Abstract
The enteric nervous system, often referred to as the second brain, is the largest assembly of neurons and glia outside the central nervous system. The enteric nervous system resides within the wall of the digestive tract and regulates local gut reflexes involved in gastrointestinal motility, blood flow and fluid transport; all these functions can be accomplished in the absence of the extrinsic innervation from the central nervous system. It is neurons and their circuitry within the enteric nervous system that govern the gut reflexes. However, it is becoming clear that enteric glial cells are also actively involved in this process through the bidirectional signaling with neurons and other cells in the gut wall. We synthesize the recently discovered modulatory roles of enteric gliotransmission in gut motility and provide our perspective for future lines of research.
Keywords: bidirectional purinergic signaling, enteric glial cells, enteric neurons, enteric nervous system, gliotransmission
Introduction
The digestive tract has both intrinsic and extrinsic innervations (reviewed in [27]). The intrinsic innervation is provided by the enteric nervous system (ENS), which resides within the gut wall [3] and can control basic gastrointestinal (GI) functions, such as motility and secretion/absorption (reviewed in [25]), in the complete absence of the extrinsic innervation [4, 85]. Consequently, the ENS has been recognized as the third division, (the other two being sympathetic and parasympathetic braches) of the autonomic nervous system [56]. Even though local ENS circuits collectively operate as an independent integration center, they normally work alongside with the extrinsic innervation, i.e., reflex loops at the level of sympathetic ganglia and the central nervous system (CNS) (reviewed in [27, 28]). The ENS is organized in two networks, myenteric (Auerbach’s) and submucosal (Meissner’s) plexuses, which in human contains ~500 million neurons, the number comparable to that of neurons in the spinal cord. The population of enteric neurons is rather diverse with approximately 20 types of functionally defined cells utilizing various transmitters common to the CNS [25]. In addition to neurons, the ENS also houses extended population of enteric glial cells (EGCs), whose number exceeds that of neurons by 6–7 times in the myenteric plexus and 1.3–1.9 times in the submucosal plexus of the human gut [32, 48]; the glia to neuron ratio, however, differs between various species. Unlike the ganglia of peripheral and autonomic nervous systems but similar to the CNS, the ENS lacks collagen and fibroblasts under normal conditions [30]. Due to the its size, complexity, structure and the ability to independently integrate information, the ENS is often called “the second brain” [33], or “the brain in the gut” [95]. Undeniably, the gut reflexes are controlled by local neuronal networks. However, relatively recent studies indicate the role of glia-neuron signaling in gut functions in health and disease (reviewed in [38, 72, 81]). In this brief review we focus on the (patho)physiological roles of enteric gliotransmission in gut motility.
Enteric glial cells: A historic perspective on this distinct and heterogeneous cell population
The concept of neuroglia was introduced in 1858 by Rudolf Virchow as a “substance… which lies between the proper nervous parts, holds them together and gives the whole its form in a greater or lesser degree” ([91], English translation in [52]). At the end of the same century, Alexandr Dogiel discovered the glial cells that reside in the intestinal wall [21], which were subsequently and temporarily classified as Schwann cells [84] due to their ability to partially separate, albeit not ensheath, nerve fibers. It was not until Giorgio Gabella that these glial cells within the gut wall were recognized as a distinct cell type [29]; subsequently, Gabella termed them enteric glial cells (EGCs) [31]. In parallel, due to their morphology (with “protoplasmic” and “fibrous” appearances) and expression of some known astrocytic markers, such as glial fibrillary acidic protein (GFAP, Figure 1A) and Ca2+ binding protein S100β [24, 50], EGCs were also temporarily considered as an astrocyte sub-type, termed “intestinal astrocytes”.
Figure 1. Enteric glial cells (EGCs) in the enteric nervous system (ENS).
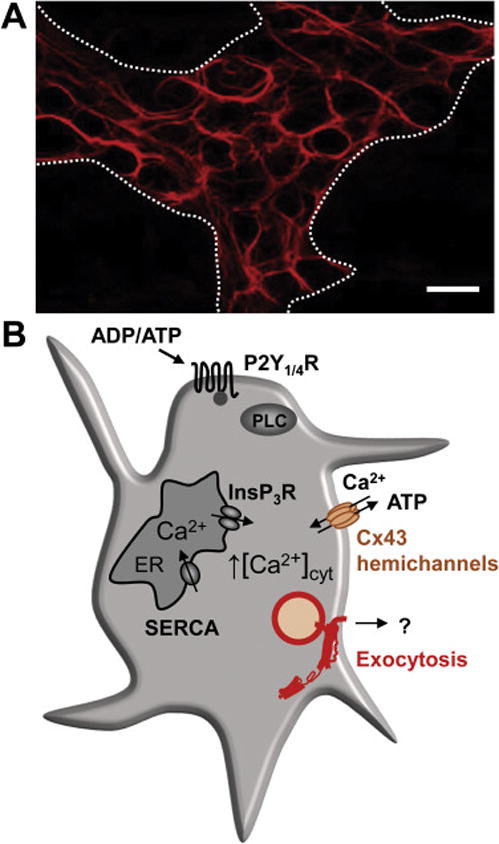
A. Expression of the EGC marker, glial fibrillary acidic protein (GFAP), in the myenteric plexus of a mouse colon. Note the close proximity between the EGCs and enteric neurons: GFAP immunoreactivity (-ir) spans across the myenteric ganglion (delineated by the dotted line) while areas within the ganglion that lack the GFAP-ir are occupied by the enteric neurons. Scale bar, 10 μm. Original unpublished image.
B. EGCs exhibit excitability based on the cytosolic Ca2+ fluctuations. Preferential binding of natural agonists, ADP or ATP, to G-protein coupled P2Y1 or P2Y4 receptors, respectively, activates phospholipase C (PLC)-mediated production of inositol 1,4,5 trisphosphate (InsP3). This second messenger induces release of Ca2+ to the cytosol from the endoplasmic reticulum (ER) upon binding to its InsP3 receptors (InsP3R). Increase in the intracellular Ca2+ concentration ([Ca2+]i) induces ATP release (and perhaps other gliotransmitters) through Cx43 hemichannels located on the plasma membrane; these channels also represent a conduit for the Ca2+ entry from the extracellular space. Increased [Ca2+]i also triggers gliotransmission via vesicular exocytosis. Store-specific Ca2+-ATPases (SERCA) pump Ca2+ to (re)fill the ER. Based on original research on enteric glia [40, 53, 64, 97].
However, there are important distinctions between EGCs of the ENS and astrocytes of the CNS. For instance, aldehyde dehydrogenase 1 family member L1, an astrocytic marker, is not expressed in EGCs [8]. Additionally, EGCs have significant transcriptional similarities not only to astrocytes, but also to oligodendrocytes and neurons of the CNS [77]. Moreover, EGCs have different embryonic being derived from the neural crest whereas astrocytes develop from the neuroectoderm. Consequently, the contemporary view is well aligned with Gabella’s ideas. Not only that EGCs are now considered as a distinct glial cell type, but are also recognized as a diverse population of cells, whose four major types are distinguished by their morphology and location within the gut wall (reviewed in [44]). Thus, (i) “protoplasmic” star-shaped cells reside in the enteric plexuses, (ii) “fibrous” elongated cells are found within fiber tracts connecting plexuses, while (iii) “intermuscular” and (iv) “mucosal” glia reflect the residential sites within the gut wall. The microbiota of the gut lumen can have a major effect on the development of the mucosal glia [51]. Nonetheless, beside specific location and variance in morphology, these EGC types differentially and variably express some of the key cell markers, such as already mentioned GFAP, S100B, as well as Sox10 [7], a neural crest transcription factor crucial for specification, maturation, and maintenance of Schwann cells and melanocytes (reviewed in [71]). Such dynamic protein expression patterns likely indicate functional diversity and plasticity of various EGCs types [7].
In summary, EGCs are juxtapositioned to enteric neurons, smooth muscle and intestinal epithelium of the gut wall, being thus strategically positioned to affect neural networks controlling gut motility and intestinal epithelial functions, as well as contribute to GI disorders (reviewed in [17, 38, 70, 72, 81]).
Cytosolic Ca2+ dynamics as a hallmark of enteric glial cell excitability
In contrast to enteric neurons capable of generating action potentials, EGCs only exhibit large “passive” currents [45] being thus electrically non-excitable cells. It was the pioneering work by Gabella which discovered “neuroglial junctions”, a specialized direct contacts between enteric neurons and glial cells [29]. These junctions represent axonal inputs (containing pre-synaptic specialization and vesicles) onto EGCs through synapse-like or synaptoid contacts lacking, however, one essential feature of synaptic contacts, the post-synaptic, i.e. EGC’s, membrane specialization. The EGS excitability is mediated by cytosolic Ca2+ signals; the first evidence showing ATP-induced increase in intracellular Ca2+ concentration of cultured EGCs [53]. Furthermore, myenteric glia in situ exhibit Ca2+ excitability after electrical field stimulation of both intrinsic, i.e originating from the ENS, and extrinsic innervation [41, 43], indicating functional neuron-to-glia signaling. It seems that EGCs, at least in the guinea pigs myenteric ganglia, predominantly respond to the sympathetic innervation [41]. Although many studies found EGCs responsive to various neurotransmitters (Table 1, see below), the main component of glial Ca2+ excitability in situ is sensitive to purinoceptor inhibitors [41, 43]. Purinergic P2Y1 and P2Y4 receptors (Table 1 and Figure 1B), both being Gq-protein coupled receptors, are the most studied in EGCs. Their activation leads to an increase in EGCs cytosolic Ca2+ concentration mediated by phospholipase C (PLC) and downstream inositol 1,4,5 trisphosphate (InsP3)-receptor signaling pathway (reviewed in [38]). Activation of InsP3-receptors located on the endomembrane of the endoplasmic reticulum (ER) leads to release of Ca2+ to the cytosol; the ER store (re)filling with Ca2+ is accomplished by ER specific Ca2+-ATPases of the SERCA (SarcoEndoplasmicReticulum ATPase) type. The P2Y1 receptor preferentially binds adenosine 5′ diphosphate (ADP), while ATP and uridine 5′ triphosphate (UTP) activate P2Y4 receptor [53]. Following neuronal and/or glial release of ATP to the extracellular space [64], this nucleotide is rapidly degraded by membrane-bound ecto-nucleosides. The triphosphate diphosphohydrolase (eNTPDase/CD39) is expressed throughout the ENS [11, 57]; eNTPDase2 (ATP is the substrate) is expressed on EGCs, while enteric neurons express eNTPDase3 (ATP and ADP are substrates) [54]. The activity of these eNTPDases underlies the extracellular production of adenosine monophosphate, which gets further hydrolyzed to adenosine by ecto-5′-nucleotidase. While adenosine A2B receptor type was detected by immunocytochemistry in the EGCs [16, 90], its functional role remains elusive. Furthermore, it is unclear whether UTP can be generated in the ENS; if so, this could occur via the exchange of Y-phosphates between adenine- and uracil-based nucleotides via nucleoside diphosphokinase (NDPK) (reviewed in [58]). It is possible that this signaling pathway gets activated during the gut infestation with parasites releasing NDPK [36].
Table 1.
Transmitter receptors on enteric glial cells. Receptor types/subunits, if determined, are parenthetically indicated. Alternatively, parentheses disclose agonists used to assess the functionality of receptors. Sensitivity of P2Y1/4 receptors to relevant natural agonists is shown parenthetically. Detection methods for the receptor expression (ICC and IHC) and/or functionality (cytosolic Ca2+ increase imaged using fluorescent indicator dyes fura-2/fluo-4, or a dose-dependent increase in the expression of specific proteins) are indicated.
| Transmitter | Receptor(subunit or agonist) | Method | Refs. |
|---|---|---|---|
| Acetylcholine | Muscarinic | fluo-4 | [6] |
| Nicotinic (α3) | fluo-4 | [12] | |
| Glutamate | mGluR5 (CHPG) | IHC/cFos & pMAPK1/2 expression | [69] |
| AMPA (GluA1 and GluA3)* | |||
| KA (GluK1)* | ICC | [93] | |
| NMDA (GluN2A, GluN2B)* | |||
| NMDA (GluN1)* | IHC | [34] | |
| Cathecholamines | Adrenergic (α2A)* | IHC | [68] |
| Nucleoside | A2B* | IHC | [90] [16] |
| Nucleotides | P2Y1 (ADP>ATP) | fluo-4 | [64] [43] [42] |
| P2Y4 (UTP≥ATP) | IHC/fluo-4 IHC ICC/fura-2 |
[43] [87] [53] |
|
| Serotonin | Not determined | fura-2 | [53] |
| 5-HT2 | fluo-4 | [6] |
Functionality of these receptors has not been demonstrated.
Abbreviations: AMPA, α-amino-3-hydroxy-5-methylisoxazole-4-propionate; ADP, adenosine 5′ diphosphate, ATP, adenosine triphosphate; CHPG, RS-2-chloro-5-hydroxyphenylglycine; ICC, immunocytochemistry; IHC, immunohistochemistry; KA, kainate; mGluR, metabotropic glutamate receptor; NMDA, N-methyl-D-aspartate; pMAPK1/2, phosphorylated form of the mitogen-activated protein kinases 1 and 2. Table is abbreviated and updated from a more comprehensive review on enteric glia [38].
Activation of muscarinic and nicotinic receptors in vitro [6] and in situ [12], respectively, along with the activation of serotonin 5-HT2 receptors in vitro [6] also leads to Ca2+ excitability in EGCs (Table 1). Activation of metabotropic glutamate receptor 5 causes a dose-dependent increase in the expression of c-Fos and the phosphorylated form of the mitogen-activated protein kinases 1 and 2 in EGCs [69]. However, expression of α2A-adrenergic and various ionotropic glutamatergic receptors in the gut wall was only detected by immunocytochemistry/immunohistochemistry [34, 68, 69, 93]; functionality and role of these enteric glial receptors is currently unknown. The additional source of Ca2+ is provided by Ca2+ entry from the extracellular space, for example through functional connexons/hemichannels composed of connexin 43 (Cx43) expressed in EGCs possess. These Cx43 hemichannels not only represent a conduit for ATP release from the EGCs cytosol to the extracellular space, mediating propagation of Ca2+ responses between EGCs in vitro [97] and in in situ [64], but also permit Ca2+ entry from the extracellular space into the cytosol of EGCs in situ [64] (Figure 1B). The pore opening of Cx43 hemichannels is regulated by Ca2+ in a biphasic fashion [20]. An initial increase in [Ca2+]i would result in cell depolarization [20] and consequently hemichannel pore opening [18]. As cytosolic Ca2+ reaches higher levels, above 0.5 μM, it decreases the pore permeability acting via Ca2+/calmodulin complexes, which directly interact with the Cx43 channel [61]. This bidirectional regulation of intracellular Ca2+ dynamics through Cx43 hemichannels could reflect the crosstalk between the two distinct modes of enteric gliotransmission, via Cx43 hemichannels and Ca2+-dependent exocytosis [40] (Figure 1B, discussed below).
Taken together, EGCs actively respond to neurotransmitters, particularly to ATP and ADP by generating Ca2+ signals. Myenteric EGCs exhibit Ca2+ responses during physiological intestinal movements, [12]. Accordingly, we next discuss bidirectional EGC to enteric neuron signaling, a heterocellular signaling pathway in the myenteric plexus, along with effects that EGCs, by their Ca2+ excitability and releasing ATP, have on the gut motility.
Enteric glia modulate motility of the gut
Enteric neuron-EGC purinergic signaling can be evoked with the neuronal P2X7 receptor (expressed on enteric neurons but not on EGCs) agonist 2′-, 3′-O-(4-benzoylbenzoyl)- ATP (BzATP). This agonist elicits robust Ca2+ responses in enteric neurons leading to cytosolic ATP release via pannexin-1 hemichannels into the extracellular space [42] (Figure 2A). Subsequently, extracellular ATP gets converted to ADP by eNTPDases. As discussed earlier, ADP activates EGCs P2Y1 receptors triggering Ca2+ signals and release of ATP via glial Cx43 hemichannels which can in turn enhance EGC Ca2+ excitability in autocrine/paracrine fashion [64, 97]. ADP-evoked Ca2+ responses from EGCs are attenuated by the mimetic peptide 43Gap26, an inhibitor of the Cx43 hemichannels (Figure 2B). The activity of Cx43 connexons in EGCs is not only important for Ca2+ excitability but is also required for physiological gut motility [64] (Figure 2C, D). Of note, key components of a reflexive gut motility patterns involve coordinated contraction and relaxation of smooth muscle proximally and distally from the reflex stimuli (for orientation see Figure 5). Interfering with glial Ca2+ signaling by inducible EGC-specific ablation of Cx43 gene reduces gut smooth muscle contractions in the ex vivo preparations of isolated colons (Figure 2C) and gut motility in vivo (Figure 2D). Electrical field stimulation of isolated mice colons that resulted in neuron-evoked muscle responses characterized by colon contractions were greatly reduced in mice with diminished expression of EGCs’ Cx43 (Figure 2C). These mice also showed an increase in colonic transit time in vivo (Figure 2D). Thus, attenuating glial Ca2+ responses by interfering with Cx43 hemichannels availability/activity weakens colonic contractions and slows colonic transit, indicating that EGCs and their Ca2+ excitability are necessary for modulation of gut motility.
Figure 2. Genetic deletion of C×43 in EGCs causes inhibition of Ca2+ signaling in EGCs and reduction of the gut motor reflexes.
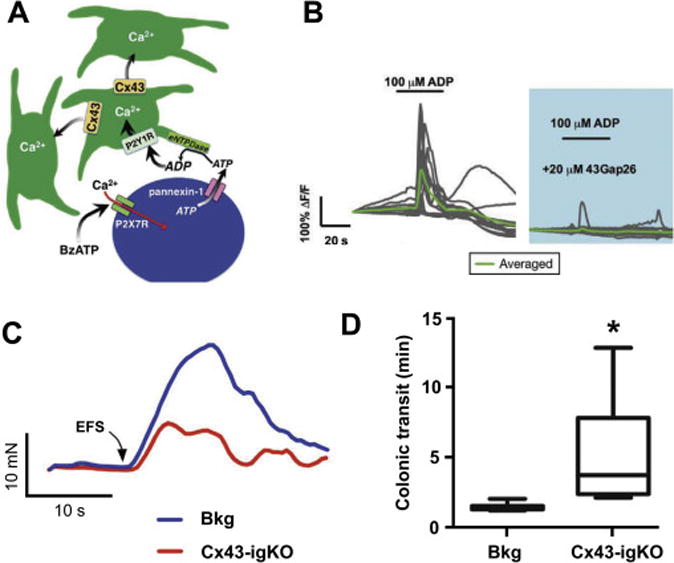
A. Model of purine-evoked Ca2+ responses through the EGC network; see text for details.
B. ADP-evoked Ca2+ responses from wild-type myenteric in situ EGCs are blocked by the connexin 43 (C×43) mimetic peptide 43Gap26, an inhibitor of the C×43 hemichannels.
C. Electrical field stimulation (EFS)-induced smooth gut muscle contractions are reduced in tamoxifen-induced glia-specific knock out of C×43 mice (C×43-igKO) and the tamoxifen treated background (Bkg) strains. Muscle relaxations were also affected (see original publication).
D. Selective genetic deletion of C×43 in EGCs increases colonic transit time, which reports on the reduction in distal colon motility in vivo. Obtained with permission for minor reformatting from [64].
Figure 5.
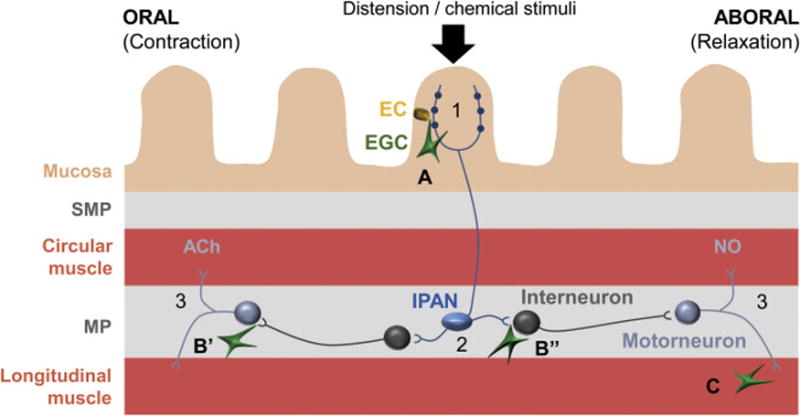
Potential sites of active EGC modulation of the enteric peristaltic reflex. The peristaltic reflex is described in detail elsewhere (reviewed in [55]) and, briefly, consists of: 1) mechanical or chemical stimulation of the intrinsic primary afferent neurons (IPANs) residing in both plexi [here shown only in myenteric plexus (MP) for clarity]; 2) IPANs activate interneurons that project both proximally and distally from the stimuli; 3) proximal (ascending) interneurons activate excitatory motoneurons that cause smooth muscle contraction by releasing acetylcholine (ACh) and neuropeptides (not shown) while distal (descending) interneurons produce relaxation by activating inhibitory motoneurons that release nitric oxide (NO), purines and other inhibitory molecules (not shown). EGC are present in all layers of the gut wall and perhaps interact with the circuit at several levels: A) at the afferent limb by modulation EC-IPAN signaling; B) integration of information in the MP by affecting the IPAN-interneuron (B’) and interneuron-motoneurons (B”) synaptic transmission; C) modulation of smooth muscle excitability by the direct interaction with the smooth muscle cells. SMP, submucosal plexus. This schematic was obtained with permission for minor reformatting from [38].
The next step was to determine whether selective stimulation of EGCs and their Ca2+ signaling are sufficient to affect gut motility (Figure 3A). This matter was addressed in experiments using Designer Receptor Exclusively Activated by Designer Drugs (DREDD) technology. Thus, GFAP::hM3Dq transgenic mice, where a fragment of human glial fibrillary acidic protein (GFAP) promoter drives expression of an engineered Gq-coupled human M3 muscarinic receptor (hM3Dq), were used [63]. Application of clozapine N-oxide (CNO), a synthetic hM3Dq agonist, increased cytosolic Ca2+ in EGCs expressing hM3Dq, but not in control wildtype EGCs (Figure 3B). Similarly, CNO caused muscle contractions in isolated mice colons; the duration and magnitude of these contractions were similar to those evoked by electrical field stimulation (Figure 3C). The effect of CNO was blocked by tetrodotoxin indicating that glia-specific effects are mediated by electrical activity of enteric neurons. Additionally GFAP::hM3Dq transgenic mice also showed decreased colonic transit time in vivo, i.e. increased gut motility, (Figure 3D). Thus, glial Ca2+ signals promote colonic contraction and accelerates transit through the gut lumen, indicating that EGCs and their Ca2+ dynamics are necessary (as seen in EGCs Cx43 ablation study) and sufficient for modulation of gut motility. The transmitter released through Cx43 hemichannels is ATP, as shown by experiments on EGCs in vitro [97] and in situ [13]. However, a variety of other transmitters could be additionally released through the Cx43 hemichannel pore (reviewed in [47]).
Figure 3. Activation of EGC Ca2+ signaling results in stimulation of gut motor reflexes.
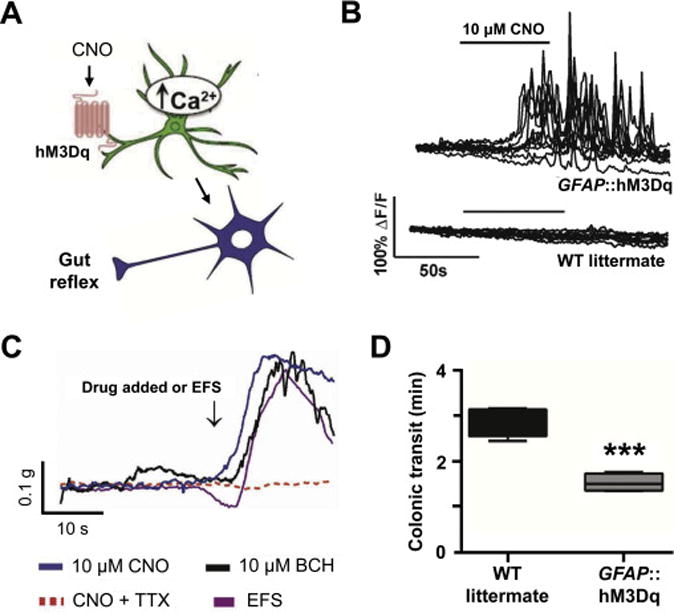
A. Experimental design using GFAP::hM3Dq transgenic mice, where a fragment of human glial fibrillary acidic protein (GFAP) promoter drives expression of an engineered Gq-coupled human M3 muscarinic receptor (hM3Dq). Activation of hM3Dq elicited by the synthetic agonist clozapine N-oxide (CNO) leads to cytosolic Ca2+ increases in EGCs.
B. CNO-evoked cytosolic Ca2+ increases are seen only in myenteric in situ EGCs expressing hM3Dq, but not in control cells from wild type (WT) littermate mice. C. CNO evokes contractions of smooth muscles in colons isolated from GFAP::hM3Dq mice. The magnitude and duration of this response is similar to those obtained from direct activation of smooth muscle or enteric neurons using bethanechol (BCH) or EFS, respectively. EGC-specific CNO effect on smooth muscle is mediated via enteric neurons since it can be blocked by tetrodotoxin (TTX). Of note, colonic muscle relaxations and colonic migrating motor complexes (CMMCs) were also affected (see original publication).
D. Selective activation of EGC Ca2+ signaling reduces colonic transit time, i.e. enhances in vivo motility of the distal colon. Obtained with minor reformatting from [63] (open access article).
Purinergic receptor- and/or Cx43 hemichannel- driven EGC Ca2+ excitability could lead to exocytotic transmitters release in the ENS, similar to that documented in astrocytes of the CNS (reviewed in [98]). Glia-selective manipulation with Cx43-hemichannel activity (Figure 4A) and Ca2+-dependent exocytosis (Figure 4F) had both distinct (Figure 4B,G; C,H) and similar (Figure 4D,I; E,J) effects on the properties of the ex vivo colonic migratory motor complexes [40]. The cross-talk between these two distinct modes of glial signaling is likely to be complex due to variations in spatiotemporal Ca2+ dynamics and presence of effector proteins having different sensitivity to Ca2+ signals [5]. Consequently, it would be important to assess the composition and characteristics of EGC exocytotic machinery.
Figure 4.
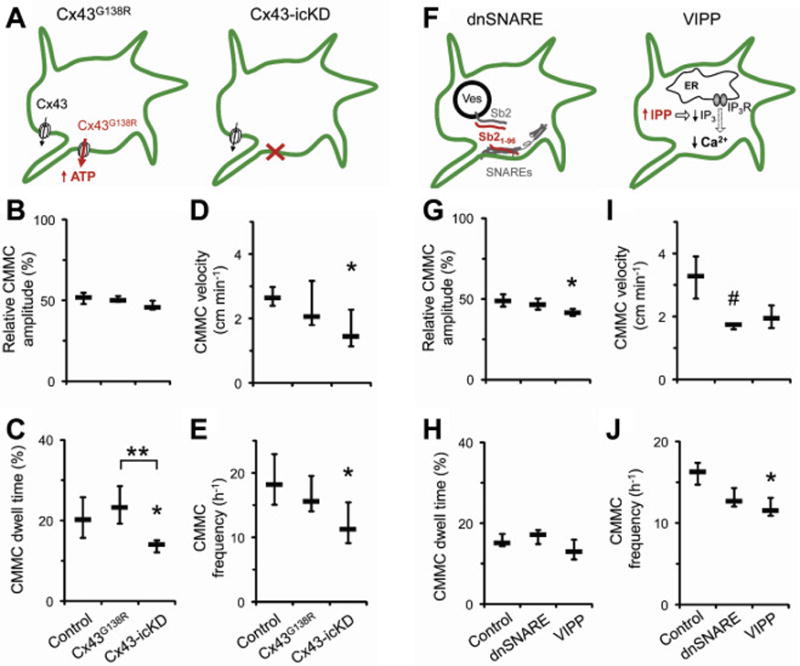
Two modes of enteric gliotransmission affect gut motility. Inducible and glia-selective transgenic mouse strains were used to modify activity of EGCs’ C×43 hemichannels (A) or Ca2+-dependent exocytosis (F) and the effect was by video recording of ex vivo colonic migratory motor complexes (CMMCs) and offline analysis of their properties (B–E and G–J, respectively). See original work for details. Note the differences and similarities between the modes of gliotransmission. Obtained with permission with minor reformatting from [40].
The ubiquitous molecular apparatus of exocytosis present in every eukaryotic cell is represented by the ternary soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (SNARE) complexes (reviewed in [49, 74]). These complexes can be composed of synaptobrevin 2, syntaxin and synaptosome-associated protein of 25 kDa or 23 kDa (SNAP25 or SNAP23), the former being present in neurons and the latter in astrocytes of the CNS [75, 88]. The ternary SNARE complex containing neuronal SNAP25B is twice as stable than that containing astrocytic SNAP23A; thus, the composition of the ternary SNARE complex endows the cells with differential ability to control the exocytotic release [65, 88]. Additionally, astrocytic vesicles contain 3–5 times less synaptobrevin 2 than the neuronal ones [82], further affecting the probability of vesicular fusion and gliotransmitter release [99]. Regardless of the exocytosis machinery composition in EGCs, it is imperative to keep in mind that the different physicochemical properties of the cell environment in situ, i.e., the gut wall along with the luminal content vs. CNS, can further affect the properties of vesicular release [1].
A perspective on how enteric glia regulate gut motility?
The basic reflex loops that regulate gut motility are formed in the intestinal wall in the neuronal circuits ENS [4, 26]. Recent studies, however, show that EGCs are important components of this reflex: enteric glia are activated during colonic motility [12] while glial activity (Figures 2, 3) [63, 64] and consequent ATP release (by Cx43 hemichannels and Ca2+-dependent exocytosis (Figure 4) [40]) are necessary and sufficient to modulate gut motility. How exactly enteric gliotransmission influences these neuronal circuits is an active area of research and here we present our views.
Conceptually, enteric motility reflex arc is represented by: (i) afferent arm, composed of sensory cells and intrinsic primary afferent neurons (IPANs); (ii) integration center, connected through neuronal synapses in the ENS; and (iii) efferent branch that includes motor neurons and smooth muscle (Figure 5) [55]. Activation of this reflex arc by distension of the gut wall and/or intraluminal chemicals leads to coordinated contraction of mooth muscle proximally and relaxation distally to the stimuli (Figure 5), resulting in gut motility that propagates intraluminal contents. Two populations of IPANs reside in either myenteric or submucosal plexus allowing the coordination between the gut motility and fluid secretion; and allowing sufficient lubrication for fecal matter propulsion. Indeed, colonic motility activates Ca2+ transients in submucosal enteric neurons [73] and distension evoked secretory responses [94]; recently, EGCs activity was found to acutely regulate secretomotor function in distal mouse colon [39].
Enterochromaffin cells, a subtype of enteroendocrine cells residing in the intestinal epithelium, are considered principal sensory cells that, in response to chemical/mechanical stimulation, secrete serotonin and activate mucosal afferents of IPANs [55]. Human mucosa is abundant in EGCs [60] and mucosal glia make connections to enteroendocrine cells [9] in close proximity to synapses between the enteroendocrine cells and IPANs [10]. Since EGCs are responsive to serotonin (Table 1) [6, 53] and release ATP [97] mucosal glial cells could modulate signaling between the principal sensory cell and IPANs. Aside ATP, EGCs could provide for additional signaling molecules, such as bradykinin, endothelin, lysophosphatidic acid, sphingosine-1-phosphate [59, 66, 79, 80, 96]. Furthermore, EGCs could get excited not only by bioactive molecules but by mechanical stimulation [59], which elicits Ca2+ responses in EGC. Together, EGCs could serve as sensory cells that contribute to activation of the enteric motility reflex (Figure 5A). Notably, astrocytes of the brainstem chemosensitive areas are primary sensory cells that control breathing reflex [37]. The ability of EGCs to sense their environment could also contribute to various integrative processes (Figure 5B). Within the myenteric plexus EGCs are closely positioned to enteric neurons (Figure 1A), similarly to astrocyte and neurons of the brain forming the multipartite synapse and astroglial cradle [2, 89]. Therefore, EGCs, through releasing various factors, may modulate synaptic connectivity and synaptic transmission in the myenteric plexus. Similar role was found for astrocytes of the hippocampus controlling synaptic networks involved in learning and memory [76].
Finally, EGCs are also positioned between the smooth muscle cells where they could affect effector arm of the motility reflex (Figure 5C). Motoneurons, by releasing neurotransmitters such as acetylcholine or ATP could also stimulate EGCs (Table 1) consequently causing release of glial signaling molecules that could modulate the excitability of motoneurons and smooth muscle cells.
In conclusion, activity of EGCs have stimulatory effect on gut motility (Figures 2, 3, 4) through modulating gut motility reflex at multiple levels (Figure 5). Exactly how and where this modulation occurs are to be clarified in by future studies. Different composition of receptors and receptor subtypes which can be expressed by EGCs may render cell-specific responses to the same stimuli. For instance, selective agonists of P2Y1, P2Y2, P2Y4 and P2Y6 agonists could be used to identify the nucleotide receptors in EGCs. Likewise, EGCs could generate distinct Ca2+ signals that can differentially regulate their functional Reponses.
Translational potential of enteric glia in GI motility disorders
As already mentioned, the selective knockout of Cx43 in EGCs reduced ATP secretion in mouse myenteric plexus in situ and suppressed the velocity of mouse colonic transit in vivo [64]. The same study also showed that normal aging causes deregulation of Cx43 expression in the gut [64]. Therefore, the change in EGS signaling could be an underlying cause of impaired intestinal transit due to normal aging as well as in idiopathic constipation.
Functional metabolic restraining of EGCs by gliotoxin fluorocitrate affected GI motility without signs of inflammation or changes in number and morphology of enteric neurons and EGCs [67]. Moreover, selective genetic ablation of EGCs led to fulminant jejunoileitis [14]. Thus, EGCs are essential for maintaining normal bowel function and for safeguarding the homeostasis in the intestines. Conceptually, EGCs are emerging as important regulators in the pathogenesis of inflammatory GI disorders (reviewed in [38, 72, 81]) and some of these mechanisms rely on the active enteric glia signaling. Inflammatory bowel diseases lead to gut dysfunction due to alterations of the ENS and loss of enteric neurons [62]. The neuron death was claimed to be mediated through the pannexin-1 pathway and downstream activation of inflammasome [42]. Inflammation also results in reactive remodeling of EGCs, characterized by an increase in GFAP expression and more “stellate” cell appearance [92], similar to that seen in reactive astrocytes [22]. Astrogliotic response includes actin cytoskeleton reorganization [46] that can enhance Ca2+ signaling systems via the cytoskeletal interactions with Cx43 channels [19]. It is possible that similar mechanism could facilitate EGC-mediated inflammation-induced neuronal death, which involves purinergic pathways and Cx43 [13]. Of note, neuronal death could also be the product of EGC interaction with the immune system (reviewed in [15]).
Expression of mGluR5 in EGCs is differentially altered in two models of colitis [69]. In the guinea pig trinitrobenzene sulfonic acid model of colitis, mGluR5 are more diffusely distributed over the colonic myenteric plexus. In the IL-10 gene knock-out mouse model of colitis, mGluR5 expression in EGCs was significantly reduced in the colonic myenteric plexus. Thus, expression of mGluR5 in EGCs and glutamatergic transmission in the ENS may represent targets for therapeutic intervention in various forms of colitis. Dysmotility is also caused by the sterile injury-induced inflammation. Postoperative ileus, a common complication of the abdominal surgery, is mediated by the ll-1 receptor1, which is primarily (but not exclusively) expressed by EGCs [83]. Interfering with the IL-1 signaling is therefore a potential treatment option for the postoperative ileus.
Lastly, EGCs are also involved in pathogenesis of infectious inflammation. Lipopolysaccharide (LPS), a component of the gram-negative bacteria cell wall, stimulates glial toll-like receptors 4 (TLR4) linked to downstream production of nitric oxide (NO) [86]. Glial release of NO release helps to fight the infection [78], but also contributes to the inflammatory process and neuronal cell death [13]. Furthermore, in vitro LPS application induces reactive phenotype in human EGCs by changing their biochemistry and affecting purinergic signaling, disrupting Ca2+ signaling and increasing ATP release by 5-folds [59]. This change in glial signaling could affect gut motility and needs to be tested in EGC in situ. On the other hand, palmitoylethanolamide (PEA) application in animal models of colitis is activates peroxisome proliferator-activated receptor α (PPAR-α)-dependent inhibition of NF-κB, consequently reducing the TLR4 dependent NO synthesis and inflammation [23]. These mechanism fo action and low toxicity make PEA suitable for potential colitis treatment.
Conclusions
The intent of this mini-review was to provide a summary of the recently discovered modulatory functions of EGCs in the gut motility. The new findings promote EGCs from their supporting cast status into a realm of an active cellular component of the ENS. For now, Cx43 hemichannels along with purinergic signaling system and underlying Ca2+ excitability have been identified as components mediating EGCs contribution to gut (patho)physiology. These molecular entities represent possible targets for medical intervention. For instance, boosting Cx43 hemichannel availability/activity in EGCs may be beneficial in bringing a relief to people suffering from age-related constipation. It should be noted, however, that Cx43 hemichannel might also be a conduit for release of metabolites and neurotransmitter precursors, such as lactate and glutamine, which can all affect normal neuronal functioning [35]. Furthermore, it is possible that beside nucleotides, other transmitters and modulators can be involved in enteric transmission and gut functions. Enteric glia sense their environment and receive inputs form neurons and perhaps other non-neuronal cells, such as smooth muscle, epithelial and immune cells, in the gut wall by a wide array of signaling molecules, including nucleotides, nucelosides, lipids, amino acids and/or peptides, opening new venues for exploring neurochemistry of the GI tract in attempt to understand its physiology and curatively intervene in pathophysiology.
Highlights.
-
►
The enteric nervous system (ENS) is the largest assembly of neurons and glia outside the central nervous system
-
►
The ENS resides within the wall of the digestive tract and regulates local gut reflexes involved in gastrointestinal motility, blood flow and fluid transport
-
►
Enteric glial cells are necessary and sufficient to modulate neuronal reflex circuits controlling gut motility
-
►
Enteric glia respond to various neurotransmitters via increase in intracellular calcium and release of neuroactive substances, such as ATP through Cx43 hemichannels or Ca2+-dependent exocytosis
-
►
Enteric glial cells are actively involved in gut motility disorders and regulation of inflammation
Acknowledgments
The authors work has been supported by the Civitan International Emerging Scholar Award at University of Alabama at Birmingham (to VG), the National Institutes of Health (The Eunice Kennedy Shriver National Institute of Child Health and Human Development award HD078678 to VP), and by the Research Agency of Slovenia (grants # P3 0310, J3 6790 and J3 7605 to RZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare no competing financial interests.
References
- 1.Amatore C, Arbault S, Guille M, Lemaitre F. The nature and efficiency of neurotransmitter exocytosis also depend on physicochemical parameters. Chemphyschem: a European journal of chemical physics and physical chemistry. 2007;8:1597–1605. doi: 10.1002/cphc.200700225. [DOI] [PubMed] [Google Scholar]
- 2.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends in neurosciences. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 3.Auerbach L. Apparat im Darmkanal der Wirbelthiere. Verlag von E Morgenstern; Breslau: 1862. Ueber einen Plexus myentericus: einer bisher unbekannten ganglio-nervösen. [Google Scholar]
- 4.Bayliss WM, Starling EH. The movements and the innervation of the large intestine. The Journal of physiology. 1900;26:107–118. doi: 10.1113/jphysiol.1900.sp000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nature reviews. Molecular cell biology. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 6.Boesmans W, Cirillo C, Van den Abbeel V, Van den Haute C, Depoortere I, Tack J, Vanden Berghe P. Neurotransmitters involved in fast excitatory neurotransmission directly activate enteric glial cells. Neurogastroenterology and motility the official journal of the European Gastrointestinal Motility Society. 2013;25:e151–160. doi: 10.1111/nmo.12065. [DOI] [PubMed] [Google Scholar]
- 7.Boesmans W, Lasrado R, Vanden Berghe P, Pachnis V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia. 2015;63:229–241. doi: 10.1002/glia.22746. [DOI] [PubMed] [Google Scholar]
- 8.Boesmans W, Rocha NP, Reis HJ, Holt M, Vanden Berghe P. The astrocyte marker Aldh1L1 does not reliably label enteric glial cells. Neuroscience letters. 2014;566:102–105. doi: 10.1016/j.neulet.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 9.Bohorquez DV, Samsa LA, Roholt A, Medicetty S, Chandra R, Liddle RA. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS One. 2014;9:e89881. doi: 10.1371/journal.pone.0089881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohorquez DV, Shahid RA, Erdmann A, Kreger AM, Wang Y, Calakos N, Wang F, Liddle RA. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. The Journal of clinical investigation. 2015;125:782–786. doi: 10.1172/JCI78361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun N, Sevigny J, Robson SC, Hammer K, Hanani M, Zimmermann H. Association of the ecto-ATPase NTPDase2 with glial cells of the peripheral nervous system. Glia. 2004;45:124–132. doi: 10.1002/glia.10309. [DOI] [PubMed] [Google Scholar]
- 12.Broadhead MJ, Bayguinov PO, Okamoto T, Heredia DJ, Smith TK. Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. The Journal of physiology. 2012;590:335–350. doi: 10.1113/jphysiol.2011.219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown IA, McClain JL, Watson RE, Patel BA, Gulbransen BD. Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell Mol Gastroenterol Hepatol. 2016;2:77–91. doi: 10.1016/j.jcmgh.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bush TG, Savidge TC, Freeman TC, Cox HJ, Campbell EA, Mucke L, Johnson MH, Sofroniew MV. Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell. 1998;93:189–201. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 15.Chow AK, Gulbransen BD. Potential roles of enteric glia in bridging neuroimmune communication in the gut. American journal of physiology. Gastrointestinal and liver physiology. 2017;312:G145–G152. doi: 10.1152/ajpgi.00384.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christofi FL, Zhang H, Yu JG, Guzman J, Xue J, Kim M, Wang YZ, Cooke HJ. Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. The Journal of comparative neurology. 2001;439:46–64. doi: 10.1002/cne.1334. [DOI] [PubMed] [Google Scholar]
- 17.Coelho-Aguiar JM, Bon-Frauches AC, Gomes AL, Verissimo CP, Aguiar DP, Matias D, Thomasi BB, Gomes AS, Brito GA, Moura-Neto V. The Enteric Glia: Identity and Functions. Glia. 2015 doi: 10.1002/glia.22795. [DOI] [PubMed] [Google Scholar]
- 18.Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotrina ML, Lin JH, Nedergaard M. Cytoskeletal assembly and ATP release regulate astrocytic calcium signaling. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1998;18:8794–8804. doi: 10.1523/JNEUROSCI.18-21-08794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Vuyst E, Wang N, Decrock E, De Bock M, Vinken M, Van Moorhem M, Lai C, Culot M, Rogiers V, Cecchelli R, Naus CC, Evans WH, Leybaert L. Ca(2+) regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell calcium. 2009;46:176–187. doi: 10.1016/j.ceca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Dogiel AS. über den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Säugetiere [German] Arch Anat Physiol Leipzig Anat Abt Jg. 1899:130–158. [Google Scholar]
- 22.Eng LF, Ghirnikar RS. GFAP and astrogliosis; 1. Brain pathology. 1994;4:229–237. doi: 10.1111/j.1750-3639.1994.tb00838.x. [DOI] [PubMed] [Google Scholar]
- 23.Esposito G, Capoccia E, Turco F, Palumbo I, Lu J, Steardo A, Cuomo R, Sarnelli G, Steardo L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-alpha activation. Gut. 2014;63:1300–1312. doi: 10.1136/gutjnl-2013-305005. [DOI] [PubMed] [Google Scholar]
- 24.Ferri GL, Probert L, Cocchia D, Michetti F, Marangos PJ, Polak JM. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature. 1982;297:409–410. doi: 10.1038/297409a0. [DOI] [PubMed] [Google Scholar]
- 25.Furness JB. The Enteric Nervous System. Wiley; 2006. [Google Scholar]
- 26.Furness JB. The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2008;20(Suppl 1):32–38. doi: 10.1111/j.1365-2982.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- 27.Furness JB. The enteric nervous system and neurogastroenterology. Nature reviews. Gastroenterology & hepatology. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 28.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 29.Gabella G. Fine structure of the myenteric plexus in the guinea-pig ileum. Journal of anatomy. 1972;111:69–97. [PMC free article] [PubMed] [Google Scholar]
- 30.Gabella G. Structure of the Autonomic Nervous System. Chapman and Hall; 1976. [Google Scholar]
- 31.Gabella G. Ultrastructure of the nerve plexuses of the mammalian intestine: the enteric glial cells. Neuroscience. 1981;6:425–436. doi: 10.1016/0306-4522(81)90135-4. [DOI] [PubMed] [Google Scholar]
- 32.Gabella G, Trigg P. Size of neurons and glial cells in the enteric ganglia of mice, guinea-pigs, rabbits and sheep. Journal of neurocytology. 1984;13:49–71. doi: 10.1007/BF01148318. [DOI] [PubMed] [Google Scholar]
- 33.Gershon M. The Second Brain: A Groundbreaking New Understanding of Nervous Disorders of the Stomach and Intestine. HarperCollins; 1999. [Google Scholar]
- 34.Giaroni C, Zanetti E, Chiaravalli AM, Albarello L, Dominioni L, Capella C, Lecchini S, Frigo G. Evidence for a glutamatergic modulation of the cholinergic function in the human enteric nervous system via NMDA receptors. Eur J Pharmacol. 2003;476:63–69. doi: 10.1016/s0014-2999(03)02147-2. [DOI] [PubMed] [Google Scholar]
- 35.Giaume C, Meme V, Koulakoff A. Astrocyte gap junctions and glutamate-induced neurotoxicity. Springer; US: 2004. [Google Scholar]
- 36.Gounaris K. Nucleotidase cascades are catalyzed by secreted proteins of the parasitic nematode Trichinella spiralis. Infect Immun. 2002;70:4917–4924. doi: 10.1128/IAI.70.9.4917-4924.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science. 2010;329:571–575. doi: 10.1126/science.1190721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grubisic V, Gulbransen BD. Enteric glia: the most alimentary of all glia. The Journal of physiology. 2017;595:557–570. doi: 10.1113/JP271021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grubisic V, Gulbransen BD. Enteric glial activity regulates secretomotor function in the mouse colon but does not acutely affect gut permeability. The Journal of physiology. 2017 doi: 10.1113/JP273492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grubisic V, Parpura V. Two modes of enteric gliotransmission differentially affect gut physiology. Glia. 2017 doi: 10.1002/glia.23121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gulbransen BD, Bains JS, Sharkey KA. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:6801–6809. doi: 10.1523/JNEUROSCI.0603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gulbransen BD, Bashashati M, Hirota SA, Gui X, Roberts JA, MacDonald JA, Muruve DA, McKay DM, Beck PL, Mawe GM, Thompson RJ, Sharkey KA. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 44.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nature reviews. Gastroenterology & hepatology. 2012;9:625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 45.Hanani M, Francke M, Hartig W, Grosche J, Reichenbach A, Pannicke T. Patch-clamp study of neurons and glial cells in isolated myenteric ganglia. American journal of physiology. Gastrointestinal and liver physiology. 2000;278:G644–651. doi: 10.1152/ajpgi.2000.278.4.G644. [DOI] [PubMed] [Google Scholar]
- 46.Hansson E. Actin Filament Reorganization in Astrocyte Networks is a Key Functional Step in Neuroinflammation Resulting in Persistent Pain: Novel Findings on Network Restoration. Neurochemical research. 2015;40:372–379. doi: 10.1007/s11064-014-1363-6. [DOI] [PubMed] [Google Scholar]
- 47.Harris AL. Connexin channel permeability to cytoplasmic molecules. Progress in biophysics and molecular biology. 2007;94:120–143. doi: 10.1016/j.pbiomolbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoff S, Zeller F, von Weyhern CW, Wegner M, Schemann M, Michel K, Ruhl A. Quantitative assessment of glial cells in the human and guinea pig enteric nervous system with an anti-Sox8/9/10 antibody. The Journal of comparative neurology. 2008;509:356–371. doi: 10.1002/cne.21769. [DOI] [PubMed] [Google Scholar]
- 49.Jahn R. Principles of exocytosis and membrane fusion. Annals of the New York Academy of Sciences. 2004;1014:170–178. doi: 10.1196/annals.1294.018. [DOI] [PubMed] [Google Scholar]
- 50.Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980;286:736–737. doi: 10.1038/286736a0. [DOI] [PubMed] [Google Scholar]
- 51.Kabouridis PS, Lasrado R, McCallum S, Chng SH, Snippert HJ, Clevers H, Pettersson S, Pachnis V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron. 2015;85:289–295. doi: 10.1016/j.neuron.2014.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kettenmann H, Verkhratsky A. Neuroglia: the 150 years after. Trends in neurosciences. 2008;31:653–659. doi: 10.1016/j.tins.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 53.Kimball BC, Mulholland MW. Enteric glia exhibit P2U receptors that increase cytosolic calcium by a phospholipase C-dependent mechanism. Journal of neurochemistry. 1996;66:604–612. doi: 10.1046/j.1471-4159.1996.66020604.x. [DOI] [PubMed] [Google Scholar]
- 54.Kukulski F, Levesque SA, Lavoie EG, Lecka J, Bigonnesse F, Knowles AF, Robson SC, Kirley TL, Sevigny J. Comparative hydrolysis of P2 receptor agonists by NTPDases 1, 2, 3 and 8. Purinergic signalling. 2005;1:193–204. doi: 10.1007/s11302-005-6217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kunze WA, Furness JB. The enteric nervous system and regulation of intestinal motility. Annu Rev Physiol. 1999;61:117–142. doi: 10.1146/annurev.physiol.61.1.117. [DOI] [PubMed] [Google Scholar]
- 56.Langley JN. The Autonomic nervous system. W. Heffer; 1921. [Google Scholar]
- 57.Lavoie EG, Gulbransen BD, Martin-Satue M, Aliagas E, Sharkey KA, Sevigny J. Ectonucleotidases in the digestive system: focus on NTPDase3 localization. American journal of physiology. Gastrointestinal and liver physiology. 2011;300:G608–620. doi: 10.1152/ajpgi.00207.2010. [DOI] [PubMed] [Google Scholar]
- 58.Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- 59.Linan-Rico A, Turco F, Ochoa-Cortes F, Harzman A, Needleman BJ, Arsenescu R, Abdel-Rasoul M, Fadda P, Grants I, Whitaker E, Cuomo R, Christofi FL. Molecular Signaling Dysfunction of the Human Reactive Enteric Glial Cell Phenotype: Implications for GI Infection, IBD, POI, Neurological, Motility, and GI Disorders. Inflamm Bowel Dis. 2016;22:1812–1834. doi: 10.1097/MIB.0000000000000854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu YA, Chung YC, Pan ST, Shen MY, Hou YC, Peng SJ, Pasricha PJ, Tang SC. 3-D imaging, illustration, and quantitation of enteric glial network in transparent human colon mucosa. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2013;25:e324–338. doi: 10.1111/nmo.12115. [DOI] [PubMed] [Google Scholar]
- 61.Lurtz MM, Louis CF. Intracellular calcium regulation of connexin43. American journal of physiology. Cell physiology. 2007;293:C1806–1813. doi: 10.1152/ajpcell.00630.2006. [DOI] [PubMed] [Google Scholar]
- 62.Mawe GM, Strong DS, Sharkey KA. Plasticity of enteric nerve functions in the inflamed and postinflamed gut. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2009;21:481–491. doi: 10.1111/j.1365-2982.2009.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McClain JL, Fried DE, Gulbransen BD. Agonist-evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell Mol Gastroenterol Hepatol. 2015;1:631–645. doi: 10.1016/j.jcmgh.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McClain JL, Grubisic V, Fried D, Gomez-Suarez RA, Leinninger GM, Sevigny J, Parpura V, Gulbransen BD. Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology. 2014;146:497–507 e491. doi: 10.1053/j.gastro.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montana V, Liu W, Mohideen U, Parpura V. Single molecule measurements of mechanical interactions within ternary SNARE complexes and dynamics of their disassembly: SNAP25 vs. SNAP23. The Journal of physiology. 2009;587:1943–1960. doi: 10.1113/jphysiol.2009.168575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murakami M, Ohta T, Otsuguro KI, Ito S. Involvement of prostaglandin E(2) derived from enteric glial cells in the action of bradykinin in cultured rat myenteric neurons. Neuroscience. 2007;145:642–653. doi: 10.1016/j.neuroscience.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 67.Nasser Y, Fernandez E, Keenan CM, Ho W, Oland LD, Tibbles LA, Schemann M, MacNaughton WK, Ruhl A, Sharkey KA. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. American journal of physiology. Gastrointestinal and liver physiology. 2006;291:G912–927. doi: 10.1152/ajpgi.00067.2006. [DOI] [PubMed] [Google Scholar]
- 68.Nasser Y, Ho W, Sharkey KA. Distribution of adrenergic receptors in the enteric nervous system of the guinea pig, mouse, and rat. The Journal of comparative neurology. 2006;495:529–553. doi: 10.1002/cne.20898. [DOI] [PubMed] [Google Scholar]
- 69.Nasser Y, Keenan CM, Ma AC, McCafferty DM, Sharkey KA. Expression of a functional metabotropic glutamate receptor 5 on enteric glia is altered in states of inflammation. Glia. 2007;55:859–872. doi: 10.1002/glia.20507. [DOI] [PubMed] [Google Scholar]
- 70.Neunlist M, Rolli-Derkinderen M, Latorre R, Van Landeghem L, Coron E, Derkinderen P, De Giorgio R. Enteric glial cells: recent developments and future directions. Gastroenterology. 2014;147:1230–1237. doi: 10.1053/j.gastro.2014.09.040. [DOI] [PubMed] [Google Scholar]
- 71.Nonaka D, Chiriboga L, Rubin BP. Sox10: a pan-schwannian and melanocytic marker. The American journal of surgical pathology. 2008;32:1291–1298. doi: 10.1097/PAS.0b013e3181658c14. [DOI] [PubMed] [Google Scholar]
- 72.Ochoa-Cortes F, Turco F, Linan-Rico A, Soghomonyan S, Whitaker E, Wehner S, Cuomo R, Christofi FL. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm Bowel Dis. 2016;22:433–449. doi: 10.1097/MIB.0000000000000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Okamoto T, Bayguinov PO, Broadhead MJ, Smith TK. Ca(2+) transients in submucous neurons during the colonic migrating motor complex in the isolated murine large intestine. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2012;24:769–778. e354. doi: 10.1111/j.1365-2982.2012.01934.x. [DOI] [PubMed] [Google Scholar]
- 74.Parpura V, Mohideen U. Molecular form follows function: (un)snaring the SNAREs. Trends in neurosciences. 2008;31:435–443. doi: 10.1016/j.tins.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 75.Parpura V, Zorec R. Gliotransmission: Exocytotic release from astrocytes. Brain research reviews. 2010;63:83–92. doi: 10.1016/j.brainresrev.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, Takano H, Moss SJ, McCarthy K, Haydon PG. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- 77.Rao M, Nelms BD, Dong L, Salinas-Rios V, Rutlin M, Gershon MD, Corfas G. Enteric glia express proteolipid protein 1 and are a transcriptionally unique population of glia in the mammalian nervous system. Glia. 2015 doi: 10.1002/glia.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schairer DO, Chouake JS, Nosanchuk JD, Friedman AJ. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence. 2012;3:271–279. doi: 10.4161/viru.20328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Segura BJ, Zhang W, Cowles RA, Xiao L, Lin TR, Logsdon C, Mulholland MW. Lysophosphatidic acid stimulates calcium transients in enteric glia. Neuroscience. 2004;123:687–693. doi: 10.1016/j.neuroscience.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 80.Segura BJ, Zhang W, Xiao L, Turner D, Cowles RA, Logsdon C, Mulholland MW. Sphingosine-1-phosphate mediates calcium signaling in guinea pig enteroglial cells. The Journal of surgical research. 2004;116:42–54. doi: 10.1016/s0022-4804(03)00281-6. [DOI] [PubMed] [Google Scholar]
- 81.Sharkey KA. Emerging roles for enteric glia in gastrointestinal disorders. The Journal of clinical investigation. 2015;125:918–925. doi: 10.1172/JCI76303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh P, Jorgacevski J, Kreft M, Grubisic V, Stout RF, Jr, Potokar M, Parpura V, Zorec R. Single-vesicle architecture of synaptobrevin2 in astrocytes. Nature communications. 2014;5:3780. doi: 10.1038/ncomms4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stoffels B, Hupa KJ, Snoek SA, van Bree S, Stein K, Schwandt T, Vilz TO, Lysson M, Veer CV, Kummer MP, Hornung V, Kalff JC, de Jonge WJ, Wehner S. Postoperative ileus involves interleukin-1 receptor signaling in enteric glia. Gastroenterology. 2014;146:176–187 e171. doi: 10.1053/j.gastro.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 84.Stohr P., Jr Synopsis of research results on the microscopic innervation of the gastrointestinal tract. Ergeb Anat Entwicklungsgesch. 1952;34:250–401. [PubMed] [Google Scholar]
- 85.Trendelenburg P. Physiologische und pharmakologische Versuche über die Dunndarmperistaltik. Vogel; 1917. [DOI] [PubMed] [Google Scholar]
- 86.Turco F, Sarnelli G, Cirillo C, Palumbo I, De Giorgi F, D’Alessandro A, Cammarota M, Giuliano M, Cuomo R. Enteroglial-derived S100B protein integrates bacteria-induced Toll-like receptor signalling in human enteric glial cells. Gut. 2014;63:105–115. doi: 10.1136/gutjnl-2012-302090. [DOI] [PubMed] [Google Scholar]
- 87.Van Nassauw L, Costagliola A, Van Op den Bosch J, Cecio A, Vanderwinden JM, Burnstock G, Timmermans JP. Region-specific distribution of the P2Y4 receptor in enteric glial cells and interstitial cells of Cajal within the guinea-pig gastrointestinal tract. Auton Neurosci. 2006;126–127:299–306. doi: 10.1016/j.autneu.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 88.Verkhratsky A, Matteoli M, Parpura V, Mothet JP, Zorec R. Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. EMBO J. 2016;35:239–257. doi: 10.15252/embj.201592705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Verkhratsky A, Nedergaard M. Astroglial cradle in the life of the synapse. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130595. doi: 10.1098/rstb.2013.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vieira C, Ferreirinha F, Silva I, Duarte-Araujo M, Correia-de-Sa P. Localization and function of adenosine receptor subtypes at the longitudinal muscle— myenteric plexus of the rat ileum. Neurochem Int. 2011;59:1043–1055. doi: 10.1016/j.neuint.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 91.Virchow R. Cellular pathology: as based upon physiological and pathological histology. First. August Hirschwald; Berlin: 1858. (Twenty lectures delivered in the pathological institute of Berlin during the months of February, March and April, 1858 [in German]). [Google Scholar]
- 92.von Boyen GB, Schulte N, Pfluger C, Spaniol U, Hartmann C, Steinkamp M. Distribution of enteric glia and GDNF during gut inflammation. BMC gastroenterology. 2011;11:3. doi: 10.1186/1471-230X-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.von Boyen GB, Steinkamp M, Adler G, Kirsch J. Glutamate receptor subunit expression in primary enteric glia cultures. J Recept Signal Transduct Res. 2006;26:329–336. doi: 10.1080/10799890600778821. [DOI] [PubMed] [Google Scholar]
- 94.Weber E, Neunlist M, Schemann M, Frieling T. Neural components of distension-evoked secretory responses in the guinea-pig distal colon. The Journal of physiology. 2001;536:741–751. doi: 10.1111/j.1469-7793.2001.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wood JD. Enteric Nervous System: (the Brain-in-the-gut) Morgan & Claypool Life Sciences. 2011 [Google Scholar]
- 96.Zhang W, Sarosi G, Jr, Barnhart D, Yule DI, Mulholland MW. Endothelin-activated calcium signaling in enteric glia derived from neonatal guinea pig. The American journal of physiology. 1997;272:G1175–1185. doi: 10.1152/ajpgi.1997.272.5.G1175. [DOI] [PubMed] [Google Scholar]
- 97.Zhang W, Segura BJ, Lin TR, Hu Y, Mulholland MW. Intercellular calcium waves in cultured enteric glia from neonatal guinea pig. Glia. 2003;42:252–262. doi: 10.1002/glia.10215. [DOI] [PubMed] [Google Scholar]
- 98.Zorec R, Araque A, Carmignoto G, Haydon PG, Verkhratsky A, Parpura V. Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route. ASN neuro. 2012;4 doi: 10.1042/AN20110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zorec R, Verkhratsky A, Rodriguez JJ, Parpura V. Astrocytic vesicles and gliotransmitters: Slowness of vesicular release and synaptobrevin2-laden vesicle nanoarchitecture. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.02.033. [DOI] [PubMed] [Google Scholar]


