Abstract
Objective
Although children’s pain memories have been shown to be a powerful predictor of subsequent pain experiences in acute procedural and experimental pain settings, little is known about the influence of children’s and parents’ pain memories on children’s future pain experiences in other painful contexts. This study used a dyadic approach to examine the roles of children’s and parents’ memories of pain on their subsequent reporting of post-surgical pain several months after the child underwent a major surgical procedure.
Methods
The sample included 66 parent-child dyads (Mage youth = 14.73 years, SD = 2.01) recruited from two tertiary level pediatric hospitals. At baseline, children and parents reported on their catastrophic thinking about the child’s pain. Parent and child reports of child pain were collected at approximately 1 month and 5 months post-surgery. At 2–4 months post-surgery, children’s and parents’ memories for post-surgical pain were assessed.
Results
Results revealed that children’s, but not parents’, pain memories were a strong predictor of subsequent pain experienced at 5 months post-surgery. Children’s and parents’ memories for pain did not influence each others’ subsequent pain reporting.
Conclusions
Findings suggest that children’s pain memories influence their continued recovery from post-surgical pain and may contribute to pain persistence. Implications for intervention and prevention are discussed.
Keywords: children, adolescents, parents, memory, pain, surgery
Memories for pain are post-injury behaviors (as indicated by recall for and avoidance of cues associated with the injury) that some have argued can be reliably observed and that alter future pain responses (de C Williams, 2016). Memories for pain are integral in pain assessment and treatment; indeed, some have conceived a goal of pain treatment as involving the extinction of pain memories (Flor, 2012). Further, it has recently been proposed that this cognitive aspect of pain be incorporated into the definition of pain (de C Williams & Craig, 2016), thereby emphasizing its critical importance in the pain experience. Research suggests that pain memories are a potential mechanism underlying the individual experience of pain trajectories and chronicity, with the hippocampus being centrally involved in the transition of pain from an acute to a chronic state (Mutso et al., 2014). Moreover, pain memories are by nature malleable and reconstructive, particularly in childhood (Jaaniste, Noel, & von Baeyer, 2016). Therefore, children’s pain memories are an underutilized and fruitful intervention target in pain management interventions to reduce distress at future pain events (Chen, Zeltzer, Craske, & Katz, 1999; Marche, Briere, & von Baeyer, 2016; Noel, 2016).
Although the importance of explicit pain memories lies in their proposed influence on subsequent pain experience (Noel, Chambers, McGrath, Klein, & Stewart, 2012a; Noel, Pavlova, McCallum, & Vinall, 2017; von Baeyer, Marche, Rocha, & Salmon, 2004), this has not been widely studied in pediatric samples. In children and adolescents (aged 3–18 years) with cancer undergoing lumbar punctures and healthy children (aged 8–12 years) undergoing experimental (cold pressor) pain, youth who developed negatively estimated memories of pain (i.e., recalled pain greater than initial report) 1–2 weeks later subsequently experienced more distress, pain, and/or fear at re-exposure to the same pain stimulus (Chen, Zeltzer, Craske, & Katz, 2000; Noel, Chambers, et al., 2012a). Moreover, children’s memories for experimental pain were found to be more predictive of subsequent pain than the initial experience of pain itself and fully mediated the relationship between initial and subsequent pain reporting (Noel, Chambers, et al., 2012a). Nevertheless, little is known about the influence of children’s pain memories on subsequent pain experiences over longer periods of time and in other painful clinical (i.e., non-needle) contexts such as surgery. Given the high prevalence (King et al., 2011) and debilitating nature of pediatric chronic pain (Palermo, 2000), and the modest, short-term effects of current psychological treatments (Eccleston, 2014), enhanced understanding of modifiable mechanisms, like memory, in the persistence of pain has important implications for intervention and prevention.
Pediatric surgery is a particularly compelling context within which to study children’s pain memory development, given that a subgroup of these youth are at risk for experiencing persistence of their postsurgical pain for months and years later (Rabbitts, Zhou, Groenewald, Durkin, & Palermo, 2015; Sieberg et al., 2013). Therefore, surgery offers a unique context within which to examine the potential role of pain memories in the transition of pain from an acute to a persistent state. To date, only one study has investigated pain memories following pediatric surgery (Noel, Rabbitts, Tai, & Palermo, 2015). Children and parents who tended to think in more catastrophic ways about child pain prior to surgery went on to develop more distressing memories of pain 2–4 months later (Noel, Rabbitts, et al., 2015). However, that study was limited in that it did not examine the relationship between children’s pain memories following surgery and their actual experience of continued recovery-related pain to address the question of whether memories may relate to persistence of pain post-surgery. The influence of parents’ memories of pain on children’s subsequent pain experience has also not been examined, despite being posited as important in influencing parent-child interactions about pain and pain cognitions (Noel, Chambers, Petter, et al., 2012; Noel, Palermo, Chambers, Taddio, & Hermann, 2015). Indeed, other parental cognitions about child pain (e.g., parental pain catastrophizing) have emerged as important predictors of persistent post-surgical pain in youth one year following surgery (Rabbitts, Zhou, et al., 2015).
The objective of this research was to examine the roles of children’s and parents’ memories of pain on their subsequent reports of continued post-surgical pain several months later. Using a dyadic analytic approach, we examined influences of pain memory on report of subsequent pain within individuals (actor effects) and between parent-child dyads (partner effects). Similar to previous research on children’s acute procedural (Chen et al., 2000) and experimental (Noel, Chambers, et al., 2012a) pain, we hypothesized that those youth who developed more negative pain memories after surgery would report higher levels of subsequent post-surgical pain, while controlling for initial pain reports. Likewise, we hypothesized that parents’ pain memories would be related to their own reporting of their children’s subsequent pain. Finally, given the posited role of pain memories on parent-child interactions about pain and pain cognitions (Noel, Chambers, Petter, et al., 2012), we tested the exploratory hypothesis that partner effects would occur wherein children’s pain memories influenced parents’ later reporting of child pain and vice versa.
Method
The data for this paper were collected as part of two larger longitudinal cohort studies examining psychological and behavioral predictors of post-surgical pain trajectories. The current paper examines a research question that is distinct from other published papers using parts of this dataset, which examined predictors of acute post-surgical pain (Rabbitts, Groenewald, Tai, & Palermo, 2015), pain trajectories over a 1-year follow-up (Rabbitts, Zhou, et al., 2015), and the role of pain catastrophizing in children’s and parents’ memories of post-surgical pain (Noel, Rabbitts, et al., 2015).
Participants and Setting
Participants included 85 parent-child dyads enrolled in one of two longitudinal studies examining predictors of post-surgical pain at pediatric hospitals in the Northwestern United States (Seattle Children’s Hospital; n = 74) and Eastern Canada (IWK Health Centre; n = 11). Ethics approval for this study was obtained from the institutional review boards (IRB) of Seattle Children’s Hospital (Seattle, Washington, United States) and the IWK Health Centre (Halifax, Nova Scotia, Canada). These 85 parents and children were enrolled and provided written consent after being identified for eligibility from the surgery schedules at the respective sites over a 4-year period. Eligibility criteria included child age of 10–18 years and undergoing major elective surgery (e.g., pectus repair, spinal fusion). These surgeries have been the focus of previous research on children’s post-surgical pain (Connelly et al., 2014; Kotzer, 2000; Page, Stinson, Campbell, Isaac, & Katz, 2012; Sieberg et al., 2013; Wong, Yuen, Chow, & Irwin, 2007) and pain memory development (Noel, Rabbitts, et al., 2015).
Exclusion criteria consisted of an inability to speak English, chronic or complex health conditions such as cancer, neurodegenerative or neurological disorders, mental health conditions, or prior history of major surgery. Of the 85 dyads, 19 dyads were missing data from either the child or parent on one or more of the covariates, predictors, or outcomes. These data were missing due to either an inability to make contact with these participants within the time period allocated for participation in the memory study (i.e., between 2–4 months following surgery) or failure to complete assessments at one of the time points. Therefore, the final sample used for the analyses included 66 youth (68.2% female; Mage = 14.73 years, SD = 2.01, Range = 10–18 years) undergoing surgery and their parents (91% mothers). These included 58 participants from Seattle Children’s Hospital and 8 participants from the IWK Health Centre site. The majority (63.6%) of participants had a household annual income greater than $70,000/year. The majority (91%) of youth were white.
Procedure
There were four key time-points captured for the purposes of the present study: the week prior to surgery (Time 0; baseline), 2–6 weeks post-surgery (Time 1; pain assessment), 2–4 months post-surgery (Time 2; memory interview), and 4–6 months post surgery (Time 3; pain assessment). At baseline, youth completed measures of catastrophic thinking about pain and pain intensity; parents completed measures of socio-demographics, parental catastrophic thinking about child pain, and parent-report of child pain intensity (described below). The pain assessments conducted at Time 1 (2–6 weeks post-surgery) and Time 3 (4–6 months post-surgery) consisted of parents and youth each completing a measure of the child’s average pain intensity. Participants received a modest honorarium for completing these assessments.
The memory interviews were conducted at Time 2 (2–4 months post-surgery; M = 78.86 days, SD = 15.96 days, Range = 52–121 days) by a researcher via telephone. Time frames of one week to one year have been used in previous research on children’s memory for pain (Badali, Pillai, Craig, Giesbrecht, & Chambers, 2000; Chen et al., 2000). The individual assessment interval between Time 2 and Time 3 was not related to pain reports or pain memories.
Measures
Demographics
Information on race/ethnicity was obtained via parent report on a demographic questionnaire. Child age and sex were obtained from medical records.
Pain Intensity – Child
Children rated their average pain intensity on an 11-point Numerical Rating Scale (NRS) ranging from 0 (“no pain”) to 10 (“worst pain possible”/ “worst pain you can imagine”). NRS measures to assess self-reported pain intensity are deemed to be both valid and reliable for use with youth as young as 8 years of age (von Baeyer et al., 2009) and in the peri-operative setting (Page, Katz, et al., 2012).
Pain Intensity – Parent
Parents’ perception of their children’s pain intensity was also rated using an 11-point numerical rating scale ranging from 0 (“no pain”) to 10 (“worst pain possible”/ “worst pain you can imagine”). The same NRS scale was used to assess parents’ ratings of child pain intensity in previous research on children’s post-operative pain (Rabbitts, Palermo, Zhou, & Mangione-Smith, 2015).
Memory
Between 2 and 4 months following surgery, a memory interview was conducted with parents and children. To avoid biasing recall, parents and youth were asked to each complete the memory interview independently in a private space. Participants were asked to remember the levels of pain that youth experienced in the first few weeks after surgery. The memory questions were directly adapted from the NRS scales administered to parents and youth during pain assessments. Prior to answering each memory question, children and parents were first asked to remember when the child was at home in the first few weeks following surgery. The memory questions required them to rate the average level of pain intensity that they remembered that they/their child experienced during that time using the same 11-point NRS. These memory questions were used in a previous research study on pain memories following pediatric surgery (Noel, Rabbitts, et al., 2015). Telephone interviews for research on children’s memory for pain have been effectively conducted with children and adolescents (Lander, Hodgins, & Fowler-Kerry, 1992; Noel, Chambers, et al., 2012a; Noel, Chambers, McGrath, Klein, & Stewart, 2012b; Noel, McMurtry, Chambers, & McGrath, 2010; Zonneveld, McGrath, Reid, & Sorbi, 1997).
Pain Catastrophizing Scale – Child and Parent versions (PCS-C and PCS-P)
The PCS-C is a 13-item self-report measure that assesses children’s catastrophic thoughts and feelings about their pain (Crombez et al., 2003). The PCS-P is a 13-item self-report measure that assesses catastrophic thoughts and feelings that parents may have when their child experiences pain (Goubert, Eccleston, Vervoort, Jordan, & Crombez, 2006). Items on both the PCS-C and PCS-P are rated on a 5-point Likert scale yielding a total score incorporating 3 domains: rumination, magnification, and helplessness. On the PCS-C, children rate the extent to which they experience each thought or feeling when they are in pain (“When I have pain I feel I can’t go on”). On the PCS-P, parents rate the extent to which they experience each thought or feeling when their child is in pain (“When my child is in pain, I feel I can’t go on like this much longer”). Lower scores indicate less rumination, magnification, or helplessness about child pain. The PCS-C and PCS-P have been found to have good validity and reliability in youth with pain (Crombez et al., 2003) and their parents (Goubert et al., 2006). For the purposes of the present study, the PCS-C and PCS-P total scores were used as covariates in the analyses.
Data Analysis
All statistical analyses were conducting using SPSS version 19. Data were evaluated for accuracy, normality, and linearity; all assumptions for the model used were met. Preliminary analyses were conducted separately for parents and children to determine the interrelationships among pain catastrophizing, memory for pain, and pain intensity. As recommended by Kenny, Kashy, and Cook (2006), Pearson product-moment correlations with dyad as the unit of analysis were conducted to examine the degree of non-independence between parents and children on major study variables (see Table 2, diagonal).
Table 2.
Intercorrelations Among Study Variables
| Variables | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| 1. Pain Catastrophizing | .32** | .25* | .17 | .06 |
| 2. Memory for Pain | .24* | .42** | .56** | .36** |
| 3. Pain Intensity T2 | .23* | .42** | .39** | .30** |
| 4. Pain Intensity T3 | .06 | .04 | .23* | .62** |
Note. Child correlations are above the diagonal, parent correlations are below the diagonal; tests for nonindependence are bolded along the diagonal.
p <.05
p <. 01
The Actor-Partner Interdependence Model (APIM) (Kenny et al., 2006) was tested using Kenny (2014) macro for APIM with distinguishable dyads. This macro tests a standard dyadic design, using multilevel modeling to estimate the effect an individual’s predictor variable has on his/her own outcome (actor effect), as well as the effect it has on his/her partner’s outcome (partner effect), and vice versa. The APIM allows for testing of actor and partner effects while simultaneously estimating the residual non-independence in outcome scores for both members of the dyad. Prior to performing these analyses, the independent variables and covariates were centered and the data were restructured into pairwise format (Popp, West, & Kenny, 2006). APIM analyses were based on 66 out of 85 dyads; 12 dyads were removed from the dataset prior to analyses based on one or more partners missing data on a covariate, and an additional 7 dyads had data missing on either the independent variable (pain memory; 2 dyads) or the dependent variable (5 dyads; see Kenny (2014). When examining demographic and other variables, participants who were excluded from the analyses due to missing data significantly differed from those included based on parental report of child pain at Time 3 (excluded: M = 4.13, SD = 2.47; included: M = 2.00, SD = 1.65; t (72) = −3.24, p <.01). To determine whether replacing the missing data would meaningfully change the results, the analyses were also performed after mean imputing missing values. Because the overall pattern and statistical significance of the results and size of effects remained unchanged, the results of the original analytic strategy are reported.
Our model estimated the actor and partner effects of memory for child pain at home on perception of child pain intensity at 4–6 months post-surgery. Parent and child pain catastrophizing and initial pain intensity ratings corresponding to each respective memory question were included as covariates in the models.
Results
Descriptive statistics for key study variables are presented in Table 1. Moderate-severe pain (NRS ≥ 4; Gerbershagen, Rothaug, Kalkman, & Meissner, 2011; Voepel-Lewis, Burke, Jeffreys, Malviya, & Tait, 2011) was reported by 30% of participants at Time 3 (4–6 months after surgery). Independent samples t-tests and chi square analyses revealed that the two research sites did not differ on any of the demographic variables: child age, child sex, or child race (ps > .05). The majority (77%) of surgeries were elective spinal fusion for idiopathic spinal deformities (idiopathic scoliosis, spondylolisthesis, or kyphosis). Preliminary analyses revealed that youth undergoing spinal fusion recalled higher levels of pain during the memory interview (M = 5.56, SD = 1.93; M = 4.29, SD = 1.49; t (63) = −2.29, p < .05) and their parents reported greater child pain at Time 3 (M = 2.24, SD = 1.62; M = 1.21, SD = 1.63; t (63) = −2.09, p < .05) than children undergoing other surgeries (pectus repair or hip/femur osteotomy). In addition, parent (but not child) memory of child pain was related to the time at which the memory interviews were completed (r = .26, p < .05). Nevertheless, when surgery type and timing of memory interviews (i.e., the number of days between the surgery and memory interviews) were entered as covariates in the models, the pattern of findings did not change. In addition, when child age was included as a covariate in the models, the pattern of results did not change. Given these patterns of results, we report analyses without these covariates.
Table 1.
Means, Standard Deviations, and Ranges for Study Variables
| Variable | M(SD) | Observed Range |
|---|---|---|
| Child Report | ||
| Pain Catastrophizing (Baseline) | 13.92 (9.49) | 0–47 |
| Pain Intensity (Time 1) | 4.06 (2.06) | 0–9 |
| Memory for Pain (Time 2) | 5.36 (2.00) | 2–9 |
| Pain Intensity (Time 3) | 2.54 (2.00) | 0–7 |
| Parent Report | ||
| Pain Catastrophizing (Baseline) | 16.57 (10.89) | 0–49 |
| Pain Intensity (Time 1) | 3.47 (2.01) | 0–9 |
| Memory for Pain (Time 2) | 4.5 (1.74) | 1–9 |
| Pain Intensity (Time 3) | 2.22 (1.86) | 0–7 |
Note: There were four key time-points captured for the purposes of the present study: pre-surgery (Time 0; baseline), 2–6 weeks post-surgery (Time 1; pain assessment), 2–4 months post-surgery (Time 2; memory interview), and 4–6 months post surgery (Time 3; pain assessment).
Correlations Among Study Variables
A series of bivariate correlations showed that children’s and parents’ pain catastrophizing, memories for pain, and pain intensity ratings at Time 1 and Time 3 were positively related to each other (Table 2). Child and parent pain catastrophizing at baseline was not related to their pain ratings 4–6 months following surgery (ps < .05). Children’s, but not parents’, pain memories were related to subsequent reporting of higher pain intensity at 4–6 months following surgery (r = .36, p < .01).
APIM: Memory for Pain Predicting Pain Intensity
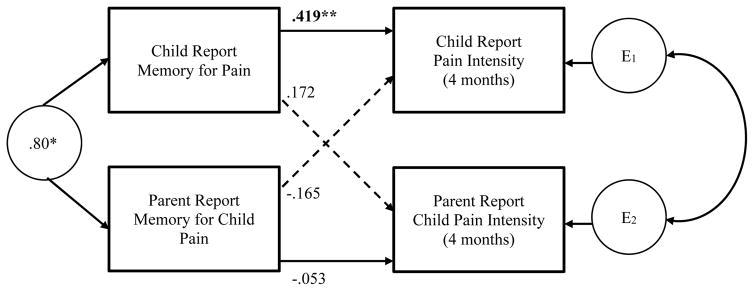
The APIM for distinguishable dyads was used to determine the dyadic effects of memory for pain on subsequent pain intensity. First, we estimated the association between an individual’s memory for child pain at home and his/her perception of child pain intensity at 4–6 months, while controlling for baseline pain catastrophizing and initial pain reports (see Figure 1, actor effects). Consistent with hypotheses, there was a significant actor effect of memory for pain on subsequent perceived pain intensity for children (b = .419, p < .003), and this was a medium effect size (β = .415). No significant actor effect was observed for parents (b = −.053, p = .708). The difference between the two effects was statistically significant (p = .027). These findings suggest that over and above baseline catastrophic thinking about pain and initial pain reports, children’s recall of higher levels of pain intensity in the acute recovery period at home (based on the memory interview) predicts higher levels of experienced pain intensity at 4–6 months post-surgery. In contrast, parents’ memory for child pain during the acute recovery period at home was not predictive of their own subsequent reporting of child pain at 4–6 months.
Figure 1.
Actor-Partner Interdependence Model: Memory for Pain and Subsequent Pain Intensity. Numbers reported are unstandardized estimates. ** p < .005
Next, we estimated the association between memory for child pain and partner’s report of child pain intensity at 4–6 months, while controlling for baseline pain catastrophizing and initial pain reports (see Figure 1, partner effects). No partner effects emerged as significant. Parents’ memory for child pain intensity was not associated with children’s self-reports of pain intensity at 4–6 months post–surgery (b = −.165, p = .252). Likewise, children’s memory for pain was not associated with their parents’ reporting of child pain at 4–6 months (b = .172, p = .171) (see Figure 1).
Post-hoc Power Analysis
At the time of study conceptualization, a power analysis algorithm had not been specifically developed for the APIM. Kenny, Kashy, and Cook (2006) have previously recommended a minimum of 25 dyads to test for non-independence. They further estimate a sample size of 80 dyads would have approximately 80% power to detect small to medium effects in the standard APIM model. Since this study was conducted, an experimental application has been developed to estimate power for the APIM with distinguishable dyads (Ackerman & Kenny, 2016). Per this program, 60 dyads are needed to have 80% power to detect average actor and partner effect sizes of .25. Conducting a post hoc analysis of power using this program revealed 95% power to detect our significant actor effect. 2,313 dyads would be needed for the parent actor effect observed in our study to reach statistical significance. 225–244 dyads would have been needed for our observed partner effects to achieve statistical significance. We are thus confident that we achieved adequate power to detect meaningful actor and partner effects in our analyses (Ackerman & Kenny, 2016; Kenny et al., 2006).
Discussion
This research is the first to examine the influence of both child and parent memories of acute post-surgical pain on subsequent pain experiences during recovery from major surgery. In line with our hypotheses and in keeping with previous research on children’s acute procedural (i.e., lumbar punctures; Chen et al. (2000) and experimental (Noel, Chambers, et al., 2012a) pain, children’s memories for pain following surgery were a strong predictor of their continued experience of post-surgical pain 4–6 months later. Specifically, over and above child and parent catastrophic thinking about pain before surgery, and initial pain reports in the acute recovery phase following surgery, more negative child pain memories were associated with more intense post-surgical pain several months later. Contrary to our hypotheses, parents’ memories of child pain were not predictive of their subsequent ratings of child pain, nor did children’s and parents’ memories for pain influence each other’s subsequent pain reporting. Given that pain memories were found to predict pain during a time when persistent post-surgical pain can develop, these findings suggest that pain memories could be a contributing factor for the transition from acute to chronic post-surgical pain in children.
The findings of this research are consistent with previous research on children’s pain memories. In both needle and experimental pain contexts, and among youth with and without chronic illness (cancer), more negatively biased memories for pain predicted more pain and greater distress at subsequent pain experiences (Chen et al., 2000; (Noel, Chambers, et al., 2012a). Youth with higher levels of anxiety were more likely to develop negatively biased pain memories (Noel, Chambers, et al., 2012a). The current findings are an important extension of this previous work by showing that once youth develop pain memories, they may influence subsequent recovery from pain following surgery. Moreover, given that none of the youth had previously undergone major surgery, this study examined the development of novel pain memories. Converging evidence across samples experiencing different types of pain in different settings underscores the robustness of the effect of children’s pain memories on subsequent pain experience. Nevertheless, the pediatric surgery context is particularly unique in that evidence suggests that some (15%) youth will go on to develop chronic pain up to several years later (Sieberg et al., 2013), thereby enabling examination of factors involved in transition to a chronic state. Conceptual models of chronic pain suggest that the influence of pain cognitions and behaviors (of the child and parent) changes as pain becomes chronic and entrenched over time (Asmundson, Parkerson, Petter, & Noel, 2012) and developmental changes in pain memory development have been proposed (Noel, Palermo, et al., 2015). Surprisingly, very little research has examined explicit autobiographical pain memories in youth with chronic pain; as such, it is unknown whether memories and their influence would be different in this population and along this spectrum of pain.
Existing memory studies in chronic pain have used word stimuli (pain related sensory, affective, and neutral words) in youth with arthritis, which revealed a pain-specific bias (Koutantji, Pearce, Oakley, & Feinmann, 1999). Similarly, retrospective ratings of pain complaints in youth with abdominal pain (Chogle et al., 2012), headache (van den Brink, Bandell-Hoekstra, & Abu-Saad, 2001) and arthritis (Stinson et al., 2014), yielded equivocal results (i.e., some studies indicated a tendency to overestimate pain whereas others suggested that youth underestimate their pain). None of these studies examined the influence of pain memories on subsequent pain experience. Future work is needed to add further support to understanding the role of pain memories on subsequent experience of chronic pain in children.
These findings can be contextualized within a developmental framework. Although children’s memories influenced their own subsequent reporting of pain at 4–6 months following surgery, parents’ memories of child pain were not predictive of subsequent pain reports of their postsurgical pain. Contrary to our exploratory hypothesis, the dyadic analyses did not reveal partner effects. The lack of parental influence on subsequent pain reporting may be due to the fact that the vast majority of youth in our sample were adolescents, a developmental period marked by increasing autonomy from parents (Piaget, 1973). Indeed, developmental frameworks of pain memory development in childhood position parental influences (e.g., cognitions, parent-child interactions) on child pain as being most important during the preschool and early school-age years and then diminishing in importance relative to peer influences by adolescence (Noel, Palermo, et al., 2015). These findings are also consistent with those found in the pediatric stress literature. Although parents have been shown to modulate the stress responses of infants and children, they have limited effect on the stress responses of adolescents (Doom, Hostinar, VanZomeren-Dohm, & Gunnar, 2015; Hostinar, Johnson, & Gunnar, 2015). In addition, research suggests that parenting responses change as pain becomes chronic, increasingly resembling parenting that is indicative of younger developmental stages (Jordan, Eccleston, & Osborn, 2007). It could be that the influence of parents’ pain memories is stronger among youth who develop post-surgical pain problems versus the majority of youth who experience a resolution, or relatively lower levels, of pain over time. Future longitudinal research is needed to determine the relative importance of parent factors on children’s post-surgical pain and at which point(s) on the pain trajectory these factors exert their greatest influence.
Children’s pain memories are a modifiable intervention target that could alter pain trajectories, reducing pain after surgery. Pain memories are fragile and have been shown to be susceptible to manipulation through post-event information (Bruck, Ceci, Francoeur, & Barr, 1995; Chen et al., 1999; Marche et al., 2016; Pickrell et al., 2007). In the context of needle procedures (e.g., lumbar punctures, anesthetic injections, vaccine injections), brief memory reframing interventions that involve discussing past painful events with children in more positive/accurate ways have been shown to reduce pain and distress at future procedures (Bruck et al., 1995; Chen et al., 1999; Noel, McMurtry, Pavlova, & Taddio, 2017; Pickrell et al., 2007). Moreover, a recent experimental study showed that having children recall positive details of a pain memory led to enhanced forgetting of the memory’s negative aspects (Marche et al., 2016). Children in this study who had a greater ability to forget negative aspects of the pain memory were less anxious prior to an experimental pain induction (cold pressor) task (Marche et al., 2016). Taken together, this suggests that brief language-based interventions designed to reframe children’s pain memories to be more positive/accurate or that facilitate forgetting of its negative aspects can alter subsequent pain experience. To our knowledge, pain memory interventions have not yet been developed for the management of children’s post-surgical pain but could offer promise for altering post-surgical pain trajectories, particularly among those at risk for developing negative memory biases. In addition to pain memory reframing interventions, there is some evidence that pain management interventions (e.g., topical anesthetics, distraction) may buffer against the development of negative biases in pain memories following vaccine injections (Cohen et al., 2001). Indeed, pain is a robust predictor of pain memory development (Noel, Chambers, et al., 2012a; Noel, Rabbitts, et al., 2015). Thus, interventions that target children’s initial pain experience in addition to subsequent memory biases are likely to be most efficacious in reducing post-surgical pain.
This study had limitations that can be addressed in future research. Assessment of pain memories only included the sensory (i.e., intensity) aspect of the pain experience and was limited to use of a single-item pain measure. Like the experience of pain, pain memories are multidimensional and include sensory, affective, and contextual aspects of the original pain experience (Ornstein, Manning, & Pelphrey, 1999). Future research should assess these various aspects of the pain memory and also incorporate assessment of free recall (i.e., a narrative of the recalled event that is elicited using open-ended prompts) to provide a richer, multifaceted account of what is remembered following surgery. Given that the data were collected across two sites, there were methodological (e.g., timing of assessments) and clinical (across patients, providers, and sites) differences that could have introduced noise to the data. On the other hand, significant results across these different contexts can also provide more confidence in the robustness of the memory-pain relationship and increase generalizability of the findings. Indeed, the relationship between children’s pain memories and subsequent pain reporting was a strong finding (i.e., medium effect size) that mirrors research on acute pain found across children, adults, and animals (Chen et al., 2000; de C Williams, 2016; Gedney & Logan, 2006; Noel, Chambers, Petter, et al., 2012). This study did not examine moderators or mediators of the memory-pain relationships. Future research should examine factors such as daily functioning (e.g., school participation, engagement in social activities), sleep, and emotional functioning/mood, which may shed light on the linkage between children’s memories and subsequent pain experience. Moreover, measures of catastrophic thinking about pain were not administered across the various time-points. Future research should examine the relationship between changes in pain beliefs and pain memories following surgery. In addition, we assessed parents’ memories of their children’s pain. It is possible that parents’ memories of their own emotional experience surrounding their children’s surgeries may influence their interactions with their children and their children’s subsequent pain experience. Similar to previous research on children’s memory for pain, we did not assess children’s current pain experience during the memory interview; therefore, it was not possible to separate recalled and current pain. The majority of participants reported a household annual income of greater than $70,000 per year, children were predominantly white and female, and the majority of parents were mothers; as such, investigation of similar processes in more diverse populations is warranted and one should hesitate before generalizing these findings to more diverse populations. Finally, although there were very few missing data and the missing data that were in the dataset were spread over several different variables, parents who reported higher child pain at Time 3 were more likely to have missing values. They (and their children) may be underrepresented in the sample; consequently, our sample and results may not include children experiencing the full range of post-surgical pain responses.
Future research is needed to shed light on the development of pediatric post-surgical pain and the relative influences of pain memories on trajectories of risk and resilience in these youth. Children’s pain memory development is dynamic and strongly influenced by cognitive and social development (Noel, Palermo, et al., 2015). As such, future research should examine post-surgical pain memories at different stages of development. It has been suggested that parent-child interactions about pain (e.g., pain narratives) following surgery, particularly for younger children, may play an important role in children’s pain cognitions and pain experiences (Noel, Chambers, Petter, et al., 2012; Simons & Sieberg, 2015). Parent-child language based interactions about pain have been shown to strongly influence children’s memories for and coping with other kinds of stressful events, including injuries (Peterson, Ross, & Tucker, 2002), and may be a more proximal predictor of children’s pain memories and post-surgical pain experience. In addition, the stability of pain memories is unknown. It could be that, like is observed in the PTSD literature, pain memories become less distressing over time for the majority of youth. Repeated assessment of pain memories over longer periods of time is needed. In addition, we assessed pain memories at one particular time point (2–4 months following surgery) and these memories were anchored to pain that youth experienced during the acute recovery phase at home. It is possible that memories assessed closer to the time of surgery and that are tied to the in-hospital time period are most susceptible to distortion.
In conclusion, this is the first study to show that children’s memories of pain following major surgery influence their subsequent experience of post-surgical pain. Over and above baseline catastrophic thinking about pain and acute post-surgical pain intensity ratings, youth who remembered pain in a more negative way went on to experience more intense post-surgical pain 4–6 months later. Parents’ memories did not influence subsequent pain reporting, suggesting that children’s cognitions may be the most important intervention target during the post-operative period for this age range. The current findings point to children’s pain memories as one potential mechanism that may underlie the persistence of pain; however, future longitudinal research is needed to further disentangle this relationship. Given the increasing prevalence (King et al., 2011) and burden (Groenewald, Wright, & Palermo, 2015) of pediatric chronic pain and its adverse impact on mental and physical health into adulthood (Noel, Groenewald, Beals-Erickson, Gebert, & Palermo, 2016; Shelby et al., 2013), this research could inform the development of cognitive-behavioral interventions to alter this course.
References
- Ackerman RA, Kenny DA. APIMPowerR: An interactive tool for Actor-Partner Interdependence Model power analysis [Computer software] 2016 Available from https://robert-a-ackerman.shinyapps.io/APIMPowerRdis/
- Asmundson GJG, Parkerson HA, Petter M, Noel M. What is the role of fear and escape/avoidance in chronic pain? Models, structural analysis, and future directions. Pain Manage. 2012;2:295–303. doi: 10.2217/pmt.12.15. [DOI] [PubMed] [Google Scholar]
- Badali MA, Pillai RR, Craig KD, Giesbrecht K, Chambers CT. Accuracy of children’s and parents’ memory for a novel pain experience. Pediatric Pain Management. 2000;5:161–168. [Google Scholar]
- Bruck M, Ceci SJ, Francoeur E, Barr R. “I hardly cried when I got my shot!” Influencing children’s reports about a visit to their pediatrician. Child Development. 1995;66:193–208. doi: 10.1111/j.1467-8624.1995.tb00865.x. [DOI] [PubMed] [Google Scholar]
- Chen E, Zeltzer LK, Craske MG, Katz ER. Alteration of memory in the reduction of children’s distress during repeated aversive medical procedures. J Consult Clin Psychol. 1999;67:481–490. doi: 10.1037//0022-006x.67.4.481. [DOI] [PubMed] [Google Scholar]
- Chen E, Zeltzer LK, Craske MG, Katz ER. Children’s memories for painful cancer treatment procedures: implications for distress. Child Development. 2000;71:933–947. doi: 10.1111/1467-8624.00200. [DOI] [PubMed] [Google Scholar]
- Chogle A, Sztainberg M, Bass L, Youssef NN, Miranda A, Nurko S, … Saps M. Accuracy of pain recall in children. J Pediatr Gastroenterol Nutr. 2012;55:288–291. doi: 10.1097/MPG.0b013e31824cf08a. [DOI] [PubMed] [Google Scholar]
- Cohen LL, Blount RL, Cohen RJ, Ball CM, McClellan CB, Bernard RS. Children’s expectations and memories of acute distress: short- and long-term efficacy of pain management interventions. J Pediatr Psychol. 2001;26:367–374. doi: 10.1093/jpepsy/26.6.367. [DOI] [PubMed] [Google Scholar]
- Connelly M, Fulmer RD, Prohaska J, Anson L, Dryer L, Thomas V, … Schwend R. Predictors of postoperative pain trajectories in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2014;39:E174–181. doi: 10.1097/BRS.0000000000000099. [DOI] [PubMed] [Google Scholar]
- Crombez G, Bijttebier P, Eccleston C, Mascagni T, Mertens G, Goubert L, Verstraeten K. The child version of the pain catastrophizing scale (PCS-C): a preliminary validation. Pain. 2003;104:639–646. doi: 10.1016/S0304-3959(03)00121-0. [DOI] [PubMed] [Google Scholar]
- de C Williams AC. What can evolutionary theory tell us about chronic pain? Pain. 2016;157:788–790. doi: 10.1097/j.pain.0000000000000464. [DOI] [PubMed] [Google Scholar]
- de C Williams AC, Craig KD. Updating the definition of pain. Pain. 2016 doi: 10.1097/j.pain.0000000000000613. [DOI] [PubMed] [Google Scholar]
- Doom JR, Hostinar CE, VanZomeren-Dohm AA, Gunnar MR. The roles of puberty and age in explaining the diminished effectiveness of parental buffering of HPA reactivity and recovery in adolescence. Psychoneuroendocrinology. 2015;59:102–111. doi: 10.1016/j.psyneuen.2015.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccleston C. Systematic review and meta-analysis of psychological therapies for children with chronic pain. Clin Pract Pediatr Psychol. 2014;39:763–782. doi: 10.1037/cpp0000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H. New developments in the understanding and management of persistent pain. Curr Opin Psychiatry. 2012;25:109–113. doi: 10.1097/YCO.0b013e3283503510. [DOI] [PubMed] [Google Scholar]
- Gedney JJ, Logan H. Pain related recall predicts future pain report. Pain. 2006;121:69–76. doi: 10.1016/j.pain.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth. 2011;107:619–626. doi: 10.1093/bja/aer195. [DOI] [PubMed] [Google Scholar]
- Goubert L, Eccleston C, Vervoort T, Jordan A, Crombez G. Parental catastrophizing about their child’s pain. The parent version of the Pain Catastrophizing Scale (PCS-P): a preliminary validation. Pain. 2006;123:254–263. doi: 10.1016/j.pain.2006.02.035. [DOI] [PubMed] [Google Scholar]
- Groenewald CB, Wright DR, Palermo TM. Health care expenditures associated with pediatric pain-related conditions in the United States. Pain. 2015;156:951–957. doi: 10.1097/j.pain.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev Sci. 2015;18:281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaaniste T, Noel M, von Baeyer CL. Young children’s ability to report on past, future and hypothetical pain states: a cognitive-developmental perspective. Pain. 2016;157:2399–2409. doi: 10.1097/j.pain.0000000000000666. [DOI] [PubMed] [Google Scholar]
- Jordan AL, Eccleston C, Osborn M. Being a parent of the adolescent with complex chronic pain: an interpretative phenomenological analysis. Eur J Pain. 2007;11:49–56. doi: 10.1016/j.ejpain.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Kenny DA. Actor-Partner Interdependence Model, Distinguishable Dyads. 2014. [Google Scholar]
- Kenny DA, Kashy DA, Cook WL. Dyadic Data Analysis. New York, NY: The Guilford Press; 2006. [Google Scholar]
- King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152:2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Kotzer AM. Factors predicting postoperative pain in children and adolescents following spine fusion. Issues Compr Pediatr Nurs. 2000;23:83–102. doi: 10.1080/01460860050121411. [DOI] [PubMed] [Google Scholar]
- Koutantji M, Pearce SA, Oakley DA, Feinmann C. Children in pain: an investigation of selective memory for pain and psychological adjustment. Pain. 1999;81:237–244. doi: 10.1016/S0304-3959(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Lander J, Hodgins M, Fowler-Kerry S. Children’s pain predictions and memories. Behav Res Ther. 1992;30:117–124. doi: 10.1016/0005-7967(92)90134-3. 0005-7967(92)90134-3 [pii] [DOI] [PubMed] [Google Scholar]
- Marche TA, Briere JL, von Baeyer CL. Children’s forgetting of pain-related memories. J Pediatr Psychol. 2016;41:220–231. doi: 10.1093/jpepsy/jsv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutso AA, Petre B, Huang L, Baliki MN, Torbey S, Herrmann KM, … Apkarian AV. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J Neurophysiol. 2014;111:1065–1076. doi: 10.1152/jn.00611.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel M. Harnessing the fragility of pain memories to help children forget: a new avenue for pediatric psychology interventions? J Pediatr Psychol. 2016;41:232–234. doi: 10.1093/jpepsy/jsv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel M, Chambers CT, McGrath PJ, Klein RM, Stewart SH. The influence of children’s pain memories on subsequent pain experience. Pain. 2012a;153:1563–1572. doi: 10.1016/j.pain.2012.02.020. [DOI] [PubMed] [Google Scholar]
- Noel M, Chambers CT, McGrath PJ, Klein RM, Stewart SH. The role of state anxiety in children’s memories for pain. J Pediatr Psychol. 2012b;37:567–579. doi: 10.1093/jpepsy/jss006. [DOI] [PubMed] [Google Scholar]
- Noel M, Chambers CT, Petter M, McGrath PJ, Klein RM, Stewart SH. Pain is not over when the needle ends: a review and preliminary model of acute pain memory development in childhood. Pain Manag. 2012;2:487–497. doi: 10.2217/pmt.12.41. [DOI] [PubMed] [Google Scholar]
- Noel M, Groenewald CB, Beals-Erickson SE, Gebert JT, Palermo TM. Chronic pain in adolescence and internalizing mental health disorders: a nationally representative study. Pain. 2016;157:1333–1338. doi: 10.1097/j.pain.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel M, McMurtry CM, Chambers CT, McGrath PJ. Children’s memory for painful procedures: the relationship of pain intensity, anxiety, and adult behaviors to subsequent recall. J Pediatr Psychol. 2010;35:626–636. doi: 10.1093/jpepsy/jsp096. [DOI] [PubMed] [Google Scholar]
- Noel M, McMurtry CM, Pavlova M, Taddio A. Brief clinical report: a systematic review and meta-analysis of pain memory reframing interventions for children’s needle procedures. Pain Practice. 2017 doi: 10.1111/papr.12572. [DOI] [PubMed] [Google Scholar]
- Noel M, Palermo TM, Chambers CT, Taddio A, Hermann C. Remembering the pain of childhood: applying a developmental perspective to the study of pain memories. Pain. 2015;156:31–34. doi: 10.1016/j.pain.0000000000000001. [DOI] [PubMed] [Google Scholar]
- Noel M, Pavlova M, McCallum L, Vinall J. Remembering the hurt of childhood: A psychological review and call for future research. Canadian Psychology-Psychologie Canadienne. 2017;58:58–68. [Google Scholar]
- Noel M, Rabbitts JA, Tai GG, Palermo TM. Remembering pain after surgery: a longitudinal examination of the role of pain catastrophizing in children’s and parents’ recall. Pain. 2015;156:800–808. doi: 10.1097/j.pain.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornstein PA, Manning EL, Pelphrey KA. Children’s memory for pain. J Dev Behav Pediatr. 1999;20:262–277. doi: 10.1097/00004703-199908000-00009. [DOI] [PubMed] [Google Scholar]
- Page MG, Katz J, Stinson J, Isaac L, Martin-Pichora AL, Campbell F. Validation of the numerical rating scale for pain intensity and unpleasantness in pediatric acute postoperative pain: sensitivity to change over time. J Pain. 2012;13:359–369. doi: 10.1016/j.jpain.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Page MG, Stinson J, Campbell F, Isaac L, Katz J. Pain-related psychological correlates of pediatric acute post-surgical pain. J Pain Res. 2012;5:547–558. doi: 10.2147/JPR.S36614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo TM. Impact of recurrent and chronic pain on child and family daily functioning: a critical review of the literature. J Dev Behav Pediatr. 2000;21:58–69. doi: 10.1097/00004703-200002000-00011. [DOI] [PubMed] [Google Scholar]
- Peterson C, Ross A, Tucker VC. Hospital emergency rooms and children’s health care attitudes. J Pediatr Psychol. 2002;27:281–291. doi: 10.1093/jpepsy/27.3.281. [DOI] [PubMed] [Google Scholar]
- Piaget J. Memory and Intelligence. New York, NY: Basic Books; 1973. [Google Scholar]
- Pickrell JE, Heima M, Weinstein P, Coolidge T, Coldwell SE, Skaret E, … Milgrom P. Using memory restructuring strategy to enhance dental behaviour. Int J Paediatr Dent. 2007;17:439–448. doi: 10.1111/j.1365-263X.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- Popp D, West T, Kenny DA. Data Restructuring using SPSS. 2006 Retrieved from http://davidakenny.net/kkc/c1/c1.htm.
- Rabbitts JA, Groenewald CB, Tai GG, Palermo TM. Presurgical psychosocial predictors of acute postsurgical pain and quality of life in children undergoing major surgery. J Pain. 2015;16:226–234. doi: 10.1016/j.jpain.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts JA, Palermo TM, Zhou C, Mangione-Smith R. Pain and health-related quality of life after pediatric inpatient surgery. J Pain. 2015;16:1334–1341. doi: 10.1016/j.jpain.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts JA, Zhou C, Groenewald CB, Durkin L, Palermo TM. Trajectories of postsurgical pain in children: risk factors and impact of late pain recovery on long-term health outcomes after major surgery. Pain. 2015;156:2383–2389. doi: 10.1097/j.pain.0000000000000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby GD, Shirkey KC, Sherman AL, Beck JE, Haman K, Shears AR, … Walker LS. Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics. 2013;132:475–482. doi: 10.1542/peds.2012-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieberg CB, Simons LE, Edelstein MR, DeAngelis MR, Pielech M, Sethna N, Hresko MT. Pain prevalence and trajectories following pediatric spinal fusion surgery. J Pain. 2013;14:1694–1702. doi: 10.1016/j.jpain.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons LE, Sieberg CB. Parents--to help or hinder pain memories in children. Pain. 2015;156:761–762. doi: 10.1097/j.pain.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson JN, Jibb LA, Lalloo C, Feldman BM, McGrath PJ, Petroz GC, … Stevens BJ. Comparison of average weekly pain using recalled paper and momentary assessment electronic diary reports in children with arthritis. Clin J Pain. 2014;30:1044–1050. doi: 10.1097/AJP.0000000000000072. [DOI] [PubMed] [Google Scholar]
- van den Brink M, Bandell-Hoekstra EN, Abu-Saad HH. The occurrence of recall bias in pediatric headache: a comparison of questionnaire and diary data. Headache. 2001;41:11–20. doi: 10.1046/j.1526-4610.2001.111006011.x. [DOI] [PubMed] [Google Scholar]
- Voepel-Lewis T, Burke CN, Jeffreys N, Malviya S, Tait AR. Do 0-10 numeric rating scores translate into clinically meaningful pain measures for children? Anesth Analg. 2011;112:415–421. doi: 10.1213/ANE.0b013e318203f495. [DOI] [PubMed] [Google Scholar]
- von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children’s memory for pain: overview and implications for practice. J Pain. 2004;5:241–249. doi: 10.1016/j.jpain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- von Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical Rating Scale (NRS-11) for children’s self-reports of pain intensity. Pain. 2009;143:223–227. doi: 10.1016/j.pain.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Wong GT, Yuen VM, Chow BF, Irwin MG. Persistent pain in patients following scoliosis surgery. Eur Spine J. 2007;16:1551–1556. doi: 10.1007/s00586-007-0361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonneveld LN, McGrath PJ, Reid GJ, Sorbi MJ. Accuracy of children’s pain memories. Pain. 1997;71:297–302. doi: 10.1016/s0304-3959(97)03379-4. S0304395997033794 [pii] [DOI] [PubMed] [Google Scholar]