Abstract
Background and Aims
The nature of cerebral edema in acute-on-chronic liver failure (ACLF) is not well studied. We aimed to characterize cerebral edema in ACLF using magnetization transfer ratio (MTR) and diffusion tensor imaging (DTI).
Methods
Forty-six patients with cirrhosis and acute decompensation were included. Patients were divided into groups A (no cerebral failure, n = 39) and B (cerebral failure, n = 7). Group A was subdivided into no-ACLF (n = 11), grade 1 (n = 10), grade 2 (n = 9) and grade 3 (n = 9) ACLF as per CANONIC study. MRI brain and plasma TNF-alpha, IL-1beta and IL-6 were measured at baseline and 7–10 days after admission. Ten age- and sex-matched healthy controls were also included.
Results
Mean diffusivity (MD) values, an MRI marker of water content, progressively increased from controls to no-ACLF to ACLF grade 1, 2 and 3 in group A in frontal white matter (FWM) and basal ganglia (P < 0.0001). MD values improved only in survivors on follow-up. MD values correlated with IL-6 levels at baseline. On multivariate analysis MELD score ≥28 and MD values (>8 × 10−9 M2/s) in FWM were independent predictors of 90-day mortality. There was no significant difference in clinical and MRI parameters between group A and B.
Conclusion
Cerebral edema increases with severity of ACLF. Correlation between MD values and IL-6 levels suggests pathogenic role of inflammation in cerebral edema. Patients with grade 3 ACLF have cerebral edema irrespective of presence of clinically evident cerebral failure. MELD score and cerebral edema have prognostic significance in ACLF.
Abbreviations: ACLF, acute-on-chronic liver failure; AIH, autoimmune hepatitis; ALIC, anterior limb of internal capsule; APASL, Asian pacific association for study of liver diseases; AUROC, area under receiver operating characteristic; BG, basal ganglia; BBB, blood–brain barrier; CANONIC, chronic liver failure (CLIF) acute-on-chronic liver failure in cirrhosis; CI, confidence interval; CLIF-SOFA, chronic liver failure-sequential organ failure assessment; CTP, Child–Turcott–Pugh; DTI, diffusion tensor imaging; FA, fractional anisotropy; FLAIR, fluid attenuation inversion recovery; FWM, frontal white matter; HBV, hepatitis B virus; HE, hepatic encephalopathy; IC, internal capsule; IL-1 beta, interleukin 1 beta; IL-6, interleukin 6; MD, mean diffusivity; MELD, model for end-stage liver disease; MRI, magnetic resonance imaging; MTR, magnetization transfer ratio; PLIC, posterior limb of internal capsule; PWM, parietal white matter; ROI, regions of interest; SIRS, systemic inflammatory response syndrome; T1W, T1 weighted; T2W, T2 weighted; TE, echo-time; TR, repetition time; TNF-alpha, tumor necrosis factor-alpha
Keywords: cerebral edema, acute-on-chronic liver failure, magnetic resonance imaging, diffusion tensor imaging
In chronic liver disease, raised blood ammonia levels cause intracellular accumulation of glutamine which disturbs brain cell volume homeostasis. Subsequently depletion of myoinositol tries to maintain intracellular osmolytes balance and reduces or limits the cellular swelling.1 This is reflected as low-grade astrocyte swelling which manifests as clinical or minimal hepatic encephalopathy. The conventional magnetic resonance (MR) imaging lacks the sensitivity for detection of mild and diffuse changes in brain water content. However, advanced MRI techniques like magnetization transfer ratio (MTR), diffusion tensor imaging (DTI) and diffusion weighted imaging (DWI) are sensitive to changes in increased brain water content and also help in differentiating the intracellular and extracellular component of brain edema. Magnetization transfer imaging has shown decrease of magnetization transfer ratio (MTR) in normal-appearing white matter and normal-appearing gray matter regions in patients with cirrhosis.2 MTR and fast fluid attenuation inversion recovery (FLAIR) sequences do point to mild brain edema but are unable to distinguish whether the edema is intracellular or extracellular. DTI assesses different parameters like mean diffusivity (MD), a marker of water movement across cell membranes thereby assessing interstitial edema, and fractional anisotropy (FA), an index of microstructural integrity of normal appearing white matter hence assessing demyelination and axonal loss.3
Systemic inflammation is induced by infection and hepatocyte cell death in liver failure. Therefore, systemic and central inflammatory cytokines acting alone or in combination with ammonia result in neuroinflammation. The raised cytokine levels can cross the blood brain barrier directly, cause microglial activation, which further leads to monocyte recruitment and increased cytokine gene expression in brain.4, 5 There is convincing evidence that neuroinflammation has a role in pathogenesis of encephalopathy in liver failure. The severity of edema seems to differ according to duration of liver failure and degree of hyperammonemia. Chronic liver failure induces low-grade interstitial brain edema and any acute insult resulting in systemic inflammation may cause increased neuroinflammation and resultant encephalopathy.6
As per chronic liver failure (CLIF) acute-on-chronic liver failure in cirrhosis (CANONIC) study, presence of organ failure and increased 28-day mortality in patients of cirrhosis with acute decompensation has been used to define acute-on-chronic liver failure (ACLF).7, 8 We have demonstrated that sepsis is the most common cause of acute decompensation in cirrhosis.9 Cerebral changes, including the degree and type of brain edema, and their pathophysiology, including the role of systemic inflammation, have not been well characterized in ACLF population.
We aimed to study various brain magnetic resonance imaging (MRI) findings [T1 weighted (T1W), T2 weighted (T2W), FLAIR, MTR and DTI] and inflammatory cytokines (TNF-alpha, IL-1beta, IL-6) in patients with different grades of ACLF and explore their role as prognostic indicator(s) of mortality along with other clinical parameters. We also compared clinical and MRI parameters between patients with grade 3 ACLF with and without cerebral failure (presence of grade III/IV hepatic encephalopathy).
Methods
Study Conduct
The Ethics Committee of Postgraduate Institute of Medical Education and Research (PGIMER), a tertiary level health care center in Chandigarh, India, approved the study (letter no. 1TRG/PG-2012/21508-520 dated 8/11/13). Each subject or their legal representatives gave written informed consent before being included into the study. The guidelines laid down by Indian Council of Medical Research (1994) and Helsinki declarations (modified 1989) were adhered to in all patients in the study. The authors had access to all study data, and reviewed and approved the final manuscript.
Patients
Eligible patients included consecutive inpatients from Department of Hepatology, PGIMER with acute decompensation of cirrhosis, defined as per Moreau et al.7 Exclusion criteria were as follows: those who refused informed consent, any neurologic diseases such as Alzheimer's disease, Parkinson's disease or stroke, chronic kidney disease, cardiopulmonary disease, pregnancy, contra-indications to MRI, and hepatocellular carcinoma.
Controls
Ten age- and sex-matched healthy subjects [mean age (range)—controls 40.2 (28–58) years, patients 41.6 (24–70) years; male: female—control 9:1, patients 35:4] were also included in study to serve as controls for MTR and DTI and for tumor necrosis factor-alpha (TNF-alpha), interleukin-1 beta (IL-1 beta) and interleukin-6 (IL-6) measurement.
Definitions
Cirrhosis of Liver
The diagnosis of cirrhosis of liver was based on liver biopsy if available or clinical, imaging, laboratory and endoscopic findings.9
Acute Decompensation
The CANONIC study criteria were used to define acute decompensation, which included acute development of ascites, hepatic encephalopathy (HE), gastrointestinal bleeding and bacterial infections.7 Alcohol consumption in last 3 months was considered as active alcoholism.7
Acute-on-Chronic Liver Failure
The CLIF-SOFA score was applied to define and grade severity of ACLF based on organ failures.7 Liver failure was defined as serum bilirubin ≥12.0 mg/dL, kidney failure as serum creatinine ≥2.0 mg/dL or patient requiring renal replacement therapy, cerebral failure as presence of grade III/IV HE defined by West Haven classification,10 coagulation failure as international normalized ratio ≥2.5 and/or platelet count ≤20,000/mm3, circulatory failure as patient requiring vasopressors to maintain blood pressure and respiratory failure as PaO2:FiO2 ≤200 or a SpO2:FiO2 ≤214 or requiring mechanical ventilation. Cerebral failure was defined as presence of grade III/IV hepatic encephalopathy.
Clinical and Laboratory Assessment
Clinical examination included a thorough general physical and systemic examination. Laboratory investigations included hemogram, serum electrolytes, renal and liver function tests and coagulogram. All patients underwent etiological work-up for cirrhosis and acute decompensation. Severity of cirrhosis was determined by Child–Turcotte–Pugh's (CTP) and model for end-stage liver disease (MELD) score. TNF-alpha, IL-1 beta, and IL-6 were measured in plasma using specific enzyme-linked immunosorbent assay kits (RayBiotech, Inc, Norcross, GA) according to manufacturer's protocol. The plate was read at 450 nm. Absorbance was converted to picograms per milliliter using a standard curve prepared with recombinant human TNF-alpha, IL-1 beta and IL-6.
Neuroimaging
Whole brain conventional MR imaging was performed on either a 3.0 Tesla Verio MR scanner (Siemens Medical Solutions, Erlangen, Germany) or on 3.0 Tesla Discovery MR750 scanner (GE medical systems, Milwaukee, USA) using standard 16/32 channel head coils. Apart from conventional sequences T2W, FLAIR and T1W volumetric sequences (MPRAGE/SPGR BRAVO), additional sequences performed were MTR and DTI. The MR findings were analyzed by two observers (CKA, NKal and NKha) blinded to clinical and biochemical characteristics and levels of inflammatory mediators.
T2W images [repetition time (TR) = 9000 ms; echo-time (TE) = 94 ms; matrix size = 320 × 320; field of view = 220 mm × 220 mm; slice thickness = 4.0 mm; interslice gap = 0.0 mm; turbo factor = 5; flip angle = 158°] and FLAIR images were acquired in axial plane covering whole brain. High-resolution T1W were collected using SPGR or MPRAGE sequence (TR = 1800 ms; TE = 3.87 ms; inversion time = 900 ms; FA = 9°; matrix size = 256 × 100; FOV = 230 × 230 mm; slice thickness = 1 mm and transverse imaging plane).
MTR was performed in transverse plane before and after application of proton saturation pulse and quantified as percentage of signal loss according to following equation: MTR = 100(S0 − SS)/S0 in which S0 is mean signal intensity for a particular region without saturation pulse and SS with saturation pulse respectively. The DTI sequence was run in axial plane using echoplanar imaging technique (TR = 3900 ms, TE = 108 ms, FoV = 220 × 220, matrix = 128 × 128, slice thickness = 4 mm with no inter slice gap). The MTR, MD and FA values were determined in regions of interest (ROI) placed in right and left frontal white matter (FWM), parietal white matter (PWM), internal capsule (IC) [average of anterior limb (ALIC), genu and posterior limb (PLIC) on each side]; and basal ganglia (BG). All values were reported as mean of right and left ROIs. ROIs size was kept constant for all regions.
Management of Patients
All patients were managed as per protocol described.8 All patients were screened for presence of infections at admission and periodically thereafter as per required. Intravenous broad spectrum antibiotics targeting gram-negative microbes were started at admission and modified according to culture reports and clinical response of patient. Renal failure, HE and ascites were managed as per standard protocols.10, 11 Patients with multiorgan failure or requiring cardiopulmonary support were managed in liver intensive care unit. Specific therapy included oral pentoxifylline 400 mg thrice daily for alcoholic hepatitis, HBV flare and AIH were managed as per AASLD guidelines.12, 13 No experimental therapy was given during the period of study and none of the patients underwent liver transplantation.
Study Design
Patients with cirrhosis with acute decompensation who fulfilled inclusion criteria were prospectively included into the study. All blood investigations including hemogram, biochemistry, coagulogram, TNF-alpha, IL-1 beta, IL-6 measurements and MRI (T1W, T2W, FLAIR, MTR, DTI) were performed in all patients on admission. They were then divided into 2 groups—groups A (no cerebral failure) and B (cerebral failure). All patients were managed according to standard protocol as described above. All investigations were repeated between 7th and 10th day after enrollment into the study in all survivors.
Statistical Analysis
Continuous data were expressed as mean [95% confidence interval (CI)] or range and categorical data as frequency (%). For a comparison of categorical variables, χ2 and Fisher exact tests were used; for continuous variables, Mann–Whitney U test for unpaired data was used. Normative data among various groups was analyzed by one-way ANOVA and post hoc analysis was done by Bonferroni test. Comparison between baseline and follow-up data was done by paired t test. Univariate analysis was done for prediction of 90-day mortality using clinical and MRI parameters and proinflammatory cytokines. Variables with P < 0.1 were entered into multivariate analysis and backward conditional logistic regression analysis was done to identify independent prognostic markers. Accuracy of these parameters was assessed using area under receiver operating characteristic (AUROC). The probability level of <0.05 was set for statistical significance. SPSS 21 (SPSS Inc, Chicago, IL) was used for statistical computations.
Results
Study Patients
Between 1 July 2013 and 31 December 2014, 78 patients with acute decompensation were screened. Forty-six (58.9%) patients who met eligibility criteria were included in study. Reasons that 32(41%) patients were excluded from study are shown in Figure 1. The patients were divided into two groups; group A (without cerebral failure, n = 39) and B (with cerebral failure, n = 7).
Figure 1.
Flow of patients in the study. Group A (without cerebral failure) and group B (with cerebral failure). HE, hepatic encephalopathy; ACLF, acute-on-chronic liver failure.
All patients in group B had ACLF grade 3. Group A was divided into no ACLF (n = 11), grade 1 (n = 10), grade 2 (n = 9) and grade 3 (n = 9) ACLF. All patients in-group B had grade 3 ACLF. The clinical and demographic characteristics of patients included in study are shown in Table 1. The predominant etiology of chronic liver disease in group A was alcohol followed by chronic hepatitis C, chronic hepatitis B, autoimmune hepatitis, Wilson disease and cryptogenic cirrhosis in 26 (66.7%), 3 (7.7%), 3 (7.7%), 3 (7.7%), 2 (5.1%) and 3 (7.7%) patients respectively (Supplementary Table 1).
Table 1.
Clinical and Demographic Characteristics of Patients in Group A (n = 39).
| Variable | aNo ACLF (n = 11) | bACLF Grade-1 (n = 10) | cACLF Grade-2 (n = 9) | dACLF Grade-3 (n = 9) | P value |
|---|---|---|---|---|---|
| Age (years) | 44.7* (26–56) | 44.6 (29–70) | 41.4 (26–55) | 41.7 (24–58) | Pabcd = NS |
| Male sex | 9† (81.8%) | 8 (80%) | 9 (100%) | 9 (100%) | Pabcd = NS |
| CTP score | 11.2 (7–14) | 10.5 (7–13) | 11.2 (9–13) | 12.2 (11–14) | Pabcd = NS |
| MELD score | 21.6 (16–31) | 20.6 (16–29) | 33.3 (30–37) | 37.2 (29–50) | Pac,ad,bc,bd = 0.001 |
| CLIF-SOFA score | 6.6 (5–8) | 8.3 (4–11) | 11.2 (8–17) | 12.1 (10–16) | Pac,ad,bd = 0.001, Pbc = 0.03 |
| APACHE II | 4.5 (3–9) | 10.7 (3–25) | 10.7 (4–20) | 14.1 (6–19) | Pad = 0.004 |
| 28-Day mortality | 0 | 0 | 3 (30%) | 5 (55.5%) | Pad,bd = 0.01 |
| 90-Day mortality | 1 (9%) | 2 (20%) | 6 (60%) | 9 (100%) | Pac = 0.005, Pad,bd < 0.0001, Pbc = 0.041 |
| TLC (103/mm3) | 10.9 (6.3–21.5) | 10.5 (5.9–30.6) | 11.4 (4–20.9) | 12.7 (2.7–32) | NSabcd |
| Bilirubin (mg/dL) | 9.8 (2.7–28.6) | 4.9 (1.1–12) | 19.7 (11.3–30.8) | 20.6 (7.3–35.9) | Pac,ad = 0.03, Pbc,bd = 0.001 |
| Platelet (103/mm3) | 103 (14.4–172) | 116 (40–369) | 110 (14.8–231) | 112 (14–225) | NSabcd |
| INR | 1.8 (1.6–2.4) | 2.1 (1.2–4.4) | 3.2 (1.6–5.2) | 2.8 (1.8–3.5) | Pac = 0.001, Pbc = 0.007 |
| Serum creatinine | 0.8 (0.4–1.3) | 2.5 (1–8.9) | 1.3 (0.6–3) | 2.7 (2.1–4.4) | Pab = 0.03, Pad = 0.01 |
Different groups have been identified by the superscript alphabets and it makes easy to give description about P value.
Mean (95% confidence interval).
N (%). ACLF, acute-on-chronic liver failure; CTP, Child–Turcotte–Pugh, MELD, model for end stage liver disease; CLIF-SOFA, chronic liver failure—sequential organ failure assessment score; APACHE II, acute physiology and chronic health evaluation II.
Inflammatory Cytokines
There were no significant differences among controls and patients with ACLF and no-ACLF in inflammatory cytokine levels studied except that IL-6 levels were significantly higher in ACLF grade 3 patients compared to controls in group A (Supplementary Table 2).
Magnetic Resonance Imaging
There were no significant differences in findings on T1W, T2W and FLAIR between various groups.
Magnetization Transfer Imaging
MTR values from FWM, PWM, PLIC, ALIC, genu and BG were significantly different between control and group A or group B (Supplementary Table 3). However, no difference could be found between no-ACLF and ACLF groups and also between various grades of ACLF.
Mean Diffusivity
MD values showed significant differences between control and various grades of ACLF in FWM, PWM and BG in group A (Table 2). MD values were significantly higher among patients with different grades of ACLF compared to controls and patients with no-ACLF (Table 2, Figure 2). There was positive correlation between MD values of PWM and IL-6 levels (r = 0.5, P = 0.005), MD values of FWM and MELD (r = 0.2, P = 0.005), CLIF-SOFA (r = 0.5, P = 0.001) and APACHE II (r = 0.4, P = 0.017) and MD values of basal ganglia and MELD (r = 0.5, P = 0.001), CLIF-SOFA (r = 0.7, P < 0.001) and APACHE II (r = 0.6, P < 0.001) respectively.
Table 2.
Baseline Mean Diffusivity Values in Controls and Various Grades of ACLF in Group A (n = 39).
| Mean diffusivity (×10−9 M2/s) | aControl (n = 10) | bNo ACLF (n = 11) | ACLF |
P value | ||
|---|---|---|---|---|---|---|
| cGrade 1 (n = 10) | dGrade 2 (n = 9) | eGrade 3 (n = 9) | ||||
| FWW | 7.4 (7.3–7.4) | 7.8 (7.6–8) | 8.4 (6.8–9.2) | 8.7 (7.8–9.4) | 9.1 (8.3–9.7) | Pac,ad,ae < 0.0001, Pbd = 0.002, Pbe = <0.0001, Pce = 0.001 |
| PWM | 7.3 (7.2–7.4) | 8.1 (7.5–8.8) | 8.4 (7.7–9.1) | 8.3 (7.4–9.1) | 8.6 (8.2–9) | Pab,ac,ad,ae = <0.0001, Pbe = 0.001 |
| ALIC | 7.3 (7.2–7.4) | 7.6 (7.2–8) | 8.1 (7.6–9) | 7.9 (7.3–9.6) | 7.9 (7.4–8.5) | Pad = 0.01 |
| Genu | 7.2 (7.2–7.4) | 7.6 (7–8.3) | 7.7 (7–8.5) | 8.2 (7.2–9.1) | 8.5 (7–9.9) | Pad,ce = 0.002, Pae,be = <0.0001 |
| PLIC | 7.2 (7.2–7.4) | 7.5 (6.9–8) | 7.6 (6.3–8.2) | 7.8 (7.2–9) | 7.9 (7.1–9.4) | Pae = NS |
| BG | 7.3 (7.2–7.4) | 7.9 (7.4–8.4) | 8.6 (8.2–9.1) | 9.3 (8.8–9.6) | 9.6 (9.3–10.2) | Pab,ac,ad,ae = <0.0001, Pbc,bd,be = <0.0001, Pcd,ce = <0.0001 |
Different groups have been identified by the superscript alphabets and it makes easy to give description about P value.
*Mean (95% confidence interval). ACLF, acute-on-chronic liver failure; FWM, frontal white matter; PWM, parietal white matter; ALIC, anterior limb of internal capsule; PLIC, posterior limb of internal capsule; BG, basal ganglia.
Figure 2.
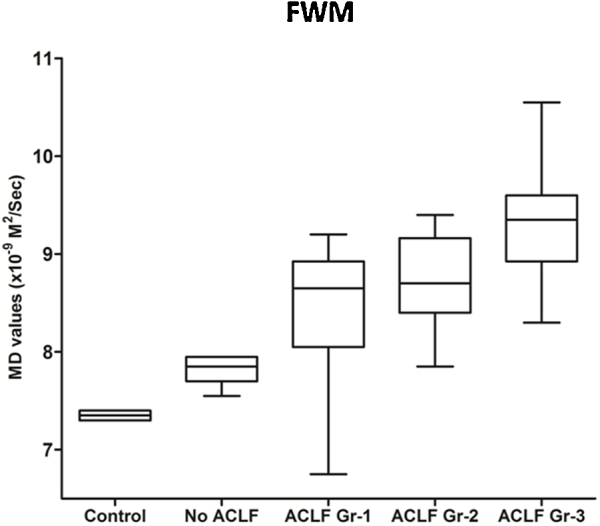
Box plot diagram of baseline MD values in frontal white matter in control, no ACLF and ACLF grade 1, 2 and 3 groups.
Fractional Anisotropy
FA values were not different among various groups (Supplementary Table 4).
Follow-up Analysis
Out of 39 patients in group A, follow-up MRI and cytokine analysis was done in 35 patients as 4 patients died before 7 days, after a mean of 8.5-days (range 7–11) of baseline study. There was significant improvement in MD values in various ROI only in patients who had survived, suggesting survival significance of this parameter (Supplementary Table 5). IL-6 decreased significantly on follow-up [179.9 pg/mL (5.4–690.7) versus 125.5 pg/mL (4.3–4934), P = 0.032], while there was no change in IL-1beta and TNF-alpha.
Factors Affecting 90-Day Survival
Univariate Analysis
All baseline parameters were compared between survivors (n = 21) and non-survivors (n = 18) at 90-days. On univariate analysis MELD, CLIF-SOFA, APACHE II, MD values in FWM, genu, PLIC and BG were significantly higher in non-survivors compared to survivors in group A (Table 3).
Table 3.
Univariate Analysis of Clinical and MRI Parameters Between Survivors and Non-Survivors in Group A (n = 39).
| Variable | Survivors (n = 21) | Non-survivors (n = 18) | Odds ratio | 95% CI | P value |
|---|---|---|---|---|---|
| Age (years) | 43.6 (26–58)* | 41.6 (24–70) | 0.965 | 0.896–1.041 | 0.481 |
| MELD | 22.8 (16–25) | 33.7 (17–50) | 1.29 | 1.115–1.512 | 0.001 |
| CLIF-SOFA | 7.7 (4–11) | 11.2 (6–17) | 1.777 | 1.226–2.577 | 0.002 |
| APACHE II | 7.7 (3–25) | 11.2 (3–27) | 1.153 | 1.024–1.299 | 0.028 |
| MD value (×10−9 M2/s) | |||||
| FWM | 8.2 (7.8–8.7) | 8.9 (7.3–10.8) | 7.858 | 2.079–29.698 | 0.002 |
| PWM | 8.3 (7.5–8.9) | 8.3 (7.8–10.9) | 1.126 | 0.273–4.642 | 0.535 |
| ALIC | 7.8 (7.1–8.7) | 7.9 (7.2–8.9) | 1.216 | 0.297–4.96 | 0.588 |
| Genu (IC) | 7.7 (7.1–8.5) | 8.3 (7.6–9.8) | 3.852 | 1.168–12.710 | 0.027 |
| PLIC | 7.5 (7.2–7.9) | 7.9 (7.1–10.8) | 3.718 | 0.977–14.149 | 0.04 |
| BG | 8.4 (8.1–8.6) | 9.2 (8.3–9.5) | 4.483 | 1.483–13.557 | 0.008 |
Mean (95% confidence interval). MELD, model for end stage liver disease; CLIF-SOFA, chronic liver failure sequential organ failure assessment score; APACHE II, acute physiology and chronic health evaluation II; MD, mean diffusivity; FWM-frontal white matter; PWM-parietal white matter; ALIC-anterior limb of internal capsule IC, internal capsule; PLIC, posterior limb of internal capsule; BG, basal ganglia.
Multivariate Analysis
Factors found significant in univariate analysis were included in multivariate analysis (Table 4). Baseline MELD score (P < 0.0001) and MD values in FWM (P = 0.001) were independent predictors of mortality. These two parameters had highest AUROC to predict 90-day mortality (Supplementary Figure 1). Further analysis demonstrated that combined MELD and MD FWM has sensitivity of 88.9% with 90.4% specificity resulting in highest diagnostic accuracy of 89.7% (Table 5).
Table 4.
Adjusted Odds Ratio of Effects of MELD, CLIF-SOFA, APACHE II, MD FWM, MD Genu (IC), MD PLIC and MD BG on 90-Day Mortality in Group A.
| Variable | Adjusted odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| MELD | 1.207 | 1.041–1.4 | 0.01 |
| CLIF-SOFA | 1.191 | 0.66–2.334 | 0.562 |
| APACHE II | 0.932 | 0.772–1.244 | 0.468 |
| MD FWM | 4.314 | 1.2941–17.234 | 0.04 |
| MD Genu (IC) | 1.047 | 0.077–10.142 | 0.973 |
| MD PLIC | 1.43 | 0.151–7.45 | 0.755 |
| MD BG | 0.594 | 0.05–23.1 | 0.669 |
*Mean (95% confidence interval). MELD, model for end stage liver disease; CLIF-SOFA, chronic liver failure sequential organ failure assessment score; APACHE II, acute physiology and chronic health evaluation II; FWM, frontal white matter; PLIC, posterior limb of internal capsule; BG, basal ganglia; MD, mean diffusivity; MELD, model for end stage liver disease; IC, internal capsule.
Table 5.
Utility of MELD and Mean Diffusivity (MD) Values (×10−9 M2/s) as Predictors of 90-Day Mortality in Group A.
| Variable | Cut off | N | Death (N) | Sensitivity | Specificity | PPV | NPV | Diagnostic accuracy |
|---|---|---|---|---|---|---|---|---|
| MELD | ≥28 | 21 | 16 | 89% | 76.2% | 76.1% | 88.9% | 82.1% |
| MD FWM | >8 | 27 | 17 | 89% | 52.3% | 62.9% | 91.6% | 71.7% |
| MELD + MD FWM | ≥28/>8 | 18 | 16 | 88.9% | 90.4% | 88.9% | 90.4% | 89.7% |
*Mean (95% confidence interval). MELD, model for end stage liver disease; MD, mean diffusivity; FWM, frontal white matter; PPV, positive predictive value; NPV, negative predictive value; AUROC, area under receiver operating curve.
Effect of cerebral failure in ACLF Grade 3
All patients with cerebral failure (Group B) had ACLF grade 3. There was no significant difference in various prognostic scores or MD values between ACLF grade 3 without cerebral failure and group B (Supplementary Table 6).
There was no significant difference in MTR, MD and FA values between ACLF patients with or without HE (Supplementary Table 7).
Discussion
This study demonstrated presence of cerebral edema in patients of ACLF, which increased in severity with increasing grades of ACLF as demonstrated on MD values. Multivariate analysis further showed that high MELD score and MD values on admission were independent predictors of 90-days mortality. Patients with grade 3 ACLF had no significant difference in cerebral edema or prognostic scores irrespective of presence or absence of clinically evident cerebral failure (grade III/IV hepatic encephalopathy).
In patients of ACLF, there are two components—underlying cirrhosis and superimposed acute component. While there is sufficient time for osmolytes/water to shift between intra-and extracellular compartments in brain resulting in osmotic balance in cirrhosis, any superimposed insult by virtue of neuroinflammation worsens the cerebral edema. Two components of cerebral edema are cytotoxic and vasogenic. Cytotoxic edema is mainly seen in ALF leading to astrocyte swelling and decreased MD values whereas vasogenic edema is seen in early part of ALF and cirrhosis resulting in increased MD values. In this study, both ACLF and no-ACLF groups demonstrated low MTR compared to healthy controls, suggesting presence of cerebral edema. We have previously demonstrated utility and clinical significance of MTR in detecting subclinical cerebral edema in cirrhosis and minimal HE.14 However, MTR values were not statistically different between grades of ACLF because while MTR indicates increase in brain-water content,2 it does not differentiate between intra-and extracellular water which may be differentially altered in various types of liver failure. To further analyze this, we studied MD values, which differed not only between patients with no-ACLF and ACLF but also between different grades of ACLF with a group-wise significant increment starting from controls to no ACLF group, to ACLF grade 1 and to grade 3, suggesting gradual increase in extracellular (vasogenic) edema with increasing severity of ACLF (Table 2) rather than intracellular (cytotoxic) edema. The normal FA values in controls and patients suggest that microstructural integrity of white matter is intact.15 Kale et al.3 also showed increased MD values and unchanged FA values in patients of cirrhosis with HE or minimal HE. Brain edema has also been demonstrated in animal models of ACLF.16 However, an experimental model of ACLF by Chavarria et al. using bile duct ligated rats challenged with lipopolysaccharide did not find increased diffusion values despite showing increased brain water content, suggesting that cerebral edema may be mixed intra- and extra-cellular.17 However, they performed MRI acutely within 3 h of lipopolysaccharide infusion when cytotoxic injury is likely to be highest, whereas in the clinical scenario MRI is done much later after onset of ACLF. A previous study has documented both extra- and intracellular water in cerebral edemain patients with ACLF.18 The predominant vasogenic edema observed by us may be due to blood brain barrier (BBB) disruption and neuroinflammation due to concomitant sepsis and cytokine storm seen in real life ACLF.
MD values increase with grades of ACLF in group A despite no clinically apparent cerebral failure. Also, patients with grade 3 ACLF had high MD values and poor prognosis and MD values and prognosis were not different between those with (Group B) and without clinically apparent cerebral failure. This suggests that even in the absence of cerebral failure there is presence of significant cerebral edema in ACLF which increases with the grade of ACLF and may have an impact on mortality. This is further borne out by the fact that follow-up imaging demonstrated improvement in MD values only in survivors reflecting its prognostic significance (Table 3). Two recent studies have demonstrated that multi-organ failure scores like CLIF-SOFA and APACHE II better predict 28-day mortality than liver specific scores.8, 19 This is likely due to multi-system involvement secondary to SIRS in ACLF. In our study MD value in FWM were found to be independent predictor of 90-day mortality, superior to multi-organ failure scores. Combination of MELD and MD values of FWM had best AUROC for predicting 90-day mortality suggesting that intra-cerebral changes influence mortality over and above liver function scores. A recent study has shown that patients of ACLF with HE had higher mortality than those without20, 21 unlike our study. This study was different from the present study as they included all grades of HE, whereas we compared those with and without cerebral failure (grade III/IV HE).
It has been shown that cytokines mediate neuroinflammation resulting in cerebral edema.5, 22, 23, 24 We found significantly elevated IL-6 levels in ACLF grade 3 and it positively correlated with MD values, suggesting increasing levels of IL-6 were associated with increasing severity of cerebral edema. This suggests that cerebral edema may be related to systemic inflammation. We also showed higher levels of IL-1 beta and TNF-alpha in ACLF patients compared to control and no-ACLF groups, however differences were not statistically significant, which is likely to be due to small number of patients in each group or possibly a differential effect of various cytokines in neuroinflammation. A previous study has also shown that inflammation and not ammonia is associated with higher grades of HE in cirrhosis.25
This study is limited by the small sample size and inability to perform MRI in some patients of grade 3 ACLF with cerebral failure as they were very sick and hence had to be excluded from the study. Also, there was no way for us to differentiate MRI changes due to septic and hepatic encephalopathy as encephalopathy is multifactorial in these patients.
In conclusion, this study has demonstrated that in patients with ACLF there is evidence of cerebral edema on DTI metrics and MTR sequences which worsens with increasing severity of ACLF. This is irrespective of the presence of clinically appreciable encephalopathy or cerebral failure. MD values correlate with IL-6 suggesting role of neuroinflammation. MELD score and MD values in FWM are predictors of 90-day mortality in patients with ACLF. Further prospective studies are required to validate these findings and to elucidate relationship between systemic inflammation, nature of cerebral edema and mortality in ACLF patients, which will allow for development of targeted therapeutic strategies.
Guarantor of Study
RKD accepts full responsibility for the conduct of the study and had access to the data and had control of the decision to publish.
Author Contributions
RKD conceived the idea for the study, wrote the protocol, performed analyses, interpreted data, and prepared the manuscript. TG wrote the protocol, performed analyses, interpreted data, and prepared the manuscript. CKA and NKha performed MRI, interpreted data, and assisted in preparing the manuscript. SA assisted in data analyses, interpreting data, and in preparing the manuscript. MC performed laboratory experiments. ST, AD and YC were involved in patient care management and assisted in the interpretation of the data and in preparing the manuscript.
Conflicts of Interest
The authors have none to declare.
Acknowledgment
Radha K. Dhiman presented this study as oral presentation at the Annual Meeting of the American Association for the Study of Liver Disease in San Francisco in November 2015.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jceh.2017.04.001.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Butterworth R.F., Giguere J.F., Michaud J., Lavoie J., Layrargue G.P. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochem Pathol. 1987;6:1–12. doi: 10.1007/BF02833598. [DOI] [PubMed] [Google Scholar]
- 2.Rovira A., Grive E., Pedraza S., Alonso J. Magnetization transfer ratio values and proton MR spectroscopy of normal-appearing cerebral white matter in patients with liver cirrhosis. AJNR Am J Neuroradiol. 2001;22:1137–1142. [PMC free article] [PubMed] [Google Scholar]
- 3.Kale R.A., Gupta R.K., Saraswat V.A. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology. 2006;43:698–706. doi: 10.1002/hep.21114. [DOI] [PubMed] [Google Scholar]
- 4.Butterworth R.F. The liver–brain axis in liver failure: neuroinflammation and encephalopathy. Nat Rev Gastroenterol Hepatol. 2013;10:522–528. doi: 10.1038/nrgastro.2013.99. [DOI] [PubMed] [Google Scholar]
- 5.Aldridge D.R., Tranah E.J., Shawcross D.L. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5:S7–S20. doi: 10.1016/j.jceh.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee G.H. Hepatic encephalopathy in acute-on-chronic liver failure. Hepatol Int. 2015;9:520–526. doi: 10.1007/s12072-015-9626-0. [DOI] [PubMed] [Google Scholar]
- 7.Moreau R., Jalan R., Gines P. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. 1437.e1–9. [DOI] [PubMed] [Google Scholar]
- 8.Olson J.C., Kamath P.S. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care. 2011;17:165–169. doi: 10.1097/MCC.0b013e328344b42d. [DOI] [PubMed] [Google Scholar]
- 9.Dhiman R.K., Agrawal S., Gupta T., Duseja A., Chawla Y. Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J Gastroenterol. 2014;20:14934–14941. doi: 10.3748/wjg.v20.i40.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blei A.T., Cordoba J. Hepatic encephalopathy. Am J Gastroenterol. 2001;96:1968–1976. doi: 10.1111/j.1572-0241.2001.03964.x. [DOI] [PubMed] [Google Scholar]
- 11.Moore K.P., Wong F., Gines P. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 12.Lok A.S., McMahon B.J. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 13.Hennes E.M., Zeniya M., Czaja A.J. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 14.Rai R., Ahuja C.K., Agrawal S. Reversal of low-grade cerebral edema after lactulose/rifaximin therapy in patients with cirrhosis and minimal hepatic encephalopathy. Clin Transl Gastroenterol. 2015;6:e111. doi: 10.1038/ctg.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rugg-Gunn F.J., Eriksson S.H., Symms M.R. Diffusion tensor imaging in refractory epilepsy. Lancet. 2002;359:1748–1751. doi: 10.1016/S0140-6736(02)08615-4. [DOI] [PubMed] [Google Scholar]
- 16.Wright G., Sharifi Y., Jover-Cobos M., Jalan R. The brain in acute on chronic liver failure. Metab Brain Dis. 2014;29:965–973. doi: 10.1007/s11011-014-9553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chavarria L., Oria M., Romero-Gimenez J., Alonso J., Lope-Piedrafita S., Cordoba J. Brain magnetic resonance in experimental acute-on-chronic liver failure. Liver Int. 2013;33:294–300. doi: 10.1111/liv.12032. [DOI] [PubMed] [Google Scholar]
- 18.Nath K., Saraswat V.A., Krishna Y.R. Quantification of cerebral edema on diffusion tensor imaging in acute-on-chronic liver failure. NMR Biomed. 2008;21:713–722. doi: 10.1002/nbm.1249. [DOI] [PubMed] [Google Scholar]
- 19.Duseja A., Choudhary N.S., Gupta S., Dhiman R.K., Chawla Y. APACHE II score is superior to SOFA, CTP and MELD in predicting the short-term mortality in patients with acute-on-chronic liver failure (ACLF) J Dig Dis. 2013;14:484–490. doi: 10.1111/1751-2980.12074. [DOI] [PubMed] [Google Scholar]
- 20.Shawcross D.L., Sharifi Y., Canavan J.B. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol. 2011;54:640–649. doi: 10.1016/j.jhep.2010.07.045. [DOI] [PubMed] [Google Scholar]
- 21.Cordoba J., Ventura-Cots M., Simon-Talero M. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF) J Hepatol. 2014;60:275–281. doi: 10.1016/j.jhep.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 22.D’Mello C., Le T., Swain M.G. Cerebral microglia recruit monocytes into the brain in response to tumor necrosis factoralpha signaling during peripheral organ inflammation. J Neurosci. 2009;29:2089–2102. doi: 10.1523/JNEUROSCI.3567-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Licinio J., Wong M.L. Pathways and mechanisms for cytokine signaling of the central nervous system. J Clin Invest. 1997;100:2941–2947. doi: 10.1172/JCI119846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharief M.K., Thompson E.J. In vivo relationship of tumor necrosis factor-alpha to blood–brain barrier damage in patients with active multiple sclerosis. J Neuroimmunol. 1992;38:27–33. doi: 10.1016/0165-5728(92)90087-2. [DOI] [PubMed] [Google Scholar]
- 25.Romero-Gomez M., Montagnese S., Jalan R. Hepatic encephalopathy in patients with acute decompensation of cirrhosis and acute-on-chronic liver failure. J Hepatol. 2015;62:437–447. doi: 10.1016/j.jhep.2014.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


