Abstract
Background
Cushing syndrome is characterized by glucose intolerance, cardiovascular disease, and an enhanced systemic inflammatory response caused by chronic exposure to excess cortisol. Eosinopenia is frequently observed in patients with adrenal Cushing syndrome, but the relationship between the eosinophil count in peripheral blood and indicators of glucose level in patients with adrenal Cushing syndrome has not been determined.
Methods
A retrospective study was undertaken of the clinical and laboratory findings of 40 patients diagnosed with adrenal Cushing syndrome at Chungnam National University Hospital from January 2006 to December 2016. Clinical characteristics, complete blood cell counts with white blood cell differential, measures of their endocrine function, description of imaging studies, and pathologic findings were obtained from their medical records.
Results
Eosinophil composition and count were restored by surgical treatment of all of the patients with adrenal Cushing disease. The eosinophil count was inversely correlated with serum and urine cortisol, glycated hemoglobin, and inflammatory markers in the patients with adrenal Cushing syndrome.
Conclusion
Smaller eosinophil populations in patients with adrenal Cushing syndrome tend to be correlated with higher levels of blood sugar and glycated hemoglobin. This study suggests that peripheral blood eosinophil composition or count may be associated with serum glucose levels in patients with adrenal Cushing syndrome.
Keywords: Cushing syndrome, Eosinophils, Hydrocortisone, Glucose level
INTRODUCTION
Cushing syndrome results from exposure of tissues to high levels of circulating cortisol for a long period. The signs and symptoms of Cushing syndrome include moon face and truncal obesity, thin skin, muscle weakness, depression, hypertension, osteoporosis, and hyperglycemia [1,2,3]. Glucocorticoid excess in patients with Cushing syndrome upregulates gluconeogenesis in the liver and inhibits insulin action in liver, muscle, and adipose tissue; thereby, contributing to the development of diabetes mellitus [4,5]. Moreover, the excess glucocorticoid alters the profile and function of immune cells, compromising the immune system and leading to a higher incidence of infectious disease [6].
A number of immune cell types have recently been implicated in the regulation of systemic glucose metabolism [7,8]. Interestingly, eosinophils are required for the correct maintenance of local and systemic glucose homeostasis in humans and mice [9,10]. Moreover, T helper type 2 (Th2) cytokines secreted by eosinophils inhibit the inflammatory response by modulating macrophage polarization in adipose tissue, leading to an amelioration of glucose intolerance in mice [9]. The eosinophil count in peripheral blood is lower in patients with Cushing syndrome compared to healthy subjects [11,12]. However, it remains to be determined whether characteristics of the eosinophil population are correlated with the level of serum glucose in patients with Cushing syndrome.
Cushing syndrome is also considered to be a chronic low-grade inflammatory state. Glucocorticoid excess stimulates leukocytosis and granulocytosis, but decreases the number of CD4+ and increases the number of CD8+ T cells, such that the CD4/CD8 ratio is lower in patients with Cushing syndrome than in age- and sex-matched healthy controls [13]. Although chronic inflammation caused by immune dysregulation in Cushing syndrome contributes to the development of diabetes, little is known regarding the impact of eosinopenia on the chronic inflammation-induced glucose intolerance. Moreover, the prevalence of type 2 diabetes is higher, but the eosinophil count in peripheral blood is lower, in patients with Cushing syndrome than in healthy subjects. Therefore, in this study, we aimed to evaluate the relationships between eosinophil count and both glucose tolerance and levels of inflammatory markers in patients with adrenal Cushing syndrome.
METHODS
Study design
A retrospective study was performed of patients with adrenal Cushing syndrome at Chungnam National University Hospital from January 2006 to December 2016. The patients' medical records were reviewed and details of their clinical and radiologic data, endocrine function, and pathologic findings were extracted. Patients with acute infectious disease or a history of parasite infection within the previous 6 months were excluded from the study. A total of 40 patients with adrenal Cushing syndrome underwent assessment of glucose homeostasis by measurement of their glycated hemoglobin and fasting plasma glucose concentrations.
Clinical and biochemical parameters
The patients were assessed for height, body mass, systolic blood pressure, and diastolic blood pressure by review of their electronic medical records. Body mass index (BMI) was calculated as the body mass (kg) divided by the square of the height (m2). A 24-hour urine free cortisol or a 1 mg overnight dexamethasone suppression test were used to screen for Cushing syndrome. A low-dose dexamethasone suppression test was then used to increase diagnostic specificity for Cushing syndrome, and to confirm the diagnosis, histopathology was undertaken on surgical biopsies.
Glycated hemoglobin was measured using high performance liquid chromatography (BioRad, Hercules, CA, USA). Plasma adrenocorticotropic hormone (ACTH, Cis-Bio International, Gif-sur-Yvette, France) and cortisol (DiaSorin Inc., Stillwater, MN, USA) were measured using radioimmunoassay kits. We also reviewed the levels of fasting plasma glucose and total cholesterol in the patients, as well as preoperative serum levels of ACTH and cortisol. The results of complete blood cell counts with white blood cell differential, including the percentages of neutrophils and eosinophils, were also recorded. Blood samples drawn 1 week before and approximately 1 month after surgery were also analyzed to confirm remission of the adrenal Cushing syndrome.
Eosinophil purification
Segments of the small intestine from male C57BL/6 mice were incubated with FACS buffer (phosphate buffered saline [PBS] containing 10% bovine serum albumin, 100 U/mL penicillin, 100 µg/mL streptomycin, 10 mmol/L ethylenediaminetetraacetic acid [EDTA]) during 30 minutes at 37℃ to remove epithelial cells and were washed extensively with PBS. Small intestinal segments were digested with 2.4 mg/mL collagenase A (Roche, Indianapolis, IN, USA) and 10 µg/mL DNase I (Roche) in RPMI 1640/10% fetal bovine serum (FBS) with continuous stirring at 37℃ for 30 minutes. EDTA was added (10 mmol/L final), and the cell suspension was incubated for an additional 5 minutes at 37℃. After washing, the cells were subjected to density-gradient centrifugation in 40%/75% Percoll gradient (GE healthcare, Buckinghamshire, United Kingdom) in PBS. The cells harvested from the interface were washed and applied to purify eosinophils. For the isolation of eosinophils, Siglec-F+ cells were separated from the harvested cells by positive magnetic cell sorting (Miltenyi Biotec, Auburn, CA, USA).
Eosinophil survival assay
Eosinophils from murine intestines were cultured at 37℃ and 5% CO2 in RPMI containing 1% penicillin/streptomycin and 10% FBS in 96-well at a cell concentration of 2.0×104 cells/well. Eosinophils were cultured in the presence of recombinant interleukin 5 (IL-5) (1 ng/mL; R&D Systems, Minneapolis, MN, USA) and dexamethasone concentration (0 to 100 nM), and survival rate in the presence of dexamethasone was compared to viability in recombinant IL-5 medium alone. Viable cells were assessed by the trypan blue exclusion method. Cell viability was observed at day 3 for all experiments and was calculated by the total cell count minus the count of nonviable or dead cells.
Statistical analysis
All of the parameters are presented as mean±standard deviation. The Student t test and one-way analysis of variance were applied. Correlations were determined by Spearman correlation to assess the relationship between eosinophil numbers and markers of glucose metabolism in the patients. P<0.05 was considered to indicate statistical significance. Statistical analyses were performed using Graph Pad PRISM 6 (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
Eosinophil numbers are restored by surgical treatment in patients with adrenal Cushing syndrome
Forty patients underwent laparoscopic or open adrenalectomy for Cushing syndrome during the study period. The demographics and baseline characteristics of the patients are summarized in Table 1. The mean age of the patients was 43.4 years (range, 24 to 62). Similar to previous studies, women were over-represented in the study population, with a female-to-male ratio of 27:13. The distribution of adrenal tumors was even between the right and left adrenal glands (45% and 55%, respectively), and bilateral tumors were not found. Tumor diameter ranged from 0.5 to 4.4 cm, with a mean of 2.8±1.0 cm. The mean BMI of the patients was 25.8±4.0 kg/m2. The prevalence of type 2 diabetes and hypertension were 40.0% and 62.5%, respectively.
Table 1. Clinical Characteristics of 40 Patients with Adrenal Cushing Syndrome.
| Characteristic | Value |
|---|---|
| Age, yr | 43.4±11.0 |
| Male sex | 32.5 |
| Site of tumor | |
| Right | 45.0 |
| Left | 55.0 |
| Both | 0 |
| Tumor size, cm | 2.8±1.0 |
| Body mass index, kg/m2 | 25.8±4.0 |
| SBP, mm Hg | 127.1±17.2 |
| DBP, mm Hg | 79.4±12.0 |
| Total cholesterol, mg/dL | 195.2±41.2 |
| Fasting glucose, mg/dL | 122.0±40.3 |
| Leukocytes, cell/mm3 | 8,395.7±1,960.8 |
| Eosinophils, cell/mm3 | 67.8±58.1 |
| Concomitant disease | |
| Hypertension | 62.5 |
| Diabetes mellitus | 35.0 |
| Anti-diabetic medication | 25.0 |
Values are expressed as mean±SD or percentage.
SBP, systolic blood pressure; DBP, diastolic blood pressure.
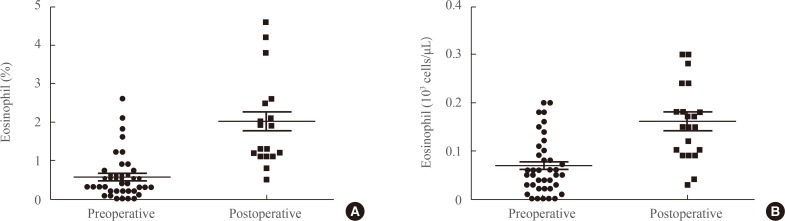
The mean preoperative eosinophil percentage was 0.52%±0.55%, whereas the postoperative count was 2.01%±1.13% (P<0.001) (Fig. 1A). Moreover, proper resection of tumor induced the restoration of eosinophil count (Fig. 1B). These data indicate that surgical resection of the adrenal gland containing the tumor led to an increase in the eosinophil count in peripheral blood.
Fig. 1. Preoperative and postoperative eosinophil population in patients with adrenal Cushing syndrome. (A) Percentage of eosinophils in peripheral blood preoperative and postoperatively. (B) Preoperative and postoperative eosinophil count in peripheral blood.
The proportion of eosinophils is inversely correlated with serum and urine cortisol in patients with adrenal Cushing syndrome
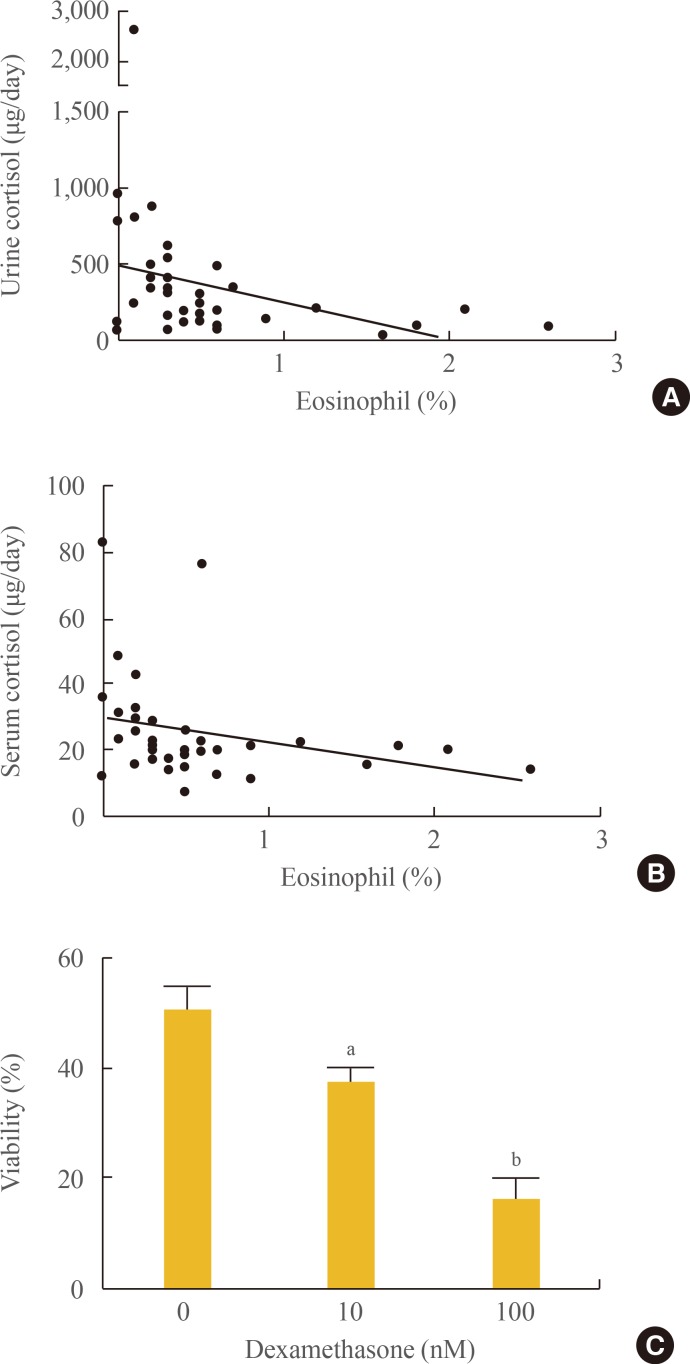
As shown in Fig. 2A, 24-hour urine free cortisol was negatively correlated with the peripheral blood eosinophil percentage (r=−0.49, P=0.001). Serum cortisol was also negatively correlated with the eosinophil percentage in peripheral blood (r=−0.37, P=0.05) (Fig. 2B). Additionally, to test the effects of steroids on eosinophil survival, eosinophils were cultured with recombinant IL-5 at 1 ng/mL. Coincubation of eosinophils with dexamethasone showed dose-dependent decrease in eosinophil survival (Fig. 2C). These data suggest that the number of eosinophils may be reduced by the higher serum cortisol level present in patients with adrenal Cushing syndrome.
Fig. 2. Serum and urine cortisol are inversely correlated with eosinophil numbers in the peripheral blood of patients with adrenal Cushing syndrome. Correlations between (A) 24-hour urine free cortisol and the percentage of eosinophils and (B) serum cortisol and the percentage of eosinophils. (C) Dexamethasone inhibited eosinophil survival at a concentration-dependent manner in vitro. Data are representative of three independent experiments and are expressed as mean±SD. aP<0.05; bP<0.01 in comparison with the corresponding controls.
Glycated hemoglobin is negatively associated with the proportion of eosinophils in patients with adrenal Cushing syndrome
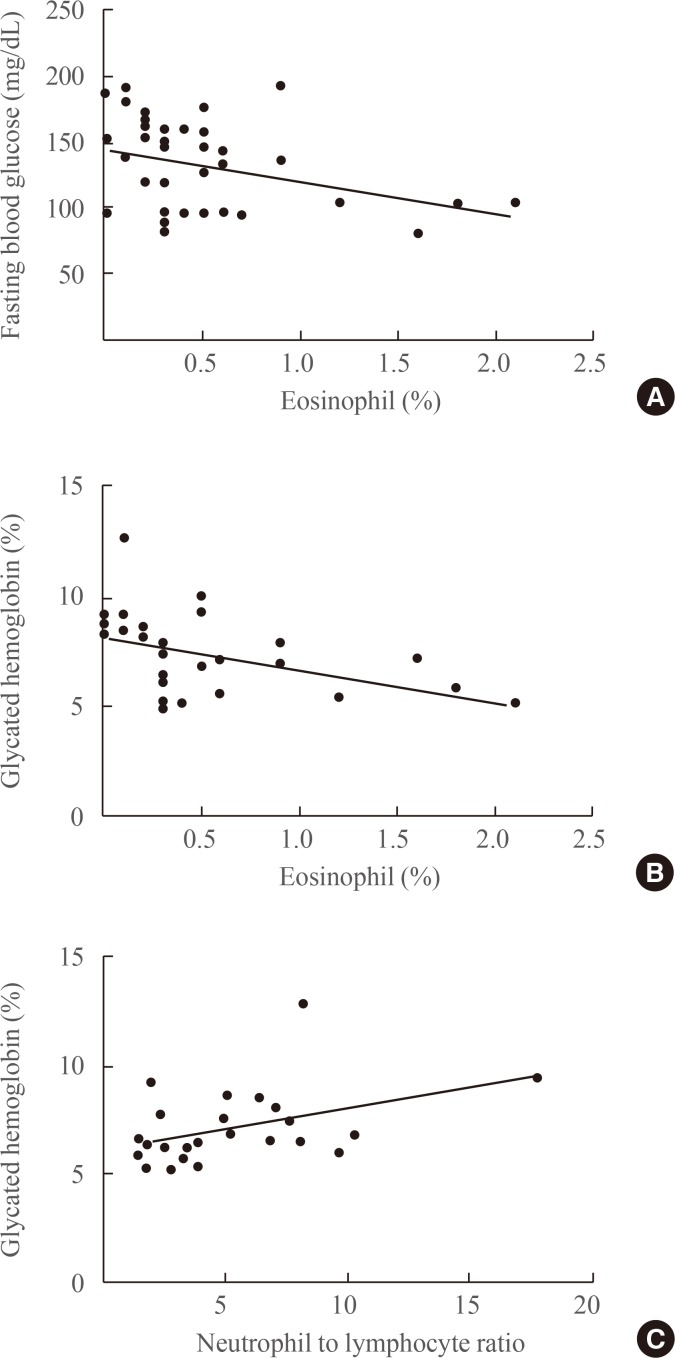
We also measured the level of glycated hemoglobin in the peripheral blood of the patients. As shown in Fig. 3A, fasting blood glucose tends to be negatively correlated (r=−0.36, P=0.08) with the eosinophil percentage. Glycated hemoglobin also showed a tendency toward negative correlation (r=−0.47, P=0.02) with the percentage of eosinophils (Fig. 3B). Moreover, as shown in Fig. 3C, the neutrophil to lymphocyte ratio was positively correlated with glycated hemoglobin (r=0.44, P=0.03).
Fig. 3. Impaired glucose metabolism in patients with adrenal Cushing syndrome is associated with the numbers of eosinophils in peripheral blood. (A) Fasting glucose levels are negatively correlated with the percentage of eosinophils. (B) Levels of glycated hemoglobin are negatively correlated with the percentage of eosinophils (C) Neutrophil-to-lymphocyte ratio is positively correlated with eosinophil population.
The number of leukocytes in peripheral blood is inversely correlated with the eosinophil count in patients with adrenal Cushing syndrome
We also found that the leukocyte count was negatively correlated with the eosinophil count in the patients with adrenal Cushing syndrome (r=−0.59, P<0.001) (Fig. 4A). As shown in Fig. 4B, the neutrophil to lymphocyte ratio was positively correlated with the eosinophil count in the patients with adrenal Cushing syndrome (r=−0.76, P<0.001).
Fig. 4. Relationship between inflammation and the number of eosinophils in patients with adrenal Cushing syndrome. (A) The leukocyte count is negatively associated with the eosinophil count in patients with adrenal Cushing syndrome. (B) The neutrophil-to-lymphocyte ratio is negatively correlated with the eosinophil count in patients with adrenal Cushing syndrome.
DISCUSSION
In this retrospective study of 40 patients with adrenal Cushing syndrome, we found that smaller eosinophil counts in the patients with adrenal Cushing syndrome tended to be correlated with higher levels of blood sugar and glycated hemoglobin. Moreover, in the current study, the leukocyte- and neutrophil-to-lymphocyte ratios were negatively associated with eosinophil population in the patients with adrenal Cushing syndrome.
The relationship between glucocorticoid concentration and eosinopenia is well-established, being supported by many experimental and clinical studies [13,14,15]. Consistent with this, our retrospective study of 40 patients with adrenal Cushing syndrome showed a lower eosinophil count in the preoperative state, but the size of the population was increased after surgical resection of the adrenal gland containing the tumor (Fig. 1). Elevated glucocorticoid levels cause eosinopenia but also suppress the adherence and migration of human eosinophils, decreasing the local accumulation of eosinophils in vitro and in vivo [16]. Moreover, glucocorticoids inhibit the survival of human eosinophils cultured with IL-3, IL-5, or granulocyte-macrophage colony-stimulating factor [15,17]. It has also been shown that glucocorticoids activate apoptosis-inducing proteases such as caspase-1 in lymphoblastic leukemia cells [18] and caspase-3 in a B cell line [19]. By contrast, IL-5 promotes the survival of human eosinophils by inhibiting apoptosis [14]. In addition, the IL-33/IL-33 receptor pathway potently enhances adhesion, CD11b expression, and survival of human eosinophils [20].
Eosinopenia has recently been implicated in the progression of insulin resistance in rodents and humans. For example, the number of eosinophils and type 2 immunity are reduced during fat accumulation, leading to a local inflammatory response in adipose tissue [21,22]. The regulatory effect of Th2 cytokines, including IL-4 and 13, on M2 macrophage polarization is critical for the maintenance of systemic glucose homeostasis [9,23,24]. Excessive cortisol is known to reduce the survival and increase apoptosis of eosinophils in vitro and in vivo [15,25], but there have been no investigations on the relationship between serum cortisol concentration and the eosinophil count in patients with Cushing syndrome. The levels of circulating proinflammatory cytokines are increased in patients with Cushing syndrome; thereby, contributing to the development of cardiovascular complications and metabolic diseases [14]. Although eosinophils are essential for the regulation of local and systemic inflammatory responses, it is poorly understood whether the smaller eosinophil population is associated with a proinflammatory state in patients with Cushing syndrome. Moreover, eosinopenia and glucose intolerance are typical findings in patients with Cushing syndrome, understanding of the relationship between eosinophil composition and indexes of impaired glucose tolerance has remained incomplete. Here, we show that the levels of glycated hemoglobin and fasting plasma glucose are negatively correlated with peripheral blood eosinophil count in patients with adrenal Cushing syndrome.
The present study has several limitations that should be acknowledged. This analysis was part of a retrospective study, making it difficult to define direct causal relationships between eosinophil numbers and glucose metabolism in the patients. Moreover, the retrospective design makes it difficult to classify whether the patients were diagnosed by work up of adrenal incidentaloma or by typical clinical symptoms of Cushing syndrome. Second, the sample size was relatively small so there may not have been sufficient statistical power to detect certain effects. The data might also have been affected by selection bias because it was collected from a cohort of patients cared for in a single center and therefore may not be generalizable. Third, eosinophil composition is affected by blood sampling time or food intake [26]. In general, blood samples were taken at 6:00 AM or 7:00 AM (pre-meal), but several samples were obtained in the afternoon or evening, which represents a confounding factor. Fourth, several patients with adrenal Cushing syndrome have already taken anti-diabetic medications before surgical resection of the adrenal tumor (Table 1). This may also have an effect on the levels of plasma glucose and eosinophils in patients with adrenal Cushing syndrome. Finally, the development of relative adrenal insufficiency after surgery may also be associated with postoperative increase of peripheral eosinophil levels (Fig. 1) rather than amelioration of insulin resistance in the patients with adrenal Cushing syndrome. However, despite these limitations, the present study provides the first data demonstrating a relationship between eosinophil numbers and markers of glucose intolerance in patients with adrenal Cushing syndrome.
In summary, we have shown that the eosinopenia that is commonly found in patients with adrenal Cushing syndrome tends to be correlated with higher levels of blood sugar and glycated hemoglobin. We also present evidence suggesting that measurements of pre- and postoperative eosinophil composition may be helpful in the assessment of whether tumors have been completely removed from patients with adrenal Cushing syndrome (Fig. 1). However, further studies are needed to determine whether eosinophil composition is helpful in determining a metabolic prognosis for patients with adrenal Cushing syndrome.
ACKNOWLEDGMENTS
This study was supported by the research fund of the Daejeon and Chungcheong Branch of Korean Endocrine Society (2016). Hyon-Seung Yi was supported by the Basic Science Research Program, through the NRF, funded by the Ministry of Science, ICT, and Future Planning, Korea (NRF-2015R1C1A1A01052432).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Chanson P, Salenave S. Metabolic syndrome in Cushing's syndrome. Neuroendocrinology. 2010;92(Suppl 1):96–101. doi: 10.1159/000314272. [DOI] [PubMed] [Google Scholar]
- 2.Di Somma C, Pivonello R, Loche S, Faggiano A, Marzullo P, Di Sarno A, et al. Severe impairment of bone mass and turnover in Cushing's disease: comparison between childhood-onset and adulthood-onset disease. Clin Endocrinol (Oxf) 2002;56:153–158. doi: 10.1046/j.0300-0664.2001.01454.doc.x. [DOI] [PubMed] [Google Scholar]
- 3.Pivonello R, De Leo M, Vitale P, Cozzolino A, Simeoli C, De Martino MC, et al. Pathophysiology of diabetes mellitus in Cushing's syndrome. Neuroendocrinology. 2010;92(Suppl 1):77–81. doi: 10.1159/000314319. [DOI] [PubMed] [Google Scholar]
- 4.Vegiopoulos A, Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol. 2007;275:43–61. doi: 10.1016/j.mce.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Mazziotti G, Gazzaruso C, Giustina A. Diabetes in Cushing syndrome: basic and clinical aspects. Trends Endocrinol Metab. 2011;22:499–506. doi: 10.1016/j.tem.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Bakker RC, Gallas PR, Romijn JA, Wiersinga WM. Cushing's syndrome complicated by multiple opportunistic infections. J Endocrinol Invest. 1998;21:329–333. doi: 10.1007/BF03350337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bronsart LL, Contag CH. A role of the adaptive immune system in glucose homeostasis. BMJ Open Diabetes Res Care. 2016;4:e000136. doi: 10.1136/bmjdrc-2015-000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lackey DE, Olefsky JM. Regulation of metabolism by the innate immune system. Nat Rev Endocrinol. 2016;12:15–28. doi: 10.1038/nrendo.2015.189. [DOI] [PubMed] [Google Scholar]
- 9.Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu L, Su T, Xu M, Xu Y, Li M, Wang T, et al. Eosinophil inversely associates with type 2 diabetes and insulin resistance in Chinese adults. PLoS One. 2013;8:e67613. doi: 10.1371/journal.pone.0067613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambrogio AG, De Martin M, Ascoli P, Cavagnini F, Pecori Giraldi F. Gender-dependent changes in haematological parameters in patients with Cushing’s disease before and after remission. Eur J Endocrinol. 2014;170:393–400. doi: 10.1530/EJE-13-0824. [DOI] [PubMed] [Google Scholar]
- 12.Greenblatt RB. Vagaries in the symptomatology of Cushing's syndrome. J Clin Endocrinol Metab. 1954;14:961–968. doi: 10.1210/jcem-14-8-961. [DOI] [PubMed] [Google Scholar]
- 13.Kronfol Z, Starkman M, Schteingart DE, Singh V, Zhang Q, Hill E. Immune regulation in Cushing's syndrome: relationship to hypothalamic-pituitary-adrenal axis hormones. Psychoneuroendocrinology. 1996;21:599–608. doi: 10.1016/s0306-4530(96)00021-2. [DOI] [PubMed] [Google Scholar]
- 14.Brode S, Farahi N, Cowburn AS, Juss JK, Condliffe AM, Chilvers ER. Interleukin-5 inhibits glucocorticoid-mediated apoptosis in human eosinophils. Thorax. 2010;65:1116–1117. doi: 10.1136/thx.2009.124909. [DOI] [PubMed] [Google Scholar]
- 15.Wallen N, Kita H, Weiler D, Gleich GJ. Glucocorticoids inhibit cytokine-mediated eosinophil survival. J Immunol. 1991;147:3490–3495. [PubMed] [Google Scholar]
- 16.Altman LC, Hill JS, Hairfield WM, Mullarkey MF. Effects of corticosteroids on eosinophil chemotaxis and adherence. J Clin Invest. 1981;67:28–36. doi: 10.1172/JCI110024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallsworth MP, Litchfield TM, Lee TH. Glucocorticoids inhibit granulocyte-macrophage colony-stimulating factor-1 and interleukin-5 enhanced in vitro survival of human eosinophils. Immunology. 1992;75:382–385. [PMC free article] [PubMed] [Google Scholar]
- 18.Geley S, Hartmann BL, Kapelari K, Egle A, Villunger A, Heidacher D, et al. The interleukin 1beta-converting enzyme inhibitor CrmA prevents Apo1/Fas- but not glucocorticoid-induced poly(ADP-ribose) polymerase cleavage and apoptosis in lymphoblastic leukemia cells. FEBS Lett. 1997;402:36–40. doi: 10.1016/s0014-5793(96)01496-2. [DOI] [PubMed] [Google Scholar]
- 19.Miyashita T, Mami U, Inoue T, Reed JC, Yamada M. Bcl-2 relieves the trans-repressive function of the glucocorticoid receptor and inhibits the activation of CPP32-like cysteine proteases. Biochem Biophys Res Commun. 1997;233:781–787. doi: 10.1006/bbrc.1997.6559. [DOI] [PubMed] [Google Scholar]
- 20.Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, et al. Interleukin-33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–1253. doi: 10.1038/labinvest.2008.82. [DOI] [PubMed] [Google Scholar]
- 21.Cildir G, Akincilar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol Med. 2013;19:487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J Endocrinol. 2014;222:R113–R127. doi: 10.1530/JOE-14-0283. [DOI] [PubMed] [Google Scholar]
- 23.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, Jouihan H, Morel CR, Heredia JE, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A. 2010;107:22617–22622. doi: 10.1073/pnas.1009152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, et al. Direct control of hepatic glucose production by interleukin-13 in mice. J Clin Invest. 2013;123:261–271. doi: 10.1172/JCI64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nittoh T, Fujimori H, Kozumi Y, Ishihara K, Mue S, Ohuchi K. Effects of glucocorticoids on apoptosis of infiltrated eosinophils and neutrophils in rats. Eur J Pharmacol. 1998;354:73–81. doi: 10.1016/s0014-2999(98)00426-9. [DOI] [PubMed] [Google Scholar]
- 26.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]