Abstract
Elevated serum ferritin level is a common finding in iron overload syndrome, autoimmune and viral hepatitis, alcoholic and nonalcoholic fatty liver diseases. High transferrin saturation is not a common finding in above diseases except for iron overload syndrome. We encountered a challenging case of 73-year-old female who presented with yellowish discoloration of skin, dark color urine and dull abdominal pain. Initial laboratory tests reported mild anemia; elevated bilirubin, liver enzymes, and transferrin saturation. We came to the final diagnosis of autoimmune hepatitis after extensive workups. Autoimmune hepatitis is a rare disease, and the diagnosis can be further complicated by a similar presentation of iron overload syndrome. Markedly elevated transferrin saturation can simulate iron overload syndrome, but a liver biopsy can guide physicians to navigate the diagnosis.
Abbreviations: AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, antimicrosomal antibody; ANA, antinuclear antibody; anti-LKM, anti-liver kidney microsomal; anti-SMA, anti-smooth muscle antibody; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CMV, cytomegalovirus; CT, computed tomography; EBV, Epstein–Barr virus; ESR, erythrocyte sedimentation rate; HHC, hereditary hemochromatosis; HLA, human leukocyte antigen; Ig, immunoglobulin; INR, international normalized ratio; LDH, lactate dehydrogenase; LFT, liver function test; MRI, magnetic resonance imaging; PT, prothrombin time; PTT, partial thromboplastin time; PTU, propylthiouracil; RBC, red blood cell; TIBC, total iron binding capacity; WBC, white blood cell
Keywords: Autoimmune hepatitis, Transferrin saturation, Iron overload syndrome, Autoimmune disease, Diagnostic dilemma
Autoimmune hepatitis (AIH) is a chronic progressive necroinflammatory liver disease of unknown cause associated with circulating autoantibodies and a high serum globulin level.1 Clinical manifestations range from merely elevated transaminases to liver cirrhosis and/or fulminant liver failure requiring liver transplantation. Here we describe a rare presentation of AIH disguised as iron overload syndrome.
Case Presentation
A 73-year-old female presented with chief complaint of progressive dark colored urine for 2 weeks, associated with dull abdominal pain, yellowish discoloration of the skin, and increased fatigability. She denied fever. She had a history of hypertension, hypothyroidism, and cholecystectomy. She denied any family history of liver diseases, recent travels, any form of complementary medicine, smoking, alcohol, or any illicit drug use.
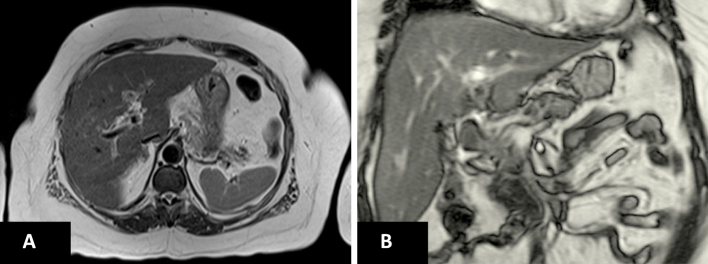
On physical examination, the patient was alert and oriented with normal range vitals. She was moderately icteric without pallor. Systemic examinations were unremarkable except for a mild tenderness in the right upper quadrant of the abdomen. Her initial laboratory analysis (Table 1) revealed markedly elevated alanine aminotransferase 829 U/L, aspartate aminotransferase 909 U/L, and total bilirubin 10.9 mg/dl with direct bilirubin 7.8 mg/dl. Viral and alcoholic hepatitis were ruled out with a negative hepatitis viral panel and negative history of alcohol consumption respectively. Cytomegalovirus (CMV) culture and Epstein–Barr virus (EBV) immunoglobulin M (IgM) were negative. Serum free T4, free T3, TSH and serum copper levels were within normal range. Urine toxicology including salicylate was negative. Anti-mitochondrial, anti-smooth muscle, and kidney-liver antibodies were negative but antinuclear antibody (ANA) was only positive with a low titer of 1:160. Therefore, AIH was considered low probability in the initial differential diagnosis. Ultrasound and computed tomography (CT) scan of abdomen reported normal findings except status post cholecystectomy. Iron profile showed a significantly elevated transferrin saturation of 91% [iron of 215 μg/dl/total iron binding capacity (TIBC) of 236 mg/dL] (Table 2), which was confirmed with a repeat test. The patient denied the history of diabetes mellitus, blood transfusion and family history of hemochromatosis. 2D echocardiogram and serum hemoglobin A1c were within normal limits. Magnetic resonance imaging (MRI) of the liver showed no evidence of iron overload (hemochromatosis) (Figure 1). Genetic testing for HFE gene was not carried out.
Table 1.
Basic Laboratory Data on Admission.
| S. no. | Tests | Values | Reference |
|---|---|---|---|
| 1. | CBCD | ||
| WBC | 6.79 × 103/mm3 | 4.50–10.9 | |
| RBC | 4.91 × 103/mm3 | 3.8–5.2 | |
| Hemoglobin | 15.6 g% | 12.2–15 | |
| Platelets | 180 × 103/mm3 | 130–400 | |
| ESR | 44 mm/h | 0–35 | |
| 2. | Chemistry | ||
| Sodium | 137 mmol/L | 136–145 | |
| Potassium | 4.6 mmol/L | 3.5–5.1 | |
| BUN | 11 mg/dL | 6–20 | |
| Creatinine | 0.7 mg/dL | 0.6–1.1 | |
| T. bilirubin | 10.9 mg/dL | 0.3–1.2 | |
| D. bilirubin | 7.8 mg/dL | 0.0–0.2 | |
| AST | 909 IU/L | 13–40 | |
| ALT | 826 IU/L | 7–35 | |
| ALP | 289 IU/L | 25–100 | |
| LDH | 464 IU/L | 100–190 | |
| Total protein | 8.7 g/dL | 6.4–8.3 | |
| Albumin | 3.5 g/dL | 3.4–4.8 | |
| Amylase | 134 U/L | 20–104 | |
| 3. | Basic coagulation profile | ||
| PT | 13.8 s | 9.70–13.20 | |
| INR | 1.21 ratio | 0.86–1.16 | |
| PTT | 35.3 s | 20.30–36.0 | |
Table 2.
Other Specific Laboratory Data.
| S. no. | Tests | Values | Reference |
|---|---|---|---|
| 1. | Serology/immunology tests | ||
| IgG | 2611 mg/dL | 694–1618 | |
| IgA | 492 mg/dL | 81–463 | |
| IgM | 317 mg/dL | 48–271 | |
| Immunofixation screen | Detected | Not-detected | |
| ANA screen | Positive | Negative | |
| ANA titer | 1:160 | 1:40 | |
| Anti-mitochondrial Ab | Negative | Negative | |
| Anti-smooth muscle Ab | Negative | Negative | |
| Cycl citrul peptide IgG | <16 units | <20 | |
| Double strand DNA ab | 1 IU/ml | ≤4 | |
| Liver/kid. microsom. tit. | ≤20 units | ≤20 | |
| Hepatitis (A, B, C, E) | Non-reactive | Non-reactive | |
| 2. | Other biochemical test | ||
| Iron | 215 μg/dL | 50–170 | |
| TIBC | 236 μg/dL | 240–450 | |
| Transferrin | 163 mg/dL | 215–380 | |
| Ferritin | 2463 ng/ml | 10–291 | |
| Transferrin saturation | 91% | <55% | |
| Hemoglobin A1c | 5.4% | 3.9–6.0 | |
Figure 1.
(A) Transverse section view and (B) coronal section view of MRI liver showing no evidence of a focal liver lesion and hemochromatosis.
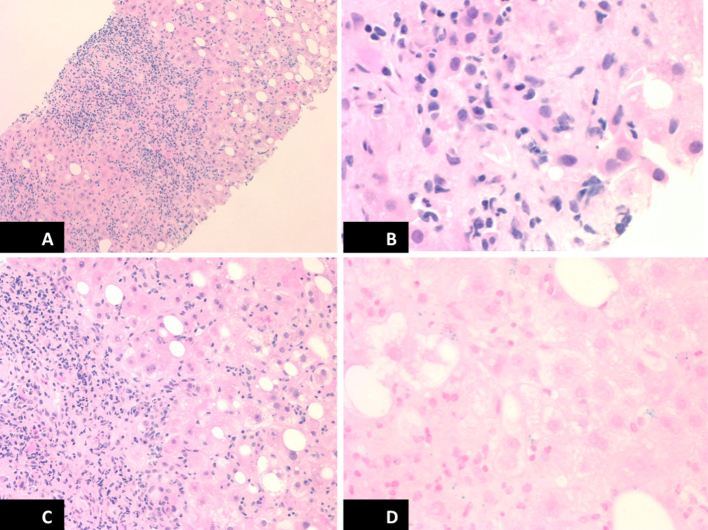
Given lacking features of hemochromatosis except for high transferrin saturation, alternative causes of hepatitis were considered and liver biopsy was performed. Liver biopsy showed dense portal inflammation with lymphocytes, plasma cells and prominent interface hepatitis with fibrosis, sparse eosinophils, interlobular spotty necrosis, diffuse hepatocellular degeneration and moderate steatosis, focal cholestasis, are apoptotic bodies, negative iron stain, which were consistent with AIH (Figure 2). The final diagnosis of AIH was made based upon simplified diagnostic criteria for AIH score of 8 (ANA titer 1:160, serum IgG 2611 mg/dL, liver histology typical of AIH and absence of viral hepatitis). The patient was immediately started on oral prednisone pulse therapy and responded well on follow up.
Figure 2.
Histopathology pictures of liver biopsy (A, B) prominent interface hepatitis with lymphocytes, plasma cell and sparse eosinophils infiltration, (C) diffuse hepatocellular degeneration on hematoxylin and eosin (H&E) stain, and (D) negative iron on iron stain.
Discussion
AIH is considered as a rare disease affecting all ages and ethnic groups with a female predominance (F:M ratio 3.6:1).2 The incidence of AIH is around 1 per 100,000 population per year.3 Given this rarity, the diagnosis can be further complicated by a similar presentation of iron overload syndrome. To our knowledge, there is only one case of AIH previously reported with a similar presentation of iron overload syndrome.4
The diagnosis of typical AIH is based on the characteristic clinical and laboratory findings with the exclusion of other causes of hepatitis. The standard laboratory assessments are elevated liver function tests (LFTs), hypergammaglobulinemia, and autoantibodies [ANA, anti-smooth muscle antibody (anti-SMA), and anti-liver kidney microsomal (anti-LKM)]. Differentials of AIH include chronic viral hepatitis (A, B, and C), primary biliary cirrhosis, primary sclerosing cholangitis, alpha-1 antitrypsin deficiency, Wilson's disease, hemochromatosis, drug-induced hepatitis [minocycline, nitrofurantoin, isoniazid, propylthiouracil (PTU), methyldopa]. Lastly, the histological findings of interface hepatitis of moderate to severe activity with or without lobular hepatitis or central portal bridging necrosis in the absence of biliary lesions or well-defined granulomas are definite features of AIH.2
Our patient presented with elevated LFTs with cholestasis picture. The patient had a transferrin saturation of more than 90%, which is considered to be pathognomonic for iron overload syndrome and is almost always found in hemochromatosis. It is known that in conditions with high levels of ferritin (but the absence of iron overload), increased serum ferritin levels are rarely associated with transferrin saturation above 55%. Serum transferrin saturation level above 55% is highly suggestive for the diagnosis of hereditary hemochromatosis (HHC) if no other causes of secondary iron overload are present.5 The patient lacked the clinical features of hemochromatosis, i.e., the absence of end-organ involvement in kidney, heart, brain and pancreas, and the MRI of the liver did not show any iron overload state. The lack of definite clue led to a need for a liver biopsy to look for an alternative diagnosis. The genetics behind the secondary iron overload remains poorly defined. More than 90% of hemochromatosis patients are homozygous in HFE-gene mutation in northern Europe.6 Heterozygosity for the HFE-gene mutation is rarely associated with liver damage due to iron alone.7 Interestingly, a number of reports suggest that the presence of heterozygous HFE-gene (C282Y) mutation is associated with increased liver iron concentration in patients with some chronic liver diseases.8 A German study showed that the prevalence of heterozygosity in HFE gene mutation was 17.2% in 64 patients with AIH.8 It would have been an academic interest to know the genetic analysis in this patient but the genetic testing for HFE gene was not pursued because of negative family history of hemochromatosis and negative iron in liver biopsy and MRI.
The mechanism of elevated transferrin saturation is not well understood in cases of AIH. However, it is possible for non-iron overload conditions to have high transferrin saturation in the presence of liver dysfunction as seen in HHC. The high transferrin saturation could be due to an increased serum iron (through hepatocellular necrosis), and a decreased transferrin synthesis (because of liver failure).9, 10, 11 Another possibility could be the dysregulation of the absorption in enterocytes.5 HHC is associated with low hepcidin level and hence consequently excessive iron release to the plasma from the enterocytes and macrophages.12 For this reason, transferrin saturation is high (>55%) with increased ferritin and non-transferrin bound iron related to toxicity to liver and other organs.13 In our case, the iron overload could have been a result of the similar mechanism such as necro-inflammatory damage to the liver causing hepatocellular necrosis and the release of serum iron to extracellular fluid and the reduction of transferrin synthesis. Nonetheless, the iron that was dispersed into the extracellular fluids and plasma does not accumulate or transport into the hepatocytes. The exact phenomenon is yet to be elucidated.
Furthermore, autoimmune diseases are usually considered in those with autoantibodies. A high ANA titer is any number greater than 1:640, while a low titer is anything less than 1:160. A titer of 1:160 can be seen in 5% of the general population.13 A high ANA titer should always increase physician's suspicious for an autoimmune disorder. In our case, the suspected iron overload condition with high transferrin saturation created a diagnostic dilemma in view of hepatitis evaluation. On top of this enigma, all the serum autoantibodies were negative (except for the low titer of ANA), which can be only seen in 7% of all AIH cases.12 The levels of autoantibody are only useful for the evaluation of disease severity and treatment response. In the cases of with negative autoantibodies are called “seronegative AIH”. These cases can present with nonstandard autoantibodies or with positive autoantibodies later in the course of the disease.12 Diagnosis of AIH becomes difficult in these cases without a positive liver biopsy. If it is recognized early, AIH is a highly treatable condition with immunosuppressive therapy. Furthermore, a reliable diagnosis of AIH can be made using simplified diagnostic criteria for AIH.14
Markedly elevated transferrin saturation can simulate iron overload syndrome, but a liver biopsy can guide physicians to navigate the correct diagnosis. It is also pertinent that clinicians do not use a low or high titer of antibody level to exclude or establish the diagnosis of AIH. When the initial workup is inconclusive in these patients, liver biopsy can guide to navigate for the diagnosis.
Consent
Informed consent is taken for publication.
Disclosure
All authors had access to the data and contributed to write the manuscript.
Conflicts of Interest
The authors have none to declare.
References
- 1.Krawitt E.L. Autoimmune hepatitis. N Engl J Med. 2006;354(1):54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 2.Czaja A.J., Freese D.K., American Association for the Study of Liver Disease Diagnosis and treatment of autoimmune hepatitis. Hepatology. 2002;36(2):479–497. doi: 10.1053/jhep.2002.34944. [DOI] [PubMed] [Google Scholar]
- 3.Jepsen P., Grønbæk L., Vilstrup H. Worldwide incidence of autoimmune liver disease. Dig Dis. 2015;33(suppl 2):2–12. doi: 10.1159/000440705. [DOI] [PubMed] [Google Scholar]
- 4.An I.C., Tiwari A.K., Ameda S., Laird-Fick H.S. Autoimmune hepatitis: diagnostic dilemma in the setting of suspected iron overload. Case Rep Gastrointest Med. 2013;2013 doi: 10.1155/2013/872987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piperno A. Classification and diagnosis of iron overload. Haematologica. 1998;83(5):447–455. [PubMed] [Google Scholar]
- 6.Feder J.N., Gnirke A., Thomas W. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 7.Bulaj Z.J., Griffen L.M., Jorde L.B., Edwards C.Q., Kushner J.P. Clinical and biochemical abnormalities in people heterozygous for hemochromatosis. N Engl J Med. 1996;335(24):1799–1805. doi: 10.1056/NEJM199612123352403. [DOI] [PubMed] [Google Scholar]
- 8.Höhler T., Leininger S., Köhler H.H., Schirmacher P., Galle P.R. Heterozygosity for the hemochromatosis gene in liver diseases—prevalence and effects on liver histology. Liver. 2000;20(6):482–486. doi: 10.1034/j.1600-0676.2000.020006482.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonkovsky H.L., Banner B.F., Lambrecht R.W., Rubin R.B. Iron in liver diseases other than hemochromatosis. Semin Liver Dis. 1996;16(1):65–82. doi: 10.1055/s-2007-1007220. [DOI] [PubMed] [Google Scholar]
- 10.Deugnier Y., Turlin B., le Quilleuc D. A reappraisal of hepatic siderosis in patients with end-stage cirrhosis: practical implications for the diagnosis of hemochromatosis. Am J Surg Pathol. 1997;21(6):669–675. doi: 10.1097/00000478-199706000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Deiss A. Iron metabolism in reticuloendothelial cells. Semin Hematol. 1983;20(2):81–90. [PubMed] [Google Scholar]
- 12.Camaschella C. Understanding iron homeostasis through genetic analysis of hemochromatosis and related disorders. Blood. 2005;106(12):3710–3717. doi: 10.1182/blood-2005-05-1857. [DOI] [PubMed] [Google Scholar]
- 13.Camaschella C., Poggiali E. Towards explaining “unexplained hyperferritinemia”. Haematologica. 2009;94(3):307–309. doi: 10.3324/haematol.2008.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennes E.M., Zeniya M., Czaja A.J. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48(1):169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]