Abstract
Objective
Infusion of angiotensin II (Ang II) induces extracellular matrix remodeling and inflammation resulting in abdominal aortic aneurysms (AAA) in normolipidemic mice. Although Ang II activates mesenchymal cells in the media and adventitia to become fibrogenic, the sentinel role of this mesenchymal population in modulating the inflammatory response and aneurysms is not known. We test the hypothesis that these ‘fibrogenic’ mesenchymal cells play a critical role in Ang II-induced aortic wall vascular inflammation and AAA formation.
Approach and Results
Ang II infusion increased phospho-Ser536-RelA and IL-6 immunostaining in the abdominal aorta. In addition, aortic mRNA transcripts of RelA-dependent cytokines IL-6 and IL-1β were significantly elevated suggesting that Ang II functionally activates RelA signaling. To test the role of mesenchymal RelA in AAA formation, we generated RelA conditional knockout (RelA-CKO) mice by administering tamoxifen to double transgenic mice harboring RelA-flox alleles and tamoxifen-inducible Col1a2 promoter-driven Cre recombinase (Col1a2-CreERT). Tamoxifen administration to Col1a2-CreERT•mT/mG mice induced Cre expression and RelA depletion in aortic smooth muscle cells and fibroblasts but not in endothelial cells. Infusion of Ang II significantly increased abdominal aortic diameter and the incidence of AAA in RelA wild-type but not in RelA-CKO mice, independent of changes in systolic blood pressure. Furthermore, mesenchymal cell-specific RelA-CKO mice exhibited decreased expression of IL-6 and IL-1β cytokines and decreased recruitment of C68+ and F4/80lo•Ly6Chi monocytes during Ang II infusion.
Conclusions
Fibrogenic mesenchymal RelA plays a causal role in Ang II-induced vascular inflammation and AAA in normolipidemic mice.
Keywords: angiotensin II, RelA, NF-κB, IL-6, abdominal aortic aneurysm, vascular inflammation
Subject Codes: vascular biology, aneurysm, vascular disease, inflammation, basic science research
Introduction
Abdominal aortic aneurysm (AAA) can be a fatal disease due to its propensity to cause aortic rupture. In the United States, the prevalence of AAA ranges from 1.3% in middle-age men to 12.5% in men older than 70 1. AAA is characterized by aortic lumen expansion, infiltration of leukocytes into the aortic media and adventitia and extracellular matrix (ECM) degradation2. The angiotensin II (Ang II) infusion model has been widely used to study the pathogenesis of AAA, a disease that often is detected only in its later stages. Ang II infusion for 7 to 28 days into normolipidemic (C57Bl/6) or hyperlipidemic (LDLR−/− or ApoE−/−) mice leads to AAA formation3–5. The incidence of AAA increases by feeding a high-fat diet to C57Bl/6 mice, but the presence of hypercholesterolemia and adiposity complicates the interpretation of Ang II effects on the aortic wall5.
Ang II infusion produces aortic aneurysms through a two-hit process, producing stromal cell activation followed by extracellular matrix remodeling mediated by recruitment and local tissue differentiation of effector macrophages6, 7. Although the mechanisms controlling macrophage recruitment and activation in the later stages of AAA formation are relatively well-understood, the primary cell type responding to Ang II is not. Ang II has potent effects on extracellular matrix remodeling – this process is mediated by activating mesenchymal cells, cells of heterogeneous lineage that secrete type I collagen composed of α1 and α2 peptides and encoded by Col1a1 and Col1a28, 9, the TGFβ growth factor10 and activated matrix metalloproteinases (MMPs)11–13. These mesenchymal cells include adventitial fibroblasts, myofibroblasts and subpopulations of Col1α-expressing “synthetic” vascular smooth muscle cells (VSMCs). Although ECM remodeling/fibrosis and vascular inflammation are thought to be largely distinct processes, recent studies have shown that extracellular matrix remodeling has significant effects on the fibroblast-myofibroblast transition, activation of intercellular adhesion molecules, formation of chemotactic molecules from the processing of the α1 peptide, and regulation of leukocyte infiltration14. These data suggest the intriguing possibility that Ang II activation of mesenchymal cells with a fibrogenic phenotype in the aortic wall may be closely coupled to vascular inflammation and AAA formation.
Following the initial trigger of mesenchymal cell activation, subsequent leukocyte activation is mediated by a coordinate secretion of chemotactic cytokines, upregulation of intercellular adhesion molecules and production of activating cytokines responsible for acquisition of effector cell functions and extracellular matrix remodeling. These genes are controlled at the level of transcription by the NF-κB transcription factor. Ang II is a potent inducer of NF-κB pathway activation6. In mesenchymal cells, Ang II activates the latent transcriptional potential of the NF-κB complex by phosphorylating the transcriptional subunit NF-κB/RelA on Ser 536 by the NF-κB-inducing kinase 15, 16. In this manner, RelA induces transcription of IL-6, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)15, 17. NF-κB is also required for priming expression of pro-IL-1β subsequently processed by the activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome and involved in Ang II-induced AAA formation18, 19. Interestingly, our previous studies showed that IL-6 was secreted by mesenchymal-like fibroblasts 20, 21, an activating cytokine that promotes macrophage activation and aortic dissections during Ang II infusion 3, 20.
Based on our previous findings that IL-6, a cytokine produced mostly by aortic adventitial fibroblasts, is a mediator of AAA formation and that RelA signaling is necessary for IL-6 transcription, we hypothesized that fibrogenic mesenchymal cells are a major trigger for Ang II-induced vascular inflammation and AAA. Knowing that the NF-κB/RelA pathway is the major intercellular signaling pathway responsible for the vascular inflammation, we generated RelA conditional knockout (RelA-CKO) mice by administering tamoxifen to transgenic mice harboring a tamoxifen-inducible Col1a2-driven CreER (Col1a2-CreERT) and RelA-floxed (RelA f/f) alleles. Knowing that Ang II-induced Col1A expression is mediated by a subpopulation of fibroblasts, SMA22+ myofibroblasts and “synthetic phenotype” VSMCs, we tested the role of fibrogenic mesenchymal NF-κB signaling on AngII-induced inflammation and dissections. To accomplish this, we bred tamoxifen inducible Col1a2-CreERT 22, 23 and RelA f/f mice, the latter containing loxP sites in intron 4 and intron 8 generated in our laboratory 24. To verify the specificity of the Col1a2-CreERT we also crossed the transgenic mouse harboring this Cre with mT/mG Cre-reporter mice. The cells of mT/mG mouse constitutively express membrane targeted dTomato (mT) and switch expression to eGFP (mG) after Cre-mediated excision of dTomato allele25. In addition, the mT/mG reporter allele is knocked into the ROSA26 locus allowing the allele to be ubiquitously expressed. Our data indicate that Col1a2-CreERT is activated in a subpopulation of mesenchymal cells, including fibroblasts and VSMCs but not in endothelial cells. Deletion of mesenchymal RelA in in the RelA-CKO mice conferred protection from Ang II-induced AAA without affecting blood pressure. These studies directly implicate for the first time a central role of RelA activation in fibrogenic mesenchymal cells in the development of Ang II-induced vascular inflammation and AAA.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Ang II infusion increased RelA signaling in the aortic wall
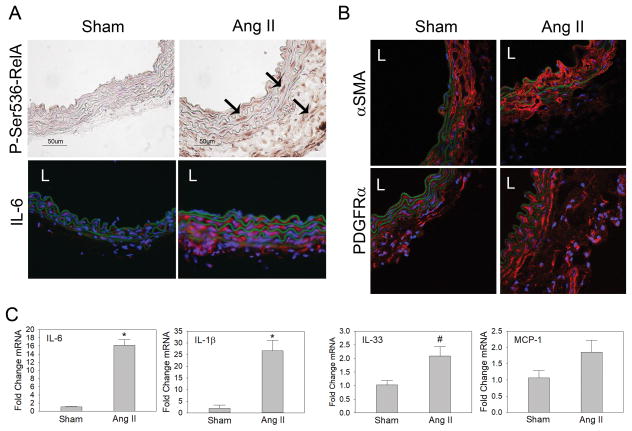
Ang II is a potent inducer of RelA-dependent cytokine IL-6 in vivo and in vitro 6. Furthermore, Ang II infusion into rats has been demonstrated to increase total aortic phospho-Ser536-RelA as measured by an ELISA assay 15. To determine which aortic cells have increased RelA signaling during Ang II infusion, we infused 3 wild type (WT) C57BL/6 mice with saline (sham) and 4 WT mice with Ang II for 7 days before isolating their aortas for immunohistochemistry and qRT-PCR analysis. While no animals developed an aortic aneurysm over the one week period, analysis of suprarenal abdominal aortas indicated a dramatic increase in phospho-Ser536 RelA staining in aortas from Ang II-infused mice, demonstrating that Ang II potently induced RelA signaling in the aortic wall in vivo (Fig. 1A; controls are in Fig. SI). Phospho-Ser536 RelA staining was observed throughout the media and the expanded adventitia suggesting that Ang II activates RelA-signaling in multiple cell-types. Since IL-6 is a downstream target of phospho-Ser536 RelA, we performed immunofluorescence analysis for IL-6 in both groups. There was slight positive immunostaining for IL-6 in aortas from the sham group but increased immunostaining was observed in the media and the adventitia in aortas from the Ang II group. These data sets complement each other and support the conclusion that Ang II activates phospho-Ser536 RelA and its pathway in medial and adventitial cells of the abdominal aorta.
Figure 1. Ang II activates NF-κB/RelA-signaling in the aortic wall.
C57Bl/6 mice were infused with saline (sham, n=3) or Ang II (n=4) for 7 days. A) Supra-renal aortas were sectioned and immunostained for phospho-Ser536-RelA and IL-6, the downstream cytokine, to detect RelA activation. Arrows indicate positive immunostaining in the media and adventitia. IL-6 was detected using AF-568-conjugated secondary antibody. Images were captured at 400x magnification. B) Aortic cross-sections were immunostained for αSMA, a SMC contractile protein and PDGFRα, a marker associated with fibroblasts. Images were captured at 630x magnification using a confocal microscope. L, vascular lumen. C) qRT-PCR was performed on aortas from sham and Ang II group to detect changes in RelA-dependent cytokine/chemokine expression. Data are presented as mean ± SEM. *P< 0.05; #P= 0.055 vs sham.
VSMCs populate the media and express high levels of smooth muscle cell α-actin (αSMA), and immunofluorescence detection of αSMA via confocal microscopy verified that medial cells were αSMA+ (Fig. 1B). In addition, there was almost complete absence of αSMA signal in the adventitia, with very few cells expressing a signal above background levels. While this suggests the majority of medial cells were αSMA+ VSMCs, myofibroblasts are also known to express αSMA and may be present in the medial and adventitial layers. Platelet derived growth factor receptor α (PDGFRα) is found on Col1a1-expressing cells in the myocardium and has been reported to be a reliable marker for cardiac fibroblasts26. However, immunofluorescent detection of PDGFRα in the aortic wall indicated its presence on cells in the media and adventitia (Fig. 1B), indicating this marker has no specificity for aortic adventitial fibroblasts and may be a more general mesenchymal marker in the aorta.
To determine whether Ang II induced functional RelA activity, expression of the NF-κB-dependent chemokines/cytokines IL-1β, MCP-1, and IL-33 was measured by quantitative-reverse transcriptase-PCR (qRT-PCR) analysis on aortas from sham and Ang II groups. Ang II infusion increased IL-6 transcripts 16-fold and IL-1β transcripts 25-fold. Furthermore, IL-33, a member of IL-1 family of cytokines, was increased 2-fold. Although the chemokine MCP-1 was slightly elevated in aortas from the Ang II group (1.8-fold vs. sham), the difference was not significant. Elevation of IL-6, IL-1β and IL-33 transcripts validates our immunohistochemistry experiments and verifies that Ang II infusion stimulates RelA signaling in the aortic wall.
Col1a2-CreERT is activated by tamoxifen in aortic mesenchymal cells
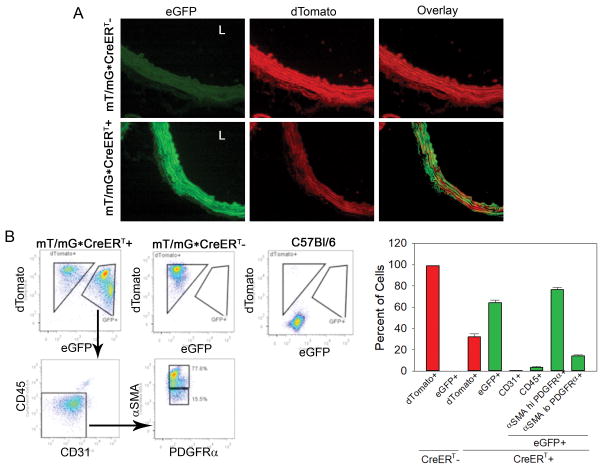
To test the role of mesenchymal RelA in aortic inflammation and AAA, we utilized Col1a2-CreERT transgenic mice in which tamoxifen administration was previously demonstrated to activate CreERT recombinase in fibrogenic mesenchymal cells (vascular fibroblasts and VSMCs). In addition to crossing the Col1α2-CreERT mouse with a RelA f/f, we also crossed the CreERT transgenic with the mT/mG Cre reporter mouse25. In mice harboring the mT/mG alleles, activation of CreERT with tamoxifen leads to excision of the dTomato allele and expression of eGFP. We subjected mT/mG•CreERT+ and mT/mG•CreERT- mice to tamoxifen treatment before isolating their aortas for characterization via fluorescence microscopy and immunophenotyping. Supra-renal aortas for mT/mG•CreERT- demonstrated a robust dTomato signal throughout the aortic wall with an almost complete absence of eGFP+ cells (Fig. 2A). In contrast, aortas from similar regions of mT/mG•CreERT+ mice expressed eGFP signal throughout the medial and adventitial layers whereas the dTomato signal was significantly diminished in regions of eGFP expression. This verified that our 10-day tamoxifen dosing regimen stimulates Col1a2-CreERT activation and also demonstrated that Col1a2-CreERT is activated in mesenchymal cells of the aortic media and adventitia.
Figure 2. Tamoxifen activates Col1α2-CreERT in aortic VSMCs and fibroblasts.
mT/mG; Col1α2-CreERT- (n=4) and mT/mG; Col1α2-CreERT+ (n=5) mice were administered tamoxifen for 10 days before aortas were isolated for characterization. Untreated C57Bl/6 (n=2) mice were used as controls. A) Red-fluorescence protein dTomato and enhanced green fluorescence protein (eGFP) signals were detected via fluorescence microscopy in abdominal aortas of CreERT+ and CreERT- mice. Only aortas from CreERT+ mice expressed eGFP which was observed in the medial and adventitial layers. L indicates aortic lumen. Images were captured at 400x magnification. B) Whole aortas from mT/mG; CreERT-, mT/mG; CreERT+ and C57Bl/6 mice were dissociated into single-cell suspension and labeled with fluorochrome conjugated antibodies raised against CD45 (hematopoietic lineage marker), CD31 (endothelial cell marker), PDGFRα and αSMA (SMC protein). Flow cytometry was used to quantify percent of eGFP+ cells that expressed the specific cellular marker. Cells that were CD45−•CD31−•αSMAhi•PDGFRα+ were considered to be VSMCs and cells that were CD45−•CD31−•αSMAlo•PDGFRα+ were considered to be aortic fibroblasts. Data are presented as mean ± SEM.
To identify cells that underwent genetic recombination after tamoxifen administration, dissociated aortic cell suspensions were labeled with antibodies targeting specific cellular markers and analyzed via flow cytometry. Consistent with results from fluorescence microscopy, almost 100% of aortic cells from mT/mG•CreERT- mice only expressed dTomato (Fig. 2B and 2C). In contrast, approximately 64% of aortic cells from mT/mG•CreERT+ mice expressed eGFP. In this group, approximately 80% were VSMCs (CD45-•CD31-•αSMAhi•PDGFRα+), 14% were fibroblasts (CD45-•CD31-•αSMAlo•PDGFRα+), 3% expressed the circulating leukocyte marker CD45, and less than 2% were endothelial cells (CD45-•CD31+). These results demonstrated that Col1a2-CreERT is mesenchymal and is mainly activated in VSMCs and fibroblasts in the aorta.
We further explored the proportion of each cell type, from the total aortic cells analyzed, that express eGFP (Fig. SII). The highest proportion of cells in the aorta were VSMCs and 40% were eGFP+. This was followed by fibroblasts, of which 25% were eGFP+. Both leukocyte and endothelial cells had minimal expression of eGFP, 2.5% and 0.1% of aortic cells, respectively. These data further confirm that Col1a2-CreERT is mostly activated in aortic VSMCs and fibroblasts.
Confirmation of functional RelA depletion in fibrogenic mesenchymal cells of RelA-CKO mice
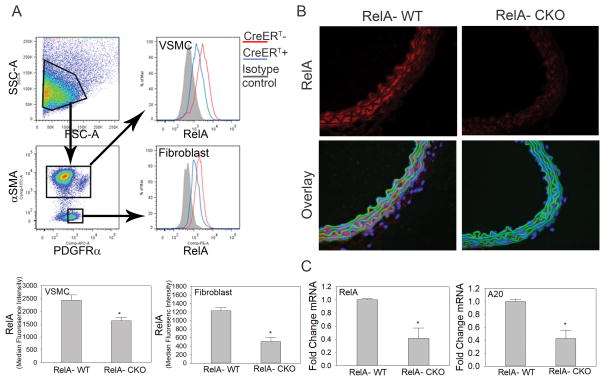
Since we observed increased RelA signaling in aortic VSMCs and fibroblasts during Ang II infusion (Fig. 1), we utilized the Col1a2-CreERT transgenic mouse to determine the contribution of aortic wall mesenchymal RelA in Ang II-induced vascular inflammation and AAA. Tamoxifen was administered to RelA f/f•Col1a2-CreERT+ and RelA f/f•Col1a2-CreERT- mice to generate RelA-CKO and RelA-WT, respectively (see Material and Methods). Aortas from RelA-CKO had a 33% decrease in RelA expression in VSMCs and a 59% decrease in RelA in aortic fibroblasts (Fig. 3A and S3A) as determined by quantitative flow cytometric analysis. Immunostaining for RelA further confirmed a decrease in RelA content in the medial and adventitial layers of the aortic wall in RelA-CKO aortas relative to RelA-WT (Fig. 3B). To further confirm RelA depletion, qRT-PCR was performed on aortic tissue for RelA and TNFAIP3/A20, a highly inducible, purely RelA-dependent gene. Aortas from RelA-CKO mice had a 60% decrease in RelA mRNA and a similar decrease in A20 mRNA (Fig. 3C), thus confirming that RelA was successfully depleted in mesenchymal cells with tamoxifen administration to RelA f/f•Col1a2-CreERT+ animals. Lastly, we confirmed genetic recombination at the RelA locus by PCR on DNA extracted from RelA-WT and RelA-CKO aortas. The 5–8Δ product that would only be observed after excision of exons 5–8 was present only in samples from RelA-CKO mice (Fig. SIIIB and SIIIC).
Figure 3. Tamoxifen induces RelA depletion in aortic VSMCs and fibroblasts from RelA-CKO mice.
A) Detection of RelA in aortic VSMCs and fibroblasts via flow cytometry. Tamoxifen was administered to RelA f/f; Col1α2-CreERT- and RelA f/f; Col1α2-CreERT+ mice to generate RelA-WT and RelA-CKO mice, respectively. Whole aortas were isolated for detection of RelA content in aortic VSMCs (αSMAhi•PDGFRα+) and fibroblasts (αSMAlo•PDGFRα+). n=3 aortas were analyzed in each group. Data are presented as the mean of RelA median fluorescence intensity ± SEM. *P< 0.05. B) Supra-renal abdominal aortas from RelA-WT and RelA-CKO mice were sectioned and immunostained for RelA, which was detected via AF-568 conjugated secondary antibody. DAPI was used to counterstain nuclei. L indicates aortic lumen. n=3 aortas were analyzed in each group. Images were captured at 400x magnification. C) qRT-PCR analysis for RelA and A20, a RelA-dependent gene, was performed on aortas from RelA-WT and RelA-CKO mice. n=3–4 mice in each group were evaluated. Data are presented as mean ± SEM. *P< 0.05.
RelA depletion in aortic fibrogenic mesenchymal cells protects from AAA
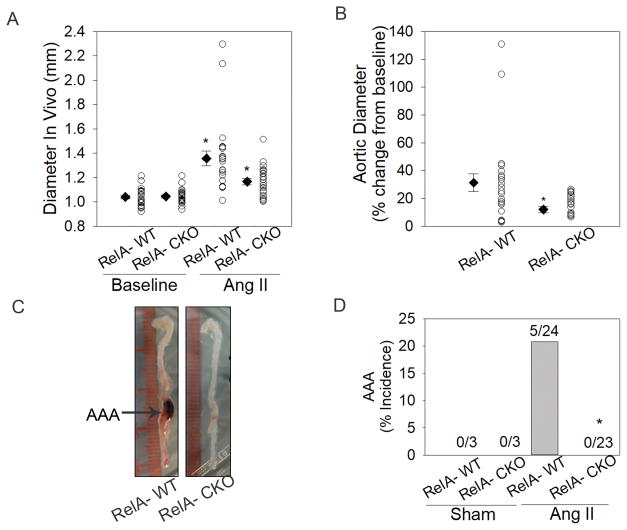
To determine the contribution of aortic mesenchymal cell RelA to development of Ang II-induced aortic dilation and AAA formation, RelA-CKO and RelA-WT mice were infused with Ang II for seven days. Abdominal aortic diameter measurements at baseline demonstrated no differences between the two groups (1.04 ± 0.01 mm vs. 1.04 ± 0.01 mm, RelA-WT vs. RelA-CKO). Ang II infusion significantly increased the mean abdominal aortic diameter in RelA-WT and in RelA-CKO mice (1.36 ± 0.06 mm vs. 1.17 ± 0.03 mm, RelA-WT vs. RelA-CKO; Fig. 4A). This corresponded to a 32 ± 6% change in RelA-WT and a 12 ± 2% change in RelA-CKO (P< 0.005; Fig. 4B). Furthermore, RelA-CKO mice were completely protected from Ang II-induced AAAs whereas there was a 20% incidence of AAA in RelA-WT mice (Figs. 4C and 4D). Since both male and female mice were utilized in this study, we further explored the role of sex on Ang II-induced changes in aortic diameter. Both male and female RelA-WT mice had similar increases in aortic diameter following Ang II infusion (31.8 ± 8% vs. 29.7 ± 3.5%, male vs. female) and both sexes of RelA-CKO mice had similar decreases in aortic diameter (12.5 ± 4% vs. 11.4 ± 3%, male vs. female). These data are shown separately for each gender in Fig. SV.
Figure 4. RelA depletion in aortic VSMCs and fibroblasts protects for AAA.
A, Abdominal aortic diameter measurements of RelA-WT (n=13) and RelA-CKO (n=14) mice were recorded at baseline and during day 6 of Ang II infusion using ultrasonogropahy. Circles represent measurement of individual mice and diamonds with error bars represent the mean ± SEM of the group. *P< 0.05 vs. RelA-WT at baseline; #P< 0.05 vs. RelA-WT with Ang II. B, Change in abdominal aortic diameter during Ang II infusion is presented as percent change from baseline. Circles represent individual mice and diamonds with error bars represent the mean ± SEM. *P< 0.05 vs. RelA-WT. C, Representative images of aortas from Ang II-infused mice. RelA-WT aortas developed AAA whereas RelA-CKO aortas remain protected from this disease. D, Percent incidence of AAA in RelA-WT and RelA-CKO infused with saline (sham) or Ang II for 7 days. Data are cumulative of three independent experiments. *P<0.05 vs. RelA-WT treated with Ang II, Fisher’s exact test.
RelA depletion in fibrogenic mesenchymal cells diminishes aortic inflammation and expansion
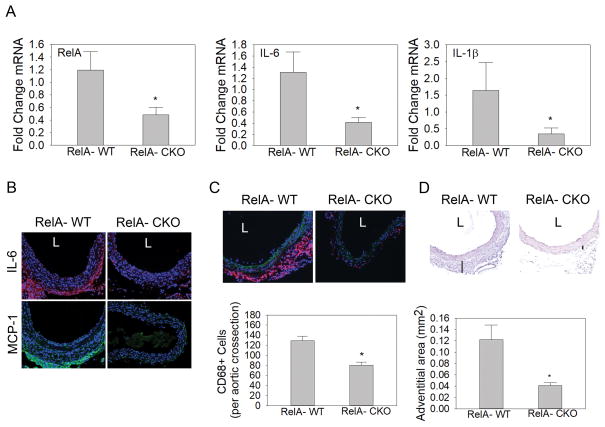
Since RelA is an essential transcriptional activator of inflammatory cytokines IL-6 and IL-1β that have been demonstrated to play a causal role in the development of AAA, we assessed if these cytokines were affected by RelA depletion. In aortas from RelA-CKO mice infused with Ang II, there was approximately a 68% decrease in the expression of RelA, IL-6 and IL-1β transcripts (Fig. 5A). This suggested that a decrease in inflammatory cytokine expression may be one mechanism by which RelA depletion protected from Ang II-induced AAAs. Immunostaining for IL-6 further indicated IL-6 accumulation in the media and adventitia of RelA-WT but not in RelA-CKO aortas (Fig. 5B). Since IL-6 and IL-1β are potent inducers of the chemokine MCP-1 that promotes monocyte/macrophage accumulation in the aortic wall, MCP-1 immunofluorescence was also performed. Immunofluorescence staining demonstrated significant MCP-1 in the adventitia of RelA-WT but almost a complete absence in RelA-CKO aortas (Fig. 5B). Similarly, CD68+ monocyte/macrophages were abundant in RelA-WT aortas but significantly diminished in RelA-CKO, indicating that the reduction in chemotactic cytokine production from depletion of RelA was sufficient to reduce monocyte/macrophage recruitment (Fig. 5C). Ang II-induced changes in the aortic wall included medial dissections and adventitial expansion. Morphometric analysis of RelA-WT and RelA-CKO aortas demonstrated that RelA-CKO aortas were protected from aortic dissections and adventitial hematomas (Fig. SIVA). Furthermore, after excluding aneurysmal samples in RelA-WT group, we found that RelA-CKO aortas still had significantly less adventitial expansion (Fig. 5D table). These results suggest that depletion of mesenchymal-restricted cellular RelA decreased aortic cytokine production, monocyte/macrophage accumulation, and limited adventitial expansion.
Figure 5. RelA depletion in aortic VSMCs and fibroblasts diminishes Ang II-induced aortic inflammation and adventitial expansion.
A, qRT-PCR analysis was performed to quantify relative abundance of RelA, IL-6 and IL-1β transcripts in aortas from RelA-WT and RelA-CKO mice infused with Ang II for 7 days. n=7 aortas from each group were evaluated.*P< 0.05 vs. RelA-WT. B, Immunofluorescence detection of IL-6 and MCP-1 in supra-renal aortic cross-sections from RelA-WT and RelA-CKO mice infused with Ang II. IL-6 was detected using AF-568-conjugated secondary antibody and MCP-1 was detected using AF-488 secondary antibody. n=5–7 abdominal aortas from each group were evaluated. Representative images from each group are shown. L indicates aortic lumen. Images were captured at 400x magnification. C, Immunofluorescence detection of CD68 monocyte/macrophages in supra-renal aortic cross sections from RelA-WT and RelA-CKO mice infused with Ang II. CD68 was detected using AF-568-conjugated secondary antibody. Representative images from n= 5–7 aortas from each group are shown. Images were captured at 400X magnification. Bottom, relative fluorescence intensity of CD68 staining was quantified using Image J, presented as mean ± SEM (n=5–7 from each group). *, P<0.05. D, Hematoxylin and eosin (H&E) staining of abdominal aortas from RelA-WT and RelA-CKO mice infused with Ang II. n=5–7 abdominal aortas were evaluated in each group. Difference in adventitial thickness (black lines) was observed between the two groups. Adventitial area was quantified using Image J software and is presented as mean ± SEM. Images were captured at 400x magnification. *, P<0.05.
RelA depletion decreases recruitment of inflammatory monocytes without affecting blood pressure
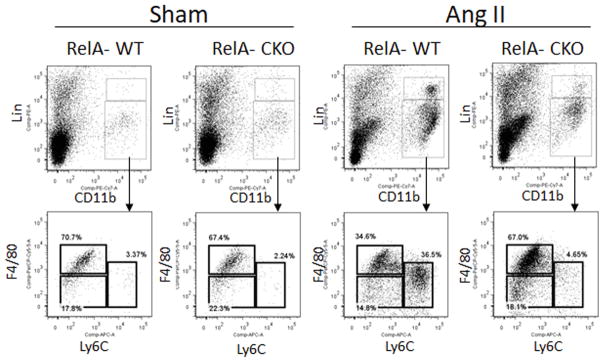
A hallmark of aortic inflammation is the recruitment of CCR2+ monocytes. In mice, up to 60% of the circulating monocytes belong to Ly6Chi •CCR2hi •CX3CR1lo subgroup 27–29. These classical or inflammatory monocytes have a short life-span and accumulate at sites of inflammation. The remaining monocytes, characterized as Ly6Clo•CCR2lo•CX3CR1hi and referred to as the non-classical subset, accumulate in low numbers while patrolling the vasculature for injury 27, 28. In the absence of the MCP-1 receptor, CCR2, Ly6Chi monocytes fail to migrate to the site of injury, reducing monocyte-induced inflammation, injury and fibrosis 3, 30–33. Since Ly6Chi monocytes are important mediators of aortic inflammation and AAA, we asked whether their abundance was affected by aortic-RelA depletion. Using a flow cytometric analysis for monocyte identification 34, we found that 67–70% of CD11b+•Linlo cells in the aortas of sham-infused mice are Ly6Clo•F4/80hi macrophages, 17–22% are Ly6Clo• F4/80lo monocytes and 2–4% are Ly6Chi•F4/80lo monocytes. During Ang II infusion, aortas from RelA-WT mice had a significant increase in Ly6Chi•F4/80lo monocytes (37% of CD11b+•Linlo cells) whereas the level of Ly6Chi monocytes in RelA-CKO aortas was similar to those observed in sham-infused mice. This suggests that activation of RelA in fibrogenic mesenchymal cells plays a critical role in the recruitment of the inflammatory subset of monocytes. We observed that RelA-WT and RelA-CKO mice, having comparable body weights at baseline, had similar increases in systolic blood pressure (SBP) and heart rate during the seven days of Ang II infusion (Table 1). Although there was a trend for slightly higher SBP in the RelA-CKO mice, this was not statistically significant.
Table 1.
Ang II-mediated effects on blood pressure and heart rate.
| Treatment | Sham | Ang II | ||
|---|---|---|---|---|
|
| ||||
| Genotype | RelA-WT | RelA-CKO | RelA-WT | RelA-CKO |
| N | 3 | 3 | 5 | 5 |
| Body weight (g) | 21.6 ± 2.0 | 25.1 ± 1.8 | 23.3 ± 1.7 | 23.6 ± 1.4 |
| SBP (mmHg) | 107 ± 2 | 111 ± 9 | 121 ± 8 | 135 ± 5 |
| Heart rate (bpm) | 690 ± 35 | 765 ± 58 | 1037 ± 92* | 1102 ± 44* |
SBP: systolic blood pressure; Data recorded using non-invasive tail-cuff method (Kent Scientific; Torrington, CT) on at least 4 consecutive days at baseline and during Ang II infusion, and are reported as mean ± SEM.
p <0.05
DISCUSSION
Vascular inflammation, a coordinated response involving activating stromal cells that trigger cytokine and chemokine secretion and leukocyte recruitment, is an important contributor to the pathogenesis of AAA6. Although the etiology of AAA remains unknown, various animal models have provided insights into the underlying mechanisms and have implicated cytokines IL-6, IL-1β and the MCP-1 chemokine receptor CCR2 in its pathogenesis 3, 18, 31. Furthermore, deficiency of Ang II type 1a receptor (AT1aR)35, pattern recognition receptor TLR4, and the adaptor protein MyD88 36 confers protection from AAA, suggesting that the downstream pro-inflammatory transcription factor NF-κB/RelA is integral to the development of AAA. In the Ang II model of AAA, it has been observed by our group and others that adventitial proliferation and inflammation precedes loss of vascular integrity and aortic dilation. Proliferating residential adventitial fibroblasts interact with infiltrating monocytes to promote cytokine amplification loops leading to additional inflammation and ECM destruction 3, 37. In this study, we sought to determine the role of aortic mesenchymal cells that are responsive to Ang II-induced signaling that leads to the development of vascular inflammation and AAA formation.
In this study, we considered the subpopulation of fibroblasts, specialized myofibroblasts, and Col1A-expressing ‘synthetic’ VSMCs that play predominant roles in fibrosis and aortic ECM formation/remodeling38, 39 as ‘fibrogenic’ mesenchymal cells. Although these cells have distinct phenotypes and roles in vascular physiology, they share the common phenotype of Col1A expression, and play an important role in Ang II-induced fibrosis and remodeling. Our studies indicate the central role of NF-κB in these fibrogenic mesenchymal cells as an upstream controller of vascular inflammation, cytokine production and leukocyte infiltration. Our data indicate that Ang II induces RelA activation and IL-6 production in both the adventitial and medial layers of the abdominal aorta. That the mesenchymal lineage is an important component of IL-6 secretion was indicated in our previous studies where IL-6 secretion predominantly originated from mesenchymal cells in the adventitia 21. In addition to the upregulation of IL-6 mRNA in the aortic wall, we also observed a significant increase in IL-1β and IL-33 transcripts during Ang II infusion, further confirming the functional activation of RelA signaling. The essential role of IL-6 and IL-1β in the development of Ang II-induced AAA has previously been determined 3, 18 but the significance of IL-33, a cytokine dependent on RelA but also a modifier of RelA signaling, remains to be explored.
To generate mesenchymal cell-derived RelA-deficient animals, we utilized the Col1a2-CreERT mouse in which the CreERT recombinase, driven by the Col1a2 promoter, is activated in cells of the mesenchymal lineage22, 23, including fibroblasts of the dermis, vasculature, intestines and membranous ossifications of the skull 40. Consistent with these publications, tamoxifen administration to Col1a2-CreERT•mT/mG Cre reporter mice demonstrated recombination not only in aortic adventitial fibroblasts, but also in aortic VSMCs. Since RelA activation was observed in both medial VSMCs and adventitial fibroblasts, we decided to utilize this mouse for investigating the role of RelA in this Col1a2-expressing fibrogenic mesenchymal population in AAA. While it would be of great interest to explore the relative role of fibroblast versus VSMC RelA in the pathogenesis of AAA, currently there is no well-documented, tightly regulated and rapidly inducible fibroblast-specific Cre transgenic mouse available. As an example, it has been demonstrated that Fibroblast Specific Protein 1 (FSP1) promoter-driven Cre is also activated in leukocytes, VSMCs and endothelial cells in the injured myocardium41. A recent study suggests that FSP1-Cre-mediated deletion of AT1aR during Ang II infusion protects from medial thickening of the ascending aorta but the group did not comment on the effect in the abdominal region which is prone to AAA, or exclude the possibility that the effect could be due to loss of leukocyte-AT1aR 42.
The mesenchymal cell restricted RelA-CKO mice were surprisingly resistant to Ang II-induced aortic dilation and AAA formation, despite a statistically insignificant trend towards higher SBP in the RelA-CKO mice. This finding argues even more strongly that the decrease in AAA was related to the decrease in aortic cytokine production and monocyte/macrophage recruitment. In this model, adventitial expansion occurs due to both fibroblast proliferation and circulating monocyte recruitment. Using flow cytometry, we demonstrated that RelA-CKO mice had decreased accumulation of the inflammatory CD11b+•F4/80lo•Ly6Chi monocyte subset during Ang II infusion. Independent investigations have shown that the decrease in circulating monocyte levels with CCR2 deficiency inhibits AAA formation 3, 31. We interpreted this to mean that fibroblast and VSMC RelA is important in Ang II-induced aortic cytokine production and monocyte recruitment that is necessary for AAA. The role of endothelial NF-κB signaling has also been demonstrated to be vital since transgenic overexpression of dominant-negative IκBα in endothelial cells protects hyperlipidemic mice from AAA 43. Authors of that study speculated that endothelial NF-κB signaling likely promotes leukocyte-endothelial interaction in the vasa vasorum leading to leukocyte extravasation into the adventitia, promoting vascular remodeling and aortic expansion 43. Interpreted together with our data, we suggest that coordinate interaction of endothelial cell-mesenchymal NF-κB signaling pathways are required for formation of Ang II-induced aneurysms and dissections.
Our work places RelA signaling in fibrogenic mesenchymal cells as an important upstream event in Ang II-induced AAAs and dissections. In addition to expressing chemokines, cytokines and adhesion factors, fibrogenic mesenchymal cells secrete and remodel the extracellular matrix through Col1A expression, fibronectin production, and matrix metalloproteinase (MMP) expression 44–47. Currently the field views ECM remodeling/fibrosis and vascular inflammation as largely distinct processes; however a number of lines of evidence suggest that inflammation and ECM remodeling are closely intertwined. ECM remodeling has significant effects on inflammation through modification of intercellular adhesion molecules, forming a basement membrane permissive for leukocyte transmigration 14 and release of bioactive and chemotactic molecules. One such protein is the MMP2-dependent production of the NH terminal fragment from the α1 procollagen of type I collagen, which has IL-8-like chemotactic activity 48. Conversely, IL-6 and MCP-1 cytokines produced in response to Ang II via RelA signaling mediate fibroblast proliferation 20, and ECM production 49. Whether these actions are mediated by distinct mesenchymal subpopulations will require further study. Our findings provide a unifying mechanistic link for the potent effects of Ang II on vascular remodeling and activation of cytokine-dependent inflammation.
In summary, this study delineates a critical role of Col1a2-expressing fibrogenic mesenchymal cells in in the development of Ang II-induced vascular inflammation and AAA. Our studies deleting RelA point to the central role of this transcription factor in production of activating cytokines and macrophage recruitment. The key finding of this study that RelA is required for Ang II-induced AAA formation provides impetus to further study how RelA signaling affects the phenotype and secretome of fibrogenic vascular mesenchymal cells. These studies will be foundational to understand some of the proximal steps in aortic inflammation and repair.
Supplementary Material
Figure 6. RelA deletion limits recruitment of inflammatory monocytes into the aortic wall.
Aortas from RelA-WT and RelA-CKO mice infused with saline (sham) or Ang II for 7 days were subjected to flow cytometric analysis for monocytes. Monocytes were identified as CD11bhi, Linlo, and F4/80lo. They were further subdivided based on their expression level of Ly6C into Ly6Chi and Ly6Clo. Macrophages were identified as having F4/80hi expression. Lin represents a combination of cell surface markers including B220, CD49b, CD90, NK1.1 and Ly6G. n=3–5 mice per group.
HIGHLIGHTS.
We generated RelA conditional knockout mice in vascular mesenchymal cells (fibroblasts and synthetic phenotype VSMC) by administering tamoxifen to double transgenic mice harboring RelA-flox alleles and tamoxifen-inducible Col1a2 promoter-driven Cre recombinase (Col1a2-CreERT).
Tamoxifen administration to Col1a2-CreERT mice induced recombination in aortic VSMC and fibroblasts but not in endothelial cells.
Infusion of Ang II significantly increased abdominal aortic diameter and the incidence of AAA in RelA wild-type but not in RelA-CKO mice.
RelA-CKO mice exhibited decreased expression of inflammatory cytokines and decreased recruitment of F4/80lo•Ly6Chi monocytes during Ang II infusion.
Acknowledgments
Core laboratory support was provided by UTMB Optical Imaging Facility and the Sealy Center for Molecular Sciences Selected Reaction Monitoring Facility. We would like to thank Dr. Satish Kumar for providing access to Kent Coda 6 blood pressure apparatus, Dr. Adrian Recinos III for helpful discussions, and Dr. Maki Wakamiya of the UTMB transgenic facility for assistance with RelA f/f mouse and cross-breeding experiments.
SOURCES OF FUNDING
This work was supported by NHLBI pre-doctoral award 1F30HL128036 (TI), UL1TR001439 (ARB), P01HL110869 (DMM), NIEHS ES006676 (ARB), and AHA Award #13GRNT17120070 (RGT).
ABBREVIATIONS
- AAA
abdominal aortic aneurysm
- Ang II
angiotensin II
- αSMA
α-smooth muscle actin
- CKO
conditional knockout
- NF-κB
nuclear factor-kappa B
- MMP
matrix metalloproteinases
- qRT-PCR
quantitative reverse transcriptase-PCR
- VSMC
vascular smooth muscle cells
- WT
wild-type
Footnotes
DISCLOSURES
None
References
- 1.Writing Group M. Mozaffarian D, Benjamin EJ, et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Bruemmer D, Daugherty A, Lu H, Rateri DL. Relevance of angiotensin ii-induced aortic pathologies in mice to human aortic aneurysms. Ann N Y Acad Sci. 2011;1245:7–10. doi: 10.1111/j.1749-6632.2011.06332.x. [DOI] [PubMed] [Google Scholar]
- 3.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial il-6/mcp1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daugherty A, Manning MW, Cassis LA. Angiotensin ii promotes atherosclerotic lesions and aneurysms in apolipoprotein e-deficient mice. J Clin Invest. 2000;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin ii-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brasier AR. The nuclear factor-kappab-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ijaz T, Tilton RG, Brasier AR. Cytokine amplification and macrophage effector functions in aortic inflammation and abdominal aortic aneurysm formation. J Thorac Dis. 2016;8:E746–754. doi: 10.21037/jtd.2016.06.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin CX, Rhaleb NE, Yang XP, Liao TD, D’Ambrosio MA, Carretero OA. Prevention of aortic fibrosis by n-acetyl-seryl-aspartyl-lysyl-proline in angiotensin ii-induced hypertension. Am J Physiol Heart Circ Physiol. 2008;295:H1253–H1261. doi: 10.1152/ajpheart.00481.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen K, Chen J, Li D, Zhang X, Mehta JL. Angiotensin ii regulation of collagen type i expression in cardiac fibroblasts: Modulation by ppar-gamma ligand pioglitazone. Hypertension. 2004;44:655–661. doi: 10.1161/01.HYP.0000144400.49062.6b. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda N, Hu WY, Kubo A, Kishioka H, Satoh C, Soma M, Izumi Y, Kanmatsuse K. Angiotensin ii upregulates transforming growth factor-beta type i receptor on rat vascular smooth muscle cells. Am J Hypertens. 2000;13:191–198. doi: 10.1016/s0895-7061(99)00152-1. [DOI] [PubMed] [Google Scholar]
- 11.Mehta PK, Griendling KK. Angiotensin ii cell signaling: Physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 12.Kim MP, Zhou M, Wahl LM. Angiotensin ii increases human monocyte matrix metalloproteinase-1 through the at2 receptor and prostaglandin e2: Implications for atherosclerotic plaque rupture. J Leukoc Biol. 2005;78:195–201. doi: 10.1189/jlb.1204715. [DOI] [PubMed] [Google Scholar]
- 13.Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, Struber M, Haverich A, Bavendiek U, Drexler H, Schieffer B. Angiotensin ii induces mmp-2 in a p47phox-dependent manner. Biochem Biophys Res Commun. 2005;328:183–188. doi: 10.1016/j.bbrc.2004.12.152. [DOI] [PubMed] [Google Scholar]
- 14.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 15.Cui R, Tieu B, Recinos A, Tilton RG, Brasier AR. Rhoa mediates angiotensin ii-induced phospho-ser536 nuclear factor kappab/rela subunit exchange on the interleukin-6 promoter in vsmcs. Circ Res. 2006;99:723–730. doi: 10.1161/01.RES.0000244015.10655.3f. [DOI] [PubMed] [Google Scholar]
- 16.Choudhary S, Lu M, Cui R, Brasier AR. Involvement of a novel rac/rhoa guanosine triphosphatase-nuclear factor-kappab inducing kinase signaling pathway mediating angiotensin ii-induced rela transactivation. Mol Endocrinol. 2007;21:2203–2217. doi: 10.1210/me.2006-0465. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Ma Y, Zhang J, Cheng J, Du J. A new cellular signaling mechanism for angiotensin ii activation of nf-kappab: An ikappab-independent, rsk-mediated phosphorylation of p65. Arterioscler Thromb Vasc Biol. 2005;25:1148–1153. doi: 10.1161/01.ATV.0000164624.00099.e7. [DOI] [PubMed] [Google Scholar]
- 18.Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, Yoshimura K, Aoki H, Tsutsui H, Noda T, Sagara J, Taniguchi S, Takahashi M. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin ii-induced aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015;35:127–136. doi: 10.1161/ATVBAHA.114.303763. [DOI] [PubMed] [Google Scholar]
- 19.Johnston WF, Salmon M, Su G, Lu G, Stone ML, Zhao Y, Owens GK, Upchurch GR, Jr, Ailawadi G. Genetic and pharmacologic disruption of interleukin-1beta signaling inhibits experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2013;33:294–304. doi: 10.1161/ATVBAHA.112.300432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tieu BC, Ju X, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Brasier AR, Tilton RG. Aortic adventitial fibroblasts participate in angiotensin-induced vascular wall inflammation and remodeling. J Vasc Res. 2011;48:261–272. doi: 10.1159/000320358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Recinos A, 3rd, LeJeune WS, Sun H, Lee CY, Tieu BC, Lu M, Hou T, Boldogh I, Tilton RG, Brasier AR. Angiotensin ii induces il-6 expression and the jak-stat3 pathway in aortic adventitia of ldl receptor-deficient mice. Atherosclerosis. 2007;194:125–133. doi: 10.1016/j.atherosclerosis.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Florin L, Alter H, Grone HJ, Szabowski A, Schutz G, Angel P. Cre recombinase-mediated gene targeting of mesenchymal cells. Genesis. 2004;38:139–144. doi: 10.1002/gene.20004. [DOI] [PubMed] [Google Scholar]
- 23.Hu B, Wu Z, Nakashima T, Phan SH. Mesenchymal-specific deletion of c/ebpbeta suppresses pulmonary fibrosis. Am J Pathol. 2012;180:2257–2267. doi: 10.1016/j.ajpath.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ijaz T, Wakamiya M, Sun H, Recinos A, 3rd, Tilton RG, Brasier AR. Generation and characterization of a novel transgenic mouse harboring conditional nuclear factor-kappa b/rela knockout alleles. BMC Dev Biol. 2016;16:32. doi: 10.1186/s12861-016-0135-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 26.Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingersoll MA, Platt AM, Potteaux S, Randolph GJ. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011;32:470–477. doi: 10.1016/j.it.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. 2013;112:1624–1633. doi: 10.1161/CIRCRESAHA.113.300890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41:21–35. doi: 10.1016/j.immuni.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for ccr2 and mcp-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daugherty A, Rateri DL, Charo IF, Owens AP, Howatt DA, Cassis LA. Angiotensin ii infusion promotes ascending aortic aneurysms: Attenuation by ccr2 deficiency in apoe−/− mice. Clin Sci (Lond) 2010;118:681–689. doi: 10.1042/CS20090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Croxford AL, Lanzinger M, Hartmann FJ, Schreiner B, Mair F, Pelczar P, Clausen BE, Jung S, Greter M, Becher B. The cytokine gm-csf drives the inflammatory signature of ccr2+ monocytes and licenses autoimmunity. Immunity. 2015;43:502–514. doi: 10.1016/j.immuni.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, Figueiredo JL, Kohler RH, Chudnovskiy A, Waterman P, Aikawa E, Mempel TR, Libby P, Weissleder R, Pittet MJ. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassis LA, Rateri DL, Lu H, Daugherty A. Bone marrow transplantation reveals that recipient at1a receptors are required to initiate angiotensin ii-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 36.Owens AP, 3rd, Rateri DL, Howatt DA, Moore KJ, Tobias PS, Curtiss LK, Lu H, Cassis LA, Daugherty A. Myd88 deficiency attenuates angiotensin ii-induced abdominal aortic aneurysm formation independent of signaling through toll-like receptors 2 and 4. Arterioscler Thromb Vasc Biol. 2011;31:2813–2819. doi: 10.1161/ATVBAHA.111.238642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin ii-infused, apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 38.Ponticos M, Smith BD. Extracellular matrix synthesis in vascular disease: Hypertension, and atherosclerosis. J Biomed Res. 2014;28:25–39. doi: 10.7555/JBR.27.20130064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsson E, McLean SE, Mecham RP, Lindahl P, Nelander S. Do two mutually exclusive gene modules define the phenotypic diversity of mammalian smooth muscle? Mol Genet Genomics. 2008;280:127–137. doi: 10.1007/s00438-008-0349-y. [DOI] [PubMed] [Google Scholar]
- 40.Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts : A potentially powerful technique for investigating gene function in fibrosis. Am J Pathol. 2002;160:1609–1617. doi: 10.1016/S0002-9440(10)61108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol Heart Circ Physiol. 2013;305:H1363–1372. doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poduri A, Rateri DL, Howatt DA, Balakrishnan A, Moorleghen JJ, Cassis LA, Daugherty A. Fibroblast angiotensin ii type 1a receptors contribute to angiotensin ii-induced medial hyperplasia in the ascending aorta. Arterioscler Thromb Vasc Biol. 2015;35:1995–2002. doi: 10.1161/ATVBAHA.115.305995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito T, Hasegawa Y, Ishigaki Y, Yamada T, Gao J, Imai J, Uno K, Kaneko K, Ogihara T, Shimosawa T, Asano T, Fujita T, Oka Y, Katagiri H. Importance of endothelial nf-kappab signalling in vascular remodelling and aortic aneurysm formation. Cardiovasc Res. 2013;97:106–114. doi: 10.1093/cvr/cvs298. [DOI] [PubMed] [Google Scholar]
- 44.Novitskiy G, Potter JJ, Rennie-Tankersley L, Mezey E. Identification of a novel nf-kappab-binding site with regulation of the murine alpha2(i) collagen promoter. J Biol Chem. 2004;279:15639–15644. doi: 10.1074/jbc.M311499200. [DOI] [PubMed] [Google Scholar]
- 45.Lee BH, Park SY, Kang KB, Park RW, Kim IS. Nf-kappab activates fibronectin gene expression in rat hepatocytes. Biochem Biophys Res Commun. 2002;297:1218–1224. doi: 10.1016/s0006-291x(02)02356-2. [DOI] [PubMed] [Google Scholar]
- 46.Raymond L, Eck S, Hays E, Tomek I, Kantor S, Vincenti M. Rela is required for il-1beta stimulation of matrix metalloproteinase-1 expression in chondrocytes. Osteoarthritis Cartilage. 2007;15:431–441. doi: 10.1016/j.joca.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: An absolute requirement for transcription factor nf-kappa b. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 48.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide cxcr ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–323. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 49.Ju X, Ijaz T, Sun H, Lejeune W, Vargas G, Shilagard T, Recinos A, 3rd, Milewicz DM, Brasier AR, Tilton RG. Il-6 regulates extracellular matrix remodeling associated with aortic dilation in a fibrillin-1 hypomorphic mgr/mgr mouse model of severe marfan syndrome. J Am Heart Assoc. 2014;3:e000476. doi: 10.1161/JAHA.113.000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.