Abstract
Introduction
X-linked myotubular myopathy (XLMTM), a devastating pediatric disease caused by the absence of the protein myotubularin, results from mutations in the MTM1 gene. While there is no cure for XLMTM, we previously reported effects of MTM1 gene therapy using adeno-associated viral (AAV) vector on muscle weakness and pathology in MTM1-mutant dogs. Here, we followed 2 AAV-infused dogs over 4 years.
Methods
We evaluated gait, strength, respiration, neurological function, muscle pathology, AAV vector copy number (VCN), and transgene expression.
Results
Four years following AAV-mediated gene therapy, gait, respiratory performance, neurological function and pathology in AAV-infused XLMTM dogs remained comparable to their healthy littermate controls despite a decline in VCN and muscle strength.
Discussion
AAV-mediated gene transfer of MTM1 in young XLMTM dogs results in long-term expression of myotubularin transgene with normal muscular performance and neurological function in the absence of muscle pathology. These findings support a clinical trial in patients.
INTRODUCTION
X-linked myotubular myopathy (XLMTM) is the most common of the centronuclear myopathies; it is estimated to affect 1 in 50,000 male births.1–3 The disease is caused by mutation of the MTM1 gene,4,5 and affected boys develop severe weakness of skeletal muscles due to a deficiency of the protein myotubularin.4,6 XLMTM presents at birth as marked hypotonia and respiratory impairment and is often fatal in the first year of life.7,8 This myopathy affects primarily males, except in cases where there is skewed X-inactivation.9,10 While there are instances of spontaneous occurrence, MTM1 mutations are often inherited from asymptomatic carrier mothers.11 The most severely affected boys become wheelchair dependent, however, a subset of patients with a milder form of the disease have been reported.12,13 These patients can survive until adulthood and have been known to remain ambulatory for a longer period of time.8,14 In Labrador retriever and mixed breed dogs, a p.N155K missense mutation in canine MTM1 results in profound skeletal muscle weakness in affected puppies analogous to the phenotype seen in boys with XLMTM.15,16 XLMTM dogs experience weakness and atrophy of skeletal muscles, causing difficulty with standing, eating, and respiratory function.17–20 The average lifespan of affected animals is 18–20 weeks of age.15,21,18 Analogous to human patients, carrier female dogs are phenotypically normal.16
As XLMTM is caused by mutations in the MTM1 gene, AAV-mediated gene replacement therapy is under intense investigation. We previously found that a direct intramuscular injection of AAV serotype 8 (AAV8) carrying a normal copy of the canine MTM1 gene to the cranial tibialis muscle of MTM1-mutant dogs restored strength of the injected limb muscle to near-normal, but contiguous non-injected limb muscles remained weakened without a commensurate improvement in overall walking gait.22 Compared to normal dogs, XLMTM dogs averaged a 46% reduction in gait speed and a 68% decrease in stride length near the end of their lifespan at 18 weeks.23 We concluded from these findings that intramuscular AAV resulted in marked strength improvement of a single paw flexor, the cranial tibialis, but targeted improvement in a single limb muscle alone is not sufficient to result in overall gait improvement.24 In contrast, transvenous limb perfusion, where the limb is isolated before administration of high-pressure perfusion, allows for AAV infusion of the entire lower limb musculature.25 Dogs treated this way demonstrated improved hind limb strength in the infused limb after treatment, but probably due to leak of AAV into the systemic circulation, effects were seen well beyond the isolated hindlimb muscles.22 This quasi-systemic delivery resulted in improved strength in contralateral untreated hindlimbs, improved respiratory function, and increased lifespan. These previous data suggested that, unlike a single targeted intramuscular injection, whole body transduction by AAV leads to overall improvement in walking gait in MTM1 mutant dogs. To address the question of long-term duration of systemic AAV delivery, we performed a battery of physiological, clinical, and biochemical analysis over a period of 4 years in AAV-treated XLMTM dogs.
Spatiotemporal measures of gait using two-dimensional video-based analysis are sensitive to changes caused by disease, as we published in XLMTM dogs.23 Quantitative gait analysis established the effects of limb muscle impairment due to neuromuscular disease26 in studies of canine models of Duchenne muscular dystrophy (DMD).27,28 Video-based kinematic analysis detects changes in range of motion (ROM) due to disease in canine models of neuromuscular disease.27 However, kinematic analysis can be time intensive and requires extensive training for appropriate accuracy. Equipment set-up, including camera placement and frame rate, can also affect accuracy and precision.29,30 Given that measures of ROM are not sufficiently sensitive to differentiate between XLMTM dogs and normal controls,23 an instrumented carpet provides an alternate method for assessing gait function. The system has been validated for use in dogs, is relatively easy to use in a reproducible fashion, and allows for quantification of some spatiotemporal measures unavailable with two-dimensional analysis, such as step width.31,30
In this study, our technical aim was to compare spatiotemporal measures from a video-based motion analysis with data collected using an instrumented carpet. We anticipated good agreement between methods, as both are validated approaches to quantify gait. Support of this idea would further justify the use of the more efficient instrumented walkway to quantify meaningful gait changes in the canine model of XLMTM. Our clinical aim was to compare gait between XLMTM dogs treated with a single intravenous infusion of AAV8-MTM1 to control dogs over a two-year time period to test the hypothesis that treatment of XLMTM dogs preserves gait function over time. This longitudinal assessment of function in the preclinical model of XLMTM is particularly informative, as gait function may indicate the overall duration of gene replacement therapy. This could inform expected patient outcomes and future treatment strategies upon translation to the clinic.
We report here the long-term follow-up of two dogs initially treated by loco-regional (quasi-systemic) perfusion with AAV8-MTM1.22 In addition to gait assessment using the instrumented carpet, we used assessment methods from previously published experiments to monitor the dogs’ health 4 years post-infusion with AAV8-MTM1.32 These assessments included muscle strength, neurological function, respiratory effort, and histological and molecular examination of muscle biopsies.19,20
METHODS
Dogs
Care and use of all laboratory animals was in keeping with the National Research Council guidelines and as approved by the Institutional Animal Care and Use Committee (IACUC). XLMTM dogs (N=2) were identified after birth by Taqman genotyping assay.15 Two groups were evaluated. For short-term assessment, XLMTM dogs (N=2) named, “Pavlov” and “Turing”, were measured prior to treatment at 8 weeks of age (T8w) and then every 4 weeks post-treatment (T12w and T16w) until 21 weeks of age (T21w) alongside wildtype male controls (N=2). For the long-term assessment, XLMTM dogs were measured 1 (T1y), 2 (T2y), 3 (T3y), and 4 (T4y) years after treatment as compared to normal carrier females (N=3 at 1 and 2 years) (N=2 at 3 and 4 years). All dogs used in the study were true littermates. Hindlimb length was recorded in anesthetized dogs with the limb against a flat surface at all time points excluding the last at 2 years after treatment, where measures were taken in awake, recumbent animals with the aid of a handler.
Treatment
XLMTM dogs were treated at 9 weeks of age via isolated limb perfusion as previously described.22 Briefly, a tourniquet was applied to the left hindlimb, and a single dose of AAV8-MTM1 (2.5 × 1013 vg/kg) was injected into the saphenous vein under high pressure.
Gait assessment
Dogs walked on a 0.9m × 7m instrumented carpet (GAITRite Electronic Walkway, CIR Systems Inc.) at 6 time points, 8-, 12- 16-, and 21 weeks of age (comparison with wild type male controls), as well as at 1 and 2 years after treatment (comparison with carrier female controls). A trained handler walked alongside leashed dogs in alternating directions parallel to the instrumented carpet at the dog’s self-selected pace. Food reward, praise, or squeaker toys were used as encouragement. Walks were selected based on consistency of gait pattern and speed and were analyzed using proprietary software (GAITFour version 4.1, CIR Systems Inc). Gait analysis with the instrumented carpet provided measures of gait speed, stride length, stride time, step length, step time, and stance time. These parameters were evaluated as outcome measures likely to display changes over time due to disease progression or subsequent recovery as a result of treatment.23
Video motion capture data were simultaneously recorded in the middle 2.4 meters of the instrumented carpet in front of a dark cloth backdrop at 8, 12, and 16 weeks of age. As previously described,23 retro-reflective tape was placed on the greater trochanter of the femur, a point equidistant between the lateral epicondyle of the femur and the fibular head, the lateral malleolus of the distal tibia, and the distolateral aspect of the fifth metatarsus. A spotlight was used during video capture for maximal marker brightness. Video was filmed with a single camera (DCR-SX63, Sony, Japan) at 30 Hz. The quality of videos was assessed based on consistency of the gait pattern and straightness of the head, body, and the walking path trajectory. Videos were digitized with MaxTRAQ software (Standard version 2.2.4.2) and exported to Visual 3D (C-Motion, Inc., Germantown, USA). Markers were filtered with a second-order bidirectional lowpass Butterworth filter with a cutoff frequency of 4 Hz, and heel-strike and toe-off events were identified for calculation of spatiotemporal measures across strides. Gait analysis with video motion capture provided measures of gait speed, stride length, and stride time.
Analysis
To compare spatiotemporal measures by the different gait analysis methods, the correlation between stride length and stride time using video motion capture and the instrumented carpet was calculated at the 16-week time point. This time point was selected due to the larger number of useful walks. At earlier time points, the young age of dogs, less experience with leash training, and smaller size of the dogs reduced the quality of video digitization. To compare gait data between XLMTM and control dogs, spatiotemporal parameters collected from the instrumented carpet were evaluated longitudinally and reported as means and standard deviations. Where relevant, measures outside of the 95% confidence intervals are indicated as deviations from control values in lieu of statistical comparisons with P-values.33,34
Strength Assessment
We followed our published protocols for dogs.35 Briefly, hindlimb torque values were measured in anesthetized dogs by placing the foot of the dog on a pedal that was attached to a motor that provides resistance to the extending or flexing muscle while also measuring the amount of torque produced by said muscle. The muscle was then stimulated using percutaneously inserted electrodes over a range of stimulation frequencies to determine torque-frequency relationships. Year 3 assessments were performed on the left limbs, and year 4 assessments were performed on the right.
Pulmonary Function
Respiratory assessment was carried out in anesthetized dogs before and after stimulation by a centrally acting stimulant, doxapram hydrochloride (1 mg/kg), as described. 36–38 Briefly, data were collected in intubated dogs using a calibrated pneumotachometer, where changes in pressure across the device determined airflow. Flow-volume loops were created by replaying experimental data through the appropriate analyzer (iox 2.8.0.13, EMKA Technologies) and capturing 10 consecutive breaths during doxapram stimulation. Software (EMKA Technologies) calculated peak inspiratory flow (PIF), peak expiratory flow (PEF), inspired volume (IV), expired volume (EV), inspiratory time (TI), and expiratory time (TE).
Neurological Function
A neurological assessment score (NAS) was assigned to each dog by a board-certified veterinary neurologist (JMS) as described39. Parameters assessed included: cranial nerve function, postural reactions, segmental spinal reflexes, gait stride length, ability to run and jump, and muscle atrophy. Dogs were observed for exercise intolerance or increased respiratory rate and effort following activity. The presence or absence of a dropped jaw (inability to hold the jaw in a closed position) was also noted. Following each examination, a neurological severity score was assigned on a scale of 10 to 1, with 10 indicating a normal examination and 1 indicating inability to maintain sternal recumbency. However, predetermined humane euthanasia criteria were usually met by an NAS score of 3. Results of neurological scoring for normal controls and for some untreated XLMTM dogs have been reported 39.
Histology
Muscle samples were collected and processed as described previously.40 Tissues from the vastus lateralis, cranialis tibilialis, and biceps brachii were collected from both wild-type control dogs and AAV8-MTM1 infused dogs. Muscle histology was performed based on staining with hematoxylin and eosin (H&E), reduced nicotinamide adenine dinucleotide (NADH), and ATPase stain performed at pH 9.4. Slides were evaluated by a board-certified anatomic pathologist and neuropathologist (M.W.L.) with respect to the full range of possible pathologies. Muscle tissue (vastus lateralis) was fixed in 2.5% glutaraldehyde, processed at the Medical College of Wisconsin electron microscopy (EM) Core Facility and evaluated at each time point using a standard approach previously described.22,41 Comprehensive reports of pathological findings at the light and EM level were prepared using an adaptation of the NINDS Common Data Elements muscle biopsy reporting form. 42
Vector copy number and transgene expression
The number of vector genomes per diploid genome (VCN) was quantified from 32 ng of total DNA by Taqman real-time PCR using a Light Cycler 480 (Roche). The canine β-glucuronidase gene was used for standardization. Primers used for vector genome amplification were: 5′-GCCTCGCCCGGACTCTA-3′ (forward), 5′-CTCAGGATCGGTGACCAGAGA-3′ (reverse) and 5′-AGGATCCAGATCTAAGC-3′ (probe). Primers and probe used for β-glucuronidase amplification were: 5′-ACGCTGATTGCTCACACCAA-3′ (forward), 5′-CCCCAGGTCTGCTTCATAGTTG-3′ (reverse) and 5′-CCCGGCCCGTGACCTTTGTGA-3′ (probe) (Applied Biosystems).
Immunoblotting
Proteins were extracted from tissues using a lysis buffer containing 10 mM Tris HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2 mM Na orthovanadate, 100 mM NaF, 4 mM sodium pyrophosphate, 1% Triton X-100, 0.5% IGEPAL, and a Protease Inhibitor Cocktail (Roche Applied Science), and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (NuPage Novex 4–12% BisTris gels, Invitrogen) and western blotting as previously described (6). Membranes were probed with a polyclonal antibody against the C-terminus of canine myotubularin (R1040, Genethon) and a mouse monoclonal antibody against GAPDH (MAB374, Millipore) as internal control. Detection was performed with a secondary antibody coupled to IRDye 680 (LI-COR) and the Odyssey infrared imaging system (LI-COR Biotechnology Inc.).
RESULTS
Dog demographics
XLMTM dogs had lower average body weights prior to treatment at 8 weeks of age (4.7 kg) as compared to their wildtype littermates (6.4 kg) despite being close in size as measured by hindlimb length (XLMTM, 12.6 cm; wildtype controls, 13.5 cm; Table 1). By 16 weeks of age, XLMTM and wildtype control dogs were of similar mass and sizes, with mean weight of 10.9 kg and limb length of 16.6 cm for XLMTM dogs, and mean weight of 10.4 kg and limb length of 17.3 cm for wildtype control animals. XLMTM and carrier control dogs also demonstrated comparable weights and hindlimb lengths at 1 and 2 years of age (Table 1). At 1 year, mean weight was 13.6 kg for XLMTM dogs and 14.1 (1.2) kg for carrier controls, and mean hindlimb length was 17.9 cm for XLMTM dogs and 17.3 (1.1) cm for carrier controls. At 2 years, mean weight was 21.0 kg for XLMTM dogs and 22.0 (2.5) kg for carrier controls, and mean hindlimb lengths were 18.5 cm for XLMTM dogs and 17.5 (0.4) cm for carrier controls. At 3 years, the mean weight was 18 kg for XLMTM dogs and 15 kg for carrier controls, and mean hindlimb lengths were 18 cm for XLMTM dogs and 15 cm for carrier controls. By 4 years, XLMTM dogs demonstrated greater body weight (22 vs. 18 kg) with longer hindlimb lengths (18.4 vs. 17 cm) compared to their age-matched carrier controls (Table 1).
Table 1.
Demographics for XLMTM dogs (N=2), wildtype controls (N=2), and carrier controls (N=3) for years 1 and 2, (N=2) for years 3 and 4.
| Genotype | XLMTM | Wildtype | Carrier | |||||
|---|---|---|---|---|---|---|---|---|
| Dog Identifier | 1 | 2 | 1 | 2 | 1 | 2 | 3 | 4 |
| Gender | M | M | M | M | F | F | F | F |
| Body mass (kg) | ||||||||
| T8w | 4.5 | 4.9 | 6.0 | 6.7 | -- | -- | -- | -- |
| T16w | 10.5 | 11.2 | 10.0 | 10.8 | -- | -- | -- | -- |
| T1y | 12.7 | 14.5 | -- | -- | 15.1 | 12.7 | 14.5 | -- |
| T2y | 19.2 | 22.7 | -- | -- | 23.1 | 19.1 | 23.7 | -- |
| T3y | 18 | 18 | -- | -- | -- | 16.7 | -- | 13.3 |
| T4y | 21.6 | 22.8 | -- | -- | -- | 18 | -- | 18 |
| Limb length (cm) | ||||||||
| T8w | 12.3 | 13.2 | 13.3 | 13.7 | -- | -- | -- | |
| T16w | 16.0 | 17.1 | 17.0 | 17.6 | -- | -- | -- | -- |
| T1y | 17.4 | 18.4 | -- | -- | 17.6 | 16.1 | 18.2 | -- |
| T2y | 18.0 | 18.9 | -- | -- | 17.9 | 17.1 | 17.4 | -- |
| T3y | 18.0 | 18.9 | -- | -- | -- | 17.5 | -- | 16.5 |
| T4y | 18.0 | 18.8 | -- | -- | -- | 17.6 | -- | 16.5 |
8 weeks of age (T8w); 16 weeks of age (T16w), 1 year (T1y), 2 years (T2y), 3 years (T3y), and 4 years of age (T4y).
Comparison of longitudinal gait changes in AAV-infused XLMTM and control dogs over 4 years
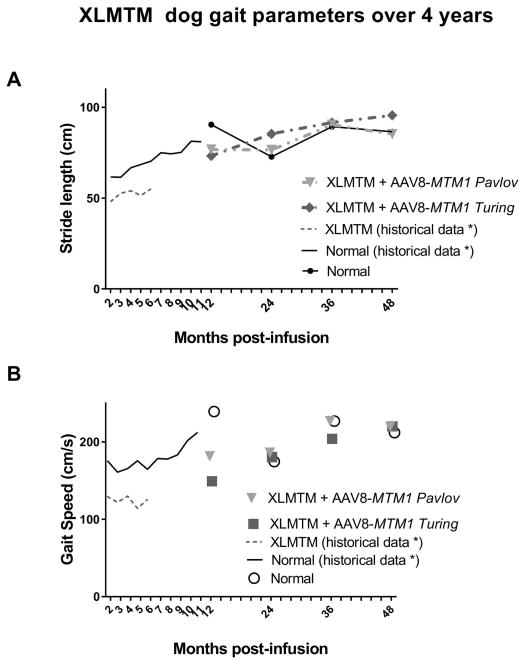
To evaluate effects of AAV on limb function and mobility, gait was evaluated while dogs walked over an instrumented carpet to capture spatiotemporal data. Four years after infusion with AAV8-MTM1, XLMTM dogs demonstrated walking gait speed and stride lengths comparable to age-matched control dogs (Figure 1). Prior to infusion, gait speed in XLMTM dogs was slightly reduced at 8 weeks of age, where XLMTM dogs walked 7% more slowly than control dogs (Table 2). However, following AAV infusion XLMTM dogs walked at speeds comparable to controls at 12, 16, and 21 weeks of age. Gait speed was maintained in XLMTM dogs over 4 years after treatment. In normal carriers, gait speed was 20% faster than XLMTM dogs at 1 year, but XLMTM gait speed was comparable to controls at 2, 3, and 4 years after treatment (Figure 1A, Table 2). After 3 years post-infusion, XLMTM dogs exhibited 10% and 0.03% slower gait speeds, respectively compared to age-matched controls. Four years post-infusion, both XLMTM dogs demonstrated 4% faster gait speeds when compared to their age-matched controls.
Figure 1.
(A) Gait speed over a 4-year period in XLMTM dogs (Pavlov and Turing) infused with AAV8-MTM1 vector remains comparable with age-matched wild-type controls. (B) Stride length over a 4-year period in XLMTM dogs infused with AAV8-MTM1 vector remains comparable with age-matched wild-type controls. Data points represent mean values.
Table 2.
Gait speed, and stride length over time for XLMTM dogs compared to normal wildtype and carrier controls.
| T8w | T12w | T16w | T21w | T1y | T2y | T3y | T4y | |
|---|---|---|---|---|---|---|---|---|
| Gait speed (cm/s) | ||||||||
| XLMTM | 110.9 (39.0) | 172.4 (15.1) | 187.5 (7.7) | 185.2 (6.6) | 186.1 (21.8) | 183.7 (6.6) | 215.5 (15.9) | 219.9 (.3) |
| Wildtype | 119.3 (10.3) | 175.4 (3.0) | 192.5 (2.4) | -- | -- | -- | -- | -- |
| Carrier | -- | -- | -- | -- | 233.4 (12.1) | 174.7 (11.6) | 226.9 (7.3) | 211.9 (46) |
| Stride length (cm) | ||||||||
| XLMTM | 42.3 (3.1) | 64.5 (1.5) | 71.5 (2.8) | 78.1 (0.9) | 84.1 (0.4) | 79.9 (5.5) | 90.9 (.8) | 90.4 (7.4) |
| Wildtype | 50.5 (1.1) | 67.8 (2.8) | 74.5 (3.5) | -- | -- | -- | -- | -- |
| Carrier | -- | -- | -- | -- | 90.1 (10.9) | 72.8 (8.7) | 89.3 (1.1) | 86.5 (12.6) |
8 weeks of age (T8w); 12 weeks of age (T12w); 16 weeks of age (T16w), 1 year (T1y), 2 years (T2y), 3 years (T3y), and 4 years of age (T4y). Data indicate mean and standard deviation (SD).
Stride lengths in XLMTM dogs were 16% shorter than controls at 10 weeks of age but were comparable to controls at all other time points as measured by the instrumented carpet (Table 2). Two years after treatment, XLMTM dogs demonstrated 10% longer strides than control dogs (Table 2). At 3 years after treatment, XLMTM dogs had 3% and 1% longer strides, respectively, than control dogs. At 4 years after treatment, 1 XLMTM dog had 2% shorter strides, while the other demonstrated 10% longer strides compared to controls (Table 2).
Respiratory function in AAV-infused XLMTM dogs compared to controls
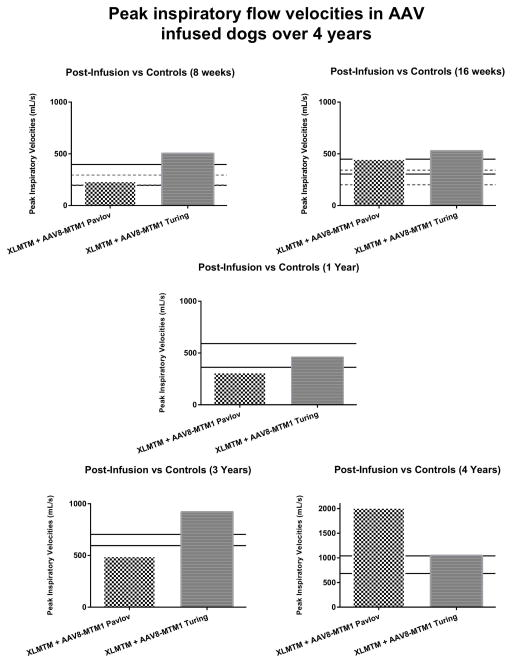
To test for effects of AAV on respiratory function, we evaluated airflow exchange in anesthetized dogs. Peak inspiratory flow (PIF) velocity appears comparable between AAV-treated XLMTM and normal controls over time (Figure 2). PIF velocity for the AAV-infused dog, Pavlov, remained lower than both the controls and the AAV-infused dog, Turing. Year 1 assessments show that Pavlov’s PIF value was approximately 35% less than the average PIF for the normal controls; this difference decreased to 22% at the 3-year assessment. During the 4-year assessment, Pavlov’s PIF was 133% greater than the controls and increased 286% when compared to his year 3 average PIF. Turing’s PIF values at the 1-year assessment were approximately 3% less than the controls, however his values surpassed the controls at years 3 and 4.
Figure 2. Peak inspiratory flow velocities in AAV infused dogs over 4 years.
PIF velocities after a single infusion of AAV8-MTM1 in XLMTM dogs measured after 2 and 4 months, and 1, 3, and 4 years compared to age-matched controls. Legend: Solid lines represents WT control SD values; dotted line represents the XLMTM untreated dogs’ SD values.
Neurological function in AAV-infused XLMTM dogs remain at near-normal levels after 4 years
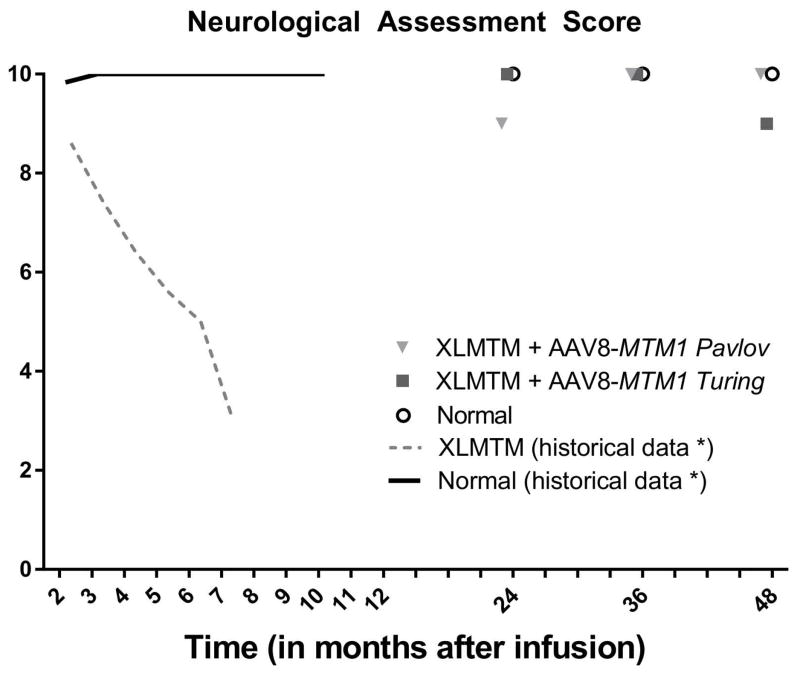
To measure changes in neurological function, we used a validated clinical scoring instrument developed for dogs, the Neurological Assessment Score (NAS). Results indicate that 2 and 3 years post-infusion, both XLMTM treated dogs achieved scores identical to controls; at 4 years one of the AAV-infused dogs (Turing) declined by 1 point (Figure 3).
Figure 3. Neurological assessment score in XLMTM dogs over 4 years.
Neurological assessment score in XLMTM dogs infused with AAV8-MTM1 remain comparable with age-matched WT controls. Legend: Solid line represents WT data; dotted line represents XLMTM untreated data. Data shown are mean values.
Limb Strength in AAV-infused XLMTM dogs approaches normal function 4 years post-infusion
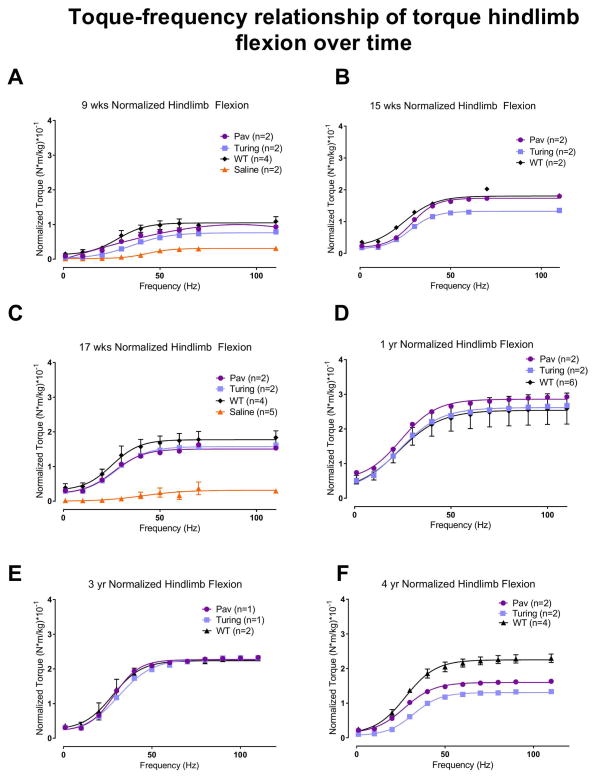
To measure strength, in vivo isometric flexion torque was repeatedly measured in the hind limbs of anesthetized dogs (Figure 4). Hindlimb flexion torque values for AAV-treated XLMTM dogs were greater than saline-infused dogs at 9- and 17 weeks, and generally comparable with WT controls at most time points up to 3 years. At the 4 year assessment, torque values of AAV-treated XLMTM dogs dropped to about half values of WT controls. Overall, hindlimb flexion torque data indicate that following AAV infusion, strength in XLMTM dogs was maintained comparable to normal age-matched controls for 3 years then began to decline.
Figure 4. Torque-frequency relationship of torque hindlimb flexion over time.
Hindlimb flexion torque values of XLMTM dogs treated with a single dose of AAV8-MTM1 and age-matched WT controls, over time. A. 9 week flexion torque; B. 15 week flexion torque; C. 17 week flexion torque D. 1 year flexion torque E. 3 year flexion torque F. 4 year flexion torque. Data shown are mean and SEM.
Muscle pathology in AAV-infused XLMTM dogs is absent 4 years following treatment
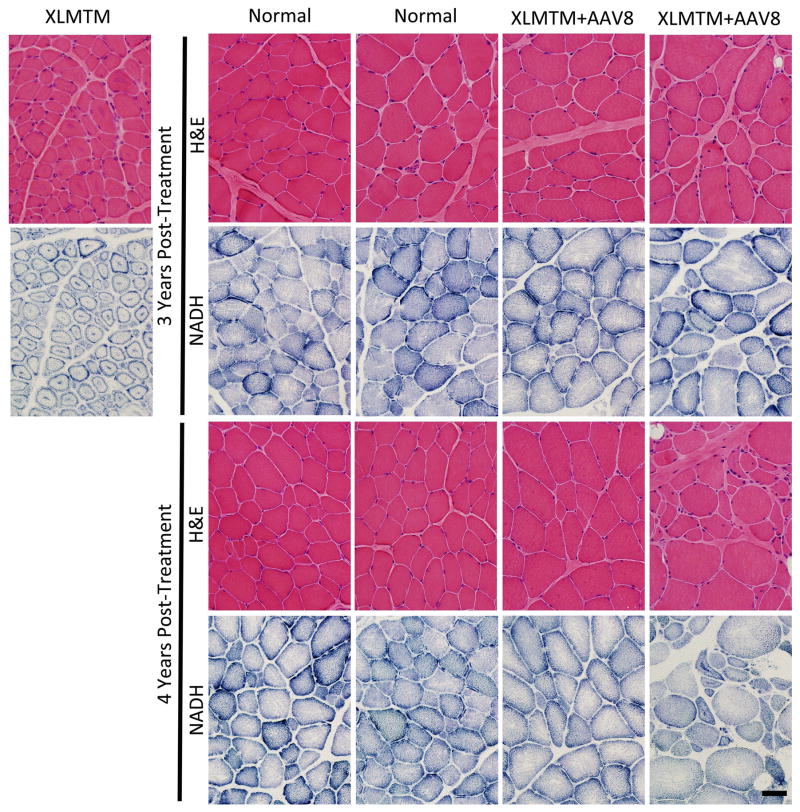
To examine effects of AAV on muscle pathology, a blinded neuropathologist examined biopsies from the vastus lateralis, cranial tibialis, and biceps brachii muscles. H&E staining of XLMTM dogs treated with AAV could not be distinguished from normal controls in almost all sampled muscles (Figure 5), with the exception of the 4 year, biceps brachii muscle of Turing. This specimen displayed myofiber smallness, internal nucleation, and organelle mislocalization consistent with XLMTM in a subset of fibers, and these changes were not apparent in the 3-year biopsy from the same muscle.43 In other sampled muscles from WT and XLMTM dogs, there was no evidence of organelle mislocalization or pathological myofiber smallness or internal nucleation. There were also occasional small fibers present in all dogs, consistent with our observation of the skeletal muscle histology of non-diseased dogs in this colony in prior studies.43,22 There was no evidence of inflammation, myonecrosis, myophagocytosis, myofiber regeneration, endomysial fibrosis, or fatty infiltration. Electron microscopy of sampled muscles revealed easily identifiable triad structures in both WT and XLMTM dog muscles, and animal genotype could not be distinguished on the basis of EM findings (not shown). Overall, the findings are consistent with a near-total reversal of XLMTM pathology at 3 and 4 years post-treatment, with a possible waning of pathological recovery in the biceps brachii from Turing at 4 years post-treatment.
Figure 5. Pathological features of XLMTM muscle.
Pathological features of XLMTM muscle remain dramatically improved in AAV8-MTM1 treated canines at 1, 3, and 4 years post-treatment. Features of XLMTM muscle pathology in untreated XLMTM canines at 22 weeks are shown (XLMTM, top left). The Wild Type condition illustrates normal histopathological features, including appropriate fiber size and peripherally located nuclei on H&E staining and an even distribution of organelles on NADH staining. The XLMTM Untreated condition illustrates histopathological findings typical for canine XLMTM, including myofiber hypertrophy, internally nucleated myofibers, and organelle mislocalization (clustering of organelles at the center and periphery of myofibers on NADH staining). A single infusion of AAV8-MTM1 resulted in correction of fiber size, nuclear placement, and organelle localization at 1, 3, and 4 years post-treatment. Black bar = 40 microns.
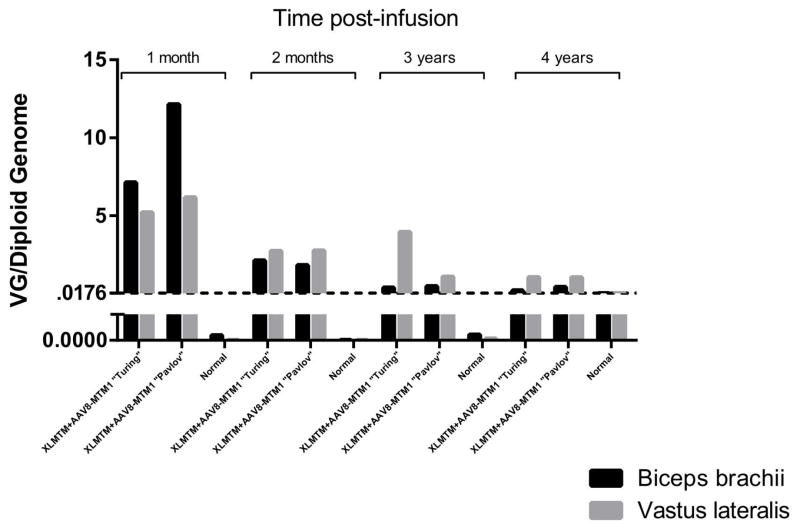
AAV vector and transgene expression
We analyzed VCN from muscle biopsies taken in anesthetized XLMTM dogs. Data over 4 years show an overall consistent decrease of VCN (Figure 6). The control group remained below the limit of detection at all time points. Myotubularin transgene expression (Supplemental Figure S1, available online) generally reflected VCN in immunoblot images. Whereas no detectable myotubularin expression bands could be seen in any XLMTM dog infused with saline, clearly defined bands could be seen in muscle lysates taken from biceps, cranial tibialis, and vastus muscles 4 years post-infusion with AAV.
Figure 6. AAV vector copy number over time.
XLMTM dogs show steady decreases in vector genome (VG)/diploid genome values over 4 years after a single infusion of AAV8-MTM1 vector. Normal indicates wildtype (non-infused) control samples. The dashed horizontal line indicates the lowest standard recorded (0.0176VG/diploid genome); Values beneath the dashed line are below the lowest standard. Legend: dark black bars, biceps brachii muscle; grey bars, vastus lateralis muscle.
DISCUSSION
Our findings indicate that a single infusion of recombinant AAV serotype 8 carrying a canine MTM1 gene driven by a desmin promoter can preserve and maintain limb function over 4 years in a large animal model of a congenital myopathy. Our findings in the p.N155K canine XLMTM model further suggest that MTM1 gene replacement using AAV8 in young patients might similarly lead to sustained preservation of muscle strength and neurologic function. Our recent report of AAV8 experiments in this canine model should inform future clinical trials with respect to dosing and timing of AAV infusion.32
The technical aim of our study addressed the comparison of spatiotemporal measures with the more time-intensive video-based motion capture analysis. Our results indicate a high correlation between measures of stride length from both video-based motion analysis simultaneous assessment with an instrumented carpet. Correlations were lower for stride time, despite similar values. This may be due to the small sample size as well as technical aspects of the video motion capture. The video motion capture in this case had relatively low temporal resolution, with a low frame rate for video capture. As stride time values are relatively small, even slight differences will reduce correlation. Both the instrumented carpet and 2-dimensional video motion capture are validated methods of quantifying spatiotemporal parameters of gait in canines.44,45,30 Unlike the instrumented carpet, video motion capture can also be used to assess kinematic or angular joint movements. With modification, video motion capture can be expanded to assess bilateral movements in 3-dimensional space to provide additional information on spatiotemporal and kinematic aspects of gait. However, video capture requires a large space to accommodate and correctly position the multiple cameras needed for appropriate capture volumes. Set up and analysis of the carpet is simpler, requires less training, and is largely automated, which allows rapid and reliable assessment of several animals with fewer resources. In contrast, multiple types of software may be necessary to convert, digitize, and analyze video capture data, resulting in more time-intensive data processing and increase potential for loss of data integrity. As there is limited additional value of hindlimb angular kinematic measurements in the XLMTM dog,23 the instrumented carpet is our preferred method. However, for canine models where changes in joint angles are observed due to disease, such as the golden retriever muscular dystrophy model,27 kinematic analysis at a higher resolution using motion capture methods may be preferable.
We previously reported that XLMTM dogs walk more slowly that their normal control littermates. They experienced a 13% decrease in speed at 10 weeks of age, and a decrease of 46% of normal by the time of humane euthanasia at 17 weeks of age.23 Strides were also reduced to 86% of normal at 10 weeks of age and 68% of normal by the terminal endpoint.23 However, normal gait involves the concerted effort of several limb muscles to maintain stability and support as the disease advances. In this study, we found that, while XLMTM dogs walk more slowly and with shorter strides prior to receiving AAV8-MTM1, whole body gene replacement therapy is associated with improved and maintained speed and stride length over 2 years, indicating preserved ambulation and limb function. This is in keeping with increased limb muscle strength and improvements in myofiber size, myotubularin expression, and in histology, particularly the sarcotubular organization in hindlimb muscles, such as the quadriceps and biceps.22 As upper limb function is of particular concern to patients, similar changes in the forelimbs are also encouraging,22 and strength and functional assessment separate from the hindlimb in the future may be useful.
In summary, our findings indicate that AAV-mediated gene replacement can preserve normal muscle and neurological function in a large animal model of XLMTM, a congenital myopathy for which there is currently no effective clinical therapy. An instrumented carpet can be useful to monitor effectiveness of investigational gene replacement on limb function in this model.
Supplementary Material
Acknowledgments
We thank K. Williams, A. Spangler Dean, Mandy Lockard, Emily Burlingame, MacKenzie Johnson, S. Browne, Shelby Hamm, Kate Bukovec and Emily Troiano for their contribution to these experiments.
Funding: Association Française contre les Myopathies (France) to A.B.-B. and M.K.C.; Myotubular Trust (UK) to A.B.-B.; Genopole d’Evry (France) to A.B.-B.; INSERM (France) to A.B.-B.; U.S. NIH grants R21 AR064503 and R01 HL115001 to M.K.C.; R01AR044345 and HD075802 to A.H.B., and K08 AR059750 to M.W.L.; Muscular Dystrophy Association MDA383249 to A.H.B. and MDA/MVP to M.K.C.; the Joshua Frase Foundation and Where There’s a Will There’s a Cure to A.H.B. and M.K.C.; Peter Khuri Myopathy Research Foundation to M.K.C.; and Senator Paul D Wellstone Muscular Dystrophy Cooperative Research Center, Seattle (NIH U54AR065139).
A.H.B. has received support from, and/or has served as a paid consultant for Audentes Therapeutics. A.B-B. has received support from, and/or has served as a paid consultant for Audentes Therapeutics. M.K.C. has received support from, and/or has served as a paid consultant for Audentes Therapeutics. M.W. L. has received support from, and/or has served as a paid consultant for Audentes Therapeutics.
Abbreviations
- XLMTM
X-linked myotubular myopathy
- AAV
adeno-associated virus
- VCN
vector copy number
- AAV8
AAV serotype 8
- DMD
Duchenne muscular dystrophy
- ROM
range of motion
- IACUC
Institutional Animal Care and Use Committee
- T8w
8 weeks
- T12w
12 weeks
- T16w
16 weeks
- T21w
21 weeks
- T1y
1 year
- T2y
2 years
- T3y
3 years
- T4y
4 years
- PIF
peak inspiratory flow
- PEF
peak expiratory flow
- IV
inspired volume
- EV
expired volume
- TI
inspiratory time
- TE
expiratory time
- NAS
neurological assessment score
- H&E
hematoxylin and eosin
- NADH
reduced nicotinamide adenine dinucleotide
- SD
standard deviation
- SEM
standard error of mean
- WB
western blot
- WT
wildtype
Footnotes
The remaining authors have no conflicts of interest.
References
- 1.Jungbluth H, Wallgren-Pettersson C, Laporte J. Centronuclear (myotubular) myopathy. Orphanet J Rare Dis. 2008;3:26. doi: 10.1186/1750-1172-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biancalana V, Beggs AH, Das S, Jungbluth H, Kress W, Nishino I, North K, Romero NB, Laporte J. Clinical utility gene card for: Centronuclear and myotubular myopathies. Eur J Hum Genet. 2012;20(10) doi: 10.1038/ejhg.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amburgey K, McNamara N, Bennett LR, McCormick ME, Acsadi G, Dowling JJ. Prevalence of congenital myopathies in a representative pediatric united states population. Ann Neurol. 2011;70(4):662–665. doi: 10.1002/ana.22510. [DOI] [PubMed] [Google Scholar]
- 4.Laporte J, Hu LJ, Kretz C, Mandel JL, Kioschis P, Coy JF, Klauck SM, Poustka A, Dahl N. A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet. 1996;13(2):175–182. doi: 10.1038/ng0696-175. [DOI] [PubMed] [Google Scholar]
- 5.Laporte J, Guiraud-Chaumeil C, Tanner SM, Blondeau F, Hu LJ, Vicaire S, Liechti-Gallati S, Mandel JL. Genomic organization of the MTM1 gene implicated in X-linked myotubular myopathy. Eur J Hum Genet. 1998;6(4):325–330. doi: 10.1038/sj.ejhg.5200189. [DOI] [PubMed] [Google Scholar]
- 6.Kim SA, Taylor GS, Torgersen KM, Dixon JE. Myotubularin and MTMR2, phosphatidylinositol 3-phosphatases mutated in myotubular myopathy and type 4B Charcot-Marie-Tooth disease. J Biol Chem. 2002;277(6):4526–4531. doi: 10.1074/jbc.M111087200. [DOI] [PubMed] [Google Scholar]
- 7.Wallgren-Pettersson C, Clarke A, Samson F, Fardeau M, Dubowitz V, Moser H, Grimm T, Barohn RJ, Barth PG. The myotubular myopathies: differential diagnosis of the X linked recessive, autosomal dominant, and autosomal recessive forms and present state of DNA studies. J Med Genet. 1995;32(9):673–679. doi: 10.1136/jmg.32.9.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McEntagart M, Parsons G, Buj-Bello A, Biancalana V, Fenton I, Little M, Krawczak M, Thomas N, Herman G, Clarke A, Wallgren-Pettersson C. Genotype-phenotype correlations in X-linked myotubular myopathy. Neuromuscul Disord. 2002;12(10):939–946. doi: 10.1016/s0960-8966(02)00153-0. [DOI] [PubMed] [Google Scholar]
- 9.Kristiansen M, Knudsen GP, Tanner SM, McEntagart M, Jungbluth H, Muntoni F, Sewry C, Gallati S, Ørstavik KH, Wallgren-Pettersson C. X-inactivation patterns in carriers of X-linked myotubular myopathy. Neuromuscul Disord. 2003;13(6):468–471. doi: 10.1016/s0960-8966(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 10.van Wijngaarden GK, Fleury P, Bethlem J, Meijer AE. Familial “myotubular” myopathy. Neurology. 1969;19(9):901–908. doi: 10.1212/wnl.19.9.901. [DOI] [PubMed] [Google Scholar]
- 11.Herman GE, Kopacz K, Zhao W, Mills PL, Metzenberg A, Das S. Characterization of mutations in fifty North American patients with X-linked myotubular myopathy. Hum Mutat. 2002;19(2):114–121. doi: 10.1002/humu.10033. [DOI] [PubMed] [Google Scholar]
- 12.Yu S, Manson J, White S, Bourne A, Waddy H, Davis M, Haan E. X-linked myotubular myopathy in a family with three adult survivors. Clin Genet. 2003;64(2):148–152. doi: 10.1034/j.1399-0004.2003.00118.x. [DOI] [PubMed] [Google Scholar]
- 13.Biancalana V, Caron O, Gallati S, Baas F, Kress W, Novelli G, D’Apice MR, Lagier-Tourenne C, Buj-Bello A, Romero NB, Mandel JL. Characterisation of mutations in 77 patients with X-linked myotubular myopathy, including a family with a very mild phenotype. Hum Genet. 2003;112(2):135–142. doi: 10.1007/s00439-002-0869-1. [DOI] [PubMed] [Google Scholar]
- 14.Hoffjan S, Thiels C, Vorgerd M, Neuen-Jacob E, Epplen JT, Kress W. Extreme phenotypic variability in a German family with X-linked myotubular myopathy associated with E404K mutation in MTM1. Neuromuscul Disord. 2006;16(11):749–753. doi: 10.1016/j.nmd.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Beggs AH, Böhm J, Snead E, Kozlowski M, Maurer M, Minor K, Childers MK, Taylor SM, Hitte C, Mickelson JR, Guo LT, Mizisin AP, Buj-Bello A, Tiret L, Laporte J, Shelton GD. MTM1 mutation associated with X-linked myotubular myopathy in Labrador Retrievers. Proc Natl Acad Sci U S A. 2010;107(33):14697–14702. doi: 10.1073/pnas.1003677107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosford KL, Taylor SM, Thompson L, Shelton GD. A possible new inherited myopathy in a young Labrador retriever. Can Vet J. 2008;49(4):393–397. [PMC free article] [PubMed] [Google Scholar]
- 17.Amoasii L, Hnia K, Chicanne G, Brech A, Cowling BS, Müller MM, Schwab Y, Koebel P, Ferry A, Payrastre B, Laporte J. Myotubularin and PtdIns3P remodel the sarcoplasmic reticulum in muscle in vivo. Journal of cell science. 2013;126(Pt 8):1806–1819. doi: 10.1242/jcs.118505. [DOI] [PubMed] [Google Scholar]
- 18.Snead EC, Taylor SM, van der Kooij M, Cosford K, Beggs AH, Shelton GD. Clinical phenotype of x-linked myotubular myopathy in labrador retriever puppies. J Vet Intern Med. 2015;29(1):254–260. doi: 10.1111/jvim.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snyder JM, Meisner A, Mack D, Goddard M, Coulter IT, Grange R, Childers MK. Validity of a Neurological Scoring System for Canine X-Linked Myotubular Myopathy. Hum Gene Ther Clin Dev. 2015;26(2):131–137. doi: 10.1089/humc.2015.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goddard MA, Mack DL, Czerniecki SM, Kelly VE, Snyder JM, Grange RW, Lawlor MW, Smith BK, Beggs AH, Childers MK. Muscle pathology, limb strength, walking gait, respiratory function and neurological impairment establish disease progression in the p. N155K canine model of X-linked myotubular myopathy. Annals of translational medicine. 2015;3(18):262–278. doi: 10.3978/j.issn.2305-5839.2015.10.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grange RW, Doering J, Mitchell E, Holder MN, Guan X, Goddard M, Tegeler C, Beggs AH, Childers MK. Muscle function in A canine model of X-linked myotubular myopathy. Muscle Nerve. 2012;46(4):588–591. doi: 10.1002/mus.23463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Childers MK, Joubert R, Poulard K, Moal C, Grange RW, Doering JA, Lawlor MW, Rider BE, Jamet T, Daniele N, Martin S, Riviere C, Soker T, Hammer C, Van Wittenberghe L, Lockard M, Guan X, Goddard M, Mitchell E, Barber J, Williams JK, Mack DL, Furth ME, Vignaud A, Masurier C, Mavilio F, Moullier P, Beggs AH, Buj-Bello A. Gene therapy prolongs survival and restores function in murine and canine models of myotubular myopathy. Sci Transl Med. 2014;6(220):220ra210. doi: 10.1126/scitranslmed.3007523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goddard MA, Burlingame E, Beggs AH, Buj-Bello A, Childers MK, Marsh AP, Kelly VE. Gait characteristics in a canine model of X-linked myotubular myopathy. J Neurol Sci. 2014;346(1–2):221–226. doi: 10.1016/j.jns.2014.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Krogt MM, Delp SL, Schwartz MH. How robust is human gait to muscle weakness? Gait Posture. 2012;36(1):113–119. doi: 10.1016/j.gaitpost.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan Z, Kocis K, Valley R, Howard JF, Chopra M, An H, Lin W, Muenzer J, Powers W. Safety and feasibility of high-pressure transvenous limb perfusion with 0. 9% saline in human muscular dystrophy. Mol Ther. 2012;20(2):456–461. doi: 10.1038/mt.2011.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott OM, Hyde SA, Goddard C, Dubowitz V. Quantitation of muscle function in children: a prospective study in Duchenne muscular dystrophy. Muscle Nerve. 1982;5(4):291–301. doi: 10.1002/mus.880050405. [DOI] [PubMed] [Google Scholar]
- 27.Marsh AP, Eggebeen JD, Kornegay JN, Markert CD, Childers MK. Kinematics of gait in golden retriever muscular dystrophy. Neuromuscul Disord. 2010;20(1):16–20. doi: 10.1016/j.nmd.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barthélémy I, Pinto-Mariz F, Yada E, Desquilbet L, Savino W, Silva-Barbosa SD, Faussat AM, Mouly V, Voit T, Blot S, Butler-Browne G. Predictive markers of clinical outcome in the GRMD dog model of Duchenne muscular dystrophy. Dis Model Mech. 2014;7(11):1253–1261. doi: 10.1242/dmm.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris GF, Wertsch JJ. Procedures for gait analysis. Arch Phys Med Rehabil. 1994;75(2):216–225. [PubMed] [Google Scholar]
- 30.Gillette RL, Angle TC. Recent developments in canine locomotor analysis: a review. Vet J. 2008;178(2):165–176. doi: 10.1016/j.tvjl.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Light VA, Steiss JE, Montgomery RD, Rumph PF, Wright JC. Temporal-spatial gait analysis by use of a portable walkway system in healthy Labrador Retrievers at a walk. Am J Vet Res. 2010;71(9):997–1002. doi: 10.2460/ajvr.71.9.997. [DOI] [PubMed] [Google Scholar]
- 32.Mack DL, Poulard K, Goddard MA, Latournerie V, Snyder JM, Grange RW, Elverman MR, Denard J, Veron P, Buscara L, Le Bec C, Hogrel JY, Brezovec AG, Meng H, Yang L, Liu F, O’Callaghan M, Gopal N, Kelly VE, Smith BK, Strande JL, Mavilio F, Beggs AH, Mingozzi F, Lawlor MW, Buj-Bello A, Childers MK. Systemic AAV8-Mediated Gene Therapy Drives Whole-Body Correction of Myotubular Myopathy in Dogs. Mol Ther. 2017 doi: 10.1016/j.ymthe.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cumming G. Replication and p Intervals p Values Predict the Future Only Vaguely, but Confidence Intervals Do Much Better. Perspectives on Psychological Science. 2008;3(4):286–300. doi: 10.1111/j.1745-6924.2008.00079.x. [DOI] [PubMed] [Google Scholar]
- 34.Goodman SN, Berlin JA. The use of predicted confidence-intervals when planning experiments and the misuse of power when interpreting results. Annals of Internal Medicine. 1994;121(3):200–206. doi: 10.7326/0003-4819-121-3-199408010-00008. [DOI] [PubMed] [Google Scholar]
- 35.Childers MK, Grange RW, Kornegay JN. In vivo canine muscle function assay. Journal of visualized experiments: JoVE. 2011;(50) doi: 10.3791/2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coglianese CJ, Peiss CN, Wurster RD. Rhythmic phrenic nerve activity and respiratory activity in spinal dogs. Respiration physiology. 1977;29(3):247–254. doi: 10.1016/0034-5687(77)90001-9. [DOI] [PubMed] [Google Scholar]
- 37.Yost CS. A new look at the respiratory stimulant doxapram. CNS drug reviews. 2006;12(3–4):236–249. doi: 10.1111/j.1527-3458.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller CJ, McKiernan BC, Pace J, Fettman MJ. The effects of doxapram hydrochloride (dopram-V) on laryngeal function in healthy dogs. J Vet Intern Med. 2002;16(5):524–528. doi: 10.1892/0891-6640(2002)016<0524:teodhd>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Snyder JM, Meisner A, Mack D, Goddard M, Coulter IT, Grange R, Childers MK. Validity of a Neurological Scoring System for Canine X-Linked Myotubular Myopathy. Hum Gene Ther Clin Dev. 2015;26(2):131–137. doi: 10.1089/humc.2015.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meng H, Janssen PM, Grange RW, Yang L, Beggs AH, Swanson LC, Cossette SA, Frase A, Childers MK, Granzier H, Gussoni E, Lawlor MW. Tissue triage and freezing for models of skeletal muscle disease. Journal of visualized experiments: JoVE. 2014;(89) doi: 10.3791/51586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawlor MW, Armstrong D, Viola MG, Widrick JJ, Meng H, Grange RW, Childers MK, Hsu CP, O’Callaghan M, Pierson CR, Buj-Bello A, Beggs AH. Enzyme replacement therapy rescues weakness and improves muscle pathology in mice with X-linked myotubular myopathy. Human molecular genetics. 2013;22(8):1525–1538. doi: 10.1093/hmg/ddt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dastgir J, Rutkowski A, Alvarez R, Cossette SA, Yan K, Hoffmann RG, Sewry C, Hayashi YK, Goebel HH, Bonnemann C, Lawlor MW. Common Data Elements for Muscle Biopsy Reporting. Archives of pathology & laboratory medicine. 2016;140(1):51–65. doi: 10.5858/arpa.2014-0453-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lawlor MW, Beggs AH, Buj-Bello A, Childers MK, Dowling JJ, James ES, Meng H, Moore SA, Prasad S, Schoser B, Sewry CA. Skeletal Muscle Pathology in X-Linked Myotubular Myopathy: Review With Cross-Species Comparisons. Journal of neuropathology and experimental neurology. 2016;75(2):102–110. doi: 10.1093/jnen/nlv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bilney B, Morris M, Webster K. Concurrent related validity of the GAITRite walkway system for quantification of the spatial and temporal parameters of gait. Gait Posture. 2003;17(1):68–74. doi: 10.1016/s0966-6362(02)00053-x. [DOI] [PubMed] [Google Scholar]
- 45.Marsolais GS, McLean S, Derrick T, Conzemius MG. Kinematic analysis of the hind limb during swimming and walking in healthy dogs and dogs with surgically corrected cranial cruciate ligament rupture. J Am Vet Med Assoc. 2003;222(6):739–743. doi: 10.2460/javma.2003.222.739. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.