Abstract
Hematuria is a cardinal symptom in IgA nephropathy, but its influence on the risk of disease progression has been scarcely investigated. We followed a cohort of 112 patients with IgA nephropathy for a mean±SEM period of 14±10.2 years, during which clinical and analytic risk factors (including urine sediment examination) were regularly recorded. According to the magnitude of time-averaged hematuria, we classified patients as those with persistent hematuria and those with negative or minimal hematuria. We also classified patients according to the magnitude of time-averaged proteinuria (>0.75 or ≤0.75 g/d). The proportion of patients reaching ESRD or a 50% reduction of renal function was significantly greater among patients with persistent hematuria than patients with minimal or negative hematuria (30.4% and 37.0% versus 10.6% and 15.2%, respectively; P=0.01). Multivariable analysis revealed time-averaged hematuria, time-averaged proteinuria, renal function at baseline, and the presence of tubulointerstitial fibrosis on renal biopsy as independent predictors of ESRD. After hematuria disappearance, which occurred in 46% of the patients, the rate of renal function decline changed from −6.45±14.66 to −0.18±2.56 ml/min per 1.73 m2 per year (P=0.001). Patients with time-averaged proteinuria >0.75 g/d had significantly poorer renal survival than those with time-averaged proteinuria ≤0.75 g/d. However, on further classification by time-averaged hematuria, only those patients with time-averaged proteinuria >0.75 g/d and persistent hematuria had significantly worse renal survival than those in the other three groups. In conclusion, remission of hematuria may have a significant favorable effect on IgA nephropathy outcomes.
Keywords: IgA nephropathy, progression of renal failure, proteinuria, hematuria
Microscopic hematuria, accompanied by variable amounts of proteinuria, is the most common clinical manifestation of IgA nephropathy (IgAN).1–3 Slow progression to ESRD occurs in a substantial proportion of patients with IgAN over long-term follow-up. Several observational retrospective studies have identified hypertension, renal function at diagnosis, and the magnitude of proteinuria during follow-up as the most important clinical predictors of ESRD.4–13 Of them, a sustained protein excretion above 0.75–1 g/d is considered as the most determinant predictor of progression, and treatment recommendations are mainly on the basis of the magnitude of proteinuria.5–15 More recently, several studies have shown that histologic lesions included in the Oxford Classification of IgAN (mesangial and endocapillary hypercellularity, segmental sclerosis, and interstitial and tubular fibrosis) are independently associated with renal outcomes and significantly improve the prediction of disease progression to ESRD.16–18
Macroscopic hematuria bouts, typically associated with pharyngeal infections, constitute one of the most frequent forms of presentation, and microscopic hematuria usually persists after the resolution of these episodes in most of the patients.1–3 The frequency of gross hematuria episodes tends to decrease over time, and microscopic hematuria can disappear in some patients, either spontaneously or after receiving immunosuppressive treatments. In other patients, however, persistent and severe microscopic hematuria is found in urine sediment examination for a long time. Despite being one of the fundamental symptoms of IgAN, few studies have systematically analyzed the influence that the persistence and magnitude of hematuria might have on the risk of ESRD in this entity.
Previous studies about the prognostic influence of hematuria on IgAN have yielded discordant results. In some of them, patients with hematuria had a worse prognosis,6,9,19,20 but this result was not confirmed in others.21–24 A common shortcoming in these studies was, however, that the analysis of hematuria was performed only at the onset of the disease or some isolated point in the evolution of patients. Likewise, in most studies, the magnitude of hematuria (i.e., the number of red blood cells [RBCs] per microscopic field) was not quantified.
Here, we present a study about the influence of hematuria on a cohort of patients with IgAN followed over a long period of time with regular and systematic visits, in which all recognized clinical and analytic risk factors for the progression of the disease (BP, proteinuria, and renal function) and analysis of the urinary sediment (presence of hematuria and its magnitude) were systematically recorded. This close and systematic monitoring of hematuria over time gives us a unique opportunity to consistently analyze the influence of hematuria on progression of the disease as well as the interaction of hematuria with proteinuria and other clinical and histologic risk factors for progression.
Results
Patients
The main characteristics of included patients are shown in Table 1. All patients presented hematuria at baseline. Patients were followed for a mean of 14±10.2 years. Patients were categorized according to the degree of time-averaged hematuria (TA-hematuria) into two groups: patients with negative or minimal hematuria (TA-hematuria of 0.2 [0–3.1] red blood cells per high-power field [RBC × hpf]; n=66) and those with persistent hematuria (TA-hematuria of 24.7 [13–71] RBC × hpf; n=46) during follow-up. Patients with persistent hematuria presented a significantly more severe hematuria at presentation than those with negative or minimal hematuria (Table 1). There were no differences between both groups in demographic characteristics, renal function, BP, or proteinuria at baseline. There was a trend for higher time-averaged proteinuria (TA-proteinuria) in patients with persistent hematuria compared with patients with negative or minimal hematuria, but it did not reach statistical significance. The proportion of patients receiving renin-angiotensin blockers and immunosuppressive treatments was similar in both groups (Tables 1 and 2).
Table 1.
Characteristics of patients categorized by the magnitude of TA-hematuria
| Characteristics | All Patients, n=112 | Minimal or Negative Hematuria during Follow-Up, n=66 | Persistent Hematuria during Follow-Up, n=46 | P Value |
|---|---|---|---|---|
| Baseline | ||||
| Men (%) | 78 (69.6) | 45 (68.2) | 33 (71.7) | 0.68 |
| Age, yra | 41.5±17.9 | 40±19 | 43±16 | 0.43 |
| White (%) | 108 (96.4) | 63 (95.4) | 45 (97.8) | 0.49 |
| Scr, mg/dla | 1.8±1.4 | 1.8±1.3 | 1.9±1.1 | 0.68 |
| eGFR, ml/min per 1.73 m2a | 58±34 | 61±40 | 50±28 | 0.12 |
| SBP, mmHga | 133±26 | 132±26 | 134±26 | 0.64 |
| DBP, mmHga | 81±18 | 80±15 | 82±21 | 0.52 |
| Proteinuria, g/db | 1.4 (0.4–2.7) | 1.5 (0.3–2.7) | 1.5 (0.7–2.4) | 0.97 |
| Hematuria, RBC × hpfb | 50 (15–100) | 24.5 (8.5–100) | 90 (31–100) | 0.02 |
| Follow-up and treatment | ||||
| Follow-up, yra | 14±10.2 | 16.2±11 | 10.9±8.2 | <0.001 |
| TA-H, RBC × hpfb | 5 (0–20) | 0.2 (0–3.1) | 24.7 (13–71) | <0.001 |
| TA-P, g/db | 0.5 (0.1–1.1) | 0.4 (0.1–1.0) | 0.7 (0.3–1.3) | 0.08 |
| TA-MBP, mmHgb | 95 (89–98) | 94 (86–98) | 95 (90–100) | 0.98 |
| RAAS blockade (%) | 94 (84) | 56 (84) | 38 (82) | 0.75 |
| Renal biopsy (%) | ||||
| M1 | 48 (42) | 23 (34) | 25 (54) | 0.04 |
| E1 | 9 (8) | 3 (4.5) | 6 (13) | 0.10 |
| S1 | 33 (29) | 15 (22) | 18 (39) | 0.06 |
| T1–T2 | 24 (21) | 14 (21) | 10 (21) | 0.94 |
Scr, serum creatinine; SBP, systolic BP; DBP, diastolic BP; TA-H, time-averaged hematuria; TA-P, time-averaged proteinuria; RAAS, renin-angiotensin-aldosterone system; M1, mesangial hypercellularity; E1, endocapillary hypercellularity; S1, segmental glomerulosclerosis; T1–T2, tubular atrophy/interstitial fibrosis >25%.
Mean±SD.
Median (interquartile range).
Table 2.
Immunosuppressive treatments
| Treatment | All Patients, n=112 | Minimal or Negative Hematuria during Follow-Up, n=66 | Persistent Hematuria during Follow-Up, n=46 | P Value |
|---|---|---|---|---|
| IS treatments (%) | 44 (39) | 23 (34) | 21 (45) | 0.24 |
| CS monotherapy | 12 (27) | 7 (30) | 5 (24) | 0.96 |
| CS + MMF | 27 (61) | 15 (65) | 12 (57) | 0.68 |
| CS + AZA | 3 (7) | — | 3 (14) | |
| CS + CYC | 2 (5) | 1 (5) | 1 (5) | 0.79 |
| Duration of CS treatment, moa | 7.3±6.6 | 6.6±1.6 | 6.8±1.5 | 0.70 |
| Cumulative dose of CS, mg/kgb | 58 (31–69) | 61 (43–67) | 49 (28–81) | 0.37 |
IS, immunosuppressive; CS, corticosteroid; MMF, mycophenolate mofetil; AZA, azathioprine; CYC, cyclophosphamide.
Mean±SD.
Median (interquartile range).
In regard to renal biopsy findings, mesangial hypercellularity was found in 34% of patients with negative or minimal hematuria compared with 54% of patients with persistent hematuria (P=0.04) (Table 1). There was a nonsignificant trend of greater prevalence of endocapillary hypercellularity and segmental glomerulosclerosis among patients with persistent hematuria compared with patients with minimal or negative hematuria (Table 1). TA-hematuria was higher among patients with a mesangial score >0.5 (10 [1–43] versus 3 [0–15] RBC × hpf in patients with a mesangial score <0.5; P=0.06), presence of segmental glomerulosclerosis (10 [0–50] versus 2 [0–19] RBC × hpf in patients with absence of segmental glomerulosclerosis; P=0.17), and endocapillary hypercellularity (10 [5–32] versus 4 [0–20] RBC × hpf in patients with absence of endocapillary hypercellularity; P=0.45), although these differences did not reach statistical significance.
Outcomes
At the end of follow-up, 21 patients (17.2%) had reached ESRD (Table 3). The rate of renal function decline was −2.34±5 ml/min per 1.73 m2 per year. As shown in Table 3, renal outcomes were better in patients with negative or minimal hematuria compared with those with persistent hematuria. Seven patients (10.6%) with minimal or negative hematuria developed ESRD compared with 14 patients (30.4%) with persistent hematuria during follow-up (P=0.01). The proportion of patients showing a 50% reduction of renal function was also significantly greater among the latter (37% versus 15.2%; P=0.01). Compared with patients with persistent hematuria, patients with negative or minimal hematuria experienced a nonsignificant slower rate of renal function decline (−1.54±3.92 versus −3.34±6.12 ml/min per 1.73 m2 per year; P=0.06).
Table 3.
Outcomes
| Outcomes | All Patients, n=112 | Minimal or Negative Hematuria during Follow-Up, n=66 | Persistent Hematuria during Follow-Up, n=46 | P Value |
|---|---|---|---|---|
| ESRD, no. (%) | 21 (17.2) | 7 (10.6) | 14 (30.4) | 0.01 |
| 50% Reduction in renal function, no. (%) | 27 (22.1) | 10 (15.2) | 17 (37) | 0.01 |
| Rate of renal function decline, ml/min per 1.73 m2 per yr | −2.34±5 | −1.54±3.92 | −3.34±6.12 | 0.06 |
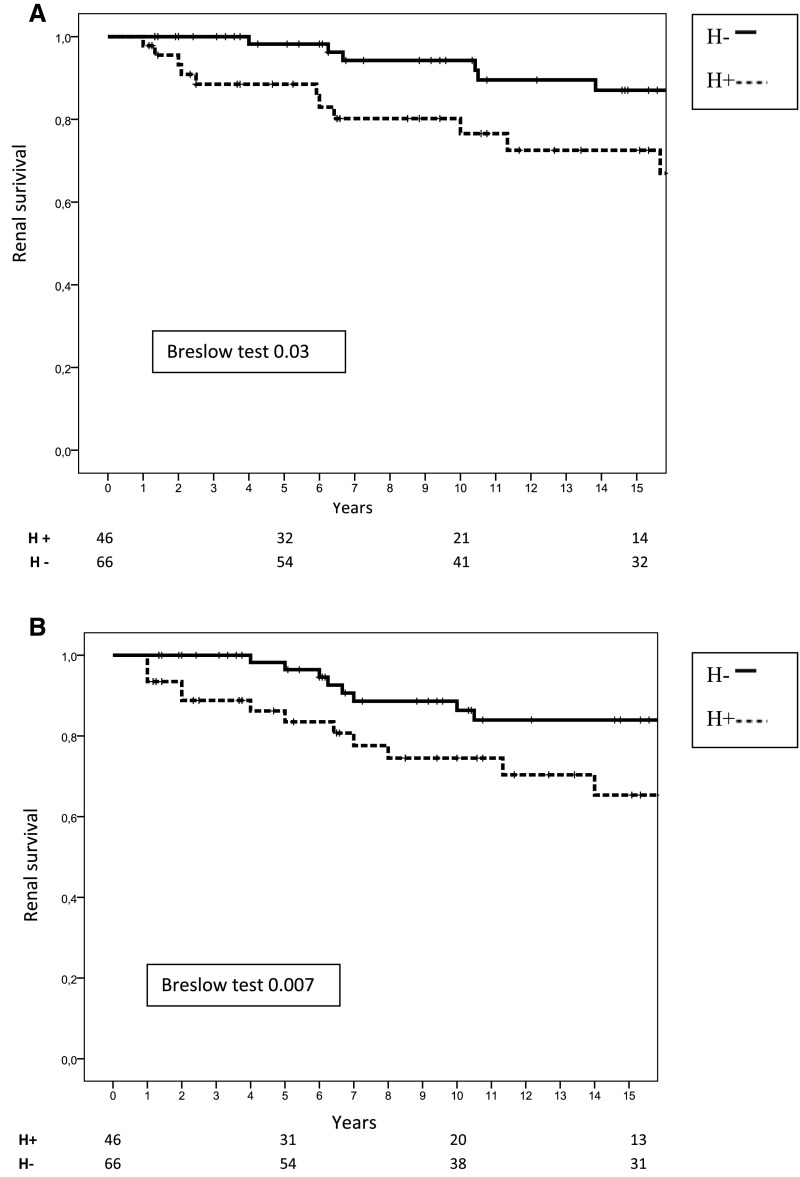
As shown in Figure 1, renal survival, defined by a status free of ESRD (Figure 1A) or a status free of 50% reduction of renal function (Figure 1B), was significantly better in patients with negative or minimal hematuria (98.2%, 94.2%, and 87% for ESRD and 96.4%, 86.3%, and 83.9% for 50% reduction of renal function after 5, 10, and 15 years, respectively) than in patients with persistent hematuria (88.5%, 76.6%, and 72.5% for ESRD and 83.5%, 74.5%, and 65.3% for 50% reduction of renal function, respectively; P=0.03 for ESRD and P=0.01 for 50% reduction of renal function).
Figure 1.
Renal survival according to TA-hematuria. (A) Renal survival (free of ESRD) according to TA-hematuria. (B) Renal survival (free of 50% reduction of renal function) according to TA-hematuria. H−, patients with TA-hematuria ≤5 RBC × hpf; H+, patients with TA-hematuria >5 RBC × hpf.
By univariate analysis, TA-hematuria, TA-proteinuria, age at diagnosis, renal function at baseline, systolic and diastolic BP at baseline, and the presence of mesangial hypercellularity and tubulointerstitial fibrosis in renal biopsy were factors significantly associated with renal survival (ESRD). In the multivariable model, TA-hematuria, TA-proteinuria, renal function at baseline, and the presence of tubulointerstitial fibrosis remained as independent predictors of ESRD (Table 4).
Table 4.
Univariate and multivariate analyses of independent prognostic factors for renal survival (ESRD)
| Risk Factor | Univariable | Multivariable | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| TA-H | 4.21 (1.66 to 10.64) | 0.02 | 2.84 (1.06 to 7.3) | 0.04 |
| TA-P | 4.88 (1.88 to 12.65) | 0.01 | 2.82 (1.04 to 7.66) | 0.04 |
| Age at baseline | 1.02 (1.01 to 1.05) | 0.05 | 0.97 (0.94 to 0.99) | 0.05 |
| Renal function at baseline | 0.96 (0.94 to 0.98) | <0.001 | 0.97 (0.95 to 0.99) | 0.03 |
| IS treatments | 1.66 (0.67 to 4.09) | 0.26 | ||
| SBP | 1.01 (1.01 to 1.02) | 0.02 | ||
| DBP | 1.01 (1 to 1.03) | 0.03 | ||
| M1 | 2.67 (1.11 to 6.45) | 0.03 | ||
| E1 | 2.16 (0.62 to 7.43) | 0.22 | ||
| S1 | 2.45 (0.98 to 6.09) | 0.06 | ||
| T1–T2 | 5.55 (2.29 to 13.33) | 0.03 | 3.19 (1.72 to 8.87) | 0.001 |
HR, hazard ratio; 95% CI, 95% confidence interval; TA-H, time-averaged hematuria; TA-P, time-averaged proteinuria; IS, immunosuppressive; SBP, systolic BP; DBP, diastolic BP; M1, present mesangial hypercellularity; E1, present endocapillary hypercellularity; S1, present segmental glomerulosclerosis; T1–T2, tubular atrophy/interstitial fibrosis >25%.
Effect of Hematuria Disappearance on the Rate of Renal Function Decline
Hematuria disappeared, according to the definition stated in Concise Methods, in 52 patients (46%). Forty-four (84.6%) of them belonged to the group of patients with negative or persistent hematuria, and eight (15.4%) belonged to the group of persistent hematuria. Median time to reach remission of hematuria was 5.98±5.92 years. As shown in Table 5, the rate of renal function decline experienced a significant slowing after hematuria disappearance (from a rate of −6.45±14.66 to −0.18±2.56 ml/min per 1.73 m2 per year; P=0.001). In patients who received immunosuppressive treatments, TA-hematuria decreased significantly from 36 (6–100) RBC × hpf before treatment to 3 (0–12) RBC × hpf after treatment (P=0.001). There was a nonsignificant trend for a shorter median time to reach hematuria remission among patients who received immunosuppressive treatments compared with among nontreated patients (5.48±5.87 versus 7.55±6.32 years, respectively; P=0.28), but it should be considered that immunosuppressive drugs were administered at different times of patient evolution. Similarly, Kaplan–Meier analysis of the probability of hematuria remission showed a nonsignificant trend for a shorter time to reach hematuria remission among treated (40%, 51%, and 69% at 5, 10, and 15 years, respectively) compared with nontreated patients (27%, 48%, and 53% at 5, 10, and 15 years, respectively; P=0.19).
Table 5.
Rate of renal function decline before and after hematuria disappearance
| Variable | Before Hematuria Disappearance | After Hematuria Disappearance | P Value |
|---|---|---|---|
| Rate of renal function decline, ml/min per 1.73 m2 per yra | −6.45±14.66 | −0.18±2.56 | 0.001 |
| Follow-up, mo | 5.98±5.92b | 10.71±9.5c | 0.003 |
Slope of eGFR.
Interval between baseline and hematuria disappearance.
Interval between hematuria disappearance and last visit.
Interaction of TA-Hematuria and TA-Proteinuria
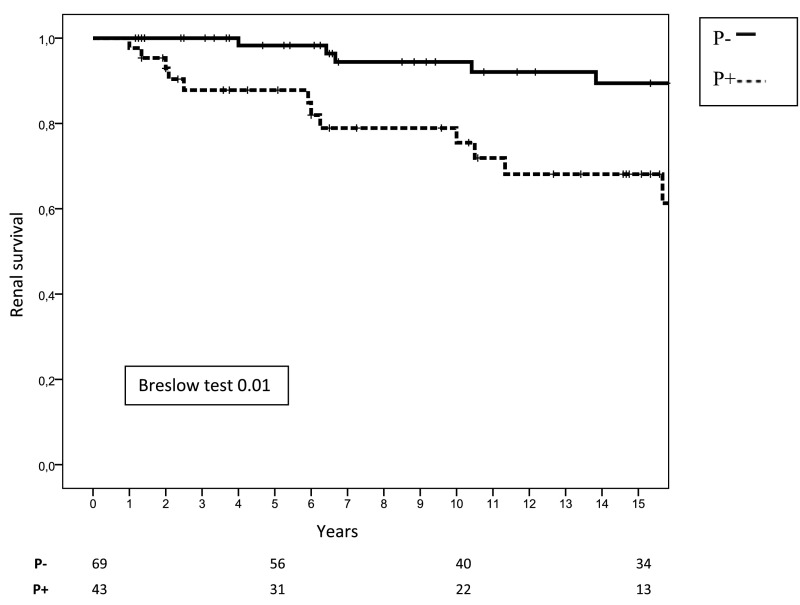
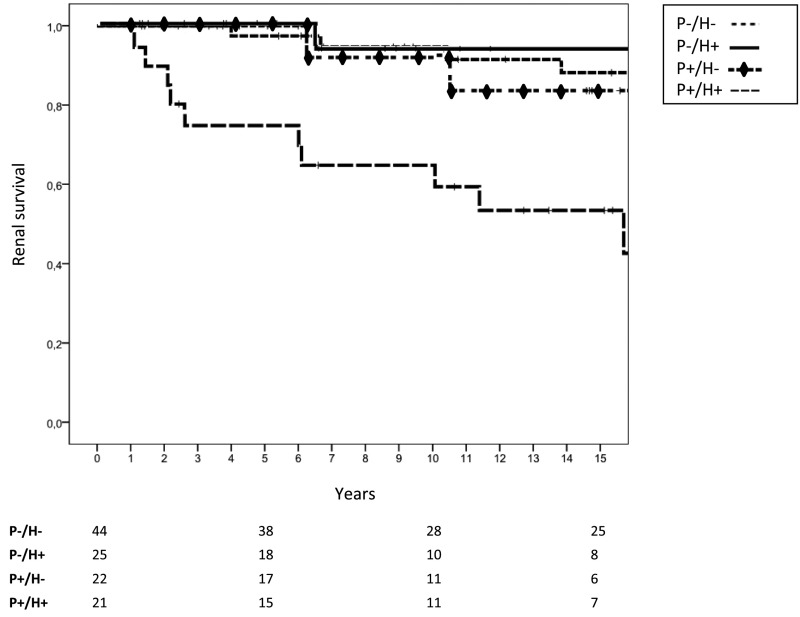
We analyzed renal survival of patients according to the amount of TA-proteinuria during follow-up. As shown in Figure 2, those patients with a TA-proteinuria ≤0.75 g/d had a significantly better renal survival (98.3%, 94.4%, and 89.4% after 5, 10, and 15 years, respectively, of follow-up) than patients with TA-proteinuria >0.75 g/d (87.8%, 75.5%, and 68.1% after 5, 10, and 15 years, respectively, of follow-up; P=0.001). Renal function decline was also significantly slower in the former (−1.08 versus −4.20 ml/min per 1.73 m2 per year; P=0.02). However, when these two groups of patients were subdivided according to the magnitude of hematuria during follow-up, we found that the patients who had maintained TA-proteinuria >0.75 g/d and persistent hematuria during follow-up (n=21) had a significantly worse renal survival than the other three groups of patients (TA-proteinuria >0.75 g/d and minimal or negative hematuria [n=22], TA-proteinuria ≤0.75 g/d and persistent hematuria [n=25], and TA-proteinuria ≤0.75 g/d and negative or minimal hematuria [n=44]), whereas no significant differences were found between the latter three groups (Figure 3).
Figure 2.
Renal survival (free of ESRD) according to TA-proteinuria. P−, patients with TA-proteinuria ≤0.75 g/d; P+, patients with TA-proteinuria >0.75 g/d.
Figure 3.
Renal survival (free of ESRD) according to TA-proteinuria and TA-hematuria. P−/H−, patients with TA-proteinuria ≤0.75 g/d and TA-hematuria ≤5 RBC × hpf; P−/H+, patients with TA-proteinuria ≤0.75 g/d and TA-hematuria >5 RBC × hpf; P+/H−, patients with TA-proteinuria >0.75 g/d and TA-hematuria ≤5 RBC × hpf; P+/H+, patients with TA-proteinuria >0.75 g/d and TA-hematuria >5 RBC × hpf.
The characteristics and outcomes of patients with TA-proteinuria >0.75 g/d and persistent hematuria during follow-up are shown in Table 6. Compared with the remaining patients, they had a greater diastolic BP at baseline, a nonsignificant trend for greater proteinuria at baseline and time-averaged mean BP (TA-MBP) during follow-up, and an almost significantly greater number of patients with mesangial hypercellularity in renal biopsy. TA-proteinuria and TA-hematuria were significantly greater than in the remaining patients, and outcomes were significantly worse: 11 patients (52%) reached ESRD and 12 (57%) showed a >50% decline in renal function compared with ten (11%) and 15 (16%), respectively, among the remaining patients (P=0.001). The rate of renal function decline was significantly faster as shown in Table 6.
Table 6.
Characteristics and outcomes of patients with TA-proteinuria >0.75 g/d and persistent hematuria
| Variable | Patients with TA-Proteinuria >0.75 g/d and Persistent Hematuria, n=21 | Other Patients, n=91a | P Value |
|---|---|---|---|
| Baseline | |||
| Men (%) | 18 (85.7) | 60 (65.9) | 0.07 |
| Age, yrb | 43±16 | 41±18 | 0.69 |
| White (%) | 20 (95.2) | 87 (96.7) | 0.75 |
| Scr, mg/dlb | 1.9±0.9 | 1.8±1.2 | 0.65 |
| eGFR, ml/min per 1.73 m2b | 48±26 | 59.±38 | 0.21 |
| SBP, mmHgb | 137±21 | 132±27 | 0.43 |
| DBP, mmHgb | 88±19 | 79±17 | 0.03 |
| Proteinuria, g/dc | 2.7 (1.2–4.4) | 1 (0.4–2.2) | 0.08 |
| Hematuria, RBC × hpfc | 50 (28–100) | 40 (10–100) | 0.60 |
| Follow-up and treatment | |||
| Follow-up, yrb | 11±8.7 | 14.7±10.5 | 0.15 |
| RAAS blockade (%) | 19 (90.5) | 75 (82.4) | 0.36 |
| IS treatments (%) | 8 (38.1) | 36 (39.6) | 0.90 |
| TA-H, RBC × hpfc | 20 (11–59) | 3 (0–15) | <0.001 |
| TA-P, g/dc | 1.7 (1–3.7) | 0.4 (0.2–0.8) | <0.001 |
| TA-MBP, mmHgc | 97 (94–104) | 93(87–98) | 0.07 |
| Kidney biopsy (%) | |||
| M1 | 13 (61.9) | 35 (38.5) | 0.05 |
| E1 | 3 (14.3) | 6 (6.6) | 0.24 |
| S1 | 8 (38.1) | 25 (27.5) | 0.33 |
| T1–T2 | 7 (33.3) | 17 (18.7) | 0.14 |
| Outcomes | |||
| ESRD no. (%) | 11 (52.4) | 10 (11) | <0.001 |
| 50% Reduction in renal function, no. (%) | 12 (57.1) | 15 (16.5) | <0.001 |
| Rate of renal function decline, ml/min per 1.73 m2 per yr | 5.7±7.2 | 1.5±4 | <0.001 |
Scr, serum creatinine; SBP, systolic BP; DBP, diastolic BP; RAAS, renin-angiotensin-aldosterone system; IS, immunosuppressive treatment; TA-H, time-averaged hematuria; TA-P, time-averaged proteinuria; M1, mesangial hypercellularity; E1, endocapillary hypercellularity; S1, segmental glomerulosclerosis; T1–T2, tubular atrophy/interstitial fibrosis>25%.
Includes patients with TA-P>0.75 g/d and minimal or negative hematuria, TA-P≤0.75 g/d and persistent hematuria, and TA-P≤0.75 g/d and negative or minimal hematuria.
Mean±SD.
Median (interquartile range).
Eight (31%) of 21 patients with TA-proteinuria >0.75 g/d and persistent hematuria received immunosuppressive treatments. As shown in Table 7, there were no differences in outcomes between treated and nontreated patients, although the former presented a nonsignificant higher proteinuria and hematuria at baseline. TA-hematuria and TA-proteinuria showed a significant decrease after immunosuppressive treatment, and there was a marked, although nonsignificant, reduction in the rate of renal function decline after treatment (Table 7).
Table 7.
Influence of immunosuppressive treatments on the outcomes of patients with TA-proteinuria >0.75 g/d and persistent hematuria
| Variable | All Patients, n=21 | Treated, n=8 | Nontreated, n=13 | P Value |
|---|---|---|---|---|
| Baseline Scr, mg/dla | 1.9±0.9 | 2±1.2 | 1.9±0.7 | 0.60 |
| Baseline proteinuria, g/db | 2.7 (1.2–4.4) | 3.3 (1.3–5.6) | 1.8 (1.2–3.3) | 0.38 |
| Baseline hematuria, RBC × hpfb | 50 (28–100) | 78 (33–100) | 36 (14–87) | 0.31 |
| ESRD, no. (%) | 11 (52) | 4 (50) | 7 (53.8) | 0.86 |
| 50% Reduction in renal function, no. (%) | 12 (57) | 4 (50) | 8 (61.5) | 0.60 |
| Rate of renal function decline, ml/min per 1.73 m2 per yra | −5.67±7.20 | −5.75±8.19 | −5.63±6.87 | 0.97 |
| TA-P before/after IS treatment, g/db | 2.9 (1.2–5.7)/1 (0.44–2.5)c | |||
| TA-H before/after IS treatment, RBC × hpfb | 75 (20–100)/12 (4–20)d | |||
| Rate of renal function decline before/after IS treatment, ml/min per 1.73 m2 per yra | −10.2±12.7/−2±5.02e | |||
| IS treatments (%) | ||||
| CS monotherapy | 0 (0) | |||
| CS + MMF | 5 (62) | |||
| CS + AZA | 2 (25) | |||
| CS + CYC | 1 (12) | |||
| Duration of CS treatment, moa | 5.62±2.97 | |||
| Cumulative dose of CS, mg/kgb | 60 (44–66) |
Scr, serum creatinine; TA-P, time-averaged proteinuria; IS, immunosuppressive treatment; TA-H, time-averaged hematuria; CS, corticosteroid; MMF, mycophenolate mofetil; AZA, azathioprine; CYC, cyclophosphamide.
Mean±SD.
Median (interquartile range).
P=0.01.
P=0.02.
P=0.12.
Discussion
Taken together, our data show that the magnitude and persistence of hematuria during follow-up have a significant influence on the progression of the disease in patients with IgAN. The probability of ESRD or a renal function decline >50% over time was significantly higher in those patients with persistent hematuria compared with patients with minimal or negative hematuria during follow-up. Almost one half of the patients (52; 46%) showed disappearance of hematuria along the follow-up. After its disappearance, a significant slowdown in the rate of loss of renal function was observed from −6.45±14.66 to −0.18±2.56 ml/min per 1.73 m2 per year (P=0.001).
Hematuria is one of the clinical hallmarks of IgAN, and its finding is virtually constant at the time of diagnosis.1–3 Despite the great clinical and diagnostic importance of hematuria in IgAN, few studies have analyzed its influence on the final outcome of the disease, and the results have been discordant. Although some studies suggested a significant influence of hematuria on the risk of developing ESRD,6,9,19,20 others could not confirm this finding.21–24 However, the robustness of these studies was reduced by the way in which hematuria was evaluated (at the onset of the disease, at the time of renal biopsy, or by cross-sectional studies) without longitudinal data.23,24 In other studies, urine dipsticks were used, and the magnitude of hematuria was not quantified.20
At the end of follow-up, 17.2% of our patients had reached ESRD. This rate of disease progression is similar to that reported in other European cohorts with similar risk factors for progression.3,4,8 Almost all of our patients were white, and some studies have suggested that patients with IgAN of Pacific Asian origin have different clinical characteristics and an increased risk of progression to ESRD.25 Hence, the influence of hematuria on the prognosis of IgAN might not be the same in non-European populations. However, the only study, as far as we know, in which hematuria was longitudinally determined and quantified, similar to our study, also showed a detrimental influence of the magnitude and persistence of hematuria on the prognosis of the patients, and it was performed in China.9 In the multivariate analysis of this study, only time-averaged values during follow-up of BP, proteinuria, and hematuria as well as renal function at the time of renal biopsy had a significant influence on renal survival. However, in this study, the interactions between TA-proteinuria and TA-hematuria were not analyzed, and histologic data were not available.9
Several studies published in recent years have shown that histologic lesions included in the Oxford Classification of IgAN (mesangial and endocapillar hypercellularity, segmental glomerulosclerosis, and tubulointerstitial fibrosis) are independent predictors of outcome.16–18 We found a significant association between TA-hematuria and the presence of mesangial hypercellularity as well as a nearly significant relationship between TA-hematuria and the presence of endocapillary hypercellularity and segmental glomerulosclerosis. These data indicate that hematuria can be considered as a biomarker of the activity of IgAN and the severity of renal histologic damage. However, data suggest that hematuria by itself actively collaborates in the progression of the disease by inducing renal damage at different nephron sites. It has been shown that hematuria induces a direct cytotoxic effect on tubular cells through the oxidative stress caused by the hemoglobin and iron released by broken RBCs into the renal tubule.26–28 This hematuria-induced renal damage might not be unique to IgAN but could also contribute to the progression of other renal diseases characterized by hematuria, which several studies have suggested.29–31
At present, therapeutic recommendations on IgAN are on the basis of the amount and persistence of proteinuria.14,15,32–34 Thus, the Kidney Disease Improving Global Outcomes glomerular guidelines recommend treatment with angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) in patients with proteinuria >1 g/d (>0.5 g/d in children).14 According to this guideline, corticosteroid treatments should be reserved for those patients who maintain levels of proteinuria above these limits after 3–6 months of optimized supportive treatment, including ACEI or ARB and adequate BP control. In agreement with previous studies, we found that a TA-proteinuria >0.75 g/d was an independent risk factor for disease progression. However, when we divided patients with TA-proteinuria >0.75 g/d between those with minimal or negative TA-hematuria and those with persistent hematuria, we found that only those patients with levels of proteinuria >0.75 g/d combined with persistent hematuria during follow-up showed a significantly worse prognosis, whereas those patients who present only proteinuria or hematuria during follow-up have a clearly better prognosis (Figure 3).
The harmful effect that the presence of proteins induces on tubular cells and podocytes has been extensively investigated.35,36 Conceivably, these detrimental effects of proteinuria could be synergistic with those induced by hemoglobin and iron released by broken RBCs as discussed above. We think that this interconnection between hematuria and proteinuria, not previously analyzed as far as we know, has important therapeutic implications for patients with IgAN. According to our data, only those patients who persistently present both severe proteinuria and hematuria during the follow-up should be considered for treatment with corticosteroids or other immunosuppressive agents, and some data of our study are in support of such possibility: as shown in Table 7, we found a significant decrease in both TA-hematuria and TA-proteinuria as well as a nonsignificant slowing in the rate of renal function decline after treatment in those patients with severe proteinuria and hematuria during follow-up who had received immunosuppressants. In addition, in the whole cohort of patients, immunosuppressive treatments induced a significant reduction in TA-hematuria that decreased from 36 (6–100) RBC × hpf before treatment to 3 (0–12) RBC × hpf after treatment (P=0.001). Obviously, prospective studies in which the presence and quantity of hematuria are evaluated at baseline are needed to show that the reduction or disappearance of the hematuria has a favorable effect on outcomes. However, failure to take into account the presence and magnitude of hematuria in patients recruited for prospective therapeutic trials on this disease may have partially distorted the results.
Our study has several limitations, because it is a retrospective study with a relatively small number of patients. However, it has evident strengths, having been carried out in a well characterized cohort of patients with IgAN, histologic data analyzed according to the Oxford Classification of IgAN, and protocolized systematic visits, in which all clinical and analytic parameters of interest were determined. In contrast with previous studies, the systematic determination and quantification of hematuria by means of urinary sediment examination performed at every visit allowed us to evaluate the influence of this parameter on renal outcomes.
In conclusion, our data show that remission of hematuria has a significantly favorable effect on IgAN progression, whereas its persistence in significant amounts is an independent risk factor for renal function loss. According to our data, patients with persistent hematuria and proteinuria during follow-up are those at risk for progression to ESRD. We suggest that these findings should be taken into account for treatment decisions and the design of therapeutic prospective trials.
Concise Methods
Patients
All of the patients with renal biopsy–proven IgAN and absence of diabetes, liver or systemic diseases, Henoch–Schönlein purpura, or any type of secondary IgAN were evaluated for the study. Twenty of 165 patients initially evaluated were excluded because of a follow-up shorter than 1 year (ESRD in six, death in three, and loss of follow-up in 11). Another 33 patients were excluded because of incomplete data (25) or loss of follow-up (eight). The 112 remaining patients were included in the study.
Patient Follow-Up and Data Collection
Regular visits at intervals of 3, 6, or 12 months were performed in every patient. Proteinuria and urine sediment were tested at every visit throughout follow-up. Urine sediment examination was manually performed by specialized skilled nurses in our renal laboratory. Urine sediment was examined in a centrifuged and concentrated sample of the first urine of the morning within 1 hour after voiding using standardized methodology. Samples were examined at low (×10) and high power (×40) on the bright-field microscope. Hematuria was quantified at every visit as RBC × hpf. Proteinuria was quantified in a 24-hour urine collection at every visit.
The following data were systematically recorded: sex, age at kidney biopsy, systolic and diastolic BP, weight, serum creatinine, eGFR, serum total cholesterol, serum total proteins, serum albumin, 24-hour proteinuria, and hematuria. All of the treatments were recorded, including antihypertensive drugs, renin-angiotensin system inhibitors (either ACEIs or ARBs), corticosteroids, and immunosuppressive drugs.
Renal Biopsy
Renal biopsies were re-evaluated by a nephropathologist (M.A.M.), and lesions were scored according to the Oxford Classification of IgAN: mesangial score <0.5 or >0.5; segmental glomerulosclerosis absent or present; endocapillary hypercellularity absent or present; and tubular atrophy/interstitial fibrosis <25%, 26%–50%, or >50%.
Definitions
Baseline was established as the time of renal biopsy performance. Follow-up was defined as the interval between renal biopsy and the last outpatient visit, death, or ESRD. ESRD was defined as an eGFR lower than 15 ml/min per 1.73 m2, a need for chronic dialysis, or renal transplantation. An average proteinuria was determined for every 6 months during follow-up for each patient. Then, TA-proteinuria was calculated for each patient, representing an average of the mean of proteinuria measurements in every 6-month period. The same methodology was applied for the calculation of TA-hematuria with the number of RBC × hpf recorded at every visit and TA-MBP with the values of mean BP. Mean BP was calculated as the diastolic BP plus one third of the pulse pressure. Urine sediments performed within an episode of gross hematuria were excluded for the calculation of TA-hematuria. eGFR was calculated by the Modification of Diet in Renal Disease abbreviated equation.
Patients were divided in two groups according to the magnitude of TA-hematuria: patients with persistent hematuria (TA-hematuria >5 RBC × hpf) and those with negative or minimal hematuria (TA-hematuria ≤5 RBC × hpf). Patients were also classified according to the magnitude of TA-proteinuria (TA-proteinuria >0.75 or ≤0.75 g/d).
Disappearance of hematuria was defined by the absence of hematuria or the presence of <5 RBC × hpf in all of the urine sediment examinations performed during at least 3 years before the last outpatient visit. For each patient meeting, this criterion (the time of hematuria disappearance) was identified and recorded.
Outcomes
The outcomes were renal survival defined by a status free of ESRD, a renal function decline higher than 50% over time, and the slope of eGFR.
Statistical Analyses
Normally distributed variables are presented as mean±SD and compared using t test, one-way ANOVA, or Pearson correlation coefficients as appropriate. Proteinuria, TA-proteinuria, TA-hematuria, and TA-MBP are expressed as medians with interquartile ranges (25th and 75th percentiles) and analyzed with mean tests. Categorical variables are expressed as frequencies and percentages and compared with Fisher and chi-squared tests. The slope of eGFR was calculated in every patient and presented as annual rates of renal function loss. The cumulative probability of developing a defined clinic event (ESRD or a >50% loss of renal function) was estimated by the Kaplan–Meier method, and survival curves were compared with the Breslow test. Survival time for each patient was computed from baseline evaluation to last follow-up. Univariate and multivariate Cox proportional hazard models were performed to explore the influence of several variables on the occurrence of ESRD. A P value <0.05 was considered significant. Statistical analysis was performed with IBM SPSS statistics 22.
Disclosures
None.
Acknowledgments
Work in this report was funded by Instituto de Salud Carlos III/Fondo Europeo de Desarrollo Regional (ISCIII/FEDER) Programa Miguel Servet grants CP10/00479 (to J.A.M.), CPII16/00017 (to J.A.M.), PI13/00802 (to J.A.M.), and PI14/00883 (to J.A.M.); the Spanish Society of Nephrology (to J.A.M.); Fundación Renal Iñigo Alvarez de Toledo (to J.A.M.); the Instituto de Salud Carlos III (to M.P.); Red de Investigacion Renal grant RD012/0021 (to M.P.); and ISCIII/FEDER grants 13/02502 (to M.P.), ICI14/00350 (to M.P.), and 16/01685 (to M.P.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Persistent Microscopic Hematuria as a Risk Factor for Progression of IgA Nephropathy: New Floodlight on a Nearly Forgotten Biomarker,” on pages 2831–2834.
References
- 1.Donadio JV, Grande JP: IgA nephropathy. N Engl J Med 347: 738–748, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Wyatt RJ, Julian BA: IgA nephropathy. N Engl J Med 368: 2402–2414, 2013 [DOI] [PubMed] [Google Scholar]
- 3.D’Amico G: Natural history of idiopathic IgA nephropathy: Role of clinical and histological prognostic factors. Am J Kidney Dis 36: 227–237, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Floege J, Feehally J: IgA nephropathy: Recent developments. J Am Soc Nephrol 11: 2395–2403, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Bartosik LP, Lajoie G, Sugar L, Cattran DC: Predicting progression in IgA nephropathy. Am J Kidney Dis 38: 728–735, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Goto M, Wakai K, Kawamura T, Ando M, Endoh M, Tomino Y: A scoring system to predict renal outcome in IgA nephropathy: A nationwide 10-year prospective cohort study. Nephrol Dial Transplant 24: 3068–3074, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reich HN, Troyanov S, Scholey JW, Cattran DC; Toronto Glomerulonephritis Registry : Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Berthoux F, Mohey H, Laurent B, Mariat C, Afiani A, Thibaudin L: Predicting the risk for dialysis or death in IgA nephropathy. J Am Soc Nephrol 22: 752–761, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le W, Liang S, Hu Y, Deng K, Bao H, Zeng C, Liu Z: Long-term renal survival and related risk factors in patients with IgA nephropathy: Results from a cohort of 1155 cases in a Chinese adult population. Nephrol Dial Transplant 27: 1479–1485, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Gutiérrez E, Zamora I, Ballarín JA, Arce Y, Jiménez S, Quereda C, Olea T, Martínez-Ara J, Segarra A, Bernis C, García A, Goicoechea M, García de Vinuesa S, Rojas-Rivera J, Praga M; Grupo de Estudio de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN) : Long-term outcomes of IgA nephropathy presenting with minimal or no proteinuria. J Am Soc Nephrol 23: 1753–1760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Liu Y, Lv J, Shi S, Liu L, Chen Y, Zhang H: Progression of IgA nephropathy under current therapy regimen in a Chinese population. Clin J Am Soc Nephrol 9: 484–489, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magistroni R, D’Agati VD, Appel GB, Kiryluk K: New developments in the genetics, pathogenesis, and therapy of IgA nephropathy. Kidney Int 88: 974–989, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao YF, Zhu L, Liu LJ, Shi SF, Lv JC, Zhang H: Measures of urinary protein and albumin in the prediction of progression of IgA nephropathy. Clin J Am Soc Nephrol 11: 947–955, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kidney Disease Improving Global Outcomes (KDIGO) Glomerulonephritis, Work Group : KDIGO clinical practice guideline for glomerulonephritis. Kidney Int Suppl 2: 209–217, 2012 [Google Scholar]
- 15.Inker LA, Mondal H, Greene T, Masaschi T, Locatelli F, Schena FP, Katafuchi R, Appel GB, Maes BD, Li PK, Praga M, Del Vecchio L, Andrulli S, Manno C, Gutierrez E, Mercer A, Carroll KJ, Schmid CH, Levey AS: Early change in urine protein as a surrogate end point in studies of IgA nephropathy: An individual-patient meta-analysis. Am J Kidney Dis 68: 392–401, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, Troyanov S, Alpers CE, Amore A, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D’Agati V, D’Amico G, Emancipator S, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo A, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hogg RJ, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Leung CB, Li LS, Li PK, Liu ZH, Mackinnon B, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H; Working Group of the International IgA Nephropathy Network and the Renal Pathology Society : The Oxford classification of IgA nephropathy: Rationale, clinicopathological correlations, and classification. Kidney Int 76: 534–545, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, Roberts IS, Morando L, Camilla R, Tesar V, Lunberg S, Gesualdo L, Emma F, Rollino C, Amore A, Praga M, Feriozzi S, Segoloni G, Pani A, Cancarini G, Durlik M, Moggia E, Mazzucco G, Giannakakis C, Honsova E, Sundelin BB, Di Palma AM, Ferrario F, Gutierrez E, Asunis AM, Barratt J, Tardanico R, Perkowska-Ptasinska A; VALIGA study of the ERA-EDTA Immunonephrology Working Group : Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney Int 86: 828–836, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbour SJ, Espino-Hernandez G, Reich HN, Coppo R, Roberts IS, Feehally J, Herzenberg AM, Cattran DC; Oxford Derivation, North American Validation and VALIGA Consortia, Oxford Derivation North American Validation and VALIGA Consortia : The MEST score provides earlier risk prediction in lgA nephropathy. Kidney Int 89: 167–175, 2016 [DOI] [PubMed] [Google Scholar]
- 19.Manno C, Strippoli GF, D’Altri C, Torres D, Rossini M, Schena FP: A novel simpler histological classification for renal survival in IgA nephropathy: A retrospective study. Am J Kidney Dis 49: 763–775, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Feng CY, Xia YH, Wang WJ, Xia J, Fu HD, Wang X, Shen HJ, Qian GL, Liu AM, Mao JH: Persistent asymptomatic isolated hematuria in children: Clinical and histopathological features and prognosis. World J Pediatr 9: 163–168, 2013 [DOI] [PubMed] [Google Scholar]
- 21.Lee H, Kim DK, Oh KH, Joo KW, Kim YS, Chae DW, Kim S, Chin HJ: Mortality of IgA nephropathy patients: A single center experience over 30 years. PLoS One 7: e51225, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi S, Lee D, Jeong KH, Moon JY, Lee SH, Lee TW, Ihm CG: Prognostic relevance of clinical and histological features in IgA nephropathy treated with steroid and angiotensin receptor blockers. Clin Nephrol 72: 353–359, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki C, Moriyama T, Tanaka K, Takei T, Nitta K: Effect of hematuria on the outcome of immunoglobulin A nephropathy with proteinuria. J Nephropathol 5: 72–78, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka K, Moriyama T, Iwasaki C, Takei T, Nitta K: Effect of hematuria on the outcome of IgA nephropathy with mild proteinuria. Clin Exp Nephrol 19: 815–821, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Barbour SJ, Cattran DC, Kim SJ, Levin A, Wald R, Hladunewich MA, Reich HN: Individuals of Pacific Asian origin with IgA nephropathy have an increased risk of progression to end-stage renal disease. Kidney Int 84: 1017–1024, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Moreno JA, Martín-Cleary C, Gutiérrez E, Rubio-Navarro A, Ortiz A, Praga M, Egido J: Haematuria: The forgotten CKD factor? Nephrol Dial Transplant 27: 28–34, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Moreno JA, Yuste C, Gutiérrez E, Sevillano ÁM, Rubio-Navarro A, Amaro-Villalobos JM, Praga M, Egido J: Haematuria as a risk factor for chronic kidney disease progression in glomerular diseases: A review. Pediatr Nephrol 31: 523–533, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Deuel JW, Schaer CA, Boretti FS, Opitz L, Garcia-Rubio I, Baek JH, Spahn DR, Buehler PW, Schaer DJ: Hemoglobinuria-related acute kidney injury is driven by intrarenal oxidative reactions triggering a heme toxicity response. Cell Death Dis 7: e2064, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vivante A, Afek A, Frenkel-Nir Y, Tzur D, Farfel A, Golan E, Chaiter Y, Shohat T, Skorecki K, Calderon-Margalit R: Persistent asymptomatic isolated microscopic hematuria in Israeli adolescents and young adults and risk for end-stage renal disease. JAMA 306: 729–736, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Yuste C, Rubio-Navarro A, Barraca D, Aragoncillo I, Vega A, Abad S, Santos A, Macias N, Mahillo I, Gutiérrez E, Praga M, Egido J, López-Gómez JM, Moreno JA: Haematuria increases progression of advanced proteinuric kidney disease. PLoS One 10: e0128575, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan M, Ware K, Qamri Z, Satoskar A, Wu H, Nadasdy G, Rovin B, Hebert L, Nadasdy T, Brodsky SV: Warfarin-related nephropathy is the tip of the iceberg: Direct thrombin inhibitor dabigatran induces glomerular hemorrhage with acute kidney injury in rats. Nephrol Dial Transplant 29: 2228–2234, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Appel GB, Waldman M: The IgA nephropathy treatment dilemma. Kidney Int 69: 1939–1944, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Boyd JK, Cheung CK, Molyneux K, Feehally J, Barratt J: An update on the pathogenesis and treatment of IgA nephropathy. Kidney Int 81: 833–843, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Floege J, Feehally J: Treatment of IgA nephropathy and Henoch-Schönlein nephritis. Nat Rev Nephrol 9: 320–327, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Abbate M, Zoja C, Remuzzi G: How does proteinuria cause progressive renal damage? J Am Soc Nephrol 17: 2974–2984, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Ohse T, Inagi R, Tanaka T, Ota T, Miyata T, Kojima I, Ingelfinger JR, Ogawa S, Fujita T, Nangaku M: Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int 70: 1447–1455, 2006 [DOI] [PubMed] [Google Scholar]