Abstract
Purpose
We evaluated the concordance between core needle biopsy (CNB) and surgical specimens on examining intrinsic biological subtypes and receptor status, and determined the accuracy of CNB as a basic diagnostic method.
Methods
We analyzed breast cancer patients with paired CNB and surgical specimen samples during 2014. We used monoclonal antibodies for nuclear staining, and estrogen receptor (ER) and progesterone receptor (PR) status evaluation. A positive test was defined as staining greater than or equal to 1% of tumor cells. Human epidermal growth factor receptor 2 (HER2) was graded by immunohistochemistry and scored as 0 to 3+ according to the recommendations of the American Society of Clinical Oncology/College of American Pathologists. Ki-67 immunostaining was performed using the monoclonal antibody Ki-67, and the results were divided at 10% intervals. The cutoff value for high Ki-67 was defined as 20%. Concordance analysis of ER, PR, HER2, Ki-67, and five intrinsic biological subtypes was performed on CNB and surgical specimens. Statistical analysis for concordance was calculated using κ-tests.
Results
We found very good agreement for ER and PR with a concordance of 96.7% for ER (κ=0.903), and 94.3% for PR (κ=0.870). HER2 and Ki-67 showed concordance rates of 84.8% (κ=0.684) and 83.5% (κ=0.647), respectively, which were interpreted as good agreement. Five subgroups analysis showed 85.8% agreement and κ-value of 0.786, also indicating good agreement.
Conclusion
CNB showed high diagnostic accuracy compared with surgical specimens, and good agreement for ER, PR, HER2, and Ki-67. Our findings reaffirmed the recommendation of CNB as an initial procedure for breast cancer diagnosis, and the assessment of receptor status and intrinsic biological subtypes to determine further treatment plans.
Keywords: Breast neoplasms, Core needle biopsy, Estrogen receptors, Human epidermal growth factor receptor 2, Immunohistochemistry
INTRODUCTION
Core needle biopsy (CNB) is widely used as a standard procedure for diagnosis of breast cancer [1,2]. However, immunohistochemistry (IHC) assessment in CNB samples may be less reliable than in surgical specimens' due to the relatively smaller sample size and tumor heterogeneity [3,4]. Several studies have reported the concordance between preoperative CNB and surgical specimens for estrogen receptor (ER), and human epidermal growth factor receptor 2 (HER2) determination [5,6,7]. A recent meta-analysis has shown that the CNB tissue could replace open excision biopsy for determining ER, progesterone receptor (PR), and HER2 status [8]. The 2015 European Society of Medical Oncology breast cancer clinical practice guideline recommends a preoperative pathological examination of the CNB, with a report on ER, PR, and HER2 status by IHC or fluorescence in situ hybridization [9].
Neoadjuvant chemotherapy (NAC) before definitive surgery can reduce the size and extent of locally advanced tumors. There is an increasing acceptance of view that a pathological complete response (pCR) following chemotherapy is important, particularly as a surrogate for prognosis [10]. The information obtained from CNB may be the only information available for determining the candidates for preoperative or neoadjuvant treatment [3]. Therefore, demand has been markedly increased for clinicians to provide prognostic information considering the determination of IHC for treatment planning. However, there are few studies that have reported concordance rates between CNB and surgical specimens, before and after NAC.
In this study, we evaluated the concordance between CNB and surgical specimens in evaluating intrinsic biological subtypes and the receptor status, and examined the accuracy of CNB as a basic diagnostic method. Second, we assessed changes in intrinsic biological subtypes of breast cancer before and after NAC comparing CNB and surgical specimens.
METHODS
Data collection
We analyzed breast cancer patients with paired CNB and surgical specimen samples during 2014 at Samsung Medical Center, Seoul, Korea. Seventeen hundred eighty-six patients underwent primary surgery or NAC prior to operation. Clinical information on patients collected from medical records included age, body mass index, operation type, NAC history, and main pathological findings that included tumor size, number, nuclear grade, TNM stage, and ER, PR, HER2, and Ki-67 status from both CNB and surgical specimens. The study was approved by the Institutional Review Board of Samsung Medical Center (approval number: 2017-01-102), Seoul, Korea.
ER, PR, HER2, and Ki-67 evaluation
We used monoclonal antibodies for nuclear staining and ER (anti-ER; clone 6F11, Novocastra, Newcastle, UK) and PR (anti-PR; clone 16, Novocastra) status evaluation. A positive test was defined as staining greater than or equal to 1% of tumor cells. A negative test was defined as staining of less than 1% of tumor cells. We used the Allred score interpretation system of intensity score (0–3) and proportion score (0–5) [6].
HER2 (anti-HER2; 4B5, BenchMark XT, Ventana, Tucson, USA) was first graded by IHC and scored as 0 to 3+ according to the recommendations of the American Society of Clinical Oncology/College of American Pathologists [11]. The scoring system defines negative as 0/1+. No observed staining or faint/barely perceptible membrane staining in <10% of tumor cells is 0. Incomplete membrane staining or faint/barely perceptible membrane staining in ≥10% of tumor cells is 1+. A weak to moderate complete membrane staining observed in >10% of tumor cells is 2+ and is interpreted as equivocal. A strong complete membrane staining observed in >10% of tumor cells is 3+ and is considered as positive. In cases of HER2 2+ surgical specimens, we conducted silver in situ hybridization (SISH) assays (INFORM DDISH™ HER2 DNA SISH probe kits; BenchMark XT) to determine HER2 amplification [11,12].
Ki-67 immunostaining was performed using the monoclonal antibody Ki-67 (clone MIB-1; Dako, Glostrup, Denmark). Ki-67 is a nuclear marker expressed in all phases of the cell cycle other than the G0 phase [13,14]. Ki-67 expression has a value between 0% and 100% and is reported at 10% intervals in our center. In this study, we classified samples as low or high expression using 20% as cutoff value.
Five intrinsic biological subtype classifications were categorized according to the 12th St. Gallen international breast cancer conference (2011): luminal A (ER and/or PR positive, HER2 negative, and Ki-67 low); luminal B/HER2 negative (ER and/or PR positive, HER2 negative, and Ki-67 high); luminal B/HER2 positive (ER and/or PR positive, any Ki-67 and HER2 positive); HER2 positive (ER and PR absent, and HER2 positive); and triple negative (ER and PR absent, and HER2 negative) [15].
Statistical analysis
Concordance analysis of ER, PR, HER2, Ki-67, and five intrinsic biological subtypes was performed on CNB and surgical specimens. Statistical analysis for concordance was calculated using κ-tests. κ-values >0.8 indicated very good agreement, between 0.6 and 0.8 indicated good agreement, between 0.4 and 0.6 were considered as moderate agreement, <0.4 as fair, and <0.2 as poor agreement. All statistical tests were two-sided and considered significant if p-value was below 0.05. We used SPSS version 22.0 (IBM Corp., Armonk, USA).
RESULTS
Patient characteristics
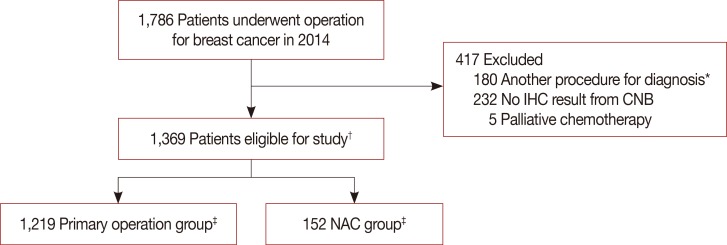
We investigated 1,786 breast cancer patients who underwent surgery during 1 year. There were 1,369 eligible patients with a median age of 49.5 years (range, 24–86 years). We excluded 180 patients who underwent vacuum-assisted biopsy, excisional biopsy, or had previous breast surgery. Moreover, 232 patients were excluded for inadequate test results; for example, CNB sent from other hospitals after diagnosis and their specimens were inadequate to conduct IHC test. Five more patients were excluded because they received palliative chemotherapy. After diagnosis by CNB, 1,219 patients underwent primary surgery and 152 received NAC before surgery (Figure 1); there were two patients with bilateral breast cancer in each group. Patient characteristics and pathology results are shown in Table 1. Eight hundred ninety-two patients (65.1%) underwent breast-conserving surgery and 479 (34.9%) underwent mastectomy. The rate of mastectomy was higher in the NAC group than in the primary surgery group.
Figure 1. Flow chart of patients selection for analysis.
CNB=core needle biopsy; IHC=immunohistochemistry; NAC=neoadjuvant chemotherapy. *Underwent vacuum-assisted biopsy, excisional biopsy or had previous breast surgery; †Two patients diagnosed both breast cancer; ‡Each 1 patients who had both breast cancer underwent operation and NAC prior to surgery.
Table 1. Patient characteristics and clinicopathological results.
| Characteristic | All (n = 1,371)* No. (%) |
Primary operation group (n = 1,219) No. (%) |
NAC group (n = 152) No. (%) |
|---|---|---|---|
| Age (yr) | |||
| ≤ 35 | 100 (7.3) | 64 (5.3) | 36 (23.8) |
| > 35, ≤ 55 | 927 (67.7) | 824 (67.7) | 103 (68.2) |
| > 55 | 342 (25.0) | 330 (27.1) | 12 (7.9) |
| BMI (kg/m2) | |||
| < 25 | 1,007 (73.6) | 907 (74.5) | 100 (66.2) |
| ≥ 25 | 362 (26.4) | 311 (25.5) | 51 (33.8) |
| Operation type | |||
| BCS | 892 (65.1) | 806 (66.1) | 86 (56.6) |
| Mastectomy | 479 (34.9) | 413 (33.9) | 66 (43.4) |
| T stage | |||
| Tis | 21 (1.5) | 4 (0.3) | 17 (11.2) |
| T0 | 19 (1.4) | 0 | 19 (12.5) |
| T1 | 813 (59.3) | 750 (61.5) | 63 (41.4) |
| T2 | 476 (34.7) | 436 (35.8) | 40 (26.3) |
| T3 | 42 (3.1) | 29 (2.4) | 13 (8.6) |
| N stage | |||
| N0 | 855 (62.5) | 775 (63.7) | 80 (52.6) |
| N1 | 387 (28.3) | 339 (27.9) | 48 (31.6) |
| N2 | 88 (6.4) | 70 (5.8) | 18 (11.8) |
| N3 | 38 (2.8) | 32 (2.6) | 6 (3.9) |
| Grade† | |||
| 1 | 317 (24.1) | 302 (25.2) | 15 (13.2) |
| 2 | 590 (44.9) | 539 (44.9) | 51 (44.7) |
| 3 | 407 (31.0) | 359 (29.9) | 48 (42.1) |
| No. of tumors | |||
| Single | 975 (71.1) | 860 (70.5) | 115 (75.7) |
| Multiple | 396 (28.9) | 359 (29.5) | 37 (24.3) |
| LN FNA‡ | |||
| Negative | 1,127 (82.2) | 1,064 (87.3) | 63 (41.4) |
| Positive | 244 (17.8) | 155 (12.7) | 89 (58.6) |
NAC=neoadjuvant chemotherapy; BMI=body mass index; BCS=breastconserving surgery; LN FNA=lymph node fine needle aspiration.
*Some mismatches were caused by two patients who underwent surgery on both breasts. See Figure 1; †Grade and number of tumors evaluated by surgical specimen; ‡Preoperative LN metastasis was determined by FNA.
Tumor pathology and IHC results
About 86% of patients were diagnosed with invasive ductal carcinoma using CNB. Expression of ER, PR, and Ki-67 was not significantly different between CNB and surgical specimens, except for HER2 in the primary surgery group. Patients receiving NAC showed a difference in the Ki-67 status. In contrast, ER, PR, and HER2 did not show significant differences (Table 2).
Table 2. Tumor pathology and IHC test results between the primary operation group and NAC group.
| NAC group (n = 152) | NAC group (n = 152) | NAC group (n = 152) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CNB, No. (%) | SS, No. (%) | p-value | CNB, No. (%) | SS, No. (%) | p-value | CNB, No. (%) | SS, No. (%) | p-value | |
| Histology | 0.001 | < 0.001 | < 0.001 | ||||||
| IDC | 1,177 (85.8) | 1,208 (89.3) | 1,036 (85.0) | 1,099 (90.2) | 141 (92.8) | 109 (82.0) | |||
| ILC | 70 (5.1) | 59 (4.4) | 65 (5.3) | 58 (4.8) | 5 (3.3) | 1 (0.8) | |||
| CIN | 55 (4.0) | 22 (1.6) | 53 (4.3) | 5 (0.4) | 2 (1.3) | 17 (12.8) | |||
| Other | 69 (5.0) | 63 (4.7) | 65 (5.3) | 57 (4.7) | 4 (2.6) | 6 (4.5) | |||
| ER | 0.663 | 0.884 | 0.983 | ||||||
| Positive | 1,069 (78.0) | 1,058 (78.7) | 989 (81.1) | 991 (81.4) | 80 (52.6) | 67 (52.8) | |||
| Negative | 302 (22.0) | 287 (21.3) | 230 (18.9) | 227 (18.6) | 72 (47.4) | 60 (47.2) | |||
| PR | 0.980 | 0.903 | 0.193 | ||||||
| Positive | 928 (67.7) | 911 (67.7) | 870 (71.4) | 872 (71.6) | 58 (38.2) | 39 (30.7) | |||
| Negative | 443 (32.3) | 434 (32.3) | 349 (28.6) | 346 (28.4) | 94 (61.8) | 88 (69.3) | |||
| HER2 (IHC) | < 0.001 | < 0.001 | 0.248 | ||||||
| Negative | 1,005 (73.3) | 869 (64.6) | 910 (74.7) | 797 (65.4) | 95 (62.5) | 72 (56.7) | |||
| Positive | 232 (16.9) | 228 (17.0) | 190 (15.6) | 194 (15.9) | 42 (27.6) | 34 (26.8) | |||
| Equivocal | 134 (9.8) | 248 (18.4) | 119 (9.8) | 227 (18.6) | 15 (9.9) | 21 (16.5) | |||
| HER2 (IHC+SISH) | < 0.001 | < 0.001 | 0.146 | ||||||
| Negative | 1,011 (73.7) | 1,016 (75.5) | 910 (74.7) | 939 (77.1) | 101 (66.4) | 77 (60.6) | |||
| Positive | 236 (17.2) | 293 (21.8) | 190 (15.6) | 254 (20.9) | 46 (30.3) | 39 (30.7) | |||
| Equivocal | 124 (9.0) | 36 (2.7) | 119 (9.8) | 25 (2.1) | 5 (3.3) | 11 (8.7) | |||
| Ki-67 | 0.471 | 0.081 | < 0.001 | ||||||
| Low | 838 (61.5) | 845 (62.8) | 813 (67.1) | 776 (63.7) | 25 (16.6) | 69 (54.3) | |||
| High | 525 (38.5) | 500 (37.2) | 399 (32.9) | 442 (36.3) | 126 (83.4) | 58 (45.7) | |||
IHC=immunohistochemistry; NAC=neoadjuvant chemotherapy; CNB=core needle biopsy; SS=surgical specimens; IDC=invasive ductal carcinoma; ILC=invasive lobular carcinoma; CIN=carcinoma in situ; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; SISH=silver in situ hybridization.
Concordance of IHC results
ER and PR expression in CNB samples showed very good agreement with results for surgical samples; overall concordance rate was 96.7% for ER (κ=0.903) and 94.3% for PR (κ=0.870). HER2 and Ki-67 expression showed good agreement between CNB and surgical specimens; overall concordance rate was 84.8% for HER2 (κ=0.684) and 83.5% for Ki-67 (κ=0.647).
We divided patients into primary surgery and NAC groups. Concordance rates and κ-values for ER, PR, and HER2 showed similar tendency between the two groups. Meanwhile, in cases of primary surgery, Ki-67 expression also showed good agreement between CNB and surgical results with a concordance of 87.0% and κ-value of 0.712. The NAC group had poor agreement between CNB and surgical results with a concordance of 50.0% and κ-value of 0.056 (p=0.397) (Table 3).
Table 3. Agreement analysis of ER, PR, HER2, and Ki-67 between CNB and surgical specimens.
| CNB | All patients (n=1,371) | Primary operation group (n=1,219) | NAC group (n=152) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of surgical specimens | Concordance rate (%) | κ-value | p-value | No. of surgical specimens | Concordance rate (%) | κ-value | p-value | No. of surgical specimens | Concordance rate (%) | κ-value | p-value | |||||||
| Pos | Neg | Equ | Pos | Neg | Equ | Pos | Neg | Equ | ||||||||||
| ER | 96.7 | 0.903 | <0.001 | 97.1 | 0.906 | <0.001 | 92.9 | 0.858 | <0.001 | |||||||||
| Pos | 1,035 | 21 | - | 972 | 16 | - | 63 | 5 | - | |||||||||
| Neg | 23 | 266 | - | 19 | 211 | - | 4 | 55 | - | |||||||||
| PR | 94.3 | 0.870 | <0.001 | 95.0 | 0.877 | <0.001 | 88.2 | 0.746 | <0.001 | |||||||||
| Pos | 878 | 43 | - | 840 | 29 | - | 38 | 14 | - | |||||||||
| Neg | 33 | 391 | - | 32 | 317 | - | 1 | 74 | - | |||||||||
| HER2 (IHC) | 84.8 | 0.684 | <0.001 | 84.6 | 0.672 | <0.001 | 86.6 | 0.762 | <0.001 | |||||||||
| Pos | 215 | 3 | 8 | 182 | 2 | 6 | 33 | 1 | 2 | |||||||||
| Neg | 7 | 833 | 147 | 6 | 766 | 138 | 1 | 67 | 9 | |||||||||
| Equ | 6 | 33 | 93 | 6 | 29 | 83 | 0 | 4 | 10 | |||||||||
| Ki-67 | Low | High | 83.5 | 0.647 | <0.001 | Low | High | 87.0 | 0.712 | <0.001 | Low | High | 50.0 | 0.056 | 0.397 | |||
| Low | 727 | 109 | 712 | 100 | 15 | 9 | ||||||||||||
| High | 112 | 389 | 58 | 341 | 54 | 48 | ||||||||||||
ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; CNB=core needle biopsy; Pos=positive; Neg=negative; Equ=equivocal; IHC=immunohistochemistry.
Concordance among intrinsic biological subtypes
We classified surgical specimens and CNB into five intrinsic biological subtypes according to the 12th St. Gallen international breast cancer conference: luminal A, luminal B (HER2 negative and positive), HER2 positive, and triple negative. Concordance among the five subtypes was 85.8% and the κ-value was 0.786 (p<0.001). Concordance rates of the subtypes were 87.5% for the primary surgery group and 69.3% for the NAC group, and the κ-values between CNB and surgical specimen results were 0.803 and 0.617, respectively (p<0.001) (Table 4).
Table 4. Agreement analysis between CNB and surgical specimens for intrinsic biological subtypes.
| CNB* | Surgical specimen (No.)* | Concordance rate (%) | κ-value | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Luminal A | Luminal B− | Luminal B+ | HER2 | TNBC | ||||
| Primary operation group | 87.5 | 0.803 | < 0.001 | |||||
| Luminal A | 0562 | 43 | 16 | 0 | 1 | |||
| Luminal B− | 29 | 96 | 9 | 0 | 6 | |||
| Luminal B+ | 1 | 0 | 96 | 9 | 0 | |||
| HER2 | 1 | 0 | 6 | 75 | 1 | |||
| TNBC | 0 | 9 | 1 | 3 | 115 | |||
| NAC group† | 69.3 | 0.617 | < 0.001 | |||||
| Luminal A | 7 | 1 | 1 | 0 | 0 | |||
| Luminal B− | 23 | 6 | 1 | 1 | 1 | |||
| Luminal B+ | 1 | 0 | 15 | 2 | 0 | |||
| HER2 | 0 | 0 | 2 | 15 | 1 | |||
| TNBC | 1 | 0 | 0 | 0 | 36 | |||
CNB=core needle biopsy; NAC=neoadjuvant chemotherapy; HER2=human epidermal growth factor receptor 2; TNBC=triple-negative breast cancer.
*Luminal A (ER+ and/or PR+, HER2-, Ki-67 low); luminal B- (ER+ and/or PR+, HER2-, Ki-67 high); luminal B+ (ER+ and/or PR+, any Ki-67, HER2+); HER2 (ER-, PR-, HER2+); and TNBC (ER-, PR-, HER2-); †Total 114 patients after excluding 38 from 152 NAC group. Thirty-eight patients were group who were HER2 2+ and did not have silver in situ hybridization results, or pathologic complete response group who did not have immunohistochemistry results after NAC. Overall concordance rate 85.8%, κ-value of 0.786 and p-value < 0.001.
pCR rate according to the intrinsic biological subtypes in NAC patients
We included both no residual disease of any sort and residual ductal carcinoma in situ (DCIS) without invasive disease and node metastasis in to define pCR. Among the 147 patients in the NAC group, the total pCR rate was 22.4% (33/147); the pCR rate of each intrinsic biological subtypes is shown in Table 5. Among the 33 patients who achieved pCR, 17 patients had no residual tumor and 16 patients had DCIS. No residual tumor patients were excluded from the comparison of the IHC results between CNB and surgical specimens because they had no available tissue sample and postoperative IHC assay could not be performed.
Table 5. pCR rate according to the intrinsic biological subtypes from CNB in NAC patients.
| Subtype* | No. of pCR patients | NAC group† | pCR rate (%) |
|---|---|---|---|
| Luminal A | 0 | 10 | 0 |
| Luminal B– | 6 | 42 | 14.3 |
| Luminal B+ | 8 | 24 | 33.3 |
| HER2 | 6 | 22 | 27.3 |
| TNBC | 13 | 49 | 26.5 |
| Total | 33 | 147 | 22.4 |
pCR=pathologic complete response; CNB=core needle biopsy; NAC=neoadjuvant chemotherapy; HER2=human epidermal growth factor receptor 2; TNBC=triple-negative breast cancer.
*Classified from CNB result. luminal A (ER+ and/or PR+, HER2-, Ki-67 low); luminal B- (ER+ and/or PR+, HER2-, Ki-67 high); luminal B+ (ER+ and/or PR+, any Ki-67, HER2+); HER2 (ER-, PR-, HER2+); and TNBC (ER-, PR-, HER2-); †Five patients were equivocal HER2 result and cannot classified subtype.
DISCUSSION
In this study, we investigated the concordance among IHC test results obtained using CNB and surgical specimens, as well as the agreement with subgroup classification. We found very good agreement for ER and PR with concordance rates of 96.7% (κ=0.903) and 94.3% (κ=0.870), respectively. HER2 and Ki-67 showed concordance rates of 84.8% (κ=0.684) and 83.5% (κ=0.647), respectively, which was interpreted as good agreement. Subgroup analysis showed 85.8% agreement and κ-value of 0.786, also indicating good agreement.
ER and PR status between CNB and surgical specimens showed very good agreement. These results were similar to those that were previously reported; the concordance rates between CNB and surgical specimens of these previous results are shown in Table 6 [3,4,6,7,16,17,18,19,20,21,22]. Hormone receptors are used as predictive factors for response to endocrine therapy and as prognostic factors [23]. We also found that overall agreement between CNB and surgical specimens is better for ER than PR. This result suggests that PR is more heterogeneously distributed in tumors [24].
Table 6. Concordance rate between CNB and surgical specimens in previous studies.
| Author (year) | No. | Concordance (%) | NAC | |||
|---|---|---|---|---|---|---|
| ER | PR | HER2 | Ki-67 | |||
| Mann et al. (2005) [16] | 100 | 86.0 | 83.0 | 80.0 | NA | No |
| Arens et al. (2005) [17] | 25 | 80.0 | 80.0 | 80.0 | 76.0 | Yes |
| Quddus et al. (2005) [18] | 39 | NA | NA | 61.5 | NA | Yes |
| Burge et al. (2006) [19] | 87 | 95.0 | 89.0 | 96.0 | NA | No |
| Arnedos et al. (2009) [3] | 336 | 98.2 | 84.5 | 98.8 | NA | No |
| Tamaki et al. (2010) [6] | 353 | 92.9 | 77.9 | 89.3 | NA | No |
| Lorgis et al. (2011) [4] | 175 | 84.0 | 78.3 | 98.3 | NA | No |
| Ough et al. (2011) [7] | 209 | 88.0 | 78.0 | 81.0 | 59.0 | No |
| Chen et al. (2013) [8] | 298 | 93.6 | 85.9 | 96.3 | 79.5 | No |
| Dekker et al. (2013) [21] | 122 | 99.1 | NA | 96.4 | NA | No |
| Seferina et al. (2013) [22] | 526 | 89.5 | 82.5 | 80.6 | NA | No |
CNB=core needle biopsy; ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; NAC=neoadjuvant chemotherapy; NA=not available.
Approximately 15% to 20% of breast cancer patients have HER2 gene amplification [11]. This study showed a significant difference when comparing results with CNB and surgical specimens for HER2 (Table 2). As a result of the IHC test, concordance rate was 84.8% and the κ-value was 0.684. Equivocal results were 9.8% and 18.4% for CNB and surgical specimens, respectively. We then performed additional SISH tests when HER2 2+ equivocal results were primarily obtained from surgical specimens, and defined HER2 positivity as SISH positive.
Ki-67 is currently the most representative marker for tumor proliferation [13]. The proliferating fraction of cells in tumors prior to treatment, as measured by the Ki-67 nuclear antigen and S-phase fraction (SPF), predict response to chemotherapy [25]. However, scoring procedures have varied, and standardization according to the specimen type is lacking [14]. In our institution, we divided the Ki-67 values at 10% intervals, and the cutoff value for high Ki-67 in this study was defined as 20%. In this study, no significant difference was observed in high and low Ki-67 values between CNB and surgical specimens, with a concordance rate of 83.5% and a κ-value of 0.647.
No significant difference was noted in the expression of hormone receptors in the NAC group. Concordance rate was 92.9% for ER (κ=0.858) and 88.2% for PR (κ=0.746). These results were slightly lower than those of the primary surgery group, but they still showed very good or good agreement as the κ-value was >0.6. HER2 results showed no significant difference between CNB and surgical specimens. Concordance rate of the IHC results was 86.6% and the κ-value was 0.762. However, the concordance rate for Ki-67 was 50.0% and the κ-value was 0.056 (p=0.397), indicating inconsistency between the two groups. These findings suggest that NAC affected proliferative activity and the chemotherapeutic agents interfered with the signal transduction pathway associated with cell division, resulting in a diminished SPF [18].
The degree of agreement of each ER, PR, and HER2 was generally good, except for results of Ki-67 in the NAC group (Table 3). The five intrinsic biological subtypes according to the 12th St. Gallen conference also showed high concordance rate in the NAC group (69.3%, κ=0.617, p<0.001) (Table 4). Notably, 20% of patients (23/114) of the Luminal B (HER2 negative) group turned out to be luminal A after NAC due to a decrease in Ki-67 expression (Table 4). A previous study has found a significant decrease in Ki-67 expression after NAC with mitoxantrone, methotrexate (±mitomycin C), and tamoxifen [25], which has been confirmed in this study.
In the current study, the pCR rate was 0% in the luminal A, 14.3% in the luminal B/HER2 negative, 33.3% in the luminal B/HER2 positive, 27.3% in the HER2, and 26.5% in the triple-negative breast cancer group. These results were similar to those of a large German series and recent studies for the same pCR definition [26,27,28]. For this reason, it is important to determine the intrinsic biological subtypes of breast cancer with CNB before NAC for predicting clinical benefits and it is recommended to some institutes that there are not conducting IHC of CNB specimens before NAC.
There are some limitations in this study. First, this was a single-center, retrospective study. Hence, the results could have selection bias and cannot be generalized. Second, many patients were initially diagnosed in other institutes and referred to our institute for surgery. In this case, CNB was not reexamined, except in cases of vague diagnoses. CNB results were exposed to the possibility of several levels of technical problems that may arise during pathological examinations at hospitals. Third, several neoadjuvant regimens were used depending on the patient, which might cause bias when evaluating the pCR rate among the intrinsic biological subtypes. Besides the limitations, to our knowledge, this study is one of the largest studies comparing concordance between CNB and surgical specimens with sufficient patients who were treated following the same guidelines in a short period. Second, we evaluated the factors of ER, PR, HER2, and Ki-67, which constitute the five intrinsic subtypes according to the 12th St. Gallen international breast cancer conference (2011) [15]. Third, we compared the results of concordance between CNB and surgical specimens of the NAC group at the same time.
In conclusion, CNB showed high diagnostic accuracy when compared with surgical specimens, and good agreement for ER, PR, HER2, and Ki-67. Similarly, patients who underwent NAC also showed good agreement for receptor status, except for Ki-67. Our findings reaffirmed the recommendation of CNB as an initial procedure for breast cancer diagnosis, as well as the assessment of the receptor status and intrinsic biological subtypes to determine further treatment plans.
Footnotes
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2015R1D1A1A01057585) and by the NRF grant funded by the Korean Government (MSIP) (2016R1A5A2945889).
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K. Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Intern Med. 2010;152:238–246. doi: 10.7326/0003-4819-152-1-201001050-00190. [DOI] [PubMed] [Google Scholar]
- 2.Pettine S, Place R, Babu S, Williard W, Kim D, Carter P. Stereotactic breast biopsy is accurate, minimally invasive, and cost effective. Am J Surg. 1996;171:474–476. doi: 10.1016/S0002-9610(96)00007-4. [DOI] [PubMed] [Google Scholar]
- 3.Arnedos M, Nerurkar A, Osin P, A'Hern R, Smith IE, Dowsett M. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC) Ann Oncol. 2009;20:1948–1952. doi: 10.1093/annonc/mdp234. [DOI] [PubMed] [Google Scholar]
- 4.Lorgis V, Algros MP, Villanueva C, Chaigneau L, Thierry-Vuillemin A, Nguyen T, et al. Discordance in early breast cancer for tumour grade, estrogen receptor, progesteron receptors and human epidermal receptor-2 status between core needle biopsy and surgical excisional primary tumour. Breast. 2011;20:284–287. doi: 10.1016/j.breast.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Al Sarakbi W, Salhab M, Thomas V, Mokbel K. Is preoperative core biopsy accurate in determining the hormone receptor status in women with invasive breast cancer? Int Semin Surg Oncol. 2005;2:15. doi: 10.1186/1477-7800-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tamaki K, Sasano H, Ishida T, Miyashita M, Takeda M, Amari M, et al. Comparison of core needle biopsy (CNB) and surgical specimens for accurate preoperative evaluation of ER, PgR and HER2 status of breast cancer patients. Cancer Sci. 2010;101:2074–2079. doi: 10.1111/j.1349-7006.2010.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ough M, Velasco J, Hieken TJ. A comparative analysis of core needle biopsy and final excision for breast cancer: histology and marker expression. Am J Surg. 2011;201:692–694. doi: 10.1016/j.amjsurg.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Yuan Y, Gu Z, Shen K. Accuracy of estrogen receptor, progesterone receptor, and HER2 status between core needle and open excision biopsy in breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;134:957–967. doi: 10.1007/s10549-012-1990-z. [DOI] [PubMed] [Google Scholar]
- 9.Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8–v30. doi: 10.1093/annonc/mdv298. [DOI] [PubMed] [Google Scholar]
- 10.Prowell TM, Pazdur R. Pathological complete response and accelerated drug approval in early breast cancer. N Engl J Med. 2012;366:2438–2441. doi: 10.1056/NEJMp1205737. [DOI] [PubMed] [Google Scholar]
- 11.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/ College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 12.Dowsett M, Bartlett J, Ellis IO, Salter J, Hills M, Mallon E, et al. Correlation between immunohistochemistry (HercepTest) and fluorescence in situ hybridization (FISH) for HER-2 in 426 breast carcinomas from 37 centres. J Pathol. 2003;199:418–423. doi: 10.1002/path.1313. [DOI] [PubMed] [Google Scholar]
- 13.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 14.Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann GB, Fahey VD, Feleppa F, Buchanan MR. Reliance on hormone receptor assays of surgical specimens may compromise outcome in patients with breast cancer. J Clin Oncol. 2005;23:5148–5154. doi: 10.1200/JCO.2005.02.076. [DOI] [PubMed] [Google Scholar]
- 17.Arens N, Bleyl U, Hildenbrand R. HER2/neu, p53, Ki67, and hormone receptors do not change during neoadjuvant chemotherapy in breast cancer. Virchows Arch. 2005;446:489–496. doi: 10.1007/s00428-005-1244-0. [DOI] [PubMed] [Google Scholar]
- 18.Quddus RM, Sung JC, Zhang C, Pasqueriello T, Eklund M, Steinhoff MM. HER-2/neu expression in locally advanced breast carcinomas: pre-and post-neoadjuvant chemotherapy. Breast Cancer. 2005;12:294–298. doi: 10.2325/jbcs.12.294. [DOI] [PubMed] [Google Scholar]
- 19.Burge CN, Chang HR, Apple SK. Do the histologic features and results of breast cancer biomarker studies differ between core biopsy and surgical excision specimens? Breast. 2006;15:167–172. doi: 10.1016/j.breast.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Sun L, Mao Y, Zhu S, Wu J, Huang O, et al. Preoperative core needle biopsy is accurate in determining molecular subtypes in invasive breast cancer. BMC Cancer. 2013;13:390. doi: 10.1186/1471-2407-13-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dekker TJ, Smit VT, Hooijer GK, Van de Vijver MJ, Mesker WE, Tollenaar RA, et al. Reliability of core needle biopsy for determining ER and HER2 status in breast cancer. Ann Oncol. 2013;24:931–937. doi: 10.1093/annonc/mds599. [DOI] [PubMed] [Google Scholar]
- 22.Seferina SC, Nap M, van den Berkmortel F, Wals J, Voogd AC, Tjan-Heijnen VC. Reliability of receptor assessment on core needle biopsy in breast cancer patients. Tumour Biol. 2013;34:987–994. doi: 10.1007/s13277-012-0635-5. [DOI] [PubMed] [Google Scholar]
- 23.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 24.Zidan A, Christie Brown JS, Peston D, Shousha S. Oestrogen and progesterone receptor assessment in core biopsy specimens of breast carcinoma. J Clin Pathol. 1997;50:27–29. doi: 10.1136/jcp.50.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11:174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 26.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30:1796–1804. doi: 10.1200/JCO.2011.38.8595. [DOI] [PubMed] [Google Scholar]
- 27.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 28.Bonnefoi H, Litière S, Piccart M, MacGrogan G, Fumoleau P, Brain E, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the EORTC 10994/BIG 1-00 phase III trial. Ann Oncol. 2014;25:1128–1136. doi: 10.1093/annonc/mdu118. [DOI] [PMC free article] [PubMed] [Google Scholar]