Abstract
Cancer patients have an increased risk of venous thromboembolism (VTE). In this review, we summarize common and cancer type–specific pathways of VTE in cancer patients. Increased levels of leukocytes, platelets, and tissue factor–positive (TF+) microvesicles (MVs) are all potential factors that alone or in combination increase cancer-associated thrombosis. Patients with lung or colorectal cancer often exhibit leukocytosis. Neutrophils could increase VTE in cancer patients by releasing neutrophil extracellular traps whereas monocytes may express TF. Thrombocytosis is often observed in gastrointestinal, lung, breast, and ovarian cancer and this could decrease the threshold required for VTE. Soluble P-selectin has been identified as a biomarker of cancer-associated thrombosis in a general cancer population and may reflect activation of the endothelium. P-selectin expression by the endothelium may enhance VTE by increasing the recruitment of leukocytes. Studies in patients with pancreatic or brain cancer suggest that elevated levels of PAI-1 may contribute to VTE. Although elevated levels of TF+ MVs have been observed in patients with different types of cancer, an association between TF+ MVs and VTE has been observed only in pancreatic cancer. Podoplanin expression is associated with VTE in patients with brain cancer and may activate platelets. Future studies should measure multiple biomarkers in each cancer type to determine whether combinations of biomarkers can be used as predictors of VTE. A better understanding of the pathways that increase VTE in cancer patients may lead to the development of new therapies to reduce the morbidity and mortality associated with thrombosis.
Introduction
Cancer is associated with an increased incidence of venous thromboembolism (VTE) (4% to 20%) and arterial thrombosis (2% to 5%).1-5 More than 50 years ago, it was proposed that alterations of coagulation factors, increased platelet adhesiveness, and a decrease in fibrinolysis were possible mechanisms to explain cancer-associated thrombosis.6 However, thromboprophylaxis is not routinely recommended for ambulatory cancer patients because the risk of VTE is not generally high enough and cancer patients are more prone to bleeding.7 Indeed, the identification of subgroups of ambulatory cancer patients who would benefit from thromboprophylaxis without an increase in bleeding remains a major goal of the field.8
Interestingly, the rates of VTE vary in different cancer types.5,6,9-12 Cancer types can be broadly divided into 3 groups according to risk of VTE: high risk (pancreatic, ovarian, brain, stomach, gynecologic, and hematologic), intermediate risk (colon and lung), and low risk (breast and prostate). This suggests that there may be cancer type–specific pathways of VTE (Table 1).
Table 1.
Proposed pathways of venous thrombosis in different types of cancer
| Tumor type | Proposed primary pathway | Reference |
|---|---|---|
| Lung and colorectal | Leukocytosis | 13, 19-24 |
| Lung | Neutrophilia and NETs | 27-29 |
| Ovarian | Thrombocytosis | 6, 43-45, 52 |
| Pancreatic | TF+ MVs | 72, 73, 78, 80, 81 |
| Brain | PDPN+ MVs | 105, 106 |
Risk assessment scores are used to identify cancer patients at high risk for VTE. The Khorana score uses various parameters, such as site of cancer, leukocytosis, and thrombocytosis, to stratify ambulatory cancer patients receiving chemotherapy according to their risk of VTE.13 The score separated patients into low risk (0.3% to 0.8%), intermediate risk (1.8% to 2.0%) and high risk (6.7% to 7.1%) of VTE.13 The PROTECT score added the use of chemotherapeutic agents (cisplatin, carboplatin, and gemcitabine) to the Khorana score.14 In addition, the biomarkers D-dimer and soluble P-selectin were used to extend the Khorana score in the Vienna Cancer and Thrombosis Study (CATS) score.15 The advantage of the D-dimer assay is that it is routinely used in the clinic. A recent study of 876 patients of which 53 (6.1%) developed VTE evaluated the different risk scores and found that they identified 13% to 34% of the VTE patients.16 The authors concluded that prediction scores were poor at predicting VTE in cancer patients and suggested that further improvements are needed before considering introduction of the scores into clinical practice.
There has been an interest in identifying biomarkers that can be used to identify cancer patients at high risk for VTE.17 The majority of studies have examined different biomarkers in pooled patient populations with different types of cancer.17 This approach may fail to detect biomarkers that are cancer type–specific because the numbers of samples for each cancer type are too small. A recent study18 summarized the results of 18 studies and 36 biomarkers used to predict thromboembolism in lung cancer. The study concluded that D-dimer and epidermal growth factor receptor (EGFR) mutation were the most reproducible predictors of thromboembolism. Future studies should focus on biomarkers and pathways of cancer-associated thrombosis in individual cancers.
Mouse studies can be used to determine the mechanisms by which a prothrombotic state, leukocytes, platelets, or other biomarkers increase venous thrombosis in cancer. Indeed, the best approach to studying pathways of cancer-associated thrombosis in different types of cancer is to integrate clinical observations with basic studies that use mouse models.
In this review, we summarize common and cancer type–specific pathways of cancer-associated thrombosis. An improved understanding of the pathways that drive thrombosis may allow for the development of novel therapies for the prevention of VTE in cancer patients.
Leukocytosis and cancer-associated thrombosis
A study in 198619 reported leukocytosis in 77 (30%) of 252 patients with different types of cancer. Leukocytosis was most frequently observed in patients with lung or colorectal tumors. The authors concluded that tumor-associated leukocytosis may be an additional tumor-associated marker of value in assessing and monitoring patients with nonhematologic malignancies. A large retrospective study found extreme leukocytosis in 758 (20%) of 3770 patients with nonhematologic cancer.20 In 69% of the patients, the etiology of the paraneoplastic leukemoid reaction was the result of myeloid growth factors.20 Another study observed leukocytosis in 33 (14%) of 227 patients with lung cancer.21 Importantly, some of the patients had increased levels of the hematopoietic cytokine granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin-6 (IL-6),21 which seemed to be expressed by the tumor. One study that included 145 patients with soft tissue sarcomas reported a high prevalence of neutrophilia (28%) and monocytosis (19%).22
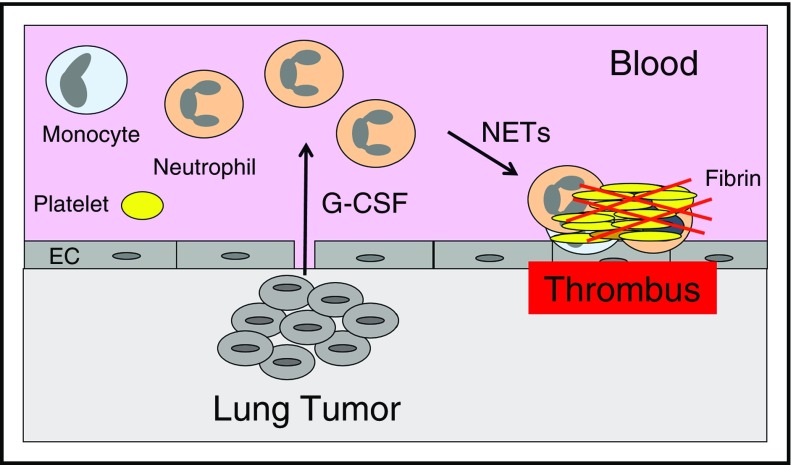
Several studies have reported that leukocytosis is associated with an increase in VTE in cancer patients,13,23,24 which suggests that leukocytosis may represent a common pathway of cancer-associated thrombosis. Neutrophils may enhance thrombosis by generating neutrophil extracellular traps (NETs),25 whereas monocytes may express the procoagulant protein tissue factor (TF).26 The mechanisms of tumor-associated leukocytosis have been studied in mouse models. One study found that the growth of the mouse mammary carcinoma 4T1 in BALB/c mice was associated with an increase in circulating neutrophils and splenomegaly.27 4T1 tumors excised from mice expressed G-CSF and GM-CSF, but only G-CSF was increased in the serum of tumor-bearing mice. Other studies observed a similar neutrophilia in mice that had 4T1 tumors.28,29 Importantly, administration of a neutralizing anti-G-CSF antibody abolished the neutrophilia in mice that had 4T1 tumors, and the injection of recombinant G-CSF into mice without tumors increased levels of circulating neutrophils.28 Both human and mouse G-CSF were present in the plasma of mice containing the human mammary tumor MDA-MB-231, suggesting that tumor cells and host cells express G-CSF.28 We observed neutrophilia in mice containing human pancreatic BxPc-3 tumors.30 These studies suggest that increased levels of hematopoietic cytokines leads to neutrophilia and that these neutrophils may be primed to release NETs that enhance thrombosis (Figure 1).
Figure 1.
Neutrophilia increases thrombosis in lung cancer. Tumor-derived G-CSF leads to increased levels of neutrophils, and these neutrophils release NETs that increase thrombosis in patients with lung cancer. EC, endothelial cell.
Leukocytes have been shown to contribute to venous thrombosis in mouse models.31,32 In a rat model of inferior vena cava (IVC) ligation, TF was expressed by adherent monocytes, neutrophils, and endothelial cells.33 NETs released from neutrophils have been shown to contribute to venous thrombosis in animal models.34-36 NETs help to capture platelets and microvesicles (MVs) that stabilize the clot.37-39 In addition, NETs increase TF activity by binding elastase and cathepsin G, which inactivates TF pathway inhibitor.40 One study found that G-CSF enhanced the ability of neutrophils to generate NETs.29
Taken together, these clinical and basic studies strongly suggest that neutrophils and monocytes contribute to cancer-associated thrombosis. Inhibition of hematopoietic cytokines or inhibition of NET formation are possibly strategies to reduce VTE in cancer patients with leukocytosis.
Thrombocytosis and cancer-associated thrombosis
Platelets play a central role in arterial thrombosis but also contribute to venous thrombosis in cancer patients.41,42 Thrombocytosis is often observed in patients with cancer, especially gastrointestinal, lung, breast, and ovarian cancer.6 Interestingly, individuals who had high platelet counts before they developed cancer had a higher rate of VTE compared with those who had low platelet counts.43 Similar results were observed when platelet counts were measured in patients with cancer.44,45
Treatment of patients with multiple myeloma who are receiving thalidomide or lenalidomide with low-dose aspirin reduced VTE to an extent similar to that of low-molecular-weight heparin, which suggests a role for platelets in venous thrombosis in these patients.46,47 In addition, aspirin use was associated with a borderline reduction in VTE in patients with ovarian cancer but not in those with breast cancer.48,49
Numerous studies have measured biomarkers of platelet activation in cancer.42 In general, these biomarkers are increased in cancer patients, but only a limited number of studies have determined whether they are predictive of VTE in this group. A recent study failed to find an association between various platelet activation markers such as platelet factor 4 (PF4) and VTE in patients with a variety of cancer types.50 However, another study of pancreatic cancer found that an elevated level of PF4 was associated with an approximately threefold increased risk of VTE.51 This difference emphasizes the importance of studying individual cancer types.
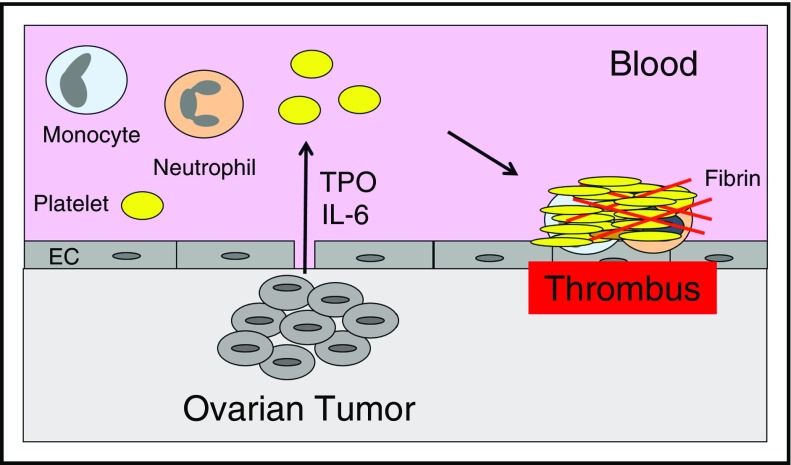
The mechanism of paraneoplastic thrombocytosis in ovarian cancer has been investigated in a mouse model. Studies showed that tumor-derived IL-6 increased hepatic thrombopoietin synthesis and thrombopoiesis52 (Figure 2). Therefore, interruption of the IL-6-thrombopoietin pathway may reduce levels of platelets and VTE in patients with ovarian cancer.
Figure 2.
Thrombocytosis increases thrombosis in ovarian cancer. Tumor-derived IL-6 stimulates hepatocytes to express thrombopoietin (TPO), which increases platelet production and enhances thrombosis in patients with ovarian cancer.
The role of platelets in thrombosis has also been studied in mouse cancer models. One study used a syngeneic orthotopic model of pancreatic cancer and found that clopidogrel reduced the binding of tumor-derived MVs to the site of thrombosis.53 We found that TF+ MVs activated platelets via thrombin and that enhancement of thrombosis in mice by injection of exogenous TF+ MVs was reduced by clopidogrel.54 Taken together, these clinical and basic studies suggest that anti-platelet drugs may be useful in preventing VTE in some cancer patients.42 Indeed, there is an ongoing clinical trial evaluating the effect of aspirin on platelet activation markers and VTE in high- and intermediate-risk cancer patients (NCT02285738).
P-selectin and cancer-associated thrombosis
Cancer patients with high levels of circulating soluble P-selectin were found to have a high rate of VTE.55 P-selectin is expressed by both platelets and endothelial cells. P-selectin expression by endothelial cells could enhance VTE by recruiting leukocytes. It has been shown that inhibition of P-selectin reduced venous thrombosis in a baboon stasis model.56 More recently, it was shown that endothelial P-selectin was responsible for the recruitment of leukocytes in a mouse model of IVC stenosis.31 Finally, inhibition of P-selectin reduced thrombosis in a mesenteric ferric chloride model in mice with pancreatic tumors without affecting thrombosis in non–tumor-bearing mice.57 These studies suggest that P-selectin may be a novel target for preventing cancer-associated thrombosis by reducing the recruitment of leukocytes and possibly the binding of MVs.
Hypofibrinolysis and cancer-associated thrombosis
Plasminogen activator inhibitor type 1 (PAI-1) inhibits fibrinolysis; therefore, elevated levels are associated with thrombosis.58 One study found that patients with deep vein thrombosis had prolonged clot lysis times compared with healthy controls,59 suggesting that hypofibrinolysis was a risk factor for VTE. Another study measured clot lysis times and different components of the fibrinolytic pathway in thrombosis patients and healthy controls and concluded that elevated levels of PAI-1 and thrombin-activatable fibrinolysis inhibitor contribute to the elevated clot lysis time observed in thrombosis patients.60 Finally, a case-control study found higher levels of active PAI-1 in VTE patients compared with controls.61
There are few studies evaluating the fibrinolytic system in cancer-associated thrombosis. A study of patients with pancreatic cancer suggested that elevated levels of PAI-1 antigen and activity may predispose patients to VTE.62 Another study found higher levels of PAI-1 in glioma patients compared with healthy controls.63
One mouse study investigated the role of PAI-1 in thrombosis in a subcutaneous xenograft model with A549 cells, a human lung adenocarcinoma cell line.64 Tumor-bearing mice had larger thrombi in a 3-hour IVC stenosis model and shorter occlusion times in a ferric chloride saphenous vein model. Administration of the anti-VEGF drug bevacizumab further increased thrombosis in both models. Interestingly, bevacizumab increased PAI-1 expression in tumors and in plasma leading to enhanced thrombosis that was reduced by a PAI-1 inhibitor. Further studies are needed to determine the role of hypofibrinolysis and PAI-1 in cancer-associated thrombosis.
TF+ MVs and venous thrombosis in pancreatic cancer
Human pancreatic tumors express high levels of TF, and expression is correlated with histologic grade.65 One study observed an association between intratumoral TF expression and VTE.66 Human pancreatic cell lines also express high levels of TF and release TF+ MVs.67
MVs (also known as microparticles [MPs] or extracellular vesicles) are small membrane vesicles released from activated or apoptotic cells.68 MVs are procoagulant because they provide a surface for the assembly of different coagulation factor complexes.69 The procoagulant activity of MVs is increased by the presence of negatively charged phospholipids such as phosphatidylserine (PSer) and TF. Platelets and red blood cells produce PSer– and PSer+ MVs, whereas activated monocytes and tumor cells release highly procoagulant PSer+ TF+ MVs.70-72
Dvorak et al73 first proposed a relationship between tumor-derived MVs and thrombosis. The authors stated that shed vesicles carry procoagulant activity that can account for the activation of the clotting system and the fibrin deposition associated with these and many other types of malignancy in animals and humans. Subsequent studies showed that the procoagulant activity of the tumor-derived MVs was a result of the presence of TF.74-76 Many types of cancer cells express TF and release TF+ MVs.72 Furthermore, circulating TF+ MVs can be detected in patients with a variety of cancers, including pancreatic, lung, gastric, breast, and brain.77-81 It is likely that these TF+ MVs are released from the tumor. For instance, 1 study in patients with pancreatic cancer found that the TF+ MVs co-expressed the epithelial tumor antigen MUC-1 and that pancreatectomy dramatically reduced the level of TF+ MVs.79
The majority of studies have examined the relationship between TF+ MVs and VTE in patients with pancreatic, brain, colorectal, or lung cancer because these patients have the highest rates of VTE.72 TF+ MVs were detected in all these cancers but patients with pancreatic cancer were found to have the highest levels of MV TF activity.80 One possible explanation for this is that the endocrine function of the pancreas provides an easy route for transporting TF+ MVs from the tumor into the blood. A longitudinal study with 11 pancreatic cancer patients found a time-dependent elevation of MV TF activity that preceded VTE in 2 patients.66 Several other prospective studies found an association between MV TF activity in patients with pancreatic cancer but not in patients with lung, gastric, colorectal, ovarian, or brain cancer78,80-82 (Raj Kasthuri, Y.H., N.M., Nigel Key, Houtan Noushmehr, Daniel J. Weisenberger, Kristin Diefes, Heidi S. Phillips, Kanan Pujara, Benjamin P. Berman, Fei Pan, Christopher E. Pelloski, Erik P. Sulman, Krishna P. Bhat, Roel G.W. Verhaak, Katherine A. Hoadley, D. Neil Hayes, Charles M. Perou, Heather K. Schmidt, Li Ding, Richard K. Wilson, David Van Den Berg, Hui Shen, Henrik Bengtsson, Pierre Neuvial, Leslie M. Cope, Jonathan Buckley, James G. Herman, Stephen B. Baylin, Peter W. Laird, Kenneth Aldape, and The Cancer Genome Atlas Research Network, unpublished data). There was also a strong association between MV TF activity and mortality in patients with pancreatic cancer.80,81 This suggests that TF+ MVs may be a biomarker of the most aggressive tumors.80,81
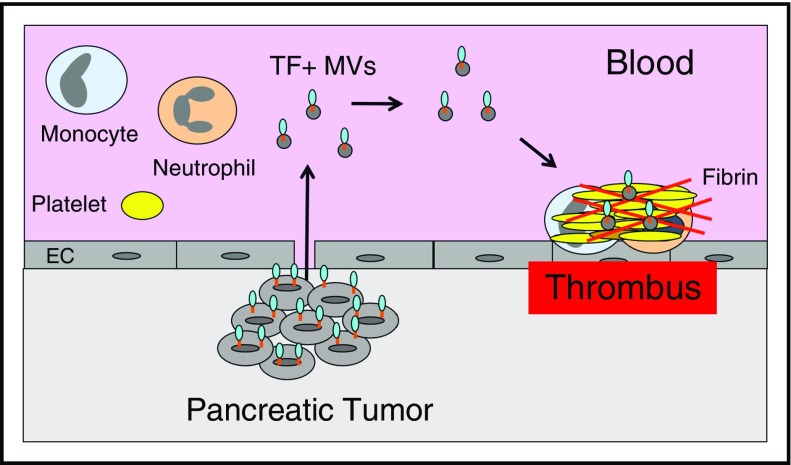
These studies suggest that TF+ MVs contribute to VTE in pancreatic cancer patients (Figure 3) and may be a useful biomarker for assessing the risk of VTE in these patients. It is likely that TF+ MVs contribute to VTE in other types of cancer, but the current studies are too small and the current assays are not sensitive or specific enough to reveal a relationship between TF+ MVs and VTE. Further development of TF+ MV assays is needed before they can be used clinically.
Figure 3.
Tumor-derived TF+MVs trigger thrombosis in pancreatic cancer. Pancreatic tumor cells release TF+ MVs into the circulation that trigger thrombosis in patients with pancreatic cancer.
Tumor allograft and xenograft mouse models have been used to study the role of tumor-derived TF+ MVs in the activation of coagulation and thrombosis (Table 2). The advantage of the allograft model is that the mice are immunocompetent; however, the number of mouse pancreatic cancer cell lines is limited. The advantage of the xenograft model is that tumor-derived human TF+ MVs can be distinguished from host-derived mouse TF, and numerous human pancreatic cancer cell lines are available. Tumors can be grown either subcutaneously or orthotopically, but most studies grow tumors subcutaneously because tumor growth can be easily measured. However, tumors grown orthotopically are in a local environment that more closely mimics the environment found in patients. The disadvantage of orthotopic tumors is that growth cannot be measured without engineering the tumor cells to express a reporter, such as firefly luciferase.
Table 2.
Mouse studies on the role of tumor-derived TF+ MVs in cancer-associated thrombosis
| Tumor type | Mouse | Tumor site | Conclusion | Reference |
|---|---|---|---|---|
| Human colorectal cancer cell lines | SCID | Subcutaneous | Human TF antigen in plasma | 83 |
| Human pancreatic cancer cell line L3.6pl | Nude | Orthotopic | Human TF MV activity and TAT in plasma | 84 |
| Mouse pancreatic cancer cell line Panc02 and lung cancer cell line LLC1 | C57BL/6J | Subcutaneous | Increased thrombosis in mesenteric ferric chloride model | 57 |
| Human pancreatic cancer cell lines HPAF-II and HPAC | Nude | Subcutaneous and orthotopic | Human TF antigen and MV activity and TAT in plasma | 67 |
| Mouse pancreatic cancer cell line Panc02 | C57BL/6J | Subcutaneous | Increased thrombosis in IVC stenosis model | 85 |
| Human pancreatic cancer cell lines BxPc-3 and L3.6pl | Nude | Orthotopic | Increased thrombosis in IVC stenosis model | 54 |
| Human pancreatic cancer cell line BxPc-3 | Nude | Orthotopic | Human TF+ MVs increase thrombosis in IVC stasis model | 30 |
TAT, thrombin-antithrombin [complex].
An early study showed that human colorectal tumors grown in SCID mice released human TF into the circulation and that the amount of TF antigen in the plasma correlated with tumor size.83 Another study found that nude mice with orthotopic human pancreatic tumors (L3.6pl) had increased MV TF activity and activation of coagulation that increased with tumor size.84 We compared the release of human TF+ MVs and activation of coagulation in nude mice with subcutaneous or orthotopic HPAF-II tumors of the same size.67 Interestingly, only mice with orthotopic tumors had detectable human TF+ MVs and activation of coagulation. This observation supports the notion that tumors in the pancreas can efficiently release TF+ MVs into the blood. Furthermore, we showed that the activation of coagulation in tumor-bearing mice was abolished by the administration of an anti-human TF monoclonal antibody,67 which indicated that tumor-derived TF was responsible for the activation of coagulation.
Thrombosis experiments have been performed in either healthy mice injected with tumor-derived TF+ MVs or in tumor-bearing mice. As expected, we and others have shown that injection of exogenous TF+ MVs derived from human and mouse pancreatic cancer cell lines into mice activated coagulation and enhanced thrombosis in various models.54,57,67,85 However, injection of exogenous TF+ MVs into healthy mice is not an ideal model for studying the role of tumor-derived TF+ MVs in cancer-associated thrombosis because TF+ MVs are likely to be only 1 of many factors that increase clot size in mice and cancer patients. Furthermore, the amount of exogenous TF+ MVs required to increase clot size is 10- to 100-fold higher than the levels of endogenous tumor-derived TF+ MVs, which suggests that TF+ MVs combine with other factors to increase clot size in mice.54,67 In addition, we and others found that the administration of TF+ MVs induced severe thrombocytopenia, shock, and death in some of the mice.54,84 For these reasons, we believe that analysis of thrombosis tumor–bearing mice is a better approach to elucidate the mechanisms of cancer-associated thrombosis in patients.
The murine pancreatic cancer cell line Panc02 has been used to study the role of tumor-derived MVs in thrombosis. Interestingly, we observed a relatively low level of TF expression in Panc02 cells compared with other murine pancreatic cell lines, such as cell lines derived from KPC mice86 (Y.H. and N.M., unpublished data). An early study showed that tumor-derived MVs localized to the site of thrombosis and that mice with Panc02 tumors had increased thrombosis in a ferric chloride mesentery vessel model.57 Thrombosis was reduced by inhibition of P-selectin, which led the authors to conclude that their results indicated that cancer cell–derived MPs bearing the ligand for P-selectin (PSGL-1) and TF play a key role in thrombus formation in vivo.57 In a follow-up study, the incidence of thrombi was increased at 3 hours in the IVC stenosis model in mice with Panc02 tumors compared with control mice.85 The authors concluded that TF expression on tumor MPs contributed to the incidence of cancer-associated venous thrombosis in mice in vivo.85 This conclusion was based on the fact that injection of MVs from a high-TF–expressing Panc02 line enhanced thrombosis to a greater extent than MVs from a low-TF–expressing Panc02 line. However, these 2 studies with Panc02 cells did not directly analyze the role of TF on the MVs in the increased thrombosis.
We examined thrombosis in a xenograft mouse model with human pancreatic cancer cell lines expressing high levels of TF.54,67 In the first study with HPAF-II orthotopic tumors, we failed to detect an increase in incidence of thrombosis at 3 hours in an IVC stenosis model.67 In a second study with BxPc-3 orthotopic tumors, we observed an increase in incidence and clot area between 3 and 24 hours in the IVC stenosis model in tumor-bearing mice compared with control mice.54 Recently, we found that mice with orthotopic BxPc-3 tumors had significantly larger clots compared with control mice at 24 and 48 hours in an IVC stasis model.30 Importantly, inhibition of human TF significantly reduced the clot size in tumor-bearing mice compared with mice receiving control immunoglobulin G.30 These studies indicate that tumor-derived, TF+ MVs are responsible for the increased clot size in tumor-bearing mice and likely contribute to VTE in patients with pancreatic cancer. Inhibition of the release of TF+ MVs from tumor cells or blocking the binding of the MVs to sites of thrombosis may be novel strategies to reduce VTE in patients with pancreatic cancer.
TF and podoplanin expression in glioblastoma multiforme subtypes and venous thrombosis
Large-scale profiling studies of glioblastoma multiforme (GBM) revealed the existence of four distinct GBM subtypes: proneural, neural, classic, and mesenchymal.87 The proneural subtype is a lower-grade GBM that occurs in younger patients. A recent study used promoter DNA methylation to identify a glioma-CpG island methylator phenotype that was most closely associated with the proneural subtype.88 In addition, mutant isocitrate dehydrogenase 1 (IDH1) GBM is largely present in the proneural subtype whereas wild-type IDH1 GBM is largely present in neural, classic, and mesenchymal subtypes.
There are significant differences in the level of TF expression in different GBM subtypes. The lowest level of TF expression is observed in the proneural subtype/glioma-CpG island methylator phenotype, and the highest level is observed in the classic subtype.89 This pattern of expression is consistent with earlier studies showing that amplification of the EGFR or expression of the mutant form of EGFR (EGFRvIII) drive TF expression in GBM cells.90-92 Interestingly, we found that the human F3 gene contains a CpG island in its promoter making it likely that F3 gene expression is regulated by methylation.93 Indeed, F3 was among the top 50 genes that were most differentially hypermethylated and downregulated in this methylator phenotype.88 Strikingly, the rate of VTE in patients with wild-type IDH1 GBM was 26% to 30% compared with 0% in patients with mutant IDH1 GBM.94 Consistent with the article by Noushmehr et al,88 another study found that the F3 promoter was hypermethylated in mutant IDH1 GBM resulting in lower TF expression.94 A recent small study found that intratumoral TF expression in patients with brain cancer was not associated with VTE.95 Similarly, we did not find a relationship between MV TF activity and VTE in brain cancer patients.80 One study reported an association between TF+ MVs and VTE in brain cancer patients by using flow cytometry to detect the TF+ MVs.96 However, there was no detectable MV TF activity in these samples,72 and we and others have failed to detect TF+ MVs in plasma by flow cytometry.97 Therefore, at present, there is no convincing data to show that elevated levels of TF+ MVs are associated with VTE in brain cancer patients. Further studies are needed to investigate the relationship between TF expression and VTE in GBM patients.
Podoplanin (PDPN) expression in GBM patients is associated with reduced survival.98 PI3K/Akt signaling is activated in many cancer types, including human GBM, and PDPN is a downstream effector of PI3K signaling.99 Interestingly, mutant IDH1 is associated with reduced PI3K signaling and PDPN expression.100 The R132H mutation in IDH1 results in a gain of enzyme function with increased production of D-2-hydroxyglutarate (D-2-HG). Moreover, PDPN was among the top 50 genes that were most differentially hypermethylated and downregulated in the glioma-CpG island methylator phenotype.88
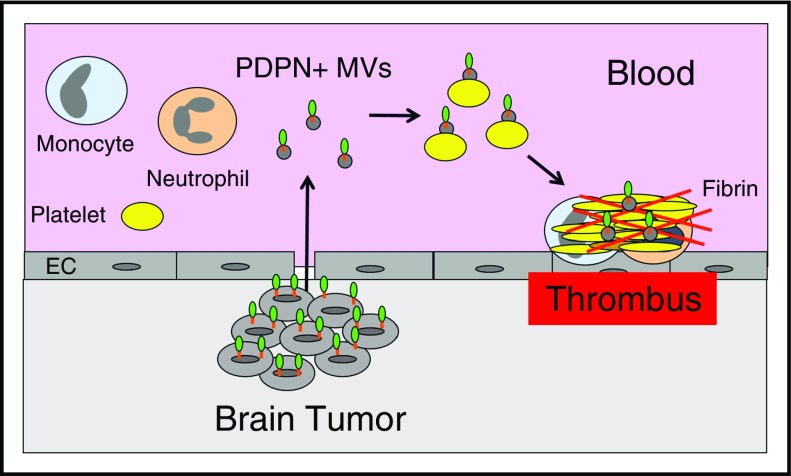
PDPN is a ligand for the platelet receptor C-type lectin receptor type-2 (CLEC-2) and induces platelet aggregation.101,102 A recent study showed that mice deficient in PDPN were protected from venous thrombosis.103 Importantly, a PDPN+ human glioblastoma cell line LN319 induced platelet aggregation in a CLEC-2–dependent manner.104 A recent study found that brain cancer patients with low platelet counts (<25% of the study population) were at increased risk of VTE.105 This observation suggests that platelets were activated by the presence of the cancer resulting in lower platelet counts. In a subsequent study, PDPN expression in brain cancer patients was shown to be inversely correlated with platelet counts and positively correlated with D-dimer.106 These data suggest that brain tumors may release PDPN+ MVs that activate circulating platelets resulting in increased VTE in patients with brain cancer (Figure 4). Additional studies are needed to determine whether PDPN+ brain tumors release PDPN+ MVs into the circulation and whether they are associated with an increased risk of VTE.
Figure 4.
Tumor-derived, PDPN+MVs trigger thrombosis in brain cancer. Brain tumor cells may release PDPN+ MVs that activate circulating platelets and increase thrombosis in patients with brain cancer.
Conclusion
Leukocytes, platelets, and MVs all seem to contribute to cancer-associated thrombosis. The current scoring systems use site of cancer, leukocyte and platelet counts, chemotherapy, and biomarkers to identify patients with all types of cancer who are at high risk of VTE. However, the identification of novel VTE-associated biomarkers in individual malignancies is required to drive the development of cancer type–specific scoring systems that have improved predictive value. Prevention and treatment of cancer-associated thrombosis may be improved by the use of conventional antithrombotics in combination with agents that reduce leukocyte and platelet counts, levels of TF+ MVs, and inhibit NET formation.
Acknowledgments
The authors thank Wolfgang Bergmeier, Steven P. Grover, Nigel S. Key, Cihan Ay, and Alisa S. Wolberg and members of the Mackman laboratory for helpful comments.
This work was supported by funds from the K.G. Jebsen Thrombosis and Expertise Center (N.M.), by a Duke/University of North Carolina pilot grant (N.M.), and by grant HL 095096 from the National Institutes of Health, National Heart, Lung, and Blood Institute (N.M.).
Authorship
Contribution: Y.H. and N.M. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nigel Mackman, Department of Medicine, Division of Hematology and Oncology, 111 Mason Farm Rd, 2312C Medical Biomolecular Research Bldg, CB#7126, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599; e-mail: nmackman@med.unc.edu.
References
- 1.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632-634. [DOI] [PubMed] [Google Scholar]
- 2.Di Nisio M, Ferrante N, Feragalli B, et al. . Arterial thrombosis in ambulatory cancer patients treated with chemotherapy. Thromb Res. 2011;127(4):382-383. [DOI] [PubMed] [Google Scholar]
- 3.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: biological and clinical aspects. J Thromb Haemost. 2013;11(2):223-233. [DOI] [PubMed] [Google Scholar]
- 4.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712-1723. [DOI] [PubMed] [Google Scholar]
- 5.Blom JW, Vanderschoot JP, Oostindiër MJ, Osanto S, van der Meer FJ, Rosendaal FR. Incidence of venous thrombosis in a large cohort of 66,329 cancer patients: results of a record linkage study. J Thromb Haemost. 2006;4(3):529-535. [DOI] [PubMed] [Google Scholar]
- 6.Levin J, Conley CL. Thrombocytosis associated with malignant disease. Arch Intern Med. 1964;114:497-500. [DOI] [PubMed] [Google Scholar]
- 7.Kuderer NM, Lyman GH. Guidelines for treatment and prevention of venous thromboembolism among patients with cancer. Thromb Res. 2014;133(Suppl 2):S122-S127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khorana AA. Cancer-associated thrombosis: updates and controversies. Hematology Am Soc Hematol Educ Program 2012;2012:626-630. [DOI] [PubMed]
- 9.Chew HK, Wun T, Harvey D, Zhou H, White RH. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med. 2006;166(4):458-464. [DOI] [PubMed] [Google Scholar]
- 10.Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Falanga A, Marchetti M, Russo L. Venous thromboembolism in the hematologic malignancies. Curr Opin Oncol. 2012;24(6):702-710. [DOI] [PubMed] [Google Scholar]
- 12.Paneesha S, McManus A, Arya R, et al. ; VERITY Investigators. Frequency, demographics and risk (according to tumour type or site) of cancer-associated thrombosis among patients seen at outpatient DVT clinics. Thromb Haemost. 2010;103(2):338-343. [DOI] [PubMed] [Google Scholar]
- 13.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111(10):4902-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verso M, Agnelli G, Barni S, Gasparini G, LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012;7(3):291-292. [DOI] [PubMed] [Google Scholar]
- 15.Ay C, Dunkler D, Marosi C, et al. . Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377-5382. [DOI] [PubMed] [Google Scholar]
- 16.van Es N, Di Nisio M, Cesarman G, et al. . Comparison of risk prediction scores for venous thromboembolism in cancer patients: a prospective cohort study [published online ahead of print on 26 May 2017]. Haematologica. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pabinger I, Thaler J, Ay C. Biomarkers for prediction of venous thromboembolism in cancer. Blood. 2013;122(12):2011-2018. [DOI] [PubMed] [Google Scholar]
- 18.Alexander M, Burbury K. A systematic review of biomarkers for the prediction of thromboembolism in lung cancer - Results, practical issues and proposed strategies for future risk prediction models. Thromb Res. 2016;148:63-69. [DOI] [PubMed] [Google Scholar]
- 19.Shoenfeld Y, Tal A, Berliner S, Pinkhas J. Leukocytosis in non hematological malignancies--a possible tumor-associated marker. J Cancer Res Clin Oncol. 1986;111(1):54-58. [DOI] [PubMed] [Google Scholar]
- 20.Granger JM, Kontoyiannis DP. Etiology and outcome of extreme leukocytosis in 758 nonhematologic cancer patients: a retrospective, single-institution study. Cancer. 2009;115(17):3919-3923. [DOI] [PubMed] [Google Scholar]
- 21.Kasuga I, Makino S, Kiyokawa H, Katoh H, Ebihara Y, Ohyashiki K. Tumor-related leukocytosis is linked with poor prognosis in patients with lung carcinoma. Cancer. 2001;92(9):2399-2405. [DOI] [PubMed] [Google Scholar]
- 22.Ruka W, Rutkowski P, Kaminska J, Rysinska A, Steffen J. Alterations of routine blood tests in adult patients with soft tissue sarcomas: relationships to cytokine serum levels and prognostic significance. Ann Oncol. 2001;12(10):1423-1432. [DOI] [PubMed] [Google Scholar]
- 23.Pabinger I, Posch F. Flamethrowers: blood cells and cancer thrombosis risk. Hematology Am Soc Hematol Educ Program. 2014;2014(1):410-417. [DOI] [PubMed] [Google Scholar]
- 24.Blix K, Jensvoll H, Brækkan SK, Hansen JB. White blood cell count measured prior to cancer development is associated with future risk of venous thromboembolism--the Tromsø study. PLoS One. 2013;8(9):e73447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brinkmann V, Reichard U, Goosmann C, et al. . Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532-1535. [DOI] [PubMed] [Google Scholar]
- 26.Gregory SA, Morrissey JH, Edgington TS. Regulation of tissue factor gene expression in the monocyte procoagulant response to endotoxin. Mol Cell Biol. 1989;9(6):2752-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DuPré SA, Hunter KW Jr. Murine mammary carcinoma 4T1 induces a leukemoid reaction with splenomegaly: association with tumor-derived growth factors. Exp Mol Pathol. 2007;82(1):12-24. [DOI] [PubMed] [Google Scholar]
- 28.Kowanetz M, Wu X, Lee J, et al. . Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci USA. 2010;107(50):21248-21255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demers M, Krause DS, Schatzberg D, et al. . Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA. 2012;109(32):13076-13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hisada Y, Ay C, Auriemma AC, Cooley BC, Mackman N. Human pancreatic tumors grown in mice release tissue factor-positive microvesicles that increase venous clot size. J Thromb Haemost. In press. [DOI] [PubMed] [Google Scholar]
- 31.von Brühl ML, Stark K, Steinhart A, et al. . Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laurance S, Bertin FR, Ebrahimian T, et al. . Gas6 promotes inflammatory (CCR2hiCX3CR1lo) monocyte recruitment in venous thrombosis. Arterioscler Thromb Vasc Biol. 2017;37(7):1315-1322. [DOI] [PubMed] [Google Scholar]
- 33.Zhou J, May L, Liao P, Gross PL, Weitz JI. Inferior vena cava ligation rapidly induces tissue factor expression and venous thrombosis in rats. Arterioscler Thromb Vasc Biol. 2009;29(6):863-869. [DOI] [PubMed] [Google Scholar]
- 34.Fuchs TA, Brill A, Duerschmied D, et al. . Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107(36):15880-15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brill A, Fuchs TA, Savchenko AS, et al. . Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10(1):136-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinod K, Demers M, Fuchs TA, et al. . Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA. 2013;110(21):8674-8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Demers M, Wagner DD. Neutrophil extracellular traps: A new link to cancer-associated thrombosis and potential implications for tumor progression. OncoImmunology. 2013;2(2):e22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demers M, Wong SL, Martinod K, et al. . Priming of neutrophils toward NETosis promotes tumor growth. OncoImmunology. 2016;5(5):e1134073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olsson AK, Cedervall J. NETosis in cancer - platelet-neutrophil crosstalk promotes tumor-associated pathology. Front Immunol. 2016;7:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massberg S, Grahl L, von Bruehl ML, et al. . Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16(8):887-896. [DOI] [PubMed] [Google Scholar]
- 41.Connolly GC, Phipps RP, Francis CW. Platelets and cancer-associated thrombosis. Semin Oncol. 2014;41(3):302-310. [DOI] [PubMed] [Google Scholar]
- 42.Riedl J, Pabinger I, Ay C. Platelets in cancer and thrombosis. Hamostaseologie. 2014;34(1):54-62. [DOI] [PubMed] [Google Scholar]
- 43.Jensvoll H, Blix K, Brækkan SK, Hansen JB. Platelet count measured prior to cancer development is a risk factor for future symptomatic venous thromboembolism: the Tromsø Study. PLoS One. 2014;9(3):e92011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khorana AA, Francis CW, Culakova E, Lyman GH. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104(12):2822-2829. [DOI] [PubMed] [Google Scholar]
- 45.Simanek R, Vormittag R, Ay C, et al. . High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost. 2010;8(1):114-120. [DOI] [PubMed] [Google Scholar]
- 46.Palumbo A, Cavo M, Bringhen S, et al. . Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol. 2011;29(8):986-993. [DOI] [PubMed] [Google Scholar]
- 47.Larocca A, Cavallo F, Bringhen S, et al. . Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012;119(4):933-939. [DOI] [PubMed] [Google Scholar]
- 48.Shai A, Rennert HS, Rennert G, Sagi S, Leviov M, Lavie O. Statins, aspirin and risk of thromboembolic events in ovarian cancer patients. Gynecol Oncol. 2014;133(2):304-308. [DOI] [PubMed] [Google Scholar]
- 49.Shai A, Rennert HS, Lavie O, et al. . Statins, aspirin and risk of venous thromboembolic events in breast cancer patients. J Thromb Thrombolysis. 2014;38(1):32-38. [DOI] [PubMed] [Google Scholar]
- 50.Riedl J, Hell L, Kaider A, et al. . Association of platelet activation markers with cancer-associated venous thromboembolism. Platelets. 2016;27(1):80-85. [DOI] [PubMed] [Google Scholar]
- 51.Poruk KE, Firpo MA, Huerter LM, et al. . Serum platelet factor 4 is an independent predictor of survival and venous thromboembolism in patients with pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19(10):2605-2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stone RL, Nick AM, McNeish IA, et al. . Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366(7):610-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mezouar S, Darbousset R, Dignat-George F, Panicot-Dubois L, Dubois C. Inhibition of platelet activation prevents the P-selectin and integrin-dependent accumulation of cancer cell microparticles and reduces tumor growth and metastasis in vivo. Int J Cancer. 2015;136(2):462-475. [DOI] [PubMed] [Google Scholar]
- 54.Geddings JE, Hisada Y, Boulaftali Y, et al. . Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J Thromb Haemost. 2016;14(1):153-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ay C, Simanek R, Vormittag R, et al. . High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood. 2008;112(7):2703-2708. [DOI] [PubMed] [Google Scholar]
- 56.Meier TR, Myers DD Jr, Wrobleski SK, et al. . Prophylactic P-selectin inhibition with PSI-421 promotes resolution of venous thrombosis without anticoagulation. Thromb Haemost. 2008;99(2):343-351. [DOI] [PubMed] [Google Scholar]
- 57.Thomas GM, Panicot-Dubois L, Lacroix R, Dignat-George F, Lombardo D, Dubois C. Cancer cell-derived microparticles bearing P-selectin glycoprotein ligand 1 accelerate thrombus formation in vivo. J Exp Med. 2009;206(9):1913-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westrick RJ, Eitzman DT. Plasminogen activator inhibitor-1 in vascular thrombosis. Curr Drug Targets. 2007;8(9):966-1002. [DOI] [PubMed] [Google Scholar]
- 59.Lisman T, de Groot PG, Meijers JC, Rosendaal FR. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005;105(3):1102-1105. [DOI] [PubMed] [Google Scholar]
- 60.Meltzer ME, Lisman T, de Groot PG, et al. . Venous thrombosis risk associated with plasma hypofibrinolysis is explained by elevated plasma levels of TAFI and PAI-1. Blood. 2010;116(1):113-121. [DOI] [PubMed] [Google Scholar]
- 61.Bollen L, Peetermans M, Peeters M, et al. . Active PAI-1 as marker for venous thromboembolism: case-control study using a comprehensive panel of PAI-1 and TAFI assays. Thromb Res. 2014;134(5):1097-1102. [DOI] [PubMed] [Google Scholar]
- 62.Andrén-Sandberg A, Lecander I, Martinsson G, Astedt B. Peaks in plasma plasminogen activator inhibitor-1 concentration may explain thrombotic events in cases of pancreatic carcinoma. Cancer. 1992;69(12):2884-2887. [DOI] [PubMed] [Google Scholar]
- 63.Sciacca FL, Ciusani E, Silvani A, et al. . Genetic and plasma markers of venous thromboembolism in patients with high grade glioma. Clin Cancer Res. 2004;10(4):1312-1317. [DOI] [PubMed] [Google Scholar]
- 64.Chen N, Ren M, Li R, et al. . Bevacizumab promotes venous thromboembolism through the induction of PAI-1 in a mouse xenograft model of human lung carcinoma. Mol Cancer. 2015;14:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kakkar AK, Lemoine NR, Scully MF, Tebbutt S, Williamson RC. Tissue factor expression correlates with histological grade in human pancreatic cancer. Br J Surg. 1995;82(8):1101-1104. [DOI] [PubMed] [Google Scholar]
- 66.Khorana AA, Ahrendt SA, Ryan CK, et al. . Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13(10):2870-2875. [DOI] [PubMed] [Google Scholar]
- 67.Wang JG, Geddings JE, Aleman MM, et al. . Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119(23):5543-5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.György B, Szabó TG, Pásztói M, et al. . Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68(16):2667-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gardiner C, Harrison P, Belting M, et al. . Extracellular vesicles, tissue factor, cancer and thrombosis - discussion themes of the ISEV 2014 Educational Day. J Extracell Vesicles. 2015;4:26901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contributions of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011;9(11):2251-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koshiar RL, Somajo S, Norström E, Dahlbäck B. Erythrocyte-derived microparticles supporting activated protein C-mediated regulation of blood coagulation. PLoS One. 2014;9(8):e104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Geddings JE, Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122(11):1873-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dvorak HF, Quay SC, Orenstein NS, et al. . Tumor shedding and coagulation. Science. 1981;212(4497):923-924. [DOI] [PubMed] [Google Scholar]
- 74.Dvorak HF, Van DeWater L, Bitzer AM, et al. . Procoagulant activity associated with plasma membrane vesicles shed by cultured tumor cells. Cancer Res. 1983;43(9):4434-4442. [PubMed] [Google Scholar]
- 75.Bastida E, Ordinas A, Escolar G, Jamieson GA. Tissue factor in microvesicles shed from U87MG human glioblastoma cells induces coagulation, platelet aggregation, and thrombogenesis. Blood. 1984;64(1):177-184. [PubMed] [Google Scholar]
- 76.Yu JL, Rak JW. Shedding of tissue factor (TF)-containing microparticles rather than alternatively spliced TF is the main source of TF activity released from human cancer cells. J Thromb Haemost. 2004;2(11):2065-2067. [DOI] [PubMed] [Google Scholar]
- 77.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5(3):520-527. [DOI] [PubMed] [Google Scholar]
- 78.Khorana AA, Francis CW, Menzies KE, et al. . Plasma tissue factor may be predictive of venous thromboembolism in pancreatic cancer. J Thromb Haemost. 2008;6(11):1983-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zwicker JI, Liebman HA, Neuberg D, et al. . Tumor-derived tissue factor-bearing microparticles are associated with venous thromboembolic events in malignancy. Clin Cancer Res. 2009;15(22):6830-6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thaler J, Ay C, Mackman N, et al. . Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10(7):1363-1370. [DOI] [PubMed] [Google Scholar]
- 81.Bharthuar A, Khorana AA, Hutson A, et al. . Circulating microparticle tissue factor, thromboembolism and survival in pancreaticobiliary cancers. Thromb Res. 2013;132(2):180-184. [DOI] [PubMed] [Google Scholar]
- 82.Cohen JG, Prendergast E, Geddings JE, et al. . Evaluation of venous thrombosis and tissue factor in epithelial ovarian cancer. Gynecol Oncol. 2017;146(1):146-152. [DOI] [PubMed] [Google Scholar]
- 83.Yu JL, May L, Lhotak V, et al. . Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood. 2005;105(4):1734-1741. [DOI] [PubMed] [Google Scholar]
- 84.Davila M, Amirkhosravi A, Coll E, et al. . Tissue factor-bearing microparticles derived from tumor cells: impact on coagulation activation. J Thromb Haemost. 2008;6(9):1517-1524. [DOI] [PubMed] [Google Scholar]
- 85.Thomas GM, Brill A, Mezouar S, et al. . Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J Thromb Haemost. 2015;13(7):1310-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hingorani SR, Wang L, Multani AS, et al. . Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469-483. [DOI] [PubMed] [Google Scholar]
- 87.Verhaak RG, Hoadley KA, Purdom E, et al. ; Cancer Genome Atlas Research Network. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noushmehr H, Weisenberger DJ, Diefes K, et al. ; Cancer Genome Atlas Research Network. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell. 2010;17(5):510-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Magnus N, Gerges N, Jabado N, Rak J. Coagulation-related gene expression profile in glioblastoma is defined by molecular disease subtype. J Thromb Haemost. 2013;11(6):1197-1200. [DOI] [PubMed] [Google Scholar]
- 90.Milsom C, Magnus N, Meehan B, Al-Nedawi K, Garnier D, Rak J. Tissue factor and cancer stem cells: is there a linkage? Arterioscler Thromb Vasc Biol. 2009;29(12):2005-2014. [DOI] [PubMed] [Google Scholar]
- 91.Rong Y, Belozerov VE, Tucker-Burden C, et al. . Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein-1 transcriptional activity. Cancer Res. 2009;69(6):2540-2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Magnus N, Garnier D, Rak J. Oncogenic epidermal growth factor receptor up-regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood. 2010;116(5):815-818. [DOI] [PubMed] [Google Scholar]
- 93.Mackman N, Morrissey JH, Fowler B, Edgington TS. Complete sequence of the human tissue factor gene, a highly regulated cellular receptor that initiates the coagulation protease cascade. Biochemistry. 1989;28(4):1755-1762. [DOI] [PubMed] [Google Scholar]
- 94.Unruh D, Schwarze SR, Khoury L, et al. . Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol. 2016;132(6):917-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thaler J, Preusser M, Ay C, et al. . Intratumoral tissue factor expression and risk of venous thromboembolism in brain tumor patients. Thromb Res. 2013;131(2):162-165. [DOI] [PubMed] [Google Scholar]
- 96.Sartori MT, Della Puppa A, Ballin A, et al. . Circulating microparticles of glial origin and tissue factor bearing in high-grade glioma: a potential prothrombotic role. Thromb Haemost. 2013;110(2):378-385. [DOI] [PubMed] [Google Scholar]
- 97.Lee RD, Barcel DA, Williams JC, et al. . Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thromb Res. 2012;129(1):80-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ernst A, Hofmann S, Ahmadi R, et al. . Genomic and expression profiling of glioblastoma stem cell-like spheroid cultures identifies novel tumor-relevant genes associated with survival. Clin Cancer Res. 2009;15(21):6541-6550. [DOI] [PubMed] [Google Scholar]
- 99.Peterziel H, Müller J, Danner A, et al. . Expression of podoplanin in human astrocytic brain tumors is controlled by the PI3K-AKT-AP-1 signaling pathway and promoter methylation. Neuro-oncol. 2012;14(4):426-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Birner P, Pusch S, Christov C, et al. . Mutant IDH1 inhibits PI3K/Akt signaling in human glioma. Cancer. 2014;120(16):2440-2447. [DOI] [PubMed] [Google Scholar]
- 101.Suzuki-Inoue K, Inoue O, Ozaki Y. Novel platelet activation receptor CLEC-2: from discovery to prospects. J Thromb Haemost. 2011;9(Suppl 1):44-55. [DOI] [PubMed] [Google Scholar]
- 102.Watson SP, Herbert JM, Pollitt AY. GPVI and CLEC-2 in hemostasis and vascular integrity. J Thromb Haemost. 2010;8(7):1456-1467. [DOI] [PubMed] [Google Scholar]
- 103.Payne H, Ponomaryov T, Watson SP, Brill A. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood. 2017;129(14):2013-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Suzuki-Inoue K, Kato Y, Inoue O, et al. . Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282(36):25993-26001. [DOI] [PubMed] [Google Scholar]
- 105.Thaler J, Ay C, Kaider A, et al. . Biomarkers predictive of venous thromboembolism in patients with newly diagnosed high-grade gliomas. Neuro-oncol. 2014;16(12):1645-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Riedl J, Preusser M, Nazari PM, et al. . Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood. 2017;129(13):1831-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]