A novel motif within the cytoplasmic tail of the type I TGF-β receptor (TβRI) controls basolateral delivery. While this element functions independent of TβRI recycling and heteromeric TGF-β receptor trafficking, it can dominantly direct an apically expressed receptor to the basolateral membrane in polarized epithelial cells.
Abstract
Delivery of biomolecules to the correct subcellular locales is critical for proper physiological function. To that end, we have previously determined that type I and II transforming growth factor beta (TGF-β) receptors (TβRI and TβRII, respectively) localize to the basolateral domain in polarized epithelia. While TβRII targeting was shown to be regulated by sequences between amino acids 529 and 538, the analogous region(s) within TβRI is unknown. To address that question, sequential cytoplasmic TβRI truncations and point mutations identified a targeting motif between residues 158 and 163 (VxxEED) required for basolateral TβRI expression. Further studies documented that receptor internalization, down-regulation, direct recycling, or Smad signaling were unaffected by motif mutations that caused TβRI mislocalization. However, inclusion of amino acids 148–217 containing the targeting motif was able to direct basolateral expression of the apically sorted nerve growth factor receptor (NGFR, p75; extracellular and transmembrane regions) in a dominant manner. Finally, coexpression of apically targeted type I and type II TGF-β receptors mediated Smad3 signaling from the apical membrane of polarized epithelial cells. These findings demonstrate that the absence of apical TGF-β signaling in normal epithelia is primarily a reflection of domain-specific receptor expression and not an inability to couple with the signaling machinery.
INTRODUCTION
Epithelial structures routinely manifest a strict asymmetric design demarcated by intercellular tight junctions that prevent the flow of solutes and macromolecules between the luminal facing apical domain and the basolateral plasma membrane, which interfaces with neighboring cells and the extracellular matrix (Mellman and Nelson, 2008; Bonifacino, 2014; Stoops and Caplan, 2014). This results in the formation of distinct membrane domains with diverse cellular functions (Odorizzi and Trowbridge, 1997; Yeaman et al., 1999; Wodarz and Nathke, 2007). To maintain this polarity, newly synthesized proteins have been shown to undergo apical/basolateral sorting at a number of subcellular locales, including, but not limited to, the cis- or trans-Golgi, recycling endosome, and/or endosomal subdomains (Miaczynska and Zerial, 2002; Ang et al., 2004; Farr et al., 2009; Stoops and Caplan, 2014). This process is regulated by distinct apical or basolateral sorting signals such as GPI-anchor and N- or O-linked glycans for apical determinants and tyrosine (e.g., NPxY) or dileucine (e.g., D/ExxLL) motifs for basolateral trafficking (Wandinger-Ness et al., 1990; Matter et al., 1992; Hunziker and Fumey, 1994; Simmen et al., 1999; Stoops and Caplan, 2014). Disruption of this system can result in a variety of developmental defects and has been implicated in the progression of numerous disease phenotypes (Stein et al., 2002; Verges, 2007; Mellman and Nelson, 2008; De Matteis and Luini, 2011; Stoops and Caplan, 2014).
Transforming growth factor beta (TGF-β) signaling is mediated via a heteromeric interaction of type I (TβRI) and type II (TβRII) receptors (Wrana et al., 1992; Anders and Leof, 1996). Ligand binding to the constitutively active TβRII promotes complex formation with TβRI, TβRI phosphorylation, and subsequent TβRI kinase activation to modulate the growth and/or differentiation of numerous cell types (Wrana et al., 1994; Blobe et al., 2000; Morikawa et al., 2016). The principal mediators of TGF-β signaling are the Smad proteins, primarily Smad2 and Smad3 (Feng and Derynck, 2005; Ross and Hill, 2008). Once phosphorylated by TβRI, they translocate to the nucleus where they function as comodulators of various transcriptional responses (Feng and Derynck, 2005; Ross and Hill, 2008). In addition to the Smad proteins, a number of Smad-independent pathways have been implicated in various aspects of TGF-β action (Hocevar et al., 1999; Moustakas and Heldin, 2005; Kang et al., 2009; Rahimi et al., 2009).
Although the in vitro as well as in vivo responsiveness of epithelial cells to TGF-β is well known, and type I, II, and III (TβRIII) TGF-β receptors (TGFβRs) have all been shown to have an obligate basolateral expression profile (Murphy et al., 2004; Yakovich et al., 2010; Meyer et al., 2014; Nallet-Staub et al., 2015), there is a relative paucity of information concerning the elements or activities regulating their spatial distribution. For TβRII, however, we previously reported that a COOH-terminal motif (529LTAxxVAxxR538) functioned to directly target TβRII to the basolateral membrane and was dominant to the apically directing signals in the influenza HA protein (Murphy et al., 2007). Subsequent studies identified the retromer Vps35 subunit as an interacting protein that might impact polarized TβRII expression (Yin et al., 2013). While retromer loss had no discernable impact on TβRII direct recycling or initial basolateral targeting, it was shown to maintain basolateral TβRII expression by controlling recycling endosome to plasma membrane delivery by way of clathrin, EEA1, and Rab11 positive compartments.
In contrast to TβRII, however, there are no reports describing analogous findings for TβRI. As the type I TGFβR is the primary mediator of TGF-β action, and the trafficking of TβRI and TβRII are known to be independently regulated (Murphy et al., 2004; Yin et al., 2013), the current study was undertaken to address that issue. Evidence is provided that 1) a dominant-acting basolateral targeting motif for TβRI resides within residues 158–163 (VxxEED); 2) the 158VxxEED163 domain has no significant impact on TβRI internalization, recycling, or down-regulation; and 3) coexpression of apically targeted type I and type II TGFβRs induces Smad3 phosphorylation and PAI-1 induction following ligand addition to the apical membrane. The latter finding demonstrates that the absence of apical TGF-β signaling in normal epithelia reflects the importance of domain-specific receptor expression and not an inability to couple with the signaling machinery.
RESULTS
A novel cytoplasmic element between amino acids 158 and 163 mediates basolateral TβRI trafficking
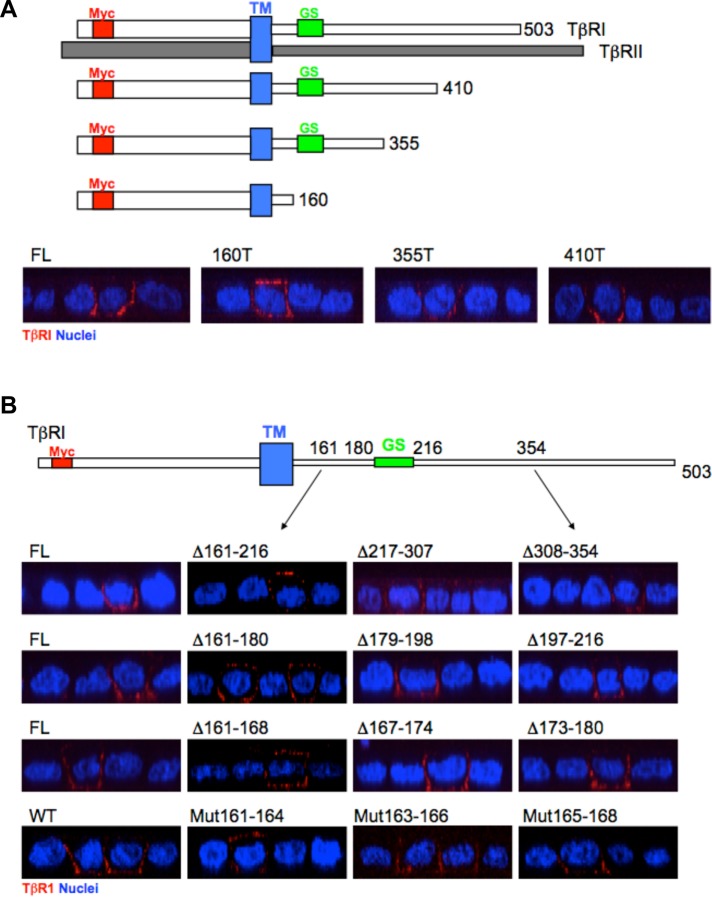
Previous studies have shown that in polarized epithelial cells type I and type II TGFβRs independently traffic to the basolateral domain adjacent to the gap junctional complex (Murphy et al., 2004; Yakovich et al., 2010; Nallet-Staub et al., 2015). While TβRII delivery is controlled by a dominant-acting motif located within amino acids 529–538 of the receptor’s C-terminal tail (Murphy et al., 2007), the presence and/or location of analogous elements in TβRI is unknown. To address that issue, the plasma membrane locale of Myc epitope-tagged TβRI full-length and truncation constructs was determined following transient transfection into polarized Madin-Darby canine kidney (MDCK) cells. While deletion of cytoplasmic residues 355–503 had no demonstrable effect on basolateral targeting, TβRI truncated at amino acid 160 showed both basolateral as well as apical expression (Figure 1A and Supplemental Figure S1A). To more critically define this activity required for basolateral delivery, additional deletions and point mutations (e.g., within the context of the full-length receptor) were made within amino acids 161–354. As shown in Figure 1B and Supplemental Figure S1, B–E (the Supplemental Data show additional transfected cells), while mutation of residues 163–168 had no significant impact on basolateral TβRI delivery, alanine mutations in amino acids 161–164 resulted in similar apical expression as previously shown by deletion of residues 160–503.
FIGURE 1:
The basolateral localizing signal of the type I TGFβR is located at the juxtamembrane region between amino acids 161 and 164. (A) Top: Depiction of full-length (FL) TβRI and TβRII as well as three TβRI truncation mutants (TM, transmembrane domain; GS, glycine/serine rich domain; Myc, epitope tag). Bottom: Polarized MDCK cells were transiently transfected with the indicated FL or COOH-terminal truncated (T) TβRI constructs and visualized by confocal microscopy following staining for the extracellular Myc tag and secondarily with Cy3 (red) as described under Materials and Methods. Images are presented as perpendicular XZ cross-sectional images. Nuclei (blue) were stained with DAPI. (B) Top: Cartoon depicting location of tested regions relative to TM and GS domains. Bottom: Immunostaining of FL TβRI and indicated serial deletions (Δ) or alanine point mutations (Mut) in polarized MDCK cells. Row 1, deletions between amino acids 161 and 354. Row 2, deletions between amino acids 161 and 216. Row 3, deletions between amino acids 161 and 180. Row 4, wild type (WT) and alanine mutations between amino acids 161 and 168. Staining and visualization was as in A.
Additional combinatorial and point mutations both within as well as upstream and downstream were generated to further define this potential motif (Figure 2 and Supplemental Figures S2 and S3). Although individual alanine mutations within the acidic EED domain were without effect (e.g., TβRI was basolaterally expressed), apical mislocalization was observed when either E161/E162 or E162/D163 were modified. However, the analogous double E161/D163 mutation was not sufficient to promote apical expression (e.g., similarly to the previously discussed individual mutations; Figure 2A and Supplemental Figure S2, A and B). Whereas various double mutations within residues 161–163 imparted significant apical staining, basolateral receptor staining (in the absence of apical) was still apparent (Supplemental Figure S2B). However, when all three amino acids (e.g., E161/E162/D163) were mutated to alanine, a more robust apical response was observed as essentially no cells expressed receptors solely at the basolateral membrane (Figure 2A and Supplemental Figure S2B).
FIGURE 2:
Basolateral targeting of TβRI is defined by a four-amino-acid motif. (A) Top: Depiction of the TβRI region examined. Bottom: Polarized MDCK cells were transfected with the indicated TβRI single (E161A, E162A, and D163A), double (E161E162/AA, E161D163/AA, and E162D163/AA), or triple (E161E162D163/AAA) point mutants and visualized for apical/basolateral expression as described in Figure 1 and under Materials and Methods. (B) Analogous studies as in A utilizing single (V158A), double (R157E161/AA, V158 E161/AA, P159E161/AA, and N160E161/AA), and quadruple (V158E161E162D163/AAAA) alanine TβRI point mutants. Two images are shown for the single and quadruple alanine constructs.
The previous data (Figures 1 and 2A and Supplemental Figure S2B) support an essential role for E161/E162/D163 in correct basolateral TβRI targeting. It does not, however, eliminate the possibility for a role of additional N- or C-terminal amino acids. To address that possibility, the adjacent four upstream (R157/V158/P159/N160) and five downstream (P164/S165/L166/D167/R168) residues were individually examined as well as in the context of E161 mutated to alanine. While mutation of R157, P159, N160 or any of the five downstream amino acids alone or with E161 had no detectable effect, the V158E161/AA construct showed both basolateral staining as well as apical mislocalization (Figure 2B and Supplemental Figures S2, C and D, and S3, A and B).
In that no significant difference in apical staining was observed with either E161/E162/D163 or V158/ E161/E162/D163 mutated to alanine (Figure 2 and Supplemental Figure S2B), yet V158 functions with E161 to promote apical TβRI delivery (Figure 2B), these data support a model whereby TβRI basolateral targeting is regulated by a hierarchical of activities within residues 158VxxEED163 (referred to as VEED motif). This is further supported by the lack of any demonstrable apical mislocalization by mutation of downstream prolines or a dileucine motif previously reported to function in TβRI internalization (Supplemental Figure S3C) (Shapira et al., 2012).
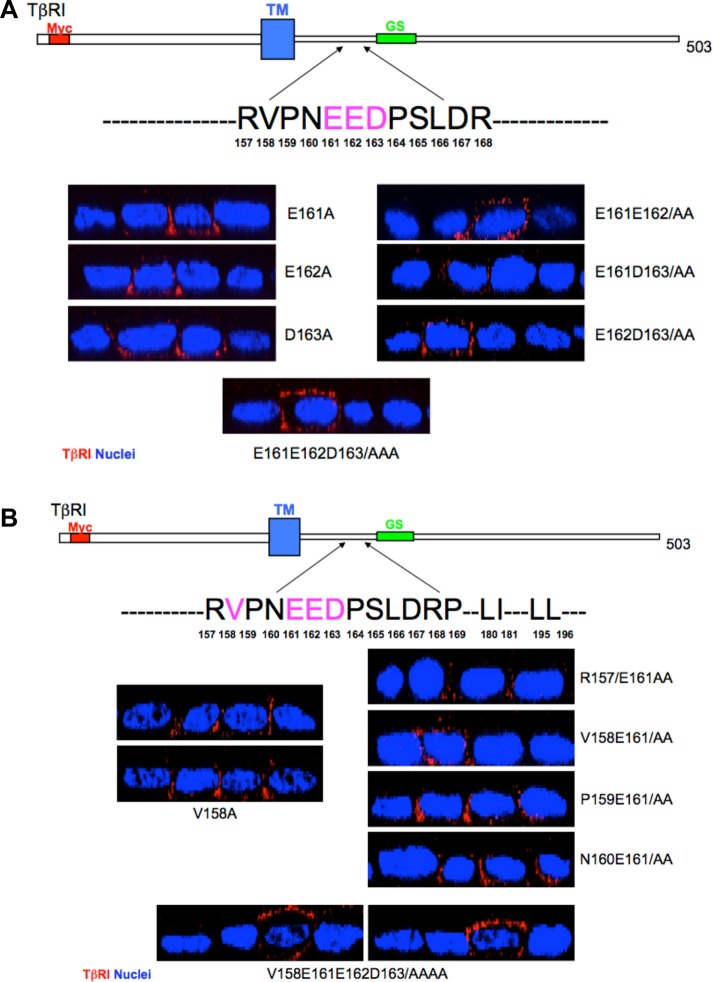
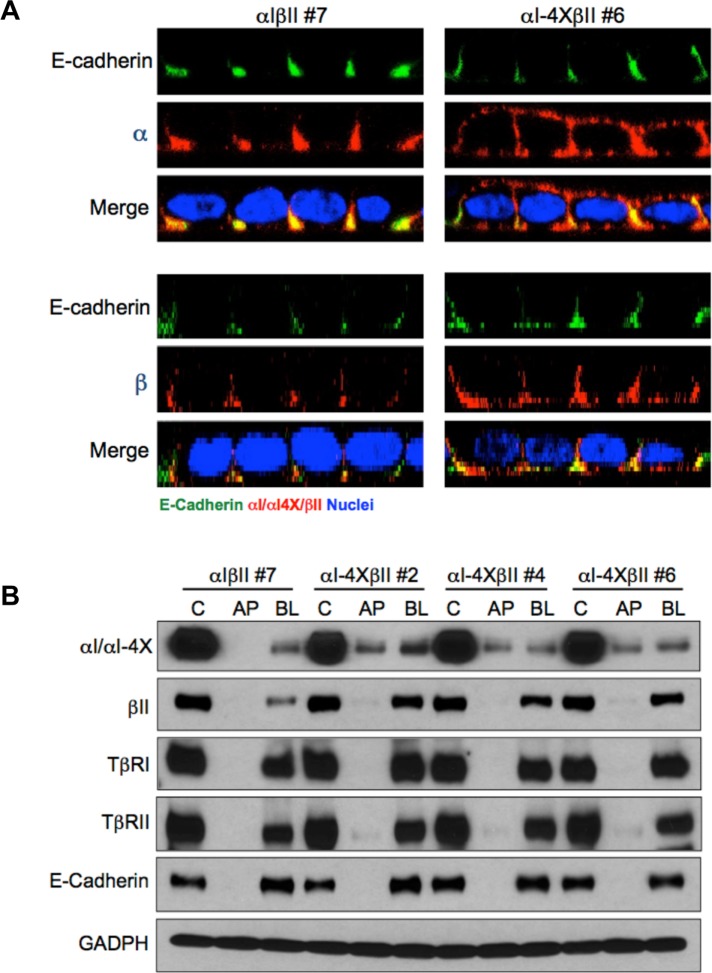
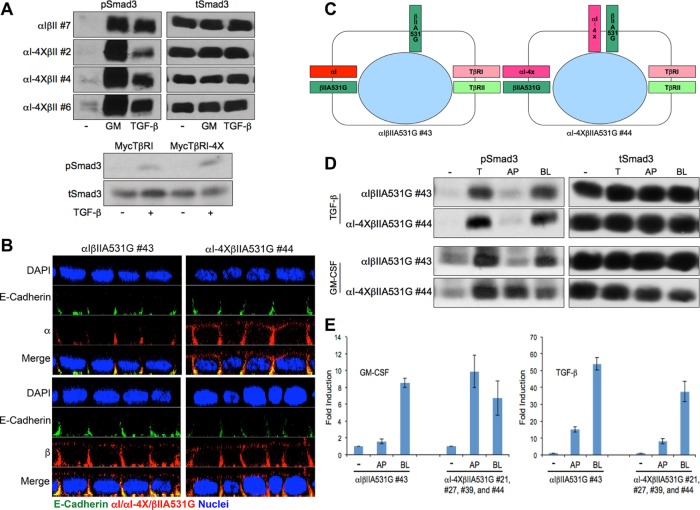
To further validate the basolateral targeting properties of the VEED motif within TβRI, stable MDCK clones expressing either wild-type chimeric type I (αI) and II (βII) TGFβRs or a wild-type chimeric type II receptor and a type I receptor in which 158VxxEED163 was mutated to 158AxxAAA163 (αI-4X) were generated (Figure 3). The chimeric system consists of the extracellular domain of the granulocyte macrophage colony-stimulating factor (GM-CSF) alpha or beta receptors fused to the transmembrane and cytoplasmic domains of TβRI or TβRII (Anders and Leof, 1996). While providing greater technical flexibility, it has previously been shown to recapitulate native TGFβR trafficking and signaling activity (Anders and Leof, 1996; Doré et al., 1998; Mitchell et al., 2004; Murphy et al., 2004; Yin et al., 2013). Analogous to that shown following transient transfection of native TβRI in Figures 1 and 2 and Supplemental Figures S1–S3, while expression of wild-type chimeric receptors is solely expressed on the basolateral plasma membrane domains, the chimeric type I receptor mutated in the VEED motif shows both basolateral as well as apical staining (Figure 3A). Furthermore, consistent with our previous determination that types I and II TGFβRs traffic independently (Murphy et al., 2004), mislocalization of the chimeric type I receptor had no impact on chimeric type II receptor basolateral targeting (Figure 3A). Additional confirmation that 158VxxEED163 is necessary for basolateral TβRI expression is shown in Figure 3B, where three independent MDCK clones stably expressing a wild-type chimeric type II and a targeting mutant type I receptor were grown on transwell dishes and exposed to biotin cross-linking from either the apical or basal chamber. Consistent with that shown by immunostaining, while the αI-4X mutant showed extensive apical biotin labeling, the wild-type βII receptor (as well as endogenous TβRI and TβRII) was only detected basolaterally.
FIGURE 3:
Chimeric TGFβRs confirm TβRI basolateral targeting motif. (A) Stable MDCK clones expressing either full-length (αIβII; clone #7) chimeric type I and type II TGFβRs or a full-length chimeric type II (βII) and a chimeric type I receptor containing alanine point mutations at residues V158, E161, E162, and D163 (αI-4X; clone #6) were transwell polarized and stained for either the extracellular GM-CSF α or β chain or E-cadherin as described (Anders and Leof, 1996; Murphy et al., 2004, 2007). Images are presented as perpendicular XZ cross-sectional images. Nuclei (blue) were stained with DAPI. (B) Biotinylation of endogenous TGFβRs and stably expressed chimeric TGFβRs in polarized MDCK cells. MDCK lines expressing wild-type βII either with αI or αIVEED/AAAA (αI-4X) were biotin labeled apically (AP) or basolaterally (BL) as described under Materials and Methods. Nonpolarized monolayer cultures were used to demonstrate total control labeling (C). Biotinylated proteins were extracted by streptavidin agarose beads and receptor specific antibodies were used for Western blotting. E-cadherin served as a basolateral marker, and GAPDH was used to confirm equal loading. Blots are representative of three separate experiments.
Basolateral delivery of TβRI is direct and regulated independent of receptor recycling, internalization, and down-regulation
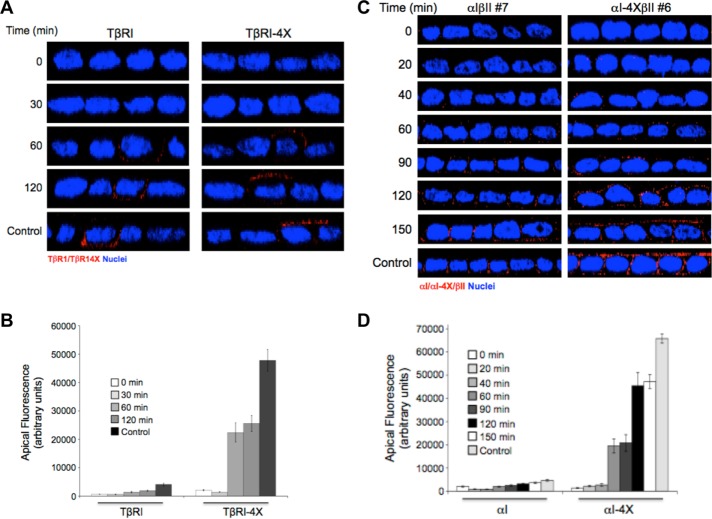
Previous studies have determined that basolateral delivery of the type II TGFβR is direct and not dependent on transient association with the apical plasma membrane (Murphy et al., 2007). In that this had not been investigated for the type I TGFβR, studies were undertaken addressing 1) a similar question for TβRI, 2) whether mutation of the VEED motif and subsequent apical expression altered the kinetics of plasma membrane delivery, and 3) whether chimeric TGFβRs showed analogous responses. To address the first two issues, following transient transfection of epitope tagged wild-type or VEED mutated native type I receptors, the kinetics of domain-specific plasma membrane expression was determined subsequent to release from a 20°C Golgi block. As shown in Figure 4A and quantitated in Figure 4B, TβRI directly traffics to the basolateral membrane and the loss of specific basolateral targeting has no significant impact on the kinetics of plasma membrane receptor expression whereby initial receptor staining is apparent by 60 min. These findings were confirmed using stable cell clones expressing analogous chimeric receptor constructs (Figure 4, C and D).
FIGURE 4:
In the absence of the VEED motif, type I TGFβRs are directly targeted to the apical membrane. (A) Polarized MDCK cells transiently transfected with either native wild-type TβRI or TβRIVEED/AAAA (TβRI-4X) for 16 h were Golgi blocked at 20°C for 3 h in serum-free DMEM. Following washing with cold PBS the apical and basal chambers were then treated with a dilute (0.05%) trypsin/PBS solution for the last 30 min of the Golgi block to remove cell surface proteins (0 min). After a PBS wash, prewarmed fresh 10% FBS/DMEM was added, and the plates were returned to 37°C and stained for the Myc-tagged type I TGFβR at the indicated times after release. (B) Quantitation of apical receptor expression as observed in A presented as arbitrary fluorescence units ± SEM of 25 cells from three independent experiments. (C) αIβII and αIVEED/AAAAβII (αI-4XβII) MDCK cell lines were polarized on 12-mm Transwell plates. Subsequent to Golgi block and trypsinization as described in A, newly expressed chimeric type I TGFβRs were visualized by immunofluorescence from 20 to 150 min after release. Cells were stained for TβRI using primary antibody to the external GM-CSF α chain and secondarily stained by Cy3 (red). Images are presented as perpendicular XZ cross-sectional images. Nuclei (blue) were stained with DAPI. (D) Quantitation as performed in B of 30 cells from three independent experiments.
While the type II TGFβR is known to undergo constitutive ligand-independent recycling (Mitchell et al., 2004; Yin et al., 2013), it is currently unknown whether TβRI is similarly regulated. To address that question as well as determine whether the basolateral targeting VEED motif impacted the response, recycling assays were performed in MDCK clones stably expressing wild-type and VEED TβRI mutant receptors. Similarly to that observed for TβRII, chimeric type I receptors recycle in the absence of ligand, and this occurs independent of the VEED domain (Figure 5A). Further support that the VEED motif is specifically controlling basolateral TβRI expression is shown in Figure 5, B and C. Both the kinetics and extent of ligand-dependent internalization and receptor down-regulation, respectively, are unaffected by VEED mutation. Thus, while the VEED motif has an obligate role in targeting the type I receptor to the basolateral membrane in polarized epithelia (Figures 2 – 4), TβRI recycling and heteromeric TGFβR complex trafficking is independently regulated (Figure 5).
FIGURE 5:
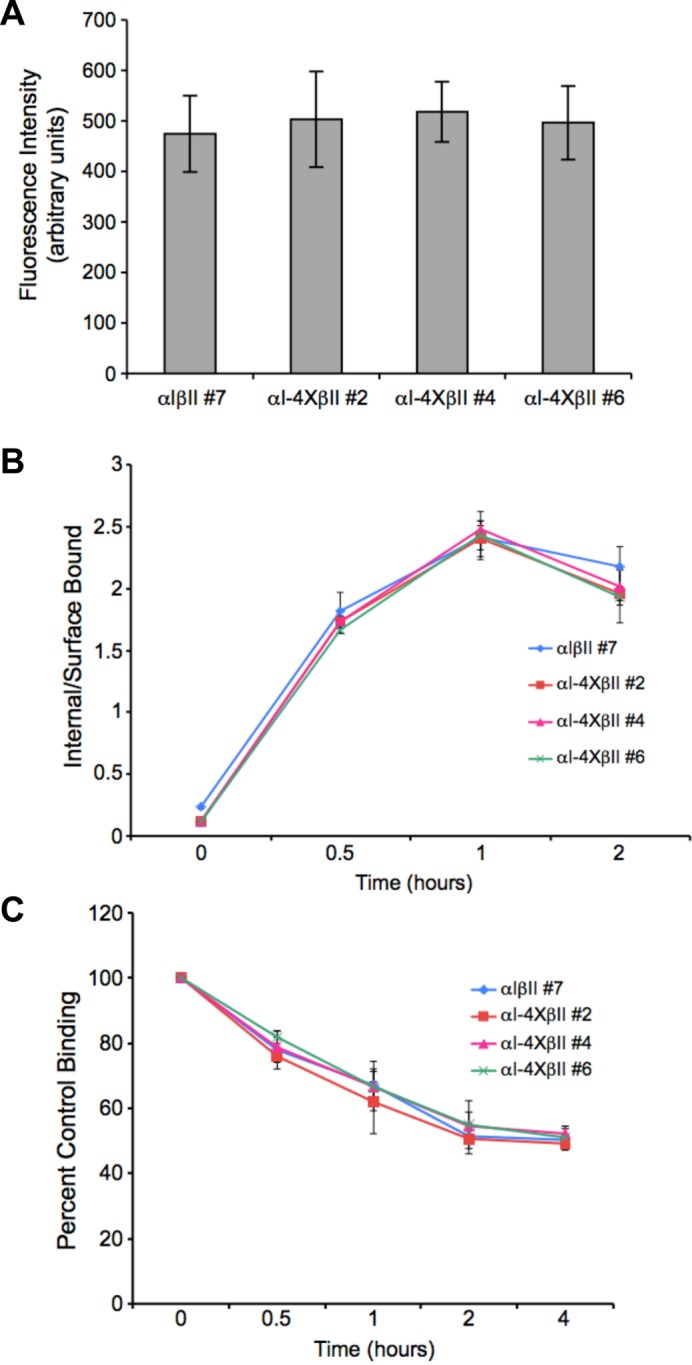
Monolayer TβRI trafficking is controlled independent of the VEED basolateral targeting signal. One MDCK cell line expressing wild-type chimeric TGFβRs (αIβII #7) and three expressing a wild-type chimeric type II in the context of a chimeric type I mutated in the VEED motif (αI-4XβII #2, #4, and #6) were assessed for effect on receptor recycling (A), internalization (B), and down-regulation (C) as described under Materials and Methods and in Anders et al. (1997), Mitchell et al. (2004), and Yin et al. (2013). (A) Direct recycling data represented as arbitrary units of fluorescence ± SD from 30 cells in each of three independent experiments. (B) Internalization of 125I-labeled GM-CSF by chimeric receptor clones. Each curve represents the mean internalized to surface bound ligand ± SD from three independent experiments. (C) Receptor down-regulation following addition of GM-CSF ligand by chimeric receptor clones. Data are presented as percentage of time zero binding following addition of GM-CSF for the indicated times and reflects the mean ± SD from three independent experiments.
The TβRI basolateral targeting domain is dominant to apical localizing elements in the nerve growth factor receptor
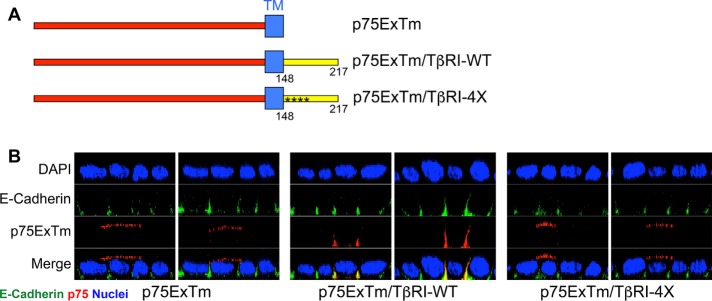
Components within the transmembrane and/or extracellular domains of the p75 nerve growth factor receptor (NGFR) direct its expression to the apical plasma membrane of polarized epithelial cells (Breuza et al., 2002; Youker et al., 2013). In that the previous data demonstrate that the VEED element targets both chimeric and native type I TGFβRs to the basolateral surface (Figures 1 – 4), it was next addressed whether it could provide similar basolateral targeting to an exogenous membrane protein. To address that question, NGFR constructs were prepared containing either the wild-type VEED domain or the identical region with VEED mutated to AAAA (Figure 6A). Following transient transfection into polarized MDCK cells, apical and basolateral receptor staining was determined. While the NGFR lacking the intracellular domain, as expected, demonstrated exclusive apical plasma membrane staining, inclusion of TβRI sequences containing the VEED motif directed the NGFR construct exclusively to the basolateral surface (Figure 6B and Supplemental Figure S4). In contrast, mutation of the TβRI VEED basolateral targeting signal to AAAA abolished the effect. These findings indicate that the VEED motif is 1) capable of directing basolateral localization in a heterologous context, 2) dominant to apical signals within the NGFR, and 3) both necessary and sufficient to funnel cargo to a defined membrane locale.
FIGURE 6:
The type I TGFβR basolateral targeting motif is dominant over the apical targeting signal in the nerve growth factor receptor (p75). (A) Depiction of the extracellular (Ex) and transmembrane domains (Tm) of p75 (p75ExTm) and chimeras also expressing TβRI amino acids 148–217 either wild type (TβRI-WT) or with alanine substitutions (*) in the VEED motif (TβRI-4X). (B) Confocal images of the indicated targets following transfection with p75ExTm, p75ExTm/TβRI-WT, or p75ExTm/TβRI-4X presented as perpendicular XZ cross-sectional images. Nuclei (blue) were stained with DAPI. Plasmids were transiently transfected into polarized MDCK cells for 16 h prior to staining.
Basolateral TGFβR targeting regulates plasma membrane domain-specific Smad signaling
The relation between TGFβR trafficking and signaling is complex, with evidence both supporting as well as not indicating a dependence (Hayes et al., 2002; Lu et al., 2002; Penheiter et al., 2002; Di Guglielmo et al., 2003; Shapira et al., 2012). Since the previous data demonstrated that the VEED domain provided an obligate signal for basolateral TβRI localization, yet was dispensable for receptor recycling, internalization, or down-regulation, we addressed two distinct questions: First, would Smad activation, per se, be regulated by the TβRI VEED motif, and, second, if Smad phosphorylation was unaffected, would Smad signaling occur independent of the plasma membrane domain where TβRI and TβRII are expressed? To address the first of these questions, chimeric TGFβR expressing MDCK cells were stimulated with either GM-CSF (e.g., activates chimeric receptor signaling) or TGF-β (e.g., activates native receptors) and Smad3 phosphorylation was determined. As shown in Figure 7A (top), VEED wild-type and mutant chimeric clones induced Smad3 phosphorylation to a similar extent as that observed for endogenous receptors stimulated with TGF-β. Analogous results were observed in polarized MDCK clones (Supplemental Figure S5) as well as with native TGF-β receptors (Figure 7A, bottom) following transient transfection of either the wild-type or VEED mutant TβRI into R1B cells that lack endogenous TβRI (Boyd and Massagué, 1989).
FIGURE 7:
Apical mislocalized TGFβRs are signaling competent. (A) Type I TGFβR basolateral targeting motif has no effect on TGF-β-induced Smad3 signaling. Top: Chimeric receptor expressing MDCK lines expressing wild-type (αIβII #7) type I and type II receptors or a wild-type type II receptor and type I receptor mutated in the VEED basolateral targeting domain (αI-4XβII #2, #4, and #6) were treated in the absence (-) or presence of GM-CSF (GM, 100 ng/ml) or TGF-β (10 ng/ml) for 1 h before being processed for Western analysis using phospho (p) or total (t) Smad3 sera. Bottom: R1B Mv1Lu cells (do not express native TβRI; Boyd and Massagué, 1989) were transiently transfected with either native wild-type TβRI or TβRI mutated in the VEED domain (TβRI-4X) directing basolateral receptor delivery. Phospho and total Smad3 was determined following 1 h stimulation ± 10 ng/ml TGF-β. (B) MDCK cells stably expressing either a wild-type chimeric type I receptor (αI) and a type II receptor (Murphy et al., 2007) mutated such that it mislocalizes to the apical membrane (αIβIIA531G #43) or chimeric type I and type II receptors that both undergo apical trafficking (αI-4XβIIA531G #44) were polarized on transwell inserts and stained for the indicated proteins. Images are presented as perpendicular XZ confocal cross-sections, and nuclei were stained with DAPI. (C) Cartoon depicting the locale of native and chimeric TGFβRs based on the chimeric receptors immunostaining data from B. (D) Chimeric clones from B were cultured in six-well transwells for 72 h. Cells were serum starved with 0.1% FBS/DMEM for 16 h and then either left untreated (-) or stimulated with TGF-β (10 ng/ml) or GM-CSF (100 ng/ml) from apical (AP), basolateral (BL), or both sides (T) at 37°C for 1 h. Equivalent protein was processed by Western blotting for phospho (p) or total (t) Smad3. Blots for A and D are representative of three separate experiments. (E) Polarized MDCK clones from D as well as three additional clones (e.g., #21, #27, and #39) expressing apically targeting chimeric TβRI and TβRII were treated as in D for 3 h with either GM-CSF (100 ng/ml; left) or TGF-β (10 ng/ml; right) and processed by RT-PCR for expression of PAI-1. Data reflect mean ± SEM from three biological replicates for control αIβIIA531G (#43) and pooled replicates for each (n = 8) of the αI-4XβIIA531G clones (#21, #27, #39, and #44).
In that the preceding data show that disrupting basolateral targeting of TβRI, per se, has no demonstrable impact on the ability of TβRI (in the context of wild-type TβRII) to activate Smad3 phosphorylation, we next investigated whether TGFβRs expressed on the apical surface would be able to similarly stimulate Smad3 activation as endogenous basolateral receptors. In other words, is receptor locale the primary determinant of TGF-β signaling or is this regulated by domain-specific ability to interact with the signaling machinery? To perform these studies, chimeric wild-type (αI) or VEED mutant (αI-4X) type I receptors were coexpressed with a type II receptor expressing a mutation at amino acid 531 (βIIA531G), which we previously demonstrated promoted apical mislocalization but was signaling competent (Murphy et al., 2007). As expected (Figure 3; Murphy et al., 2004, 2007), confocal immunostaining of polarized cultures showed that αI had an obligate basolateral locale while αI-4X and βIIA531G showed both apical as well as basolateral expression (Figure 7B). In that both native and chimeric TGFβR signaling requires the formation of a heteromeric complex of type I and type II receptors, MDCK clones expressing αI-4X and βIIA531G (e.g., as they are both signaling competent) provided the first opportunity to determine whether apically expressed TGFβRs are capable of activating/coupling with the Smad signaling machinery (model depicted in Figure 7C). This question was directly addressed in Figure 7, D and E, where polarized cultures were stimulated with TGF-β or GM-CSF from either the apical or basolateral transwell chamber and Smad3-dependent activity assessed. While apical ligand delivery was unable to induce Smad3 phosphorylation from 1) endogenous TGFβRs irrespective of the chimeric receptor profile or 2) chimeric receptors if only one was apically expressed (e.g., αIβIIA531G clone), when both chimeric receptors were present on the apical surface (e.g., αI-4XβIIA531G clone) there was similar Smad3 phosphorylation regardless of the plasma membrane domain stimulated (Figure 7D). Analogous findings were observed by reverse transcriptase-PCR (RT-PCR) assessment of the Smad3 target gene PAI-1 in multiple clones expressing apical TGFβRs (Figure 7E).
DISCUSSION
A primary role of epithelial cells is to direct proteins to distinct plasma membrane surfaces, as polarity plays a fundamental role in defining their response to various environmental cues (Bonifacino, 2014; Stoops and Caplan, 2014). This occurs through the development of intercellular tight junctions that selectively regulate the transfer of material between the apical luminal facing plasma membrane and the basolateral domain in contact with neighboring cells and the basal lamina (Bryant and Mostov, 2008; Mellman and Nelson, 2008; Apodaca et al., 2012). This physical demarcation results in domain-specific functional differences due to the asymmetric distribution of cargo to either the apical or basolateral surfaces. For this to occur, the sorting and domain-specific delivery of transmembrane proteins in polarized epithelia is routinely mediated by carriers arising from the trans-Golgi network and/or recycling endosome (Sheff et al., 1999; Brown and Breton, 2000; Ang et al., 2004; Mellman and Nelson, 2008; Bonifacino, 2014; Stoops and Caplan, 2014). For most basolateral-destined cargo, sorting is dependent on distinct determinants encoded within the protein’s cytosolic domain such as tyrosine (e.g., NPxY or YxxF)- or dileucine-based (e.g., D/ExxxLL) motifs that often also serve as endocytosis signals. In contrast, apical determinants are quite variable, localized throughout the protein, and can be associated with various components, including amino acids, carbohydrates, and lipids (Brewer and Roth, 1991; Mellman and Nelson, 2008; Youker et al., 2013; Bonifacino, 2014; Stoops and Caplan, 2014).
Although TGFβRs have been extensively investigated as to their signaling activity and role(s) in a variety of diseases (Blobe et al., 2000; Feng and Derynck, 2005; Massagué, 2012), the associated trafficking itinerary and regulatory components have not received similar attention. To that end, we previously determined that in nonpolarized cultures 1) TGFβRs undergo ligand-mediated internalization and down-regulation dependent on clathrin and TβRII kinase activity (Anders et al., 1997, 1998); 2) TβRI and TβRII bind AP2 via the trunk domain of the β2 subunit (Yao et al., 2002); and 3) TβRII undergoes constitutive recycling via a Rab11-, Dab2-, and retromer-dependent, Rab4-independent mechanism(s) (Mitchell et al., 2004; Penheiter et al., 2010; Yin et al., 2013). Moreover, as trafficking to the appropriate membrane domain in polarized epithelia is the initial event necessary for regulating epithelial cell growth and altered in a number of diseases (Stein et al., 2002; Verges, 2007; Mellman and Nelson, 2008), additional studies have investigated whether TGFβRs show distinct apical/basolateral membrane expression in polarized epithelial cultures. As such, evidence has been generated that types I, II, and III TGFβRs all show obligate basolateral localization (Murphy et al., 2004, 2007; Yakovich et al., 2010; Meyer et al., 2014). Furthermore, basolateral targeting of TβRII and TβRIII occurs independent of the aforementioned canonical signals and is regulated by the sequence 529LTAxxVAxxR538 or proline 826, respectively (Murphy et al., 2007; Meyer et al., 2014). Since analogous information has not been reported for TβRI, the current study addressed two fundamental questions: First, was TβRI basolateral expression similarly controlled by a defined cis-acting element, and if so, second, is TGF-β signaling dictated by domain-specific association with the signaling machinery or would apical mislocalization of both TβRI and TβRII respond to TGF-β ligand delivered from the apical surface?
To address the first of these questions, a number of cytoplasmic domain truncations and point mutations were made in both native and chimeric TβRI and their effect on basolateral targeting was examined. Immunostaining of transiently transfected as well as stable MDCK clones identified a four-amino-acid motif (158VxxEED163) responsible for directing basolateral TβRI expression, which was additionally confirmed by plasma membrane domain-specific surface biotinylation (Figures 1 – 3 and Supplemental Figures S1–S3). Further analysis determined that the VEED motif directly targeted TβRI to the basolateral domain and that it functioned independent of any detectable impact on TβRI recycling or ligand-dependent TGFβR internalization and down-regulation (Figures 4 and 5). This lack of any effect on TGFβR endocytosis is consistent with a previous report showing that while mutation of the acidic EED sequence is partially effective in inhibiting TβRI internalization in COS7 cells, it has only a very minor effect in mink lung R1B-L17 cells (Shapira et al., 2012). Together with our results in MDCK cells, such findings indicate the importance of cell context (e.g., perhaps reflecting differences in the repertoire of adaptor proteins) in defining receptor endocytic/trafficking activity.
In that 1) TGFβR basolateral targeting is not impacted by factors mediating basolateral delivery of other cargo such as Clostridium toxin B, μ1B, or pharmacologic disruption of cytoskeletal actin or microtubules (data not shown) and 2) the VEED motif is not similar to previously reported basolateral targeting signals (Aroeti et al., 1998; Bonifacino, 2014; Stoops and Caplan, 2014), the current findings reflect both the uniqueness and need for further investigation of this enigmatic receptor complex.
As mentioned previously, a number of diseases have been associated with defects in altered trafficking or cell polarity (Stein et al., 2002; Verges, 2007; Mellman and Nelson, 2008). Although similar examples have not (as yet) been reported for pathologies dependent on TGF-β, apically activated receptors could easily generate a variety of concerns associated with luminal ligand stimulating inappropriate proliferation/growth inhibition, development, and/or induction of an epithelial/mesenchymal transition. However, for this to be a concern, it would first need to be determined whether apically mislocalized TGFβRs were even capable of activating a signaling response, as it has previously been determined that various receptors can show differential signaling and/or endocytic activity in polarized epithelia depending on plasma membrane domain-specific expression (Denning and Welsh, 1991; Becker et al., 1995; Kuwada et al., 1998). Furthermore, since TGFβR activity requires the formation of a heteromeric complex of type I and type II receptors (Wrana et al., 1992; Anders and Leof, 1996), apical expression of both receptors is necessary. This was directly tested in Figure 7 where polarized MDCK cells expressing mislocalized chimeric TβRI and TβRII were shown to similarly induce Smad3 phosphorylation and PAI-1 induction when stimulated from either the apical or basolateral transwell chamber. In that TGFβRs are capable of coupling to the cellular signaling machinery regardless of their overall membrane locale, such findings clearly indicate the importance of domain-specific expression in order to appropriately respond to environmental cues and maintain normal homeostasis.
MATERIALS AND METHODS
Cell culture and transfection
MDCK and R1B cells were maintained in DMEM (Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS) (HyClone Laboratories, Logan, UT) at 37°C and 5% CO2. For transwell culture, cells were plated at a density of 7.5 × 104 cells in 0.5 ml of culture medium or 5 × 105 cells in 1.5 ml of culture medium in 12 and 24 mm Costar polycarbonate membranes (Corning, Corning, NY), respectively. Medium was changed every day, and polarization was achieved after 72 h (Murphy et al., 2004, 2007).
MDCK clones stably expressing chimeric type I and type II TGFβRs were maintained as above with the addition of 500 μg/ml G418 (Mediatech, Manassas, VA) and 250 μg/ml hygromycin (Invitrogen, Carlsbad, CA). The designations αI and βII refer to chimeric receptors expressing the extracellular ligand-binding domain of the GM-CSF α or β receptor coupled to the transmembrane and cytoplasmic domain of the TGF-β type I and type II receptors, respectively (Anders and Leof, 1996). We have previously determined that analogous signaling and trafficking activity is observed with chimeric and native TGFβRs (Anders and Leof, 1996; Anders et al., 1997; Yao et al., 2002; Mitchell et al., 2004; Murphy et al., 2004, 2007; Yin et al., 2013)
Transfection of cells cultured in transwell dishes was performed 48 h following seeding using Lipofectamine 2000 (Life Technologies). Briefly, culture medium was changed to Opti-MEM (Life Technologies) prior to addition of 2 μl Liopfectamine 2000 diluted in 50 μl Opti-MEM with 0.2 μg receptor DNA and 0.4 μg of empty vector in 50 μl Opti-MEM (100 μl total). Following room temperature incubation for 20 min, the DNA and Lipofectamine 2000 mixture was then added into the apical transwell chamber for 3 h at 37°C. The medium was removed and replaced with 10% FBS/DMEM, and immunostaining (see below) was performed subsequent to an additional 16 h 37°C incubation. For cells in monolayer dishes, transfection was carried out similarly as above, except cells were seeded for 24 h prior to transfection, no empty vector DNA was used, the DNA and Lipofectamine 2000 mixture was removed following 6 h treatment, and cultures were incubated for 24 h prior to use.
Plasmid construction
The human type I TGFβR with a Myc tag between amino acids 27 and 28 (provided by Yoav Henis) was cloned into pcDNA3.1(+) (Invitrogen) between the NotI and HindIII sites. All mutations and deletions were generated using the QuikChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA) for both native and chimeric TGFβRs.
To examine the effect of the TβRI basolateral-targeting sequence on apically directed nerve growth factor receptor trafficking, the extracellular and transmembrane domain of the human NGFR was first cloned into pcDNA3.1(+) at KpnI and XhoI. A BamHI site was introduced right after the transmembrane domain where TβRI sequences from amino acids 148 to 217 expressing either wild-type or the BL targeting mutant VEED/AAAA were inserted.
Direct recycling assay
This was previously described in detail (Fraile-Ramos et al., 2001; Mitchell et al., 2004). Briefly, an antibody recognizing the extracellular receptor domain is visualized through 1.5 cycles of recycling. Since the fluorescent secondary antibody binds only those receptors that return to the cell surface with attached primary antibody, intracellular fluorescence is observed following an additional internalization event.
Immunostaining and microscopy
Transwell cultures were rinsed with filter-sterilized 0.2% bovine serum albumin (BSA)/phosphate-buffered saline (PBS: 137 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 8.1 mM Na2HPO4, pH 7.4; wash buffer) and incubated with primary antibody diluted in ice-cold 5% normal donkey serum (NDS; Vector Laboratories, Burlingame, CA)/0.2%BSA/PBS (blocking buffer) on ice for 1 h. Supplemental Table S1 lists source and use of all antibodies. After washing with ice-cold wash buffer (3 × 5 min), the cultures were fixed with 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS at room temperature for 20 min and subsequently quenched with 50 mM NH4Cl/PBS on ice for 10 min before incubation (room temperature, 30 min) with secondary antibodies and 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes, Eugene, OR) diluted in blocking buffer. Slides were mounted using Vectashield (Vector Laboratories) following washing (3 × 10 min) in wash buffer.
For monolayer staining, cells were fixed as above, permeablized (0.1% Triton X-100/PBS for 10 min), and incubated for 1 h in blocking buffer (5% normal goat serum, 1% glycerol, 0.1% BSA, 0.1% fish skin gelatin, 0.04% sodium azide, PBS, pH 7.2) prior to addition of primary antibodies (in blocking buffer) for 1 h. Slides were washed with PBS (3 × 10 min) and then incubated with secondary antibodies in blocking buffer for 30 min. After PBS wash (3 × 10 min), slides were mounted using Vectashield. All treatments were performed at room temperature.
Fluorescence internalization images were acquired at room temperature using an AX-70 Olympus microscope (100×/1.35 NA oil immersion objective) equipped with a C4742-95-12NR camera (Hamamatsu, Japan). For confocal microscopy a 100× objective (1.3 NA oil lens) on a Zeiss LSM 510 confocal system was used (Carl Zeiss, Jena, Germany). No two-dimensional deconvolution of nearest neighbors or three-dimensional reconstructions, surface or volume rendering, or gamma adjustments were performed. Quantitation was done using MetaMorph, version 7.3.2 (Molecular Devices, Sunnyvale, CA).
Cell-surface receptor biotinylation
Cell-surface TGFβRs were detected by biotinylation essentially as described (Yin et al., 2013). Briefly, following plating of 5 × 105 cells in 24-mm transwells for 72 h and daily medium (10% FBS/DMEM) change, sulfo-NHS-SS biotin/Hanks balanced salt solution (HBSS) (1 mg/ml; Thermo Scientific, Rockford, IL) was added to the apical (1 ml) or basolateral (1.5 ml) surfaces of polarized transwell cultures for 1 h at 4°C. To assess total (T) labeling, 1.5 × 106 cells were seeded (10% FBS/DMEM) into 10-cm2 culture plates for 24 h. Biotin labeling was similarly performed using 3 ml of 1 mg/ml sulfo-NHS-SS biotin/HBSS. Cells were lysed by addition of modified RIPA buffer (50 mM Tris-HCl, pH 7.4, 1% Triton X-100, 0.25% deoxycholate, 150 mM NaCl, 1 mM EDTA, 10 mM NaF) with protease inhibitors (Roche, Indianapolis, IN) and biotinylated proteins (0.75 mg) precipitated by addition of streptavidin–agarose (150 μl; Thermo Scientific) in 1 ml of total volume for 3 h at 4°C. Following elution with 150 μl 4× Laemmli buffer (70°C for 20 min), target proteins were detected by Western blotting using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific).
Western blotting
Monolayer and polarized chimeric receptor MDCK cell lines were serum starved with 0.1% FBS/DMEM for 16 h before induction with either GM-CSF (100 ng/ml) or TGF-β1 (10 ng/ml; R&D Systems, Minneapolis, MN) at 37°C for 1 h. R1B cells transfected with either TβRI or TβRIVEED/AAAA (TβRI4X) were serum starved for 2 h and induced with TGF-β at 37°C for 1 h. Cells were lysed in modified RIPA including protease inhibitor cocktail on ice for 1 h. Clarified lysate (16,200 × g for 15 min) was resolved on SDS–PAGE, transferred to polyvinylidene fluoride membranes (Millipore, Billerica, MA), blocked with 5% nonfat milk in 150 mM NaCl/10 mM Tris (pH 7.4)/0.1% Tween 20, incubated overnight at 4°C with primary antibodies, and, following addition of horseradish peroxidase–conjugated secondary antibodies (1 h, room temperature), processed as described above in cell-surface biotinylation.
Internalization and down-regulation assays
Internalization and down-regulation assays with chimeric TGFβRs were performed as previously described (Anders et al., 1997). Briefly, for internalization studies, MDCK clones were plated in six-well dishes (9.6 cm2/well) at 1.5 × 105 cells/well for 24 h. Following incubation with 125I-labeled GM-CSF (100 pM; Perkin-Elmer, Waltham, MA) for 2 h at 4°C in the presence or absence of 25-fold excess unlabeled GM-CSF (2.5 nM) and removal of unbound ligand, cells were placed at 37°C for the indicated times. Remaining surface-bound ligand was removed/counted by acid stripping (PBS, pH 3.0) and internalized ligand determined by cell solubilization in 0.2 N NaOH/40 μg/ml salmon sperm DNA.
To determine receptor down-regulation, cells were treated at 37°C with 10 ng/ml GM-CSF for the indicated times. Following acid stripping (PBS, pH 3.0) to remove any remaining bound ligand, specific surface binding of 125I-labeled GM-CSF (100 pM) was determined following 2 h incubation at 4°C in the presence or absence of 25-fold excess unlabeled GM-CSF (2.5 nM).
RT-PCR analysis
MDCK clones expressing endogenous and the indicated wild-type or targeting defective chimeric TGFβRs were cultured in 24-mm transwells for 72 h before serum starvation with 0.1% FBS/DMEM for 16 h. The polarity of the cells was determined by measurement of transepithelial resistance. GM-CSF or TGF-β1 were diluted with DMEM at concentrations of 100 or 10 ng/ml, respectively, and applied to either the apical (1 ml) or basolateral (1.5 ml) chamber. Serum-free DMEM was placed in the opposite transwell chambers and also used as a negative control. Following 3 h incubation at 37°C, cultures were washed with ice-cold PBS and processed for RNA extraction with the RNeasy mini kit (Qiagen, Valencia, CA), where 2 μg was reverse transcribed using Maxima Reverse Transcriptase (Life technologies). Quantitative RT-PCR analysis for expression of PAI-1 was performed using Sybr green (Clontech, Mountain View, CA) and the ABI 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA). Results were normalized to Smad2 mRNA expression and the data plotted as the mean (±SEM) fold induction of either GM-CSF or TGF-β1 stimulation to unstimulated levels. Primers used were as follows: canine PAI-1, 5′-GCCTCCTGGTTCTGCCTAAG-3′ (forward) and 5′-CTTGAGAAGTCCGCCAGGTT-3′ (reverse); canine Smad2, 5′-AATTTGCTGCTCTCCTGGCT-3′ (forward) and 5′-CGGTATTCTGCTCCCCATCC-3′ (reverse).
Supplementary Material
Acknowledgments
We thank Yoav Henis (Tel Aviv University) for providing Myc-tagged TβRI. This work was supported by Public Health Service grants GM-55816 and GM-54200 from the National Institute of General Medical Sciences, a pilot grant from the Mayo Brain SPORE (CA-108961), the Caerus Foundation, and the Mayo Foundation to E.B.L.
Abbreviations used:
- αI
chimeric type I TGF-β receptor
- βII
chimeric type II TGF-β receptor
- AP
apical
- BL
basolateral
- GM-CSF
granulocyte macrophage colony-stimulating factor
- NGFR
nerve growth factor receptor
- TGF-β
transforming growth factor beta
- TβRI
type I transforming growth factor beta receptor
- TβRII
type II transforming growth factor beta receptor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E17-05-0334) on August 2, 2017.
REFERENCES
- Anders RA, Arline SL, Doré JJE, Leof EB. Distinct endocytic responses of heteromeric and homomeric transforming growth factor β receptors. Mol Biol Cell. 1997;8:2133–2143. doi: 10.1091/mbc.8.11.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders RA, Doré JEJ, Arline SA, Garamszegi N, Leof EB. Differential requirements for type I and type II TGFβ receptor kinase activity in ligand-mediated receptor endocytosis. J Biol Chem. 1998;273:23118–23125. doi: 10.1074/jbc.273.36.23118. [DOI] [PubMed] [Google Scholar]
- Anders RA, Leof EB. Chimeric granulocyte/macrophage colony-stimulating factor/transforming growth factor-β (TGF-β) receptors define a model system for investigating the role of homomeric and heteromeric receptors in TGF-β signaling. J Biol Chem. 1996;271:21758–21766. doi: 10.1074/jbc.271.36.21758. [DOI] [PubMed] [Google Scholar]
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apodaca G, Gallo LI, Bryant DM. Role of membrane traffic in the generation of epithelial cell asymmetry. Nat Cell Biol. 2012;14:1235–1243. doi: 10.1038/ncb2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aroeti B, Okhrimenko H, Reich V, Orzech E. Polarized trafficking of plasma membrane proteins: emerging roles for coats, SNAREs, GTPases and their link to the cytoskeleton. Biochim Biophys Acta. 1998;1376:57–90. doi: 10.1016/s0304-4157(98)00005-7. [DOI] [PubMed] [Google Scholar]
- Becker BN, Cheng HF, Burns KD, Harris RC. Polarized rabbit type 1 angiotensin II receptors manifest differential rates of endocytosis and recycling. Am J Physiol. 1995;269:C1048–C1056. doi: 10.1152/ajpcell.1995.269.4.C1048. [DOI] [PubMed] [Google Scholar]
- Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Eng J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS. Adaptor proteins involved in polarized sorting. J Cell Biol. 2014;204:7–17. doi: 10.1083/jcb.201310021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd FT, Massagué J. Transforming growth factor-β inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J Biol Chem. 1989;264:2272–2278. [PubMed] [Google Scholar]
- Breuza L, Garcia M, Delgrossi MH, Le Bivic A. Role of the membrane-proximal O-glycosylation site in sorting of the human receptor for neurotrophins to the apical membrane of MDCK cells. Exp Cell Res. 2002;273:178–186. doi: 10.1006/excr.2001.5442. [DOI] [PubMed] [Google Scholar]
- Brewer CB, Roth MG. A single amino acid change in the cytoplasmic domain alters the polarized delivery of influenza virus hemagglutinin. J Cell Biol. 1991;114:413–421. doi: 10.1083/jcb.114.3.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D, Breton S. Sorting proteins to their target membranes. Kidney Int. 2000;57:816–824. doi: 10.1046/j.1523-1755.2000.00920.x. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis MA, Luini A. Mendelian disorders of membrane trafficking. N Eng J Med. 2011;365:927–938. doi: 10.1056/NEJMra0910494. [DOI] [PubMed] [Google Scholar]
- Denning GM, Welsh MJ. Polarized distribution of bradykinin receptors on airway epithelial cells and independent coupling to second messenger pathways. J Biol Chem. 1991;266:12932–12938. [PubMed] [Google Scholar]
- Di Guglielmo GM, Leroy C, Goodfellow AF, Wrana JL. Distinct endocytic pathways regulate TGF-β receptor signaling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- Doré JJE, Jr, Edens M, Garamszegi N, Leof EB. Heteromeric and homomeric transforming growth factor-β receptors show distinct signaling and endocytic responses in epithelial cells. J Biol Chem. 1998;273:31770–31777. doi: 10.1074/jbc.273.48.31770. [DOI] [PubMed] [Google Scholar]
- Farr GA, Hull M, Mellman I, Caplan MJ. Membrane proteins follow multiple pathways to the basolateral cell surface in polarized epithelial cells. J Cell Biol. 2009;186:269–282. doi: 10.1083/jcb.200901021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X-H, Derynck R. Specificity and versatility in TGF-β signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Fraile-Ramos A, Kledal TN, Pelchen-Matthews A, Bowers K, Schwartz TW, Marsh M. The human cytomegalovirus US28 protein is located in endocytic vesicles and undergoes constitutive endocytosis and recycling. Mol Biol Cell. 2001;12:1737–1749. doi: 10.1091/mbc.12.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S, Chawla A, Corvera S. TGFß receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J Cell Biol. 2002;158:1239–1249. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH. TGF-β induces fibronectin synthesis through a c-Jun N-termial kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W, Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. Embo J. 1994;13:2963–2969. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol. 2009;19:385–394. doi: 10.1016/j.tcb.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Kuwada SK, Lund KA, Li XF, Cliften P, Amsler K, Opresko LK, Wiley HS. Differential signaling and regulation of apical vs. basolateral EGFR in polarized epithelial cells. Am J Physiol. 1998;275:C1419–C1428. doi: 10.1152/ajpcell.1998.275.6.C1419. [DOI] [PubMed] [Google Scholar]
- Lu Z, Murray JT, Luo W, Li H, Wu X, Xu H, Backer JM, Chen YG. Transforming growth factor beta activates Smad2 in the absence of receptor endocytosis. J Bio Chem. 2002;277:29363–29368. doi: 10.1074/jbc.M203495200. [DOI] [PubMed] [Google Scholar]
- Massagué J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AE, Gatza CE, How T, Starr M, Nixon AB, Blobe GC. Role of TGF-beta receptor III localization in polarity and breast cancer progression. Mol Biol Cell. 2014;25:2291–2304. doi: 10.1091/mbc.E14-03-0825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaczynska M, Zerial M. Mosaic organization of the endocytic pathway. Exp Cell Res. 2002;272:8–14. doi: 10.1006/excr.2001.5401. [DOI] [PubMed] [Google Scholar]
- Mitchell H, Choudhury A, Pagano RE, Leof EB. Ligand-dependent and -independent transforming growth factor-beta receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol Biol Cell. 2004;15:4166–4178. doi: 10.1091/mbc.E04-03-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa M, Derynck R, Miyazono K. TGF-beta and the TGF-beta family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 2016;8 doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. Non-Smad TGF-beta signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, Doré JJE, Edens M, Coffey RJ, Barnard JA, Mitchell H, Wilkes M, Leof EB. Differential trafficking of transforming growth factor-ß receptors and ligand in polarized epithelial cells. Mol Biol Cell. 2004;15:2853–2862. doi: 10.1091/mbc.E04-02-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SJ, Shapira KE, Henis YI, Leof EB. A unique element in the cytoplasmic tail of the type II TGFß receptor controls basolateral delivery. Mol Biol Cell. 2007;18:3788–3799. doi: 10.1091/mbc.E06-10-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nallet-Staub F, Yin X, Gilbert C, Marsaud V, Ben Mimoun S, Javelaud D, Leof EB, Mauviel A. Cell density sensing alters TGF-beta signaling in a cell-type-specific manner, independent from Hippo pathway activation. Dev Cell. 2015;32:640–651. doi: 10.1016/j.devcel.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odorizzi G, Trowbridge IS. Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J Cell Biol. 1997;137:1255–1264. doi: 10.1083/jcb.137.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penheiter SG, Mitchell H, Garamszegi N, Edens M, Doré JJE, Jr, Leof EB. Internalization-dependent and -independent requirements for transforming growth factor β receptor signaling via the Smad pathway. Mol Cell Biol. 2002;22:4750–4759. doi: 10.1128/MCB.22.13.4750-4759.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penheiter SG, Singh RD, Repellin CE, Wilkes MC, Edens M, Howe PH, Pagano RE, Leof EB. Type II transforming growth factor-beta receptor recycling is dependent upon the clathrin adaptor protein Dab2. Mol Biol Cell. 2010;21:4009–4019. doi: 10.1091/mbc.E09-12-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi R, Andrianifahanana M, Wilkes MC, Edens ME, Kottom TJ, Blenis J, Leof EB. Distinct roles for mammalian target of rapamycin complexes in the fibroblast response to transforming growth factor-β. Cancer Res. 2009;69:84–93. doi: 10.1158/0008-5472.CAN-08-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross S, Hill CS. How the Smads regulate transcription. Int J Bioch Cell Biol. 2008;40:383–408. doi: 10.1016/j.biocel.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Shapira KE, Gross A, Ehrlich M, Henis YI. Coated pit-mediated endocytosis of the Type I transforming growth factor-beta (TGF-beta) receptor depends on a di-leucine family signal and is not required for signaling. J Biol Chem. 2012;287:26876–26889. doi: 10.1074/jbc.M112.362848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheff DR, Daro EA, Hull M, Mellman I. The receptor recycling pathway contains two distinct populations of early endosomes with different sorting functions. J Cell Biol. 1999;145:123–139. doi: 10.1083/jcb.145.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen T, Nobile M, Bonifacino JS, Hunziker W. Basolateral sorting of furin in MDCK cells requires a phenylalanine-isoleucine motif together with an acidic amino acid cluster. Mol Cell Biol. 1999;19:3136–3144. doi: 10.1128/mcb.19.4.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Wandinger-Ness A, Roitbak T. Altered trafficking and epithelial cell polarity in disease. Trends Cell Biol. 2002;12:374–381. doi: 10.1016/s0962-8924(02)02331-0. [DOI] [PubMed] [Google Scholar]
- Stoops EH, Caplan MJ. Trafficking to the apical and basolateral membranes in polarized epithelial cells. J Am Soc Nephrol. 2014;25:1375–1386. doi: 10.1681/ASN.2013080883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verges M. Retromer and sorting nexins in development. Front Biosci. 2007;12:3825–3851. doi: 10.2741/2355. [DOI] [PubMed] [Google Scholar]
- Wandinger-Ness A, Bennett MK, Antony C, Simons K. Distinct transport vesciles mediate the delivery of plasma membrane proteins to the apical and basolateral domains of MDCK cells. J Cell Biol. 1990;111 doi: 10.1083/jcb.111.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Nathke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–1024. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Yakovich AJ, Huang Q, Du J, Jiang B, Barnard JA. Vectorial TGFbeta signaling in polarized intestinal epithelial cells. J Cell Physiol. 2010;224:398–404. doi: 10.1002/jcp.22135. [DOI] [PubMed] [Google Scholar]
- Yao D, Ehrlich M, Henis YI, Leof EB. Transforming growth factor-beta receptors interact with AP2 by direct binding to beta2 subunit. Mol Biol Cell. 2002;13:4001–4012. doi: 10.1091/mbc.02-07-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman C, Grindstaff KK, Nelson WJ. New perspectives on mechanisms involved in generating epithelial cell polarity. Physiol Rev. 1999;79:73–98. doi: 10.1152/physrev.1999.79.1.73. [DOI] [PubMed] [Google Scholar]
- Yin X, Murphy SJ, Wilkes MC, Ji Y, Leof EB. Retromer maintains basolateral distribution of the type II TGF-beta receptor via the recycling endosome. Mol Biol Cell. 2013;24:2285–2298. doi: 10.1091/mbc.E13-02-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youker RT, Bruns JR, Costa SA, Rbaibi Y, Lanni F, Kashlan OB, Teng H, Weisz OA. Multiple motifs regulate apical sorting of p75 via a mechanism that involves dimerization and higher-order oligomerization. Mol Biol Cell. 2013;24:1996–2007. doi: 10.1091/mbc.E13-02-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.