Abstract
Background
There is no published data of treating hepatitis C in thalassemia major patients with any sofosbuvir based direct acting antivirals (DAAs). This study was performed to determine the efficacy and safety of these regimes using generic drugs in the thalassemia major population.
Methods
In this observational study, 902 patients of thalassemia major from five transfusion centres in Mumbai were screened for HCV antibody. Of the 120 positive patients, HCV RNA was detected in 50%. The first 29 patients were enrolled for evaluating the efficacy and safety of generic sofosbuvir based DAAs.
Results
The 29 patients’ had a mean age of 24 years with genotype 1 in 17, genotype 3 in 11patients, while 1 patient's genotype could not be classified. Six patients had compensated cirrhosis and 8 patients were treatment experienced. SVR 12 was achieved in 100% of patients. There was significant increase in PRC (packed red cell) requirements (P = 0.0003) during treatment. At 12 weeks post-treatment, PRC requirements returned to baseline with a significant fall in serum ferritin (P = 0.03). Headache, fatigue and diarrhoea were the most common side effects. The difference in side effects including anaemia between patients who received ribavirin (19/29) and those who did not receive ribavirin (10/29) was not significant. Presence of diabetes, splenectomy, high ferritin or liver or heart iron overload on MRI T2* did not affect the efficacy of treatment.
Conclusion
Generic DAAs are safe in thalassemia major patients with hepatitis C with efficacy of 100%. Serum ferritin falls significantly after treatment despite an increase in transfusion requirements during treatment.
Abbreviations: PRC, packed red blood cells; HIV, human immunodeficiency virus; DAAs, direct acting antivirals; SVR, sustained virological response; RNA, ribonucleic acid; VAS, visual analogue scale; RVR, rapid virological response; EBR, elbasvir; GZR, grazoprevir; SD, standard deviation; AST, aspartate amonotransferase; ALT, alanine aminotransferases; ALP, alkaline phosphatase; WCC, white cell count; Hb, haemoglobin; ETR, end of treatment response
Keywords: sofosbuvir, ledipasvir, daclatasvir, haemoglobinopathy, India
Haemoglobinopathies are the commonest single gene disorder in India.1 The carrier rate ranges from 3 to 17%2, 3 with higher incidence in communities like Sindhis, Kutchhi, Bhanushalis and Punjabis from Western and Northern India.4 Packed red cells (PRC) transfusion is the mainstay of therapy.5 Thalassemia major was considered a fatal condition with most patients succumbing to effects of severe anaemia,6 however with improved availability of PRC, these children now live into adulthood.7 Repeated PRC transfusions are associated with hazards like iron overload and risk of acquiring transfusion-transmitted infections i.e. human immunodeficiency virus (HIV), hepatitis B and hepatitis C (HCV).8 Hepatitis C antibody testing in transfusion products was made mandatory in India only in June 2001,9 Hence patients transfused before 2001 had a high risk of acquiring hepatitis C.10 Even after 2001, the risk albeit small, remains as the donor can be in the window period or testing kits have a low sensitivity. Indian studies have found anti HCV antibodies in 16–43% of thalassemic patients,11, 12 with prevalence of 8–50% in other parts of the world. Although hepatitis C has surpassed HIV as a cause of death,13 successful treatment of HCV can delay or prevent the development of end stage liver disease.
In the last decade, the only treatment available for hepatitis C was a combination of pegylated interferon and ribavirin which was not well tolerated, especially by thalassemic children.14 These drugs required weekly injections for prolonged duration, had significant side effects and therefore a lower compliance with an SVR of up to 70% only.15 These drugs were costly and beyond the reach of a majority of patients as these are not available through the public health system and a majority of patients pay out of pocket. After the introduction of direct acting antivirals (DAAs), the standard of care for treatment of hepatitis C has changed worldwide. Present therapy entails short course therapy (12–24 weeks), high SVR (>90%), and minimal side effects. In India, sofosbuvir became available in March 201516 while ledipasvir and daclatasvir became available from January 2016.17 Due to pressure from patients rights groups and policy intervention, which promoted local generics these drugs are available at a low cost The entire therapy in India would cost about US $300 as compared to around US $64,80018 in the USA. Worldwide, there has been no prospective study on the use of sofosbuvir based DAAs in thalassemic patients. We undertook this study to determine the efficacy and safety of generic DAAs in adult patients with thalassemia major.
Method
Study Design and Patients
This was a prospective study in which HCV positive thalassemia major patients from five transfusion centres in Mumbai were included. Approval was obtained from the institutional ethics committee and an informed consent was taken from the patient before enrolling them for treatment. Thalassemia major patients >18 years of age with detectable hepatitis C virus RNA (ribonucleic acid, Cobas Taqman PCR) were included. Patients with other co-infections like tuberculosis, HBsAg and HIV, as well as malignancy were excluded.
Patient Evaluation
All patients were evaluated at baseline (pre-transfusion) by haemogram, liver profile, renal profile, serum ferritin, blood sugar, thyroid stimulating hormone, HCV genotype, fibroscan and ultrasound of the abdomen. A history of frequency and volume of PRC, details of chelation and other drugs was obtained. MRI T2* scan for determining iron load in the heart and liver was performed in all patients. Haemogram, liver profile, renal profile and HCV RNA which was done at baseline, was repeated at 4 weeks, end of treatment and 12 weeks post-treatment. The change in volume of PRC required was noted during and after completion of therapy. On fibroscan (ECHOSENS, Paris), the median value was recorded (after 10 successful readings) and success rate of more than 90% was accepted as a valid reading. A score of >8 kPa was considered to be significant fibrosis. Cirrhosis was diagnosed by ultrasonography evidence of cirrhosis or portal hypertension in terms of liver echotexture, dilated portal vein, ascites or presence of collaterals or a fibroscan value >15 kPa.
Treatment
The treatment protocol is listed in Table 1.
Table 1.
The Treatment Protocol for Patients With Thalassemia Major and Hepatitis C.
| Genotype | Characteristics of patients | Direct acting antivirals | Duration (weeks) | Number of patients in the present studya |
|---|---|---|---|---|
| GT1 | Treatment naïve and fibroscan ≤8 kPa | Sofosbuvir + ledipasvir | 12 | 5 |
| Treatment naïve/experienced and fibroscan >8 kPa | Sofosbuvir + ledipasvir + ribavirin or sofosbuvir + ledipasvir | 12 24 |
12 | |
| GT3 | Treatment naïve and fibroscan ≤8 kPa | Sofosbuvir + daclatasvir | 12 | 1 |
| Treatment naïve and fibroscan > 8 kPa or treatment experienced | Sofosbuvir + daclatasvir + ribavirin or sofosbuvir + daclatasvir | 12 24 |
10 |
The sum of patients is 28 as one patient had undetectable genotype and was treated with sofosbuvir/daclatasvir based therapy. Fibroscan score of 8 kPa was considered as a cut-off for presence of significant fibrosis and these patients were treated as compensated cirrhosis.
Assessment of Efficacy
The primary aim was to determine the efficacy in terms of a sustained virological response (SVR) at 12 weeks after starting DAAs. Analysis was done with an intention to treat and any individual who received even a single dose of DAAs was included in the analysis. Virologic failure was defined as non-response (detectable HCV RNA at end of treatment); rebound (>1 log10 IU/ml increase in HCV RNA from nadir while on treatment); breakthrough (detectable HCV RNA on treatment after previously having undetectable HCV RNA); or relapse (HCV RNA undetectable at end of treatment, then HCV RNA became detectable during follow-up). MRI was not repeated at the end of therapy.
Assessment of Safety
The secondary aim was to evaluate the safety of the DAAs in this subset of patients. Adverse events were graded as per the visual analogue scale (VAS). Each patient was asked to grade the adverse effects from a scale of 0–10 with 10 being most severe. A note of any side effects during and after completion of therapy was made. Insomnia, nausea, skin rash, pruritus, fatigue, arthralgia, myalgia, diarrhoea, headache, flu-like symptoms, oral ulcers, anorexia, fever, weight loss and other side effects (if any) were specifically asked for during each visit.
The interval between transfusions was recorded and pre-therapy haemoglobin level was kept >8 g/dl. The secondary outcomes aimed to correlate response to treatment with parameters such as serum ferritin, presence or absence of diabetes, cirrhosis, treatment experienced in the past; comparison of ferritin and liver function tests, pre and post-treatment.
Statistics
SPSS version 20 was used for all analyses. Mean and standard deviation was calculated for continuous variables. All quantitative data was analysed using t test (paired and unpaired). Qualitative data was analysed using Pearson's chi square test. P value <0.05 was considered as significant.
Results
Baseline Characteristics
During the study period, 902 patients of thalassemia major from five transfusion centres in Mumbai were screened with anti HCV antibody. Of these, 120 (13.3%) were anti HCV positive. HCV RNA was detected in 50% (60 of the 120 patients). The initial 29 of the 60 patients in whom treatment was started and the have completed 12 weeks follow up post-treatment completion were enrolled for the study. The demographic details, clinical and biochemical parameters, fibroscan and MRI T2* values, chelation drug details and genotype as well as treatment experience have been elaborated upon in Table 2.
Table 2.
Baseline Characteristics of patients with Hepatitis C and Thalassemia Major Treated With Directly Acting Antivirals (DAA).
| Age in years (mean ± SD) | 24 ± 5.1 |
| Gender (M:F) | 16:13 |
| Body mass index (kg/m2) (mean ± SD) | 19.4 ± 2.9 |
| Splenectomy (n) | 9 |
| Diabetes (n) | 6 |
| Hypothyroidism (n) | 4 |
| Thrombocytopenia (<150 × 109/l) | 4 |
| Chelation (15 patients received 2 chelators) | |
| Deferoxamine (n) | 10 |
| Weekly vials (mean ± SD) | 10.1 ± 5.1 |
| Deferiprone (n) | 13 |
| Daily dose (mg) (mean ± SD) | 2807 ± 878 |
| Deferasirox (n) | 21 |
| Daily dose (mg) (mean ± SD) | 1533 ± 502 |
| Transfusion requirement (ml/month) (mean ± SD) | 896 ± 199 |
| T2* MRI heart iron overload (n = 24) score ≥2 | 6 |
| T2* MRI Liver iron overload (n = 24) score ≥2 | 16 |
| Serum ferritin (ng/ml) (mean ± SD) | 5381 ± 4198 |
| Fasting sugar (mg/dl) (mean ± SD) | 98 ± 25 |
| 2 h post-prandial sugar (mg/dl) (mean ± SD) | 117 ± 39 |
| Hepatitis C RNA (IU/ml) (mean ± SD) | 1.82 ± 3.05 × 106 |
| Genotype 1 (n) (a/b) | 17 (7/10) |
| Treatment naïve (n) (a/b) | 10 (5/5) |
| Treatment experienced (n) (a/b) | 7(2/5) |
| Genotype 3 (n) | 11 |
| Treatment naïve (n) | 10 |
| Treatment experienced (n) | 1 |
| Treatment experienced (n) | 8 |
| Partial responder (n) | 3 (all genotype 1) |
| Null responder (n) | 1 (genotype 3) |
| Relapse (n) | 4 (all genotype 1) |
| Recurrence (n) | 0 |
| Cirrhosis (n) | 6 |
| Fibroscan (kPA) (mean—range) | 14.12 (3.3–29.5) |
| AST (IU/l) (mean ± SD) | 64 ± 49 |
| ALT (IU/l) (mean ± SD) | 59 ± 33 |
| ALP (IU) (mean ± SD) | 107 ± 81 |
| Hb (g/dl) (mean ± SD) | 8.8 ± 1.0 |
| Platelets (per litre) (mean ± SD) | 298 ± 151 × 109 |
| Albumin (g/dl) (mean ± SD) | 4 ± 0.4 |
| Creatinine (mg%) (mean ± SD) | 0.6 ± 0.1 |
| Bilirubin (mg/dl) (mean ± SD) | 1.7 ± 0.8 |
T2* MRI score >2 implies significant iron overload.
SD, standard deviation; AST, aspartate amonotransferase; ALT, alanine aminotransferases; ALP, alkaline phosphatase; WCC, white cell count; Hb, haemoglobin.
1 patient had a unclassified genotype and was treated with sofosbuvir and daclatasvir and was treatment naïve.
Two patients were on amiodarone for complications related to cardiac iron overload. This was stopped 1 month prior to start of DAA therapy.
Hepatitis C Treatment
Sofosbuvir and ledipasvir were given in 17 patients (genotype 1) and sofosbuvir with daclatasvir was given in 12 patients (genotype 3 and undetectable genotype). Ribavirin was used in 19 patients and mean ribavirin dose was 10.2 mg/kg/day (range 7.1–15.1 mg/kg). Ribavirin was started at a low dose of 200 mg/day and increased as tolerated. All drugs were generic in nature as were manufactured in India. The duration of therapy was 12 weeks in 21 patients, 24 weeks in 6 patients. One patient received 20 weeks of therapy (planned 24 weeks) while another received 8 weeks of therapy (planned 12 weeks) as these two patients discontinued their treatment. Discontinuation was not related to side effects of the drugs. Details of treatment received in different sub groups are mentioned in Table 1.
Efficacy of Therapy
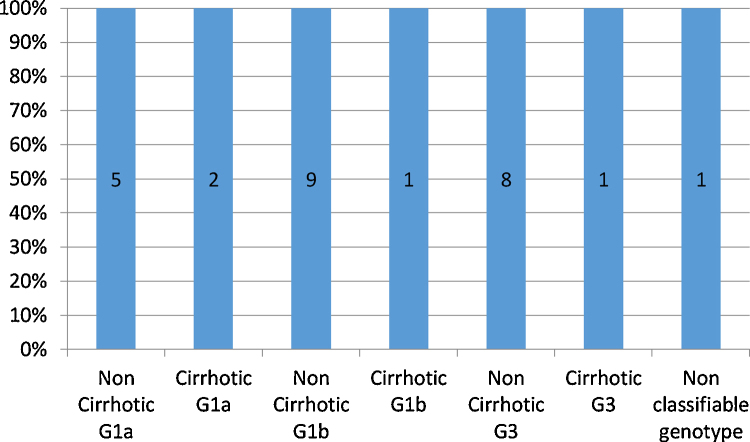
Twenty-eight patients achieved rapid virological response (RVR) after 1 month of therapy. The SVR rate was 100% (Figure 1). There was no relapse or breakthrough during and after the treatment respectively. None of the patients decompensated on treatment. Mean ALT, AST, ALP, bilirubin and albumin at baseline, during treatment and at 12 weeks post-treatment are shown in Table 3.
Figure 1.
Bar diagram showing efficacy of direct acting antivirals in different subgroups of patients. The numbers in the bar diagram denote the number of patients in that subgroup.
Table 3.
Change in Parameters During Treatment and at SVR 12 of Patients With Hepatitis C and Thalassemia Major With Direct Acting Antivirals Compared to Baseline.
| At onset of treatment (mean ± SD) | Peak/nadir values during/end of the treatment (mean ± SD) | P value | At SVR 12 (mean ± SD) | P value | |
|---|---|---|---|---|---|
| Mean AST (IU/l) | 64 ± 49 | 30 ± 15 (ETR) | <0.0001 | 40 ± 21 | 0.2 |
| Mean ALT (IU/l) | 59 ± 33 | 36 ± 19 (ETR) | 0.0005 | 35 ± 17 | <0.0001 |
| Mean ALP (IU) | 107 ± 81 | 126 ± 106 (ETR) | 0.08 | 110 ± 97 | 0.55 |
| Mean Hb (g/dl) | 8.8 ± 1.0 | 7.5 ± 1.5 (nadir) | <0.0001 | 9.1 ± 1.3 | 0.28 |
| Mean ferritin (ng/ml) | 5381 ± 4198 | 4249 ± 3106 (ETR) | 0.08 | 4703 ± 2781 | 0.03 |
| Mean WCC (per cumm) | 10,340 ± 12,987 | 6838 ± 4739 (nadir) | 0.06 | 8975 ± 6991 | 0.4 |
| Mean platelets (per litre ×109) | 298 ± 151 | 266 ± 174 (nadir) | 0.02 | 327 ± 203 | 0.14 |
| Mean albumin (g/dl) | 4 ± 0.4 | 3.9 ± 0.5 (nadir) | 0.3 | 4.0 ± 0.6 | 0.97 |
| Mean bilirubin (mg/dl) | 1.7 ± 0.8 | 2.3 ± 0.8 (peak) | 0.00027 | 1.6 ± 0.5 | 0.8 |
| Deferoxamine—mean weekly vials | 10.1 ± 5.1 | 10.2 ± 4.7 (ETR) | 0.08 | 10.5 ± 5.1 | 0.86 |
| Deferiprone—mean daily dose (mg) | 2807 ± 878 | 2884 ± 984 (ETR) | 0.33 | 3000 ± 1040 | 0.61 |
| Deferasirox—mean defirox dose | 1533 ± 502 | 1542 ± 497 (ETR) | 0.32 | 1516 ± 509 | 0.91 |
| Mean transfusion requirement (ml/month) | 896 ± 199 | 1074 ± 256 (ETR) | 0.0003 | 937 ± 197 | 0.06 |
| Serum creatinine (mg%) | 0.6 ± 0.1 | – | – | 0.6 ± 0.1 | 0.25 |
| Mean HCV RNA (IU/ml) | 1,820,907 ± 3,059,612 | – | – | 0 | 0.001 |
P < 0.05 was considered as significant. Significant p values are shown in bold. The ETR values were taken on completion of treatment. The peak and nadir values were the highest and the least values at any point during the entire course of treatment until the completion of therapy. SD, standard deviation; AST, aspartate amonotransferase; ALT, alanine aminotransferases; ALP, alkaline phosphatase; WCC, white cell count; ETR, end of treatment response.
Safety
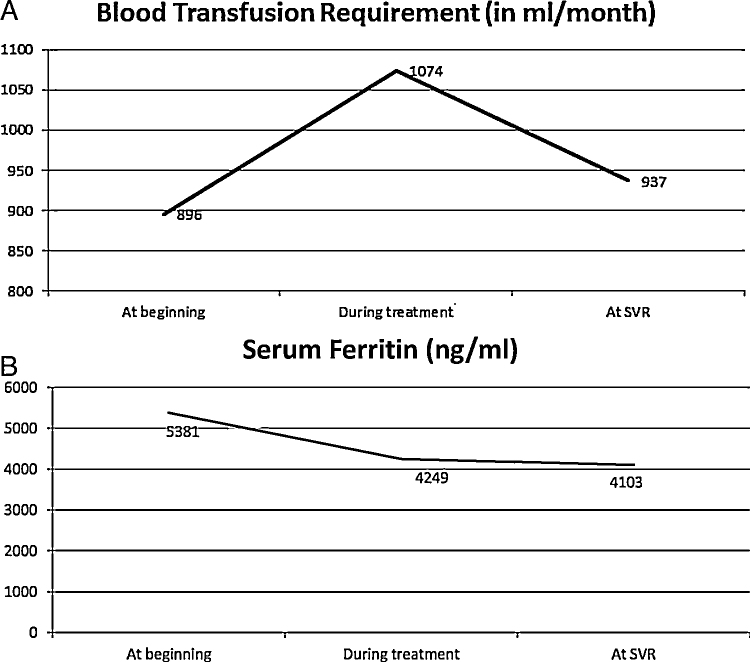
Mean PRC requirement (per month) before, during treatment and 12 weeks after completion of therapy was 896 ml, 1074 ml and 937 ml respectively. Increased requirement was observed in 18 patients overall (62%). There was statistically significant rise in PRC requirement during the treatment (P = 0.0003). But this increase started to normalise during the time of SVR and although higher, difference between the requirement at baseline and at SVR was not statistically significant. The mean ± SD PRC requirement per month during treatment in the ribavirin group was 1121 ± 264 ml versus 981 ± 286 ml in the non-ribavirin group which was not statistically different (P = 0.08).
There was no significant change in the requirement of chelating agents between baseline, during and at SVR12. Haemoglobin, white cell count, platelets, albumin or creatinine at SVR12 compared to the baseline values were similar. However, when we considered the peak bilirubin during the treatment and nadir values of platelets and haemoglobin during the treatment, there was significant change in these parameters during the treatment. Simultaneously, there was a rise in PRC requirements during the course of treatment despite fall in ferritin levels (Figure 2a and b).
Figure 2.
(a) The mean values of transfusion requirement before, during and after treatment with direct acting antivirals. (b) Serum ferritin values (in mean) before, during and after treatment with direct acting antivirals.
Side effects were seen in 13 of 29 patients. The adverse effects graded as severe by the patient interview (>5 severity) on VAS were arthralgia in 2 patients, headache, anorexia, oral ulcer and insomnia in 1 each. Other side effects which were seen included fatigue, nausea, vomiting, diarrhoea, breathlessness, body ache, irritability, weight loss and dry skin. None of the side effects were more common in individuals taking ribavirin, or needed cessation of DAAs.
Discussion
Hepatitis C in patients with thalassemia major is particularly difficult to treat as poor efficacy and adverse effects pose a major challenge. In the best reported results by Sood et al.,19 SVR was attained in 40% patients with monotherapy with pegylated interferon α2b and 70% with combination therapy.
The treatment of hepatitis C has recently undergone a paradigm change and has moved on to all oral regimens with or without ribavirin. With the advent of DAAs, it is expected to change the management criteria in thalassemics similar to the non-thalassemic population, although no prospective sofosbuvir based study has so far been done in this population. Since a significant number of our thalassemic patients suffer from hepatitis C (13.3% vs. 1%) in our general population,20 we felt the need to look for efficacy of the DAAs in this population. In the non-thalassemic population, studies in genotype 1 patients have demonstrated that 12 weeks of ledipasvir/sofosbuvir without ribavirin was an effective treatment.21, 22 Genotype 3 patients, when compared to other genotypes, have relatively faster rate of fibrosis progression, severe steatosis and higher incidence of hepatocellular carcinoma.23 In the current era, genotype 3 has been labelled as a difficult virus to treat and so needs more aggressive treatment. We anticipated that our genotype 3 patients would have a poor efficacy as majority of our patients had iron overload with high mean baseline value of serum ferritin being >5000 ng/ml. Considering that, all patients with significant fibrosis—fibroscan values >8 kPa, were offered ribavirin based therapy rather than giving ribavirin free regimens. The mean fibroscan values were 14.12 and therefore majority of our patients ended up receiving ribavirin. However, irrespective of iron overload or fibroscan values, all our patients achieved SVR12. In the future, it would be worthwhile studying the efficacy of ribavirin free regimens in patients with thalassemia and significant iron overload.
In a study by Zachou et al.24 in thalassemics, 30 patients received IFN-based therapy with or without ribavirin with SVR in 46.7%. Eleven patients (9 non-responders to IFN-based therapies, 1 relapser and 1 naïve) received treatment with Sofosbuvir based DAAs (SVR: 100%). 3/11 patients had an increase in PRC requirements while 1/11 reported mild arthralgias. Our study results shows that the efficacy of generic DAA in thalassemic population is equivalent to the non-thalassemic population. About 30% of our study population was treatment experienced, 20% had evidence of cirrhosis and 75% had significant fibrosis. Irrespective of the severity and previous therapy, DAAs in our study showed 100% efficacy with 96.5% patients achieving RVR and 100% attaining end of treatment response and SVR12. The one person who did not achieve RVR was a 21-year-old male with baseline ferritin of 8714 ng/ml, genotype 1b, had cirrhosis and was treatment experienced. He cleared the virus by end of treatment and achieved SVR12. Even the presence of significant iron overload, splenectomy and diabetes did not affect the efficacy of treatment which was similar to the non-thalassemic population. In terms of tolerability, DAAs appear to be safe in patients with thalassemia major as none of our patients developed any severe adverse reactions and all side effects were managed conservatively.
To our knowledge, the only other prospective study is a multicenter study from 31 study sites across the world in patients with various haemoglobinopathies (156 patients) using a different DAA combination of elbasvir/grazoprevir (EBR/GZR) for 12 weeks in which 97.6% thalassemic patients achieved SVR12.25 Our study used a different DAAs (sofosbuvir based) of a generic make in a developing country.
One of the major advantages of DAAs over pegylated interferon is tolerability and safety in difficult to treat populations. Apart from PRC requirement which went up, none of the other untoward effects required any change in therapy. The number of patients who were given ribavirin did not experience higher side effects as compared to those not given ribavirin. So, it seems plausible that it is the combination of pegylated interferon and ribavirin rather than ribavirin alone that is responsible for the side effects and intolerance.
An interesting finding in the present study was that even though the PRC requirements were significantly higher in those treated for hepatitis C, overall serum ferritin values decreased sequentially. Elevated serum ferritin level is independently associated with advanced liver fibrosis, hepatic steatosis, and poor response to interferon-alpha-based therapy.26, 27 The serum ferritin levels have significantly decreased in our patients even though the PRC requirement increased. This corroborates with the belief that the virus is responsible for rise in serum ferritin and with the inflammation declining, the ferritin levels begins to decline. Our study attempted to look at iron overload not only in the form of serum ferritin but also T2* MRI for iron stores in the heart and liver. Change in serum ferritin was not evaluated in the study by Hezode et al.25 Also, this multicentre trial did not look at the splenectomy status or presence of diabetes in their patients. We had aimed to correlate these as well as the iron parameters with response rates but did not do so as there was a 100% SVR.
Two of our patients stopped treatment prematurely. The non-compliance was not related to adverse effects. Both these patients achieved SVR and therefore shorter duration of therapy may also be attempted in subgroups of the thalassemics as in the non-thalassemic population.28
In the developing world, questions are often raised about the therapeutic equivalence of generic drugs compared to preparations from the original manufacturer often a multinational. A significant point of this study was that the DAAs used were all generics made in India. This supports the data published by an Australian group that generic drugs available in India are equally efficacious to the much costlier drugs available in the West.29 The significantly lower costs of the Indian generics hugely impacts on access in a country like India with a large number of poor patients and where most health care expenditure is out of pocket. In case of thalassemics, the financial challenges are even more as the family has often spent well beyond their means for the continuous PRC transfusions, chelation therapy and frequent investigations.
A major strength of this study is that it is a prospective evaluation of a difficult to manage hepatitis C population. This could serve as a pilot for other large studies in thalassemia major population. A limitation of the study is that there were only 6 patients who had compensated cirrhosis and the decompensated group was not studied. This sub group needs study in the future. Also, the sample size was small and the study was carried out in adults. A large number of thalassemia major patients are diagnosed with hepatitis C during their adolescent age with significant fibrosis.
Conclusion
Generic DAAs are safe and effective in adults with thalassemia major and hepatitis C. The sustained virological response rate at 12 weeks is 100%. PRC requirement rises significantly during the course of treatment but normalizes after completion of therapy. Serum ferritin levels fall significantly during and after treatment implying the role of Hepatitis C virus in inducing a rise in ferritin. Presence of diabetes, splenectomy or iron overload do not adversely affect the outcome of treatment.
Conflicts of Interest
The authors have none to declare.
Acknowledgements
Mr. Vinay Shetty—Think Foundation, a Non-Governmental Organisation running transfusion centres for patients of Thalassemia Major in Mumbai.
Bhatia Hospital Institutional Research Board.
References
- 1.Mohanty D., Colah R.B., Gorakshakar A.C. Prevalence of beta-thalassemia and other haemoglobinopathies in six cities in India: a multicentre study. J Community Genet. 2013;4:33–42. doi: 10.1007/s12687-012-0114-0. [PMID: 23086467] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Philip J., Jain N. Resolution of alloimmunization and refractory autoimmune hemolytic anemia in a multi-transfused beta-thalassemia major patient. Asian J Transfus Sci. 2014;8:128–130. doi: 10.4103/0973-6247.137454. [PMID: 25161355] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panigrahi I., Marwaha R.K. Mutational spectrum of thalassemias in India. Indian J Hum Genet. 2007;13:36–37. doi: 10.4103/0971-6866.32034. [PMID: 21957341] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colah R.B., Gorakshakar A.C., Nadkarni A.H. Invasive & non-invasive approaches for prenatal diagnosis of haemoglobinopathies: experiences from India. Indian J Med Res. 2011;134:552–560. [PMID: 22089620] [PMC free article] [PubMed] [Google Scholar]
- 5.Amarapurkar D.N., Kumar A., Vaidya S. Frequency of hepatitis B, C and D and human immunodeficiency virus infections in multi-transfused thalassemics. Indian J Gastroenterol. 1992;11(2):80–81. [PMID: 1428037] [PubMed] [Google Scholar]
- 6.Rechavi G., Rivella S. Regulation of iron absorption in hemoglobinopathies. Curr Mol Med. 2008;8:646–662. doi: 10.2174/156652408786241401. [PMID: 18991651] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Dreuzy E., Bhukhai K., Leboulch P., Payern E. Current and future alternative therapies for beta-thalassemia major. Biomed J. 2016;39:24–38. doi: 10.1016/j.bj.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vidja P.J., Vachhani J.H., Sheikh S.S., Santwani P.M. Blood transfusion transmitted infections in multiple blood transfused patients of beta thalassaemia. Indian J Hematol Blood Transfus. 2011;27:65–69. doi: 10.1007/s12288-011-0057-3. [PMID: 22654294] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra D., Pensi T. Transfusion associated hepatitis C in multi-transfused thalassemic children. Indian Pediatr. 2004:287–288. [PubMed] [Google Scholar]
- 10.Jain R., Perkins J., Johnson S.T. A prospective study for prevalence and/or development of transfusion-transmitted infections in multiply transfused thalassemia major patients. Asian J Transfus Sci. 2012;6:151–154. doi: 10.4103/0973-6247.98919. [PMID: 22988380] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal M.B., Malkan G.H., Bhave A.A. Antibody to hepatitis-C virus in multi-transfused thalassaemics—Indian experience. J Assoc Physicians India. 1993;41:195–197. [PMID: 8270554] [PubMed] [Google Scholar]
- 12.Choudhury N., Saraswat S., Naveed M. Serological monitoring of thalassaemia major patients for transfusion associated viral infections. Indian J Med Res. 1998;107:263–268. [PMID: 9701894] [PubMed] [Google Scholar]
- 13.Jafroodi M., Davoudi-Kiakalayeh A., Mohtasham-Amiri Z., Pourfathollah A.A., Haghbin A. Trend in prevalence of hepatitis C virus infection among beta-thalassemia major patients: 10 years of experience in Iran. Int J Prev Med. 2015;6:89. doi: 10.4103/2008-7802.164832. [PMID: 26445636] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Marco V., Lo Iacono O., Capra M. Alpha-Interferon treatment of chronic hepatitis C in young patients with homozygous beta-thalassemia. Haematologica. 1992;77:502–506. [PMID: 1289187] [PubMed] [Google Scholar]
- 15.Aminizadeh E., Alavian S.M., Akbari Sari A., Ebrahimi Daryani N., Behnava B. Efficacy of adding ribavirin to interferon or peginterferon in treatment of hepatitis C infection in patients with thalassemia: a systematic review on randomized controlled trials. Hepat Mon. 2016;16:e28537. doi: 10.5812/hepatmon.28537. [PMID: 27226796] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puri P., Saraswat V.A., Dhiman R.K. Indian National Association for Study of the Liver (INASL) guidance for antiviral therapy against HCV infection: update 2016. J Clin Exp Hepatol. 2016;6:119–145. doi: 10.1016/j.jceh.2016.07.001. [PMID: 27493460] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham P. Treatment for hepatitis C virus infection in India: promising times. Indian J Med Microbiol. 2016;34:273–274. doi: 10.4103/0255-0857.188312. [PMID: 27514946] [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal R., Chen Q., Goel A. Cost-effectiveness of hepatitis C treatment using generic direct-acting antivirals available in India. PLOS ONE. 2017;12(5):e0176503. doi: 10.1371/journal.pone.0176503. eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sood A., Sobti P., Midha V. Efficacy and safety of pegylated IFN alfa 2b alone or in combination with ribavirin in thalassemia major with chronic hepatitis C. Indian J Gastroenterol. 2010;29:62–65. doi: 10.1007/s12664-010-0014-3. [DOI] [PubMed] [Google Scholar]
- 20.Puri P., Anand A.C., Saraswat V.A. Consensus Statement of HCV Task Force of the Indian National Association for Study of the Liver (INASL). Part I: status report of HCV infection in India. J Clin Exp Hepatol. 2014;4:106–116. doi: 10.1016/j.jceh.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundaram V., Kowdley K.V. Role of ledipasvir/sofosbuvir combination for genotype 1 hepatitis C virus infection. Hepat Med. 2016;8:75–80. doi: 10.2147/HMER.S63125. [PMID: 27418860] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gane E.J., Stedman C.A., Hyland R.H. HCV therapeutics: new agents. Hepatology. 2013;58(S1):243A–246A. [Google Scholar]
- 23.Kanwal F., Kramer J.R., Ilyas J., Duan Z., El-Serag H.B. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60:98–105. doi: 10.1002/hep.27095. [PMID: 24615981] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zachou K., Arvaniti P., Gatselis N.K. Patients with haemoglobinopathies and chronic hepatitis C: a real difficult to treat population in 2016? Mediterr J Hematol Infect Dis. 2017;9(1):e2017003. doi: 10.4084/MJHID.2017.003. [PMID: 28101309] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hezode C., Colombo M., Bourliere M. Elbasvir/grazoprevir for patients with hepatitis C virus infection and inherited blood disorders: a phase III study. Hepatology. 2017 doi: 10.1002/hep.29139. [DOI] [PubMed] [Google Scholar]
- 26.Milic S., Mikolasevic I., Orlic L. The role of iron and iron overload in chronic liver disease. Med Sci Monit. 2016;22:2144–2151. doi: 10.12659/MSM.896494. [PMID: 27332079] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lange C.M., Kutalik Z., Morikawa K. Serum ferritin levels are associated with a distinct phenotype of chronic hepatitis C poorly responding to pegylated interferon-alpha and ribavirin therapy. Hepatology. 2012;55:1038–1047. doi: 10.1002/hep.24787. [DOI] [PubMed] [Google Scholar]
- 28.Kowdley K.V., Sundaram V., Jeon C.Y. Eight weeks of ledipasvir/sofosbuvir is effective for selected patients with genotype 1 hepatitis C virus infection. Hepatology. 2017;65:1094–1103. doi: 10.1002/hep.29005. [DOI] [PubMed] [Google Scholar]
- 29.Freeman J., Sallie R., Kennedy A. High sustained virological response rates using generic direct acting antiviral treatment for hepatitis C, imported into Australia. J Hepatol. 2016;64(2):S209. [Google Scholar]