Abstract
Objective
To evaluate, through biomechanical testing, the resistance to and energy required for the occurrence of proximal femoral fracture in synthetic bone after removal of a proximal femoral nail model (PFN), comparing the results obtained with a reinforcement technique using polymethylmethacrylate (PMMA).
Methods
Fifteen synthetic bones were used: five units for the control group (CG), five for the test group without reinforcement (TGNR), and five for the test group with reinforcement (TGR). The biomechanical analysis was performed simulating a fall on the trochanter using a servo-hydraulic machine. In the GC, the assay was performed with the PFN intact. In the TGNR and TGR groups, a model of PFN was introduced and the tests were performed in the TGNR, after simple removal of the synthesis material, and in the TGR, after removal of the same PFN model and filling of the cavity in the femoral neck with PMMA.
Results
All groups presented a basicervical fracture. The CG presented a mean of 1427.39 Newtons (N) of maximum load and 10.14 Joules (J) of energy for the occurrence of the fracture. The TGNR and TGR presented 892.14 N and 1477.80 N of maximum load, and 6.71 J and 11.99 J of energy, respectively. According to the Kruskal–Wallis ANOVA, there was a significant difference in the maximum load (p = 0.009) and energy (p = 0.007) between these groups.
Conclusion
The simple removal of a PFN in synthetic bone showed a significant reduction of the maximum load and energy for the occurrence of fracture, which were re-established with a reinforcement technique using PMMA.
Keywords: Hip, Hip fractures, Osteoporosis, Polymethylmethacrylate
Resumo
Objetivo
Avaliar, por meio de ensaio biomecânico, a resistência e a energia necessária para ocorrência de fratura do fêmur proximal em osso sintético após retirada de um modelo de haste de fêmur proximal (PFN) e comparar os resultados obtidos com técnica de reforço com polimetilmetacrilato (PMMA).
Métodos
Foram usados 15 ossos sintéticos: cinco unidades para o grupo controle (GC), cinco para o grupo teste sem reforço (GTS) e cinco para grupo teste com reforço (GTC). A análise biomecânica foi feita e simulou queda sobre o trocânter com máquina servo-hidráulica. No GC, o ensaio foi feito com sua integridade intacta. Nos grupos GTS e GTC, foi introduzido um modelo de PFN e os ensaios foram feitos no GTS, após simples retirada do material de síntese, e no GTC, após retirada do mesmo modelo de haste e preenchimento do pertuito no colo com PMMA.
Resultado
Todos os grupos apresentaram fratura basocervical. O grupo GC apresentou média 1.427,39 Newtons (N) de carga máxima e 10,14 Joules (J) de energia para a ocorrência da fratura. Os grupos GTS e GTC apresentaram 892,14 N e 1.477,80 N de carga máxima e 6,71 J e 11,99 J de energia, respectivamente. Segundo a Anova de Kruskal–Wallis, existe diferença significativa na carga máxima (p = 0,009) e na energia (p = 0,007) entre esses grupos.
Conclusão
A simples retirada de um PFN em osso sintético apresentou redução significativa da carga máxima e da energia para a ocorrência de fratura, que foram reestabelecidas com uma técnica de reforço com PMMA.
Palavras-chave: Quadril, Fraturas de quadril, Osteoporose, Polimetilmetacrilato
Introduction
Proximal femoral fractures are one of the most common problems among the elderly, representing an important cause of morbidity and mortality in this age group. Due to the increase in life expectancy, they will become increasingly more frequent.1, 2
The goal in treating such fractures is to allow the patient to return to normal activities as soon as possible by using fixation of the fracture either through rods, plates and/or screws, or arthroplasty of the hip, to reduce the possibility of complications associated to patient immobility.2
Synthesis implant removal is indicated in cases of persistent pain in the gluteal and thigh region caused by the prominence of the synthetic material, implant failure, or infection.2, 3 After proximal femur fractures are healed, removing the implants can cause complications such as possible fractures of the femoral neck or intertrochanteric region.3
In unstable transtrochanteric fractures, there is a tendency to use intramedullary osteosynthesis, especially in poor quality bone, due to its better biomechanical and clinical results.4
In elderly patients with reduced bone mineral density, the removal of intramedullary implants from the proximal femur should be carefully assessed. Considering the need for physical activities and the presence of comorbidities, implant removal used to be reserved for younger patients. However, nowadays, due to prolonged life expectancy and the practice of sports activities among the elderly, the need for removal will become a growing trend.5, 6
Considering the trend to use intramedullary nails in the treatment of unstable transtrochanteric fractures and the elderly population growth expected over the next 20 years, describing the results of a static flexion test simulating a fall onto the trochanter in synthetic femurs after proximal femoral nail (PFN) removal with the presence and absence of an augmentation technique may provide results that guide the development of clinical trials to more carefully establish indications for PFN removal.
Material and methods
Fifteen Brazilian-made synthetic femurs with a 12-mm medullary canal from the same batch and of the same model were divided into three groups, each consisting of five units (Fig. 1). The control group (CG) consisted of synthetic femurs with intact external and internal integrity.
Fig. 1.
Test specimens before assays.
In the test group without reinforcement (TGNR) and in the test group with reinforcement (TGR), a PFN with a sliding screw of 12 mm in diameter, without previous fractures, was implanted and removed soon thereafter.
In the TGNR, the biomechanical assay was performed shortly after removal of the implant, and no reinforcement technique was used.
In the TGR, after the implant was removed, the samples were reinforced with polymethylmethacrylate (PMMA) in the cavity of the sliding screw, introduced anterogradely with the aid of a syringe (Fig. 2).
Fig. 2.
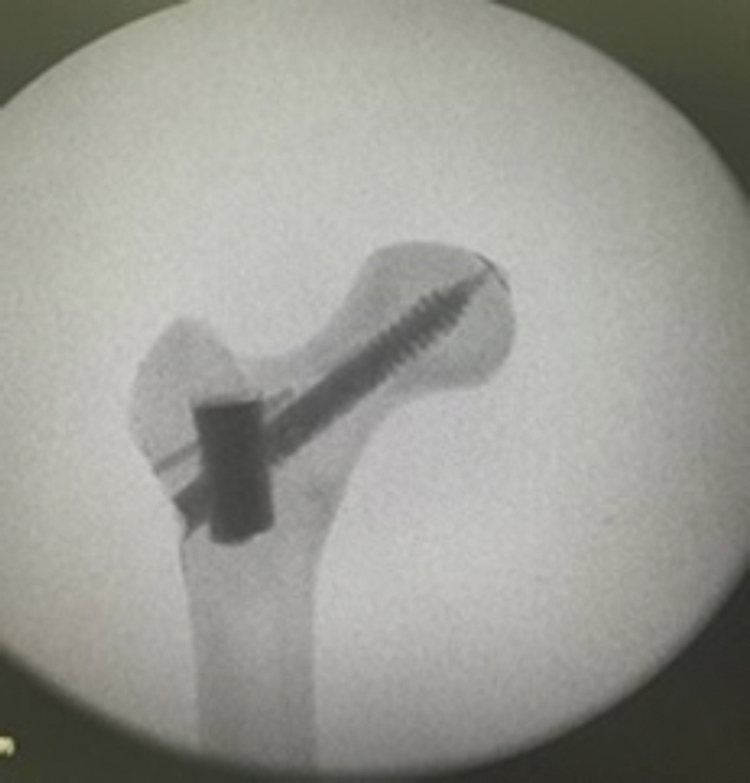
Radiographs of a synthetic model after PMMA reinforcement.
All samples were sent to the biomechanical assay laboratory, which were performed statically in flexion using the servohydraulic test machine MTS model 810 – FlexTest 40 – with a capacity of 100 kN.
The femur was fixated to the test device with 150 mm of its proximal length outside the device and toward the hydraulic piston. The proximal femur was placed at the base of the testing machine at 10° horizontal inclination and 15° internal rotation measured by a digital goniometer. The greater trochanter was supported by a silicon disk of 8 × 2 cm diameter (Fig. 3A). A 40 N preload was applied at a speed of 2 mm/s; subsequently, load was applied on the femoral head until fracture (Fig. 3B). The values of maximum load were recorded in N and maximum energy in J.
Fig. 3.
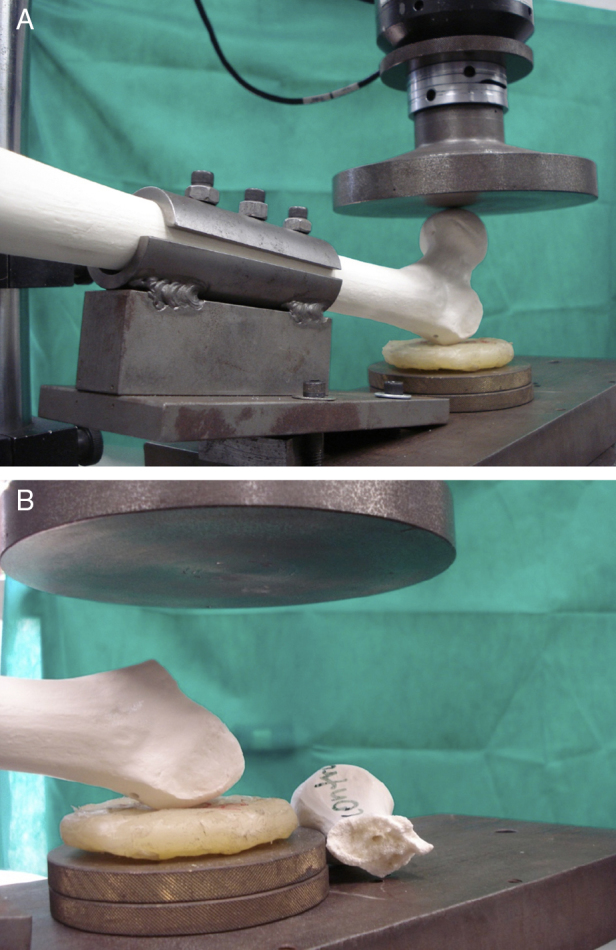
(A) Femur fixated to the device during the test prior to the occurrence of the fracture. (B) Fractured femur after the test.
Descriptive analysis was used to present the observed data, expressed by median and interquartile range (Q1–Q3) according to the experiment group, in tables and illustrative graphs. The inferential analysis was made using Kruskal–Wallis ANOVA and Dunn's multiple comparisons test. The adopted criterion for significance level was 5%. Statistical analysis was performed using SPSS version 20.0 software.
Results
All the specimens tested presented basicervical fracture (Fig. 4).
Fig. 4.
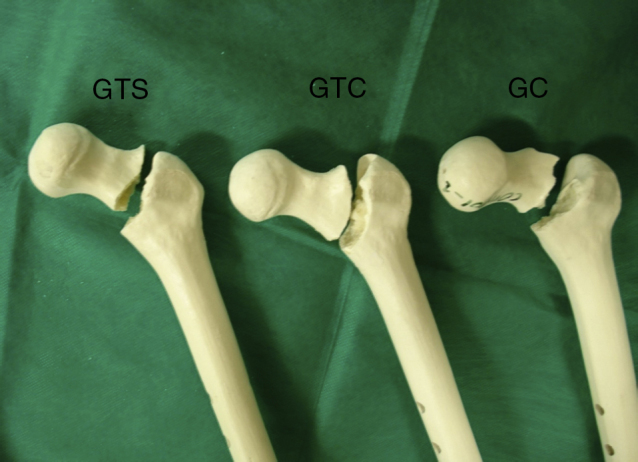
Test specimens from the CG, TGNR, and TGR, showing the fracture pattern (basicervical fracture).
Table 1 presents the median and interquartile range (Q1–Q3) of maximum load-to-fracture (N) and energy-to-fracture (J) in the CG, TGNR, and TGR, with the corresponding descriptive level (p-value).
Table 1.
Maximum load values, energy, and p-value for fracture.
| Variable | Group | Median | IQR | p-Valuea | Significant ≠b | ||
|---|---|---|---|---|---|---|---|
| Maximum load (N) | Control | 1337 | 1243 | – | 1657 | 0.009 | Control ≠ uncemented; cemented ≠ uncemented |
| Cemented | 1346 | 1224 | – | 1798 | |||
| Uncemented | 928 | 780 | – | 986 | |||
| Energy for fracture (J) | Control | 10.8 | 8.9 | – | 11.0 | 0.007 | Control ≠ uncemented; cemented ≠ uncemented |
| Cemented | 12.4 | 9.2 | – | 14.6 | |||
| Uncemented | 6.6 | 6.2 | – | 7.3 | |||
IQR, interquartile range (Q1–Q3).
Kruskal–Wallis ANOVA.
Dunn's multiple comparison test, at 5%.
In the inferential analysis, Kruskal–Wallis ANOVA was used to assess whether there was a significant difference between the groups, and Dunn's multiple comparison test to identify which groups differed significantly at the 5% level.
In the ANOVA Kruskal–Wallis test, a significant difference was observed in maximum load-to-fracture (p = 0.009) and maximum energy-to-fracture (p = 0.007) between the groups.
In the Dunn test, it was observed that, at the 5% level, the CG and the TGR presented a significantly higher maximum load- and energy-to-fracture than the TGNR. No statistically significant difference at the level of 5% was observed between the CG and the TGR.
Discussion
The removal of considerably large implants such as PFNs after fracture healing has relatively high rates of complications, such as femoral neck fracture; removal is recommended only in cases of deep and chronic infection.6
The literature presents experimental results in the use of PMMA bone reinforcement after implant removal; there is concern regarding the volume used, due to local thermal reaction.7, 8
The mechanism of trauma adopted in the present study (fall on the great trochanter), was chosen because this is the most common injury of the proximal femur in elderly patients.
The choice of synthetic bones was adopted to ensure comparable biomechanical properties between groups and eliminate further variables. Thus, possible alterations inherent in human bones that, would hinder the methodological evaluation due to their non-uniform characteristics (bone density, length, and diameter) were eliminated.9
Although the absolute values were not comparable to those observed in experimental studies with cadaver bones due to the structural and biomechanical differences of cadaveric and synthetic bones, the results were compatible regarding the increase in strength when PMMA reinforcement was used.10, 11, 12 The presence of the same fracture pattern in groups, especially in the TGR, demonstrates that the reinforcement acted specifically on the load increase, and that there was no biomechanical pattern change for the occurrence of fracture.
Conclusion
The results presented herein may serve as a motivation for the development of clinical trials that improve the level of evidence of the biomechanical benefits of bone reinforcement in the removal of implants such as PFNs in elderly patients.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Study conducted at Hospital Ortopédico e Medicina Especializada (HOME), Instituto de Pesquisa e Ensino (IPE), Brasília, DF, Brazil.
References
- 1.Gullberg B., Johnell O., Kanis J.A. World-wide projections for hip fracture. Osteoporos Int. 1997;7(5):407–413. doi: 10.1007/pl00004148. [DOI] [PubMed] [Google Scholar]
- 2.Yang J.H., Jung T.G., Honnurappa A.R., Cha J.M., Ham C.H., Kim T.Y. The analysis of biomechanical properties of proximal femur after implant removal. Appl Bionics Biomech. 2016;2016:4987831. doi: 10.1155/2016/4987831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kukla C., Pichl W., Prokesch R., Jacyniak W., Heinze G., Gatterer R. Femoral neck fracture after removal of the standard gamma interlocking nail: a cadaveric study to determine factors influencing the biomechanical properties of the proximal femur. J Biomech. 2001;34(12):1519–1526. doi: 10.1016/s0021-9290(01)00157-9. [DOI] [PubMed] [Google Scholar]
- 4.Mahaisavariya B., Sitthiseripratip K., Suwanprateeb J. Finite element study of the proximal femur with retained trochanteric gamma nail and after removal of nail. Injury. 2006;37(8):778–785. doi: 10.1016/j.injury.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Kröger H., Kettunen J., Bowditch M., Joukainen J., Suomalainen O., Alhava E. Bone mineral density after the removal of intramedullary nails: a cross-sectional and longitudinal study. J Orthop Sci. 2002;7(3):325–330. doi: 10.1007/s007760200055. [DOI] [PubMed] [Google Scholar]
- 6.Eberle S., Wutte C., Bauer C., von Oldenburg G., Augat P. Should extramedullary fixations for hip fractures be removed after bone union? Clin Biomech. 2011;26(4):410–414. doi: 10.1016/j.clinbiomech.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Strauss E.J., Pahk B., Kummer F.J., Egol K. Calcium phosphate cement augmentation of the femoral neck defect created after dynamic hip screw removal. J Orthop Trauma. 2007;21(5):295–300. doi: 10.1097/BOT.0b013e3180616ba5. [DOI] [PubMed] [Google Scholar]
- 8.Heini P.F., Franz T., Fankhauser C., Gasser B., Ganz R. Femoroplasty-augmentation of mechanical properties in the osteoporotic proximal femur: a biomechanical investigation of PMMA reinforcement in cadaver bones. Clin Biomech. 2004;19(5):506–512. doi: 10.1016/j.clinbiomech.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 9.Cristofolini L., Viceconti M., Cappello A., Toni A. Mechanical validation of whole bone composite femur models. J Biomech. 1996;29(4):525–535. doi: 10.1016/0021-9290(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 10.Fliri L., Sermon A., Wähnert D., Schmoelz W., Blauth M., Windolf M. Limited V-shaped cement augmentation of the proximal femur to prevent secondary hip fractures. J Biomater Appl. 2013;28(1):136–143. doi: 10.1177/0885328212443274. [DOI] [PubMed] [Google Scholar]
- 11.Basafa E., Murphy R.J., Otake Y., Kutzer M.D., Belkoff S.M., Mears S.C. Subject-specific planning of femoroplasty: an experimental verification study. J Biomech. 2015;48(1):59–64. doi: 10.1016/j.jbiomech.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckmann J., Ferguson S.J., Gebauer M., Luering C., Gasser B., Heini P. Femoroplasty—augmentation of the proximal femur with a composite boné cement—feasibility, biomechanical properties and osteosynthesis potential. Med Eng Phys. 2007;29(7):755–764. doi: 10.1016/j.medengphy.2006.08.006. [DOI] [PubMed] [Google Scholar]