Abstract
Objective
Blood vessel wall damage often results in the formation of a fibrin clot that traps inflammatory cells, including monocytes. The effect of clot formation and subsequent lysis on the expression of monocyte-derived genes involved in the development and progression of ischemic stroke and other vascular diseases, however, is unknown. Determine if clot formation and lysis regulates the expression of human monocyte-derived genes that modulate vascular diseases.
Approach and Results
We performed Next Generation RNA-sequencing on monocytes extracted from whole blood clots and using a purified plasma clot system. Numerous mRNAs were differentially expressed by monocytes embedded in clots compared to unclotted controls, and interleukin 8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) were among the upregulated transcripts in both models. Clotted plasma also increased expression of IL-8 and MCP-1, which far exceeded responses observed in LPS-stimulated monocytes. Upregulation of IL-8 and MCP-1 occurred in a thrombin-independent, but fibrin-dependent manner. Fibrinolysis initiated shortly after plasma clot formation (i.e., 1–2 hours) reduced the synthesis of IL-8 and MCP-1, while delayed fibrinolysis was far less effective. Consistent with these in vitro models, monocytes embedded in unresolved thrombi from patients undergoing thrombectomy stained positively for IL-8 and MCP-1.
Conclusions
These findings demonstrate that clots are potent inducers of monocyte gene expression, and that timely fibrinolysis attenuates inflammatory responses, specifically IL-8 and MCP-1. Dampening of inflammatory gene expression by timely clot lysis may contribute to the clinically-proven efficacy of fibrinolytic drug treatment within hours of stroke onset.
Keywords: thrombolysis, fibrin, monocytes, inflammation, fibrinogen, thrombin
Introduction
Coagulation contributes to the pathogenesis of many disease processes including sepsis, myocardial infarction, and ischemic stroke1, 2. Upon vessel injury, blood is exposed to tissue factor, resulting in the formation of thrombin that stems blood loss by activating coagulation proteases and platelets3–5. Thrombin also induces the conversion of fibrinogen to fibrin, resulting in the formation of clots that seal wounds6, 7.
When clots form, they trap numerous blood cells including monocytes. Monocytes play an important role in bridging hemostasis and inflammation because they generate tissue factor and inflammatory cytokines in response to external stimuli8–10. Perhaps the most well-known stimulus of monocyte gene expression is lipopolysaccharide (LPS)11, 12. However, LPS is not present in most clinical situations where clots form, and individual clotting factors such as tissue factor, thrombin13, 14, and fibrin15–17 have variable and generally mild stimulatory effects on monocyte gene expression. This suggests that clots are ineffective triggers of gene expression by monocytes, but this assumption has not been rigorously tested. Accordingly, we determined if intact clots alter gene expression patterns in monocytes using human models of whole blood and plasma clot formation. Our data demonstrate that clots induce marked changes in the monocyte transcriptome, including increased expression of IL-8 and MCP-1. Remarkably, these shifts were more intense than changes in gene expression induced by LPS.
After fibrin formation occurs under physiologic conditions, tissue-type plasminogen activator (tPA) is generated and immediately begins breaking down the fibrin matrix18. Iatrogenic fibrinolysis is utilized as a therapeutic intervention in pathologic thrombosis19, 20. In the setting of stroke, tPA is administered at high levels to quickly dissolve the clot and improve blood flow to ischemic brain. In either setting, the effect of fibrinolysis on cytokine expression has not been examined. Our results demonstrate fibrinolysis significantly down-regulates IL-8 and MCP-1 synthesis by monocytes, depending on when lysis was initiated. These findings have significant implications in ischemic stroke as timely administration of tPA after stroke dictates outcomes.
Material and Methods
Please see online supplemental for materials and methods.
Results
Whole Blood Clots Trigger Inflammatory Gene Expression in Monocytes
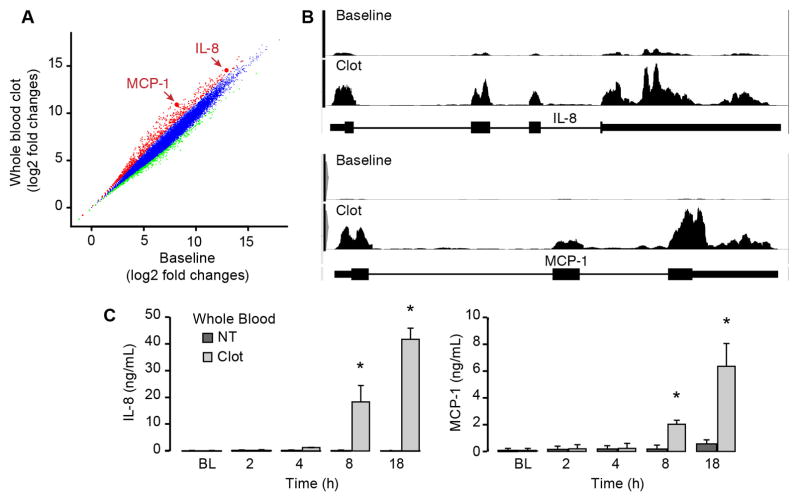
To test whether clots trigger inflammatory gene expression, whole blood was clotted with recombinant tissue factor in the presence of calcium for two hours and gene expression patterns were examined in purified monocytes. Isolated monocytes were mostly CD14+C16− (>90%) and this population did change significantly after whole blood clot formation (Supplemental Figure I). RNA-seq revealed that monocytes expressed over 10,000 transcripts, and nearly 1000 were differentially expressed (i.e., greater than 4-fold) in clot-retrieved monocytes compared to baseline monocytes (Figure 1A). Among the differentially expressed transcripts were Interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1), which were increased by more than 6 and 48-fold, respectively, in clot-retrieved monocytes (Figure 1A and 1B). Increased mRNA expression was confirmed by real-time PCR (Supplemental Figure II) and was associated with elevated levels of IL-8 and MCP-1 protein, which accumulated in a time-dependent fashion (Figure 1C). In addition to IL-8 and MCP-1, 13 other proteins involved in inflammation were increased after whole blood clot formation, and mRNA for these genes correlated with their protein expression levels (Supplemental Figure III). These cytokines were also expressed in thrombi extracted from patients (Supplemental Figure IV) indicating that in vivo clot formation is also associated with the generation of inflammatory proteins
Figure 1. Whole blood clot formation markedly alters gene expression patterns in monocytes.
(A) RNA sequencing was performed in baseline or clot-retrieved monocytes and relative expression of individual genes were plotted against each other. Red and green indicate increased and decreased gene expression, respectively, between clotted compared to baseline (4-fold difference or greater). IL-8 and MCP-1 mRNA expression compared to other genes is highlighted by arrows. (B) Distribution of RNA-seq reads across MCP-1 and IL-8 transcripts (with intron/exon structures depicted below each plot) in monocytes from unclotted (baseline) blood and clotted whole blood. (C) Protein for IL-8 and MCP-1 protein was measured in the plasma of clotted whole blood at specific times post-clot formation. The bars in this graph indicate mean±SEM of three independent experiments (n=3). The asterisk indicates p<0.05 compared to freshly-isolated monocytes that were processed immediately (BL = baseline).
Plasma Clot Formation Induce Inflammatory Gene Expression in Monocytes
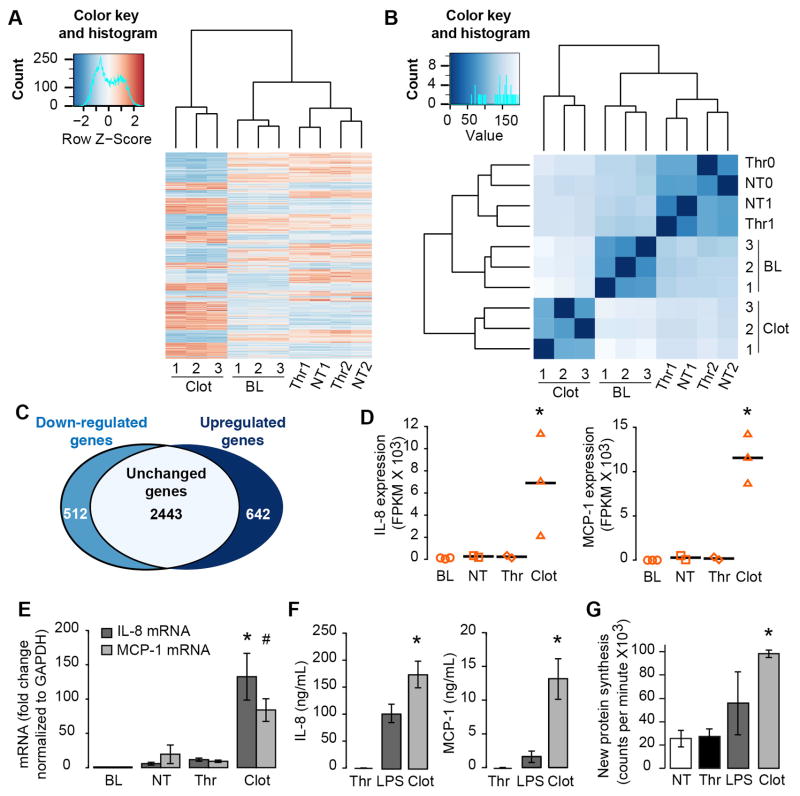
Next we determined if clotted plasma triggers gene expression in a manner similar to whole blood. For these studies, recombinant tissue factor was added to re-calcified, cell-free plasma and allowed to clot before addition of purified autologous monocytes (Supplemental Figure V). Changes in monocyte gene expression in response to clot formation were then assessed. RNA-seq analyses revealed that mRNAs were differentially expressed in monocytes embedded within clots compared to freshly-isolated monocytes (baseline), unstimulated monocytes (untreated) that were left in suspension culture for equivalent time periods, or thrombin (Thr) stimulated monocytes (Figure 2A). Similarity matrices demonstrated that RNA patterns for freshly-isolated, untreated and thrombin-stimulated, and clot-retrieved monocytes clustered in distinct nodes (Figure 2A). More than 1100 transcripts were differentially expressed by more than 4-fold between the clotted and control groups (Figure 2A). In addition, patterns of gene expression in monocytes isolated from whole blood and plasma clots were moderately correlated with one another (Supplemental Figure VI, R=0.364 and p = 0.0008) demonstrating that the reduced plasma model clot system mimics responses observed in a whole blood clotting model.
Figure 2. Monocytes embedded in plasma fibrin clots undergo significant changes in gene expression.
(A) Monocytes were embedded in plasma clots for 18 hours (CLOT) and gene expression patterns were compared to control samples. The control samples included freshly-isolated monocytes (BL – baseline), monocytes left in suspension M199 culture for 18 hours (NT – not treated), or monocytes in M199 stimulated with 0.1 U/ml of thrombin (Thr). Heatmaps of gene expression patterns and global sample distance clustering of monocytes that were retrieved from clots compared to control samples. Upregulated and downregulated genes (p<0.05 and greater than 4-fold difference) in clot-retrieved monocytes compared to monocytes left in suspension culture. Expression of IL-8 and MCP-1 mRNA in monocytes retrieved from clots versus control samples, as measured by RNA-sequence analysis (B) or) real-time PCR (C). (D) Synthesis of IL-8 and MCP-1 protein by monocytes embedded in plasma clots versus thrombin or LPS stimulated monocytes.
Monocytes embedded in plasma clots displayed increased expression for IL-8 and MCP-1 mRNA compared to all control groups as measured by RNA-seq (Figure 2B). Quantitative real-time PCR confirmed increases in IL-8 and MCP-1 (Figure 2C) and demonstrated a time-dependent change in mRNA expression (Supplemental Figure VII). Consistent with changes in mRNA, high levels of IL-8 and MCP-1 protein were detected in plasma clot samples (Figure 2D). In contrast, protein for IL-8 and MCP-1 was not observed in untreated, or thrombin-stimulated samples resuspended in M199 (Figure 2D). Monocytes embedded within clots also produced more IL-8 and MCP-1 protein than LPS-stimulated monocytes (Figure 2D), and monocytes stained similarly for IL-8 and MCP-1 as compared to thrombi isolated from human patients (Supplemental Figure VIII). Global protein synthesis was also markedly higher in clotted samples when compared to NT and LPS-treated samples (Supplemental Figure IX).
MCP-1 and IL-8 Expression are Regulated at the Transcriptional Level
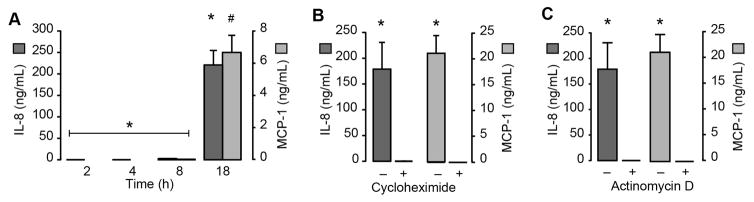
Protein for IL-8 and MCP-1 was markedly increased after 18 hours, but not before then (Figure 3A). Accumulation of IL-8 and MCP-1 protein was completely blocked in the presence of cycloheximide, a global inhibitor of protein synthesis (Figure 3B and Supplemental Figure X). Actinomycin D also prevented monocytes from generating IL-8 and MCP-1 protein (Figure 3C). To further examine transcriptional regulation of IL-8 and MCP-1 induced by plasma clots, we treated monocytes with a NFκB inhibitor. Inhibition of NFκB blocked IL-8 and MCP-1 synthesis (Supplemental Figure XI). Taken together these results demonstrate that the synthetic events were primarily controlled at the transcriptional level
Figure 3. Synthesis of IL-8 and MCP-1 occurs at the transcriptional level.
(A) Time-dependent secretion of IL-8 and MCP-1 protein. (B,C) IL-8 and MCP-1 protein levels (18 hours post-clot formation) in the presence of cycloheximide, an inhibitor of global protein synthesis (B), or actinomycin D, a transcriptional inhibitor (C). The bars in the panels represent the mean±SEM of 3 independent experiments for each group. The asterisk indicates p<0.05 compared to 2, 4, and 8 hour timepoints (panel A), cycloheximide treatment (panel B), or actinomycin treatment (panel C).
Fibrin Formation is Required for Cytokine Synthesis
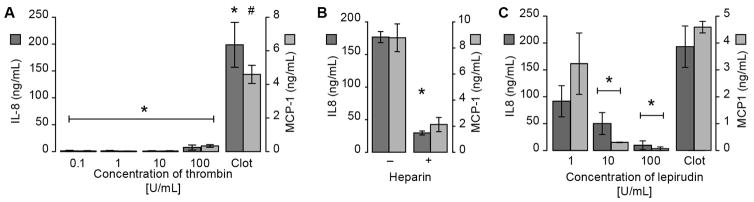
Results displayed in Figures 2C and 2D demonstrate that treatment of purified monocytes (i.e not in plasma) with low concentrations of thrombin (0.1 U/ml) did not have appreciable effects on IL-8 and MCP-1 mRNA or protein levels, and thrombin did not increase global protein synthesis (Supplemental Figure IX). We also found that increasing concentrations of purified thrombin had no appreciable effect on IL-8 or MCP-1 synthesis (Figure 4A). However, pretreatment of plasma with heparin, which blunts thrombin generation and thereby clot formation, significantly reduced IL-8 and MCP-1 synthesis (Figure 4B). The specific thrombin inhibitor lepirudin also reduced cytokine synthesis in a dose-dependent manner (Figure 4C). These studies indicated that thrombin generation, but not thrombin itself, is critical for clot-dependent cytokine production.
Figure 4. The role of thrombin in the induction of inflammatory responses in monocytes.
(A) Monocytes were embedded in plasma clots triggered by tissue factor and calcium or stimulated in the absence of plasma with increasing concentrations of thrombin. (B) Monocytes were embedded in plasma clots in the presence or absence of heparin. (C) Monocytes were embedded in plasma clots that were formed in the presence of the direct thrombin inhibitor lepirudin. In all treatment conditions, cell-free supernatants were harvested after 18 hours and IL-8 and MCP-1 levels were assessed. The bars in the panels represent the mean±SEM of 3 independent experiments for each group. The asterisk indicates p>0.05 compared to all thrombin concentrations (panel A), heparin-treated samples (panel B), and 10 or 100 U/ml of lepirudin (panel C).
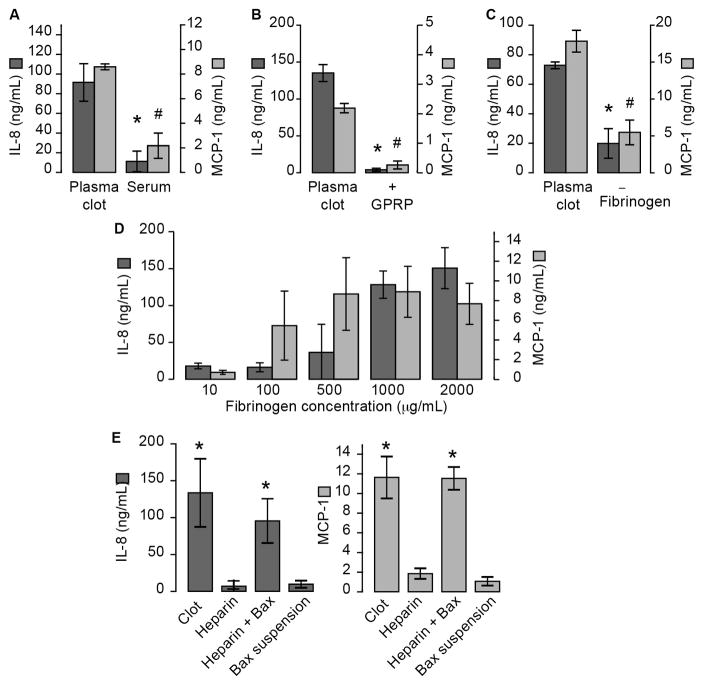
Because thrombin converts fibrinogen to fibrin, we determined if mediators released into the clotted milieu or fibrin formation are required for cytokine synthesis. The addition of fibrin-free serum did not induce IL-8 or MCP-1 synthesis (Figure 5A), suggesting that fibrin formation is the primary instigator of these synthetic events. To examine this postulate in more detail, we added Gly-Pro-Arg-Pro (GPRP) to thrombin-treated plasma to see if inhibition of fibrin polymerization prevents MCP-1 and IL-8 synthesis. As shown in Figure 5B, the addition of GPRP blocked IL-8 and MCP-1 synthesis. The concentration of GPRP to inhibit fibrin formation had no effect on LPS stimulated monocytes (Supplemental Figure XII). We also found that the addition of tissue factor and calcium to fibrinogen-deficient plasma did not induce a robust cytokine response as compared to the presence of fibrinogen (Figure 5C). In addition, tissue factor, alone, had little effect on cytokine production (Supplemental Figure XIII). Reintroduction of fibrinogen to fibrinogen-deficient plasma, however, triggered IL-8 and MCP-1 synthesis in a concentration-dependent manner (Figure 5D). When thrombin generation was blocked by the presence of heparin, but fibrin formation was allowed to proceed by the addition of batroxobin - a snake venom capable of cleaving fibrinogen - monocytes synthesized IL-8 and MCP-1 (Figure 5E). Fibrin stabilization through Factor (F) XIII may play a critical role inducing monocyte derived IL-8 and MCP-1. To address the role of FXIII, we formed plasma clots in the presence and absence of a specific FXIII inhibitor, T101. Plasma clots formed in the presence of T101 had reduced fibrin cross-linking, but had no effect on cytokine production (Supplemental Figure XIV). In addition, plasma clots were generated in the presence and absence of FXIII using FXIII-deficient plasma. In the absence of FXIII, we observed decreased fibrin crosslinking, but no change in IL-8 and MCP-1 synthesis (Supplemental Figure XIV). We next examined if purified fibrinogen and thrombin alone were sufficient to induce IL-8 and MCP-1 synthesis. Interestingly, purified fibrinogen and thrombin did not recapitulate the plasma system (Supplemental Figure XV). However, the addition of serum to purified fibrinogen and thrombin induced robust IL-8 and MCP-1 secretion. Taken together, these results indicate that fibrin formation in the setting of plasma induces inflammatory gene expression in monocytes.
Figure 5. Fibrin Formation induces the inflammatory response in monocytes.
(A) Purified, cell-free plasma clots were triggered with recombinant tissue factor and calcium. After two hours, serum was removed from the plasma clots by centrifuging the sample at 12,000 x g for 20 minutes. Monocytes were then incubated with plasma clots or in residual serum. (B) Monocytes were embedded into plasma fibrin clots formed in the presence of GPRP, a fibrin polymerization inhibitor or vehicle (water). (C) Monocytes were embedded in plasma clots that were generated from normal plasma or fibrinogen-deficient plasma (left). (D) Monocytes were embedded in fibrinogen-deficient plasma that was reconstituted with increasing concentrations of fibrinogen (right). (E) Plasma fibrin clots were formed in the presence of heparin to prevent thrombin generation, and in the presence or absence of batroxobin (1 U/mL), which generates fibrin in a thrombin-independent manner. As a control, monocytes were resuspended in M199 and stimulated with batroxobin. In all treatment conditions, cell-free supernatants were harvested after 18 hours and IL-8 and MCP-1 levels were assessed. The bars in the panels represent the mean±SEM of 3 independent experiments for each group. The asterisk indicates p>0.05 in clot samples versus their comparative controls.
Fibrinolysis Blunts Clot-induced Cytokine Synthesis in a Time-dependent Manner
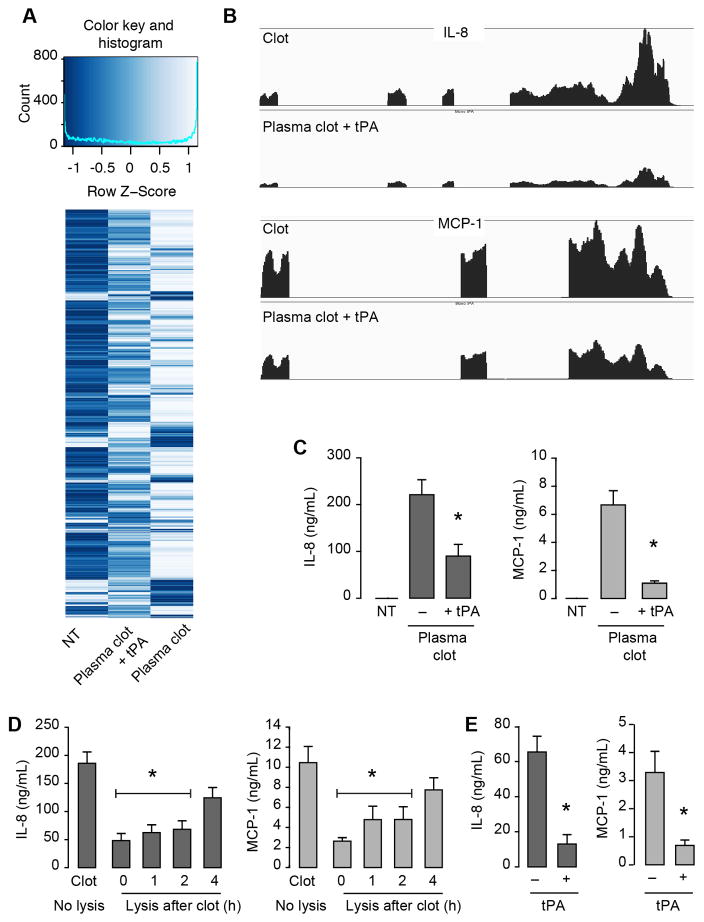
Next we determined if fibrinolysis (breakdown of the fibrin clot) affects clot-induced cytokine synthesis. Monocyte mRNA expression patterns were examined in plasma clots that were lysed with tPA 30 minutes after clot formation and the addition of monocytes. As shown in Figure 6A, mRNA expression patterns were markedly different in clot-retrieved monocytes compared to untreated monocytes, and these changes were blunted in the presence of tPA. Furthermore, patterns of gene expression in monocytes isolated from plasma clots and plasma clots plus tPA demonstrated a negative correlation with one another (R = 0.807, p<0.001, Supplemental Figure XVI) demonstrating that the fibrinolysis significantly blunted inflammatory gene responses. Monocytes isolated from lysed samples had reduced expression of IL-8 and MCP-1 mRNA when compared to clot-retrieved monocytes (Figure 6B), and protein for these cytokines was similarly reduced (Figure 6C). Decreases in IL-8 and MCP-1 were time-dependent and not durable when tPA-treatment was delayed by more than 2 hours after addition of monocytes (Figure 6D). tPA treatment similarly reduced IL-8 and MCP-1 protein levels when it was administered to whole blood 1 hour after clots were formed (Figure 6C).
Figure 6. Timely fibrinolysis of clots blunts the inflammatory response in monocytes.
(A) Monocytes were left in suspension culture (NT – not treated), embedded in plasma fibrin clots, or embedded in plasma fibrin clots for 30 minutes and then lysed with tPA. After 18 hours, monocytes were harvested and prepared for RNA-sequencing. (A) Heatmaps of mRNA expression patterns (4-fold change or greater) in NT, Clot, and Clot + tPA samples. (B) Distribution of RNA-sequencing reads for IL-8 and MCP-1 in NT, Clot, and Clot + tPA samples. (C) IL-8 and MCP-1 protein levels in NT, Clot, and Clot + tPA samples. Whole blood was clotted in the presence (added 1-hour post-clot formation) or absence of tPA and after 18 hours, IL-8 and MCP-1 protein levels were determined. The asterisk indicates p>0.05 in clot samples versus their comparative controls. Monocytes were embedded in plasma clots and subsequently treated with tPA at different times post-clot formation. IL-8 and MCP-1 protein was assessed after 18 hours (D). The bars in Panels C–D represent the mean±SEM of 3 independent experiments.
Discussion
Monocytes play prominent roles in driving coagulation and inflammation, in part through de novo synthesis of thromboinflammatory proteins upon stimulation. Here, we determined for the first-time intact clots trigger robust, global expression of inflammatory genes in monocytes, including IL-8 and MCP-1. Thrombi extracted from patients demonstrated similar patterns of cytokine expression, suggesting in vivo clot formation can also induce the generation of IL-8 and MCP-1 (Supplemental Figure IV). The ability of the clot to activate multiple inflammatory cytokines (IL-8, MCP-1 and others (Supplemental Figure XVII) and pathways underscores the complexity of the interaction between coagulation reactions and inflammation. Furthermore, these data suggest that dysregulation of clot formation may significantly impact downstream processes such as wound healing, and could contribute to ongoing coagulation responses since genes such as tissue factor display increased expression in clot-retrieved monocytes (Supplemental Figure XVIII).
RNA-seq analysis of monocytes derived from whole blood clots demonstrated robust differential gene expression compared to monocytes isolated immediately after whole blood was drawn. To further verify specific changes in the monocyte transcriptome due to plasma clot formation, we performed additional RNA-seq analysis from monocytes embedded in a purified plasma clot system. RNA-seq analysis revealed that monocytes embedded in plasma clots mounted a robust gene expression response, and differentially expressed mRNAs resembled observed changes in the more complex whole blood clot (Supplemental Figure VI, R = 0.364, p value = 0.0008). Plasma clots induced robust synthesis of IL-8 and MCP-1 mRNA and protein, and a marked increase in global protein synthesis. Consistent with an increase in global protein synthesis, ribosomes attached to endoplasmic reticulum were more frequently observed in clot-embedded monocytes compared to unstimulated monocytes (Supplemental Figure IX). The upregulation of mRNAs followed by subsequent synthesis of their corresponding proteins (Supplemental Figure XVII) in clot-retrieved monocytes suggests that the synthesis of numerous genes is regulated at the transcriptional level. In this regard, transcriptional inhibitors completely abrogated the synthesis of IL-8 and MCP-1 demonstrating that clots induce the transcription and then translation of both mRNAs.
The data indicate that generation of fibrin is essential for plasma clot-induced changes in gene expression. We first demonstrated using heparin, a clinically useful anticoagulant, a significant reduction in cytokine protein expression. To focus on the role of thrombin, we next used the direct thrombin inhibitor lepirudin21, which also blocked the production of cytokines. We did not observe IL-8 and MCP-1 synthesis when increasing concentrations of thrombin were introduced to purified monocytes in the absence of plasma, despite the fact that the higher doses utilized exceeded previously reported peak levels of thrombin generation22, 23. We also found that thrombin did not increase global protein synthesis by monocytes, and mRNA patterns in thrombin-stimulated monocytes clustered with mRNA expression in unstimulated monocytes. These results are similar to those produced by Nieuwenhuizen and colleagues13 who demonstrated that high concentrations of thrombin did not induce cytokine synthesis, but contrast a report showing that thrombin regulates the expression of inflammatory mRNAs14. The reasons for these differences are not obvious, but may be due to the techniques (RNA-sequencing versus microarray analysis) and/or the experimental milieus employed. Other have used genetics models to examine the role of thrombin in regulating inflammation. For example, deletion of protease activator receptor 1, the major receptor for thrombin signaling, had no effect on macrophage cytokine synthesis24. Taken together, these findings indicate only a small role for thrombin in regulating these inflammatory responses.
Although thrombin did not directly modulate gene expression in purified, plasma-free monocytes, our data demonstrates that thrombin is still indirectly necessary for cytokine responses by acting on fibrinogen. Our data suggests that thrombin exerts its effect by inducing fibrin formation, which directly triggers gene expression pathways in monocytes. Indeed, pretreatment of plasma with the peptide GPRP, which inhibits fibrin polymerization by blocking ‘A:a’ interactions, significantly reduced cytokine synthesis25, 26. Likewise, fibrinogen-deficient plasma failed to support cytokine production, but reintroduction of fibrinogen to plasma restored clot formation and cytokine synthesis. Batroxobin27, which cleaves fibrinogen into fibrin independent of thrombin, also restored clot formation and supported IL-8 and MCP-1 production in heparinized plasma. These data demonstrate that fibrin formation elicits gene expression responses in monocytes. However, the absence of fibrinogen did not completely abolish cytokine formation (Figure 5C) and purified fibrin generated by thrombin was unable to induce IL-8 and MCP-1 synthesis (Supplemental Figure XV). These findings suggest additional factors circulating in the plasma or generated during clot formation are necessary in addition to fibrin to induce robust cytokine response in monocytes. While the current study focused on the role of fibrin in the setting of plasma, additional studies are warranted to determine other critical factors important in regulating this response.
The exact mechanism by which fibrinogen/fibrin drive IL-8, MCP-1 and other inflammatory gene expression is not known. Previous studies have demonstrated fibrin(ogen) is capable of inducing synthesis of IL-8, MCP-1, IL-6, IL-1β and other cytokines in immune cells and vascular endothelial cell in vitro and in vivo24, 28–31. The mechanism behind fibrinogen and/or fibrin driven cytokine responses was thought to be through toll-like receptor-4 (TLR4) on macrophages32, 33. However, inhibition of TLR434, 35 had no effect on cytokine production (Supplemental Figure XIX), suggesting that ligation of TLR4 by fibrinogen is not the mechanism for induction of cytokine synthesis in our experiments29. In addition, these data indicate that low levels of LPS in clots are not responsible for increased IL-8 and MCP-1 synthesis. The generation of FXIIIa and its role in stabilizing fibrin through cross-linking may also play a role in driving the cytokine response in monocytes. Previous studies have demonstrated FXIIIa is capable of binding to αvβ3 on monocytes and upregulating proliferation and migration while preventing apoptosis36. Using pharmacologic inhibition and FXIII-deficient plasma, we observed minimal changes in IL-8 and MCP-1 synthesis (Supplemental Figure XIV). Another possibility is CD11b/CD18, which binds fibrinogen/fibrin with high affinity through the c-terminal region of fibrinogen’s gamma chain37. It has been shown that this site becomes exposed upon conversion of fibrinogen to fibrin38, 39, and previous publications have shown that interactions of fibrin with CD11b/CD18 regulate NFκB-dependent cytokine production. Interestingly, pharmacologic inhibition of NFκB blunts clot-induced synthesis of IL-8 and MCP-1 (Supplemental Figure XI). However, an inhibitory antibody against CD11b blocked fibrinogen binding, but had little effect on cytokine production (Supplemental Figure XX). Additional studies using mice deficient in CD11b/CD18 are necessary to fully examine the role CD11b/CD18 in plasma clot-mediated IL-8 and MCP-1 synthesis as studies using CD11b/C18 knockout mice demonstrated they are protected from cerebral ischemia reperfusion injury40, suggesting that interactions of fibrin with CD11b/CD18 may regulate inflammatory processes during stroke.
While the ability of fibrin to interact with vessels and cells is important in stemming blood loss, fibrin deposition and its prompt removal are necessary in wound repair41. The removal of fibrin from a thrombus is regulated by the generation of plasmin from its pro-form, plasminogen. Plasminogen binding to fibrin in conjunction with tPA results in plasmin generation and the subsequent degradation of the fibrin clot18; therefore, a delicate balance is needed under physiologic conditions between pro-coagulant and fibrinolytic processes to prevent bleeding or thrombosis and to ensure proper wound repair. Previous studies in plasminogen deficient mice have demonstrated exacerbated inflammatory diseases associated with arthritis, nerve damage and osteoporosis42–45. Genetic or pharmacologic depletion of fibrinogen rescued the associated phenotype suggesting that fibrin and its subsequent lysis modulates the disease process42, 44, 45. Additionally, Cole et al. demonstrated that plasminogen-deficient animals have elevated levels of IL-646. These studies, together with our findings, demonstrate that the balance between fibrin formation and lysis critically regulates IL-8, MCP-1 and other inflammatory cytokine production by monocytes.
As many as 795,000 individuals experience a new or recurrent stroke in the United States each year47. Intravenous fibrinolytic therapy involving the use of tPA for acute stroke is widely agreed upon to be beneficial if administered within 3–4.5 hours of symptom onset48. This demonstrates that timely administration of tPA is necessary for maximal benefit to the patients. While fibrinolytics allow for reperfusion in the ischemic area, our data suggest an added benefit of timely fibrinolysis is modulation of cytokine production in the ischemic area. Specifically, addition of tPA to clots within two hours significantly reduces the synthesis of IL-8 and MCP-1. Increased inflammation is a hallmark finding in stroke, as evidenced by increased levels of IL-8, MCP-1, IL-1β, and other cytokines in the blood – which associate with poor outcomes49–51. While these studies suggest certain cytokines are important in regulating outcomes in stroke, they have not examined the role of fibrinolytic therapy in regulating cytokine expression after stroke. Our findings demonstrate for the first time and strongly suggest that the positive benefits of timely tPA administration in stroke is due, in part, to an attenuated inflammatory response.
Supplementary Material
Highlights.
Clot formation induces a robust inflammatory response in monocytes specifically through fibrin-dependent mechanisms.
Timely fibrinolysis blunts monocyte inflammatory responses revealing a potential novel mechanism of fibrinolytic therapy in stroke.
Acknowledgments
We like to thank Dr. Tammy Smith for input on experimental design and data collection in reference to Figures 1 and supplemental Figure III and IX as well as critically reviewing the manuscript. We thank Ms. Diana Lim for her excellent preparation of the figures. We would like to Thank Drs. Tim LaPine and Tom Martins at ARUP for their help with the multiplex analysis. In addition, we would like to thank the High-Throughput Genomics and Bioinformatics Analysis and the Electron Microscopy cores at the University of Utah.
Sources of Funding
This work was funded by the following: HL066277 and HL112311 (ASW), AG040631 and HL092161 (MTR), R37HL044525 (GAZ), Utah Stroke Net Fellowship 4U10NS086606 (RAC) and the American Heart Association 11POST7290019 (RAC).
Footnotes
Disclosures
None of the authors have any disclosures.
References
- 1.Foley JH, Conway EM. Cross talk pathways between coagulation and inflammation. Circ Res. 2016;118:1392–1408. doi: 10.1161/CIRCRESAHA.116.306853. [DOI] [PubMed] [Google Scholar]
- 2.Lindsberg PJ, Grau AJ. Inflammation and infections as risk factors for ischemic stroke. Stroke. 2003;34:2518–2532. doi: 10.1161/01.STR.0000089015.51603.CC. [DOI] [PubMed] [Google Scholar]
- 3.Monroe DM, Hoffman M, Roberts HR. Transmission of a procoagulant signal from tissue factor-bearing cell to platelets. Blood Coagul Fibrinolysis. 1996;7:459–464. doi: 10.1097/00001721-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Campbell RA, Overmyer KA, Bagnell CR, Wolberg AS. Cellular procoagulant activity dictates clot structure and stability as a function of distance from the cell surface. Arterioscler Thromb Vasc Biol. 2008;28:2247–2254. doi: 10.1161/ATVBAHA.108.176008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell RA, Overmyer KA, Selzman CH, Sheridan BC, Wolberg AS. Contributions of extravascular and intravascular cells to fibrin network formation, structure, and stability. Blood. 2009;114:4886–4896. doi: 10.1182/blood-2009-06-228940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolberg AS, Campbell RA. Thrombin generation, fibrin clot formation and hemostasis. Transfus Apher Sci. 2008;38:15–23. doi: 10.1016/j.transci.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisel JW. Structure of fibrin: Impact on clot stability. J Thromb Haemost. 2007;5(Suppl 1):116–124. doi: 10.1111/j.1538-7836.2007.02504.x. [DOI] [PubMed] [Google Scholar]
- 8.Hogg N. Human monocytes are associated with the formation of fibrin. J Exp Med. 1983;157:473–485. doi: 10.1084/jem.157.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Bruhl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tezono K, Sarker KP, Kikuchi H, Nasu M, Kitajima I, Maruyama I. Bioactivity of the vascular endothelial growth factor trapped in fibrin clots: Production of il-6 and il-8 in monocytes by fibrin clots. Haemostasis. 2001;31:71–79. doi: 10.1159/000048047. [DOI] [PubMed] [Google Scholar]
- 11.Guha M, Mackman N. Lps induction of gene expression in human monocytes. Cell Signal. 2001;13:85–94. doi: 10.1016/s0898-6568(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 12.Sharif O, Bolshakov VN, Raines S, Newham P, Perkins ND. Transcriptional profiling of the lps induced nf-kappab response in macrophages. BMC Immunol. 2007;8:1. doi: 10.1186/1471-2172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieuwenhuizen L, Falkenburg WJ, Schutgens RE, Roosendaal G, van Veghel K, Biesma DH, Lafeber FP. Stimulation of naive monocytes and pbmcs with coagulation proteases results in thrombin-mediated and par-1-dependent cytokine release and cell proliferation in pbmcs only. Scand J Immunol. 2013;77:339–349. doi: 10.1111/sji.12033. [DOI] [PubMed] [Google Scholar]
- 14.Lopez ML, Bruges G, Crespo G, Salazar V, Deglesne PA, Schneider H, Cabrera-Fuentes H, Schmitz ML, Preissner KT. Thrombin selectively induces transcription of genes in human monocytes involved in inflammation and wound healing. Thromb Haemost. 2014;112:992–1001. doi: 10.1160/TH14-01-0034. [DOI] [PubMed] [Google Scholar]
- 15.Lee ME, Kweon SM, Ha KS, Nham SU. Fibrin stimulates microfilament reorganization and il-1beta production in human monocytic thp-1 cells. Mol Cells. 2001;11:13–20. [PubMed] [Google Scholar]
- 16.Flick MJ, Du X, Degen JL. Fibrin(ogen)-alpha m beta 2 interactions regulate leukocyte function and innate immunity in vivo. Exp Biol Med (Maywood) 2004;229:1105–1110. doi: 10.1177/153537020422901104. [DOI] [PubMed] [Google Scholar]
- 17.Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphambeta2/mac-1 is critical for host inflammatory response in vivo. J Clin Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cesarman-Maus G, Hajjar KA. Molecular mechanisms of fibrinolysis. Br J Haematol. 2005;129:307–321. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 19.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, Boysen G, Bluhmki E, Hoxter G, Mahagne MH, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The european cooperative acute stroke study (ecass) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 20.Adams HP, Jr, del Zoppo G, Alberts MJ, et al. Guidelines for the early management of adults with ischemic stroke: A guideline from the american heart association/american stroke association stroke council, clinical cardiology council, cardiovascular radiology and intervention council, and the atherosclerotic peripheral vascular disease and quality of care outcomes in research interdisciplinary working groups: The american academy of neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38:1655–1711. doi: 10.1161/STROKEAHA.107.181486. [DOI] [PubMed] [Google Scholar]
- 21.Gosselin RC, Dager WE, King JH, Janatpour K, Mahackian K, Larkin EC, Owings JT. Effect of direct thrombin inhibitors, bivalirudin, lepirudin, and argatroban, on prothrombin time and inr values. Am J Clin Pathol. 2004;121:593–599. doi: 10.1309/D79K-4YG7-8NTN-YY38. [DOI] [PubMed] [Google Scholar]
- 22.Machlus KR, Colby EA, Wu JR, Koch GG, Key NS, Wolberg AS. Effects of tissue factor, thrombomodulin and elevated clotting factor levels on thrombin generation in the calibrated automated thrombogram. Thromb Haemost. 2009;102:936–944. doi: 10.1160/TH09-03-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allen GA, Wolberg AS, Oliver JA, Hoffman M, Roberts HR, Monroe DM. Impact of procoagulant concentration on rate, peak and total thrombin generation in a model system. J Thromb Haemost. 2004;2:402–413. doi: 10.1111/j.1538-7933.2003.00617.x. [DOI] [PubMed] [Google Scholar]
- 24.Szaba FM, Smiley ST. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 2002;99:1053–1059. doi: 10.1182/blood.v99.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laudano AP, Doolittle RF. Synthetic peptide derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. Proc Natl Acad Sci U S A. 1978;75:3085–3089. doi: 10.1073/pnas.75.7.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laudano AP, Doolittle RF. Studies on synthetic peptides that bind to fibrinogen and prevent fibrin polymerization. Structural requirements, number of binding sites, and species differences. Biochemistry. 1980;19:1013–1019. doi: 10.1021/bi00546a028. [DOI] [PubMed] [Google Scholar]
- 27.Stocker K, Barlow GH. The coagulant enzyme from bothrops atrox venom (batroxobin) Methods Enzymol. 1976;45:214–223. doi: 10.1016/s0076-6879(76)45021-8. [DOI] [PubMed] [Google Scholar]
- 28.Qi J, Kreutzer DL. Fibrin activation of vascular endothelial cells. Induction of il-8 expression. J Immunol. 1995;155:867–876. [PubMed] [Google Scholar]
- 29.Kuhns DB, Priel DA, Gallin JI. Induction of human monocyte interleukin (il)-8 by fibrinogen through the toll-like receptor pathway. Inflammation. 2007;30:178–188. doi: 10.1007/s10753-007-9035-1. [DOI] [PubMed] [Google Scholar]
- 30.Lee ME, Rhee KJ, Nham SU. Fragment e derived from both fibrin and fibrinogen stimulates interleukin-6 production in rat peritoneal macrophages. Mol Cells. 1999;9:7–13. [PubMed] [Google Scholar]
- 31.Perez RL, Roman J. Fibrin enhances the expression of il-1 beta by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J Immunol. 1995;154:1879–1887. [PubMed] [Google Scholar]
- 32.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 33.Al-ofi E, Coffelt SB, Anumba DO. Fibrinogen, an endogenous ligand of toll-like receptor 4, activates monocytes in pre-eclamptic patients. J Reprod Immunol. 2014;103:23–28. doi: 10.1016/j.jri.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Belcher JD, Chen C, Nguyen J, Milbauer L, Abdulla F, Alayash AI, Smith A, Nath KA, Hebbel RP, Vercellotti GM. Heme triggers tlr4 signaling leading to endothelial cell activation and vaso-occlusion in murine sickle cell disease. Blood. 2014;123:377–390. doi: 10.1182/blood-2013-04-495887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rondina MT, Schwertz H, Harris ES, Kraemer BF, Campbell RA, Mackman N, Grissom CK, Weyrich AS, Zimmerman GA. The septic milieu triggers expression of spliced tissue factor mrna in human platelets. J Thromb Haemost. 2011;9:748–758. doi: 10.1111/j.1538-7836.2011.04208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dardik R, Krapp T, Rosenthal E, Loscalzo J, Inbal A. Effect of fxiii on monocyte and fibroblast function. Cell Physiol Biochem. 2007;19:113–120. doi: 10.1159/000099199. [DOI] [PubMed] [Google Scholar]
- 37.Ugarova TP, Solovjov DA, Zhang L, Loukinov DI, Yee VC, Medved LV, Plow EF. Identification of a novel recognition sequence for integrin alpham beta2 within the gamma-chain of fibrinogen. J Biol Chem. 1998;273:22519–22527. doi: 10.1074/jbc.273.35.22519. [DOI] [PubMed] [Google Scholar]
- 38.Lishko VK, Kudryk B, Yakubenko VP, Yee VC, Ugarova TP. Regulated unmasking of the cryptic binding site for integrin alpha m beta 2 in the gamma c-domain of fibrinogen. Biochemistry. 2002;41:12942–12951. doi: 10.1021/bi026324c. [DOI] [PubMed] [Google Scholar]
- 39.Sitrin RG, Pan PM, Srikanth S, Todd RF., 3rd Fibrinogen activates nf-kappa b transcription factors in mononuclear phagocytes. J Immunol. 1998;161:1462–1470. [PubMed] [Google Scholar]
- 40.Soriano SG, Coxon A, Wang YF, Frosch MP, Lipton SA, Hickey PR, Mayadas TN. Mice deficient in mac-1 (cd11b/cd18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke. 1999;30:134–139. doi: 10.1161/01.str.30.1.134. [DOI] [PubMed] [Google Scholar]
- 41.Clark RA. Fibrin and wound healing. Ann N Y Acad Sci. 2001;936:355–367. doi: 10.1111/j.1749-6632.2001.tb03522.x. [DOI] [PubMed] [Google Scholar]
- 42.Bugge TH, Kombrinck KW, Flick MJ, Daugherty CC, Danton MJ, Degen JL. Loss of fibrinogen rescues mice from the pleiotropic effects of plasminogen deficiency. Cell. 1996;87:709–719. doi: 10.1016/s0092-8674(00)81390-2. [DOI] [PubMed] [Google Scholar]
- 43.Hultman K, Cortes-Canteli M, Bounoutas A, Richards AT, Strickland S, Norris EH. Plasmin deficiency leads to fibrin accumulation and a compromised inflammatory response in the mouse brain. J Thromb Haemost. 2014;12:701–712. doi: 10.1111/jth.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kao WW, Kao CW, Kaufman AH, Kombrinck KW, Converse RL, Good WV, Bugge TH, Degen JL. Healing of corneal epithelial defects in plasminogen- and fibrinogen-deficient mice. Invest Ophthalmol Vis Sci. 1998;39:502–508. [PubMed] [Google Scholar]
- 45.Akassoglou K, Kombrinck KW, Degen JL, Strickland S. Tissue plasminogen activator-mediated fibrinolysis protects against axonal degeneration and demyelination after sciatic nerve injury. J Cell Biol. 2000;149:1157–1166. doi: 10.1083/jcb.149.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole HA, Ohba T, Nyman JS, Hirotaka H, Cates JM, Flick MJ, Degen JL, Schoenecker JG. Fibrin accumulation secondary to loss of plasmin-mediated fibrinolysis drives inflammatory osteoporosis in mice. Arthritis Rheumatol. 2014;66:2222–2233. doi: 10.1002/art.38639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: A report from the american heart association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 48.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 49.Kostulas N, Kivisakk P, Huang Y, Matusevicius D, Kostulas V, Link H. Ischemic stroke is associated with a systemic increase of blood mononuclear cells expressing interleukin-8 mrna. Stroke. 1998;29:462–466. doi: 10.1161/01.str.29.2.462. [DOI] [PubMed] [Google Scholar]
- 50.Whiteley W, Jackson C, Lewis S, Lowe G, Rumley A, Sandercock P, Wardlaw J, Dennis M, Sudlow C. Inflammatory markers and poor outcome after stroke: A prospective cohort study and systematic review of interleukin-6. PLoS Med. 2009;6:e1000145. doi: 10.1371/journal.pmed.1000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: Pathophysiology and role of inflammatory mediators. FEBS J. 2009;276:13–26. doi: 10.1111/j.1742-4658.2008.06766.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.