Key Points
Low-dose hypomethylating agents are safe and effective in patients with lower-risk MDS and MDS/MPN.
Decitabine was associated higher response rates compared with azacitidine, especially in patients with higher-risk features.
Abstract
Hypomethylating agents (HMAs) improve survival in patients with higher-risk myelodysplastic syndromes (MDS) but are less well-studied in lower-risk disease. We compared the safety and efficacy of low-dose decitabine vs low-dose azacitidine in this group of patients. Adults with low- or intermediate 1-risk MDS or MDS/myeloproliferative neoplasm (MPN), including chronic myelomonocytic leukemia, according to the International Prognostic Scoring System, were randomly assigned using a Bayesian adaptive design to receive either azacitidine 75 mg/m2 intravenously/subcutaneously daily or decitabine 20 mg/m2 intravenously daily for 3 consecutive days on a 28-day cycle. The primary outcome was overall response rate (ORR). Between November 2012 and February 2016, 113 patients were treated: 40 (35%) with azacitidine and 73 (65%) with decitabine. The median age was 70 years; 81% of patients were intermediate 1-risk patients. The median number of cycles received was 9. The ORRs were 70% and 49% (P = .03) for patients treated with decitabine and azacitidine, respectively. Thirty-two percent of patients treated with decitabine became transfusion independent compared with 16% of patients treated with azacitidine (P = .2). Cytogenetic response rates were 61% and 25% (P = .02), respectively. With a median follow-up of 20 months, the overall median event-free survival was 18 months: 20 and 13 months for patients treated with decitabine and azacitidine, respectively (P = .1). Treatment was well tolerated, with a 6-week mortality rate of 0%. The use of low-dose HMAs is safe and effective in patients with lower-risk MDS and MDS/MPN. Their effect on the natural history of lower-risk disease needs to be further studied. This trial was registered at clinicaltrials.gov (identifier NCT01720225).
Introduction
Myelodysplastic syndromes (MDSs) are a heterogeneous group of myeloid malignancies with variable prognoses.1,2 Patients can be generally divided into lower- and higher-risk groups.3-5 The outcome of patients with lower-risk MDS (low- and intermediate 1-risk by the International Prognostic Scoring System [IPSS]) is heterogeneous.3,6 For instance, using the MD Anderson lower-risk scoring system, patients can be separated in subsets with distinct survival outcomes, ranging from a 4-year survival rate of 65% for patients with more favorable disease to only 7% for patients with poor-risk features.6
Until now, most strategies in lower-risk MDS have focused on improving transfusion requirements (eg, hematopoietic growth factors) or are only active in a small subset of patients (eg, lenalidomide in patients with deletion of chromosome 5q).7 Given the poor prognosis of a fraction of patients with so-called lower-risk MDS, strategies that could potentially alter the natural history of this disease and improve overall survival (OS) are needed. The hypomethylating agents (HMAs) azacitidine and decitabine have been shown to improve survival or delay disease progression in patients with higher-risk MDS.8-11 However, the use of HMAs is less well-studied in patients with lower-risk disease.
The standard dose/schedules of decitabine (20 mg/m2/day × 5 days) and azacitidine (75 mg/m2/day × 7 days) that are commonly used for patients with higher-risk MDS tend to be myelosuppresive and may have a less favorable risk-benefit balance in patients with lower-risk MDS. Several studies have previously suggested that low doses of HMAs administered using shorter treatment schedules are active in lower-risk MDS.12-14 Low-dose decitabine (20 mg/m2 daily × 3 days) showed promising results in a small trial, with an objective response rate of 23% and a transfusion independency rate of 67%.12 Furthermore, a phase 1 study of oral azacitidine (CC-486) indicated that lower drug doses with more prolonged exposure may have an improved toxicity profile.13 An objective response rate of 73% was documented in patients with previously untreated MDS with oral azacitidine.
We conducted a randomized study to assess response rates and event-free survival (EFS) in patients with lower-risk MDS treated with low-dose HMAs, the risk-benefit profile of which is better aligned with less severe MDS.
Patients and methods
Patients
Adult patients with low- or intermediate 1-risk MDS or chronic myelomonocytic leukemia (CMML), as per IPSS, were eligible for this study. The IPSS was used to classify both patients with MDS and patients with CMML to have a consistent set of inclusion criteria across disease subgroups. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 to 2 and adequate liver function (bilirubin <2 mg/dL) and renal function (creatinine <2 mg/dL). Nursing and pregnant women were excluded. Patients of childbearing potential were required to practice effective methods of contraception. Other exclusions included active and uncontrolled infection and uncontrolled intercurrent illnesses. Patients could not have received any prior therapy with HMAs, although patients who had received other prior treatments for MDS, including growth factors, were eligible. Hydroxyurea was permitted to control white blood cell counts before study treatment. All patients signed informed consent according to institutional guidelines and in accordance with the Declaration of Helsinki. This trial was registered at clinicaltrials.gov (identifier NCT01720225).
Study design and therapy
A Bayesian response-adaptive design was used to assign patients to 1 of the 2 treatment arms: arm A, decitabine 20 mg/m2 intravenously (IV) over the course of 1 hour daily for 3 days; or arm B, azacitidine 75 mg/m2 IV over the course of 1 hour or subcutaneously daily for 3 days.15,16 Courses were repeated every 4 weeks when possible. Dose reductions for grade 3 and 4 toxicities were allowed for decitabine (to 15 mg/m2 and 10 mg/m2) and azacitidine (to 50 mg/m2 and 25 mg/m2 daily). Responding patients were allowed to continue therapy indefinitely. Bone marrow aspiration and/or biopsy were performed at the end of course 2 and every 3 months during the first year, and then every 3 to 6 months thereafter.
Statistical considerations and response criteria
An adaptive randomization design was used to assign patients to treatment based on response rate after the second cycle of therapy.15,16 Response after 2 cycles was used to facilitate adaptive randomization, although overall response rate (ORR) was the primary endpoint of the study. Initially, 20 patients were assigned to each arm with equal assignment probability. Then, as efficacy data accumulated, patient assignment to each treatment arm depended on response rates. A sample size of 120 patients was planned, which can provide 90% power to detect a difference in the ORR between the 2 treatment arms.
Responses were coded according to the modified 2006 International Working Group criteria.17 Differences among variables were evaluated by the χ-square test and Mann–Whitney U test for categorical and continuous variables, respectively.18 Cytogenetic responses were assessed in patients with abnormal karyotype at the time of enrollment. Complete cytogenetic response was defined as the achievement of a diploid karyotype among at least 20 metaphases analyzed. Partial cytogenetic response was defined as the reduction of 50% of the abnormal metaphases without the acquisition of any new abnormality among at least 20 metaphases analyzed.19 EFS was defined as the time between the start of therapy and the date of determination of lack of response, loss of response, transformation to acute myeloid leukemia, or death, whichever occurred first. Leukemia-free survival (LFS) was calculated as the number of months from treatment initiation to transformation to leukemia. OS was defined as the time between the start of therapy and death. Patients who were alive at the last follow-up date were censored in survival analyses. Survival probabilities were calculated by using the Kaplan-Meier method and were assessed from the time of therapy initiation; they were compared by the log-rank test.20 Univariate Cox proportional hazards were used to identify association between survival and potential risk factors. Statistical analysis was performed using SPSS version 22 statistical software (IBM Corporation). Generalized linear models were used to study the association of ORR, complete response (CR), and risk factors. The ORR and adverse events were monitored during the study, and all data were used to update the prior distributions for toxicity and efficacy parameters.
Gene sequencing
We performed mutation analysis using a 53- or 28-gene panel, as previously described.21-23 Briefly, genomic DNA was extracted from bone marrow aspirates or peripheral blood. Amplicon-based next-generation sequencing targeting the entire coding regions of a panel of 53 or 28 genes (supplemental Methods, available on the Blood Web site) associated with myeloid neoplasms was performed using a MiSeq platform (Illumina, San Diego, CA). A sequencing library was prepared using 250 ng DNA template. Equal quantities of DNA from purified sequencing libraries were used for TruSeq paired-end sequencing on the MiSeq sequencer, using the MiSeq Reagent Kit v2 (500 cycles). Variant calling was performed with Illumina MiSeq Reporter Software, using human genome build 19 (hg 19) as a reference. For clinical reporting, a minimum sequencing coverage of 250× (bidirectional true paired-end sequencing) was required. The analytical sensitivity was established at 5% mutant reads in a background of wild-type reads. Previously described somatic mutations registered at the Catalogue of Somatic Mutations in Cancer (COSMIC: http://cancer.sanger.ac.uk/cosmic) were considered as potential driver mutations. In addition, sequencing of SF3B1 (exons 14 and 15), SRSF2 (exon 1), U2AF1 (exons 2 and 6), and ZRSR2 (exons 1-11) was performed on whole-bone marrow DNA by Sanger sequencing on a total of 52 patients.
Results
Patient characteristics
In total, 113 patients were enrolled and treated between November 2012 and February 2016 (supplemental Figure 1). The median follow-up was 20 months (range, 2-43 months). Seventy-three patients were randomly assigned to receive decitabine, and 40 were randomly assigned to receive azacitidine. The imbalance of the arms was a result of a higher response rate seen with decitabine, which contributed to the skewed randomization. The median time from diagnosis to therapy with decitabine and azacitidine was 6 weeks (range, 1-216 weeks) and 4 weeks (range, 1-271 weeks), respectively. Patient characteristics are summarized in Table 1. Treatment arms were well-balanced for patient and disease characteristics. The median age of the enrolled population was 70 years (range, 44-84 years). The majority of patients had MDS; 18% had therapy-related MDS, 6 (5%) had an overlap MDS/myeloproliferative neoplasm (MPN) other than CMML, and 16 (14%) had CMML. One-half of patients had 2 to 3 cytopenias, and 53% were transfusion dependent at enrollment. Twenty-eight percent of patients had 5% or more bone marrow blasts, and 90% of patients had good or intermediate-risk cytogenetics by the IPSS classification.3 In both arms, most patients had intermediate 1-risk MDS by IPSS. By the MD Anderson lower-risk scoring system, most patients had intermediate- or high-risk disease. Twenty-two patients (19% of the entire cohort) had received prior growth factors, including erythropoietin-stimulating agents (ESAs) or granulocyte colony-stimulating factor.
Table 1.
Patient characteristics
| Parameter n (%)/median [range] | Overall (n = 113) | Decitabine (N = 73) | Azacitidine (N = 40) |
|---|---|---|---|
| Age, years | 70 [44-88] | 70 [44-88] | 70 [53-84] |
| WHO diagnosis | |||
| RCUD | 15 (13) | 10 (14) | 5 (13) |
| RCMD | 40 (35) | 29 (40) | 11 (28) |
| MDS with ringed sideroblasts | 3 (3) | 2 (3) | 1 (3) |
| MDS-EB | 25 (22) | 14 (19) | 11 (28) |
| 5q- | 2 (2) | 0 | 2 (5) |
| MDS-U | 6 (5) | 6 (8) | 0 |
| MDS/MPN-U | 6 (5) | 2 (3) | 4 (10) |
| CMML | 16 (14) | 10 (14) | 6 (15) |
| Therapy-related MDS | 20 (18) | 13 (18) | 7 (18) |
| ≥2 cytopenias | 68 (50) | 34 (47) | 22 (55) |
| Transfusion dependence | 59 (52) | 39 (53) | 20 (50) |
| BM blasts percentage | 3 [0-10] | 2 [0-10] | 3 [0-10] |
| Blasts ≥5% | 32 (28) | 21 (29) | 11 (27) |
| Cytogenetic risk (IPSS) | |||
| Good | 69 (61) | 43 (63) | 26 (65) |
| Intermediate | 30 (27) | 17 (23) | 13 (33) |
| Poor | 11 (10) | 10 (14) | 1 (3) |
| IPSS risk group | |||
| Low | 22 (19) | 16 (22) | 6 (15) |
| Intermediate-1 | 91 (81) | 57 (78) | 34 (85) |
| IPSS-R risk group | |||
| Very low | 14 (12) | 10 (14) | 4 (10) |
| Low | 41 (36) | 25 (34) | 16 (40) |
| Intermediate | 34 (30) | 21 (29) | 13 (33) |
| High | 23 (20) | 16 (22) | 7 (18) |
| Very high | 1 (1) | 1 (1) | 0 |
| MDACC LR-MDS score | |||
| Low | 13 (11) | 10 (14) | 3 (8) |
| Intermediate | 52 (46) | 35 (48) | 14 (43) |
| High | 48 (42) | 28 (38) | 20 (40) |
| Prior therapy | |||
| Growth factors | 22 (19) | 12 (16) | 10 (25) |
| Others | 9 (8) | 5 (7) | 4 (10) |
| Time from diagnosis, weeks | 5 [1-271] | 6 [1-216] | 4 [1-271] |
BM, bone marrow; MDACC, MD Anderson Cancer Center; MDS-EB, MDS with excess blasts; MDS-U, MDS, unclassified; MPN-U, myeloproliferative neoplasm, unclassified; RCMD, refractory cytopenia with multilineage dysplasia; RCUD, refractory cytopenia with unilineage dysplasia; WHO, World Health Organization.
Molecular alterations
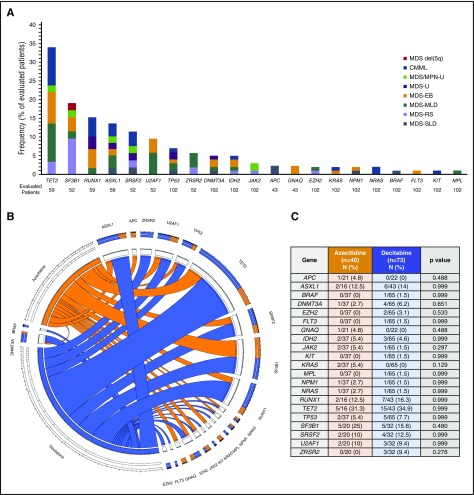
Baseline targeted next-generation sequencing was performed in 102 patients (90%; 59 with the 28-gene panel, 43 with the 53-gene panel). In addition, sequencing analysis of splicing genes (SF3B1, SRSF2, U2AF1, and ZRSR2) was available in 52 patients (46%). Mutations are detailed in Figure 1 and supplemental Table 1. A total of 64 patients (63%) had at least 1 detectable mutation. Characteristics of patients with no detectable mutations are detailed in supplemental Table 2. The median number of detectable mutations was 1 (range, 0-4). The most frequently detected mutations included TET2 (n = 20/59; 34%), SF3B1 (n = 10/52; 19%), RUNX1 (n = 9/59, 15%), ASXL1 (n = 8/59, 14%), and SRSF2 (n = 6/52, 12%), all present in more than 10% of evaluated patients. No significant differences in the distribution of mutations in evaluated genes were observed between both treatment arms (Figure 1B-C).
Figure 1.
Mutation landscape of enrolled patients. (A) Frequency of detected mutations based on World Health Organization 2016 subtype. MDS-EB, MDS with excess blasts; MDS-MLD, MDS with multilineage dysplasia; MDS/MPN-U, MDS/MPN unclassifiable; MDS-SLD, MDS with single lineage dysplasia. Frequencies are expressed for total number of patients studied for each particular mutation. (B-C) Circos plot and table describing frequencies of identified mutations based on treatment group.
Response to therapy
Overall response
One hundred nine patients (96% of enrolled patients) received at least 2 cycles of HMAs and were evaluable for response (Table 2). The ORR for the entire cohort was 62% (68/109), with 37% of patients (40/109) achieving a CR. Marrow CR (mCR) was seen in 9% (10/109), and hematologic improvement was seen in 18% (20/109). Overall, 25% of patients (15/59) became transfusion independent.
Table 2.
Response
| Parameter | Overall, n (%) | Decitabine, n (%) | Azacitidine, n (%) | P |
|---|---|---|---|---|
| Morphologic response, N | 109 | 70 | 39 | |
| CR | 40 (37) | 26 (37) | 14 (36) | |
| mCR | 8 (7) | 6 (9) | 2 (5) | |
| HI | 20 (18) | 17 (24) | 3 (8) | |
| Overall | 68 (62) | 49 (70) | 19 (49) | .03 |
| Transfusion response, N | 57 | 38 | 19 | |
| RBC | 11/46 (24) | 8/29 (28) | 3/17 (18) | |
| Platelets | 3/5 (60) | 3/4 (75) | 0/1 | |
| RBC + Platelets | 1/6 (17) | 1/5 (20) | 0/1 | |
| Overall | 15 (26) | 12 (32) | 3 (16) | .2 |
| Cytogenetic response, N | 44 | 28 | 16 | |
| Complete | 8 (18) | 7 (25) | 1 (6) | |
| Partial | 13 (30) | 10 (36) | 3 (19) | |
| Overall | 21 (48) | 17 (61) | 4 (25) | .02 |
| Morphologic response (BM blasts ≥5%), N | 32 | 21 | 11 | |
| CR | 14 (44) | 12 (57) | 2 (18) | |
| mCR | 8 (25) | 6 (29) | 2 (18) | |
| HI | 3 (9) | 3 (14) | 0 | |
| Overall | 25 (78) | 21 (100) | 4 (36) | <.001 |
| Hematologic response (BM blasts <5%), N | 72 | 45 | 27 | |
| ≥1 lineage | 29 (40) | 16 (36) | 13 (48) | |
| All lineages | 17 (24) | 10 (22) | 7 (26) |
HI, hematologic improvement; RBC, red blood cell.
The response rate after 2 cycles of therapy was 60% for decitabine and 38% with azacitidine (P = .26). The ORR was 70% with decitabine and 49% with azacitidine (P = .03). The CR rates were similar, at 37% and 36%, respectively (P = .90). Hematologic improvement rates were 24% for decitabine and 8% with azacitidine. The median time to best response was 2 months (range, 1-20 months). The median number of cycles received was 9 (range, 1-41 cycles). The median duration of response in both arms was 18 months (range, 2-40+ months in patients treated with decitabine and 2-30+ in patients treated with azacitidine). No early mortality (defined as death within 6 weeks) was observed.
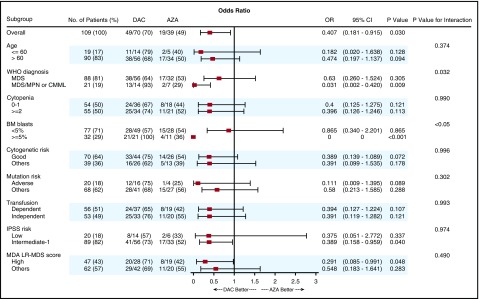
We evaluated whether there were any subgroups of patients, including based on age, number of cytopenias, cytogenetics, molecular mutation status, transfusion dependency, and disease risk categories, that were associated with a higher response rate within a particular treatment arm (Figure 2). Patients with MDS/MPN, a higher percentage of bone marrow blasts (5% and more), intermediate 1-risk disease by IPSS, and high-risk disease by the MD Anderson Cancer Center lower-risk scoring were associated with significantly higher response rates in patients who received decitabine compared with azacitidine.
Figure 2.
Subgroup analysis: a forest plot showing the odds ratios for overall response of various subgroups by treatment group.
Transfusion independency
A total of 57 patients were transfusion-dependent at baseline, and therefore evaluable for transfusion response: 38 patients treated with decitabine and 19 with azacitidine. Overall, 32% of patients treated with decitabine became transfusion independent compared with 16% of patients treated with azacitidine (P = .20; Table 2).
Baseline serum erythropoietin (EPO) levels were available in 52 of 72 patients who had a hemoglobin level lower than 10 g/dL and/or were RBC transfusion-dependent at enrollment; 14 of these patients had prior ESA exposure. The EPO levels and transfusion requirements of these 38 ESA-naive patients are shown in supplemental Table 3.
In a post hoc analysis, we attempted to correlate EPO levels with erythroid hematologic improvement. Pretreatment EPO level was available on 36 patients with RBC transfusion dependency at the time of enrollment. Nine of these patients (25%) became transfusion independent with HMA therapy (2 in the azacitidine arm and 7 in the decitabine arm). EPO levels were lower in those who achieved transfusion independency (median, 108 IU/L [range, 23.5-1141 IU/L]) than in those who did not (median, 337.9 IU/L [range, 22.9-2684 IU/L]; P = .11). In the subset of patients with transfusion dependency at baseline, 16 had been treated with prior ESA. Three patients (19%) with prior ESA exposure achieved transfusion independence. The EPO levels of these patients were 48 IU/L (decitabine), 386.9 IU/L (decitabine), and not tested (azacitidine).
Cytogenetic response
A total of 44 patients had abnormal cytogenetics at baseline and were evaluable for cytogenetic response: 28 were treated with decitabine and 16 with azacitidine. A total of 18% of patients (8/44) achieved a complete cytogenetic response. Sixty-one percent of patients treated with decitabine had at least a partial cytogenetic response, with 25% achieving a complete cytogenetic response. In contrast, 25% of patients (n = 4) treated with azacitidine achieved at least a partial cytogenetic response, with 1 patient only achieving a complete cytogenetic response (P = .02).
Response by bone marrow blasts ≥5% vs <5%
We assessed responses by baseline blast percentage. Only those with at least 5% blasts can be assessed for CR and mCR, whereas those with less than 5% blasts can at best have a hematologic improvement. All patients with bone marrow blasts at least 5% treated with decitabine responded for an ORR of 100% compared with 36% of patients treated with azacitidine (P < .001). The CR rates were 57% and 18%, respectively, in this subset of patients.
Among patients with baseline bone marrow blast lower than 5% and cytopenias, there was no difference in the rates of hematologic improvement: 36% and 48% of patients treated with decitabine and azacitidine had a hematologic improvement in at least 1 lineage, respectively. Twenty-two percent of patients treated with decitabine and 26% of those treated with azacitidine had an improvement in all lineages.
Response by mutations
To determine the effect of mutations in response outcomes, we evaluated likelihood of response based on identified mutations. None of the detected mutations significantly predicted for overall response, although the number of patients with each mutation was small, limiting power to detect a difference (supplemental Table 4).
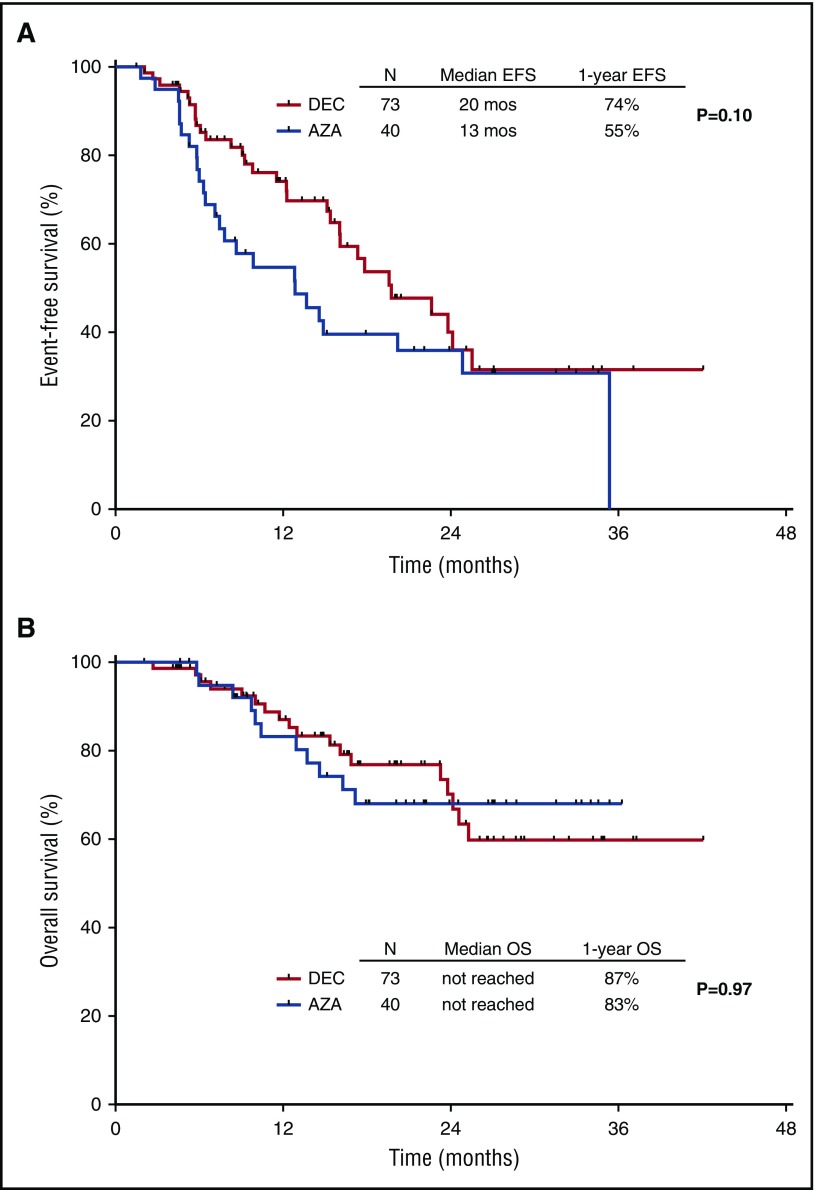
Survival
The median EFS for the entire cohort was 18 months (95% CI, 14-22 months), and the median OS has not yet been reached. The median EFS was 20 months and 13 months for patients treated with decitabine and azacitidine, respectively (P = .10). The 1-year EFS rates were 74% and 55%, respectively (Figure 3A). The median survival has not been reached for either arm. The 1-year OS rates were 87% and 83%, respectively (Figure 3B). A total of 12 patients had transformation to acute myeloid leukemia after a median of 11 months (range, 4-23 months).
Figure 3.
Kaplan-Meier curves for EFS. By treatment group (A) and survival by treatment arm (B).
Effect of low-dose HMA therapy on outcome of patients with lower-risk MDS
We evaluated whether there were any subgroups of patients who preferentially benefitted from early intervention with low-dose HMA (either arm). By univariate analysis for OS, transfusion dependence before enrollment (4-year OS rate, 71% vs 55%; HR, 2.3 [95% CI 1.1-4.9]; P = .033) and presence of 2 or more cytopenias (4-year OS rate, 72% vs 56%; HR, 2.2 [95% CI, 1.0-4.9]; P = .046] affected survival unfavorably, whereas achievement of CR affected survival favorably (4-year OS rate, 78% vs 67%; HR, 0.38 [95% CI, 0.2-0.9]; P = .026). Age group (<60 vs ≥60 years), World Health Organization subgroup (MDS vs MDS/MPN), bone marrow blast percentage (<5% vs ≥5%), cytogenetic risk (good vs others), IPSS (low vs intermediate 1), IPSS-R (very low, low, or intermediate vs higher or very high), nor MDA lower-risk prognosis scoring system category (high vs others) significantly affected survival of patients. In addition, mutations in DNMT3A (median OS, 13 months vs not reached [NR]; HR, 5.7 [95% CI, 1.6-20.2]; P = .007), TP53 (median OS, 13.7 months vs NR; HR, 3.7 [95% CI, 1.2-11.0]; P = .02), and ZRSR2 (median OS, 6.8 months vs NR; HR, 45.5 [95% CI, 2.8-727.3]; P = .007) affected OS unfavorably (Table 3; supplemental Table 5).
Table 3.
Univariate and multivariate analysis for OS (overall population)
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.948 | 0.590-6.438 | .274 | |||
| ≤60 y | ||||||
| >60 y | ||||||
| WHO diagnosis | 1.343 | 0.594-3.034 | .478 | |||
| MDS | ||||||
| MDS/MPN | ||||||
| Cytopenias | 2.230 | 1.015-4.899 | .046 | 4.197 | 0.340-51.865 | .264 |
| 0-1 | ||||||
| ≥2 | ||||||
| BM blasts | 1.393 | 0.657-2.957 | .387 | |||
| <5% | ||||||
| ≥5 | ||||||
| Cytogenetic risk | 0.500 | 0.213-1.170 | .110 | |||
| Good | ||||||
| Others | ||||||
| Transfusion | 2.304 | 1.070-4.959 | .033 | 1.242 | 0.186-8.301 | .823 |
| Dependent | ||||||
| Independent | ||||||
| IPSS risk | 1.144 | 0.398-3.293 | .803 | |||
| Low | ||||||
| Intermediate 1 | ||||||
| IPSS-R risk | 1.263 | 0.519-3.188 | .406 | |||
| High or very high | ||||||
| Others | ||||||
| MDA LR-MDS score | 1.258 | 0.607-2.606 | .537 | |||
| High (category 3) | ||||||
| Others | ||||||
| Response | 0.378 | 0.161-0.890 | .026 | 0.045 | 0.002-0.995 | .047 |
| CR | ||||||
| Non-CR | ||||||
| DNMT3A | 5.719 | 1.621-20.181 | .007 | 25.930 | 0.904-743.842 | .057 |
| Wild-type | ||||||
| Mutated | ||||||
| TP53 | 3.682 | 1.228-11.035 | .020 | 136.764 | 5.292-3534.38 | .003 |
| Wild-type | ||||||
| Mutated | ||||||
| ZRSR2 | 45.475 | 2.843-727.310 | .007 | 94.865 | 2.528-3559.721 | .014 |
| Wild-type | ||||||
| Mutated | ||||||
By multivariate analysis for OS, achievement of CR (favorable) and presence of TP53 or ZRSR2 mutations (adverse) retained their significant effect in survival (Table 3).
When evaluating LFS, achievement of a CR (4-year LFS rate of 71% vs 65%; HR, 0.5 [95% CI, 0.2-0.9]; P = .018) and mutations in DNMT3A (median LFS, 13 months vs NR; HR, 4.5 [95% CI, 1.3-15.5]; P = .018), TP53 (median LFS, 13.7 months vs NR; HR, 3.9 [95% CI, 1.5-10.7]; P = .006), and ZRSR2 (median LFS, 6.8 months vs NR; HR, 22.8 [95% CI, 2.1-251.6]; P = .011) were associated with significantly shorter LFS by univariate analysis (supplemental Table 6). By multivariate analysis for LFS, only DNTM3A, TP53, and ZRSR2 retained their significant effect in LFS (supplemental Table 7).
Toxicity
The treatment was overall well-tolerated in both arms, with most adverse events being of grade 1 to 2 (Table 4). Grade 3 and higher nonhematologic adverse events were rare. Infection or neutropenic fever occurred in 7% and 5% of patients treated with decitabine and azacitidine, respectively. Grade 3 adverse events were rare, and no grade 4 adverse events were observed. More myelosuppression was encountered in patients treated with decitabine, resulting in cycle delays and dose reductions. Cycle delays and dose reduction were required in 38% and 12% of patients treated with decitabine and 20% and 5% of patients treated with azacitidine. These dose or schedule modifications were usually a result of myelosuppression.
Table 4.
Nonhematologic adverse events observed in at least 3% of patients
| Parameter | N (%) | |||
|---|---|---|---|---|
| Decitabine (n = 73) | Azacitidine (n = 40) | |||
| All Grades | Grade 3 | All Grades | Grade 3 | |
| Nausea | 11 (15) | 0 | 6 (15) | 0 |
| Fatigue | 6 (8) | 0 | 4 (10) | 0 |
| Infection/Neutropenic fever | 5 (7) | 4 (5) | 2 (5) | 0 |
| Constipation | 3 (4) | 0 | 6 (15) | 0 |
| Diarrhea | 2 (3) | 0 | 3 (8) | 0 |
Discussion
Outcomes of patients with lower-risk MDS are heterogeneous, ranging from a 4-year survival rate of 65% for patients with more favorable risk disease to only 7% for patients with poor-risk features.6 Some patients with “lower risk” MDS have a survival that is inferior to patients with metastatic breast cancer that has failed first-line therapy. Limited treatment options are available for these patients: HMA therapy is often offered for patients with higher-risk disease, and sometimes used for lower-risk disease, but is poorly studied in the latter group.7-10 Allogeneic stem cell transplantation can be considered for patients with higher-risk disease and for those with lower-risk disease at the time of progression.24,25
Our study indicates that the use of HMA at low dose is relatively safe, as no treatment-related early deaths or high-grade adverse events were encountered. Low-dose HMA therapy was effective in this patient population, with more than half the patients benefiting, a third achieving a complete response, and the vast majority being alive and event-free at 1-year landmark (1-year EFS rate, 65%; 1-year survival rate, 85%). This benefit was more pronounced in patients with higher-risk features. In our analysis and in a multivariate setting, the achievement of CR independently predicted for a better survival, whereas the presence of TP53 or ZRSR2 mutations were unfavorable factors. Interestingly, neither the IPSS category nor the MDA lower-risk prognosis scoring system category predicted the prognostic outcomes of these HMA-treated patients. Although this may be in part because the population is skewed toward higher-risk categories (48% of patients with category 2/intermediate risk and 38% with category 3/high risk), one could hypothesize that it may be a result the therapy improving the outcomes of these higher-risk patients, and therefore negating the effect of the adverse biological features.
In our comparison of 2 low-dose regimens of decitabine and azacitidine, decitabine was found to be superior mainly in patients with advanced features, including patients with at least 5% bone marrow blasts, adverse mutation profile, and higher-risk disease by the MD Anderson Cancer Center lower-risk scoring system. Decitabine induced higher rates of overall response (70% vs 49%), transfusion independency (32% vs 16%), and cytogenetic response (61% vs 25%). It should be noted that in the current study, the 2 drugs are not used at equivalent doses. Decitabine was used at two-thirds of the approved dose, whereas azacitidine was being used at lower doses (approximately 45% of the original dose of 75 mg/m2 daily for 7 days). As such, the underperformance of azacitidine could be a result of the lower dose used. A 5-day regimen may improve its efficacy, mainly in patients with higher disease burden.
Whether early intervention with low-dose HMA therapy will affect the outcome of these patients remains to be confirmed. Even though promising responses were seen in these lower-risk patients, it is unknown whether this translates to meaningful changes in survival or quality of life compared with intervening when patients have higher-risk disease. One concern of this approach is that HMAs are no longer available to patients at the time of disease progression, although if progression is delayed by HMA treatment, that may be less of concern. Because of the nature of our tertiary centers, patients with lower-risk MDS are usually referred after being failed by supportive care or concern that they are higher risk than suggested by the IPSS, so a comparison with historical cohorts not treated was not possible. The skewing of this study’s patient population to a relatively “higher risk” group of IPSS lower-risk patients may also limit the generalizability of these results to the broader population of lower-risk patients with MDS. A randomized study (NCT02269280) comparing supportive care strategy with early intervention is ongoing and will help addressing whether HMA therapy of lower-risk patients improves survival.
Previous studies have reported the adverse prognostic effect of several genomic abnormalities in patients with lower-risk MDS.26-31Contrary to previous studies, we did not observe an adverse prognostic effect of EZH2, ASXL1, or RUNX1 mutations, but as previously reported, we also observed unfavorable survival outcomes in patients with TP53 and ZRSR2 mutations, although these results should be interpreted with caution, as the number of patients harboring each of these mutations was small. In addition, the presence of DNMT3A, TP53, and ZRSR2 mutations was associated with a higher risk for transformation into acute myeloid leukemia. Although there are several limitations in our sequencing methodology that could have affected the observed outcomes, our results may suggest early intervention and close monitoring may be justified in lower-risk patients with MDS with higher-risk mutations. New scoring systems that take into account molecular profiling of the patients with lower-risk disease in addition to the clinical and biological features may better identify patients with lower-risk disease at higher risk who may benefit from early intervention with HMA therapy.2,6,26-28,32
This study is limited by its open-label design, the small number of patients enrolled, and its primary endpoint being ORR. These factors may limit the interpretation and extrapolation of the results to the larger population of adult patients with low- or intermediate 1-risk MDS. As a consequence, larger studies are needed to confirm these data and to further understand the molecular effects of the intervention. A larger multicenter study assessing the benefit of early intervention is ongoing. This study will address the role of early intervention (HMA therapy vs supportive care only) and the best schedule of HMA therapy (3 days of decitabine vs 3 days of azacitidine vs 5 days of azacitidine). Furthermore, response assessment based on the 2006 modified International Working Group criteria may not be applicable for patients with low-risk disease with less than 5% marrow blasts and those with CMML, particularly when assessed after 2 cycles, an endpoint selected for convenience.17 This endpoint may have affected the Bayesian adaptive randomization design, and specifically the construction of predictive probability, which is the continuity of the trend in data observed up to the decision point. The ongoing confirmatory study will overcome this concern by its larger size and by having EFS rate as primary endpoint. Finally, different sequencing platforms were used to evaluate the presence of somatic mutations, leading to variability in the total number of genes interrogated within the included study population. Use of a homogenous sequencing technique to evaluate all the known recurrently mutated genes in MDS would be required and is being performed in the ongoing confirmatory study.
A better understanding of the disease biology, the mechanisms of resistance to HMA therapy, and dysregulated signaling pathways involved has generated a plethora of novel insights into the treatment of patients with MDS with several promising strategies in late-stage development.24,33,34 A combination of the oral cytidine deaminase inhibitor (E7727) with oral decitabine in patients with de novo and relapsed MDS was shown to be safe and effective with a more favorable pharmacokinetic profile compared with decitabine.34 If confirmed, these compounds and others in a late stage of development alone or in combination may play an important role in the early management of patients with lower-risk disease and, as such, may change the natural history of this disease, and oral agents may also allow exploration of schedules of therapy that would be difficult with parenteral azacitidine or decitabine.
In summary, in this study, the use of low-dose HMAs appeared to be active and safe in patients with lower-risk MDS and MDS/MPN. Using a low-dose schedule, decitabine appeared to result in improved response rates compared with azacitidine, especially in patients with higher-risk features. The treatment of these patients with lower-risk MDS remains an unmet medical need. A larger confirmatory study is ongoing and may help to clarify whether low-dose HMA therapy in patients with lower-risk MDS can alter the natural history of this disease.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
This work is supported in part by the University of Texas MD Anderson Cancer Center Support Grant CA016672, the University of Texas MD Anderson MDS/AML Moon Shot, and the MDS Clinical Research Consortium, which is funded by the Edward P. Evans Foundation.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.J. and G.G.-M. designed the study, contributed patients, analyzed data, and wrote the manuscript; X.H. and W.Q. designed the study and analyzed data; N.J.S., G.M.-B., and K.S. analyzed data and wrote the manuscript; C.B.-R., H.Y., and C.Z. performed pathologic and genomic analysis; T.K., G.B., N.P., Z.E., J.C., F.R., Y.A., and H.K. contributed patients and reviewed the manuscript; and R.K., M.A.S., D.P.S., A.D., and G.R. designed the study and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elias Jabbour, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Box 428, Houston, TX 77030; e-mail: ejabbour@mdanderson.org.
References
- 1.Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;361(19):1872-1885. [DOI] [PubMed] [Google Scholar]
- 2.Bejar R, Levine R, Ebert BL. Unraveling the molecular pathophysiology of myelodysplastic syndromes. J Clin Oncol. 2011;29(5):504-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberg P, Cox C, LeBeau MM, et al. . International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89(6):2079-2088. [PubMed] [Google Scholar]
- 4.Greenberg PL, Tuechler H, Schanz J, et al. . Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kantarjian H, O’Brien S, Ravandi F, et al. . Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113(6):1351-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia-Manero G, Shan J, Faderl S, et al. . A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22(3):538-543. [DOI] [PubMed] [Google Scholar]
- 7.List A, Dewald G, Bennett J, et al. ; Myelodysplastic Syndrome-003 Study Investigators. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355(14):1456-1465. [DOI] [PubMed] [Google Scholar]
- 8.Silverman LR, Demakos EP, Peterson BL, et al. . Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429-2440. [DOI] [PubMed] [Google Scholar]
- 9.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. ; International Vidaza High-Risk MDS Survival Study Group. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantarjian H, Issa JP, Rosenfeld CS, et al. . Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794-1803. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H, Oki Y, Garcia-Manero G, et al. . Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109(1):52-57. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Manero G, Jabbour E, Borthakur G, et al. . Randomized open-label phase II study of decitabine in patients with low- or intermediate-risk myelodysplastic syndromes. J Clin Oncol. 2013;31(20):2548-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Manero G, Gore SD, Cogle C, et al. . Phase I study of oral azacitidine in myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia. J Clin Oncol. 2011;29(18):2521-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filì C, Malagola M, Follo MY, et al. . Prospective phase II study on 5-days azacitidine for treatment of symptomatic and/or erythropoietin unresponsive patients with low/INT-1-risk myelodysplastic syndromes. Clin Cancer Res. 2013;19(12):3297-3308. [DOI] [PubMed] [Google Scholar]
- 15.Thall PF, Simon RM, Estey EH. New statistical strategy for monitoring safety and efficacy in single-arm clinical trials. J Clin Oncol. 1996;14(1):296-303. [DOI] [PubMed] [Google Scholar]
- 16.Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat Med. 1995;14(4):357-379. [DOI] [PubMed] [Google Scholar]
- 17.Cheson BD, Greenberg PL, Bennett JM, et al. . Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419-425. [DOI] [PubMed] [Google Scholar]
- 18.Agresti A. Categorical Data Analysis, 2nd ed Hoboken, NJ: John Wiley & Sons Inc.; 1990. [Google Scholar]
- 19.Issa JP, Garcia-Manero G, Huang X, et al. . Results of phase 2 randomized study of low-dose decitabine with or without valproic acid in patients with myelodysplastic syndrome and acute myelogenous leukemia. Cancer. 2015;121(4):556-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaplan EL, Maier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1965;53(282):457-481. [Google Scholar]
- 21.Patel KP, Ravandi F, Ma D, et al. . Acute myeloid leukemia with IDH1 or IDH2 mutation: frequency and clinicopathologic features. Am J Clin Pathol. 2011;135(1):35-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh RR, Bains A, Patel KP, et al. . Detection of high-frequency and novel DNMT3A mutations in acute myeloid leukemia by high-resolution melting curve analysis. J Mol Diagn. 2012;14(4):336-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luthra R, Patel KP, Reddy NG, et al. . Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica. 2014;99(3):465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Manero G. Myelodysplastic syndromes: 2015 update on diagnosis, risk-stratification and management. Am J Hematol. 2015;90(9):831-841. [DOI] [PubMed] [Google Scholar]
- 25.Jabbour EJ, Garcia-Manero G, Strati P, et al. . Outcome of patients with low-risk and intermediate-1-risk myelodysplastic syndrome after hypomethylating agent failure: a report on behalf of the MDS Clinical Research Consortium. Cancer. 2015;121(6):876-882. [DOI] [PMC free article] [PubMed]
- 26.Bejar R. Implications of molecular genetic diversity in myelodysplastic syndromes. Curr Opin Hematol. 2017;24(2):73-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papaemmanuil E, Gerstung M, Malcovati L, et al. ; Chronic Myeloid Disorders Working Group of the International Cancer Genome Consortium. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bejar R, Stevenson KE, Caughey BA, et al. . Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J Clin Oncol. 2012;30(27):3376-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu SJ, Kuo YY, Hou HA, et al. . The clinical implication of SRSF2 mutation in patients with myelodysplastic syndrome and its stability during disease evolution. Blood. 2012;120(15):3106-3111. [DOI] [PubMed] [Google Scholar]
- 30.Makishima H, Yoshizato T, Yoshida K, et al. . Dynamics of clonal evolution in myelodysplastic syndromes. Nat Genet. 2017;49(2):204-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damm F, Kosmider O, Gelsi-Boyer V, et al. ; Groupe Francophone des Myélodysplasies. Mutations affecting mRNA splicing define distinct clinical phenotypes and correlate with patient outcome in myelodysplastic syndromes. Blood. 2012;119(14):3211-3218. [DOI] [PubMed] [Google Scholar]
- 32.Nazha A, Narkhede M, Radivoyevitch T, et al. . Incorporation of molecular data into the Revised International Prognostic Scoring System in treated patients with myelodysplastic syndromes. Leukemia. 2016;30(11):2214-2220. [DOI] [PubMed] [Google Scholar]
- 33.Giagounidis A, Platzbecker U, Germing U, et al. Luspatercept treatment leads to long term increases in hemoglobin and reductions in transfusion burden in patients with low or intermediate-1 risk myelodysplastic syndromes (MDS): preliminary results from the phase 2 PACE-MDS extension study [abstract]. Blood. 2015;126. Abstract 92.
- 34.Garcia-Manero G, Odenike O, Amrein PC, et al. Successful emulation of IV decitabine pharmacokinetics with an oral fixed-dose combination of the oral cytidine deaminase inhibitor (CDAi) E7727 with oral decitabine, in subjects with myelodysplastic syndromes (MDS): final data of phase 1 study [abstract]. Blood. 2016;128. Abstract 114.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.