Abstract
Glomerular sclerotic lesions develop when the glomerular filtration surface area exceeds the availability of podocyte foot process coverage, but the mechanisms involved are incompletely characterized. We evaluated potential mechanisms using a transgenic (podocin promoter-AA-4E-BP1) rat in which podocyte capacity for hypertrophy in response to growth factor/nutrient signaling is impaired. FSGS lesions resembling human FSGS developed spontaneously by 7 months of age, and could be induced earlier by accelerating kidney hypertrophy by nephrectomy. Early segmental glomerular lesions occurred in the absence of a detectable reduction in average podocyte number per glomerulus and resulted from the loss of podocytes in individual glomerular capillary loops. Parietal epithelial cell division, accumulation on Bowman’s capsule, and tuft invasion occurred at these sites. Three different interventions that prevented kidney growth and glomerular enlargement (calorie intake reduction, inhibition of mammalian target of rapamycin complex, and inhibition of angiotensin-converting enzyme) protected against FSGS lesion development, even when initiated late in the process. Ki67 nuclear staining and unbiased transcriptomic analysis identified increased glomerular (but not podocyte) cell cycling as necessary for FSGS lesion development. The rat FSGS-associated transcriptomic signature correlated with human glomerular transcriptomes associated with disease progression, compatible with similar processes occurring in man. We conclude that FSGS lesion development resulted from glomerular growth that exceeded the capacity of podocytes to adapt and adequately cover some parts of the filtration surface. Modest modulation of the growth side of this equation significantly ameliorated FSGS progression, suggesting that glomerular growth is an underappreciated therapeutic target for preservation of renal function.
Keywords: glomerular disease, podocyte, progression of renal failure, hypertrophic stress, growth
The podocyte depletion hypothesis posits that a mismatch between the glomerular filtration surface area to be served and the podocyte foot process coverage available in any individual glomerulus determines whether proteinuria and glomerulosclerosis supervene.1,2 Relative podocyte depletion can be caused by a podocyte deficit (reduction in number, size, or function), by enlargement of the glomerular filtration surface area, or by some combination of these mechanisms.3 The reason why glomerular sclerotic lesions are characteristically focal and segmental is not well understood.
In the podocin-AA-4E-BP1 transgenic (TG) Fischer344 rat every cell of the body except the podocyte has normal capacity to maintain structure/function and respond to growth signaling cues.1 The intrinsic machinery of the podocyte itself is also normal in the sense that no rat protein is knocked down or genetically modified. The only abnormality present is that a modified version of the human 4E-BP1 protein of the mTORC1 pathway is expressed specifically by podocytes under the control of the human podocin promoter that becomes active in late glomerular development. The modified human 4E-BP1 protein used as a transgene has two threonine residues changed to alanine (AA-4EBP1) so that they cannot be phosphorylated. This prevents the mTORC1 kinase from phosphorylating them as is necessary to release the CAP-binding protein eIF4e to initiate transcription. Expression of the AA-4E-BP1 transgene by podocytes thereby blunts the growth response to mTORC1 activation by growth factors and nutrients.4,5 The intact homozygous TG rat at young age has normal-appearing glomeruli, normal podocyte number, normal kidney function, grows normally, and reproduces normally.1 If one kidney is removed so that hypertrophic stress is imposed on the remaining kidney, podocytes are less able to cope with hypertrophic demand resulting in proteinuria and progression to ESRD over 12 weeks.1 In this report, we extend previous work to better understand the effect of various interventions on the development of FSGS lesions as well as investigate the mechanisms of FSGS development.
Results
Mature FSGS Lesions in the AA-4E-BP1 TG Rat
Homozygous male and female TG rats develop increased proteinuria by 7 months of age (300 g body wt) by which time 16%±7% of glomeruli contain typical mature FSGS lesions (Figure 1A) that closely resemble mature human FSGS lesions (Figure 1B). No increase in proteinuria or glomerulosclerosis occurs in wild-type Fischer344 rats by 7 months of age,6 thereby confirming that FSGS lesion development is dependent on the transgene expressed specifically by podocytes.
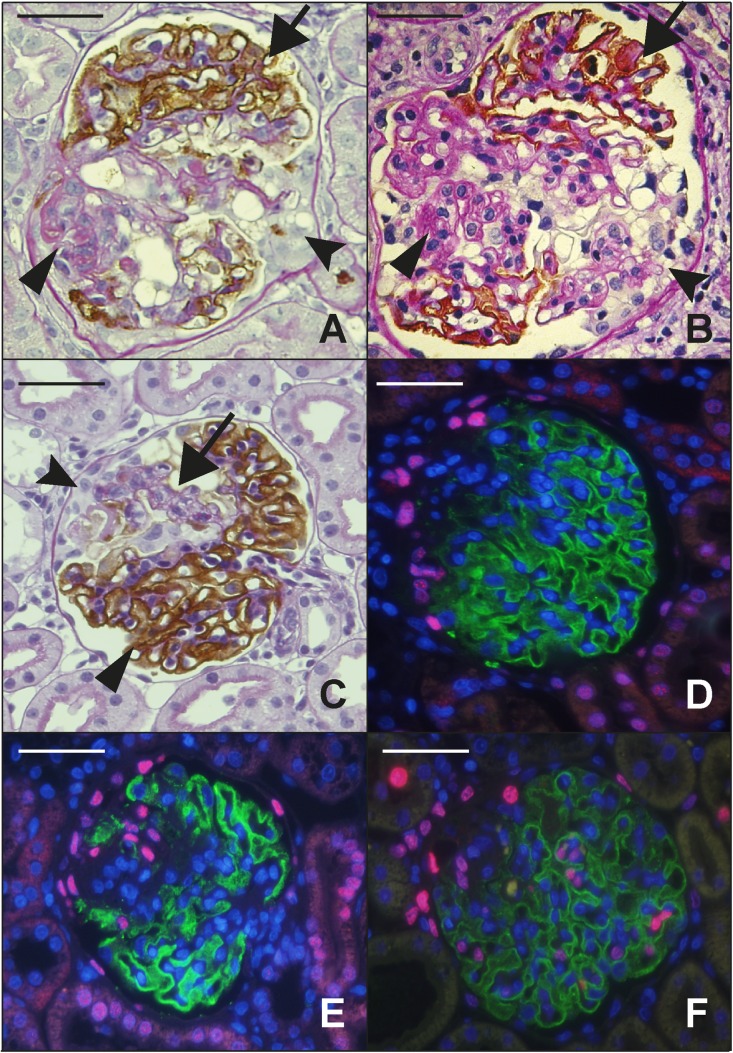
Figure 1.
Structural features related to FSGS lesion development. (A) TG rat glomerulus showing a mature FSGS lesion at 7 months by Glepp1 peroxidase counter-stained with PAS. Podocytes are absent from the sclerotic area (arrowhead) in contrast to the remainder of the tuft which shows a normal distribution of brown peroxidase positive Glepp1-stained podocytes (arrow). An adhesion joining Bowman’s capsule to the glomerular tuft is also present (fluted arrowhead). (B) A mature human FSGS lesion that is identical to the rat FSGS lesion shown in (A). The arrows point to the same features as emphasized for (A). (C) Early segmental FSGS lesion present at 3 weeks after nephrectomy of the AA-4E-BP1 rat model. A naked glomerular capillary loop (arrow) is devoid of the brown-staining Glepp1-peroxidase product that is present in the remaining tuft area (arrowhead). PECs accumulate on Bowman’s capsule adjacent to the segmental lesion and some PECS appear to have invaded the tuft within the segmental lesion. (D and E) PECs, identified by their pink Pax8-positive nuclei. In (D), Pax8-positive cells have accumulated opposite an early developing FSGS lesion that is losing its podocytes (as indicated by loss of green Glepp1-stained cytoplasm). In (E), the Pax8-positive PECs are invading the glomerular tuft at a site of absent podocytes (as shown by absence of Glepp1 green fluorescence). Nuclei are shown by blue DAPI staining. (F) Ki67 immunofluorescence to identify dividing cells. In association with development of the early FSGS lesion as indicated by loss of Glepp1-positive green signal, Ki67-positive nuclei representing cycling cells (pink nuclei) are present on Bowman’s capsule (PECs), within the glomerular tuft (intrinsic glomerular cells), in the peri-glomerular and interstitial compartments, and in tubules. Double-labeling studies for WT1 and Ki67 showed no double-positive cells, indicating that cells identified as podocytes on the basis of anatomic location were not WT1-positive podocytes. Class II staining showed no increase in class II positivity in association with FSGS lesion formation. Scale bars, 100 μm.
Early FSGS Lesion Development
TG rats at 100 g body wt have normal-appearing glomeruli as judged by light microscopy (H and E, PAS, and silver staining, Glepp1 immune-peroxidase) as well as by transmission and scanning electron microscopy.1 If these 100 g TG rats are nephrectomized in order to induce hypertrophy in the remaining kidney, by 3 weeks after nephrectomy the contralateral kidney has increased in weight by 63%, glomerular volume has increased by 48%, the urine protein/creatinine ratio has increased to 7.3, 15%±8% of glomeruli have developed segmental glomerular lesions as judged by Glepp1 immuno-peroxidase (Figure 1C), and 5% have developed adhesions to Bowman’s capsule as detected by Masson trichrome staining (Table 1). In association with 48% glomerular enlargement and development of FSGS lesions neither the average podocyte nuclear number per glomerulus nor the total podocyte volume per glomerulus (Glepp1-positive glomerular volume) has changed (Table 2), the rate of podocyte detachment measured in urine has not increased significantly (Table 1), and no podocytes can be identified free in Bowman’s space or in tubular lumens. Therefore, by 3 weeks after nephrectomy glomerular volume enlargement had not yet resulted in measurable podocyte loss. However, as a consequence of the glomerular volume enlargement and in association with FSGS lesion development in some glomeruli the podocyte nuclear density (number per volume) had decreased by 49.8% and the podocyte cell density (Glepp1 area density) had decreased by 30.3%, and the average Glepp1-negative glomerular volume (representing the nonpodocyte component of the glomerulus) had doubled (Table 2). In the early segmental lesions, individual glomerular capillary loops (shown by the arrow in Figure 1C) have become completely devoid of their normal podocyte covering (as previously also noted by scanning electron microscopy1). No increased proteinuria and no FSGS lesions were observed in wild-type rats subjected to the same hypertrophic stress conditions. Figure 1C also shows cells that appear to be parietal epithelial cells (PECs) accumulating on Bowman’s capsule at the site of podocyte absence and invading the tuft. This is further supported by using Pax8 as a PEC marker and demonstrating similar accumulation of cells on Bowman’s capsule adjacent to areas of segmental podocyte depletion (Figure 1D). In some glomeruli, Pax8-positive cells can be seen within the podocyte-depleted regions of the glomerular tuft (Figure 1E).
Table 1.
Phenotypic Analysis
| Groups | Body Weight, g | Kidney Weight, g | Urine Protein/Creatinine Ratio | Serum Creatinine, mg% | Adhesions, % | Urine Podocin mRNA/Creat Ratio, units/g Creatinine |
|---|---|---|---|---|---|---|
| TG.1K.ALD (FSGS) | 202±7 | 1.8±0.2 | 7.3±1.6 | 1.4±0.1 | 5.6±2.6 | 3.1±2.1 |
| TG.2K.ALD | 202±15 | 1.1±0.2a | 2.4±0.5a | 1.1±0.2 | 0.0a | 0.7±0.3 |
| TG.1K.CRD | 144±9a | 0.9±0.1a | 0.9±0.3a | 1.3±0.2 | 0.0a | 1.1±0.2 |
| TG.1K.ALD.Rapa | 145±9a | 1.1±0.1a | 1.1±0.1a | 1.7±0.3 | 0.0a | 0.3±0.2b |
| TG.1K.ALD.ACEi | 196±8 | 1.3±0.1a | 1.4±0.2a | 1.4±0.2 | 0.0a | 4.4±3.3 |
Phenotyping for TG rat groups 3 weeks after nephrectomy or sham nephrectomy. Data for the control group before surgery at time 0 is shown at top. The remaining groups were evaluated 3 weeks after surgery (n=5–7 per group). FSGS with adhesions to Bowman’s capsule identified on Masson trichrome–stained sections was observed only in the nephrectomized ad lib–fed group and was associated with greater kidney weight gain and higher level proteinuria. FSGS lesions did not develop if rats did not undergo nephrectomy that caused compensatory kidney hypertrophy, or were prevented from gaining body weight by either calorie reduction or rapamycin treatment, or were treated with ACE inhibitor that did not prevent body weight gain but did prevent kidney enlargement. The rate of podocyte detachment as measured by the urine podocin mRNA/creatinine ratio was not significantly increased by 3 weeks in any group. The statistical comparisons shown compare the four groups that did not develop FSGS to the nephrectomized ad lib–fed group that did develop FSGS. Statistical comparisons by ANOVA with Bonferroni correction. Data are mean±SD. Creat, creatinine; TG, TG rats; 1K/2K, nephrectomized/sham-nephrectomized; ALD, ad lib diet; CRD, 40% calorie reduced diet; rapa, rapamycin-treated; ACEi, treated with angiotensin II inhibitor enalapril.
P<0.01.
P<0.05.
Table 2.
Podometric Analysis
| Group | Glomerular Volume, ×106 µm3 | Podocyte No. per Tuft, n | Podocyte Nuc Density, per 106 µm | Podocyte Volume, ×103 µm3 | Glepp1 Area, % | Glepp1+ve Glom Vol, ×103 µm3 | Glepp1–ve Glom Vol, ×103 µm3 |
|---|---|---|---|---|---|---|---|
| TG.1K.ALD (FSGS) | 1.2±0.1 | 145±15 | 120±11 | 2.7±0.2 | 32.6±2.7 | 379±80 | 817±105 |
| TG.2K.ALD | 0.8±0.2a | 142±31 | 178±38a | 2.7±0.5 | 46.7±1.5a | 393±46 | 429±78a |
| TG.1K.CRD | 0.7±0.2a | 146±21 | 212±21a | 2.1±0.2b | 44.7±2.3a | 313±68 | 392±110a |
| TG.1K.ALD.Rapa | 0.5±0.1a | 126±19 | 248±15a | 1.8±0.1a | 44.2±1.3a | 225±25a | 285±43a |
| TG.1K.ALD.ACEi | 0.8±0.1a | 131±17 | 212±29b | 2.7±0.4 | 41.8±1.4a | 345±40 | 483±81a |
| WT.2K.ALD | 0.6±0.1a | 130±19 | 241±73a | 2.1±0.6 | 46.8±1.6a | 271±43 | 263±51a |
| WT.1K.ALD | 0.6±0.1a | 136±19 | 272±73a | 1.9±0.7 | 46.8±1.1a | 252±50 | 309±45a |
Podometric analysis for TG or WT rat groups 3 weeks after nephrectomy (1K) or sham nephrectomy (2K). All groups (n=5–7) were evaluated 3 weeks after surgery. Statistical comparisons by ANOVA with Bonferroni correction are shown for all groups in comparison to the nephrectomized ad lib–fed group that developed FSGS (TG.1K.ALD) (top row). There was no reduction in podocyte number per glomerulus in TG.1K.ALD rats that developed FSGS. On the other hand, the glomerular volume was increased resulting in reduced podocyte nuclear density and Glepp1 area density and an increase in the Glepp1-negative (nonpodocyte) glomerular volume compared with other groups. Prevention of FSGS by calorie-reduced diet, rapamycin, or ACE inhibitor were all associated with maintenance of smaller glomerular volume and higher podocyte nuclear density and Glepp1 area density. WT nephrectomized or sham-nephrectomized rats on an ad lib diet did not develop increased proteinuria or FSGS lesions and maintained podometric values that were not significantly different from the TG sham-nephrectomized group that did not develop FSGS. Data are mean±SD. Nuc, nuclear; Glepp1+ve Glom Vol, glomerular podocyte volume; Glepp1–ve Glom Vol , glomerular volume not occupied by podocytes; TG, Fischer344 TG rats; 1K/2K, nephrectomized/sham-nephrectomized; ALD, ad lib diet; CRD, 40% calorie reduced diet; Rapa, rapamycin-treated; ACEi, treated with the angiotensin II inhibitor enalapril; WT, wild-type Fischer344 rats.
P<0.01.
P<0.05.
Taken together, these data support the hypothesis that in this model, nephrectomy resulted in compensatory glomerular enlargement that could not be accommodated by TG podocytes, thereby giving rise to naked glomerular capillary loops, proteinuria, and accumulation of PECs adjacent to areas of podocyte depletion that subsequently invade the tuft and will contribute to forming mature segmentally sclerotic lesions.
Prevention of FSGS Lesion Development by Slowing Glomerular Growth Rate
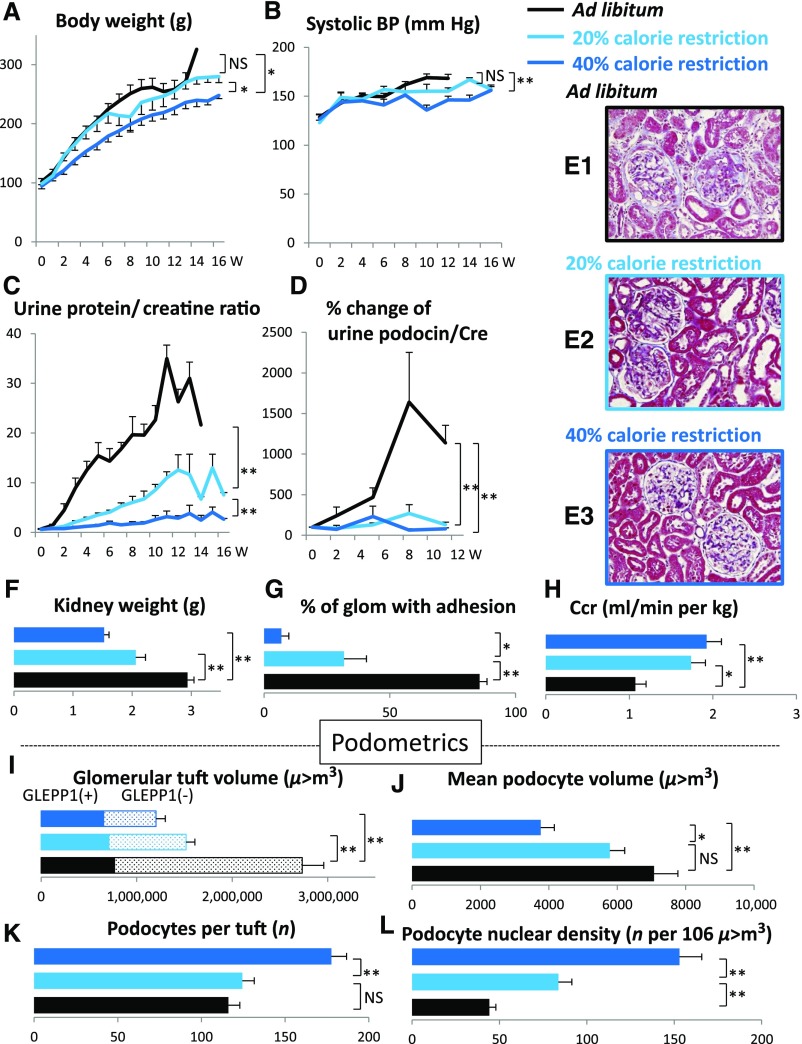
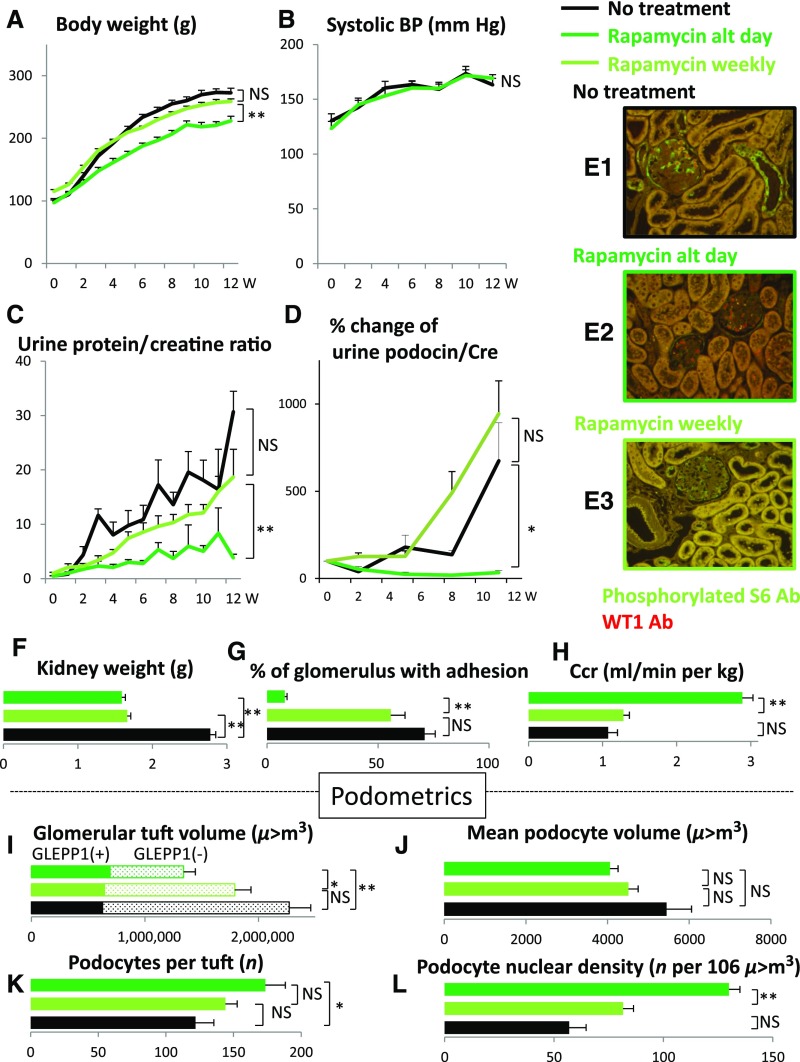
If the above hypothesis is correct then slowing the rate of growth of the glomerulus would be predicted to prevent FSGS lesion development. The mTORC1 complex senses and integrates growth factor signaling and nutrient supply (amino acids and glucose) that controls growth of cells and organs.4,5 As shown in Figures 2 and 3, slowing the rate of growth of the TG rat either by reducing calorie intake or by inhibiting the mTORC1 kinase pathway directly (by rapamycin) had identical effects to slow the rate of body weight gain, reduce kidney weight gain, reduce glomerular enlargement, prevent podocyte detachment measured in urine, prevent podocyte depletion from glomeruli, prevent proteinuria, prevent development of glomerulosclerosis, and prevent reduction in renal function. This occurred in a dose-dependent fashion as shown by modest calorie intake reduction or lower-dose rapamycin. This result confirms both the mTORC1-dependent and the growth-dependent nature of development of FSGS lesions and progression to ESRD in the model.
Figure 2.
Dietary calorie intake reduction prevents growth-induced glomerular failure in the AA-4E-BP1 model in a dose-dependent manner. Dietary calorie intake reduction (40% reduction>20% reduction) has the following protective effects: body weight gain was slowed (A); kidney weight gain was reduced (F); glomerular tuft volume was reduced (I); rate of podocyte detachment was reduced (D); reduction of podocyte number per glomerular tuft was prevented (K); reduction in podocyte density was prevented (L); the increase in podocyte cell volume was prevented (J); urine protein/creatinine ratio was reduced (C); percentage of glomeruli with adhesions and amount of glomerulosclerosis were reduced (G and E1, 2, and 3); decreased creatinine clearance was prevented (H); and systolic BP increase with age was prevented (B). Data shown as the mean±SEM. Glomerular tuft volume (μm3), mean podocyte volume (μm3), podocytes per tuft (n) and podocyte nuclear density (n per 106 μm3). *P<0.05, **P<0.01. Ccr, creatinine clearance; Cre, urine creatinine; glom, glomerulus; W, weeks.
Figure 3.
The mTORC1 inhibitor rapamycin prevents growth-induced glomerular failure in the AA-4E-BP1 model. Rapamycin (delivered at 10 mg/kg by IP injection on alternate days [dark green line] or at low dose once per week [light green line]) inhibited mTORC1 kinase activity as demonstrated by completely preventing phosphorylation of ribosomal S6 in glomeruli and tubules shown by green immunofluorescence (E1 versus E2). Podocyte nuclei are shown by red WT1 immunofluorescent staining. In contrast, lower-dose rapamycin (delivered as 10mg/kg by IP injection once per week) did not fully inhibit ribosomal S6 phosphorylation (E1 versus E3). Rapamycin treatment had the following protective effects: body weight gain was slowed (A); kidney weight gain was reduced (F); glomerular tuft volume was reduced (I); rate of podocyte detachment was reduced (D); reduction of podocyte number per glomerular tuft was prevented (K); reduction in podocyte density was prevented (L); there was no significant change in podocyte cell volume (J); urine protein/creatinine ratio was reduced (C); percentage of glomeruli with adhesions and amount of glomerulosclerosis were reduced (G); decreased creatinine clearance was prevented (H); and systolic BP increase with age was not prevented (B). Data shown as the mean±SEM. Glomerular tuft volume (μm3), mean podocyte volume (μm3), podocytes per tuft (n) and podocyte nuclear density (n per 106 μm3). *P<0.05, **P<0.01. Ab, antibody; alt, alternate; Ccr, creatinine clearance; Cre, urine creatinine; W, weeks.
Supplemental Table 1 shows the effect of modulating individual components of the diet in the heterozygous fully grown TG rat. Protection of progression was achieved by 40%>20% calorie intake reduction and by low-protein diet. High-fat diet, possibly because it has low protein and carbohydrate content, was also partially protective, although this protection could be neutralized by adding extra carbohydrate in the form of 10% sucrose in the drinking water (to simulate the typical sucrose content of commercially available beverages). These data are compatible with the concept that hypertrophy-induced glomerular injury can be modulated by diets that effect energy sensing through the mTORC1 pathway.
Angiotensin II Blockade Also Prevents Kidney and Glomerular Growth and FSGS Lesion Development
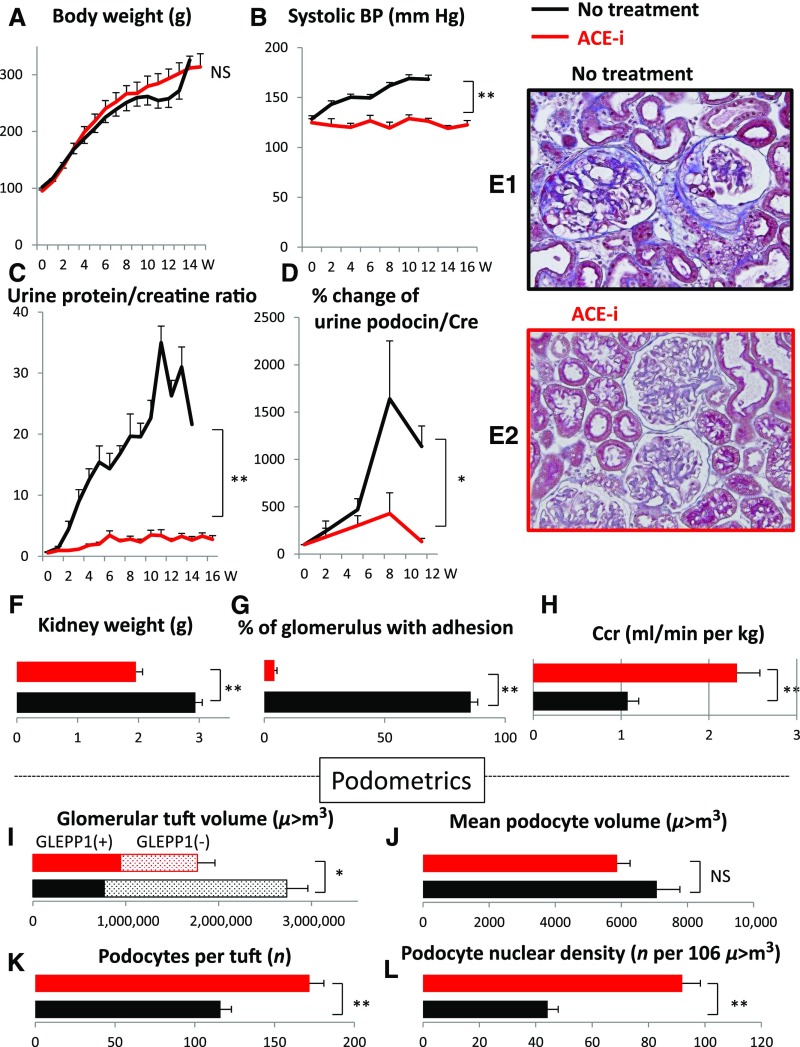
Figure 4 shows that the ACE inhibitor enalapril did not slow body growth rate but did slow kidney growth rate and downstream consequences including glomerular volume enlargement, proteinuria, increased rate of podocyte detachment, glomerular sclerosis, and reduction in renal function. Enalapril also lowered BP in the model. Thus, angiotensin II blockade had the same effect on reducing kidney and glomerular growth and its downstream consequences as did calorie intake reduction and mTORC1 inhibition shown in Figures 2 and 3, but did not reduce the rate of body growth.
Figure 4.
ACE inhibition prevented growth-induced glomerular failure in the AA-4E-BP1 model. The following protections were observed by ACE inhibitor treatment with enalapril (10 mg/kg per day in the drinking water): body weight gain was not changed (A); kidney weight gain was reduced (F); glomerular tuft volume was reduced (I); rate of podocyte detachment was reduced (D); reduction of podocyte number per glomerular tuft was prevented (K); reduction in podocyte density was prevented (L); the increase in podocyte cell volume was not significantly reduced (J); urine protein/creatinine ratio was reduced (C); percent of glomeruli with adhesions and amount of glomerulosclerosis were reduced (G, E1, and E2); decreased creatinine clearance was prevented (H); and systolic BP increase with age was prevented (B). Data shown as the mean±SEM. Glomerular tuft volume (μm3), mean podocyte volume (μm3), podocytes per tuft (n) and podocyte nuclear density (n per 106 μm3). *P<0.05, **P<0.01. ACE-i, angiotensin-converting enzyme inhibitor; Ccr, creatinine clearance; Cre, urine creatinine; W, weeks.
Timing of Intervention
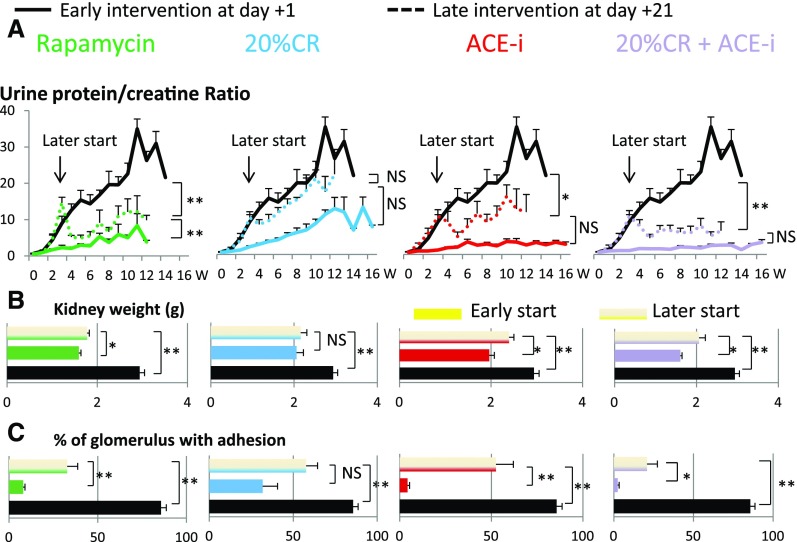
To simulate interventions in real life situations, the three treatment strategies were initiated 3 weeks after nephrectomy at a time when the urine protein/creatinine ratio had reached a value of about seven and early FSGS lesions were already present (Figure 5). Rapamycin (even at once per week dosing) was significantly effective, 20% calorie reduction alone was not significantly effective, and ACE inhibitor was significantly effective at reducing injury and proteinuria. The combination of modest 20% calorie reduction plus ACE inhibitor appeared to be more effective than either strategy alone.
Figure 5.
Later intervention 3 weeks after nephrectomy when the urine protein/creatinine ratio was ten was partially effective in preventing growth-induced glomerular failure in the AA-4E-BP1 model. Rapamycin (10 mg/kg by IP injection) was started either on day 1 (solid green line) or day 21 (dotted green line or striped bar) after nephrectomy. Modest 20% calorie reduction was started either on day 1 (solid blue line) or day 21 (dotted blue line or striped bar) after nephrectomy. ACE inhibitor (enalapril at 10 mg/kg per day) was started either on day 1 (solid red line) or day 21 (dotted red line or striped bar) after nephrectomy. The combination of calorie reduction (20%) and ACE inhibitor (enalapril 10 mg/kg per day) was started either on day 1 (solid purple line) or day 21 (dotted purple line or striped bar) after nephrectomy. Data are shown for the urine protein/creatinine ratio (A), the kidney weight (B), and percentage of glomeruli with adhesions (C). In each case, the later intervention was less effective than the earlier intervention at preventing kidney weight change and glomerular injury. Modest calorie intake reduction in combination with ACE inhibition appeared more effective than either treatment alone. Data shown as the mean±SEM. *P<0.05, **P<0.01. ACE-i, angiotensin-converting enzyme inhibitor; CR, creatinine clearance; W, weeks.
Relationships of Body Weight, Kidney Weight, and Glomerular Volume
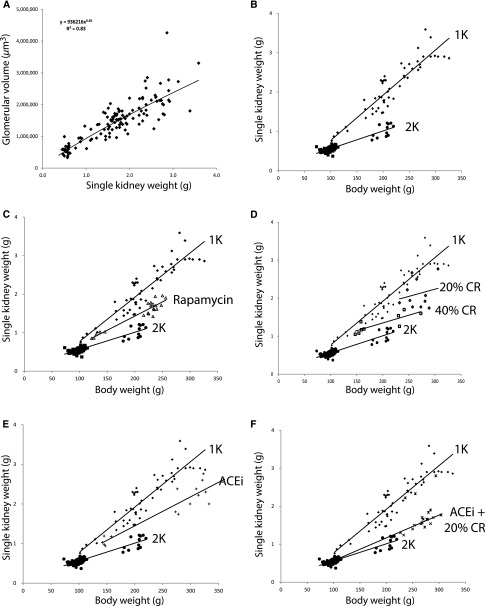
Figure 6 shows how glomerular volume is related to kidney weight and body weight in the presence and absence of nephrectomy, and how these parameters are modulated by treatments that prevent development of FSGS. Glomerular volume is directly proportional to single kidney weight under all conditions tested, including nephrectomy and sham nephrectomy (Figure 6A). There is also a linear relationship between single kidney weight and body weight, as shown (Figure 6B). However, the steepness of the slope of this relationship depends on kidney mass. After removal of one kidney, the slope relating body weight to kidney weight doubled such that for every incremental increase in body weight there was twice the increase in kidney weight (and therefore in glomerular volume). Therefore, body weight gain in the setting of reduced kidney mass imposes greater hypertrophic glomerular stress. The interventions that were protective of development of FSGS all modified the relationships such that the effect of nephrectomy on kidney weight gain in relation to body weight gain was reduced by about 50% (Figure 6, C–E). The combination of ACE inhibitor and moderate calorie restriction almost eliminated the accelerating effect of nephrectomy on kidney hypertrophy (Figure 6F).
Figure 6.
Relationship between glomerular volume, kidney weight, and body weight. (A) Glomerular volume was directly proportional to kidney weight under all conditions and diets tested. Kidney weight (mass) is therefore a surrogate for glomerular volume. (B) As previously reported7 the slope of the relationship between single kidney weight (SKW) and body weight (BW) doubles when one kidney is removed (2K to 1K), and the SKW/BW ratio relationship remains constant as the animal continues to increase weight thereby creating increased podocyte hypertrophic stress after nephrectomy. (C) Rapamycin reduced the slope SKW/BW ratio by about 50%. SKW is therefore preferentially reduced compared with BW by mTORC1 inhibition. (D) Calorie reduction (CR) reduced the SKW/BW ratio in relation to the degree of CR (40% CR [open squares] >20% CR [open diamonds]). SKW is therefore also preferentially reduced compared with BW by CR. (E) ACE inhibitor (ACEi) reduced the SKW/BW ratio by about 50%. SKW is therefore again preferentially reduced compared with BW by ACEi treatment. (F) The combination of CR and ACEi (10mg/kg per day) reduced the SKW/BW ratio almost back to the normal 2K state.
Cell Cycling
If glomerular growth is truly a key determinant of FSGS we would expect that cell cycling would be increased in nonpodocyte cells of the glomerulus in association with glomerular enlargement. Accordingly, we performed Ki67 immunofluorescence to identify cells which had entered the cell cycle (Figure 1F, Table 3). Rats that developed FSGS lesions (TG.1K.ALD) had significantly increased rates of Ki67-positive cells in all kidney compartments examined, including in glomeruli. Sham-nephrectomized TG rats maintained on the ad libitum diet (that did not develop FSGS lesions by 3 weeks after nephrectomy but did develop FSGS lesions by 7 months of age in the intact rat) also had increased intraglomerular Ki67-positive cells, although the number per glomerulus was less than in the nephrectomized rats that developed FSGS lesions by 3 weeks after nephrectomy. In contrast, conditions that prevented development of FSGS lesions (calorie intake reduction, rapamycin, and ACE inhibition) were all associated with reduced rates of cell cycling in all kidney cortex compartments (i.e., growth was slowed). Ki67-positive PECs increased in rats that developed FSGS lesions, but not in other groups. These data are consistent with the growth data shown above and demonstrate that increased cell cycling is necessary, but not alone sufficient, for development of FSGS lesions in the model. If cell cycling was slowed by any of three mechanisms, then FSGS lesions did not occur in spite of nephrectomy.
Table 3.
Ki67 Cell Cycle Analysis
| Group | Ki67-Positive Nuclei per Field | ||||
|---|---|---|---|---|---|
| Intraglomerular | Podocyte | PEC | Periglomerular/Interstitial | Tubular | |
| TG.1K.ALD (FSGS) | 10.3±1.7a | 0.01±0.1 | 2.8±0.7a | 8.2±2.2a | 4.0±1.1a |
| TG.2K.ALD | 6.4±0.9a | 0.1±0.1 | 0.9±0.4 | 3.2±0.5 | 2.0±0.4 |
| TG.1K.CRD | 1.9±1.2 | 0.0±0.0 | 0.6±0.3 | 1.7±0.9 | 0.7±0.4 |
| TG.1K.ALD.Rapa | 2.0±1.2 | 0.0±0.0 | 0.4±0.2 | 2.3±0.8 | 1.1±0.7 |
| TG.1K.ALD.ACEi | 2.8±0.6 | 0.0±0.0 | 0.7±0.3 | 2.7±1.0 | 1.3±0.5 |
| WT.2K.ALD | 4.5±1.0a | 0.0±0.0 | 0.7±0.4 | 3.2±0.8 | 2.5±0.9 |
| WT.1K.ALD | 6.7±1.0b | 0.0±0.0 | 0.6±0.3 | 2.6±0.2 | 2.8±1.1 |
Ki67-positive cell nuclei in different kidney compartments in nephrectomized (1K) or sham-nephrectomized (2K) TG and WT rat groups under different conditions. Group descriptors (n=5–7) are shown at left. Statistical indicators using ANOVA with Bonferroni adjustment compare each group with the reduced calorie intake group (TG.1K.CRD). All rats were the same age, weight, and sex at start of study 3 weeks before analysis. There were no changes in podocyte Ki67-positive nuclei in any group, and in a parallel study no Ki67 cell nuclei were identified that were also WT1-positive (data not shown) and no binucleate WT1-positive cells were identified, indicating that cells designated as podocytes on the basis of their anatomic localization were probably PECs. In the TG.1K.ALD group that developed FSGS (top row), Ki67-positive nuclei were statistically increased in all other kidney compartments. In the Sham nephrectomized TG group (TG.2K.ALD) and WT nephrectomized and sham-nephrectomized groups only intraglomerular Ki67-positive cells were significantly increased above the reduced calorie intake (TG.1K.CRD) group, but these three groups also had significantly fewer intraglomerular Ki67-positive cells than the TG.1K.ALD (FSGS) group (P<0.01). Prevention of FSGS development by three different methods (CRD, Rapamycin, or ACE inhibition) all showed the similar Ki67 data demonstrating low cell division in all kidney compartments indicating that cell division rate was similarly slowed by all three strategies. These data are consistent with the concept that growth represented by Ki67-positive cells was required for FSGS development. If cell division/growth was prevented (by calorie reduction, rapamycin, or ACE inhibitor) or was not sufficiently rapid (sham-nephrectomy), or the susceptibility transgene was absent (WT rats), FSGS did not occur within the 3-week time frame of study. Statistical comparisons by ANOVA with Bonferroni correction. Data are mean±SD. TG, Fischer344 TG rats; 1K/2K, nephrectomized/sham-nephrectomized; ALD, ad libitum diet; CRD, 40% calorie reduced diet; rapa, rapamycin-treated; ACEi, treated with the angiotensin II inhibitor enalapril; WT, wild-type Fischer344 rats.
P<0.01.
P<0.05.
Podocytes Do Not Enter the Cell Cycle
Podocyte cell cycling was assessed by a double label study using WT1 as a podocyte nuclear marker and Ki67 as a nuclear marker of cell cycling. In the TG.1K.ALD group that developed FSGS, 57 glomeruli in six rats were imaged containing 787 WT1-positive nuclei and 534 Ki67-positive nuclei. One cell was identified with both a WT1- and Ki67-positive nucleus (<0.02% of Ki67-positive intraglomerular cells). Therefore, in spite of hypertrophic stress induced by nephrectomy that causes cell cycling in glomerular cells, podocytes (as defined by WT1-positive nuclei) did not enter the cell cycle.
Transcriptomic Signal Associated with FSGS Lesion Development
To obtain an unbiased perspective of early events driving the FSGS phenotype we performed Affymetrix-based transcriptomic analysis of isolated glomerular RNA obtained 3 weeks after nephrectomy for the groups shown in Tables 1–3. To identify major pathways involved in FSGS lesion development, we compared gene expression profiles from the group that developed FSGS lesions (TG.1K.ALD) to those that were prevented from developing FSGS lesions by treatments in spite of nephrectomy (TG.1K.CRD, TG.1K.ALD.Rapa, and TG.1K.ALD.ACEi). Genes whose expression level changed significantly (P<0.05) and in the same direction (n=538) were subjected to Ingenuity Canonical Pathway Analysis. The top 35 candidates in the Diseases or Functions annotations were either “cell cycle” or various cancers. The top four candidates designated as various aspects of “cell cycle” are shown in Table 4, supporting the concept that differences in cell cycling were a major component of whether FSGS lesions occurred.
Table 4.
Ingenuity Pathway Transcript Analysis
| IPA Rank Order | Categories | Diseases or Functions Annotation | P Value | Molecules | Molecule Number |
|---|---|---|---|---|---|
| 1 | Cell cycle, | Segregation of | 7.4E-25 | ATRX,AURKB,BUB1,CCNA2,CCNB1,CCNB2,CDC14A, | 34 |
| cellular assembly | chromosomes | CENPE,CENPF,CENPT,ECT2,HJURP,HMMR,KIF11,KIF2C, | |||
| and organization, | KMT5B,KNSTRN,LATS1,LMNA,NCAPD2,NCAPD3,NCAPG, | ||||
| DNA replication, | NDC80,NINL,NUF2,NUSAP1,PLK1,PTTG1,SGO1,STAG1, | ||||
| recombination, | STAG2,TOP2A,TPX2,ZWINT | ||||
| and repair | |||||
| 2 | Cell cycle | Arrest in mitosis | 1.6E-17 | AURKB,BUB1,BUB1B,CDC20,CDK1,CENPE,CENPI,CSNK1A1, | 23 |
| DCTN2,KIF11,KIF4A,KNL1,KNTC1,MYBL2,NDC80,NUF2, | |||||
| PDLIM7,PLK1,PURA,RACGAP1,SGO1,TPX2,ZWINT | |||||
| 3 | Cell cycle | Mitosis | 1.9E-17 | AURKB,BCL2,BMP2,BUB1,BUB1B,CCNA2,CCNB1,CDC14A, | 57 |
| CDC20,CDC25C,CDK1,CENPE,CENPF,CENPI,CENPT, | |||||
| CLASP2,CSNK1A1,DCTN2,DLGAP5,DYNLT3,FBXW5,FOXM1, | |||||
| GADD45B,JUN,KIF11,KIF18A,KIF18B,KIF2C,KIF4A,KNL1, | |||||
| KNTC1,LATS1,LATS2,MYBL2,NDC80,NINL,NRG1,NUF2, | |||||
| NUSAP1,PDGFRA,PDLIM7,PHB2,PHIP,PLK1,PTHLH,PTTG1, | |||||
| PURA,RACGAP1,SGO1,SLC9A3R1,SPP1,TNC,TOP2A,TPX2, | |||||
| TRIM33,YWHAE,ZWINT | |||||
| 4 | Cell cycle | M phase | 1.1E-16 | AURKB,BCL2,BUB1B,CCNB1,CD2AP,CDC14A,CDC20,CDK1, | 37 |
| CENPE,DIAPH3,ECT2,GIPC1,HMMR,KIF14,KIF20A, | |||||
| KIF20B,KIF23,KIF4A,KLHL9,LATS1,LATS2,LMNA,mir-23, | |||||
| NCAPD2,NDC80,NUF2,NUSAP1,PFN1,PLK1,PRC1,PTTG1, | |||||
| RAB11FIP3,RACGAP1,RHOC,STAG1,TOP2A,TOPAZ1 |
Ingenuity Pathway Analysis of glomerular genes. Five hundred thirty-eight genes (shown in Supplemental Table 2) were expressed significantly differently (either up or down) between the TG.1K.ALD rats that developed FSGS and the three groups that were prevented by treatment from developing FSGS (TG.1K.CRD, TG.1K.Rapa, and TG.1K.ACEi). Ingenuity Pathway Analysis of these genes in the Diseases or Functions annotation showed that the top 35 were designated either as cell cycle or various cancers. The top four candidates, all designated as different aspects of cell cycle, with overlapping genes, are shown. Transcript levels for the 84 genes identified in Table 5 relative to the TG.1K.ALD group are shown in Supplemental Table 3. IPA, Ingenuity Pathway Analysis.
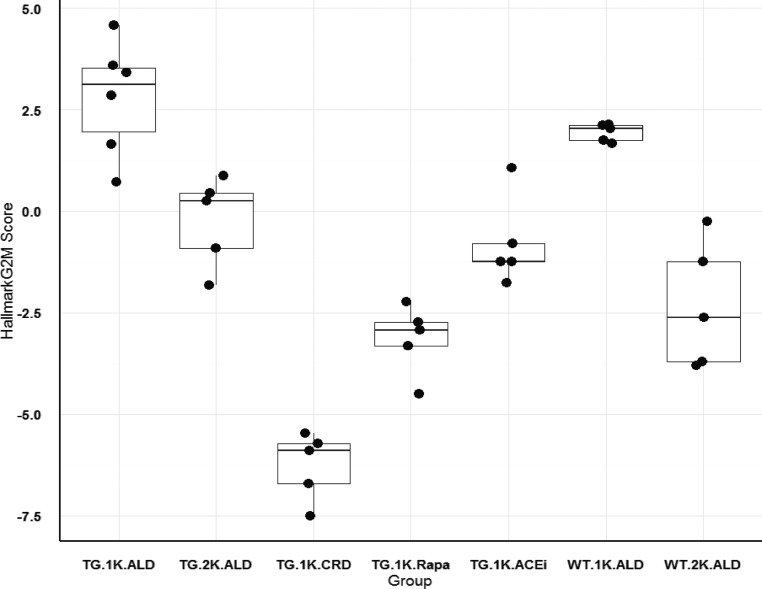
To compare the relative level of cell cycle gene expression in the different rat groups the G2M checkpoint was selected as a key cell cycle event. The HALLMARK_G2M_CHECKPOINT list of 200 genes from the Broad Institute was used to derive a score for each rat glomerular transcriptome as shown in Figure 7 (see Concise Methods). Both the TG and WT nephrectomized ad libitum–fed rat groups (TG.1K.ALD and WT.1K.ALD) had high G2M scores whereas TG nephrectomized rats treated to prevent FSGS development (TG.1K.CRD, TG.1K.ALD.Rapa, and TG.1K.ALD.ACEi) had significantly lower scores. Non-nephrectomized TG and WT groups had intermediate scores. These data independently confirm the Ki67 data (Table 3), and again support the concept that high-level cell cycling was necessary, but not sufficient, for FSGS lesion formation.
Figure 7.
Glomerular transcriptional HALLMARK_G2M_CHECKPOINT cell cycle signature for rat groups. Each rat glomerular Affymetrix transcriptome was used to obtain a G2M signature value derived from the “Hallmark_G2M” gene list downloaded from the GSEA/MSigDB molecular signatures database at the Broad Institute (see Concise Methods). Data were normalized as shown on the y axis. Individual points for each rat are shown according to groupings defined according to whether they were TG or WT, nephrectomized (1K) or sham-nephrectomized (2K), and whether they were maintained on an ad libitum diet (ALD) or a calorie intake reduction diet (CRD). Data for groups are shown as box plots with a mean and interquartile range. The rat group that developed FSGS lesions (TG.1K.ALD) was significantly different from all groups (P<0.01) except the WT.1K.ALD group. TG rat groups that were prevented from developing FSGS in spite of undergoing nephrectomy (TG.1K.ALD, TG.1K.ALD.Rapa, and TG.1K.ALD.ACEi) had significantly lower G2M values. Note that 98% of glomeruli purified by sieving for Affymetrix analysis do not include Bowman’s capsule and therefore PECs are not represented. The group distribution is similar to the Ki67 data shown in Table 3. Rapa, rapamycin.
Podocyte Transcriptomic Signatures Associated with FSGS Development
If hypertrophic podocyte stress is indeed necessary for the FSGS phenotype under the conditions tested then this may be reflected in the podocyte transcriptomic signature. Table 5 provides parallel podometric and transcriptomic parameters for the different groups shown as a percentage of wild-type rat values. As noted above, podocyte number per glomerulus did not decrease in association with FSGS lesion development although podocyte density (measured by two different methods) did decrease due to increased nonpodocyte cell cycling resulting in increased glomerular volume. As would be expected, this podocyte “dilution” effect was also reflected by a significant reduction in podocyte-specific transcript expression as exemplified by WT1, podocin, nephrin, and ptpro (Glepp1) (Table 5). Therefore, podocyte transcript gene expression levels per se could not be interpreted as evidence of podocyte stress. However, differential podocyte expression of genes that are specific to the podocyte can be used to examine this question. Nephrin is preferentially downregulated in stressed podocytes.6,7 The podocin/nephrin ratio increased significantly in association with FSGS lesion development in the TG.1K.ALD rat group that developed FSGS compared with other groups, compatible with podocyte hypertrophic stress occurring in association with FSGS lesion development.
Table 5.
Podocyte Transcript Analysis
| Group | Podometric Parameters | Glomerular Transcript Values Expressed as % Compared with WT.2K.ALD | ||||||
|---|---|---|---|---|---|---|---|---|
| Podocyte Number per Glom | Podocyte Nuclear Density | Glepp1 Area Density | WT1 | Podocin | Nephrin | Ptpro (Glepp1) | Podocin/Nephrin Ratio | |
| TG.1K.ALD | 106.6±10.7 | 49.9±4.7a | 69.6±5.8a | 60.3±13.1a | 67.6±11.1b | 50.8±9.4a | 51.6±8.1a | 132.7±3.2a |
| TG.2K.ALD | 104.5±23.0 | 74.0±15.9 | 99.8±3.1 | 107.1±17.8 | 99.5±16.0 | 93.0±14.5 | 121.5±16.4 | 106.5±4.0 |
| TG.1K.CRD | 107.4±15.7 | 87.9±12.0 | 95.5±4.8 | 101.1±18.5 | 83.6±14.9 | 87.9±15.4 | 91.4±12.2 | 94.8±8.9 |
| TG.1K.ALD.Rapa | 92.9±13.8 | 102.7±6.4 | 94.5±2.7 | 30.4±10.1a | 33.1±9.0a | 24.3±8.9a | 34.5±11.9a | 140.9±18.5a |
| TG.1K.ALD.ACEi | 96.1±12.2 | 76.6±13.8 | 89.4±3.1 | 68.3±13.4b | 66.4±14.5b | 62.4±12.1a | 63.9±13.4a | 105.4±7.0 |
| WT.1K.ALD | 95.8±14.0 | 113±31.2 | 99.9±2.4 | 88.7±4.5 | 94.9±5.3 | 86.9±6.7 | 77.9±9.6 | 108.9±3.5 |
| WT.2K.ALD | 100±14.0 | 100±30.2 | 100±3.4 | 100±11.7 | 100±5.7 | 100±13.0 | 100±8.0 | 100±8.5 |
Comparison of glomerular podometric and transcriptomic parameters. All data are from isolated glomeruli obtained 3 weeks after nephrectomy or sham nephrectomy for the same groups as shown in Tables 1–3. Data shown in comparison with the wild-type sham-nephrectomized rats maintained on an ad libitum diet (WT.2K.ALD in bottom row at 100%). Podometric parameters are shown at left, relative transcriptomic expression in the middle, and podocyte transcript ratios at right. Nephrectomized TG rats maintained on an ad libitum diet (TG.1K.ALD) that developed FSGS are shown in the top row. In this group, although podocyte number per glomerulus was unchanged, podocyte nuclear density and Glepp1 cell area density were both decreased due to glomerular enlargement partially compensated for by podocyte cell hypertrophy. Podocyte transcripts (WT1, podocin, nephrin, and ptpro [Glepp1]) were all decreased in approximate proportion to the decreased podocyte density. The ratios of podocyte transcripts (podocin/nephrin previously), shown to be related to podocyte hypertrophic stress,6,7 were also increased in the TG.1K.ALD group that developed FSGS, but not in other groups. Rapamycin treatment itself markedly reduced podocyte transcript expression, thereby making interpretation of transcriptomic data in the setting of rapamycin difficult. ACE inhibition may have had minor effects on transcript levels that were not significant in the ratio data. These data show that there were two different reasons for podocyte transcript changes: (1) podocyte density was decreased due to accumulation of nonpodocyte glomerular cells, and (2) relative expression of podocyte genes was also altered compatible with podocytes undergoing hypertrophic stress in association with FSGS lesion development. Reduction in the podocyte transcriptomic signal was not due to preferential loss of podocytes from glomeruli. Data are shown as the mean±1 SD. Comparisons were performed by ANOVA using the Bonferroni correction. Glom, glomerulus; TG, Fischer344 TG rats; 1K/2K, nephrectomized/sham-nephrectomized; ALD, adlib diet; CRD, 40% calorie reduced diet; rapa, rapamycin-treated; ACEi, treated with the angiotensin II inhibitor enalapril; WT, wild-type Fischer344 rats.
P<0.05.
P<0.01.
Comparison of the Rat FSGS Transcriptomic Signature to Progressive Human Glomerular Diseases Transcriptomic Signatures
If the gene expression signatures identified for rat FSGS lesion development do represent events taking place in association with progression in humans, then we would expect that these altered patterns in gene expression might also be present in human progressive glomerular diseases. As shown in Table 6, a rat “FSGS gene expression signature” correlated highly with expression signatures for progressive human glomerular diseases reported in the Nephroseq database.8
Table 6.
Nephroseq Comparisons of the Rat versus Human Datasets
| Reference | Comparison Disease | P Value | Q Value | Gene No. |
|---|---|---|---|---|
| Increasers (n=376 genes) | ||||
| Ju | Lupus nephritis versus HLD | 2.6E-40 | 3.3E-37 | 105 |
| Ju | Diabetic neph versus HLD | 2.0E-30 | 1.3E-27 | 92 |
| Ju | Membranous neph versus HLD | 3.1E-26 | 1.8E-23 | 86 |
| Ju | Vasculitic neph versus HLD | 3.1E-24 | 1.4E-21 | 83 |
| Ju | IgA neph versus HLD | 8.2E-16 | 2.7E-13 | 69 |
| Ju | FSGS versus HLD | 3.3E-14 | 7.8E-12 | 66 |
| Decreasers (n=239 genes) | ||||
| Hodgin | Collapsing FSGS versus NLD | 1.5E-8 | 1.5E-5 | 34 |
| Hodgin | FSGS versus NLD | 4.2E-6 | 0.002 | 46 |
| Ju | IgA neph versus HLD | 3.8E-7 | 2.4E-4 | 37 |
| Ju | Lupus neph versus HLD | 1.1E-6 | 6.2E-4 | 27 |
| Ju | Diabetic neph versus HLD | 2.3E-6 | 0.002 | 27 |
Comparison of the rat FSGS transcriptomic profile with human glomerular transcriptomic profiles derived from the Nephroseq database. There were 800 genes differently expressed (1.5-fold change, P<0.05) between glomeruli in which FSGS developed (TG.1K.ALD) versus the TG.1K.CRD where FSGS did not occur. The TG.1K.ALD.Rapa and TG.1K.ALD.ACEi groups were not included because of potential drug effects on the gene expression profiles. Of these 800 rat genes 185 were not recognized by the Nephroseq database, leaving 615 genes for analysis of which 376 were significantly increased and 239 were significantly decreased in association with FSGS lesion formation. These two gene sets were then used to search the Nephroseq database for correlations to previously reported glomerular disease datasets for increasing and decreasing genes associated with glomerular diseases. Ju and Hodgin refer to Nephroseq datasets.8 NLD and HLD refer to normal living donor as a source for comparison to pathologic glomeruli. HLD, human living donor kidney; Neph, nephropathy; NLD, normal living donor kidney.
Discussion
In the TG rat model, acceleration of kidney growth rate (induced by contralateral nephrectomy) resulted in FSGS lesion development that progressed to ESRD within 12 weeks of nephrectomy. In contrast, slowing the kidney and glomerular growth rate by three different methods (calorie intake reduction, inhibiting the mTORC1 pathway [by rapamycin], or angiotensin II blockade [by ACE inhibition]) all prevented FSGS lesion development. The key effect of growth in nonpodocyte glomerular cells was further confirmed by Ki67 analysis to quantitate cycling cells in various kidney compartments, and by unbiased glomerular transcriptomic analysis. In spite of a general increase in cell cycling, mature podocytes themselves (identified by WT1-positive nuclei) did not enter the cell cycle. In parallel studies, no FSGS lesions developed in wild-type rats thereby demonstrating that podocyte susceptibility (conferred by the transgene) was also required for FSGS lesion development. We conclude that two different but complementary factors were both necessary for FSGS lesion development, although neither alone was sufficient. These are (1) enhanced cycling of glomerular cells (but not podocytes) under the influence of growth factors and nutrients that cause glomerular enlargement, and (2) the inability of podocytes to adapt to these growth-induced stresses. Importantly, slowing the glomerular growth rate, even by quite modest amounts and after proteinuria was already present and when early FSGS lesions had already developed, slowed progression.
Across all mammalian species, from the smallest to the largest, body weight is directly proportional to glomerular volume.9 Similarly, glomerular volume increases in relation to body weight increase during normal growth.1 Body weight is therefore a surrogate for glomerular volume, although, as shown in Figure 6, if nephron number (mass) becomes reduced for any reason (e.g., by 50% after nephrectomy) the relationship between rate of glomerular volume increase and weight gain doubles, thereby accounting for the increased podocyte hypertrophic stress that caused FSGS lesion development in the model system. Importantly, angiotensin II blockade prevented kidney weight increase, but not body weight increase, suggesting that normal kidney and glomerular growth both require angiotensin II whereas body growth as a whole does not. Angiotensin II blockade therefore serves to disassociate kidney growth and glomerular enlargement from body growth, thereby accounting for its protective effect. This effect of angiotensin II blockade parallels its well established role in utero where it retards normal fetal kidney growth and development.10
Is this report relevant to FSGS as it occurs in man? The mature FSGS lesion in the rat model system was indistinguishable from a human FSGS lesion, and the rat FSGS-associated transcriptomic signature correlated with diverse progressive human glomerular diseases. Normal glomerular growth, particularly in the early years of life, requires podocytes to undergo remarkable size increases to maintain the filtration surface.11 Although FSGS phenotypes represent a diverse collection of underlying genetic and other causes, they typically present to the clinician during phases of life associated with rapid body growth. This is strikingly apparent for early childhood and adolescence, but also occurs in adults in association with increased body size (large body size itself, obesity, acromegaly, dietary supplement intake, and similar conditions).12 Indeed, the prevalence of FSGS has increased in parallel with the spread of the obesity epidemic, first reported in the United States and later observed in China, India, Brazil, and elsewhere12–21 in parallel with population access to essentially unlimited calorie intake and a sedentary life-style that has affected body size.22 The question of whether dietary intervention can slow the rate of CKD progression remains controversial although obesity is associated with worse outcome in all CKD, and bariatric surgery is shown to reduce proteinuria and slow the rate of kidney function decline.23–26 Growth factor overexpression drives glomerulosclerosis in model systems, although reported data from humans treated with exogenous growth factors is so far limited.27 We focused on calorie intake because our prior work examined factors that affect the aging process where calorie intake reduction is well known to prolong life-span.6,28 However, viewed through the prism of mTORC1 as the major transducer of growth signaling in all cells, one would expect that glucose, amino acids, and growth factors would all be integrated through the mTORC1 pathway to drive growth and cell cycling in nonpodocyte glomerular cells and thereby promote podocyte hypertrophic stress, FSGS development, and accelerated progression to ESRD in susceptible individuals.
The glomerulus is a complex machine containing several cell types each with common housekeeping and some more or less specific pathways that comprise its characteristic transcriptome. Therefore, interpretation of the global glomerular transcriptome in terms of individual pathways is challenging. In this study, an increase in the proportion of glomerular cells that were not podocytes resulted in a relative decrease in podocyte-specific transcripts (WT1, nephrin, podocin, or ptpro [Glepp1]) even though the number of podocytes did not change measurably. These transcript changes per se therefore could not be interpreted as evidence of modulation of transcription by podocytes. In contrast, ratios of two podocyte-specific transcripts (e.g., podocin and nephrin) demonstrate that modulated transcription did occur in association with FSGS lesion development, as previously reported.6,7
From the FSGS mechanistic viewpoint, glomerular enlargement in the TG rat model was associated with development of segmental lesions devoid of podocytes, as also occurs in man. At early time points these lesions could be seen to be related to glomerular capillary loops that had lost their podocyte covering as judged by both light microscopy and SEM.1 At the same time, the average number of podocytes per glomerulus was not measurably decreased and there was no increase in rate of podocyte detachment detected in the urine pellet. This is in contrast to the diphtheria toxin receptor model we previously reported, where FSGS lesion development was driven specifically by podocyte loss.29 Nagata and Kriz also reported FSGS lesion development in the absence of reduced podocyte number per glomerulus.30
What mechanisms could account for the observed segmental absence of podocytes apparently occurring without loss of podocytes from glomeruli? One possibility is that PECs invade the glomerular tuft and displace podocytes. In the model used, podocyte-depleted tuft segments were present before and in the absence of invasion by Pax8-positive cells (PECs). Accumulation of PECs (some of which were Ki67 positive) occurred on Bowman’s capsule opposite the podocyte-devoid tuft segments, and by the 3-week time point in some glomeruli Pax8-positive cells had invaded podocyte-depleted areas. Therefore, in this model although PEC tuft invasion was not the primary initiator of FSGS lesion development, the data are entirely compatible with a model in which PECs play an essential role in driving FSGS lesion sclerosis as reported by Smeets et al.31–33 A second possibility is that podocytes detached from glomerular capillary loops in response to hypertrophic stress analogous to the “mitotic podocyte catastrophe” model suggested by Lasagni et al.34 and similar to what we previously observed in aging human glomeruli.35 Although we were not able to identify detached podocytes in Bowman’s space or tubular lumens by histology or by urine podocyte assay, and the podocyte number per glomerulus was not measurably decreased in association with FSGS lesion development, it is possible that the methods used were not sensitive enough to detect these events occurring in a subset of glomeruli. A third possibility is that in the setting of glomerular capillary loop enlargement that exceeds the capacity of podocytes to provide coverage, the default response of the podocyte population is to cover whatever territory it can and abandon territory that cannot be covered. The capacity of mature podocytes to migrate is already demonstrated by several investigators.36–38 Under this “Abandoned Capillary Loop Hypothesis” it would make sense that the abandoned unit would be an individual capillary loop whose blood flow could be independently regulated to reduce protein loss. This hypothesis appears to best fit the data although further studies will be required to test it.
In summary, FSGS lesion development can be conceptualized as an adaptive glomerular response to the inability of one member of the glomerular team, the podocyte, to comply with the hypertrophic demand imposed by the other members of the glomerular team under the influence of nutrients and growth factors.
Concise Methods
Rat studies were approved by the University of Michigan Use and Care of Animals Committee (IACUC) (PRO00006209).
Reagents
GLEPP1 mouse mAb (1B4) was raised against the recombinant rat GLEPP1 extracellular domain and used as described previously.39 WT1 (SC-7385 monoclonal IgG1) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA); Phospho-S6 antibody (no. 2215) was purchased from Cell Signaling Technology (Beverley, MA); Pax8 antibodies were purchased from Abcam (Cambridge, MA); ab191870 rabbit mAb; MHC Class II antibody was purchased from from AbD Serotec (Raleigh, NC); MCA46GA mouse mAb and Ki67 rabbit monoclonal antibody was purchased from Novus Biologicals (Littleton, CO) NB600–1252.
Rat Nephrectomy Model
Wild-type and TG Fischer344 rats (expressing the AA-4E-BP1 transgene under the control of the human podocin promoter) were used for study as previously reported.1 Both homozygous and heterozygous TG rats were used for different studies. Male rats underwent nephrectomy or sham nephrectomy at 100 g body wt. One day after nephrectomy they were placed on various interventions as outlined below. Rats were weighed once per week and one day per week they were placed into a metabolic cage for overnight (17 hours) urine collection. In some experiments (Figures 2–5) rats were kept for 12–14 weeks after nephrectomy and then euthanized with perfusion-fixation of kidneys using PLP buffer (containing paraformaldehyde [2%], lysine [1.37%], and periodate [0.2%] at +4°C at 100 mmHg). In other experiments, rats were kept for 3 weeks before euthanization with ketamine/diazepam and kidneys were harvested and weighed. Once per week body weight and BP were measured, and timed overnight urine collection for protein, creatinine, urea, and urine mRNAs was collected.
FSGS Prevention Studies
On the day after nephrectomy 100 g male rats were randomly assigned to various interventions. For calorie intake reduction studies rats were maintained in individual cages so that the amount of food delivered to each rat could be monitored. For all other studies rats were fed an ad libitum regular chow diet. For mTORC1 inhibitor studies rapamycin (LC Laboratories, Woburn, MA) was dissolved in ethanol at 100 mg/ml and then diluted to 1 mg/ml in vehicle (distilled water containing 5% Tween-80 and 5% PEG). Rapamycin was delivered by intraperitoneal injection (10 mg/kg in vehicle) every 2 days or once a week. Control rats received vehicle alone. There were no differences noted between vehicle-treated and control ad libitum–fed rats. For ACE inhibitor studies, enalapril (Sigma, St. Louis, MO) was delivered at 10 mg/kg per day in the drinking water as previously described.39 Calorie reduction studies were performed as previously described using National Institute on Aging rat protocol (NIH31/NIA Fortified for CR feeding) and pellets6 either as 40% calorie restriction or 20% calorie restriction. For other dietary studies the following diets were used: 6% protein diet (TD.90016) and 40% protein diet (TD.90018) purchased from Harlan Laboratories (Madison, WI); and 45% fat diet (D12451) purchased from Research Diets Inc. (New Brunswick, NJ). In some studies, sucrose 10% was added to drinking water to simulate the sugar content of Coca-Cola.
Histologic Analysis
At nephrectomy, 1-mm slices of kidney were placed in 10% paraformaldehyde overnight and then soaked in 10% ethanol before storage and subsequent sectioning at 3 μm thickness for further study. Masson trichrome staining was performed for quantitation of adhesions and scarring by a blinded observer.
Podometric Analysis
Immunofluorescence using WT1 mAb in 3 μm thick paraformaldehyde-fixed sections was used to identify and measure podocyte nuclear number and size by ImagePro software in 25 consecutive glomerular tufts as previously described.40 True podocyte nuclear density and size were estimated using the quadratic equation developed to correct for section thickness and nuclear shape using a downloadable spread-sheet.40 The coverslip of the immunofluorescently stained section was removed and the section restained by Glepp1 immunoperoxidase and counterstained by PAS. The percentage of the glomerular tuft that was Glepp1-positive, representing podocytes in the same glomeruli imaged for WT1 podocyte nuclear counting, was estimated using imaging software as previously described.40 Average glomerular volume in the same 25 consecutive glomerular tuft profiles was estimated from the average glomerular tuft area using the method of Weibel as previously reported.12,40,41 The proportion of tuft made up of podocytes was estimated by multiplying the average tuft volume by the %glepp1-positive area. The podocyte nuclear number per tuft was estimated by dividing the average glomerular volume by the podocyte nuclear density. The average podocyte cell volume was estimated by dividing the total podocyte volume by the number of podocyte nuclei per glomerular tuft.
Ki67 Analysis
Double immunofluorescent labeling using rabbit mAb against Ki67 (red) and a mouse mAb against Glepp1(green) with blue DAPI staining was used to identify dividing cells and glomerular and tubular structure in 3-μm paraformaldehyde-fixed sections. Compound images from the red, green, and blue channels for 12 consecutive glomerular profiles were made for each rat at ×20 magnification with a glomerulus in the center of the field. Ki67-positive cell nuclei in each cortical compartment were counted including PECs, podocytes, intraglomerular cells, periglomerular and interstitial cells, and tubular cells. Averaged values for the 12 consecutive images were used for each rat. In parallel studies, double label immunofluorescence was performed using Ki67 (rabbit monoclonal) and WT1 (mouse monoclonal) to identify Ki67-positive podocytes. Class II immunofluorescence was also performed and quantitated as above.
Glomerular Isolation
Rat glomeruli were isolated by sieving from kidney cortex derived from euthanized rats in which kidneys had been perfused with phosphate buffered saline at 4°C. Purity of glomeruli was >95% and 98% of isolated glomeruli did not have Bowman’s capsules. RNAlater was added to the glomerular preparation for storage at −80°C before RNA purification using the RNAeasy kit. The quality of the purified RNA was >8 as judged by the RIN scale.
Transcriptomic Studies
Preliminary studies showed that, in ad libitum–fed TG rats, by 3 weeks after nephrectomy there were only minor adhesions developed and glomeruli could be quantitatively isolated by sieving. Wild-type and TG rats at 100 g were either nephrectomized or sham-nephrectomized. They were divided into seven groups as shown in Tables 1–4. Specifically, the TG nephrectomized ad libitum–fed group that developed FSGS lesions was compared with the TG nephrectomized groups that were prevented from developing FSGS lesions by calorie intake reduction, rapamycin treatment, or ACE inhibitor treatment. Three weeks after nephrectomy, glomeruli were isolated from renal cortex by sieving. Rat Gene ST 2.1 Affymetrix gene arrays were developed by the University of Michigan Affymetrix Core. Genes that changed significantly (P<0.05) in the same direction in all three prevention groups (TG.1K.CRD, TG.1K.ALD.Rapa, and TG.1K.ALD.ACEi) compared with the group that developed FSGS (TG.1K.ALD) were identified (n=538). This gene list was used for Ingenuity Pathway Analysis as reported in the results to find that cell cycling was the major pathway common to all three treatment strategies for preventing FSGS. Transcriptomic data will be available through Nephroseq at the next iteration.
Transcriptomic Scoring for Comparison of Cell Cycle Gene Activity between Groups
The HALLMARK_G2M_CHECKPOINT gene expression signature list of 200 genes associated with the G2/M cell cycle checkpoint was downloaded from the GSEA/MSigDB resource at the Broad Institute (http://software.broadinstitute.org/gsea/msigdb). Orthologs were found in the rat data set for 176 of these genes. An expression matrix was constructed from these genes across all samples which was then transformed using the singular value decomposition (SVD). The first principal component (PC1) of the resulting rotated matrix accounted for 46.6% of the total variance and correlated well (r=0.80) with the mean Z-normalized expression of member genes, and was thus selected to represent the signature over the sample space. The effective weighting of the component genes is implicitly nonuniform, with 16 of the 176 genes accounting for >50% of the sum of squared weights and 44 genes for >90%.
Urine Processing and mRNA Analysis
Rats were placed in freshly washed metabolic cages in which the collection pan had been rinsed with 100% ethanol as previously reported.42 Food was removed. A timed overnight urine collection (average 17 hours) was collected into a plastic 50-ml centrifuge tube. Urine (up to 50 ml in a sterile 50-ml plastic centrifuge tube) was centrifuged at 4°C for 15 minutes at 4000 rpm (3200 × g) on a table-top centrifuge. Two 2-ml aliquots of the supernatant were removed and stored at −20°C for protein, creatinine, and other measurements. The urine pellet was suspended in 750 μl of cold diethylpyrocarbonate-treated PBS (pH 7.4) at 4°C using a sterile disposable polystyrene transfer pipette and then transferred to a labeled 1.7-ml plastic centrifuge tube. A second 750 μl of PBS was used to wash the bottom of the 50-ml centrifuge tube to recover any remaining pellet material, and added to the 1.7-ml tube. The transferred pellet material now in 1.5 ml PBS was then centrifuged at 12,000 rpm in a mini-centrifuge for 5 minutes at 4°C. The supernatant was discarded. To the centrifuged material (“washed” urine pellet) was added 350 μl of RLT buffer containing β-mercaptoethanol at 10 ml/ml of RLT buffer according to the RNeasy Qiagen protocol (Germantown, MD). The pellet was suspended in RLT/β -mercaptoethanol buffer and then frozen at −80°C for assay. RNA was purified by RNAeasy column and reverse transcribed before assay using TaqMan primers for NPHS2 spanning exons 3–4 (Applied Biosystems cat. No. Rn00709834). A glomerular RNA preparation was used as a standard for every assay. Data were expressed per g of urine creatinine as arbitrary units.
Statistical Analyses
For rat studies shown in Figures 2–5 all results are presented as mean±SEM. For all other studies, data are shown as the mean±1 SD. Means of variables in two or more independent groups were compared by the unpaired t test and ANOVA with Bonferroni correction.
Disclosures
None.
Supplementary Material
Acknowledgments
R.C.W. acknowledges the support of the National Institutes of Health (grants R01 DK 46073 and R01 DK 102643) and the University of Michigan O’Brien Kidney Translational Core Center P30 DK081943.
The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Podocyte Growing Pains in Adaptive FSGS,” on pages 2825–2827.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2017020174/-/DCSupplemental.
References
- 1.Fukuda A, Chowdhury MA, Venkatareddy MP, Wang SQ, Nishizono R, Suzuki T, Wickman LT, Wiggins JE, Muchayi T, Fingar D, Shedden KA, Inoki K, Wiggins RC: Growth-dependent podocyte failure causes glomerulosclerosis. J Am Soc Nephrol 23: 1351–1363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kriz W: Glomerular diseases: Podocyte hypertrophy mismatch and glomerular disease. Nat Rev Nephrol 8: 618–619, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 4.Dibble CC, Manning BD: Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol 15: 555–564, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grahammer F, Wanner N, Huber TB: mTOR controls kidney epithelia in health and disease. Nephrol Dial Transplant 29[Suppl 1]: i9–i18, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Wiggins JE, Goyal M, Sanden SK, Wharram BL, Shedden KA, Misek DE, Kuick RD, Wiggins RC: Podocyte hypertrophy, “adaptation,” and “decompensation” associated with glomerular enlargement and glomerulosclerosis in the aging rat: Prevention by calorie restriction. J Am Soc Nephrol 16: 2953–2966, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Fukuda A, Wickman LT, Venkatareddy MP, Wang SQ, Chowdhury MA, Wiggins JE, Shedden KA, Wiggins RC: Urine podocin: Nephrin mRNA ratio (PNR) as a podocyte stress biomarker. Nephrol Dial Transplant 27: 4079–4087, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nephroseq Research Edition. Available at: https://www.nephroseq.org/. Accessed December 17, 2016
- 9.Maluf NS, Gassman JJ: Kidneys of the killerwhale and significance of reniculism. Anat Rec 250: 34–44, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Vinturache AE, Smith FG: Angiotensin type 1 and type 2 receptors during ontogeny: Cardiovascular and renal effects. Vascul Pharmacol 63: 145–154, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi M, Wickman L, Rabah R, Wiggins RC: Podocyte number and density changes during early human life. Pediatr Nephrol 32: 823–834, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Agati VD, Chagnac A, de Vries AP, Levi M, Porrini E, Herman-Edelstein M, Praga M: Obesity-related glomerulopathy: Clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol 12: 453–471, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Bonilla-Felix M, Parra C, Dajani T, Ferris M, Swinford RD, Portman RJ, Verani R: Changing patterns in the histopathology of idiopathic nephrotic syndrome in children. Kidney Int 55: 1885–1890, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Gulati S, Sharma AP, Sharma RK, Gupta A: Changing trends of histopathology in childhood nephrotic syndrome. Am J Kidney Dis 34: 646–650, 1999 [DOI] [PubMed] [Google Scholar]
- 15.Braden GL, Mulhern JG, O’Shea MH, Nash SV, Ucci AA Jr, Germain MJ: Changing incidence of glomerular diseases in adults. Am J Kidney Dis 35: 878–883, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Filler G, Young E, Geier P, Carpenter B, Drukker A, Feber J: Is there really an increase in non-minimal change nephrotic syndrome in children? Am J Kidney Dis 42: 1107–1113, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Dragovic D, Rosenstock JL, Wahl SJ, Panagopoulos G, DeVita MV, Michelis MF: Increasing incidence of focal segmental glomerulosclerosis and an examination of demographic patterns. Clin Nephrol 63: 1–7, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Swaminathan S, Leung N, Lager DJ, Melton LJ 3rd, Bergstralh EJ, Rohlinger A, Fervenza FC: Changing incidence of glomerular disease in Olmsted County, Minnesota: A 30-year renal biopsy study. Clin J Am Soc Nephrol 1: 483–487, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Borges FF, Shiraichi L, da Silva MP, Nishimoto EI, Nogueira PC: Is focal segmental glomerulosclerosis increasing in patients with nephrotic syndrome? Pediatr Nephrol 22: 1309–1313, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Zhou FD, Zhao MH, Zou WZ, Liu G, Wang H: The changing spectrum of primary glomerular diseases within 15 years: A survey of 3331 patients in a single Chinese centre. Nephrol Dial Transplant 24: 870–876, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Das U, Dakshinamurty KV, Prayaga A: Pattern of biopsy-proven renal disease in a single center of south India: 19 years experience. Indian J Nephrol 21: 250–257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, Farzadfar F, Riley LM, Ezzati M; Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index) : National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet 377: 557–567, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clase CM, Smyth A. Chronic kidney disease: diet. Systematic review 2004. BMJ Clinical Evidence. Available at http://clinicalevidence.bmj.com/x/systematic-review/2004/overview.html. Accessed January, 2017
- 24.Nehus EJ, Khoury J, Inge T, Xiao N, Jenkins T, Moxey-Mims M, Mitsnefes M. Kidney outcomes three years after bariatric surgery in severely obese adolescents. Kidney Int 91: 451–458, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li K, Zou J, Ye Z, Di J, Han X, Zhang H, Liu W, Ren Q, Zhang P: Effects of bariatric surgery on renal function in obese patients: A systematic review and meta analysis. PLoS One 11: e0163907, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nehus EJ, Khoury JC, Inge TH, Xiao N, Jenkins TM, Moxey-Mims MM, Mitsnefes MM: Kidney outcomes three years after bariatric surgery in severely obese adolescents. Kidney Int 91: 451–458, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doublier S, Seurin D, Fouqueray B, Verpont MC, Callard P, Striker LJ, Striker GE, Binoux M, Baud L: Glomerulosclerosis in mice transgenic for human insulin-like growth factor-binding protein-1. Kidney Int 57: 2299–2307, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Wiggins JE, Patel S, Shedden K, Goyal M, Wharram B, Martini S, Kretzler M, Wiggins RC: NFkB in the pro-inflammatory, pro-coagulant, pro-fibrotic aging glomerulus. J Am Soc Nephrol 21: 587–597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Nagata M, Kriz W: Glomerular damage after uninephrectomy in young rats. II. Mechanical stress on podocytes as a pathway to sclerosis. Kidney Int 42: 148–160, 1992 [DOI] [PubMed] [Google Scholar]
- 31.Smeets B, Kuppe C, Sicking EM, Fuss A, Jirak P, van Kuppevelt TH, Endlich K, Wetzels JF, Gröne HJ, Floege J, Moeller MJ: Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. J Am Soc Nephrol 22: 1262–1274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smeets B, Stucker F, Wetzels J, Brocheriou I, Ronco P, Gröne HJ, D’Agati V, Fogo AB, van Kuppevelt TH, Fischer HP, Boor P, Floege J, Ostendorf T, Moeller MJ: Detection of activated parietal epithelial cells on the glomerular tuft distinguishes early focal segmental glomerulosclerosis from minimal change disease. Am J Pathol 184: 3239–3248, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuppe C, van Roeyen C, Leuchtle K, Kabgani N, Vogt M, Van Zandvoort M, Smeets B, Floege J, Gröne HJ, Moeller MJ. Investigations of glucocorticoid action in GN. J Am Soc Nephrol 28: 1408–1420, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasagni L, Lazzeri E, Shankland SJ, Anders HJ, Romagnani P: Podocyte mitosis - A catastrophe. Curr Mol Med 13: 13–23, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodgin JB, Bitzer M, Wickman L, Afshinnia F, Wang SQ, O’Connor C, Yang Y, Meadowbrooke C, Chowdhury M, Kikuchi M, Wiggins JE, Wiggins RC: Kidney aging and focal global glomerulosclerosis. A podometric perspective. J Am Soc Nephrol 26: 3162–3178, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hackl MJ, Burford JL, Villanueva K, Lam L, Suszták K, Schermer B, Benzing T, Peti-Peterdi J: Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat Med 19: 1661–1666, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schulte K, Berger K, Boor P, Jirak P, Gelman IH, Arkill KP, Neal CR, Kriz W, Floege J, Smeets B, Moeller MJ: Origin of parietal podocytes in atubular glomeruli mapped by lineage tracing. J Am Soc Nephrol 25: 129–141, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lasagni L, Angelotti ML, Ronconi E, Lombardi D, Nardi S, Peired A, Becherucci F, Mazzinghi B, Sisti A, Romoli S, Burger A, Schaefer B, Buccoliero A, Lazzeri E, Romagnani P: Podocyte regeneration driven by renal progenitors determines glomerular disease remission and can be pharmacologically enhanced. Stem Cell Rep 5: 248–263, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fukuda A, Wickman LT, Venkatareddy MP, Sato Y, Chowdhury MA, Wang SQ, Shedden KA, Dysko RC, Wiggins JE, Wiggins RC: Angiotensin II-dependent persistent podocyte loss from destabilized glomeruli causes progression of end stage kidney disease. Kidney Int 81: 40–55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Venkatareddy M, Wang S, Yang Y, Patel S, Wickman L, Nishizono R, Chowdhury M, Hodgin J, Wiggins PA, Wiggins RC: Estimating podocyte number and density using a single histologic section. J Am Soc Nephrol 25: 1118–1129, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weibel ER: Stereologic Methods: Practical Methods for Biologic Morphometry, London, Academic Press, Inc., pp 40–116, 415, 1979 [Google Scholar]
- 42.Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, Chang JW, Wiggins JE, Lienczewski C, Kretzler M, Wiggins RC: Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol 20: 1041–1052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.