Abstract
Cardiovascular risk remains high in kidney transplant recipients (KTRs) despite improved kidney function after transplant. Urinary markers of kidney fibrosis and injury may help to reveal mechanisms of this risk. In a case-cohort study among stable KTRs who participated in the FAVORIT trial, we measured 4 urinary proteins known to correlate with kidney tubulointerstitial fibrosis on biopsy (urine alpha 1 microglobulin [α1m], monocyte chemoattractant protein-1 [MCP-1], procollagen type I [PINP] and type III [PIIINP] N-terminal amino peptide) and evaluated associations with cardiovascular disease (CVD) events (N=300) and death (N=371). In adjusted models, higher urine α1m (hazard ratio [HR] per doubling of biomarker 1.40 [95% CI 1.21, 1.62]), MCP-1 (HR 1.18 [1.03, 1.36]), and PINP (HR 1.13 [95% CI 1.03, 1.23]) were associated with CVD events. These 3 markers were also associated with death (HR per doubling α1m 1.51 [95% CI 1.32, 1.72]; MCP-1 1.31 [95% CI 1.13, 1.51]; PINP 1.11 [95% CI 1.03, 1.20]). Higher concentrations of urine α1m, MCP-1, and PINP may identify KTRs at higher risk for CVD events and death. These markers may identify a systemic process of fibrosis involving both the kidney and cardiovascular system, and give new insights into mechanisms linking the kidney with CVD.
Introduction
Chronic kidney disease (CKD) is an important risk factor for cardiovascular disease (CVD) and death.(1) In kidney transplant recipients (KTRs), despite improved kidney function after transplant, the incidence of CVD remains high (2) and is the leading cause of death.(3) As in non-transplanted CKD patients, urine albumin to creatinine ratio (ACR) and level of estimated glomerular filtration rate (eGFR) are important independent predictors of adverse risk in KTRs. Higher ACR predicts graft loss and death,(4) while lower eGFR is associated with CVD risk.(5, 6)
While ACR indicates predominantly glomerular injury, markers indicating injury of kidney tubules may provide new insights into mechanisms of kidney injury and have been a focus of recent research. Understanding tubular function as well as glomerular function is likely to add prognostic and mechanistic information about overall kidney health. Kidney fibrosis may be of particular relevance in KTRs, as interstitial fibrosis and tubular atrophy (IFTA) on kidney allograft biopsy are strongly predictive of future graft loss, independent of eGFR and ACR.(7) The degree of kidney tubulointerstitial fibrosis is not well captured by eGFR and ACR(8) and therefore is invisible to clinicians in the absence of a kidney biopsy. Although kidney biopsies are performed more frequently in KTRs than in non-transplanted CKD patients, they are still invasive, carry risk of bleeding, and are used primarily for diagnostic purposes; they are only rarely repeated to monitor responses to change in therapy.
We evaluated urine concentrations of alpha 1 microglobulin (α1m), monocyte chemoattractant protein-1 (MCP-1), and procollagen amino-terminal pro-peptides of type I and type III collagen (PINP and PIIINP) in stable KTRs. We chose these markers as they have been associated with the severity of tubulointerstitial fibrosis on kidney biopsy in prior studies. Briefly, α1m is a low molecular weight protein freely filtered at the glomerulus but reabsorbed by proximal tubular epithelial cells under healthy conditions.(9) With kidney tubule dysfunction, elevated urine α1m levels indicate decreased proximal tubular reabsorptive capacity as seen after prolonged cold ischemia times (10, 11) and higher urine α1m concentrations correlate with IFTA on biopsy.(7) MCP-1 is a potent chemokine expressed by renal tubular epithelial cells, which induces recruitment of macrophages and renal interstitial fibroblasts and leads to both interstitial and mesangial fibrosis.(12) Higher urine concentrations have been associated with greater fibrosis in diabetic nephropathy and with disease progression.(13) PINP and PIIINP are cleaved from type 1 and type 3 collagen fibrils during collagen deposition, which is an important step in fibrogenesis.(14) Urine PIIINP is the N-terminal fragment of type III collagen and is released during newly deposited collagen type III and is correlated with interstitial fibrosis (11) and kidney function decline(15) in patients with CKD of different etiologies (16) and in KTRs.(11)
When evaluating risk of allograft failure, we recently showed that urine α1m and MCP-1 were strongly associated with future allograft failure, independent of eGFR, ACR, or other risk factors in FAVORIT, whereas PINP and PIIINP were not.(17) Whether or not these markers of tubular fibrosis may give insights to the link between the kidney and CVD above and beyond the classical glomerular markers of eGFR and ACR in KTRs is uncertain. We designed this study to evaluate associations between non-invasive urine markers of tubulointerstitial fibrosis and long-term CVD events and death in the FAVORIT trial. Our main goal is to provide new insights into possible pathways and mechanisms of disease supported by biomarker associations. The FAVORIT trial is uniquely positioned to address this question given the large sample size, long-term follow-up, and availability of adjudicated CVD endpoints, which were the primary outcomes of the trial. We hypothesized a priori that higher urine concentrations of each marker would be associated with risk of CVD events and death independent of CKD and CVD risk factors, baseline eGFR, and ACR.
Materials and Methods
Study Population
This study is an ancillary study of the Folic Acid for Vascular Outcomes Reduction in Transplantation (FAVORIT) Trial (clinicaltrials.gov: NCT00064753), a multi-center double-blind randomized controlled trial to determine whether lowering homocysteine levels with vitamin therapy reduced CVD events in stable KTRs. The FAVORIT trial protocol was approved by the Institutional Review Boards at the participating institutions and all participants provided written informed consent. The trial design and primary results have been described elsewhere.(18-21) Briefly, between August 2002 and January 2007, 4,110 KTRs aged 35 to 75 years who were at least 6 months post-kidney transplant were enrolled at 30 transplant centers in the United States, Canada, and Brazil. Participants were randomized to either a standard multivitamin with high doses of folic acid, vitamin B6 and B12 or a multivitamin containing no folic acid and low doses of vitamin B6 and vitamin B12. Entry criteria included elevated serum homocysteine level (≥ 11 μmol/L for women; ≥ 12 μmol/L for men) and stable kidney function, defined by an estimated creatinine clearance ≥ 30 mL/min in men and ≥ 25 mL/min in women. Follow-up contacts occurred every six months through January 31, 2010 to obtain study related outcomes through June 24, 2009. The primary outcome was pooled incident or recurrent CVD events. As reported previously, there was no significant difference between treatment groups for primary or secondary outcomes.(20)
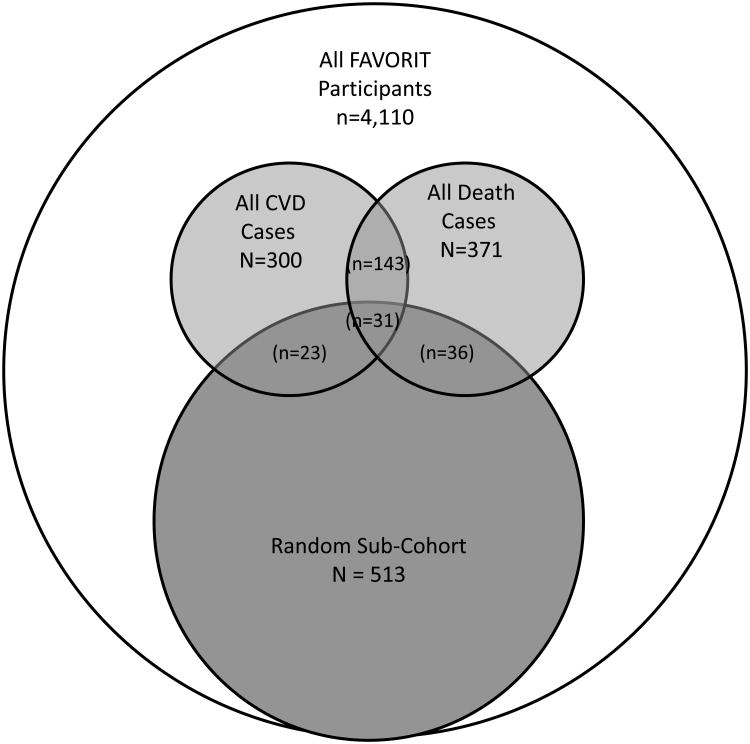
We designed this analysis as a case-cohort study to minimize specimen needs and expense while retaining statistical power. Per standard case-cohort design, members of the sub-cohort were selected at random irrespective of whether or not they experienced CVD or death during follow-up. Only individuals with adjudicated events (22) were included, for a total of 319 CVD cases and 405 deaths. We selected a random sub-cohort of 513 participants in coordination with a prior FAVORIT analysis that had measured urine injury biomarkers in the same subsample.(23) After excluding participants who were missing urine samples (N=53) or key covariates (N=51) at baseline, the final study sample consisted of 513 participants in the random sub-cohort, 300 individuals who experienced CVD events and 371 who died. 23 CVD events and 36 deaths occurred among members of the sub-cohort (with 31 participants experiencing both). Among the cases there were 143 who had both CVD events and death during follow-up, resulting in a final analytic sample of 759 for the analysis of CVD events and 817 for the analysis of death (Figure 1).
Figure 1.
Venn Diagram Describing Sampling Strategy.
Urine Markers of Fibrosis
Urine α1m, MCP-1, PINP, and PIIINP were measured at the University of Vermont in spot urine samples obtained at the baseline study visit, which had been stored at -80°C until measurement. Specimens had been thawed once previously for measurement of urine injury biomarkers including neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule (KIM)-1, interleukin (IL)-18 and liver-type fatty acid binding protein (L-FABP).(23) There was no variability in freeze-thaw cycles among the samples. All measurements were performed in 2015. To improve precision, we measured each fibrosis marker twice in each urine specimen and averaged results. Urine α1m was measured on a Siemens BNII nephelometer, Munich, Germany. The lower limit of detection was 0.5 mg/dL, and our estimates of the inter-assay coefficients of variation (CVs) ranged from 1.87 to 5.03%. Urine MCP-1 was measured using an ELISA (R&D Systems, Minneapolis, MN) after diluting urine samples 1:2. The acceptable analytic range was between 2 and 4000 pg/mL, and inter-assay CVs were between 5.9 and 9.2% across the analytic range. Urine PINP was measured by a radio-immunoassay (RIA) from ORION Diagnostica (Espoo, Finland). The lower limit of detection was 0.1 mcg/L and inter-assay CVs ranged from 6.8 to 9.2%. Similarly, we used a RIA (ORION Diagnostica, Espoo, Finland) to measure urine PIIINP.(15) The lower limit of detection was 0.02 mcg/L and inter-assay CVs ranged from 11.0 to 16.3%. When urine samples were assayed but the biomarker concentration was found to be below the detectable range, we imputed the lower limit of detection. Among the 4 urine fibrosis biomarkers, 13.3% (n=145) had α1m levels below the detectable range. Corresponding numbers for urine MCP-1, PINP and PIIINP were 0.5% (n=5), 11.4% (n=124), and 2.4% (n=26) respectively.
Outcomes
The primary endpoint of the FAVORIT trial was CVD events, which was adjudicated by the FAVORIT clinical endpoints committee. Events were defined as a composite of CVD death, myocardial infarction, resuscitated sudden death, and stroke.(24) Death was identified by review of medical records, regular participant contact, and contact with family. Time to event was considered from randomization to CVD event, death, last follow-up visit, or end of the study period.
Other Measurements
Demographics (age, sex, race, country of origin); smoking status (current, former or never); past medical history (CVD, diabetes mellitus); transplant characteristics (living donor kidney, time since transplant [“vintage”]); physical examination findings (body mass index [BMI], systolic and diastolic blood pressure); and standard laboratory measurements including serum creatinine and urine ACR, which were obtained at time of study enrollment. Race was recorded as white, black, or other. Baseline blood pressure was the average of two measurements. Diabetes was defined by the use of insulin, oral hypoglycemic medications, or participant self-report. Prior history of CVD was determined by self-report at baseline and included prior myocardial infarction, coronary artery revascularization, stroke, carotid arterial revascularization, abdominal or thoracic aortic aneurysm repair, and/or lower extremity arterial revascularization or amputation above the ankle. BMI was calculated using the formula: weight [kg]/ height [m]2. Serum creatinine was measured using an alkaline picrate kinetic method on an Olympus AU 400e (Olympus America Inc., Center Valley, PA) instrument that was calibrated to an isotope dilution mass spectrometry traceable standard, and was used to determine the eGFR value using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) 2009 equation.(25) Urine albumin and creatinine were measured in spot urine samples to calculate ACR. Urine albumin was measured using an immunoturbidimetric assay. Intra-assay CV was 2% and inter-assay CV was 4%.
Statistical Methods
Baseline characteristics were assessed in the sub-cohort and the subgroup with each outcome. Within the sub-cohort, we calculated the Spearman correlation coefficients among the 4 urine fibrosis markers, eGFR, urine ACR, and the previously measured urine injury biomarkers (urine NGAL, KIM-1, IL-18 and L-FABP).
To account for the case-cohort study design, weighted Cox proportional hazards regression was used to examine the association between baseline urine fibrosis markers and time to CVD event or death.(26, 27) To provide an equal comparison across biomarkers, we evaluated each marker as a continuous independent variable. Given right skewed distributions, we transformed each on the log-base-2 scale such that coefficients could be interpreted as “per doubling” of each biomarker. We also evaluated each marker across quartiles setting the lowest quartile as the reference category. The proportions with CVD events and death in each quartile were tabulated; event rates (per 100 person years [PY]) were calculated among individuals in the sub-cohort as they represent a random sample of the overall cohort. A series of multivariable adjusted models were tested for each biomarker. Model 1 adjusted for urine creatinine (to account for urine tonicity), age, sex, race, country, and randomized treatment arm. Model 2 additionally adjusted for diabetes, systolic blood pressure, prevalent CVD, LDL and HDL cholesterol, BMI, smoking status, allograft vintage, and living or deceased donor status. Model 3 additionally adjusted for eGFR and urine albumin to allow assessment of the degree of attenuation by clinically available measures of kidney health. Model 3 was considered our final model. In exploratory analyses, we additionally adjusted for urinary NGAL, IL-18, KIM-1, and L-FABP, to determine whether each urine fibrosis biomarker was associated with outcomes independent of previously assessed markers of kidney tubule cell injury (Model 4a); we also adjusted Model 3 variables for the 3 other urine fibrosis markers simultaneously to determine unique contributions of each with outcomes (Model 4b).
In order to assess longitudinally whether changes in eGFR were a major confounder in the associations with CVD events and death, we performed sensitivity analyses using time-varying eGFR measurements. Prevalent CVD was not an exclusion criterion in FAVORIT, thus analysis of the primary endpoint in the FAVORIT trial included patients with and without prevalent CVD; we constructed our main analysis similarly. In order to evaluate the influence of prevalent CVD on our associations of interest, we conducted a sensitivity analysis excluding those with prevalent CVD at baseline for the CVD endpoint. (See Supplemental Results)
Since our predictors were moderately correlated with one another, we used a data-driven regularization method to select a parsimonious set of biomarkers using least absolute shrinkage and selection operator (LASSO) for automatic variable selection.(28) The 4 urine fibrosis markers, eGFR, urine ACR, and the previously measured urine injury biomarkers (urine NGAL, KIM-1, IL-18 and L-FABP) were entered into the LASSO analysis. This regression method penalizes the absolute size of the regression coefficients. A LASSO penalty with leave-one-out cross-validation was used to estimate the penalty parameter and results were reported using the penalty that gave the best cross-validated fit. The identified set of predictors was entered into the unadjusted and adjusted model. (See Supplemental Results)
Finally, in order to determine the relative discriminatory value of these biomarkers to predict risk of CV outcomes and mortality, we calculated the discrimination (C-statistics) and quantified each prediction model's ability to separate those who experience a specific outcome from those who do not. For comparison, we used Models 2 and 3 and an approximated Framingham model based on age, gender, smoking, LDL, HDL, SBP and DM. (The FAVORIT cohort did not assess family history of heart disease, total cholesterol or BP med use.) We also assessed calibration, which evaluates how closely the predicted outcome corresponds with the observed event.(29) We utilized Nam and D'Agostino's modified Hosmer-Lemeshow chi-square statistic.(30) As the FAVORIT trial cohort is the only one of its kind with available biomarker measurements and long-term adjudicated CVD events, we are unable to externally validate our findings. Thus, we used resampling model calibration with bootstrapping to get bias-corrected (overfitting- corrected) estimates of predicted vs. observed values based on subsetting predictions into intervals. The resampling validation which also uses bootstrapping provided bias-corrected indexes specific to each type of model (C-statistic).
All analyses were conducted using R, version 3.2.1. P values < 0.05 were considered statistically significant for all analyses.
Results
Baseline Characteristics
Among the 513 sub-cohort participants selected at random, mean age was 51 ± 9 years, 38% were women, 24% were non-white, and 32% were recruited at centers located outside the US. Mean eGFR at baseline was 46 ± 18 ml/min/1.73m2, median graft vintage was 3.9 years, 41% had received kidneys from living donors, 19% had CVD, and 37% had diabetes. As expected, the random sub-cohort had similar characteristics to the overall FAVORIT population (Supplemental Table 1). The distributions of all 4 urine fibrosis markers were right skewed, with medians (interquartile ranges [IQR]) of 1.60 [0.8-3.8] mg/dL for α1m, 183 [84-351] pg/mL for MCP-1, 2.4 [1.2-3.8] mcg/L for PINP, and 3.6 [2.1-6.2] mcg/L for PIIINP. During a median 3.46 years of follow-up, there were 300 adjudicated CVD events and 371 deaths. The median [IQR] time to outcome was 3.31 [2.24, 4.89] years for CVD and 3.43 [2.45, 4.95] years for death.
Table 1 shows the characteristics of the study population in the sub-cohort and in those sampled as cases. Compared to the random sub-cohort, those who had a CVD event during follow-up were less likely to have received a living donor kidney, more likely to have prevalent CVD, more likely to have diabetes, and had a higher SBP. Similarly, those who died were also less likely to have received a living donor kidney and more likely to have had prevalent CVD, diabetes, and higher SBP.
Table 1. Baseline Characteristics by Study Group among Kidney Transplant Recipients in FAVORIT*.
| Range | Random Sub-cohort | CVD Event cases | Death cases |
|---|---|---|---|
| N | 513 | 300 | 371 |
| Age ± SD | 51 ± 9 | 55 ± 9 | 56 ± 10 |
| Female, n (%) | 197 (38%) | 103 (34%) | 136 (37%) |
| Race | |||
| White | 386 (76%) | 226 (75%) | 269 (73%) |
| Black | 86 (17%) | 54 (18%) | 76 (21%) |
| Other | 38 (7%) | 20 (7%) | 26 (7%) |
| Treatment group | |||
| High dose vitamin | 257 (50%) | 147 (49%) | 183 (49%) |
| Low dose vitamin | 256 (50%) | 153 (51%) | 188 (51%) |
| Country, n (%) | |||
| US | 351 (68%) | 239 (80%) | 298 (80%) |
| Canada | 70 (14%) | 38 (12%) | 37 (10%) |
| Brazil | 92 (18%) | 23 (8%) | 36 (10%) |
| Graft vintage, median [IQR] | 3.90 [1.72, 7.25] | 4.47 [1.89, 7.88] | 4.42 [1.76, 7.89] |
| Living donor kidney, n (%) | 212 (41%) | 99 (33%) | 106 (29%) |
| Calcineurin inhibitor use, n (%) | 429 (88%) | 265 (91%) | 316 (88%) |
| Sirolimus use, n (%) | 53 (10%) | 25 (8%) | 41 (11%) |
| CVD at baseline, n (%) | 96 (19%) | 124 (41%) | 126 (34%) |
| Diabetes, n (%) | 189 (37%) | 193 (64%) | 216 (58%) |
| Smoking, n (%) | |||
| Never | 254 (50%) | 133 (44%) | 142 (38%) |
| Current | 61 (12%) | 41 (14%) | 53 (14%) |
| Past | 192 (37%) | 125 (42%) | 174 (47%) |
| Body mass index (kg/m2) ± SD | 29.0 ± 5.9 | 29.7 ± 6.4 | 29.5 ± 6.4 |
| SBP (mmHg) ± SD | 136 ± 20 | 143 ± 21 | 141 ± 20 |
| DBP (mmHg) ± SD | 79 ± 12 | 78 ± 12 | 77 ± 12 |
| LDL cholesterol (mg/dl) ± SD | 104 ± 33 | 98 ± 37 | 99 ± 37 |
| HDL cholesterol (mg/dl) ± SD | 47 ±14 | 45 ± 14 | 45 ±15 |
| eGFR (ml/min/1.73m2) ± SD | 46 ± 18 | 44 ± 18 | 45 ± 18 |
| Urine ACR (mg/g), median [IQR] | 24.7 [9.6, 106.4] | 46.4 [13.6, 238.6] | 58.0 [15.0, 240.3] |
| Urine NGAL (ng/ml), median [IQR] | 20.2 [8.2, 50.4] | 25.0 [10.7, 60.5] | 28.8 [13.5, 80.9] |
| Urine IL 18 (pg/ml), median [IQR] | 28.8 [11.5,60.5] | 28.9 [11.2, 60.6] | 34.0 [14.0, 80.8] |
| Urine KIM-1 (pg/ml), median [IQR] | 653.0 [310.9, 1363.2] | 746.0 [353.3, 1668.7] | 941.6 [430.8, 1764.9] |
| Urine L FABP (ng/ml), median [IQR] | 6.1 [3.0, 17.6] | 7.8 [3.7, 22.6] | 8.2 [3.8, 24.7] |
| Urine α1 microglobulin (mg/dl), median [IQR] | 1.6 [0.8, 3.7] | 2.3 [1.1, 4.6] | 2.4 [1.2, 5.0] |
| Urine MCP-1 (pg/ml), median [IQR] | 178.2 [83.8, 349.2] | 218.0 [84.7, 448.5] | 234.9 [108.2, 466.8] |
| Urine PINP (mcg/L), median [IQR] | 2.3 [1.2, 3.8] | 2.4 [1.2, 4.0] | 2.4 [1.3, 4.0] |
| Urine PIIINP (mcg/L), median [IQR] | 3.5 [2.1, 6.0] | 3.8 [2.0, 6.2] | 3.7 [1.9, 6.3] |
CVD = cardiovascular disease; SBP = systolic blood pressure; DBP = diastolic blood pressure; LDL = low-density lipoprotein; HDL = high-density lipoprotein; eGFR = estimated glomerular filtration rate; ACR = albumin to creatinine ratio
Supplemental Table 2 shows the correlations of the 4 urine fibrosis markers with one another, in addition to eGFR, urine ACR, and 4 urinary tubular injury markers. We observed the strongest correlation between urine α1m and liver fatty acid binding protein (L-FABP) which is one of the urine injury biomarkers measured previously (ρ=0.77). The remainder of the correlations were weak to moderate in strength. The correlations of the four urine fibrosis markers with eGFR ranged from -0.08 to -0.25, while those with urine ACR ranged from 0.21 to 0.51.
Risk of Cardiovascular Disease Events
Table 2 shows the associations of urine fibrosis markers with risk of CVD events. In minimally adjusted linear models, each doubling (log2) in urine α1m concentration was associated with a 43% higher risk of CVD (Model 1). We also observed a graded relationship between increasing quartiles of urine α1m and CVD events. These associations remained strong and minimally altered after adjustment for CVD risk factors and comorbidities (Model 2), and for eGFR and ACR (Model 3).
Table 2. Association of Urine Fibrosis Markers with Risk of Cardiovascular Disease Events.
| Range | # at risk | # CVD Events | Event Rate (per 100 person-years) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| Urine α1 Microglobulin | ||||||||
| Per Log2 | Increase | 1.43 (1.26, 1.62) | 1.47 (1.28, 1.68) | 1.40 (1.21, 1.62) | ||||
| Q1 | < 0.79 | 177 | 56 | 1.48 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |
| Q2 | 0.79 - 1.60 | 169 | 53 | 1.86 | 1.06 (0.65, 1.70) | 0.98 (0.58, 1.67) | 0.95 (0.56, 1.63) | |
| Q3 | 1.61 - 3.78 | 217 | 99 | 2.73 | 2.25 (1.44, 3.52) | 2.17 (1.32, 3.57) | 1.98 (1.20, 3.27) | |
| Q4 | > 3.78 | 193 | 91 | 5.43 | 3.06 (1.87, 5.00) | 3.05 (1.74, 5.33) | 2.56 (1.43, 4.59) | |
| Urine MCP-1 | ||||||||
| Per Log2 | Increase | 1.32 (1.16, 1.51) | 1.32 (1.16, 1.51) | 1.18 (1.03, 1.36) | ||||
| Q1 | < 84.18 | 183 | 72 | 3.01 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |
| Q2 | 84.18 - 183.27 | 178 | 58 | 1.33 | 0.83 (0.55, 1.40) | 0.87 (0.55, 1.40) | 0.81 (0.48, 1.35) | |
| Q3 | 183.28 - 351.38 | 182 | 71 | 2.45 | 1.47 (0.89, 2.44) | 1.47 (0.89, 2.44) | 1.02 (0.57, 1.81) | |
| Q4 | ≥351.39 | 194 | 90 | 4.31 | 2.11 (1.23, 3.62) | 2.11 (1,23, 3.62) | 1.23 (0.67, 2.27) | |
| Urine PINP | ||||||||
| Per Log2 | Increase | 1.11 (1.03, 1.20) | 1.15 (1.06, 1.25) | 1.13 (1.03, 1.23) | ||||
| Q1 | <1.25 | 199 | 76 | 1.26 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |
| Q2 | 1.25 - 2.36 | 192 | 73 | 2.03 | 1.03 (0.67, 1.58) | 1.48 (0.90, 2.45) | 1.48 (0.89, 2.44) | |
| Q3 | 2.37 - 3.83 | 183 | 67 | 2.21 | 1.33 (0.85, 2.07) | 1.91 (1.14, 3.21) | 1.82 (1.07, 3.09) | |
| Q4 | ≥ 3.83 | 185 | 84 | 5.99 | 2.04 (1.31, 3.17) | 2.68 (1.63, 4.41) | 2.27 (1.35, 3.80) | |
| Urine PIIINP | ||||||||
| Per Log2 | Increase | 1.11 (1.01, 1.22) | 1.07 (0.97, 1.19) | 1.05 (0.95, 1.16) | ||||
| Q1 | < 2.10 | 206 | 83 | 1.43 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | |
| Q2 | 2.10-3.63 | 181 | 60 | 1.96 | 0.91 (0.58, 1.41) | 0.88 (0.54, 1.43) | 0.89 (0.55, 1.44) | |
| Q3 | 3.64 - 6.15 | 191 | 80 | 2.99 | 1.44 (0.93, 2.23) | 1.33 (0.82, 2.14) | 1.26 (0.79, 2.03) | |
| Q4 | > 6.15 | 197 | 76 | 4.99 | 1.57 (0.98, 2.51) | 1.20 (0.71, 2.04) | 1.02 (0.59, 1.74) |
Model 1: Adjusted for urine creatinine, age, sex, race, country, randomized treatment.
Model 2: above plus diabetes, SBP, CVD, LDL, HDL, BMI, smoking, graft vintage, living donor.
Model 3: plus eGFR and urine albumin
Urine MCP-1 concentrations were also significantly associated with CVD events. In models adjusted for demographics, each doubling of MCP-1 was associated with approximately 32% higher risk of CVD events. The association of MCP-1 was moderately attenuated but retained significance after adjustment for CVD risk factors and kidney function.
Urine PINP was also strongly associated with CVD events in all models. In the linear analysis, each doubling of urine PINP was associated with a 13% higher risk of CVD after adjustment for comorbidities and kidney function, and associations were graded with ascending PINP quartiles in the fully adjusted model. In contrast, urine PIIINP was not associated with CVD events in any model, in either linear analyses or by quartiles.
To provide a frame of reference for the strengths of the adjusted associations, we compared the highest quartile of each biomarker with that of the lowest quartiles of eGFR and highest quartile of ACR (i.e. those representing worst kidney function) in the final model (Figure 2a). The point estimates for the association of the highest quartiles of α1m and PINP were comparable in strength to those of baseline eGFR and ACR, whereas MCP-1 had a weaker association.
Figure 2.
(A) Associations of biomarkers with CVD events. (B) Associations of biomarkers with death.
Risk of Death
We next evaluated the associations of the four urine fibrosis biomarkers with risk of death (Table 3). In linear models, each doubling of urine α1m was associated with a 50% higher risk of death after demographic adjustment, which remained similar when adjusted for CVD risk factors (Model 2). After additional adjustment for eGFR and urine albumin, α1m remained associated with 51% higher death risk. The association also appeared strong and graded across α1m quartiles.
Table 3. Association of Urine Fibrosis Markers with Risk of Mortality.
| Range | # at risk | # Death Events | Event Rate (per 100 person-years) | Model 1 HR (95% CI) | Model 2 HR (95% CI) | Model 3 HR (95% CI) | |||
|---|---|---|---|---|---|---|---|---|---|
| Urine α1 Microglobulin | |||||||||
| Per Log2 | Increase | 1.50 (1.33, 1.69) | 1.55 (1.37, 1.76) | 1.51 (1.32, 1.72) | |||||
| Q1 | < 0.79 | 184 | 66 | 1.99 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| Q2 | 0.79 - 1.60 | 177 | 62 | 1.97 | 1.04 (0.65, 1.67) | 0.96 (0.58, 1.60) | 0.90 (0.54, 1.51) | ||
| Q3 | 1.61 - 3.78 | 221 | 111 | 4.16 | 2.29 (1.47, 3.57) | 2.32 (1.46, 3.70) | 2.16 (1.35, 3.46) | ||
| Q4 | > 3.78 | 231 | 129 | 5.07 | 3.53 (2.21, 5.64) | 3.58 (2.14, 6.01) | 3.19 (1.86, 5.46) | ||
| Urine MCP-1 | |||||||||
| Per Log2 | Increase | 1.46 (1.28, 1.66) | 1.39 (1.22, 1.60) | 1.31 (1.13, 1.51) | |||||
| Q1 | < 84.18 | 183 | 77 | 3.88 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| Q2 | 84.18 - 183.27 | 188 | 69 | 1.45 | 0.96 (0.60, 1.52) | 0.88 (0.54, 1.44) | 0.86 (0.53, 1.40) | ||
| Q3 | 183.28 - 351.38 | 197 | 86 | 2.32 | 1.63 (0.99, 2.68) | 1.28 (0.75, 2.19) | 1.17 (0.68, 2.01) | ||
| Q4 | > 351.39 | 223 | 125 | 5.07 | 2.86 (1.68, 4.89) | 2.40 (1.36, 4.23) | 1.96 (1.08, 3.55) | ||
| Urine PINP | |||||||||
| Per Log2 | Increase | 1.11 (1.03, 1.19) | 1.14 (1.05, 1.23) | 1.11 (1.03, 1.20) | |||||
| Q1 | <1.25 | 203 | 90 | 2.96 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| Q2 | 1.25 - 2.36 | 212 | 94 | 2.13 | 1.06 (0.70, 1.61) | 1.12 (0.71, 1.76) | 1.09 (0.70, 1.72) | ||
| Q3 | 2.37 - 3.83 | 198 | 84 | 2.49 | 1.28 (0.83, 1.97) | 1.38 (0.87, 2.20) | 1.29 (0.81, 2.07) | ||
| Q4 | > 3.83 | 204 | 103 | 5.69 | 2.03 (1.32, 3.12) | 2.45 (1.58, 3.82) | 2.05 (1.30, 3.23) | ||
| Urine PIIINP | |||||||||
| Per Log2 | Increase | 1.10 (1.01, 1.21) | 1.11 (1.01, 1.22) | 1.09 (0.99, 1.20) | |||||
| Q1 | <2.10 | 215 | 99 | 2.59 | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||
| Q2 | 2.10 - 3.63 | 200 | 84 | 2.85 | 1.00 (0.65, 1.53) | 1.17 (0.75, 1.83) | 1.16 (0.74, 1.81) | ||
| Q3 | 3.64 - 6.15 | 198 | 89 | 3.26 | 1.26 (0.82, 1.93) | 1.27 (0.80, 2.03) | 1.20 (0.76, 1.91) | ||
| Q4 | > 6.15 | 202 | 98 | 4.43 | 1.50 (0.96, 2.34) | 1.64 (1.02, 2.64) | 1.40 (0.87, 2.27) |
Model 1: Adjusted for urine creatinine, age, sex, race, country, randomized treatment.
Model 2: above plus diabetes, SBP, CVD, LDL, HDL, BMI, smoking, graft vintage, living donor.
Model 3: plus eGFR and urine albumin
The association between urine MCP-1 and death was also significant in the demographic-adjusted model (Model 1), but was more strongly attenuated with adjustment for CVD risk factors, eGFR, and urine albumin (Table 3). Nonetheless, MCP-1 remained significantly associated with death in the final model.
Urine PINP was also associated with death across the sequence of adjusted models, although the association was weaker relative to α1m and MCP-1. Each doubling of PINP was associated with 11% higher risk of death in the final model (Table 3). Across quartiles, the association appeared relatively flat across quartiles 1 to 3 and increased substantially in quartile 4. Finally, each doubling of urine PIIINP was associated with a 9% higher death risk, which was not quite statistically significant in the final model (p=0.051).
We again compared strengths of adjusted associations of the highest quartile of each fibrosis biomarker relative to those of eGFR and urine ACR for the death outcome (Figure 2b). The associations of α1m, MCP-1, and PINP were all stronger than that of eGFR and comparable to that of ACR, despite being adjusted for eGFR and ACR in these models.
Discriminatory ability of biomarkers
To assess the predictive value of the biomarkers, we calculated C-statistics. These revealed discriminatory ability of all biomarkers together, relative to the Framingham score for the CVD outcome (C-statistic 0.724 [95% CI 0.695, 0.753] compared to 0.700 [95% CI 0.669, 0.729]) (Supplemental Table 3). The inclusion of all four biomarkers also resulted in significant change in discriminatory ability for the outcome of mortality relative to Framingham (C-statistic 0.698 [0.669, 0.727] compared to 0.677 [0.648, 0.706]). The calibration chi-square indicated good calibration for all models (p> 0.05) (Supplemental Table 3).
Discussion
Among stable kidney transplant recipients, higher urine concentrations of several proteins that indicate tubulointerstitial fibrosis, including urine α1m, MCP-1 and PINP, are strongly and independently associated with CVD events and death. These associations are independent of eGFR, ACR, and traditional CKD and CVD risk factors. Associations of all 3 biomarkers with either end-point were stronger than those of eGFR and comparable in strength to those of ACR.
To our knowledge, this is the first study to evaluate associations of urine fibrosis biomarkers with non-kidney endpoints in KTRs. We have recently shown that higher urine α1m and MCP-1 are also strongly associated with risk of kidney allograft failure in FAVORIT.(17) In a prior FAVORIT analysis evaluating the kidney tubule injury biomarkers (NGAL, KIM-1, IL-18 and LFABP), only urine NGAL was associated CVD events and death, while KIM-1 and IL-18 were the only markers associated with death.(31) The associations we describe here, using fibrosis markers, were notably stronger relative to NGAL in fully adjusted models. For example, the association of the highest quartile of urine NGAL was 1.79 (hazard ratio; 95% CI 0.95 to 3.34) for CVD events and 3.12 (1.73 to 5.64) for death in FAVORIT, whereas those of α1m were 2.56 (1.43, 4.59) and 3.19 (1.86, 5.46) in our study using similarly adjusted models. Thus, whether comparing to NGAL, eGFR, or urine ACR, the urine fibrosis markers consistently had strong associations with CVD and death in this study. As these associations remained strong even after adjustment for eGFR and ACR, they may give insight into kidney tubule health above and beyond clinically available measures of kidney function and may have utility to identify KTRs at higher risk of both kidney (17) and cardiovascular end-points.
Why would urine concentrations of fibrosis markers be so strongly associated with CVD events and death in KTRs? The mechanisms are uncertain, but we hypothesize that induction of fibrosis in the kidney may be indicative of broader, systemic fibrotic processes that may also involve the vascular system contributing to CVD. Indeed, vascular diseases including hypertension (32) and calcineurin inhibitor induced vasoconstriction and ischemia(33) are strongly associated with tubulointerstitial fibrosis on kidney biopsy. Thus common pathways of vascular disease may simultaneously promote fibrosis, CVD events, and kidney disease progression, which were captured in our study by higher concentrations of fibrosis markers in the urine. Alternatively, these biomarkers may be indicative of more severe kidney disease that is not measured by eGFR and ACR, which in turn may promote CVD. Finally, tubulointerstitial disease may reflect defects in vitamin D metabolism,(34) erythropoietin production,(35) and acid-base regulation,(36) which may all play a role in promoting CVD and death risk.
Prior studies using α1m, MCP-1, and PIIINP in KTRs have primarily focused on kidney biopsy findings, where higher urine concentrations of each have been associated with greater tubulointerstitial fibrosis.(11, 37, 38) In contrast, to our knowledge PINP has not previously been studied in KTR populations. Like PIIINP, type 1 collagen is abundantly expressed in renal fibrosis. There is a growing body of literature demonstrating that kidney tubule health can be measured non-invasively and can provide insights about CKD progression, above and beyond “glomerular” markers of kidney health (eGFR and ACR).(15) Evidence is accumulating that markers of tubular health may provide information about risk of non-kidney-health outcomes as well. Ischemia associated with kidney transplantation induces both tubular and glomerular injury, but glomerular structures recover more quickly while tubular dysfunction persists.(9) Injury to the tubules may thus represent a more subtle but prolonged disease process. Multiple factors in KTRs including BK nephropathy, chronic allograft nephropathy, and drug toxicity primarily induce tubular rather than glomerular injury. The ability to capture abnormal tubular function that persists irrespective of glomerular injury may be part of the reason that α1m is the most sensitive of our biomarkers.(9) Another important reason may be due to its stability and precision in measurement.(39, 40) Similarly, MCP-1 is secreted by proximal tubular cells (41) and may play a role in systemic disease given its role as a chemokine upregulated in response to reactive oxygen species.
PINP and PIIINP are generated in the interstitium between adjacent renal tubules during collagen deposition. Thus, they mark tubulointerstitial fibrosis rather than tubule function per se, and this may be one reason for different associations with outcomes observed in this study. Urine PINP was also associated with CVD and death, while PIIINP was not. Neither PINP nor PIIINP were associated with allograft loss in our previous study in KTRs.(17) This may reflect the fact that pro-collagen processes occur during a finite time period only.
Strengths of our study include the large sample of KTRs at least 6 months post transplantation from multiple centers across the Americas. As the study population comprised participants in a clinical trial with CVD as its primary endpoint, there were well-adjudicated CVD and death events and reasonably long follow-up, providing substantial statistical power. We also had excellent characterization of baseline glomerular function, ACR, and CVD risk factors. Although the study was not designed to address discriminatory ability of biomarkers, we demonstrated with the use of the C-statistic that the use of the four biomarkers significantly improved prediction of both CVD and mortality. As expected, though, this association was not as strong in the internally validated subset of the cohort. We lack data from an external validation cohort, thus were unable to validate our findings. This study also has important limitations. We measured the urine fibrosis markers at one time point only on spot urine samples collected at baseline, and kidney biopsy pathologic data, pre-transplant dialysis vintage, HLA status, and auto-antibody status are not available. Also, blood levels of these biomarkers or other systemic markers of fibrosis are not available, although our previous work evaluating urine markers of fibrosis also measured plasma PIIINP and this had no substantial effect on models adjusted for other cardiovascular comorbidities and confounders.(15) As with any observational study, we cannot exclude the possibility of residual confounding, although we believe that the strengths of association and the consistency of findings across multiple biomarkers all measuring tubulointerstitial fibrosis makes this less likely.
We demonstrate for the first time that urine markers of tubulointerstitial fibrosis are strongly and independently associated with CVD events and death risk in stable KTRs, independent of eGFR and urine ACR. Collecting urine for clinical measurements is less invasive than performing kidney biopsies and may facilitate repeating measurements in individual patients to determine trajectories of risk and/or responses to changes in treatment. If these findings are confirmed, measurement of α1m, MCP-1, and PINP may provide an opportunity to monitor KTRs serially and non-invasively and to identify those at higher risk of CVD events, in whom closer surveillance and targeted CVD prevention therapies may be warranted.
Supplementary Material
Table S1: Comparison of Characteristics in the Random Sub-cohort versus the Original FAVORIT Cohort
Table S2. Spearman Correlation Matrix of Urine Fibrosis Markers, eGFR, urine ACR, and Urine Injury Markers in FAVORIT†
Table S3: Model Discrimination and Calibration for CVD and All-cause Mortality
Table S4. Association of Urine Fibrosis Markers with Risk of Cardiovascular Disease Events - excluding prevalent CVD (sensitivity analysis) (N=545)
Table S5: Association of Urine Fibrosis Markers with Risk of Cardiovascular Disease and Mortality Events After Adjustment for Tubular Injury Biomarkers
Table S6: LASSO Analysis
Table S7: Association of Urine Fibrosis Markers with Risk of Cardiovascular Disease and Mortality Events using eGFR as a Time-Dependent Covariate
Acknowledgments
We thank the participants in the FAVORIT trial and their families.
This work was supported by Meyeon Park's NIH National Institute of Diabetes and Digestive and Kidney Diseases K23 DK099238 and Doris Duke Charitable Foundation Clinical Scientist Development Award (P0506533) and by an American Heart Association (AHA) Established Investigator Award (EIA 18560026 to JHI), and grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R01 DK098234 to JHI and MGS and K24 DK110427 to JHI). The biomarker measurements were funded by the AHA. The FAVORIT Trial was supported by cooperative agreement U01 DK61700 from the NIDDK (to AB), and by the Office of Dietary Supplements at the National Institutes of Health.
Abbreviations
- α1m
alpha 1 microglobulin
- ACR
albumin to creatine ratio
- BMI
body mass index
- CKD
chronic Kidney Disease
- CKD-EPI
chronic kidney disease epidemiology collaboration
- CVD
cardiovascular disease
- CVs
coefficients of variation
- eGFR
estimated glomerular filtration rate
- FAVORIT
Folic Acid for Vascular Outcomes Reduction in Transplantation
- HR
hazard ratio
- IL-18
interleukin 18
- KIM-1
kidney injury molecule 1
- KTR
kidney transplant recipient
- LASSO
least absolute shrinkage and selection operator
- L-FABP
liver-type fatty acid binding protein
- MCP-1
monocyte chemoattractant protein-1
- NGAL
neutrophil gelatinase-associated lipocalin
- RIA
radio-immunoassay
- PINP
procollagen type I
- PIIINP
type III N-terminal amino peptide
- PY
person year
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Supporting Information: Additional Supporting Information may be found in the online version of this article.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Chakkera HA, Roel J. Explained and Unexplained Ischemic Heart Disease Risk after Renal Transplantation. Journal of the American Society of Nephrology. 2000;11(9):1735–43. doi: 10.1681/ASN.V1191735. [DOI] [PubMed] [Google Scholar]
- 3.Briggs JD. Causes of death after renal transplantation. Nephrology Dialysis Transplantation. 2001;16(8):1545–9. doi: 10.1093/ndt/16.8.1545. [DOI] [PubMed] [Google Scholar]
- 4.Halimi JM, Buchler M, Al-Najjar A, Laouad I, Chatelet V, Marliere JF, et al. Urinary albumin excretion and the risk of graft loss and death in proteinuric and non-proteinuric renal transplant recipients. Am J Transplant. 2007;7(3):618–25. doi: 10.1111/j.1600-6143.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- 5.Abbott KC, Yuan CM, Taylor AJ, Cruess DF, Agodoa LYC. Early Renal Insufficiency and Hospitalized Heart Disease after Renal Transplantation in the Era of Modern Immunosuppression. Journal of the American Society of Nephrology. 2003;14(9):2358–65. doi: 10.1097/01.asn.0000083008.25305.67. [DOI] [PubMed] [Google Scholar]
- 6.Weiner DE, Carpenter MA, Levey AS, Ivanova A, Cole EH, Hunsicker L, et al. Kidney Function and Risk of Cardiovascular Disease and Mortality in Kidney Transplant Recipients: The FAVORIT Trial. American Journal of Transplantation. 2012;12(9):2437–45. doi: 10.1111/j.1600-6143.2012.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amer H, Lieske JC, Rule AD, Kremers WK, Larson TS, Franco Palacios CR, et al. Urine high and low molecular weight proteins one-year post-kidney transplant: relationship to histology and graft survival. Am J Transplant. 2013;13(3):676–84. doi: 10.1111/ajt.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, et al. The Association Between Age and Nephrosclerosis on Renal Biopsy Among Healthy Adults. Annals of Internal Medicine. 2010;152(9):561–7. doi: 10.1059/0003-4819-152-9-201005040-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akerstrom B, Logdberg L, Berggard T, Osmark P, Lindqvist A. alpha(1)-Microglobulin: a yellow-brown lipocalin. Biochimica et biophysica acta. 2000;1482(1-2):172–84. doi: 10.1016/s0167-4838(00)00157-6. [DOI] [PubMed] [Google Scholar]
- 10.Weber MH, Verwiebe R. Alpha 1-microglobulin (protein HC): features of a promising indicator of proximal tubular dysfunction. European journal of clinical chemistry and clinical biochemistry : journal of the Forum of European Clinical Chemistry Societies. 1992;30(10):683–91. [PubMed] [Google Scholar]
- 11.Teppo AM, Tornroth T, Honkanen E, Gronhagen-Riska C. Urinary amino-terminal propeptide of type III procollagen (PIIINP) as a marker of interstitial fibrosis in renal transplant recipients. Transplantation. 2003;75(12):2113–9. doi: 10.1097/01.TP.0000066809.60389.48. [DOI] [PubMed] [Google Scholar]
- 12.Tesch GH. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. American journal of physiology Renal physiology. 2008;294(4):F697–701. doi: 10.1152/ajprenal.00016.2008. [DOI] [PubMed] [Google Scholar]
- 13.Nadkarni GN, Rao V, Ismail-Beigi F, Fonseca VA, Shah SV, Simonson MS, et al. Association of Urinary Biomarkers of Inflammation, Injury, and Fibrosis with Renal Function Decline: The ACCORD Trial. Clin J Am Soc Nephrol. 2016 doi: 10.2215/CJN.12051115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boor P, Floege J. Renal allograft fibrosis: biology and therapeutic targets. Am J Transplant. 2015;15(4):863–86. doi: 10.1111/ajt.13180. [DOI] [PubMed] [Google Scholar]
- 15.Ix JH, Biggs ML, Mukamal K, Djousse L, Siscovick D, Tracy R, et al. Urine Collagen Fragments and CKD Progression-The Cardiovascular Health Study. J Am Soc Nephrol. 2015;26(10):2494–503. doi: 10.1681/ASN.2014070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghoul BE, Squalli T, Servais A, Elie C, Meas-Yedid V, Trivint C, et al. Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist. Clin J Am Soc Nephrol. 2010;5(2):205–10. doi: 10.2215/CJN.06610909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ix JH, Katz R, Bansal N, Foster M, Weiner DE, Tracy R, et al. Urine Fibrosis Markers and Risk of Allograft Failure in Kidney Transplant Recipients: A Case-Cohort Ancillary Study of the FAVORIT Trial. Am J Kidney Dis. 2016 doi: 10.1053/j.ajkd.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bostom AG, Carpenter MA, Hunsicker L, Jacques PF, Kusek JW, Levey AS, et al. Baseline characteristics of participants in the Folic Acid for Vascular Outcome Reduction in Transplantation (FAVORIT) Trial. Am J Kidney Dis. 2009;53(1):121–8. doi: 10.1053/j.ajkd.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Pfeffer MA, Levey AS, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J. 2006;152(3):448 e1–7. doi: 10.1016/j.ahj.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Bostom AG, Carpenter MA, Kusek JW, Levey AS, Hunsicker L, Pfeffer MA, et al. Homocysteine- lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation. 2011;123(16):1763–70. doi: 10.1161/CIRCULATIONAHA.110.000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiner DE, Carpenter MA, Levey AS, Ivanova A, Cole EH, Hunsicker L, et al. Kidney function and risk of cardiovascular disease and mortality in kidney transplant recipients: the FAVORIT trial. Am J Transplant. 2012;12(9):2437–45. doi: 10.1111/j.1600-6143.2012.04101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarolim P, Claggett BL, Conrad MJ, Carpenter MA, Ivanova A, Bostom AG, et al. B-Type Natriuretic Peptide and Cardiac Troponin I Are Associated With Adverse Outcomes in Stable Kidney Transplant Recipients. Transplantation. 2017;101(1):182–90. doi: 10.1097/TP.0000000000001080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bansal N, Carpenter MA, Weiner DE, Levey AS, Pfeffer M, Kusek JW, et al. Urine Injury Biomarkers and Risk of Adverse Outcomes in Recipients of Prevalent Kidney Transplants: The Folic Acid for Vascular Outcome Reduction in Transplantation Trial. J Am Soc Nephrol. 2016;27(7):2109–21. doi: 10.1681/ASN.2015030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bostom AG, Carpenter MA, Kusek JW, Hunsicker LG, Pfeffer MA, Levey AS, et al. Rationale and design of the Folic Acid for Vascular Outcome Reduction In Transplantation (FAVORIT) trial. Am Heart J. 2006;152(3):448 e1–7. doi: 10.1016/j.ahj.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50(4):1064–72. [PubMed] [Google Scholar]
- 27.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. Journal of clinical epidemiology. 1999;52(12):1165–72. doi: 10.1016/s0895-4356(99)00102-x. [DOI] [PubMed] [Google Scholar]
- 28.Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16(4):385–95. doi: 10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Borra SDCA. Measuring the prediction error: a comparison of cross-validation, bootstrap and covariance penalty methods. Computational Statistics and Data Analysis. 2010;54(12):2976–89. [Google Scholar]
- 30.D'Agostino RB, Nam BH. Evaluation of the performance of survival models: discrimination and calibration measures. Handbook of Statistics. 2004;23:1–25. [Google Scholar]
- 31.Bansal N, Carpenter MA, Weiner DE, Levey AS, Pfeffer M, Kusek JW, et al. Urine Injury Biomarkers and Risk of Adverse Outcomes in Recipients of Prevalent Kidney Transplants: The Folic Acid for Vascular Outcome Reduction in Transplantation Trial. Journal of the American Society of Nephrology. 2015 doi: 10.1681/ASN.2015030292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zucchelli P, Zuccalá A. Progression of renal failure and hypertensive nephrosclerosis. Kidney International. 1998;54(68):S55–S9. doi: 10.1046/j.1523-1755.1998.06814.x. [DOI] [PubMed] [Google Scholar]
- 33.Divella C, Rossini M, Loverre A, Schena A, Maiorano A, Gesualdo V, et al. Immunohistochemical characterization of glomerular and tubulointerstitial infiltrates in renal transplant patients with chronic allograft dysfunction. Nephrology Dialysis Transplantation. 2010;25(12):4071–7. doi: 10.1093/ndt/gfq377. [DOI] [PubMed] [Google Scholar]
- 34.Kusunoki Y, Matsui I, Hamano T, Shimomura A, Mori D, Yonemoto S, et al. Excess 25-hydroxyvitamin D3 exacerbates tubulointerstitial injury in mice by modulating macrophage phenotype. Kidney Int. 2015;88(5):1013–29. doi: 10.1038/ki.2015.210. [DOI] [PubMed] [Google Scholar]
- 35.Hu L, Yang C, Zhao T, Xu M, Tang Q, Yang B, et al. Erythropoietin ameliorates renal ischemia and reperfusion injury via inhibiting tubulointerstitial inflammation. The Journal of surgical research. 2012;176(1):260–6. doi: 10.1016/j.jss.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 36.Nath KA, Hostetter MK, Hostetter TH. Pathophysiology of chronic tubulo-interstitial disease in rats. Interactions of dietary acid load, ammonia, and complement component C3. J Clin Invest. 1985;76(2):667–75. doi: 10.1172/JCI112020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teppo AM, Honkanen E, Finne P, Tornroth T, Gronhagen-Riska C. Increased urinary excretion of alpha1-microglobulin at 6 months after transplantation is associated with urinary excretion of transforming growth factor-beta1 and indicates poor long-term renal outcome. Transplantation. 2004;78(5):719–24. doi: 10.1097/01.tp.0000131816.51366.6b. [DOI] [PubMed] [Google Scholar]
- 38.Cornec-Le Gall E, Audrezet MP, Chen JM, Hourmant M, Morin MP, Perrichot R, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24(6):1006–13. doi: 10.1681/ASN.2012070650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kido T, Honda R, Yamada Y, Tsuritani I, Ishizaki M, Nogawa K. alpha 1-Microglobulin determination in urine for the early detection of renal tubular dysfunctions caused by exposure to cadmium. Toxicology letters. 1985;24(2-3):195–201. doi: 10.1016/0378-4274(85)90057-8. [DOI] [PubMed] [Google Scholar]
- 40.Donaldson MD, Chambers RE, Woolridge MW, Whicher JT. Stability of alpha 1-microglobulin, beta 2-microglobulin and retinol binding protein in urine. Clinica chimica acta; international journal of clinical chemistry. 1989;179(1):73–7. doi: 10.1016/0009-8981(89)90024-7. [DOI] [PubMed] [Google Scholar]
- 41.Prodjosudjadi W, Gerritsma JS, Klar-Mohamad N, Gerritsen AF, Bruijn JA, Daha MR, et al. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995;48(5):1477–86. doi: 10.1038/ki.1995.437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Comparison of Characteristics in the Random Sub-cohort versus the Original FAVORIT Cohort
Table S2. Spearman Correlation Matrix of Urine Fibrosis Markers, eGFR, urine ACR, and Urine Injury Markers in FAVORIT†
Table S3: Model Discrimination and Calibration for CVD and All-cause Mortality
Table S4. Association of Urine Fibrosis Markers with Risk of Cardiovascular Disease Events - excluding prevalent CVD (sensitivity analysis) (N=545)
Table S5: Association of Urine Fibrosis Markers with Risk of Cardiovascular Disease and Mortality Events After Adjustment for Tubular Injury Biomarkers
Table S6: LASSO Analysis
Table S7: Association of Urine Fibrosis Markers with Risk of Cardiovascular Disease and Mortality Events using eGFR as a Time-Dependent Covariate