Abstract
Background
The impact on the timing of renal replacement therapy (RRT) initiation on clinical outcomes for patients with acute kidney injury (AKI) remains controversial.
Materials and methods
We searched the Cochrane Library, EMBASE, Global Health, MEDLINE, PubMed, the International Clinical Trials Registry Platform, and Web of Science.
Results
We included 49 studies involving 9698 patients. Pooled analysis of 5408 critically ill patients with AKI showed that early RRT was significantly associated with reduced mortality compared to late RRT [odds ratio (OR), 0.40; 95% confidential intervals (CI), 0.32 - 0.48; I2, 50.2%]. For 4290 non-critically ill patients with AKI, there was no statistically significant difference in the risk of mortality between early and late RRT (OR, 1.07; 95% CI, 0.79 - 1.45; I2, 73.0%). Early RRT was markedly associated with shortened intensive care units (ICU) length of stay (LOS) and hospital LOS compared to late RRT in both critically ill and non-critically ill patients with AKI.
Conclusions
Early RRT probably reduce the mortality, ICU and hospital LOS in critically ill patients with AKI. Inversely, early RRT in non-critically ill patients with AKI did not decrease the mortality, but shortened the ICU and hospital LOS.
Keywords: acute kidney injury, renal replacement therapy, mortality, meta-analysis
INTRODUCTION
Acute kidney injury (AKI) is increasingly common and associated with adverse clinical outcomes, including excess mortality and morbidity, and prolonged hospital length of stay (LOS) [1–4]. Renal replacement therapy (RRT) is the cornerstone for the treatment of severe AKI. Although RRT provokes a considerable escalation in the complexity of therapy, the optimal timing of initiation of RRT in patients with AKI has been the focus of those debates [5, 6]. Conflicting results from clinical trials and systematic reviews have not resolved the debates, leaving clinicians to select the timing of initiation of RRT based on suboptimal evidence.
Studies aimed at determining the optimal time for starting RRT have evaluated the various arbitrary cut-offs for time from Intensive Care Unit (ICU) admission [7–9] or development of a biochemical “start time” [10, 11], AKI stage [12, 13], serum urea [14, 15], urine output [16, 17], fluid balance [18], and serum creatinine [15, 19, 20]. However, the arbitrary cut-offs often differentiated between early and late RRT. Some data suggested that early compared with late RRT reduced the mortality with better renal recovery. Early initiation of RRT may produce benefits by avoiding hypervolemia, eliminating of uremic toxins, establishing acid-base homeostasis, and preventing other complications such as gastric hemorrhage and metabolic encephalopathy [7, 13, 16]. Late RRT may allow time for the stabilization of a patient’s condition before RRT and may even avoid the RRT [12, 21–23]. Gaudry et al. showed that the mortality was lower in patients who never received RRT than those received RRT early or late (37.1% vs. 48.5% or 61.8%), and the patients with late RRT were the most severely ill at baseline [13]. Thus, we hypothesized that the different severity of illness for patients with AKI who received early RRT may produce distinct effects on mortality. Therefore, we firstly performed a meta-analysis according to the severity of illness for patients with AKI to investigate the opportunity of RRT initiation.
3 earlier meta-analyses (Seabra et al. [24] identified 23 studies, Karvellas et al. [25] identified 15 studies and Wang et al. [26] included 51 trials) showed that early RRT could confer a survival benefit. 11 trials performed before 1985 in Seabra et al. and Wang et al. were excluded, and the addition of 10 recently published studies have been included in the present meta-analysis. However, a recent meta-analysis found no significant difference in mortality between early and late RRT [27], but included only nine “high-quality” studies. Furthermore, the included studies were limited with high heterogeneity. In the present study, we firstly made a definition of early RRT based on time-based cutoffs for patients with AKI to investigate the optimal timing of initiation of RRT.
RESULTS
Study enrolment and characteristics
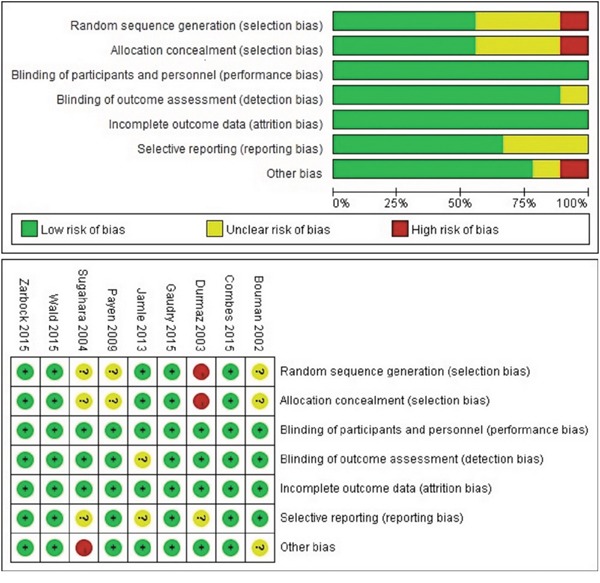
Figure 1 outlines the process for study selection. 49 studies including 9 RCTs [10, 12, 13, 15, 16, 19, 21–23] and 40 observational studies [7–9, 11, 14, 17, 18, 20, 28–59] were included in our meta-analysis. The eligible studies were conducted from 1985 to 2016 with 9698 patients evaluated the timing of initiation of RRT in patients with AKI. The characteristics of the articles were listed in Table 1, and the details of risk of bias for RCTs were showed in Figure 2.
Figure 1. Flow diagram for the selection of studies inclusion in the meta-analysis.
Table 1. The fundamental characteristics and patient demographic data of included studies reporting data on early RRT versus late RRT.
| Auther, Year | Country | Study Design | Population | Early Mortality | Late Mortality | Severity ofIllness | Early RRT Criteria | Late RRT Criteria | Quality |
|---|---|---|---|---|---|---|---|---|---|
| Early time to RRT <12 h | |||||||||
| Bouman2002 | Netherlands | RCT | Multisystem | 20/70 | 9/36 | Early: SOFA 10.3;Late: SOFA 10.6 | Time to RRT<12 h | Time to RRT>12h | M |
| Piccinni2006 | Italy | Retrospective | Sepsis; ICU | 18/40 | 29/40 | Early: APACHE2=27.2;Late: APACHE2=27.8 | Time to RRT <12 h | No RRT | 7 |
| Andrade2007 | Brazil | Retrospective | Multisystem;Leptospirosis | 3/18 | 10/15 | Early: APACHE2=24.5;Late: APACHE2=26 | Mean time to RRT = 4.4hrs | Mean time to RRT = 27.3hrs | 5 |
| Wu VC2007 | China | Retrospective | Acute LiverFailure;Surgical ICU | 34/54 | 22/26 | Early: APACHE2=18;Late: APACHE2=19 | Mean time from ICU admit to RRT =4.4hrs; BUN<80 mg/dL ANDtraditional indications present | Mean time from ICU admit to RRT =11.1hrs; BUN>80 mg/dL ANDtraditional indications present | 6 |
| Manche2008 | Malta | Retrospective | Post CardiacSurgery | 14/56 | 13/15 | NR | Mean RRT start 8.6hrs post-op; Oliguria unresponsive to med mgmt | Mean RRT start 41.2hrs post-op; Oliguria refractory to med mgmt | 6 |
| Ji2011 | China | Retrospective | Post CardiacSurgery | 3/34 | 9/24 | Early: APACHE3= 69;Late: APACHE3= 88.2p<0.001 | Time from urine output <0.5ml/kg/h to RRT <12h; Mean oliguria to start of RRT 8.4hrs | Time from urine output <0.5ml/kg/h to RRT >12h; Mean oliguria to start of RRT21.5hrs | 6 |
| Shum2013 | China | Retrospective | Multisystem;Sepsis | 43/89 | 15/31 | Early: SOFA 13;Late: SOFA 12P=0.011 | Mean time from ICU admit to RRT= 10.8hrs (RIFLE criteria:‘Injury’ or ‘Failure’ criteria) | Mean time from ICU admit to RRT =20.7hrs (RIFLE criteria:‘pre- Risk’ or ‘Risk’ criteria) | 6 |
| Serpytis2014 | Lithuania | Retrospective | Multisystem;Sepsis | 30/42 | 39/43 | NR | Time from anuria to RRT <12hrs | Time from anuria to RRT >12hrs | 5 |
| Wald2015 | Canada | RCT | Multisystem | 16/48 | 19/52 | Early: SOFA 13.3;Late: SOFA 12.8 | Mean time to RRT = 9.7hrs | Meantime to RRT = 32hrs;Classic indications for RRT | H |
| Crescenzi2015 | Italy | Prospective | Post CardiacSurgery | 28/46 | 10/13 | NR | Time from urine output <0.5ml/kg/hto RRT <12h | Time from urine output <0.5ml/kg/h to RRT >12h | 6 |
| Zarbock2015 | Germany | RCT | Multisystem | 44/112 | 65/119 | Early: SOFA 15.6;Late: SOFA 16.0 | Time to RRT <8h; KDIGO stage 2 | Time to RRT <12h; Stage 3 AKIor no initiation | H |
| Gaudry2015 | France | RCT | Multisystem | 150/311 | 153/308 | Early: SOFA 10.9;Late: SOFA 10.8 | Time to RRT <6h; Stage 3 AKI | Classic indications for RRT; Oliguria or anuria >72hrs after randomization | H |
| Early time to RRT <24 h | |||||||||
| Elahi2004 | UK | Retrospective | Post Cardiacsurgery | 8/36 | 12/28 | NR | Mean RRT start 0.78 days;Low urine output <100ml within 8h after surgery | Mean RRT start 2.5 days; Traditional indications: Urea≥30mmol/L, Cr ≥250mmol/L, K >6.0mEq/L | 6 |
| Demirkilic2004 | Turkey | Retrospective | Post CardiacSurgery | 8/34 | 15/27 | NR | Mean RRT start 0.88 days;Low urine output <100ml within 8hrs post-op; | Mean RRT start 2.56 days;Cr ≥5mg/dL, or K >5.5 mEq/L | 6 |
| Boussekey2012 | France | Retrospective | Multisystem | 28/67 | 28/43 | Early: SOFA: 11.1;Late: SOFA 8.8;p=0.002 | Time from RIFLE- ‘Injury’ to RRT< 16hrs; Mean time to RRT=6hrs | Time from RIFLE- ‘Injury’ to RRT > 16hrs; Mean time to RRT=64hrs | 7 |
| Chon2012 | Korea | Retrospective | Multisystem;Sepsis | 7/36 | 9/19 | Early: SOFA 13.5;Late: SOFA 12 | Time to RIFLE ‘Injury’/‘Failure’< 24hrs; Mean time to RRT=12.5hrs | Time to RIFLE ‘Injury’/‘Failure’> 24hrs; Mean time to RRT= 42.2hrs | 7 |
| Leite2013 | Brazil | Retrospective | Multisystem | 33/64 | 67/86 | Early: APACHE2=19.2;Late: APACHE2=18.7 | Time from AKIN 3 diagnosis to RRT <24hrs | Time from AKIN 3 diagnosis to RRT >24hrs | 7 |
| Jun2014 | Australia | Prospective | Multisystem;Sepsis | 82/219 | 84/220 | Early: SOFA: 2.0;Late: SOFA 2.1 | Time from AKI diagnosis to RRT <17.6hrs | Time from AKI diagnosis to RRT>17.6hrs | 6 |
| Combes2015 | France | RCT | Post CardiacSurgery | 40/112 | 40/112 | Early: SOFA 11.5;Late: SOFA 12.0 | RRT initiated <24hrs and continuedfor min of 48hrs | Traditional indications for RRT | H |
| Yang2016 | China | Retrospective | Post CardiacSurgery | 20/59 | 80/154 | Early: APACHE2=21.4.;Late: APACHE2=23.1 | AKI in absence of traditional indications for RRT; persistence of hypotension (for more than 6 h) despite preload optimization; | Traditional indications for RRT | 7 |
| Early time to RRT <48 h | |||||||||
| Durmaz2003 | Turkey | RCT | Post CardiacSurgery | 1/21 | 7/23 | NR | Cr rise >10% from pre-op levelwithin 48hrsof surgery | Cr rise >50%from pre-op level;or Urine output <400ml/24hrs | L |
| Lyem2009 | Turkey | Prospective | Post CardiacSurgery | 5/95 | 6/90 | NR | Low urine output triggering RRT started <48hrs; Evidence of 50% increase in BUN, | Time >48hrs to start of RRT for similar markers of renal failure managed medically for minimum 48hrs | 7 |
| Bagshaw2009 | Multicountries | Prospective | Multisystem | 462/785 | 304/442 | Early: SOFA 10.9;Late: SOFA 10.7p=0.04 | RRT started <2d from ICU admission | RRT started >2d from ICU admission | 7 |
| Perez2012 | Spain | Prospective | MultisystemSepsis | 71/135 | 78/109 | Early: SOFA 12;Late: SOFA 11 | Time from ICU admission to RRT < 48h | Time from ICU admission to RRT > 48h | 5 |
| Lim2014 | Singapore | Prospective | Multisystem | 37/56 | 36/84 | Early: SOFA 11;Late: SOFA 7;p=0.001 | RRT started < 2d from admission;Traditional indications for RRT | RRT started > 2d from admission; AKIN stage 1 or 2 with indication or AKIN stage3 | 6 |
| Hyung2016 | Korea | Retrospective | MultisystemSepsis | 9/30 | 17/30 | Early: APACHE2=22.9;Late: APACHE2=21.1 | Time to RRT <26.4 h | Time to RRT >26.4 h | 6 |
| Early time to RRT <72 h | |||||||||
| Sugahara2004 | Japan | RCT | Post CardiacSurgery | 12/14 | 2/14 | Early: APACHE2=18;Late: APACHE2=19 | Mean time to RRT start 1.7d±0.8 post op; UOP <20ml/hrs ×2hrs + OR UOP <500ml/day | Mean time to RRT start 18d±0.9 post op; UOP <30ml/hrs ×3hrs ORUOP <750ml/day | L |
| Sabater2009 | Spain | Prospective | Multisystem | 21/44 | 68/104 | Early: APACHE2=26;Late: APACHE2=24 | Mean RRT start 2.2d post ICU admit (RIFLE criteria: RISK & INJURY) | Mean RRT start 6.4d post ICU admit (RIFLE criteria: FAILURE) | 7 |
| Fernandez2011 | Spain | Retrospective | Post CardiacSurgery | 59/111 | 74/92 | NR | RRT started <3d after cardiac surgery | RRT started >3d after cardiac surgery | 5 |
| Shiao2012 | China | Retrospective | Surgical | 236/436 | 143/212 | Early: SOFA 11.4;Late: SOFA 11.3 | Time to development of traditional RRT indications <3d; Mean time to start of RRT 1.4d | Traditional RRT indications AND start of RRT >3 d; Mean time to start of RRT 18d | 6 |
| Early time to RRT >72 h | |||||||||
| Gettings1999 | USA | Retrospective | Multisystem;Trauma | 25/41 | 47/59 | Early ISS = 33.0;Late ISS = 37.2 | Mean RRT start post admission10d; BUN <60mg/dl AND Oliguria, Vol overload, Electrolytes, Uremia; | Mean RRT start post admission 19d; BUN >60 mg/dL AND Oliguria, Electrolytes, Uremia; | 5 |
| Shiao2009 | China | Prospective | MajorAbdominalSurgery | 22/51 | 34/47 | Early: SOFA 8.3;Late: SOFA 8.5 | Mean Time to RRT from ICU Admit =7.3d (RIFLE criteria:RISK or pre-RISK criteria) | Mean Time to RRT from ICU Admit = 8.4d (RIFLE criteria:INJURY or FAILURE criteria) | 7 |
| Chung2009 | US | Retrospective | Severe BurnedPatients | 9/29 | 24/28 | Early: SOFA 13;Late: SOFA 13 | Mean time from admit to RRT =17 days; AKIN stage2(+shock)/3 | Mean time from admit to AKIN stage 2(+shock)/3 but not dialyzed = 23 days | 6 |
| Carl2010 | US | Retrospective | Multisystem;Sepsis | 44/85 | 42/62 | Early: APACHE2=24.8;Late: APACHE2=24.7 | Mean ICU stay prior to RRT = 6.3d;BUN <100mg/dL + AKIN stage >2; | Mean ICU stay prior to RRT = 12.3d; BUN > 100mg/dL + AKIN stage >2; | 7 |
| Hyung2012 | Korea | Retrospective | Multisystem | 75/105 | 81/105 | Early: SOFA 14.4;Late: SOFA 14.4 | Time from ICU admission to RRT =4.7d | Time from ICU admission to RRT =4.8d | 7 |
| RRT initiated base on biochemical indicators; Meantime to initiation of RRT not specified | |||||||||
| Kresse1999 | Germany | Retrospective | Multisystem | 83/141 | 102/128 | NR | BUN≤34mmol/L, sCr 380umol/L, and urine output 924 ml/24h | BUN >34mmol/L, sCr 477umol/L, and urine output 525 ml/24h | 7 |
| Splendiani2001 | Italy | Retrospective | Multisystem | 6/14 | 3/13 | NR | BUN≤ 33mmol/L | BUN> 59 mmol/L and/or severe electrolyte disturbances | 5 |
| Tsai2005 | China | Retrospective | Multisystem | 42/67 | 30/31 | NR | BUN< 29 mmol/L | BUN> 29 mmol/L | 5 |
| Liu2006 | Multicountries | Prospective | Multisystem | 43/122 | 50/121 | NR | Azotemia defined by BUN < 76mg/dL | Azotemia defined by BUN > 76mg/dL | 6 |
| Payen2009 | France | RCT | Multisystem | 20/37 | 17/39 | Early: SOFA 11.6;Late: SOFA 10.4 | RRT × 96hrs w/diagnosis of ‘sepsis’ | No RRT; unless metabolic renal failure & classic indications for RRT present | M |
| Elsevivrs2010 | Belgium | Prospective | Multisystem | 379/653 | 280/650 | Early: SOFA 9.9;Late: SOFA 8.5p=0.001 | Serum Cr >2mg/dL | No RRT | 5 |
| Konopka2011 | Poland | Retrospective | Multisystem | 17/25 | 11/12 | NR | As soon as AKI was diagnosed | After full treatment for HF and unsuccessful pharmacological treatment of complicating AKI | 5 |
| Chou2011 | China | Retrospective | Sepsis;Surgery ICU | 135/192 | 124/178 | Early: SOFA 10.8;Late: SOFA 11.6 | RIFLE criteria: RISK or pre-RISK | RIFLE criteria: INJURY or FAILURE | 6 |
| Nascimento2012 | Brazil | Retrospective | Multisystem | 9/23 | 43/63 | Early: APACHE 2= 21;Late: APACHE 2= 28 | BUN ≤26.7 mmol/L | BUN>26.7 mmol/L | 6 |
| Wu SC2012 | China | Retrospective | MultisystemSurgery | 10/20 | 45/53 | Early: SOFA 9.5;Late: SOFA 10.0 | RIFLE criteria: RISK | RIFLE criteria: INJURY or FAILURE | 5 |
| Hu2013 | China | Retrospective | Multisystem | 20//36 | 8/13 | Early: SOFA 9.3;Late: SOFA 11.5 | AKIN 1and 2 (Cr >200-300%baseline &Urine<0.5cc/kg/h for >12h) | AKIN 3 (Cr ≥354μmol/L or Cr >300% baseline & urine <0.3cc/kg/h for 24h or anuria >12h) | 5 |
| Jamle2013 | India | RCT | Multisystem | 21/102 | 13/106 | Early: SOFA 7.3;Late: SOFA 8.2 | Cr >618μmol/L | Traditional indications for RRT | M |
| Gaudry2014 | France | Retrospective | Multisystem;Sepsis | 44/91 | 29/112 | Early: SOFA 9;Late: SOFA 8P<0.01 | RRT criteria: Cr ≥300μmol/L, Urea >25mmol/L, K >6.5mmol/L,pH <7.2, Oliguria, Vol overload, | No RRT | 5 |
| Tian(461)2014 | China | Retrospective | Multisystem;Sepsis | 5/23 | 11/26 | Early: SOFA 7.6;Late: SOFA 8.4 | AKIN 1 (Cr ≥26.4μmol/L or >150- 200% baseline & urine < 0.5cc/kg/h for >6h) | No RRT | 6 |
| Tian(462)2014 | China | Retrospective | Multisystem;Sepsis | 12/31 | 14/21 | Early: SOFA 9.3;Late: SOFA 9.6 | AKIN 2 (Cr >200-300% baseline &Urine <0.5cc/kg/h for >12h) | No RRT | 6 |
| Tian(463)2014 | China | Retrospective | Multisystem;Sepsis | 31/46 | 11/13 | Early: SOFA 10;Late: SOFA 11.2 | AKIN 3 (Cr ≥354μmol/L or Cr >300% baseline & urine < 0.3cc/kg/h for 24h or anuria >12h) | No RRT | 6 |
LEGEN: AKI Acute kidney injury, RRT renal replacement therapy, Cr Creatinine, UOP Urine output, ICU Intensive Care Unit, AKIN Acute Kidney Injury Network, RIFLE Risk, Injury, Failure, Loss and End-stage, KDIGO Kidney Disease: Improving Global Outcomes, RCTs randomized clinical trials, Quality Score: The Cochrane Collaboration Risk of Bias tool for RCTs and Newcastle-Ottawa Scale for observational studies, H High quality: low risk of bias, M Medium quality: unclear risk of bias, L Low quality: high risk of bias, APACHE Acute Physiology and Chronic Health Evaluation, SOFA Sequential Organ Failure Assessment, NR Not reported.
Figure 2. Risk of bias summary of early versus late RRT initiation on mortality in patients with AKI on randomized controlled trial.
Meta-analysis results
Primary outcomes
Pooled analysis of 5408 critically ill patients with AKI showed that early RRT was markedly associated with reduced mortality compared to late RRT (OR, 0.40; 95% CI, 0.32 - 0.48; I2, 50.2%, Figure 3). For 4290 non-critically ill patients with AKI, there was no statistically significant difference in the risk of mortality between early and late RRT (OR, 1.07; 95% CI, 0.79 - 1.45; I2, 73.0%, Figure 3).
Figure 3.
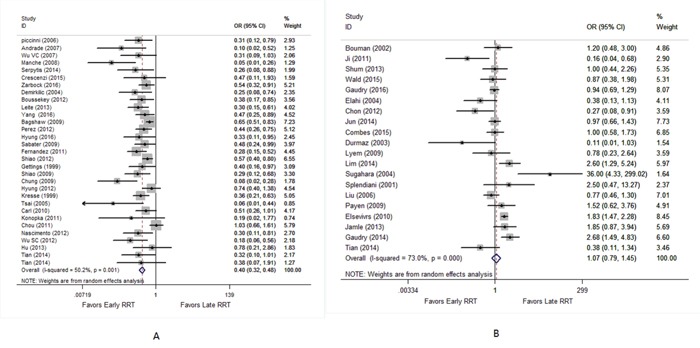
Forest plot shows the effect of early versus late RRT on mortality in critically ill (A) and non-critically ill patients with AKI (B).
Subgroup analysis of critically ill patients was firstly conducted in the present study by using the definition of early according to time criteria versus biochemical indicators. The significant association between early RRT and reduced mortality was also found under the studies that defined early by time criteria [early RRT within 12 hours (OR, 0.28; 95% CI, 0.16 - 0.49; I2, 44.8%), within 24 hours (OR, 0.37; 95% CI, 0.25 - 0.54; I2, 0.0%), within 48 hours (OR, 0.55; 95% CI, 0.39 - 0.77; I2, 30.8%), within 72 hours (OR, 0.45; 95% CI, 0.29 - 0.69; I2, 48.2%), and after 72 hours (OR, 0.32; 95% CI, 0.14 - 0.74; I2, 71.4%)], and by biochemical parameters (OR, 0.40; 95% CI, 0.25 - 0.64; I2, 58.9%). Subgroup analysis of non-critically ill patients depending on the definition of early showed no significant subgroup survival benefits from early RRT.
Subgroup analysis of critically ill patients was based on the type of ICU admission. Early RRT was significantly associated with reduced mortality compared to late RRT among surgical group (OR, 0.33; 95% CI, 0.22 - 0.48; I2, 47.9%) and mixed group (OR, 0.43; 95% CI, 0.34 - 0.54; I2, 49.8%). Subgroup analysis of non-critically ill patients based on ICU admission type showed no evidence of survival advantage in early RRT.
Subgroup analysis of critically ill patients was also performed according to RRT modality [continuous renal replacement therapy (CRRT), intermittent hemodialysis (IHD) or Mixed]. We found a markedly significant reduce in mortality in critically ill patients assigned to early RRT in the CRRT group (OR, 0.40; 95% CI, 0.30 - 0.54; I2, 28.4%), IHD group (OR, 0.11; 95% CI, 0.03 - 0.43; I2, 56.9%) and Mixed group (OR, 0.45; 95% CI, 0.35 - 0.57; I2, 53.6%) when compared to late RRT. Subgroup analysis of non-critically ill patients according to RRT modality showed that early RRT could not confer a survival benefit (Table 2).
Table 2. Outcomes measures of early versus late RRT initiation.
| Outcome or Subgroup | Group A: critically ill patients with AKI | Group B: non-critically ill patients with AKI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Studies | No. of Patients | Study Reference No | Effect Estimate (95% CI) | p | Studies | No. of Patients | Study Reference No | Effect Estimate (95% CI) | p | |
| Primary Outcomes: early versus late RRT initiation on mortality | ||||||||||
| All studies | 31 | 5408 | 7-9,12,18,28-30,32,34,35,38-41,43,44, 462,463,47,48,50-59 | OR, 0.40 (0.32 to 0.48) | 0.001 | 20 | 4290 | 10,11,13-17,19-23,31,33,36,37,42,45,461,49 | OR, 1.07 (0.79 to 1.45) | 0.000 |
| Subgroup stratified by the definition of early according to time criteria and biochemical indicators on mortality | ||||||||||
| Time: Early RRT <12h | 7 | 639 | 9,12,28-30,32,56 | OR, 0.28 (0.16 to 0.49) | 0.093 | 5 | 1003 | 10,13,21,31,42 | OR, 0.86 (0.58 to 1.29) | 0.201 |
| Time: Early RRT <24h | 4 | 534 | 34,35,53,54 | OR, 0.37 (0.25 to 0.54) | 0.691 | 4 | 782 | 11,22,33,36 | OR, 0.72 (0.43 to 1.19) | 0.097 |
| Time: Early RRT <48h | 3 | 1531 | 7,55,57 | OR, 0.55 (0.39 to 0.77) | 0.236 | 3 | 368 | 17,19,37 | OR, 0.82 (0.18 to 3.79) | 0.012 |
| Time: Early RRT <72h | 3 | 999 | 18,38,58 | OR, 0.45 (0.29 to 0.69) | 0.145 | 1 | 28 | 16 | OR, 36.0 (4.33 to 299.02) | NE |
| Time: Early RRT >72h | 4 | 465 | 8,39,40,52 | OR, 0.32 (0.14 to 0.74) | 0.015 | 0 | NE | NE | NE | NE |
| Biochemicl indicators | 10 | 1240 | 41,43,44, 462,463-48,50,51,59 | OR, 0.40 (0.25 to 0.64) | 0.009 | 7 | 2109 | 14,15,20,23,45, 461,49 | OR, 1.46 (0.96 to 2.23) | 0.008 |
| Subgroup stratified by surgical versus mixed medical admissions on mortality | ||||||||||
| Surgical | 9 | 1506 | 8,9,18,30,32,34,38,44,54 | OR, 0.33 (0.22 to 0.48) | 0.053 | 6 | 602 | 16,17,19,22,31,33 | OR, 0.71 (0.24 to 2.07) | 0.000 |
| Mixed medical | 22 | 3902 | 7,12,28,29,35,39,41,43,462,463-48,50-53,55-59 | OR, 0.43 (0.34 to 0.54) | 0.004 | 14 | 3688 | 10,11,13-15,20,21,23,36,37,42,45,461,49 | OR, 1.22 (0.91 to 1.63) | 0.000 |
| Subgroup stratified by RRT modality on mortality | ||||||||||
| Mixed | 14 | 3442 | 7,9,12,28,29,35,38,41,43,48,53,54,55,57 | OR, 0.45 (0.35 to 0.57) | 0.009 | 6 | 2495 | 13,14,20,21,45,49 | OR, 1.32 (0.86 to 2.03) | 0.000 |
| CRRT | 14 | 1771 | 8,18,32,34,39,40,44,462,463,47,50,52,55,58 | OR, 0.40 (0.30 to 0.54) | 0.152 | 12 | 1544 | 10,11,15-17,22,31,33,36,37,42, 461 | OR, 0.92 (0.58 to 1.46) | 0.017 |
| IHD | 3 | 255 | 30,51,59 | OR, 0.11 (0.03 to 0.43) | 0.098 | 2 | 251 | 19,23 | OR, 0.56 (0.04 to 8.73) | 0.000 |
| Secondary outcomes: ICU and Hospital LOS | ||||||||||
| ICU LOS | 8 | 862 | 28,34,35,38,41, 462,463,53 | MD, −0.41 (−0.55 to −0.27) | 0.000 | 4 | 336 | 17,19,31, 461 | MD, −1.47 (−1.71 to −1.22) | 0.000 |
| Hospital LOS | 6 | 755 | 8,28,34,38,39,54 | MD, −0.36 (−0.51 to −0.21) | 0.000 | 3 | 287 | 17,19,31 | MD, −1.07 (−1.31 to −0.82) | 0.415 |
LEGEN: OR odds ratio, 95% CI confidence interval, P Test for Heterogeneity, MD mean difference, RRT renal replacement therapy, ICU Intensive Care Unit, CRRT continuous renal replacement therapy, IHD intermittent hemodialysis, Mixed CRRT and/or IHD and/or other RRT modality, LOS length of stay, NE not evaluable.
Secondary outcomes
For critically ill patients with AKI, as showed in Table 2, early RRT significantly shortened ICU (MD, −0.41; 95% CI, −0.55 to −0.27; I2, 87.0%) and hospital LOS (MD, −0.36; 95% CI, −0.51 to −0.20; I2, 94.7%) compared to late RRT. Similar results were obtained in non-critically ill patients with AKI in ICU (MD, −1.47; 95% CI, −1.71 to −1.22; I2, 89.3%) and hospital LOS (MD, −1.07; 95% CI, −1.31 to −0.82; I2, 0%).
Sensitivity, meta-regression analyses
Statistically similar results were obtained after omitting each study of critically ill patients with AKI, and the results of the sensitivity analyses were robust. Sensitivity analyses showed that Elsevivrs et al. [20] was the main source of heterogeneity for the studies of non-critically ill patients with AKI, and the heterogeneity was significantly decreased by omitting the study. For non-critically ill patients with AKI, there was no statistically significant difference in the risk of mortality between early and late RRT with the study (OR, 1.07; 95% CI, 0.79 - 1.45; I2, 73.0%) or without the study (OR, 1.02; 95% CI, 0.74-1.40; I2, 66.8%). Elsevivrs et al. was a large sample trial with 1303 patients when compared to other articles including not more than 619 subjects (Figure 4).
Figure 4.
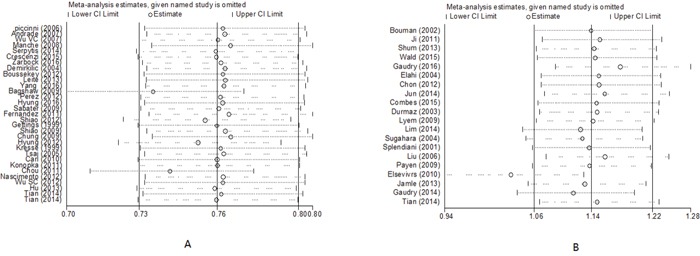
Sensitivity analyses of early versus late RRT on mortality in critically ill (A) and non-critically ill patients with AKI (B).
With the meta-regression, we did not find a correlation between patient mortality and study design (RCT vs. observational), RRT modality (CRRT, IHD vs. Mixed), study quality score, severity of illness [Sequential Organ Failure Assessment (SOFA) score], ICU admission type (surgical vs. mixed medical admissions). However, we find a correlation between patient mortality and sample size (n ≥ 100 vs. n < 100, P = 0.001) in critically ill patients with AKI.
Publication bias
No potential publication bias was observed in non-critically ill patients with AKI (P = 0.347 for the Begg test, and P = 0.169 for the Egger test). Publication bias was seen in critically ill patients with AKI (P = 0.001 for the Begg test, and P = 0.000 for the Egger test, Figure 5).
Figure 5.
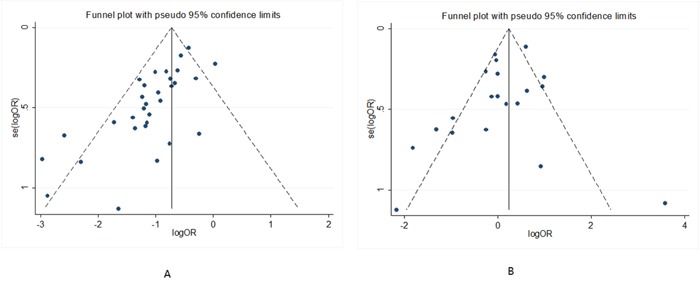
Begg’s funnel plots of early versus late RRT on mortality in critically ill (A) and non-critically ill patients with AKI (B).
DISCUSSION
We identified 49 studies reported data on the timing of RRT initiation among 9698 patients with AKI, and we found that early RRT significantly reduced the mortality compared to late RRT in critically ill patients with AKI. In addition, no significant survival benefits associated with early RRT were seen in non-critically ill patients with AKI. Early RRT was markedly associated with shortened ICU and hospital LOS compared to late RRT in both critically ill and non-critically ill patients with AKI.
Regardless of the definition of early RRT (according to time criteria or biochemical indicators), ICU admission type (surgical vs. mixed) or RRT modality (CRRT, IHD vs. Mixed), subgroup analyses of critically ill patients with AKI did reveal survival benefits from early RRT. Furthermore, subgroup analyses of non-critically ill patients with AKI showed that no evidence of survival advantage in early RRT.
In the present study, we firstly performed the meta-analysis according to the severity of illness and definition of early RRT based on time-based cutoffs for patients with AKI to investigate the time of RRT initiation. We accepted a broad definition of “critically ill patients with AKI” based on AKI with multiple-organ dysfunction syndrome [60], septic shock [40], RIFLE criteria (failure, loss of function, and end-stage kidney disease) [37, 43, 44], AKIN stages 3 [41, 42, 46] or Kidney Disease: Improving Global Outcomes (KDIGO) stage 3 [12, 61].
By the meta-regression, we found sample size was one of the sources of heterogeneity. In contrast to previous meta-analyses, we found a lower heterogeneity among studies on this topic, especially in the subgroup. We noted those critically ill patients in early RRT within 12 hours (I2, 44.8%), 24 hours (I2, 0.0%), 48 hours (I2, 30.8%), and 72 hours (I2, 48.2%) showed the lower heterogeneities, indicating that the heterogeneity may be partially explained by the definition of early RRT timing. However, we could not account for the observed heterogeneity by meta-regression according to study design, RRT modality, the study quality score, severity of the illness, and ICU admission type. Thereby, the heterogeneity observed is most likely explained by the differences in definitions for early RRT timing, the inability to account for heterogeneity in clinical practice and critical care patterns, many confounding factors that affect the mortality, publication bias, sample size and the inclusion of retrospective, prospective and RCTs.
The present systematic review has some limitations. Firstly, definitions for AKI are to some extent different in the included studies. Secondly, the definition of early RRT based on various arbitrary cut-offs for time, which ultimately downgraded the strength of evidence. Thirdly, there were publication bias and significant heterogeneity in the present study. Many confounding factors affect the mortality, and meta-regression may not be enough to verify this issue. Lastly, the association with mortality is largely dependent on observational studies and might have been affected by allocation or selection bias. Thus, further high-quality RCTs focused on mortality according to the optimal time for starting RRT are necessary to fully understand the effects of early RRT for patients with AKI.
MATERIALS AND METHODS
Participants, interventions and outcome measures
We included studies that evaluated the timing of initiation of RRT in patients with AKI. For the review, early and late RRT were defined based on criteria used by the authors in their studies. early and late RRT were defined as extended time-based cutoffs (arbitrary cut-offs for time from ICU admission or development of a biochemical “start time”), or biochemical indicators [serum creatinine, serum urea, RIFLE (risk, injury, failure, loss of function, and end-stage kidney disease) classifications, Acute Kidney Injury Network (AKIN) stages, urine output, and fluid balance]. Late RRT criteria also included conventional RRT indications (hyperkalemia, acidosis or fluid overload) and expectant care (no RRT initiated). The primary outcome was mortality, and the secondary outcomes were ICU and hospital LOS.
Searching strategies
We searched the Cochrane Library, EMBASE, Global Health, MEDLINE, PubMed, the International Clinical Trials Registry Platform, and Web of Science from January 1985 to November 2016. Owing to a low likelihood of relevance to modern RRT and critical care practices, studies published before 1985 were excluded in the present study. Keywords include acute renal failure/acute kidney injury/renal insufficiency, mortality, renal replacement therapy/renal dialysis/hemodialysis/dialysis. The related research references were also reviewed.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) randomized clinical trials (RCTs) and/or observational cohort studies; (2) studies evaluating the timing of initiation of RRT in patients with AKI with direct effect on mortality; (3) complete data available to calculate odds ratio (OR) or mean difference (MD) with 95% confidence interval (CI); (4) clear definitions of AKI stated. Exclusion criteria were as follows: (1) data from the studies could not be extracted and analyzed; (2) duplicate publications; (3) non-human experimental studies.
Study selection and data extraction
Two investigators (Kaiping Luo and Shufang Fu) independently performed the study selection. All the disagreements were resolved by discussion. Data extraction included first author, year of publication, country, study design, sample size, age, sex, RRT modality, mortality, ICU LOS, hospital LOS, and definitions of early and late RRT.
Dr. Gaudry and colleagues [13] showed that the mortality was lower in the patients who never received RRT than those received RRT early or late. Patients who received RRT late were the most severely ill at baseline, and patients who never received it were less ill at baseline. More than 50% mortality in critically ill patients with AKI received RRT was confirmed by many randomized controlled trials [1, 3, 4, 60]]. Thus, we hypothesized that critically ill patients with AKI who receive early RRT may decrease mortality, non-critically ill patients with AKI may confer survival benefits without early RRT. Subjects were identified as being of “critically ill patients” if the late RRT group with high mortality rates (≥ 50%), or “non-critically ill patients” if the late RRT group with low mortality rates (< 50%).
Quality assessment
The Cochrane Collaboration Risk of Bias tool was used to assess RCTs [62]. This tool consists of 6 domains and assesses 5 specific biases. A judgment of low risk, unclear risk, or high risk was provided for each domain. The Newcastle-Ottawa Scale (NOS) was used in the assessment of quality of cohort studies [63]. NOS quality assessment scale ranges from 0 to 9 stars. The star evaluates 3 main categories: selection, comparability, and outcome.
Statistical analysis
Statistical analysis was performed using Review Manager (version 5.3) and STATA statistical software (version 12.0). We calculated OR with 95% CI for dichotomous data and MD with 95% CI for continuous data. Statistical heterogeneity of the data was quantified using the I2 test, and the I2> 50% indicated significant statistical heterogeneity. Sensitivity analysis, meta-regression analyses and subgroup analysis were conducted to investigate the potential sources of heterogeneity. Publication bias was assessed by constructing a funnel plot and using the Egger regression test and the Begg rank correlation test. A P value less than 0.05 was considered statistically significant.
CONCLUSIONS
Our data suggest that early RRT probably reduce the mortality, ICU and hospital LOS in critically ill patients with AKI. Inversely, early RRT in non-critically ill patients with AKI did not decrease the mortality, but shorted the ICU and hospital LOS.
Abbreviations
- RRT
renal replacement therapy
- AKI
acute kidney injury
- OR
odds ratio
- CI
confidential intervals
- ICU
intensive care units
- LOS
length of stay
- RIFLE
risk, injury, failure, loss of function, and end-stage kidney disease
- AKIN
Acute Kidney Injury Network
- RCTs
randomized clinical trials
- MD
mean difference
- NOS
Newcastle-Ottawa Scale
- CRRT
continuous renal replacement therapy
- IHD
intermittent hemodialysis
- SOFA
Sequential Organ Failure Assessment
- KDIGO
Kidney Disease: Improving Global Outcomes
Footnotes
CONFLICTS OF INTEREST
The authors have no competing interests to declare.
REFERENCES
- 1.Palevsky PM, Zhang JH, O'Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, et al. VA/NIH Acute Renal Failure Trial Network Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16:3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestel C, Su S, RENAL Replacement Therapy Study Investigators Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–38. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 4.Schiffl H, Lang SM, Fischer R. Daily hemodialysis and the outcome of acute renal failure. N Engl J Med. 2002;346:305–10. doi: 10.1056/NEJMoa010877. [DOI] [PubMed] [Google Scholar]
- 5.Ronco C, Ricci Z, De Backer D, Kellum JA, Taccone FS, Joannidis M, Pickkers P, Cantaluppi V, Turani F, Saudan P, Bellomo R, Joannes-Boyau O, Antonelli M, et al. Renal replacement therapy in acute kidney injury: controversy and consensus. Crit Care. 2015;19:146. doi: 10.1186/s13054-015-0850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.James M, Bouchard J, Ho J, Klarenbach S, LaFrance JP, Rigatto C, Wald R, Zappitelli M, Pannu N. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:673–85. doi: 10.1053/j.ajkd.2013.02.350. [DOI] [PubMed] [Google Scholar]
- 7.Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators Timing of renal replacement therapy and clinical outcomes in critically ill patients with severe acute kidney injury. J Crit Care. 2009;24:129–40. doi: 10.1016/j.jcrc.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 8.Shiao CC, Wu VC, Li WY, Lin YF, Hu FC, Young GH, Kuo CC, Kao TW, Huang DM, Chen YM, Tsai PR, Lin SL, Chou NK, et al. National Taiwan University Surgical Intensive Care Unit-Associated Renal Failure Study Group Late initiation of renal replacement therapy is associated with worse outcomes in acute kidney injury after major abdominal surgery. Crit Care. 2009;13:R171. doi: 10.1186/cc8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu VC, Ko WJ, Chang HW, Chen YS, Chen YW, Chen YM, Hu FC, Lin YH, Tsai PR, Wu KD. Early renal replacement therapy in patients with postoperative acute liver failure associated with acute renal failure: effect on postoperative outcomes. J Am Coll Surg. 2007;205:266–76. doi: 10.1016/j.jamcollsurg.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med. 2002;30:2205–11. doi: 10.1097/00003246-200210000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Jun M, Bellomo R, Cass A, Gallagher M, Lo S, Lee J, Randomized Evaluation of Normal Versus Augmented Level of Replacement Therapy (RENAL) Study Investigators Timing of renal replacement therapy and patient outcomes in the randomized evaluation of normal versus augmented level of replacement therapy study. Crit Care Med. 2014;42:1756–65. doi: 10.1097/CCM.0000000000000343. [DOI] [PubMed] [Google Scholar]
- 12.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstädt H, Boanta A, GerΔ J, Meersch M. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA. 2016;315:2190–99. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 13.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, Boyer A, Chevrel G, Lerolle N, Carpentier D, de Prost N, Lautrette A, Bretagnol A, et al. AKIKI Study Group Initiation Strategies for Renal-Replacement Therapy in the Intensive Care Unit. N Engl J Med. 2016;375:122–33. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 14.Liu KD, Himmelfarb J, Paganini E, Ikizler TA, Soroko SH, Mehta RL, Chertow GM. Timing of initiation of dialysis in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2006;1:915–19. doi: 10.2215/CJN.01430406. [DOI] [PubMed] [Google Scholar]
- 15.Payen D, Mateo J, Cavaillon JM, Fraisse F, Floriot C, Vicaut E, Hemofiltration and Sepsis Group of the Collège National de Réanimation et de Médecine d’Urgence des Hôpitaux extra-Universitaires Impact of continuous venovenous hemofiltration on organ failure during the early phase of severe sepsis: a randomized controlled trial. Crit Care Med. 2009;37:803–10. doi: 10.1097/CCM.0b013e3181962316. [DOI] [PubMed] [Google Scholar]
- 16.Sugahara S, Suzuki H. Early start on continuous hemodialysis therapy improves survival rate in patients with acute renal failure following coronary bypass surgery. Hemodial Int. 2004;8:320–25. doi: 10.1111/j.1492-7535.2004.80404.x. [DOI] [PubMed] [Google Scholar]
- 17.Iyem H, Tavli M, Akcicek F, Büket S. Importance of early dialysis for acute renal failure after an open-heart surgery. Hemodial Int. 2009;13:55–61. doi: 10.1111/j.1542-4758.2009.00347.x. [DOI] [PubMed] [Google Scholar]
- 18.Shiao CC, Ko WJ, Wu VC, Huang TM, Lai CF, Lin YF, Chao CT, Chu TS, Tsai HB, Wu PC, Young GH, Kao TW, Huang JW, et al. National Taiwan University Hospital Study Group on Acute Renal Failure (NSARF) U-curve association between timing of renal replacement therapy initiation and in-hospital mortality in postoperative acute kidney injury. PLoS One. 2012;7:e42952. doi: 10.1371/journal.pone.0042952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durmaz I, Yagdi T, Calkavur T, Mahmudov R, Apaydin AZ, Posacioglu H, Atay Y, Engin C. Prophylactic dialysis in patients with renal dysfunction undergoing on-pump coronary artery bypass surgery. Ann Thorac Surg. 2003;75:859–64. doi: 10.1016/s0003-4975(02)04635-0. [DOI] [PubMed] [Google Scholar]
- 20.Elseviers MM, Lins RL, Van der Niepen P, Hoste E, Malbrain ML, Damas P, Devriendt J, SHARF investigators Renal replacement therapy is an independent risk factor for mortality in critically ill patients with acute kidney injury. Crit Care. 2010;14:R221. doi: 10.1186/cc9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wald R, Adhikari NK, Smith OM, Weir MA, Pope K, Cohen A, Thorpe K, McIntyre L, Lamontagne F, Soth M, Herridge M, Lapinsky S, Clark E, et al. Canadian Critical Care Trials Group Comparison of standard and accelerated initiation of renal replacement therapy in acute kidney injury. Kidney Int. 2015;88:897–904. doi: 10.1038/ki.2015.184. [DOI] [PubMed] [Google Scholar]
- 22.Combes A, Bréchot N, Amour J, Cozic N, Lebreton G, Guidon C, Zogheib E, Thiranos JC, Rigal JC, Bastien O, Benhaoua H, Abry B, Ouattara A, et al. Early High-Volume Hemofiltration versus Standard Care for Post-Cardiac Surgery Shock. The HEROICS Study. Am J Respir Crit Care Med. 2015;192:1179–90. doi: 10.1164/rccm.201503-0516OC. [DOI] [PubMed] [Google Scholar]
- 23.Jamale TE, Hase NK, Kulkarni M, Pradeep KJ, Keskar V, Jawale S, Mahajan D. Earlier-start versus usual-start dialysis in patients with community-acquired acute kidney injury: a randomized controlled trial. Am J Kidney Dis. 2013;62:1116–21. doi: 10.1053/j.ajkd.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Seabra VF, Balk EM, Liangos O, Sosa MA, Cendoroglo M, Jaber BL. Timing of renal replacement therapy initiation in acute renal failure: a meta-analysis. Am J Kidney Dis. 2008;52:272–84. doi: 10.1053/j.ajkd.2008.02.371. [DOI] [PubMed] [Google Scholar]
- 25.Karvellas CJ, Farhat MR, Sajjad I, Mogensen SS, Leung AA, Wald R, Bagshaw SM. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15:R72. doi: 10.1186/cc10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Lv LS, Huang H, Guan J, Ye Z, Li S, Wang Y, Lou T, Liu X. Initiation time of renal replacement therapy on patients with acute kidney injury: A systematic review and meta-analysis of 8179 participants. Nephrology (Carlton) 2017;22:7–18. doi: 10.1111/nep.12890. [DOI] [PubMed] [Google Scholar]
- 27.Wierstra BT, Kadri S, Alomar S, Burbano X, Barrisford GW, Kao RL. The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Crit Care. 2016;20:122. doi: 10.1186/s13054-016-1291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piccinni P, Dan M, Barbacini S, Carraro R, Lieta E, Marafon S, Zamperetti N, Brendolan A, D’Intini V, Tetta C, Bellomo R, Ronco C. Early isovolaemic haemofiltration in oliguric patients with septic shock. Intensive Care Med. 2006;32:80–86. doi: 10.1007/s00134-005-2815-x. [DOI] [PubMed] [Google Scholar]
- 29.Andrade L, Cleto S, Seguro AC. Door-to-dialysis time and daily hemodialysis in patients with leptospirosis: impact on mortality. Clin J Am Soc Nephrol. 2007;2:739–44. doi: 10.2215/CJN.00680207. [DOI] [PubMed] [Google Scholar]
- 30.Manché A, Casha A, Rychter J, Farrugia E, Debono M. Early dialysis in acute kidney injury after cardiac surgery. Interact Cardiovasc Thorac Surg. 2008;7:829–32. doi: 10.1510/icvts.2008.181909. [DOI] [PubMed] [Google Scholar]
- 31.Ji Q, Mei Y, Wang X, Feng J, Cai J, Zhou Y, Sun Y, Xie S, Hu D. Timing of continuous veno-venous hemodialysis in the treatment of acute renal failure following cardiac surgery. Heart Vessels. 2011;26:183–89. doi: 10.1007/s00380-010-0045-9. [DOI] [PubMed] [Google Scholar]
- 32.Crescenzi G, Torracca L, Pierri MD, Rosica C, Munch C, Capestro F. ‘Early’ and ‘late’ timing for renal replacement therapy in acute kidney injury after cardiac surgery: a prospective, interventional, controlled, single-centre trial. Interact Cardiovasc Thorac Surg. 2015;20:616–21. doi: 10.1093/icvts/ivv025. [DOI] [PubMed] [Google Scholar]
- 33.Elahi MM, Lim MY, Joseph RN, Dhannapuneni RR, Spyt TJ. Early hemofiltration improves survival in post-cardiotomy patients with acute renal failure. Eur J Cardiothorac Surg. 2004;26:1027–31. doi: 10.1016/j.ejcts.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 34.Demirkiliç U, Kuralay E, Yenicesu M, Cağlar K, Oz BS, Cingöz F, Günay C, Yildirim V, Ceylan S, Arslan M, Vural A, Tatar H. Timing of replacement therapy for acute renal failure after cardiac surgery. J Card Surg. 2004;19:17–20. doi: 10.1111/j.0886-0440.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 35.Boussekey N, Capron B, Delannoy PY, Devos P, Alfandari S, Chiche A, Meybeck A, Georges H, Leroy O. Survival in critically ill patients with acute kidney injury treated with early hemodiafiltration. Int J Artif Organs. 2012;35:1039–46. doi: 10.5301/ijao.5000133. [DOI] [PubMed] [Google Scholar]
- 36.Chon GR, Chang JW, Huh JW, Lim CM, Koh Y, Park SK, Park JS, Hong SB. A comparison of the time from sepsis to inception of continuous renal replacement therapy versus RIFLE criteria in patients with septic acute kidney injury. Shock. 2012;38:30–36. doi: 10.1097/SHK.0b013e31825adcda. [DOI] [PubMed] [Google Scholar]
- 37.Lim CC, Tan CS, Kaushik M, Tan HK. Initiating acute dialysis at earlier Acute Kidney Injury Network stage in critically ill patients without traditional indications does not improve outcome: a prospective cohort study. Nephrology (Carlton) 2015;20:148–54. doi: 10.1111/nep.12364. [DOI] [PubMed] [Google Scholar]
- 38.García-Fernández N, Pérez-Valdivieso JR, Bes-Rastrollo M, Vives M, Lavilla J, Herreros J, Monedero P, GEDRCC Timing of renal replacement therapy after cardiac surgery: a retrospective multicenter Spanish cohort study. Blood Purif. 2011;32:104–11. doi: 10.1159/000324195. [DOI] [PubMed] [Google Scholar]
- 39.Gettings LG, Reynolds HN, Scalea T. Outcome in post-traumatic acute renal failure when continuous renal replacement therapy is applied early vs. late. Intensive Care Med. 1999;25:805–13. doi: 10.1007/s001340050956. [DOI] [PubMed] [Google Scholar]
- 40.Chung KK, Lundy JB, Matson JR, Renz EM, White CE, King BT, Barillo DJ, Jones JA, Cancio LC, Blackbourne LH, Wolf SE. Continuous venovenous hemofiltration in severely burned patients with acute kidney injury: a cohort study. Crit Care. 2009;13:R62. doi: 10.1186/cc7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carl DE, Grossman C, Behnke M, Sessler CN, Gehr TW. Effect of timing of dialysis on mortality in critically ill, septic patients with acute renal failure. Hemodial Int. 2010;14:11–17. doi: 10.1111/j.1542-4758.2009.00407.x. [DOI] [PubMed] [Google Scholar]
- 42.Shum HP, Chan KC, Kwan MC, Yeung AW, Cheung EW, Yan WW. Timing for initiation of continuous renal replacement therapy in patients with septic shock and acute kidney injury. Ther Apher Dial. 2013;17:305–10. doi: 10.1111/j.1744-9987.2012.01147.x. [DOI] [PubMed] [Google Scholar]
- 43.Chou YH, Huang TM, Wu VC, Wang CY, Shiao CC, Lai CF, Tsai HB, Chao CT, Young GH, Wang WJ, Kao TW, Lin SL, Han YY, et al. NSARF Study Group Impact of timing of renal replacement therapy initiation on outcome of septic acute kidney injury. Crit Care. 2011;15:R134. doi: 10.1186/cc10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu SC, Fu CY, Lin HH, Chen RJ, Hsieh CH, Wang YC, Yeh CC, Huang HC, Huang JC, Chang YJ. Late initiation of continuous veno-venous hemofiltration therapy is associated with a lower survival rate in surgical critically ill patients with postoperative acute kidney injury. Am Surg. 2012;78:235–42. [PubMed] [Google Scholar]
- 45.Gaudry S, Ricard JD, Leclaire C, Rafat C, Messika J, Bedet A, Regard L, Hajage D, Dreyfuss D. Acute kidney injury in critical care: experience of a conservative strategy. J Crit Care. 2014;29:1022–27. doi: 10.1016/j.jcrc.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Tian H, Sun T, Hao D, Wang T, Li Z, Han S, Qi Z, Dong Z, Lv C, Wang X. The optimal timing of continuous renal replacement therapy for patients with sepsis-induced acute kidney injury. Int Urol Nephrol. 2014;46:2009–14. doi: 10.1007/s11255-014-0747-5. [DOI] [PubMed] [Google Scholar]
- 47.Hu ZJ, Liu LX, Zhao CC. [Influence of time of initiation of continuous renal replacement therapy on prognosis of critically ill patients with acute kidney injury] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2013;25:415–19. doi: 10.3760/cma.j.issn.2095-4352.2013.07.012. Influence of time of initiation of continuous renal replacement therapy on prognosis of critically ill patients with acute kidney injury. [DOI] [PubMed] [Google Scholar]
- 48.Kresse S, Schlee H, Deuber HJ, Koall W, Osten B. Influence of renal replacement therapy on outcome of patients with acute renal failure. Kidney Int Suppl. 1999;56:S75–78. [PubMed] [Google Scholar]
- 49.Splendiani G, Mazzarella V, Cipriani S, Pollicita S, Rodio F, Casciani CU. Dialytic treatment of rhabdomyolysis-induced acute renal failure: our experience. Ren Fail. 2001;23:183–91. doi: 10.1081/jdi-100103490. [DOI] [PubMed] [Google Scholar]
- 50.Konopka A, Banaszewski M, Wojtkowska I, Stępińska J. Early implementation of continuous venovenous haemodiafiltration improves outcome in patients with heart failure complicated by acute kidney injury. Kardiol Pol. 2011;69:891–96. [PubMed] [Google Scholar]
- 51.do Nascimento GV, Balbi AL, Ponce D, Abrão JM. Early initiation of dialysis: mortality and renal function recovery in acute kidney injury patients. J Bras Nefrol. 2012;34:337–42. doi: 10.5935/0101-2800.20120022. [DOI] [PubMed] [Google Scholar]
- 52.Oh HJ, Shin DH, Lee MJ, Koo HM, Doh FM, Kim HR, Han JH, Park JT, Han SH, Yoo TH, Choi KH, Kang SW. Early initiation of continuous renal replacement therapy improves patient survival in severe progressive septic acute kidney injury. J Crit Care. 2012;27:743. doi: 10.1016/j.jcrc.2012.08.001. e9–18. [DOI] [PubMed] [Google Scholar]
- 53.Leite TT, Macedo E, Pereira SM, Bandeira SR, Pontes PH, Garcia AS, Militão FR, Sobrinho IM, Assunção LM, Libório AB. Timing of renal replacement therapy initiation by AKIN classification system. Crit Care. 2013;17:R62. doi: 10.1186/cc12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang XM, Tu GW, Gao J, Wang CS, Zhu DM, Shen B, Liu L, Luo Z. A comparison of preemptive versus standard renal replacement therapy for acute kidney injury after cardiac surgery. J Surg Res. 2016;204:205–12. doi: 10.1016/j.jss.2016.04.073. [DOI] [PubMed] [Google Scholar]
- 55.Oh HJ, Kim MH, Ahn JY, Ku NS, Park JT, Han SH, Choi JY, Han SH, Yoo TH, Song YG, Kang SW, Kim JM. Can early initiation of continuous renal replacement therapy improve patient survival with septic acute kidney injury when enrolled in early goal-directed therapy? J Crit Care. 2016;35:51–56. doi: 10.1016/j.jcrc.2016.04.032. [DOI] [PubMed] [Google Scholar]
- 56.Serpytis P, Smigelskaite A, Katliorius R, Griskevicius A. The early use of hemofiltration increases survival in patients with acute renal failure in the setting of intensive cardiac care unit. Glob Heart. 2014;9:e70. abstract PM046. [Google Scholar]
- 57.Perez X, Sabater J, Huguet M, et al. Early timing in septic shock patients. Intensive Care Med. 2012;38:S263. abstract. [Google Scholar]
- 58.Sabater J, Perez XL, Albertos R, Gutierrez D, Labad X. Acute renal failure in septic shock: should we consider different continuous renal replacement therapies on each RIFLE score stage? Intensive Care Med. 2009;35:S239. abstract 0924. [Google Scholar]
- 59.Tsai HW, Yang M, Lin Y, Ko W, Wu K. Outcome in the acute liver failure patients treated with renal replacement therapy for acute renal failure: comparison between early or late dialysis. J Am Soc Nephrol. 2005;16:540A. abstract. [Google Scholar]
- 60.Vinsonneau C, Camus C, Combes A, Costa de Beauregard MA, Klouche K, Boulain T, Pallot JL, Chiche JD, Taupin P, Landais P, Dhainaut JF, Hemodiafe Study Group Continuous venovenous haemodiafiltration versus intermittent haemodialysis for acute renal failure in patients with multiple-organ dysfunction syndrome: a multicentre randomised trial. Lancet. 2006;368:379–85. doi: 10.1016/S0140-6736(06)69111-3. [DOI] [PubMed] [Google Scholar]
- 61.Wald R, Gallagher M, Bagshaw SM. Shedding New Light on an Old Dilemma: Two Trials Examining the Timing of Renal Replacement Therapy Initiation in Acute Kidney Injury. Am J Kidney Dis. 2017;69:14–17. doi: 10.1053/j.ajkd.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods Group, and Cochrane Statistical Methods Group The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–05. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]


