Abstract
Objective
Adequate platelet reactivity is required for maintain hemostasis. However, excessive platelet reactivity can also lead to the formation of occlusive thrombi. 12-lipoxygenase (12-LOX), an oxygenase highly expressed in the platelet, has been demonstrated to regulate platelet function and thrombosis ex vivo, supporting a key role for 12-LOX in the regulation of in vivo thrombosis. However, the ability to pharmacologically target 12-LOX in vivo has not been established to date. Here, we studied the effect of the first highly selective 12-LOX inhibitor, ML355, on in vivo thrombosis and hemostasis.
Approach and Results
ML355 dose-dependently inhibited human platelet aggregation and 12-LOX oxylipin production, as confirmed by mass spectrometry. Interestingly, the antiplatelet effects of ML355 were reversed following exposure to high concentrations of thrombin in vitro. Ex vivo flow chamber assays confirmed that human platelet adhesion and thrombus formation at arterial shear over collagen were attenuated in whole blood treated with ML355 comparable to aspirin. Oral administration of ML355 in mice showed reasonable plasma drug levels by pharmacokinetic assessment. ML355 treatment impaired thrombus growth and vessel occlusion in FeCl3-induced mesenteric and laser-induced cremaster arteriole thrombosis models in mice. Importantly, hemostatic plug formation and bleeding following treatment with ML355 was minimal in mice in response to laser ablation on the saphenous vein or in a cremaster microvasculature laser-induced rupture model.
Conclusions
Our data strongly supports 12-LOX as a key determinant of platelet reactivity in vivo and inhibition of platelet 12-LOX with ML355 may represent a new class of antiplatelet therapy.
Keywords: platelets −12 LOX, platelet reactivity, thrombosis, hemostasis
Introduction
Platelet adhesion and aggregation at the site of vascular injury are essential to maintaining normal hemostasis and preventing blood loss. However, the same processes can also lead to the development of arterial thrombosis and vessel occlusion when the integrity of the vessel wall is compromised by rupture of an atherosclerotic plaque.1 Excessive platelet activation and aggregation may lead to the formation of occlusive thrombi and result in severe consequences such as myocardial infarction, ischemic stroke, and pulmonary embolism, which are the predominant causes of morbidity and mortality worldwide.2 Antiplatelet therapy is considered a gold standard for its effectiveness in preventing aberrant platelet activation and pivotal in the treatment of cardiovascular atherothrombotic events to reduce morbidity and mortality.3 Currently approved antiplatelet therapies inhibit platelet function by targeting platelet enzymes, receptors, and glycoproteins.4 While these therapeutic approaches limit platelet function, they often result in a concomitant increased risk of bleeding. Therefore, a need exists for the identification of novel antiplatelet therapeutic targets that limit bleeding.
Platelet 12(S)-lipoxygenase (12-LOX), an oxygenase predominantly expressed in human platelets, utilizes arachidonic acid (AA) released from the phospholipids as a substrate to form bioactive metabolites (12-(S)-hydroperoxyeicosatetraenoic acid (12-HpETE) and 12-(S)-hydroxyeicosatetraenoic acid (12-HETE)) that have been shown to regulate biological processes such as integrin activation, vascular hypertension, and progression of certain types of cancer.5, 6 The bioactive lipid products resulting from 12-LOX oxidation have been shown to play a role in platelet activation, granule secretion, and clot retraction in vitro suggesting an important role of 12-LOX in regulation of platelet function.4 However, the role of 12-LOX in regulating platelet function, thrombus formation, and hemostasis in vivo is poorly understood due to a lack of highly selective inhibitors to 12-LOX.7, 8 With the recent development of a new class of selective platelet 12-LOX inhibitors, a key role for 12-LOX activity has been identified in regulation of protease-activated receptor (PAR)4- and GPVI-mediated signaling pathways in the platelet.9–11 Further support for development of the 12-LOX inhibitor ML355 for prevention of thrombosis was recently identified and has been well characterized in vitro. ML355 has shown >50-fold selectivity vs. the paralogs, 5-human lipoxygenase, reticulocyte 15-human lipoxygenase type-1, and epithelial 15-human lipoxygenase type-2, and >100-fold selectivity vs. bovine cyclooxygenase (COX)-1 and human COX-2. Further, 12-LOX was recently reported to be essential for FcγRIIa-mediated platelet activation suggesting 12-LOX may also be a potential therapeutic target to limit immune-mediated thrombosis.10 While recent research has indicated an important role for 12-LOX in regulation of platelet function ex vivo, the ability to pharmacologically target 12-LOX as an anti-thrombotic therapy and its impact on hemostasis in vivo have not been established to date.
In our current study, in addition to in vitro and ex-vivo easement using human platelets, we take advantage of the advanced intravital microcopy imaging of in vivo thrombosis in mice and show the first evidence of ML355 protective actions using quantitative approaches for both in vivo thrombosis and hemostasis models in micro and macro vascular injury, we comprehensively assessed the impact of pharmacological inhibition of 12-LOX on thrombosis and hemostasis. We assessed inhibition of platelet 12-LOX as a potential antiplatelet therapeutic target and its safety profile by comparing the effect of ML355 on thrombotic/hemostatic clot composition, bleeding time and blood loss with other existing widely used anti-platelet regents such as aspirin.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
ML355 inhibits human platelet aggregation induced by thrombin
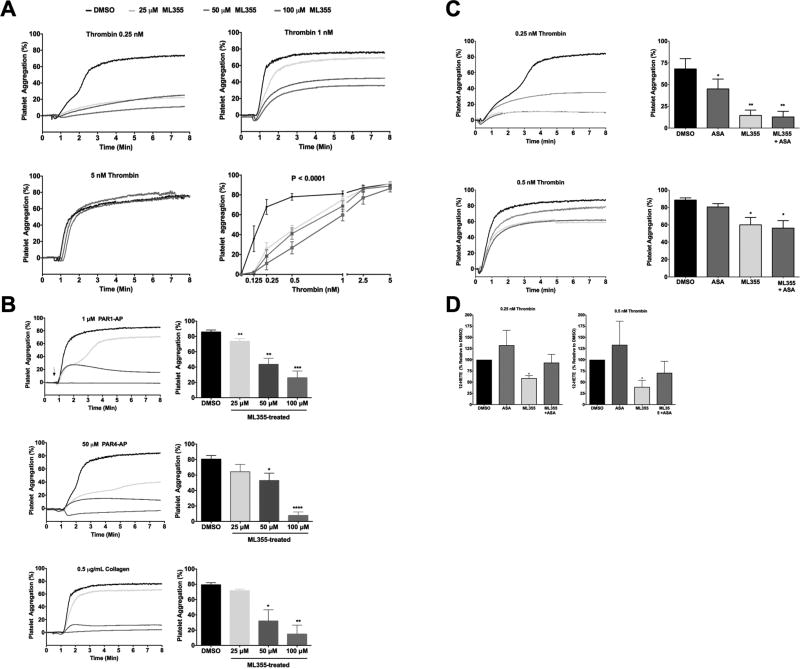
Pharmacologic inhibition of 12-LOX has been shown to attenuate GPVI- and PAR4-mediated platelet activation.11 Further, pharmacologic inhibition of 12-LOX by ML355 was shown to attenuate FcγRIIa-mediated human platelet aggregation via modulation of the proximal signaling components of the pathway.12 To identify if 12-LOX is a key regulator of platelet activation following activation of the coagulation pathway, the effect of ML355 on platelet aggregation induced by thrombin was assessed. Platelet aggregation in washed human platelets was measured following stimulation with multiple concentrations of thrombin (Figure 1A). For each thrombin concentration, platelets were pretreated with increasing concentrations of ML355 (25, 50, and 100 µM). Platelet aggregation was fully blocked at the lower concentration of thrombin (0.25 nM) with all concentrations of ML355. ML355 treatments inhibit about 50–60% platelet aggregation at 0.5nM thrombin and inhibit about 25% aggregation at medium and lower doses by 1 nM thrombin. Interestingly, ML355 inhibition of platelet function can be overcome by high concentrations of thrombin (>2.5 nM) indicating the inhibitory effect of ML355 on platelet 12-LOX is reversible, which may be beneficial for hemostasis in the event of severe injury. In order to test if ML355 inhibition of platelet aggregation showed selectivity towards specific receptor pathways in the platelet, platelet aggregation response was assessed for PAR1-activating peptide (AP) (1 µM), PAR4-AP (50 µM), and collagen (0.5 µg/mL) with or without ML355 (Figure 1B). High concentration of ML355 (100 µM) strongly inhibited platelet aggregation induced by all agonists and the inhibitory effect on platelet function was similarly diminished with decreasing concentration of ML355.
Figure 1. ML355 inhibits human platelet aggregation induced by various agonists.
ML355 dose-dependently inhibits human platelet aggregation induced by various platelet agonists. Washed human platelets were incubated with 25–100 µM of ML355 or DMSO for 5 min and platelet aggregation was measured following stimulation with various platelet agonists. A) Thrombin-induced platelet aggregation (n=7 independent experiments). B) Platelet aggregation induced by PAR1-AP (1 µM), PAR4-AP (50 µM) and collagen (0.5 µg/mL). N=4 independent experiments. *, P <0.05; **, P < 0.01). C) ML355 inhibits human platelet aggregation in the presence of COX-1 inhibitor ASA. Washed human platelets were incubated with DMSO, 25 µM ML355, 100 µM ASA, or ML355 + ASA prior to aggregation assays. ML355 showed stronger inhibition when compared with ASA alone. D) ML355 treatment inhibited 12-HETE production in thrombin-stimulated human platelets. Human platelets (3×108 platelets/mL) treated with DMSO, 25 µM ML355, 100 µM ASA or ML355 + ASA prior to activation by 0.25 nM thrombin (left panel) and 0.5 nM thrombin (right panel). 12-HETE production (ng/1×106 platelets) was determined by LC/MS/MS.
To determine if ML355 had similar inhibitory potential to aspirin (ASA), the most widely used anti-platelet agent on platelet activation, the effect of ML355 on platelet aggregation was compared to treatment with ASA. As expected, treatment of platelets with either ASA (100 µM) or ML355 (25 µM) inhibited platelet aggregation in response to thrombin (Figure 1C). In comparison to ASA, ML355 seem to more potently inhibit thrombin-mediated platelet aggregation. Interestingly, in response to thrombin stimulation, platelets incubated with both ML355 and ASA had a decrease in aggregation compared to platelets treated with either the vehicle or ASA, but not ML355. These data suggest ML355 is a more potent inhibitor of human platelet aggregation compared to ASA and dual inhibition of COX and 12-LOX does not result in an additive inhibitory effect. Further, mass spectrometry analysis (Figure 1D) showed ML355 treatments effectively decreased 12-HETE formation when compared to control. In contrast, ASA treatment increased the production of 12-HETE compared to control. The increase in 12-HETE in the presence of ASA is expected based on previous work showing that an inhibition of COX-1 leads to an increase in the available AA substrate for oxidation by 12-LOX.13 Platelets treated with both ML355 and ASA also resulted in a decrease in thrombin-induced 12-HETE formation. These results indicate ML355 concentrations that inhibit thrombin-mediated platelet aggregation also attenuate 12-HETE formation.
ML355 attenuates human platelet adhesion and aggregation on collagen under arterial shear forces in whole blood
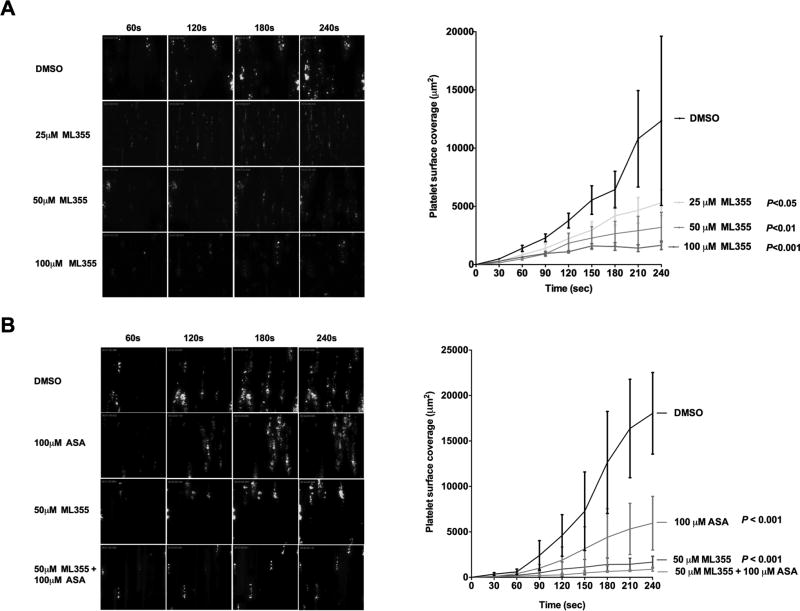
In order to assess the effect of ML355 on human platelet adhesion and aggregation under flow, heparin-anticoagulated whole blood was incubated with ML355 (25 µM, 50 µM, or 100 µM) or an equivalent volume of DMSO for 5 min followed by perfusion at arterial shear (1,800 sec−1) over a collagen-coated surface. As shown in Figure 2A, platelets adhere, aggregate, and form a stable thrombus over the course of perfusion in DMSO-treated whole blood. In contrast, whole blood treated with ML355 exhibited an attenuation of platelet adhesion, aggregation, and thrombus formation in a dose-dependent manner. The adhered fluorescent platelets and platelet aggregates were observed to be unstable and visibly smaller in size compared to DMSO-treated whole blood. Quantitative dynamics of surface area covered by fluorescent platelets were analyzed throughout the course of perfusion. As shown in Figure 2B, the dynamics of platelet surface coverage was significantly reduced in ML355-treated whole blood compared to controls (25 µM: P<0.05; 50 µM: P<0.01; and 100 µM: P<0.001). Further, ML355 showed stronger platelet inhibition when compared to ASA (100 µM), and combination of ML355 with ASA showed a synergistic inhibitory effect on platelet function compared to ASA or ML355 treatment alone (P<0.0001) (Figure 2C).
Figure 2. ML355 potently inhibits platelet function ex vivo under arterial shear.
A) ML355 attenuates platelet adhesion, aggregation, and thrombus formation under arterial flow conditions. Human whole blood was pre-incubated with 25–100 µM of ML355 or DMSO for 5 min prior to being perfused over a collagen-coated surface at arterial shear rate (1800/s) for 4 min. Representative pictures of platelet adhesion and aggregation at the end of each minute of perfusion (upper panel). Dynamics of platelet surface coverage in each 30-sec interval (lower panel). N=8. P value determined by ANOVA analysis compared to DMSO condition. B) ML355 inhibits platelet adhesion and aggregation in the presence of COX-1 inhibitor ASA. Human whole blood was pre-incubated with DMSO, 25 µM ML355, 100 µM ASA, or ML355 + ASA prior to undergoing perfusion as described above.
In vivo ML355 pharmacokinetics in mice
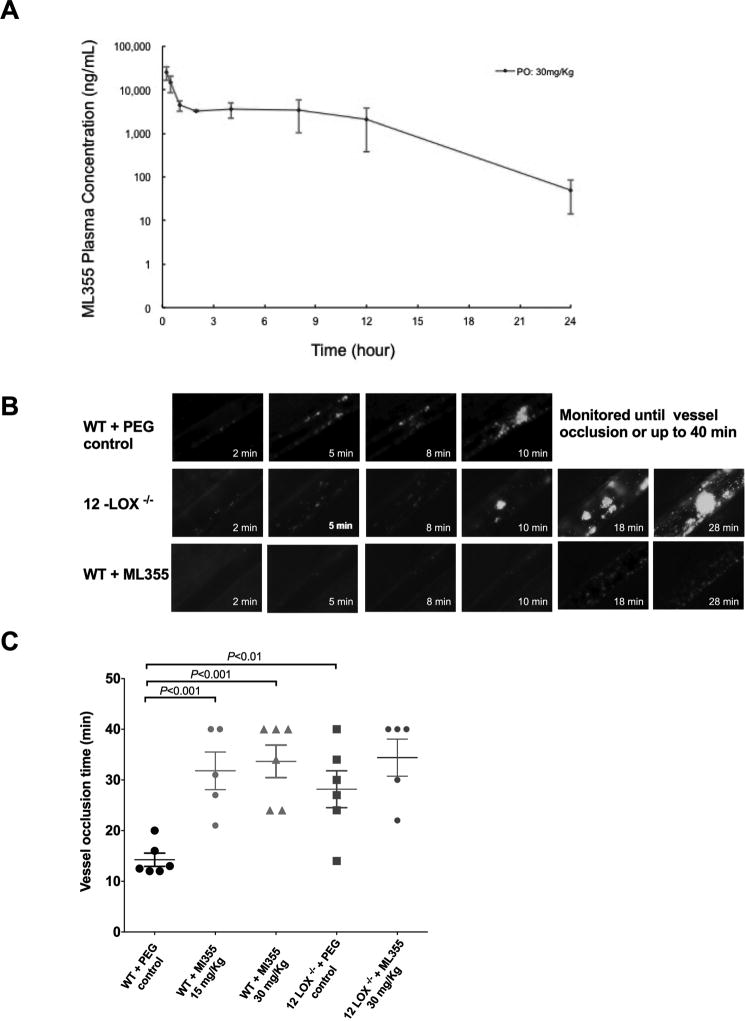
Oral administration of ML355 in mice results in favorable plasma concentration. ML355 Cmax in plasma was ~ 57 µM and the concentration of ML355 in plasma was ~5 µM at 12 hours which shows sufficient drug in circulation in mice used for in vivo studies (Figure 3A)
Figure 3. ML355 treatment inhibited the formation of an occlusive thrombus in mesenteric artery in vivo.
A) Concentrations of ML355 in mice plasma over time following 30mg/kg via oral gavage (PO). Values represent mean and standard deviation; N=3 per time point. Pharmacological inhibition of 12-LOX by ML355 impairs thrombus formation and vessel occlusion in FeCI3-induced mesenteric arteriole thrombosis model. B) Representative images of platelet adhesion, aggregation, and thrombus formation after application of FeCI3 on mesenteric arterioles in WT (upper), 12-LOX−/− (middle) treated with PEG control, and WT mice after orally administered with ML355 (30 mg/kg twice a day for 2 days; bottom). Time after FeCI3 injury is indicated. C) Vessel occlusion time in mesenteric arterioles in WT mice (red) with PEG control or treated with ML355 (15 mg/Kg 30 mg/kg) and 12-LOX −/− mice (grey) with or without ML355 treatments (30mg/Kg) (5 to 6 mice each group). Thrombus formation was recorded up to complete vessel occlusion or stopped at 40 min if occlusion did not occur following the topical application of FeCI3.
ML355 impairs thrombus growth and vessel occlusion in vivo
The effect of ML355 on thrombus formation and vessel occlusion in vivo was assessed using intravital microscopy models in different vascular beds. In the FeCl3-induced mesenteric arteriole injury model, thrombosis was assessed in response to injury in WT mice treated orally with ML355 (15mg/Kg and 30 mg/Kg) or PEG300 alone twice per day for two days with FeCl3 being applied to the vessel at the end of the second day. As shown in Figure 3B (supplementary videos 1A–C), FeCl3 treatment to the mesenteric vessel in WT mice treated with PEG300 caused platelets to adhere to and form visible aggregates and thrombus at the site of injury. The thrombus continuously grew resulting in a stable vessel occlusion in all PEG300-treated vessels. In contrast, platelet adhesion, aggregation, and thrombus formation in all WT mice treated with ML355 were impaired, unstable, and frequently embolized. As shown in Figure 3C, 3 of the 6 ML355-treated mice failed to form vessel occlusion during the 40 min recording time. The remaining ML355-treated mice were able to form a vessel occlusion that was significantly delayed compared to WT + PEG300. Further, the thrombi that did form in the ML355-treated mice were unstable and embolized quickly following occlusion. Consistent with the observed impairment of thrombus formation following inhibition of 12-LOX, thrombus formation in 12-LOX−/− mice was similarly impaired resulting in a delay in platelet adhesion, aggregation, and thrombus formation. ML355 treatments in 12-LOX−/− mice seem to not further impair thrombus formation in 12-LOX−/− mice. Thrombi formation and vessel occlusion were observed to be delayed and unstable. As shown in Figure 3B, the time for complete vessel occlusion was significantly longer in both ML355-treated and 12-LOX−/− mice when compared to WT + PEG300. (Mean vessel occlusion time: WT + PEG300 =14.3 ± 1 min; ML355-treated mice= =28.2 ± 4 min, P <0.001, 12-LOX−/− mice=33.7 ± 3 min, P <0.01).
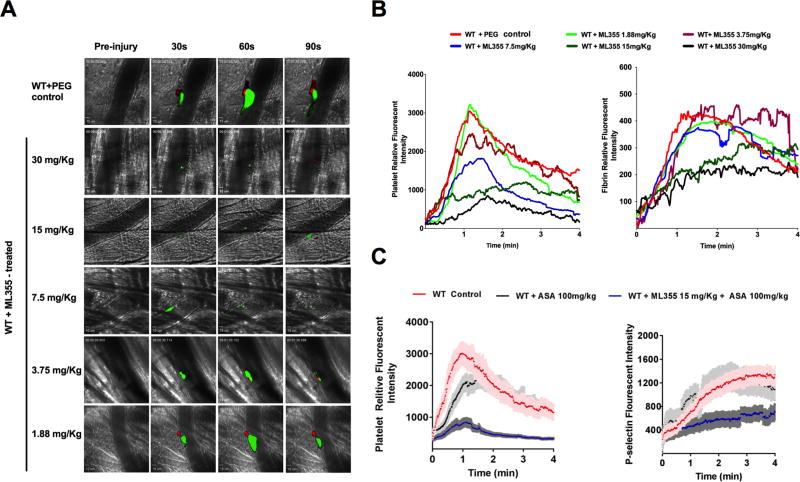
To determine the efficacy of ML355 on limiting thrombus formation in vivo, we studied the dynamics of platelet accumulation and fibrin formation in growing thrombi using the laser injury cremaster arterial thrombosis model. Cremaster arteriole thrombus formation in response to laser injury was quantitatively assessed by real-time measurement of platelet recruitment and fibrin formation at the site of injury in WT mice pretreated with ML355 (30, 15, 7.5, 3.75, or 1.88 mg/kg) or vehicle only, via oral gavage twice a day for two days. As shown in representative pictures of thrombus formation in Figure 4A (and video supplement 2A–F), the thrombus formation in mice was strongly inhibited by higher doses of ML355 treatments compared to WT (vehicle) controls. The thrombi were smaller, unstable, and detached more readily in mice treated with 3.5 mg/kg of ML355 or higher. The inhibitory effect of ML355 was less notable in mice pretreated with 1.88 mg/kg of ML355 while both platelet recruitment and fibrin formation at the site of injury were comparable to vehicle-treated control mice. Quantitative analysis of dynamic platelet recruitment and fibrin formation (Figure 4B) within thrombi revealed that both platelet recruitment and fibrin formation were strongly inhibited in mice treated with higher doses of ML355 (15, 30 mg/kg). Interestingly, while platelet inhibition was observed at lower doses of ML355 (7.5, 3.75 mg/kg), fibrin content in thrombi at these doses of ML355 was not significantly affected compared to control conditions. Finally, platelet recruitment and fibrin content at the lowest dose of ML355-treated mice (1.88 mg/kg) were comparable to the thrombi in PEG300-treated mice. The effect of ML355 on platelet recruitment and platelet P-selectin expression in thrombi was also tested in mice treated with control buffer, ASA 100 mg/kg or 15mg/kg ML355 plus ASA. Compare to the controls, ASA treatment decreased the platelet recruitment without notably effect the P-selectin positive platelet within thrombi. Dual treatment of ML355 and ASA strongly inhibited platelet recruitment and P-selectin expression on platelet surface within thrombi.
Figure 4. 12-LOX inhibition impairs thrombus formation in laser-induced cremaster arteriole thrombosis models.
A) Representative images of platelet accumulation (green) and fibrin formation (red) in growing thrombi in cremaster arterioles in WT control treated with PEG (control, upper panel) and WT treated with ML355 (30 to 1.88 mg/kg, twice a day for 2 days, lower panels). B) Dynamics of platelet accumulation and fibrin formation in thrombi analyzed by change in fluorescent intensity. The kinetic curves represent the mean fluorescence intensity of platelet (left) and fibrin (right). Platelet recruitment was significantly inhibited with all of the doses of ML355 treatments above 1.88 mg/kg (P<0.001) while inhibition of fibrin was significant at only higher doses of ML355 (15, 30 mg/kg, (P<0.001) when compared to PEG-treated control (8–10 thrombi in each mouse, 3 mice in each group. Standard error (SEM) was not shown in the figures for clarity. C) ASA (100 mg/Kg) treatment impaired platelet recruitment without significantly effect P-selectin positive platelets within thrombi. However, 15 mg/kg ML355 and ASA dual treatments significantly inhibited platelet recruitment and platelet surface P-selectin expression in thrombi (P<0.001).
ML355 inhibition of platelet function is specific to 12-LOX in vivo
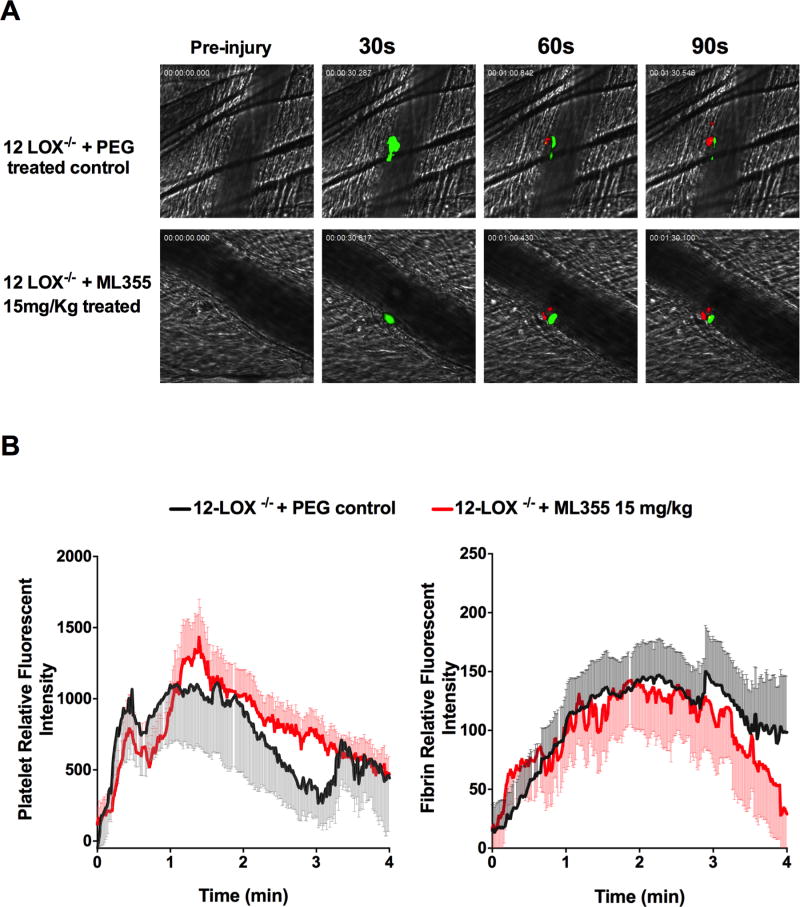
The pharmacological inhibition of platelet function and thrombus formation by ML355 in vivo is consistent with the decreased platelet activation and aggregation in vitro11 and impaired thrombus formation in vivo in 12-LOX−/− mice from our recent studies.14 To determine if the observed inhibition of thrombus formation by ML355 in vivo was 12-LOX-dependent, thrombus formation was measured in 12-LOX−/− mice treated with 15 mg/kg ML355. As shown in Figure 5A and in agreement with recently published work,14 thrombus formation in 12-LOX-null mice was impaired and formed unstable smaller thrombi. Interestingly, the residual thrombus in 12-LOX-null mice was not further reduced following treatment with ML355. No significant differences were observed in the dynamics of platelet recruitment and fibrin formation within thrombi in 12-LOX−/− mice with or without ML355 treatment (Figure 5B, P>0.05). The inability of ML355 to further reduce thrombus formation in 12-LOX−/− mice strongly supports that the inhibition of thrombus formation by ML355 is specific to 12-LOX.
Figure 5. ML355 inhibition of thrombus formation requires platelet 12-LOX.
A) Representative images of laser-induced cremaster arteriole thrombosis in 12-LOX−/− mice treated with PEG (control, upper panel) or treated with ML355 (15mg/kg, lower panel) twice a day for two days. Platelet accumulation is shown in green and fibrin formation is shown in red. Time after injury is indicated above. B) Dynamics of fluorescent intensity of platelet (right) and fibrin (left) in residual thrombi in 12-LOX−/− mice treated with PEG (control, shown in black) or treated with ML355 (15mg/kg, shown in red). The kinetic curves represent the mean fluorescence intensity of platelets and fibrin and the shaded regions are representative of the standard error (SEM) (8–10 thrombi in each mouse and 3 mice in each group, P>0.05).
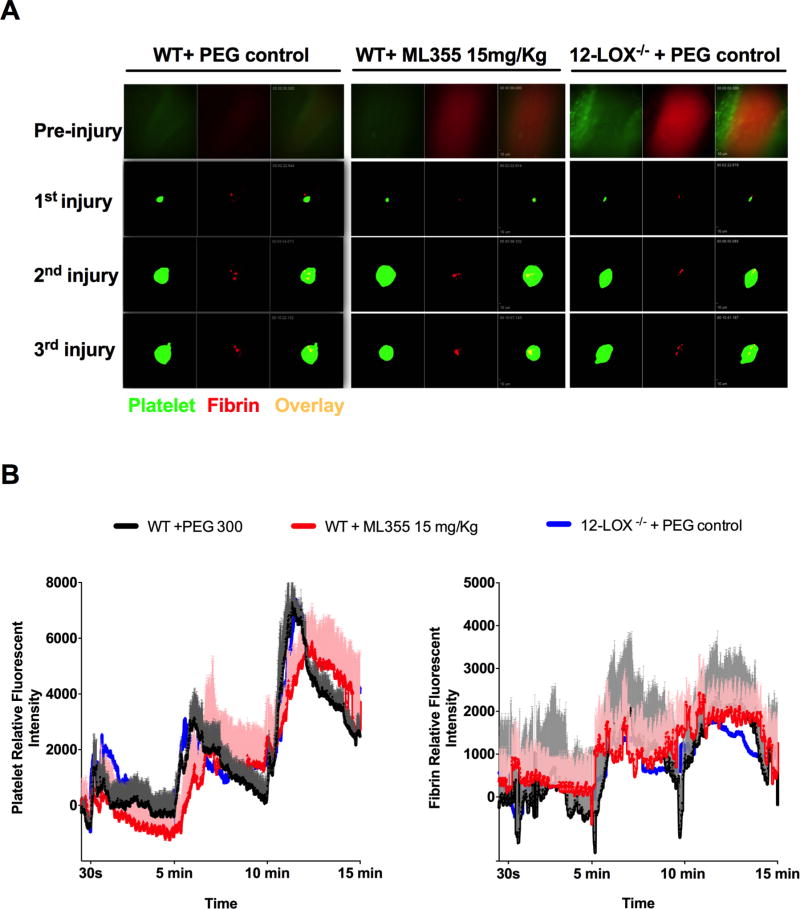
ML355 treatment minimally impacts hemostasis in vivo
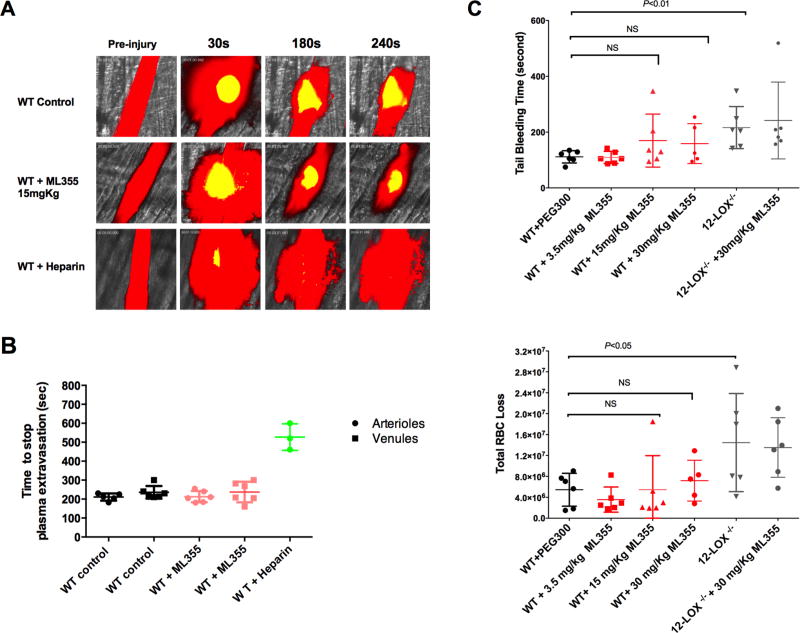
The potential impact of ML355 treatment on hemostasis was assessed using several vessels in the mouse model. Firstly, hemostasis was assessed by measuring the formation of a hemostatic clot and bleeding using a recently developed intravital microscopy laser ablation model of hemostasis in real-time in 12-LOX−/− or WT mice treated with ML355 (15 mg/kg) or vehicle. Hemostatic clot formation at the site of vascular injury was assessed in the saphenous vein hemostasis model where platelet plug and fibrin formation were visualized and quantitated following laser-induced rupture of the saphenous vein wall under intravital microscopy as previously described15. Immediately following laser injury, fluorescent platelets were seen adhering to the site of injury and forming a visible platelet plug with fibrin ring observed at the edges of the vascular insult. The laser injury was repeated at 5 min and 10 min intervals at the same site of injury resulting in formation of serially larger and more robust stable hemostatic plugs. As shown in Figure 6A, no significant alteration in hemostatic clot formation was observed in WT mice treated with ML355 when compared with vehicle-treated WT mice. Furthermore, hemostatic plug formation in 12-LOX−/− mice was not significantly impaired under the same experimental conditions. Quantitative analysis of the dynamics of the platelet plug and fibrin formation within the hemostatic clot in response to repetitive injury did not significantly differ between WT with or without ML355 treatments and 12-LOX−/− mice (Figure 6B). A second measurement of altered hemostasis was also performed in the cremaster vessels by assessing time required for the cessation of plasma extravasation in the cremaster microvasculature following laser-induced vascular wall rupture. Cremaster microvasculature blood flow was visualized by intravenous injection of FITC-dextran prior to injury followed by a vascular wall rupture of either the cremaster arteriole or venule wall using a laser under confocal intravital microscopy as described previously14, 16. Immediately following rupture of the vascular wall, the FITC-dextran was observed leaking out of the vessel at the site of injury. Following the formation of a hemostatic plug, dextran leakage from the vessel decreased over time and cessation of vascular leakage was observed after formation of a stable hemostatic plug at the site of injury. FITC-Dextran plasma extravasation and time required for cessation were assessed for both arterioles and venules. As shown in Figure 7A (video supplements 3A–C), plasma extravasation and cessation of FITC-dextran extravasation following platelet plug formation in cremaster arterioles was similar in WT mice treated with ML355 (15 mg/kg) or vehicle as well as 12-LOX−/− mice. Analysis of sequential images in the cremaster vessel rupture model showed no notable difference in the time required for the cessation of plasma extravasation in either arterioles or venules in WT mice with or without ML355 or in 12-LOX−/− mice (Figure 7B). Under the same experimental conditions, heparin treatment in WT mice resulted in a significant impairment of hemostatic plug formation and delay in cessation of FITC-dextran extravasation. These results are consistent with the observation that no difference was observed in platelet plug formation with ML355 in the saphenous vein hemostasis model. Lastly, we assessed the impact of ML355 treatment on tail-bleeding time and blood loss. Tail-bleeding in mice treated with ML355 at a doses of 3.5 mg/kg,15 mg/kg, and 30 mg/kg for 2 days did not significantly prolonged the tail-bleeding time and red blood cell loss when compared to PEG300 treated control mice (P>0.05) while 12-LOX−/− displayed an increased bleeding time and ML355 treatment did not further deteriorated bleeding in 12-LOX−/− mice (Figure 7C).
Figure 6. ML355 treatment did not impair hemostatic plug formation in laser ablation saphenous vein hemostasis model.
A) Representative images of platelet accumulation (green) and fibrin formation (red) within hemostatic plug forming at the injured site of repeated vascular injury on saphenous vein of WT mice treated with PEG (control) or with ML355 (15 mg/kg,) and 12 LOX−/− mice treated with PEG control twice a day for 2 days. B) Quantitative analysis of platelet accumulation and fibrin formation after laser ablation at 30 sec and repeated at 5 min and 10 min time points. The kinetic curves represent the mean platelet (left) and fibrin (right) fluorescence intensity and the shaded regions are representative of the standard error (SEM). ML355 treatment in WT mice attenuated platelet adhesion and accumulation (P<0.001) when compared to WT mice or 12 LOX−/− mice treated with PEG N= 6 (2 independent injuries in each mouse, 3 mice in each group).
Figure 7. ML355 treatment did not significantly increase bleeding as assessed by plasma extravasation following laser-induced rupture of cremaster microvasculature and tail bleeding assays.
A) Fluorescein isothiocyanate-dextran (10,000 MW) was infused prior to laser injury in order to visualize the blood flow (plasma shown in red) in cremaster arteriole of WT mice treated with PEG control (upper panel), WT mice treated with ML355 (15 mg/kg, twice a day for 2 days; middle panel) and WT intravenously injected with 25U heparin 10 min prior to microscopy. The cremaster muscle arteriole wall was exposed to a high intensity laser pulse to puncture a hole, which resulted in plasma extravasation and subsequent formation of a platelet hemostatic plug (shown in yellow) leading to cessation of extravasation of plasma. B) The time required for the cessation of plasma dextran extravasation from arterioles (shown in circle), venules (shown in square) in PEG-treated WT control mice (shown in black), and ML355-treated WT mice (shown in red). Heparin-treated WT mice control shown in green. Data from 1–2 independent injuries per mouse, 3 mice in each group. P>0.05. C) Tail bleeding time and total red blood cell loss was assessed in WT treated with PEG control (Black), WT mice treated with ML355 (3.5 mg/kg,15 mg/kg and 30 mg/kg twice a day for 2 days; red) and 12 LOX−/− mice with or without ML355 (30 mg/kg) treatment (grey). Compared to PEG 300 treated control mice, ML355 treatment mice did not significantly increase tail-bleeding time all tested doses (P>0.05, upper panel) and total blood loss (lower panel, P>0.05) while 12 LOX−/− mice has increased tail bleeding time (P<0.01) and blood loss (P<0.05).
Discussion
Regulation of platelet function is essential for the prevention and treatment of cardiovascular atherothrombotic events such as coronary artery disease and stroke. While current antiplatelet drugs effectively inhibit platelet function and prevent thrombotic complications, these treatments often increase the risk of unwanted bleeding. Therefore, there is an unmet need for the identification of novel antiplatelet targets that block unnecessary platelet activation in disease conditions and reverse platelet inhibition with a minimal risk of bleeding. Here we report that the highly specific 12-LOX inhibitor ML355 is a viable option for antiplatelet therapy. We have investigated the impact of genetic ablation of 12-LOX on injury-induced thrombus formation in 12-LOX−/− mice. More importantly, we studied the therapeutic effect of pharmacologically targeting 12-LOX with ML3559, 11, 17. Inhibition of 12-LOX in human platelets dose-dependently inhibited platelet aggregation initiated through either the thrombin- or collagen-mediated activation pathways. Additionally, inhibition of 12-LOX in human whole blood resulted in attenuation of platelet adhesion, aggregation, and thrombus formation under arterial shear conditions. Finally, using complementary intravital microscopy models of thrombosis and hemostasis, we found that orally dosed ML355 was able to attenuate thrombosis without notably impairing hemostasis.
Platelet reactivity is an important functional index for assessing platelet response to vascular injury. Using selective 12-LOX inhibitors such as NCTT-956, 12-LOX was previously shown to be important in the regulation of platelet reactivity.18 Therefore, targeting 12-LOX activation with a selective, orally bioavailable 12-LOX inhibitor may represent a viable antiplatelet therapy. With development of the first-in-class highly selective, orally bioavailable 12-LOX inhibitor, ML355, the current study strongly supports primary observations that 12-LOX plays an important role in platelet reactivity. Here, 12-LOX inhibition is shown to dose-dependently inhibit platelet aggregation induced by thrombin, the most potent platelet agonist in vitro and in vivo. Interestingly, the inhibitory effect of ML355 on thrombin-induced platelet aggregation ex vivo diminishes with the increasing concentration of thrombin and is completely overcome by the presence of high concentration of thrombin. This observation supports that ML355-treated platelets are still reactive and able to form platelet aggregates and hemostatic clots when thrombin reaches high concentrations locally at the site of vascular injury where maintenance of hemostasis is essential to prevention of unwanted bleeding. Additionally, data from ex vivo flow chamber studies (Figure 2) suggests that ML355 is able to attenuate platelet adhesion, aggregation, and thrombus formation under high shear conditions. Together, the data support 12-LOX is an important regulator of platelet reactivity.
COX-1, one of the major oxygenases in the platelet also produces oxylipin products from fatty acids similar to 12-LOX. ASA is a widely used and effective antiplatelet therapy that works by irreversibly inhibiting COX-1 to prevent production of thromboxane A2. As we assess the potency and utility of inhibiting 12-LOX with ML355 as a potential therapeutic, it is important to determine if ML355 is effective as an antiplatelet monotherapy or needs to be used as a dual therapy in conjunction with ASA. The results from mono and dual inhibition with ML355 and ASA strongly support that combination of ML355 may more potent effect in inhibiting platelet adhesion, aggregation, and thrombosis. (Figs 1, 2, and 4). It will be important in the future to determine how inhibition of 12-LOX compares to other antiplatelet approaches currently in use including targeting of the P2Y12 receptor, PAR1, as well as the integrin receptor αIIbβ3. While each of these approaches has been shown to be effective as dual therapeutic approaches in the clinic, it will be important to determine if inhibition of 12-LOX alone or in conjunction with these inhibitory approaches offers a better protection with reduced risk of bleeding.
While the role of 12-LOX and its metabolites in regulation of platelet function has been a topic of significant interest for years, the ability to regulate platelet activation and clot formation in vivo through modulation of 12-LOX has been elusive. Both inhibitory and supportive roles of platelet 12-LOX have been reported.8, 11, 18–23 Recent work from our lab and others has found that 12-LOX plays an important role in human platelet activation and positively regulates PAR4- and GPVI-mediated platelet reactivity.7, 11 Additionally, the development of a more selective inhibitor against 12-LOX has enabled the observation that pharmacologically inhibiting 12-LOX results in attenuation of human platelet aggregation, integrin αIIbβ3 activation, and dense granule secretion.11 Together, these findings strongly indicate 12-LOX positively regulates platelet function and might be an important regulator of thrombosis in vivo. Administration of ML355 to mice shows promising pharmacokinetics and favorable drug concentration in blood circulation 12 hours following oral administration. Using well-established complementary intravital microscopy thrombosis and hemostasis models, ML355 given orally is shown to dose-dependently inhibit thrombosis formation and vessel occlusion in WT mice. The impairment of thrombosis is also evident in 12-LOX−/− mice and cannot be further abolished by ML355 treatment, which supports that the inhibitory effect of ML355 is specific to 12-LOX pathways in vivo.
One of the primary concerns with targeting pro-coagulant pathways in the platelet is the risk for increased bleeding.3 Intriguingly, while ML355 treatment significantly inhibited thrombosis formation in WT mice in several in vivo thrombosis models, it was observed to have only a minimal effect on hemostasis although 12-LOX deficient mice were shown to have prolonged tail bleeding times. Using two newly developed in vivo hemostatic assays to assess both hemostatic plug formation and bleeding measured by plasma extravasation following the rupture of the vascular wall in real-time, hemostasis was able to be quantitatively assessed in large and small vessels in the mouse following treatment with ML355. In our hemostasis models (saphenous vein and cremaster arteriole/venule models by laser injury), the injury is intensive and results in full penetration and rupture of the vascular wall at the site of injury resulting in blood component leakage (bleeding) out of the vessel and into the extravascular space. The bleeding is limited by the formation of a hemostatic plug at the site of injury that leads to cessation of bleeding. In severe vascular injury conditions in these models of hemostasis, ML355-treated WT mice and 12-LOX null mice were able to form hemostatic plugs at the site of injury and confine the leakage of blood components in a similar kinetic manner as observed for WT mice. Although these in vivo hemostatic models may mimic the hemostasis process, each model has its own set of limitations and may not fully reflect hemostasis in the human vessel. Overall, the data presented here clearly show that while ML355 is inhibitory to platelet activation and clot formation in vivo, vascular injury resulting in complete rupture of the vessel wall, likely resulting in significant activation of the coagulation pathway and thrombin generation, can overcome the inhibitory effects of ML355. This threshold for platelet activation in the presence of ML355 at high concentrations of endogenous agonists in the pro-coagulatory conditions appears to play an important role in restoring normal clotting and hemostasis. These data taken together indicate that ML355 has the potential to function as a safer antiplatelet therapy that minimally impacts hemostasis.
In summary, this is the first in vivo study showing that ML355 is an important regulator of thrombosis and pharmacologically targeting 12-LOX is a viable antiplatelet approach. ML355 dose-dependently inhibits platelet function in vitro, under ex vivo flow conditions, and in in vivo thrombosis. Finally, ML355 treatment in mice has now been shown to protect against injury-induced thrombosis without notably impairing hemostasis.
Supplementary Material
Highlights.
12-LOX as a key determinant of platelet reactivity in vivo
ML355, a platelet 12-LOX inhibitor, potently inhibits human platelet function
Orally administered ML355 dose-dependably inhibits thrombus formation
Inhibition of 12-LOX by ML355 does not alter hemostasis
Acknowledgments
We would like to thank Izabela Holinstat for carefully reviewing the manuscript and helpful suggestions. We would like to thank Dr. Rodney M. Camire at Children's Hospital of Philadelphia for providing anti-mouse fibrin antibody for in vivo studies.
Sources of Funding: This study was supported in part by the National Institutes of Health Grants HL114405 (MH), GM105671 (MH), AG047986 (TRH and MH), HL129491 (BT), and HL129481 (JY). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 12-HETE
12-(S)-hydroxyeicosatetraenoic acid
- 12-HpETE
12-(S)-hydroperoxyeicosatetraenoic acid
- 12-LOX
platelet 12(S)-lipoxygenase
- 12-LOX−/−
12-LOX null
- AA
arachidonic acid
- ANOVA
analysis of variance
- COX
cyclooxygenase
- PAR
protease-activated receptor
- PAR4-AP
protease-activated receptor protease-activated receptor 4-activating peptide
- PEG 300
Polyethylene Glycol 300
- WT
wild-type
Footnotes
Disclosures: Several authors are co-inventors for a patent filed with the USPTO for ML355 (DKL, AJ, AS, DJM, TRH, and MH). Otherwise, authors have nothing to disclose.
References
- 1.Ruggeri ZM. Platelets in atherothrombosis. Nature medicine. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics C, Stroke Statistics S. Executive summary: Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 3.Steg PG, Dorman SH, Amarenco P. Atherothrombosis and the role of antiplatelet therapy. J Thromb Haemost. 2011;9(Suppl 1):325–332. doi: 10.1111/j.1538-7836.2011.04277.x. [DOI] [PubMed] [Google Scholar]
- 4.Ikei KN, Yeung J, Apopa PL, Ceja J, Vesci J, Holman TR, Holinstat M. Investigations of human platelet-type 12-lipoxygenase: Role of lipoxygenase products in platelet activation. J Lipid Res. 2012;53:2546–2559. doi: 10.1194/jlr.M026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, Sigman CC, Kelloff GJ. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol Biomarkers Prev. 1999;8:467–483. [PubMed] [Google Scholar]
- 6.Ghosh J, Myers CE. Inhibition of arachidonate 5-lipoxygenase triggers massive apoptosis in human prostate cancer cells. Proc Natl Acad Sci U S A. 1998;95:13182–13187. doi: 10.1073/pnas.95.22.13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffey MJ, Jarvis GE, Gibbins JM, Coles B, Barrett NE, Wylie OR, O'Donnell VB. Platelet 12-lipoxygenase activation via glycoprotein vi: Involvement of multiple signaling pathways in agonist control of h(p)ete synthesis. Circ Res. 2004;94:1598–1605. doi: 10.1161/01.RES.0000132281.78948.65. [DOI] [PubMed] [Google Scholar]
- 8.Nyby MD, Sasaki M, Ideguchi Y, Wynne HE, Hori MT, Berger ME, Golub MS, Brickman AS, Tuck ML. Platelet lipoxygenase inhibitors attenuate thrombin- and thromboxane mimetic-induced intracellular calcium mobilization and platelet aggregation. J Pharmacol Exp Ther. 1996;278:503–509. [PubMed] [Google Scholar]
- 9.Kenyon V, Rai G, Jadhav A, et al. Discovery of potent and selective inhibitors of human platelet-type 12-lipoxygenase. J Med Chem. 2011;54:5485–5497. doi: 10.1021/jm2005089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luci DK, Jameson JB, 2nd, Yasgar A, et al. Synthesis and structure-activity relationship studies of 4-((2-hydroxy-3-methoxybenzyl)amino)benzenesulfonamide derivatives as potent and selective inhibitors of 12-lipoxygenase. J Med Chem. 2014;57:495–506. doi: 10.1021/jm4016476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeung J, Apopa PL, Vesci J, Stolla M, Rai G, Simeonov A, Jadhav A, Fernandez-Perez P, Maloney DJ, Boutaud O, Holman TR, Holinstat M. 12-lipoxygenase activity plays an important role in par4 and gpvi-mediated platelet reactivity. Thrombosis and haemostasis. 2013;110:569–581. doi: 10.1160/TH13-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeung J, Tourdot BE, Fernandez-Perez P, Vesci J, Ren J, Smyrniotis CJ, Luci DK, Jadhav A, Simeonov A, Maloney DJ, Holman TR, McKenzie SE, Holinstat M. Platelet 12-lox is essential for fcgammariia-mediated platelet activation. Blood. 2014;124:2271–2279. doi: 10.1182/blood-2014-05-575878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holinstat M, Boutaud O, Apopa PL, Vesci J, Bala M, Oates JA, Hamm HE. Protease-activated receptor signaling in platelets activates cytosolic phospholipase a2alpha differently for cyclooxygenase-1 and 12-lipoxygenase catalysis. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:435–442. doi: 10.1161/ATVBAHA.110.219527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeung J, Tourdot BE, Adili R, Green AR, Freedman CJ, Fernandez-Perez P, Yu J, Holman TR, Holinstat M. 12(s)-hetre, a 12-lipoxygenase oxylipin of dihomo-gamma-linolenic acid, inhibits thrombosis via galphas signaling in platelets. Arterioscler Thromb Vasc Biol. 2016;36:2068–2077. doi: 10.1161/ATVBAHA.116.308050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Getz TM, Piatt R, Petrich BG, Monroe D, Mackman N, Bergmeier W. Novel mouse hemostasis model for real-time determination of bleeding time and hemostatic plug composition. Journal of thrombosis and haemostasis : JTH. 2015;13:417–425. doi: 10.1111/jth.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welsh JD, Muthard RW, Stalker TJ, Taliaferro JP, Diamond SL, Brass LF. A systems approach to hemostasis: 4. How hemostatic thrombi limit the loss of plasma-borne molecules from the microvasculature. Blood. 2016;127:1598–1605. doi: 10.1182/blood-2015-09-672188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luci D, Jameson JB, II, Yasgar A, et al. Probe reports from the nih molecular libraries program. Bethesda (MD): 2010. Discovery of ml355, a potent and selective inhibitor of human 12-lipoxygenase. [PubMed] [Google Scholar]
- 18.Yeung J, Apopa PL, Vesci J, Kenyon V, Rai G, Jadhav A, Simeonov A, Holman TR, Maloney DJ, Boutaud O, Holinstat M. Protein kinase c regulation of 12-lipoxygenase-mediated human platelet activation. Mol Pharmacol. 2012;81:420–430. doi: 10.1124/mol.111.075630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson EN, Brass LF, Funk CD. Increased platelet sensitivity to adp in mice lacking platelet-type 12-lipoxygenase. Proc Natl Acad Sci U S A. 1998;95:3100–3105. doi: 10.1073/pnas.95.6.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dioszeghy V, Rosas M, Maskrey BH, Colmont C, Topley N, Chaitidis P, Kuhn H, Jones SA, Taylor PR, O'Donnell VB. 12/15-lipoxygenase regulates the inflammatory response to bacterial products in vivo. J Immunol. 2008;181:6514–6524. doi: 10.4049/jimmunol.181.9.6514. [DOI] [PubMed] [Google Scholar]
- 21.Morgan LT, Thomas CP, Kuhn H, O'Donnell VB. Thrombin-activated human platelets acutely generate oxidized docosahexaenoic-acid-containing phospholipids via 12-lipoxygenase. Biochem J. 2010;431:141–148. doi: 10.1042/BJ20100415. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Zhang W, Giroux C, Cai Y, Ekambaram P, Dilly AK, Hsu A, Zhou S, Maddipati KR, Liu J, Joshi S, Tucker SC, Lee MJ, Honn KV. Identification of the orphan g protein-coupled receptor gpr31 as a receptor for 12-(s)-hydroxyeicosatetraenoic acid. J Biol Chem. 2011;286:33832–33840. doi: 10.1074/jbc.M110.216564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kapoor R, Huang YS. Gamma linolenic acid: An antiinflammatory omega-6 fatty acid. Curr Pharm Biotechnol. 2006;7:531–534. doi: 10.2174/138920106779116874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.