Abstract
Previous studies have shown that protein kinase M zeta (PKMζ), a brain-specific isoform of protein kinase C, is involved in the central processing of nociception in several pain models by using a synthetic zeta inhibitory peptide. In the present study, we investigated whether PKMζ contributes to the pathogenesis of postsurgical pain using both conditional and conventional PKMζ knockout mice. Our results showed that the expression of PKMζ in anterior cingulate cortex, but not spinal cord, of the conditional PKMζ knockout mice was inhibited following tamoxifen injection. And the conditional PKMζ knockout mice displayed similar plantar incision-produced postsurgical pain responses as those in wild-type mice. Moreover, the expression of PKMζ was inhibited in both anterior cingulate cortex and spinal cord of the conventional PKMζ knockout mice. And there were no significant differences in the development of postsurgical pain among wild-type, heterozygous and homozygous conventional PKMζ knockout mice. These data suggest that PKMζ is not required for the development of postsurgical pain after plantar incision.
Keywords: PKMζ, postsurgical pain, anterior cingulate cortex, spinal cord, plantar incision, synaptic plasticity
Introduction
It has been shown that protein kinase M zeta (PKMζ), a brain-specific, autonomously active isozyme of protein kinase C, is necessary and sufficient for maintaining the late-phase of synaptic potentiation in hippocampal slices [1]. Many forms of long-term memory in a wide variety of neural circuits were also maintained by the persistent activity of PKMζ in the central nervous system (CNS) [2–5]. However, most of previous studies supporting the involvement of PKMζ in long-term potentiation (LTP) and long-term memory rely heavily on pharmacological inhibition of PKMζ. By using genetic knockout (KO) mouse models, a recent study from Dr. Richard Huganir Laboratory at Johns Hopkins University School of Medicine demonstrates that PKMζ is not required for hippocampal synaptic plasticity, learning and memory [6]. In that study, both conventional and conditional PKMζ KO mice show normal synaptic transmission and LTP at hippocampal synapses, and have no deficits in several hippocampal-dependent learning and memory tasks [6]. Another study using PKCζ/PKMζ null mice has also shown that absence of PKMζ does not impair learning and memory in mice [7]
It is widely believed that long-lasting changes (e.g., LTP) in central sensitization of the CNS serve as a neural basis of chronic pain development. Thus, it is interesting to know whether PKMζ contributes to nociceptive plasticity and central mechanisms underlying pain transmission. Previous studies using pharmacological inhibition of PKMζ have shown that PKMζ may play a role in the central processing of nociception [8–15]. These studies employed a synthetic zeta inhibitory peptide (ZIP) to inhibit the activities of PKMζ in the CNS, but it has been indicated that the effect of ZIP may be independent of PKMζ, because ZIP still reverses LTP in PKMζ KO mice [6, 7]. Therefore, it remains to be illuminated whether PKMζ is involved in pain processing. To further clarify this issue, we used both conventional and conditional PKMζ KO mice to investigate the effect of PKMζ deletion on postsurgical pain in the present study.
Materials and Methods
Animals
Both conditional and conventional PKMζ KO mice [6] with C57BL/6 background were obtained from Dr. Richard Huganir Laboratory at Johns Hopkins University School of Medicine. Male mice were used in this study and all animal procedures were carried out in accordance with the National Institutes of Health guide for the care and use of laboratory animals, and were approved by Texas A&M University College of Dentistry Institutional Animal Care and Use Committee. All animals weighing 25–30 g were housed up to four per cage on a standard 12-h light/dark cycle, with water and food pellets available ad libitum. All efforts were made to minimize pain or discomfort and to reduce the number of animals used.
The conditional PKMζ KO mice were generated by crossing PKMζfl/fl mice with CaMKII-CreERT2 mice [6], which express Cre recombinase that becomes active only upon addition of tamoxifen driven by the CaMKII promoter. Thus, we induced PKMζ KO by intraperitoneal injection of tamoxifen (Sigma, 2 mg per day for 5 days) dissolved in sunflower seed oil. Control mice were either PKMζfl/fl mice injected with tamoxifen (Tamoxifen control) or PKMζfl/fl; CaMKII-CreERT2 mice injected with sunflower seed oil (Vehicle control). The conventional PKMζ KO mice were generated by breeding to a CMV-Cre transgenic mouse line [6]. PCR amplification for genotyping of PKMζ KO mice was performed as described previously [6].
Plantar incision
A plantar incision was made in the left hind paw according to previous studies [16, 17]. In brief, mice were anesthetized with 1.5–2% isoflurane. After antiseptic preparation of the left hindpaw, a 5-mm longitudinal incision was made through the skin and fascia of the plantar foot. The incision was started 2 mm from the proximal edge of the heel and extended toward the toes. The underlying muscle was elevated with a curved forceps, leaving the muscle origin and insertion intact.
Pain behavior testing
Mechanical and thermal pain hypersensitivities were measured as described in our previous studies [16, 18–20] at 1 day before incision surgery as baseline and 1, 3, 5, 7, 10, 15, 20, and 30 days after surgery. For mechanical pain testing, mice were placed on an elevated wire mesh floor and were covered with a clear Plexiglas chamber. After acclimation for 30 min, paw withdrawal responses to mechanical stimuli were determined using calibrated von Frey filaments (0.08, 0.15, 0.25, 0.41, 0.75, 1.2, and 2.0 g). Each monofilament was applied five times to the plantar side of the hind paws for approximately 1 s with a 10-s interval, starting with a force of 0.08 g and continuing in ascending order. A stimulus-related withdrawal was considered a positive response. The paw withdrawal threshold was calculated as the force at which the positive response occurred in three out of five stimuli. For thermal pain testing, mice were placed in a Plexiglas chamber on a glass plate under which a light box was located. A radiant heat stimulus was applied by aiming a beam of light through a hole in the light box to each hind paw through the glass plate. The light beam was turned off when the animal lifted the paw, allowing the measurement of time between start of the light beam and the paw lift. This time was defined as the paw withdrawal latency. The stimulus intensity was adjusted to give 10–12 s withdrawal latency in the normal mouse (baseline). A cutoff time of 20 s was used to avoid tissue damage to the hind paw. For all mice, both ipsilateral (incision) and contralateral (non-incision) hind paws were tested five times at 5-min intervals to obtain an average response latency for each hind paw. All pain behavioral tests were done by an investigator blinded to animal treatment groups.
Western blotting
The mice were sacrificed, and lumbar spinal cord and anterior cingulate cortex (ACC) tissues were harvested. PKMζ expression in the spinal cord and ACC was analyzed with quantitative Western blotting. The primary antibody against PKMζ (1:1000, anti-rabbit, Thermo Fisher Scientific) was used in our experiments. The intensity of Western blots was quantified with densitometry.
Statistical analysis
Data are expressed as mean ± SEM. Comparisons among groups were performed by one-way analysis of variance followed by the Student—Newman—Keuls method. Differences with P < 0.05 were considered statistically significant.
Results
Conditional PKMζ KO mice showed specifically PKMζ expression deficiency in the ACC after tamoxifen injection
Western blot analysis was used to assess the expression of PKMζ protein in the lumbar spinal cord and ACC of different groups of mice. Tissues from control mice were harvested at 2 weeks after sunflower seed oil (Vehicle control) or tamoxifen (Tamoxifen control) injection. As shown in Fig.1a, PKMζ protein was expressed in both spinal cord and ACC of control and conditional PKMζ KO mice. The expression of PKMζ in the ACC significantly decreased at 2 weeks, but not 3 and 6 weeks, after tamoxifen injection compared to control groups (Fig.1b, *P < 0.05 vs. control). In the spinal cord, no significant difference of PKMζ protein expression was shown among different groups (Fig.1c).
Figure 1. Decreased PKMζ expression in the ACC of conditional PKMζ KO mice after tamoxifen injection.
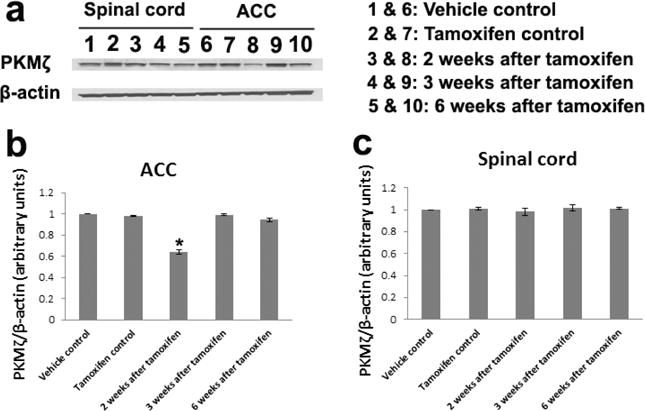
a Western blot analysis showed that PKMζ protein was expressed in both spinal cord and ACC of control and conditional PKMζ KO mice. The expression of PKMζ in the ACC, but not spinal cord, significantly decreased at 2 weeks after tamoxifen injection compared to control groups. At 3 and 6 weeks after tamoxifen, no significant change in PKMζ expression was observed in the conditional PKMζ KO mice. β-actin served as a loading control. The data shown are representative of three independent experiments. b Statistical analysis of the PKMζ expression in the ACC. n = 3 for each group; *P < 0.05 vs. control groups. c Statistical analysis of the PKMζ expression in the spinal cord. n = 3 for each group. There was no significant difference among different groups.
PKMζ in the ACC is not required for pain processing following plantar incision
To investigate if PKMζ protein in the ACC is involved in postsurgical pain, we prepared a plantar incision-induced postsurgical pain model in conditional PKMζ KO mice at 2 weeks after tamoxifen injection. We found that plantar incision decreased paw withdrawal threshold and paw withdrawal latency in the ipsilateral hind paws of the conditional PKMζ KO mice in a similar pattern as those in both tamoxifen and vehicle control mice (Fig. 2a,b). The time course of postsurgical pain in the conditional PKMζ KO mice was not significantly different from that in the control mice (Fig. 2). In the contralateral hind paws, no mechanical and thermal pain behaviors were observed in all the mice (Data not shown).
Figure 2. Tamoxifen-induced PKMζ deficiency in the ACC has no effect on postsurgical pain in conditional PKMζ KO mice.
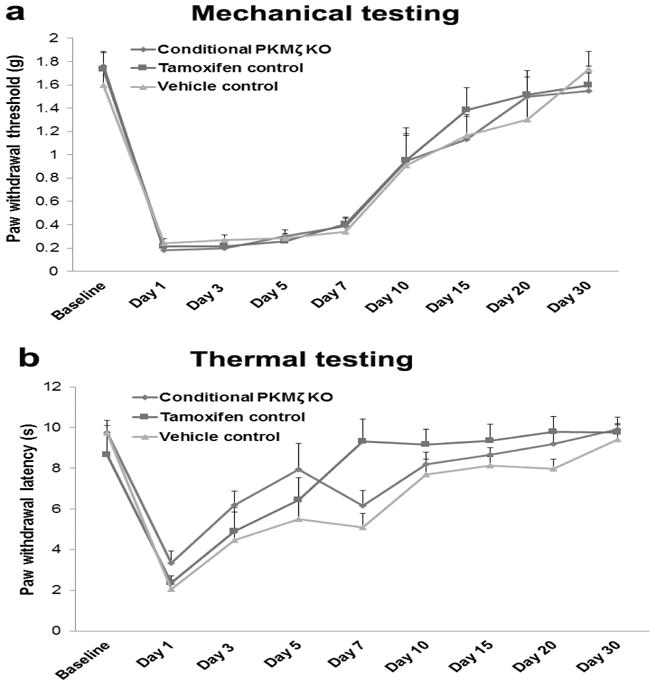
a Mechanical testing: Plantar incision decreased paw withdrawal threshold in the ipsilateral hind paws of the conditional PKMζ KO mice with tamoxifen injection in a similar pattern as those in both tamoxifen and vehicle control mice. The time course of postsurgical mechanical pain in the conditional PKMζ KO mice was not significantly different from that in the control mice. b Thermal testing: Plantar incision decreased paw withdrawal latency in the ipsilateral hind paws of the conditional PKMζ KO mice with tamoxifen injection in a similar pattern as those in both tamoxifen and vehicle control mice. The time course of postsurgical thermal pain in the conditional PKMζ KO mice was not significantly different from that in the control mice.
Conventional PKMζ KO mice showed PKMζ protein deletion in both ACC and spinal cord
To further investigate the possible role of PKMζ in pain processing, we used conventional PKMζ KO mice in our study. We observed that PKMζ protein was deleted completely in both ACC and spinal cord of the conventional PKMζ KO (Homo) mice (Fig. 3). In the heterozygotes (Het) of the KO mice, PKMζ was down-regulated in the ACC and spinal cord compared to wild-type (WT) mice (Fig. 3).
Figure 3. Conventional PKMζ KO mice display PKMζ protein deletion in both ACC and spinal cord.
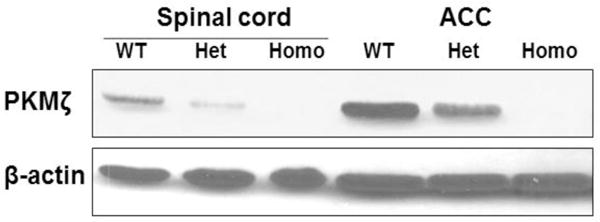
Note that PKMζ protein was deleted completely in both ACC and spinal cord of the conventional PKMζ KO (Homo) mice. In the heterozygotes (Het) of the KO mice, PKMζ was down-regulated in the ACC and spinal cord compared to wild-type (WT) mice. β-actin served as a loading control. The data shown are representative of three independent experiments.
PKMζ in the spinal cord is also not required for plantar incision-induced postsurgical pain
As shown above, PKMζ protein deletion occurred in both ACC and spinal cord of the conventional PKMζ KO mice. Our study using conditional PKMζ KO mice has indicated that PKMζ in the ACC is not required for pain processing following plantar incision. To further clarify if PKMζ in the spinal cord is involved in postsurgical pain, we prepared a plantar incision-induced postsurgical pain model in conventional PKMζ KO mice. We found that plantar incision decreased paw withdrawal threshold and paw withdrawal latency in the ipsilateral hind paws of WT, Het, and Home PKMζ KO mice in a similar pattern (Fig. 4a,b). The time course of postsurgical pain in these mice was not significantly different (Fig. 4).
Figure 4. Conventional PKMζ KO mice show similar postsurgical pain as that in WT mice.
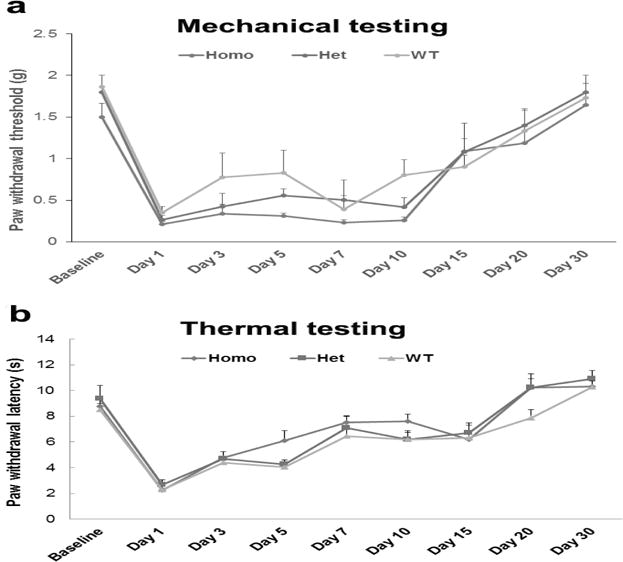
a Mechanical testing: Plantar incision decreased paw withdrawal threshold in the ipsilateral hind paws of WT, Het, and Home PKMζ KO mice in a similar pattern. The time course of postsurgical mechanical pain in these mice was not significantly different. b Thermal testing: Plantar incision decreased paw withdrawal latency in the ipsilateral hind paws of WT, Het, and Home PKMζ KO mice in a similar pattern. The time course of postsurgical thermal pain in these mice was not significantly different.
Discussion
Although pharmacological studies using PKMζ inhibitors have shown that PKMζ may contribute to the maintenance of LTP in the CNS [1–5, 21, 22], recent studies using transgenic mouse models demonstrate that PKMζ is not required for learning and memory [6, 7]. Thus, it remains uncertain whether PKMζ plays a role in synaptic plasticity of the CNS. By using ZIP (a synthetic zeta inhibitory peptide), previous studies have shown that PKMζ in both spinal cord and brain may be involved in the modulation of neuropathic and inflammatory pain [8–15, 23–25]. However, ZIP is not specific for inhibiting the activities of PKMζ in the CNS and the inhibitor can still reverses LTP in PKMζ KO mice [6, 7], indicating that the effect of ZIP is independent of PKMζ inhibition.
In the present study, we employed the conditional and conventional PKMζ KO mice [6] from Dr. Richard Huganir Laboratory. We investigated the effect of PKMζ deficiency in the spinal cord and anterior cingulate cortex (ACC, a pain-related brain area) on plantar incision-induced postsurgical pain. By using conditional PKMζ KO mice, we showed that the expression of PKMζ in the ACC, but not spinal cord, is significantly decreased after tamoxifen induction. And the PKMζ deficiency in the ACC of the conditional KO mice does not affect the development of postsurgical pain. We observed that the expression of PKMζ in the ACC of the conditional KO mice returns to normal level after 3 weeks following tamoxifen injection. The recovery of PKMζ expression could be related to the degradation of tamoxifen or potential mosaic recombination in the ACC. Moreover, by using conventional PKMζ KO mice, we showed that the expression of PKMζ in both ACC and spinal cord is completely deleted in the Homo mice and markedly decreased in the Het mice compared to that in WT mice. And the PKMζ deletion in both ACC and spinal cord of the conventional KO mice also does not affect the development of postsurgical pain. It is possible that other PKC subtypes may compensate PKMζ function in the conventional PKMζ KO mice. Thus, we used both conditional and conventional PKMζ KO mice to confirm each other in the present study. Taken together, our results suggest that PKMζ in both ACC and spinal cord is not required for the pathogenesis of postsurgical pain.
The recent study using the same conditional and conventional PKMζ KO mice as described in the present study supports our findings by showing that PKMζ is not required for synaptic plasticity, learning and memory [6], because central sensitization (a type of synaptic plasticity) of the CNS plays a critical role in pain transmission and chronification. Regarding the role of PKMζ in pain processing, we found that PKMζ in both ACC and spinal cord is not necessary during the development of postsurgical pain, but previous studies using a synthetic zeta inhibitory peptide ZIP show that PKMζ in the CNS contributes to pain mechanisms in different animal models [8–15, 23–25]. The controversy between our findings and these previous pain studies using ZIP is because ZIP is not a specific inhibitor for PKMζ and it may cause non-specific effects on pain transmission, which is independent of PKMζ activities in the CNS. In our study, we used tamoxifen-induced knockdown and conventional knockout mouse models. These genetic approaches allow us to examine the specific role of PKMζ in pain processing. Our results indicate that PKMζ in both ACC and spinal cord is not involved in plantar incision-induced postsurgical pain. We may need to use our transgenic mouse models to clarify if PKMζ in the CNS contributes to other types of pain in the future.
In conclusion, our findings in the present study demonstrate that PKMζ is not required for development of postsurgical pain, which is consistent with recent studies on the role of PKMζ in learning and memory [6, 7]. Those studies show that absence of PKMζ does not impair learning and memory in mice, and that ZIP can reverse LTP and erase memory even when PKMζ is not present [6, 7], suggesting that PKMζ is not required for synaptic plasticity in the CNS and the effect of ZIP is independent of PKMζ. Therefore, genetic approach-based transgenic models should be used to investigate the specific role of PKMζ in different types of pain.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 DE022880 (F.T.) and K02 DE023551 (F.T.) as well as National Natural Science Foundation of China 81500962 (S.L.). The authors thank Dr. Richard Huganir (Johns Hopkins University School of Medicine) for providing conditional and conventional PKMζ KO mice.
References
- 1.Ling DS, Benardo LS, Serrano PA, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–296. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- 2.Hardt O, Migues PV, Hastings M, Wong J, Nader K. PKMzeta maintains 1-day- and 6-day-old long-term object location but not object identity memory in dorsal hippocampus. Hippocampus. 2010;20:691–695. doi: 10.1002/hipo.20708. [DOI] [PubMed] [Google Scholar]
- 3.Li YQ, Xue YX, He YY, Li FQ, Xue LF, Xu CM, Sacktor TC, Shaham Y, Lu L. Inhibition of PKMzeta in nucleus accumbens core abolishes long-term drug reward memory. J Neurosci. 2011;31:5436–5446. doi: 10.1523/JNEUROSCI.5884-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migues PV, Hardt O, Wu DC, Gamache K, Sacktor TC, Wang YT, Nader K. PKMzeta maintains memories by regulating GluR2-dependent AMPA receptor trafficking. Nat Neurosci. 2010;13:630–634. doi: 10.1038/nn.2531. [DOI] [PubMed] [Google Scholar]
- 5.Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini C, Kelley AE, Maren S, Rudy JW, et al. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–2706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volk LJ, Bachman JL, Johnson R, Yu YL, Huganir RL. PKM-zeta is not required for hippocampal synaptic plasticity, learning and memory. Nature. 2013;493:420–U169. doi: 10.1038/nature11802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AM, Kanter BR, Wang D, Lim JP, Zou ME, Qiu C, McMahon T, Dadgar J, Fischbach-Weiss SC, Messing RO. Prkcz null mice show normal learning and memory. Nature. 2013;493:416–419. doi: 10.1038/nature11803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An K, Zhen C, Liu ZH, Zhao Q, Liu HP, Zhong XL, Huang WQ. Spinal protein kinase M zeta contributes to the maintenance of peripheral inflammation-primed persistent nociceptive sensitization after plantar incision. European Journal of Pain. 2015;19:39–47. doi: 10.1002/ejp.517. [DOI] [PubMed] [Google Scholar]
- 9.Asiedu MN, Tillu DV, Melemedjian OK, Shy A, Sanoja R, Bodell B, Ghosh S, Porreca F, Price TJ. Spinal protein kinase M zeta underlies the maintenance mechanism of persistent nociceptive sensitization. J Neurosci. 2011;31:6646–6653. doi: 10.1523/JNEUROSCI.6286-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen A, Bao C, Tang Y, Luo X, Guo L, Liu B, Lin C. Involvement of protein kinase zeta in the maintenance of hippocampal long-term potentiation in rats with chronic visceral hypersensitivity. J Neurophysiol. 2015;113:3047–3055. doi: 10.1152/jn.00929.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han J, Kwon M, Cha M, Tanioka M, Hong SK, Bai SJ, Lee BH. Plasticity-Related PKMzeta Signaling in the Insular Cortex Is Involved in the Modulation of Neuropathic Pain after Nerve Injury. Neural Plast. 2015;2015:601767. doi: 10.1155/2015/601767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laferriere A, Pitcher MH, Haldane A, Huang Y, Cornea V, Kumar N, Sacktor TC, Cervero F, Coderre TJ. PKM zeta is essential for spinal plasticity underlying the maintenance of persistent pain. Molecular Pain. 2011;7 doi: 10.1186/1744-8069-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330:1400–1404. doi: 10.1126/science.1191792. [DOI] [PubMed] [Google Scholar]
- 14.Marchand F, D’Mello R, Yip PK, Calvo M, Muller E, Pezet S, Dickenson AH, McMahon SB. Specific involvement of atypical PKCzeta/PKMzeta in spinal persistent nociceptive processing following peripheral inflammation in rat. Mol Pain. 2011;7:86. doi: 10.1186/1744-8069-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melemedjian OK, Tillu DV, Asiedu MN, Mandell EK, Moy JK, Blute VM, Taylor CJ, Ghosh S, Price TJ. BDNF regulates atypical PKC at spinal synapses to initiate and maintain a centralized chronic pain state. Mol Pain. 2013;9:12. doi: 10.1186/1744-8069-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Yang Y, Liu S, Fang H, Zhang Y, Furmanski O, Skinner J, Xing Y, Johns RA, Huganir RL, et al. Stress induces pain transition by potentiation of AMPA receptor phosphorylation. J Neurosci. 2014;34:13737–13746. doi: 10.1523/JNEUROSCI.2130-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pogatzki EM, Raja SN. A mouse model of incisional pain. Anesthesiology. 2003;99:1023–1027. doi: 10.1097/00000542-200310000-00041. [DOI] [PubMed] [Google Scholar]
- 18.Tao F, Su Q, Johns RA. Cell-permeable peptide Tat-PSD-95 PDZ2 inhibits chronic inflammatory pain behaviors in mice. Mol Ther. 2008;16:1776–1782. doi: 10.1038/mt.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao F, Tao YX, Mao P, Zhao C, Li D, Liaw WJ, Raja SN, Johns RA. Intact carrageenan-induced thermal hyperalgesia in mice lacking inducible nitric oxide synthase. Neuroscience. 2003;120:847–854. doi: 10.1016/s0306-4522(03)00362-2. [DOI] [PubMed] [Google Scholar]
- 20.Tao F, Tao YX, Zhao C, Dore S, Liaw WJ, Raja SN, Johns RA. Differential roles of neuronal and endothelial nitric oxide synthases during carrageenan-induced inflammatory hyperalgesia. Neuroscience. 2004;128:421–430. doi: 10.1016/j.neuroscience.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 21.Serrano P, Yao Y, Sacktor TC. Persistent phosphorylation by protein kinase Mzeta maintains late-phase long-term potentiation. J Neurosci. 2005;25:1979–1984. doi: 10.1523/JNEUROSCI.5132-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Q, Zhang L, Shu R, Wang C, Yu Y, Wang H, Wang G. Involvement of Spinal PKMzeta Expression and Phosphorylation in Remifentanil-Induced Long-Term Hyperalgesia in Rats. Cell Mol Neurobiol. 2016 doi: 10.1007/s10571-016-0401-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Y, Chen A, Chen Y, Guo L, Dai H, Huang Y, Chen Q, Lin C. Zeta Inhibitory Peptide as a Novel Therapy to Control Chronic Visceral Hypersensitivity in a Rat Model. PLoS One. 2016;11:e0163324. doi: 10.1371/journal.pone.0163324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King T, Qu CL, Okun A, Melemedjian OK, Mandell EK, Maskaykina IY, Navratilova E, Dussor GO, Ghosh S, Price TJ, et al. Contribution of PKM zeta-dependent and independent amplification to components of experimental neuropathic pain. Pain. 2012;153:1263–1273. doi: 10.1016/j.pain.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


