Abstract
Purpose
Subsequent to the American College of Surgeons Oncology Group (ACOSOG) Z0011 and After Mapping of the Axilla: Radiotherapy or Surgery (AMAROS) trials, complete axillary lymph node dissection is not routinely performed, even in cases where metastatic sentinel lymph nodes are detected. We investigated the percentage of N2 or N3 stages in T1–2 invasive breast cancer patients with no lymphadenopathy and developed a nomogram to predict the possibility of N2 or N3 stages in these patients.
Methods
We retrospectively reviewed the charts of invasive breast cancer patients who were clinically N0 stage, but had a positive sentinel or non-sentinel lymph node detected on sentinel lymph node biopsy. The association of potential risk factors with known outcomes (N2 or N3 stages) was tested using logistic regression analysis. Variables with p<0.05 in the multivariate analysis were included in the nomogram. Internal performance validation was carried out using a 5-fold cross validation method.
Results
Among a total of 1,437 patients, 1,355 patients had stage N1 disease (94.3%), while 82 had stage N2 or N3 disease (5.7%). Multivariate stepwise logistic regression analysis revealed lymphovascular invasion (p=0.008), T2 stage (p=0.026), metastatic lymph node ratio (p<0.001), and perinodal extension (p<0.001) as independent predictors of N2 or N3 stages. A nomogram was developed based on these factors. The area under the curve estimated from the receiver operating characteristic graph was 0.8050 in the model set and 0.8246 in the test set.
Conclusion
Our nomogram can be employed for the prediction of N2 or N3 stage among cases fulfilling the ACOSOG Z0011 or AMAROS criteria.
Keywords: Breast neoplasms, Lymph node excision, Nomograms, Sentinel lymph node biopsy
INTRODUCTION
The involvement of axillary lymph node (LN) in patients with invasive breast cancer is one of the most influential prognostic factors [1]. Lately, complete axillary dissection is not routinely performed, even in cases where metastatic sentinel LNs (SLNs) are detected [2]. In the American College of Surgeons Oncology Group (ACOSOG) Z0011 trial, axillary LN dissection (ALND) and axillary radiotherapy after detection of positive SLNs provided excellent and comparable axillary control for patients with T1–2 primary breast cancer and no palpable lymphadenopathy, and results in significantly less morbidity than does complete axillary dissection [2]. In After Mapping of the Axilla: Radiotherapy or Surgery (AMAROS) trial, SLN biopsy (SLNB) without ALND offered excellent regional control and this regimen may be a reasonable option for the management for selected patients with early-stage breast cancer treated with breast-conserving therapy and adjuvant systemic therapy [3].
According to the seventh edition of the American Joint Committee for Cancer (AJCC), breast cancer staging N2 with T1–2 falls into stage IIIA, while N3 with any T falls into stage IIIC. Stages IIIA and IIIC refer to locally advanced breast cancer [4]. One might question why many doctors agree that it is safe not to perform complete ALND (cALND) in locally advanced breast cancer. We investigated the percentage of N2 or N3 stages in T1–2 invasive breast cancer patients with no lymphadenopathy, and developed a nomogram for the prediction of N2 or N3 stage in these patients.
METHODS
Study population
We performed a retrospective chart review of 1,820 invasive breast cancer patients who were clinically N0, but had a positive SLN or non-SLNs (NSLNs) during SLNB. All patients underwent ALND between January 2005 and January 2015 at Samsung Medical Center. Patients were excluded if they had stage T3 or higher disease and had undergone neoadjuvant chemotherapy (NAC) due to high false-negative rate of SLNB after NAC [5]. Those with only 1 or 2 metastatic LNs during SLNB were included. A total of 1,437 patients were ultimately included (Figure 1). All electronic medical records and pathology reports were reviewed. This study adhered to the tenets of the Declaration of Helsinki. This study was approved by the Institutional Review Board of Samsung Medical Center (IRB number: 2016-09-117).
Figure 1. Flow chart of the patient inclusion process.
SLN=sentinel lymph node; SLNB=sentinel lymph node biopsy; LN=lymph node.
Sentinel lymph node mapping
We conducted lymphatic mapping using technetium-99m (99mTc) sulfur colloid diluted in normal saline solution and/or vital blue dye (1% indigo carmine). The site and timing of administration of the mapping agent were at the physician's discretion. The radiolabeled colloid was injected 1 to 6 hours preoperatively, and/or 1 to 3 mL 1% indigo carmine was injected periareolarly and massaged into the breast tissue for 5 minutes. A handheld gamma detection probe (Neoprobe 2000; Neoprobe Corp., Dublin, USA) was used to scan the axilla transcutaneously and identify the most radioactive area. All radioactive and/or vital blue dye mapped LNs were excised and submitted as SLNs while palpable LNs without uptake of radioactive and/or vital blue dye were excised and submitted as NSLNs.
Histopathological evaluation
The dissected LNs were measured. Serial sections of 2-mm thickness were prepared from a portion of the frozen, dissected LNs. The remaining tissue was fixed in 10% formalin, embedded in paraffin blocks, and stained with hematoxylin and eosin (H&E). Pathologic evaluation of the sections was performed. If the H&E-stained sections were negative for malignancy, additional sections were immunohistochemically stained for keratin using the monoclonal, anti-human cytokeratin antibody (clone AE1/AE3; Dako, Carpinteria , USA).
Data analysis
We retrospectively evaluated the following clinicopathologic factors: age, menopausal status, body mass index (BMI), family history of breast cancer, type of surgery, laterality, multiplicity, histopathological type of breast cancer, estrogen receptor (ER) status, progesterone receptor status, human epidermal growth factor receptor 2 (HER2) status, lymphovascular invasion (LVI), perinodal extension (PNE), extensive intraductal component, nuclear grade, Ki-67 status, pathological T stage according to the seventh edition of the AJCC classification, subtype; luminal A-like was group showing positive ER and negative HER2 expression. Luminal B-like was group showing not only positive for both ER and HER2 expression but also positive ER and negative HER2 expression and more than 14% ki-67. HER2 was group showing negative ER and positive HER2 expression. Triple-negative breast cancer (TNBC) was group showing negative for both ER and HER2 expression, method of SLN mapping (radiolabeled colloid and/or vital blue dye), number of removed LNs, and number of metastatic LNs observed on SLNB. Proportion analysis was conducted using the chi-square or Fisher exact tests. The association of potential risk factors with outcomes (N2 or N3) was tested using logistic regression analysis. In the case of rare events, we applied a logistic regression model using the Firth's penalized maximum likelihood estimation method. Variables with p<0.05 in a univariate analysis were included in a multivariate analysis. Stepwise selection methods were applied to identify covariates for the logistic regression model. Variables with p<0.05 in the multivariate analysis were included in the nomogram. The adjusted area under the receiver operating characteristic (ROC) curve (AUC) was calculated in order to quantify the ability to rank patients by risk. Internal performance validation was performed using the 5-fold cross validation method. Statistical analyses were performed using the statistical software SAS version 9.4 (SAS Institute Inc., Cary, USA). A nomogram was formulated based on the results of the stepwise multivariate analysis using the software R 3.0.3 (R foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/).
RESULTS
Proportion of N2 or N3 stages
Among the 1,437 patients, 1,355 patients had stage N1 disease (94.3%), while 82 had stage N2 or N3 disease (5.7%) (Table 1). The proportion of stages N2 or N3 was higher (14.3%) when there were two metastatic LNs (3.2%), as opposed to when there was just one (p<0.001). There were no significant differences by breast cancer subtype.
Table 1. Proportion of N stage in T1–2 invasive breast cancer patients with clinical N0 but metastatic lymph node as sentinel lymph node biopsy.
| Characteristic | Total (n = 1,437) | N1 (n = 1,355) No. (%) | N2 or N3 (n = 82) No. (%) | p-value |
|---|---|---|---|---|
| No. of metastatic LN | < 0.001 | |||
| Metastatic LN = 1 | 1,109 | 1,074 (96.8) | 35 (3.2) | |
| Metastatic LN = 2 | 328 | 281 (85.7) | 47 (14.3) | |
| Metastatic LN = 1 | 0.128 | |||
| S1N0* | 1,004 | 975 (97.1) | 29 (2.9) | |
| S0N1† | 105 | 99 (94.2) | 6 (5.8) | |
| Metastatic LN = 2 | 0.107 | |||
| S1N1 | 108 | 87 (80.6) | 21 (19.4) | |
| S2N0 | 204 | 181 (88.7) | 23 (11.3) | |
| S0N2 | 16 | 13 (81.3) | 3 (18.7) | |
| Subtype | 0.295 | |||
| Luminal A-like | 1,056 | 996 (94.3) | 60 (5.7) | |
| Luminal B-like | 135 | 126 (91.9) | 9 (8.1) | |
| HER2 | 107 | 100 (93.5) | 7 (6.5) | |
| TNBC | 139 | 135 (97.2) | 4 (2.9) | |
| Luminal A-like | < 0.001 | |||
| Metastatic LN = 1 | 809 | 785 (97.0) | 24 (3.0) | |
| Metastatic LN = 2 | 249 | 213 (85.5) | 36 (14.5) | |
| Luminal B-like | 0.125 | |||
| Metastatic LN = 1 | 104 | 98 (94.2) | 6 (5.8) | |
| Metastatic LN = 2 | 31 | 26 (83.8) | 5 (16.2) | |
| HER2 | 0.664 | |||
| Metastatic LN = 1 | 82 | 77 (93.9) | 5 (6.1) | |
| Metastatic LN = 2 | 25 | 23 (92.0) | 2 (8.0) | |
| TNBC | 0.001 | |||
| Metastatic LN = 1 | 114 | 114 (100) | 0 | |
| Metastatic LN = 2 | 25 | 21 (84.0) | 4 (16.0) |
N1=nodal stage 1; N2=nodal stage 2; N3, nodal stage3; LN=lymph node; S, sentinel node; N, non-sentinel node; HER2=human epidermal growth factor receptor 2; TNBC=triple-negative breast cancer.
*S1N0, 1 metastatic sentinel lymph node and no metastatic non-sentinel lymph node during sentinel lymph node biopsy; †S0N1, no metastatic sentinel lymph node and 1 metastatic non-sentinel lymph node during sentinel lymph node biopsy.
The proportional differences with regard to the number of metastatic LNs are also listed in Table 1. In luminal A-like type, the proportion of stages N2 or N3 was higher when there were two metastatic LNs as opposed to when there was just one. In case of TNBC, there were no N2 or N3 cases observed, when there was only one metastatic LN; however, there were N2 or N3 cases when there were two metastatic LNs. For the luminal B-like subtype and HER2(+), the number of metastatic LNs did not affect the proportion of N stages.
We performed a sub-classification of the N stage ratios based on all the cases of SLNs and NSLNs (Table 1). The ratio of N2 or N3 tended to be higher in the S0N1 group than in the S1N0 group among cases with 1 metastatic LN, although the difference was not significant. Among cases with two metastatic LNs, the proportion of N2 or N3 tended to be higher in cases in which the NSLNs were metastatic than in which they were nonmetastatic. However, this difference was also not significant.
Patient characteristics and factors associated with N2 or N3 stages
Patient characteristics and N stage proportions are listed in Table 2. The following parameters had no association with N stage: age, BMI, menopause, family history, surgery type, laterality, multiplicity, histologic tumor type, extensive intraductal component, nuclear grade, Ki-67 status, subtype, and SLNs detection method.
Table 2. Univariable and multivariable analysis of related factors to nodal stage in T1–2 breast cancer patients with no lymphadenopathy.
| Characteristic | Total no. | N1 No. (%) |
N2 or N3 No. (%) |
Multivariable | Multivariable | ||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||||
| Age (yr) | 0.439 | ||||||
| ≤ 35 | 96 | 91 (94.7) | 5 (5.3) | Reference | |||
| > 35, ≤ 55 | 1,020 | 957 (93.8) | 63 (6.2) | 1.196 (0.469–3.050) | |||
| > 55 | 321 | 307 (95.6) | 14 (4.3) | 0.827 (0.290–2.358) | |||
| Age (yr)* | 43.5 ± 12.0 | 48.8 ± 9.7 | 49.2 ± 9.7 | 0.769 | |||
| Histologic type | 0.571 | ||||||
| IDC | 1,311 | 1,236 (94.3) | 75 (5.7) | Reference | |||
| ILC | 62 | 59 (95.2) | 3 (4.8) | 0.839 (0.257–2.740) | |||
| IDC+ILC | 51 | 47 (92.2) | 4 (7.8) | 1.404 (0.493–4.002) | |||
| Others | 13 | 13 (100) | 0 | ||||
| LVI | 0.004 | 0.008 | |||||
| Yes | 729 | 671 (92.0) | 58 (8.0) | 2.138 (1.274–3.580) | 1.987 (1.192–3.312) | ||
| No | 704 | 680 (96.5) | 24 (3.5) | Reference | Reference | ||
| Unknown | 4 | 4 (100) | 0 | ||||
| NG | 0.515 | ||||||
| Low | 189 | 175 (92.6) | 14 (7.4) | Reference | |||
| Intermediate | 831 | 784 (94.3) | 47 (5.7) | 0.749 (0.403–1.393) | |||
| High | 417 | 396 (94.9) | 21 (5.1) | 0.659 (0.327–1.327) | |||
| Perinodal extension | < 0.001 | < 0.001 | |||||
| Positive | 581 | 519 (89.3) | 62 (10.7) | 4.063 (2.368–6.973) | 4.310 (2.520–7.371) | ||
| Negative | 845 | 826 (97.8) | 19 (2.2) | Reference | Reference | ||
| Unknown | 11 | 10 (94.3) | 1 (5.7) | ||||
| Ki-67 (%) | 0.275 | ||||||
| ≤ 25 | 796 | 749 (94.1) | 47 (5.9) | Reference | |||
| > 25, ≤ 50 | 62 | 61 (98.4) | 1 (1.6) | 0.261 (0.035–1.925) | |||
| > 50, ≤ 75 | 466 | 437 (93.8) | 29 (6.2) | 1.055 (0.654–1.701) | |||
| > 75, ≤ 100 | 108 | 104 (96.3) | 4 (3.7) | 0.607 (0.214–1.719) | |||
| Unknown | 5 | 4 (80.0) | 1 (20.0) | ||||
| pT stage | 0.02 | 0.03 | |||||
| T1 | 793 | 764 (96.3) | 29 (3.7) | Reference | Reference | ||
| T2 | 644 | 591 (91.8) | 53 (8.2) | 1.798 (1.095–2.953) | 1.752 (1.069–2.870) | ||
| Subtype | 0.264 | ||||||
| Luminal A-like | 1,056 | 996 (94.3) | 60 (5.7) | Reference | |||
| Luminal B-like | 135 | 124 (91.8) | 11 (8.2) | 1.475 (0.755–2.881) | |||
| HER2 | 107 | 100 (93.5) | 7 (6.5) | 1.164 (0.518–2.615) | |||
| TNBC | 139 | 135 (97.1) | 4 (2.9) | 0.492 (0.176–1.377) | |||
| No. of removed LNs | 0.001 | ||||||
| 1 | 72 | 61 (84.7) | 11 (15.3) | Reference | |||
| 2 | 291 | 269 (92.4) | 22 (7.6) | 0.454 (0.209–0.985) | |||
| 3 | 329 | 314 (95.4) | 15 (4.5) | 0.265 (0.116–0.604) | |||
| ≥4 | 745 | 711 (95.4) | 34 (4.5) | 0.265 (0.128–0.549) | |||
| No. of removed LNs* | 3.96 ± 1.9 | 3.86 ± 1.7 | 3.30 ± 1.7 | 0.006 | |||
| No. of metastatic LNs | < 0.001 | ||||||
| 1 | 1,109 | 1,074 (96.8) | 35 (3.2) | Reference | |||
| 2 | 328 | 281 (85.7) | 47 (14.3) | 5.110 (3.236–8.068) | |||
| Metastatic LN ratio | < 0.001 | < 0.001 | |||||
| ≤ 0.25 | 565 | 555 (98.2) | 10 (1.8) | Reference | Reference | ||
| > 0.25, ≤ 0.50 | 663 | 624 (94.1) | 39 (5.9) | 3.402 (1.618–7.153) | 3.495 (1.664–7.340) | ||
| > 0.50, ≤ 0.75 | 86 | 73 (84.9) | 13 (15.1) | 8.719 (3.500–21.717) | 8.479 (3.422–21.006) | ||
| > 0.75, ≤ 1 | 123 | 103 (83.7) | 20 (16.3) | 8.707 (3.748–20.227) | 8.778 (3.809–20.230) | ||
N1=nodal stage 1; N2=nodal stage 2; N3=nodal stage3; OR=odds ratio; CI=confidence interval; IDC=invasive ductal carcinoma; ILC=invasive lobular carcinoma; LVI=lymphovascular invasion; NG=nuclear grade; HER2=human epidermal growth factor receptor 2; TNBC=triple-negative breast cancer; LNs=lymph nodes.
*Mean±SD.
Multivariate stepwise logistic regression showed LVI (p=0.008), PNE (p<0.001), T2 stage (p=0.026) and metastatic LN rate (p<0.001) were independent predictors of N2 or N3. There was an association between the number of metastatic LNs and the metastatic LN rate. Therefore, we included only the metastatic LN rate in the multivariate analysis.
Nomogram
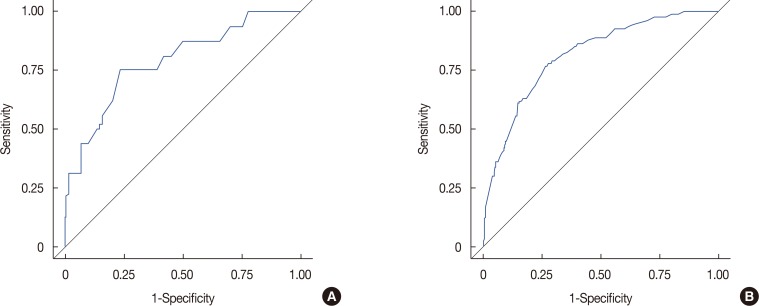
This nomogram was developed based on the following four variables: metastatic LN ratio, PNE, LVI, and T stage (Figure 2). The total sum of each variable was located on a total point line. This line was drawn with a negative slope to calculate the probability of N2 or N3 stage. The AUC of the ROC graph was 0.8050 in the model set (Figure 3A) and 0.8246 in the test set (Figure 3B). We also developed another nomogram using size of metastatic tumor (Figure 4). The AUC of the ROC graph was 0.8129 in the model set and 0.7905 in the test set. Uni- and multi-variable analyses of metastasis size related to nodal stage are shown in Table 3.
Figure 2. Nomogram to predict likelihood of N2 or N3 stage in clinical T1–2N0M0 breast cancer patients using four variables (metastasis node ratio, perinodal extension, lymphovascular invasion, and T stage).
Figure 3. The area under the curve of the receiver operating characteristic graph. (A) In the model set (0.8050) and (B) in the test set (0.8246).
Figure 4. Nomogram to predict likelihood of N2 or N3 stage in clinical T1–2N0M0 breast cancer patients including metastasis size data (metastasis size, metastasis node ratio, lymphovascular invasion, and T stage).
Table 3. Univariable and multivariable analysis of metastatic size related to nodal stage in T1–2 breast cancer patients with no lymphadenopathy.
| Characteristic | Total no. | N1 No. (%) |
N2 or N3 No. (%) |
Univariable | Multivariable | ||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||||
| LN metastasis | < 0.001 | 0.001 | |||||
| Micro | 365 | 361 (98.9) | 4 (1.1) | Reference | Reference | ||
| Macro | 1,072 | 994 (92.7) | 78 (7.3) | 7.079 (2.574–19.470) | 5.300 (1.910–14.720) | ||
| Metastatic size (cm) | |||||||
| > 0.2, ≤ 0.5 | 383 | 375 (97.9) | 8 (2.1) | 1.925 (0.575–6.449) | 0.288 | 1.582 (0.468–5.350) | 0.461 |
| > 0.5, ≤ 1.0 | 480 | 439 (91.5) | 41 (8.5) | 8.428 (2.991–23.750) | < 0.001 | 5.967 (2.090–17.020) | 0.008 |
| > 1.0, ≤ 1.5 | 125 | 105 (84.0) | 20 (16.0) | 17.190 (5.749–51.390) | < 0.001 | 12.680 (4.170–38.520) | < 0.001 |
| > 1.5, ≤ 2.0 | 37 | 32 (86.5) | 5 (13.5) | 14.100 (3.606–55.130) | 0.001 | 7.215 (1.760–29.550) | 0.006 |
| >2 | 8 | 5 (62.5) | 3 (37.5) | 54.150 (9.527–307.800) | < 0.001 | 44.790 (7.010–286.200) | < 0.001 |
N1=nodal stage 1; N2=nodal stage 2; N3=nodal stage3; OR=odds ratio; CI=confidence interval; LN=lymph node; Micro=micrometastasis, meta size ≤0.2 cm; Macro=macrometastasis, meta size >0.2 cm.
Machine learning analysis
We also developed a predictive model using the support vector classifier (SVC) by scikit-learn, a machine learning library [6]. We analyzed the same data used in logistic regression analysis. Among the 1,437 cases, 932 cases were used for modeling and 505 cases used for testing. The average accuracy of prediction was 0.840 (Table 4). The predictive model using the SVC was not superior to the nomogram evaluated using logistic regression analysis.
Table 4. Prediction by machine learning, support vector classification (total 505 patients)*.
| Category | 1 | Precision† | Recall‡ | F-1 score§ | |
|---|---|---|---|---|---|
| N1 | N2 or N3 | ||||
| Predicted | |||||
| N1 | 384 | 40 | 0.910 | 0.910 | 0.910 |
| N2 or N3 | 39 | 42 | 0.520 | 0.510 | 0.520 |
| Average | 0.840 | 0.840 | 0.840 | ||
N1=nodal stage 1; N2=nodal stage 2; N3=nodal stage 3.
*Among 1,437 cases, 932 cases were used for modeling and 505 cases for testing; †Precision, positive predictive value=true positive/true positive+false positive; ‡Recall, sensitivity=true positive/true positive+false negative; §F-1 score, accuracy.
Prospective validation
We conducted a prospective validation with 100 patients who developed ALND after SLNB and T1–2 stage in 2016, at Samsung Medical Center. When we applied a predictive value of 0.3 to our nomogram, the accuracy was 0.96 (Table 5).
Table 5. Prospective validation (total 100 cases).
| Predicted value | 1 | False | Specificity* | Sensitivity† | Precision‡ | Accuracy§ | ||
|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |||||
| Predicted value 0.2ǁ | ||||||||
| No. of cases | 69 | 25 | 1 | 5 | 0.960 | 0.930 | 0.980 | 0.940 |
| Predicted value 0.3ǁ | ||||||||
| No. of cases | 71 | 25 | 2 | 2 | 0.920 | 0.970 | 0.970 | 0.960 |
*Specificity, true negative rate=true negative/true negative+false positive; †Sensitivity, true positive rate=true positive/true positive+false negative; ‡Precision, positive predictive value=true positive/true positive+false positive; §Accuracy=true positive+true negative/total; ∥Each predicted value of our nomogram was applied.
DISCUSSION
Among the 1,437 cases, there were 82 cases (5.7%) of N2 or N3 stages, with 73 cases of N2 and nine of N3. These cases made up a low percentage of the total number of cases; nonetheless, it was important to analyze them carefully because T1–2 with N2 breast cancer belongs in stage IIIA, while any T with N3 belongs in stage IIIC.
There were several studies about nomogram for prediction of axillary lymph node metastasis [7,8]. Previous studies reviewed the records of breast cancer patients who underwent cALND and developed nomogram for prediction of four or more metastatic lymph node [7,8]. Our nomogram was different from previous nomogram in that prediction of N2 or N3 stage patients among cases fulfilling the ACOSOG Z0011 or AMAROS criteria.
In the ACOSOG Z0011 trial, cases with 1 to 2 metastatic SLNs were included. In contrast, we included cases with 1 to 2 metastatic LNs (SLNs or NSLNs) identified during SLNB, because the accuracy of SLN detection is not always ideal, and NSLNs are occasionally metastatic. The false-negative rate of SLN detection is approximately 4% to 11% [9]. In the B-32 trial, which was designed to establish whether SLNs resection could achieve the same therapeutic goals as cALND, 3.9% of cases were identified by palpation alone (515 of 13,171) [10]. If we had enrolled only 1 to 2 metastatic SLNs cases in our sample pool, there would be 179 N2 or N3 cases (N2, 151 and N3, 28), i.e., 12.5% of the total 1,426 cases.
We collected data from both patients treated with breast-conserving surgery (BCS) and those treated with total mastectomy (TM), unlike the ACOSOG Z0011 trial, which only included patients treated with BCS. We intended to determine the actual percentage of N2 or N3 cases in breast cancer with T1–2 (and clinically N0). In this study, the T1–2 settings were the same as that in the ACOSOG Z0011 study. In addition, the type of surgery (BCS or TM) was not a significant factor influencing N2 or N3 stages.
In the ACOSOG Z0011 trial, there were no differences in survival or disease-free survival at 5 years [2]. According to new long-term results from the ACOSOG Z0011 trial (a median follow-up of 9.25 years), there were also no significant differences in local recurrence-free survival [11]. Several studies have appraised the results of the ACOSOG Z0011 trial [12,13,14]. Of all the enrolled patients, approximately 70% had T1 disease, 83% had ER-positive, and 35% had only micro metastases to the SLN [12]. There also may have been bias related to patient selection. Leitch et al. [13] reported that 69% of patients with node-positive disease who did not enroll in the Z0011 trial were treated with cALND. Additional axillary metastases were identified in 27% of patients who underwent ALND, indicating that 73% of patients who underwent ALND had only 1 to 2 metastatic LNs [14]. These deliberations are important for interpreting the Z0011 data and using them in clinical practice. If these trials had included more cases with a higher disease burden, survival results would have been different.
The metastatic node ratio, LVI, PNE, and metastasis size, as established by several mathematical models, are important factors for predicting additional nodal disease [15,16]. In particular, the T2 stage has been shown to be a valuable predictor in previous studies. For example, Hwang et al. [17] found that size of the primary tumor >2 cm was an independent predictor of positive NSLNs (odds ratio, 4.1; 95% confidence interval, 1.42–11.89; p=0.009).
In addition, metastasis size has been found to be an important factor that predicts additional nodal involvement in previous studies [18,19]. We did analyze metastasis size with more segmented grouping than previous studies. In particular, groups of 1.0 to 1.5 cm and more than 2.0 cm were closely correlated with N2 or N3 stage. We assumed that the relatively lower odds ratio of the 1.5 to 2.0 cm group than that of the 1.0 to 1.5 cm group was caused by the small number of 1.5 to 2.0 cm cases. If there were more number of cases, the prediction accuracy of our nomogram would have improved.
The SVC has shown comparable performance in the few studies on medical data sets [20,21]. In our results, the predictive model using the SVC was not superior to those obtained with the nomogram, using logistic regression analysis. Logistic regression models are useful to test the statistical significance of the coefficients in the model and it has a linear nature [22]. Currently, the SVC builds optimal separating boundaries between data sets and produce dichotomous results [22]. Logistic regression is more suitable for the prediction of N stage. However, the SVC also showed reasonable predictive results (the average accuracy was 0.840).
Our study has several limitations. First, we used results from permanent tissue for analysis. In previous studies, there is no mention of clear references regarding the results of LVI from preoperative biopsies or permanent tissues [15,23]. In order to apply our nomogram clinically, we would need data from pre- or intra-operative biopsies. Some authors have found that core needle biopsy (CNB) is unable to accurately detect LVI [24,25]. In contrast, in a study of 500 cases of invasive carcinoma diagnosed by CNB, Harris et al. [25] found a 69% concordance rate between CNB and surgical specimens, in LVI detection. PNE on frozen section is not only used in breast cancer [26], but also in prostate cancer research [27]. Therefore, it is possible that the PNE results from frozen sections during SLNB apply to our nomogram too. In the future, we plan to develop a new nomogram that includes only preor intraoperative biopsy results. Second, we did not collect complication data including wound infections, axillary seromas, lymphedema, and paresthesias that could have been increased by ALND than by SLNB [28]. Third, we did not conduct external validation. We tried to obtain external data but we could not find detailed data that included metastatic results of SLN or NSLN. Hence, further external validation is needed for improving the value of the nomogram in clinical use.
There is a growing tendency to avoid ALND in patients with clinical T1–2 N0M0 stage breast cancer. In addition, SLNB is not acceptable in stage IIIA or IIIC patients as much as it is in early-stage breast cancer patients. Our nomogram could be used for the selection of N2 or N3 stage patients, among cases fulfilling the ACOSOG Z0011 or AMAROS criteria.
Footnotes
This article was supported by a grant from the Korea Health Technology R&D project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI17C1142).
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Bevilacqua JL, Kattan MW, Fey JV, Cody HS, 3rd, Borgen PI, Van Zee KJ. Doctor, what are my chances of having a positive sentinel node? A validated nomogram for risk estimation. J Clin Oncol. 2007;25:3670–3679. doi: 10.1200/JCO.2006.08.8013. [DOI] [PubMed] [Google Scholar]
- 2.Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donker M, van Tienhoven G, Straver ME, Meijnen P, van de, Mansel RE, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15:1303–1310. doi: 10.1016/S1470-2045(14)70460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esteva FJ, Hortobagyi GN. Locally advanced breast cancer. Hematol Oncol Clin North Am. 1999;13:457–472. doi: 10.1016/s0889-8588(05)70065-4. [DOI] [PubMed] [Google Scholar]
- 5.Han A, Moon HG, Kim J, Ahn SK, Park IA, Han W, et al. Reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013;16:378–385. doi: 10.4048/jbc.2013.16.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y, Lee CK. Classification of multiple cancer types by multicategory support vector machines using gene expression data. Bioinformatics. 2003;19:1132–1139. doi: 10.1093/bioinformatics/btg102. [DOI] [PubMed] [Google Scholar]
- 7.Katz A, Smith BL, Golshan M, Niemierko A, Kobayashi W, Raad RA, et al. Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol. 2008;26:2093–2098. doi: 10.1200/JCO.2007.11.9479. [DOI] [PubMed] [Google Scholar]
- 8.Unal B, Gur AS, Beriwal S, Tang G, Johnson R, Ahrendt G, et al. Predicting likelihood of having four or more positive nodes in patient with sentinel lymph node-positive breast cancer: a nomogram validation study. Int J Radiat Oncol Biol Phys. 2009;75:1035–1040. doi: 10.1016/j.ijrobp.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 9.Harlow SP, Krag DN, Julian TB, Ashikaga T, Weaver DL, Feldman SA, et al. Prerandomization Surgical Training for the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 trial: a randomized phase III clinical trial to compare sentinel node resection to conventional axillary dissection in clinically node-negative breast cancer. Ann Surg. 2005;241:48–54. doi: 10.1097/01.sla.0000149429.39656.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Ashikaga T, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 11.Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016;264:413–420. doi: 10.1097/SLA.0000000000001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caudle AS, Hunt KK, Kuerer HM, Meric-Bernstam F, Lucci A, Bedrosian I, et al. Multidisciplinary considerations in the implementation of the findings from the American College of Surgeons Oncology Group (ACOSOG) Z0011 study: a practice-changing trial. Ann Surg Oncol. 2011;18:2407–2412. doi: 10.1245/s10434-011-1593-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leitch AM, McCall L, Beitsch P, Whitworth P, Reintgen D, Blumencranz P, et al. Factors influencing accrual to ACOSOG Z0011, a randomized phase III trial of axillary dissection vs. observation for sentinel node positive breast cancer. J Clin Oncol. 2006;24:601. [Google Scholar]
- 14.Gatzemeier W, Mann GB. Which sentinel lymph-node (SLN) positive breast cancer patient needs an axillary lymph-node dissection (ALND): ACOSOG Z0011 results and beyond. Breast. 2013;22:211–216. doi: 10.1016/j.breast.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Van Zee KJ, Manasseh DM, Bevilacqua JL, Boolbol SK, Fey JV, Tan LK, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–1151. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Scomersi S, Da Pozzo F, Torelli L, Zanconati F, Tonutti M, Dore F, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary lymphnodes in breast cancer patients: is axillary dissection always indicated? Ann Ital Chir. 2010;81:335–341. [PubMed] [Google Scholar]
- 17.Hwang RF, Krishnamurthy S, Hunt KK, Mirza N, Ames FC, Feig B, et al. Clinicopathologic factors predicting involvement of nonsentinel axillary nodes in women with breast cancer. Ann Surg Oncol. 2003;10:248–254. doi: 10.1245/aso.2003.05.020. [DOI] [PubMed] [Google Scholar]
- 18.Schrenk P. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Eur Surg. 2005;37:175–177. doi: 10.1097/01.sla.0000150255.30665.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Straver ME, Meijnen P, van Tienhoven G, van de, Mansel RE, Bogaerts J, et al. Sentinel node identification rate and nodal involvement in the EORTC 10981-22023 AMAROS trial. Ann Surg Oncol. 2010;17:1854–1861. doi: 10.1245/s10434-010-0945-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang RF, Wu WJ, Moon WK, Chou YH, Chen DR. Support vector machines for diagnosis of breast tumors on US images. Acad Radiol. 2003;10:189–197. doi: 10.1016/s1076-6332(03)80044-2. [DOI] [PubMed] [Google Scholar]
- 21.Dreiseitl S, Ohno-Machado L, Kittler H, Vinterbo S, Billhardt H, Binder M. A comparison of machine learning methods for the diagnosis of pigmented skin lesions. J Biomed Inform. 2001;34:28–36. doi: 10.1006/jbin.2001.1004. [DOI] [PubMed] [Google Scholar]
- 22.Dreiseitl S, Ohno-Machado L. Logistic regression and artificial neural network classification models: a methodology review. J Biomed Inform. 2002;35:352–359. doi: 10.1016/s1532-0464(03)00034-0. [DOI] [PubMed] [Google Scholar]
- 23.Viale G, Maiorano E, Pruneri G, Mastropasqua MG, Valentini S, Galimberti V, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg. 2005;241:319–325. doi: 10.1097/01.sla.0000150255.30665.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usami S, Moriya T, Kasajima A, Suzuki A, Ishida T, Sasano H, et al. Pathological aspects of core needle biopsy for non-palpable breast lesions. Breast Cancer. 2005;12:272–278. doi: 10.2325/jbcs.12.272. [DOI] [PubMed] [Google Scholar]
- 25.Harris GC, Denley HE, Pinder SE, Lee AH, Ellis IO, Elston CW, et al. Correlation of histologic prognostic factors in core biopsies and therapeutic excisions of invasive breast carcinoma. Am J Surg Pathol. 2003;27:11–15. doi: 10.1097/00000478-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 26.van der Loo EM, Sastrowijoto SH, Bril H, van Krimpen C, de Graaf PW, Eulderink F. Less operations required due to perioperative frozen section examination of sentinel nodes in 275 breast cancer patients. Ned Tijdschr Geneeskd. 2001;145:1986–1991. [PubMed] [Google Scholar]
- 27.Cheng L, Pisansky TM, Ramnani DM, Leibovich BC, Cheville JC, Slezak J, et al. Extranodal extension in lymph node-positive prostate cancer. Mod Pathol. 2000;13:113–118. doi: 10.1038/modpathol.3880019. [DOI] [PubMed] [Google Scholar]
- 28.Lucci A, McCall LM, Beitsch PD, Whitworth PW, Reintgen DS, Blumencranz PW, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group Trial Z0011. J Clin Oncol. 2007;25:3657–3663. doi: 10.1200/JCO.2006.07.4062. [DOI] [PubMed] [Google Scholar]