake T, Iso M, Kato F, Yokoi H, Suzuki Y, Tsubouchi K, Nakagomi Y, Kikuchi N, Horikawa R, Ogata T: GATA3 abnormalities in six patients with HDR syndrome. Endocr J 58: 117–121, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K: GATA3 haplo-insufficiency causes human HDR syndrome. Nature 406: 419–422, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Montenegro LR, Silveira LF, Tusset C, de Castro M, Versiani BR, Latronico AC, Mendonca BB, Trarbach EB: Combined use of multiplex ligation-dependent probe amplification and automatic sequencing for identification of KAL1 defects in patients with Kallmann syndrome. Fertil Steril 100: 854–859, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Hardelin JP, Levilliers J, del Castillo I, Cohen-Salmon M, Legouis R, Blanchard S, Compain S, Bouloux P, Kirk J, Moraine C, Chaussain JL, Weissenbach J, Petit C: X chromosome-linked Kallmann syndrome: Stop mutations validate the candidate gene. Proc Natl Acad Sci U S A 89: 8190–8194, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Asakura Y, Toyota Y, Muroya K, Kurosawa K, Fujita K, Aida N, Kawame H, Kosaki K, Adachi M: Endocrine and radiological studies in patients with molecularly confirmed CHARGE syndrome. J Clin Endocrinol Metab 93: 920–924, 2008 [DOI] [PubMed] [Google Scholar]
- 50.Spruijt L, Hoefsloot LH, van Schaijk GH, van Waardenburg D, Kremer B, Brackel HJ, de Die-Smulders CE: Identification of a novel EYA1 mutation presenting in a newborn with laryngomalacia, glossoptosis, retrognathia, and pectus excavatum. Am J Med Genet A 140: 1343–1345, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.