Abstract
PA-MSHA and BPIFB1 play especially important roles in triggering innate immune responses by inducing production of pro- or anti-inflammatory cytokines in the oral cavity and upper airway. We found that PA-MSHA had a strong ability to activate pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α. However, BPIFB1 alone did not express a directly inductive effect. With incubation of PA-MSHA and BPIFB1, the combination can activate the CD14/TLR4/MyD88 complex and induce secretion of subsequent downstream cytokines. We used a proteome profiler antibody array to evaluate the phosphokinases status with PA-MSHA and BPIFB1 treatment. The results showed that the activation of MAPK, STAT, and PI-3K pathways is involved in PA-MSHA-BPIFB1 treatment, and that the related pathways control the secretion of targeting cytokines in the downstream. When we assessed the content changes of cytokines, we found that PA-MSHA-BPIFB1 treatment increased the production of pro-inflammatory cytokines in the early phase of treatment and induced the increase of IL-4 in the late phase. Our observations suggest that PA-MSHA-BPIFB1 stimulates the release of pro-inflammatory cytokines, and thereby initiates the innate immune system against inflammation. Meanwhile, the gradual release of anti-inflammatory cytokine IL-4 by PA-MSHA-BPIFB1 can also regulate the degree of inflammatory response; thus the host can effectively resist the environmental risks, but also manipulate inflammatory response in an appropriate and adjustable manner.
Keywords: PA-MSHA, BPIFB1, Innate immune, CD14/TLR4/MyD88
1. Introduction
Oral surfaces are continually exposed to bacteria, fungi, and viruses. Yet, under normal conditions, the host survives from external infection because epithelial defense plays an especially important role in the oral cavity and upper airway [1]. Epithelial defense systems usually can be categorized into two broad groups: the constitutive, innate immune system and the antigen-recognizing or adaptive immune system. Innate immunity provides the first line of defense against pathogenic microorganisms. The efficient recognition and appropriate response to pathogenic ligands are critical for innate immune system [2].
Bactericidal/Permeability-Increasing protein (BPI) functions as innate immune sensors for the lipopolysaccharide (LPS) component of Gram-negative bacterial cell walls [3-4]. BPI fold-containing family B, member 1 (BPIFB1) belongs to a family of proteins that have considerable structural similarity with BPI and are responsible for hose defense in oral and upper airway epithelia [5]. It has been reported that BPIFB1 can transfer inflammatory signals via the family of Toll-like receptors (TLRs) with stimulation by LPS and initiate host’s early immune reaction, ultimately avoiding potentially fatal consequences for the host. Therefore, BPIFB1 may play a role in protection against bacterial infection [6-7].
The Pseudomonas aeruginosa mannose sensitive hemagglutination (PA-MSHA) is derived from the P. aeruginosa strain with MSHA fimbriae by modern molecular biology technology [8-9]. It can activate a Toll-like receptor (TLR) pathway that serves as a Gram-negative pathogen analog to initiate an inflammation reaction [10-11]. However, the mechanisms in inflammation induced by PA-MSHA have not yet been elucidated. Furthermore, in recent years some laboratory and clinical studies have demonstrated that high-dose usage of antibacterials could cause unwanted side effects, poor stability, and different degrees of resistance in long-term use [12]. Therefore, it will be more promising to search for a novel substitute or strategy for clinical usage that can effectively give rise to immune responses against inflammation in a low-dose and low-toxicity manner.
The purpose of this study is to characterize the effect of combination of PA-MSHA with BPIFB1, and to evaluate whether this combination could enhance non-specific immune ability in the innate immune system. All will be beneficial to understand the mechanisms to these molecules and their effects in therapeutic treatments.
2. Method
2.1. Reagents and antibodies
Human BPIFB1 protein was obtained from Sino Biological (Beijing, China). PA-MSHA was purchased from Wanter Bio-pharmaceutical (Beijing, China). Human TNF-α Quantikine ELISA Kits was purchased from R&D Systems (Minneapolis, MN). Human THP-1 cell line was purchased from American Type Culture Collection (ATCC) (Manassas, VA). Fetal bovine serum (FBS), RPIM-1640 medium, ECL Western blot stripping buffer, a BCA protein assay kit, and penicillin-streptomycin cocktails were from Thermo Scientific (Rockford, IL). Monoclonal anti-CD14 antibody, polyclonal anti-MyD88 antibody, polyclonal anti-TLR4 antibody, polyclonal anti-TNF-α antibody, polyclonal anti-IL-1β antibody, polyclonal anti-IL-4 antibody, polyclonal anti-IL-6 antibody, and anti-β-actin antibody were obtained from Abcam Inc (Cambridge, MA). Polyclonal anti-rabbit horseradish peroxidase (HRP) conjugate was from Bio-Rad Lab. (Hercules, CA). The human phosphokinase antibody array was obtained from R&D Systems (Minneapolis, MN). TLR4 inhibitor TAK-242 was from Merck Millipore (Hayward, CA). Phorbol 12-myristate 13-acetate (PMA), protease inhibitor, LPS (extracted from P. aeruginosa serotype 10), phosphatase inhibitor cocktails, and other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Cell culture
The human monocytic leukemia cell line THP-1 was maintained in RPMI 1640 medium supplemented with 10% FBS, 100 U/ml penicillin, 2 mM L-glutamine and 100 µg/ml streptomycin.
For THP-1 differentiation into macrophage-like subsets, we proceeded as described previously [13]. The cells were plated (at density of 1×106 cell/ml) in RPMI 1640 medium and treated with 100ng/ml PMA for 48 hours.
2.3. PA-MSHA and BPIFB1 Dose Assay
For the PA-MSHA dose assay, differentiated THP-1 cells were incubated with a medium containing PA-MSHA (0, 1×107, 2×107, 4×107, 8×107, 20×107, and 40×107 bacteria/ml) for 24 hours. For BPIFB1, purified protein (0, 1, 2, 3, 4, 5, and 6 mg/ml) was added into the differentiated THP-1cells with or without 10 ng/ml of LPS for 24 hours. Culture supernatants were used to assess PA-MSHA or BPIFB1 dose-response effects using Quantikine TNF-α ELISA Assay kit following the manufacturer’s instructions.
2.4. RNA Extraction and Real-time PCR
5×105/ml differentiated THP-1 cells were treated with 2×107/ml of PA-MSHA and 1 mg/ml BPIFB1 for 24 hours. RNA was extracted using RNeasy Mini kit. First-strand cDNA was synthesized from total RNA using the SuperScript First-strand Synthesis system according to the manufacturer’s protocols. The cDNA was used as a template in real-time PCR reactions with Power SYBR Green PCR Master mix and was run on an Applied Biosystems 7500 real-time PCR system. The 25 µl real-time quantitative PCR reaction mixture consisted of 1x SYBR Green Supermix, 0.25 mmol/L forward and reverse primers, and 10 ng cDNA. The PCR conditions were 50°C for 2 minutes, 95°C for 2 minutes, followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Relative gene expression quantifications were calculated according to the comparative Ct method using GAPDH as an endogenous control. Final results were determined by the 2ΔΔCt formula. The primers used in the experiments are summarized in Table 1.
Table 1.
Primers used in the experiments.
| Fragment | Primer Orientation | Sequence |
|---|---|---|
| CD14 | Forward | 5’-AGCCTAGACCTCAGCCACAA-3’ |
| Reverse | 5’-CTTGGCTGGCAGTCCTTTAG-3’ | |
| TNF-α | Forward | 5’-CCTGTGAGGAGGACGAACAT-3’ |
| Reverse | 5’-GGTTGAGGGTGTCTGAAGGA-3’ | |
| TLR4 | Forward | 5’-CGGAGGCCATTATGCTATGT-3’ |
| Reverse | 5’-TCCCTTCCTCCTTTTCCCTA-3’ | |
| MyD88 | Forward | 5’-GCACATGGGCACATACAGAC-3’ |
| Reverse | 5’-TAGCTGTTCCTGGGAGCTGT-3’ | |
| IL-1β | Forward | 5’-TCGCAGCAGCACATCAACAA-3’ |
| Reverse | 5’-TGGAAGGTCCACGGGAAAGA-3’ | |
| IL-4 | Forward | 5’-CCATATCCACGGATGCGACA-3’ |
| Reverse | 5’-ATGGCGTCCCTTCTCCTGTG-3’ | |
| IL-6 | Forward | 5’-TGGAGCCCACCAAGAACGAT-3’ |
| Reverse | 5’-CCGTGGTTGTCACCAGCATC-3’ | |
| GAPDH | Forward | 5’-ACCCAGAAGACTGTGGATGG-3’ |
| Reverse | 5’-CACATTGGGGGTAGGAACAC-3’ |
2.5. Western blot analysis
Differentiated THP-1 cells were cultured to approximately 40% confluence in 100mm tissue culture dishes (Falcon) and then treated with PA-MSHA and BPIFB1 as described above. Total protein was extracted using a B-PER lysis buffer and protease inhibitors at 4°C for 1 hour. Cell lysates were then sonicated on ice for 30 seconds, centrifuged at 14,000 g for 10 minutes at 4°C, and the protein concentration of the supernatant was quantified using the BCA Protein Assay Kit. 20 µg proteins were loaded per lane and electrophoresis/electroblotting using anti-IL-1β, anti-IL-4, anti-IL-6, anti-TNF-α, anti-CD14, anti-TLR4, or anti-MyD88 antibody. β-actin protein was used as the loading control. Protein bands were detected using SuperSignal West Pico Chemiluminescent Substrate and exposed on DNR MF-Chemi Bio-Imaging Systems. Intensity of the bands was analyzed by ImageJ (National Institutes of Health, NIH) to quantitatively show the expression levels of proteins.
2.6. Human Phospho-kinase antibody array
Approximately 1×107 cells with PA-MSHA and BPIFB1 treatment were solubilized in lysis buffer and centrifuged at 14,000 g for 5 minutes. Protein concentrations of the resulting lysates were measured using a BCA protein assay kit. Next, each of antibody-coated array membranes was placed into the provided dish and incubated with block buffer for 1 hour. 300 ug of prepared cell lysates and 20 µl reconstituted detection antibody cocktail were mixed to incubate for 1 hour. The block buffer was aspirated from the wells of the provided dish, and the prepared sample/antibody mixtures were added to incubate at 4°C with gentle shaking overnight. The membranes were washed with wash buffer and then were incubated with horseradish peroxidase-conjugated streptavidin for 30 minutes. After a final wash, membrane intensity was acquired using chemiluminescence and pixel densities were analyzed using Gelpro Analyzer software (Media Cybernetics, Rockville, MD). Densities were measured as percentage of the positive controls included on each membrane. After subtracting background signals and normalization to positive controls, comparison of signal intensities among array images were used to determine relative differences in expression levels of each protein between groups.
2.7. TLR-4 specific kinase inhibitors treatment
Differentiated THP-1 cells were cultured as described above and co-incubated PA-MSHA and BPIFB1 with 10 µM TLR-4 specific kinase inhibitors (TAK-242) for 24 hours. The control group was treated with 10 µM DMSO as control. The cell lysates were used to determine TNF-α production by Western blot.
2.8. Data analysis
Student’s t-test was used for data analysis. Data were presented as means±SEM. Values for p < 0.05 were considered statistically significant. The model included the main effects of treatments and replicates.
3. Result
3.1. PA-MSHA stimulates TNF-α production directly
To demonstrate the inductive activities of PA-MSHA and BPIFB1, we set up a range of concentrations of PA-MSHA or BPIFB1 to incubate with differentiated THP-1 cells. The ELISA result showed that TNF-α production was increased observably by any experimental concentrations of PA-MSHA, and 2×107/ml PA-MSHA had the maximum effect (Figure 1A). For the BPIFB1, there was any effect on the treatment alone (Figure 1B). However, when LPS was added, BPIFB1 significantly enhanced the TNF-α production (Figure 1C). This indicates that PA-MSHA alone can trigger the innate immune response directly, and BPIFB1 inductive effect needs LPS stimulation.
Figure 1.
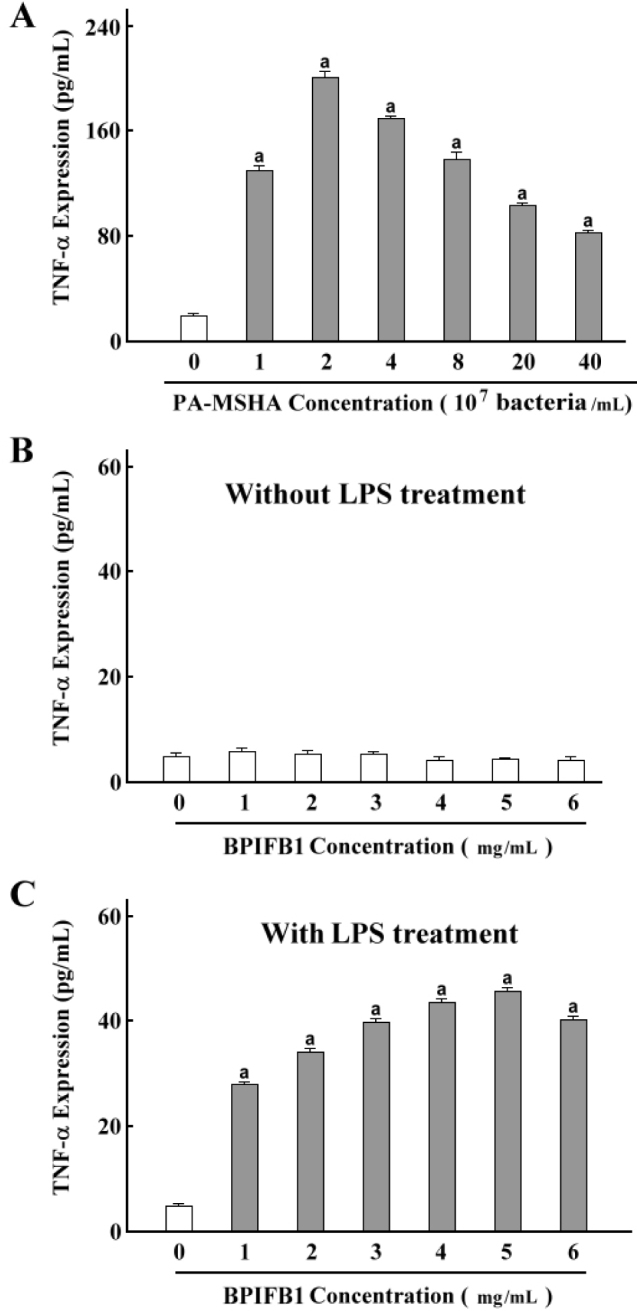
PA-MSHA and BPIFB1 dose dependent assay. A. The human monocytic leukemia cell line THP-1 was treated with 100 ng/ml PMA for 48 hours. Differentiated THP-1 cells were incubated with medium containing PA-MSHA (0, 1×107, 2×107, 4×107, 8×107, 20×107, and 40×107 bacteria/ml) for 24 hours. B and C. Purified BPIFB1 protein (0, 1, 2, 3, 4, 5, and 6 mg/ml) was added into the differentiated THP-1cells with or without 10 ng/ml of LPS for 24 hours. Culture supernatants were used to assess PA-MSHA or BPIFB1 dose-response effects using the Quantikine TNF-α ELISA Assay kit. The results shown represent the mean±SEM of at least three independent experiments. a: p<0.05 compared with none PA-MSHA treatment.
3.2. CD14/TLR4/MyD88 complex contributes to the PA-MSHA-BPIFB1signaling transduction
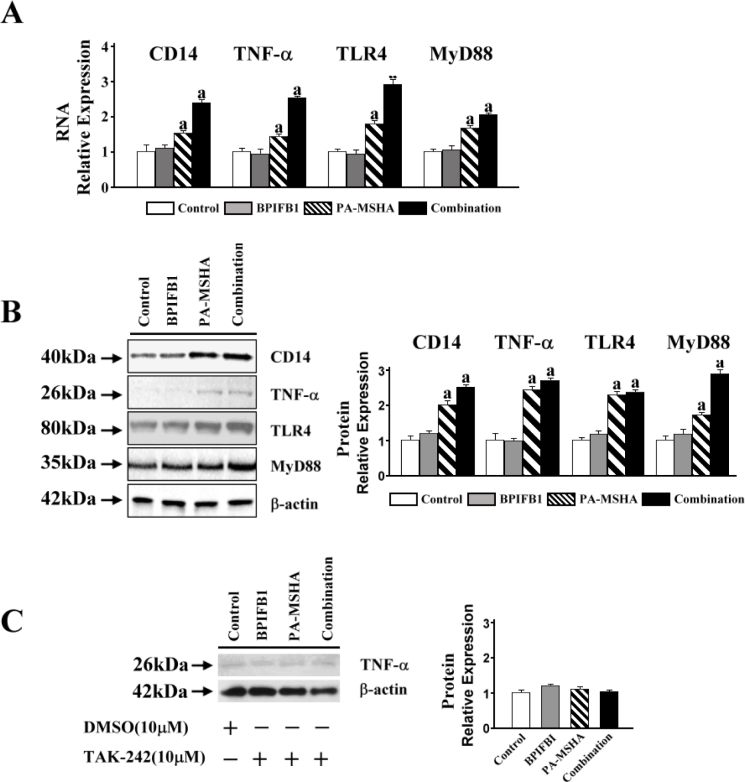
In THP-1 cells treatment with BPIFB1 alone, no significant changes in both the mRNA and protein levels of CD14 were detected compared with the control. However, the production of CD14 with induction by PA-MSHA was significantly increased, whereas the combination of PA-MSHA with BPIFB1 vigorously enhanced the effect. While assessing whether PA-MSHA-BPIFB1induction was dependent on the TLR4 complex, and MyD88-mediated signal was associated with, we measured the expression levels of TLR4 and MyD88. The results showed that PA-MSHA stimulated the expression of TLR4 and MyD88, and that PA-MSHA-BPIFB1treatment substantially enhanced this effect (Figure 2A, 2B).
Figure 2.
CD14/TLR4/MyD88 pathway contributed to the PA-MSHA-BPIFB1 signaling transduction. A. 5×105/ml differentiated THP-1 cells were treated with 2×107/ml of PA-MSHA and 1 mg/ml BPIFB1 for 24 hours. RNA was extracted and first-strand cDNA was synthesized using SuperScript First-strand Synthesis system. The cDNA was used as a template in real-time PCR reactions to analyze the expressions of CD14, TNF-α, TLR-4, and MyD88. B. The protein expressions of CD14, TNF-α, TLR-4, and MyD88 were detected by Western blot. β-actin protein was used as the loading control. Protein bands were detected using SuperSignal West Pico Chemiluminescent Substrate; the intensity of the bands was analyzed by ImageJ to show the expression levels of proteins quantitatively. C. Differentiated THP-1 cells were co-incubated with BPIFB1, PA-MSHA and TLR-4 specific kinase inhibitors (TAK-242, 10 µM) for 24 hours. 10 µM DMSO was added into the control group. The cell lysates were used to determine TNF-α production by western blot. Data representative results derived from a minimum of 3 independent experiments. a: p<0.05 compared with none PA-MSHA treatment.
Because TAK-242 was shown to effectively inhibit TLR-4-mediated signaling events, we used this compound to knock down the activity of TLR-4 and then assessed TNF-α production to determine whether cytokine secretion could be affected by PA-MSHA and BPIFB1. Figure 2C shows that TNF-α was not produced in any response to the PA-MSHA and BPIFB1 stimulation after incubation with TAK-242. In comparison, with normal activity of TLR-4 in THP-1 cells, PA-MSHA and BPIFB1 stimulated a strong cytokine induction (Figure 2A, 2B). All the data demonstrate that CD14/TLR4/MyD88 is an receptor complex to essential activation of the PA-MSHA and BPIFB1 induction pathway.
3.3. Phosphorylation of kinases is involved in PA-MSHA-BPIFB1induction
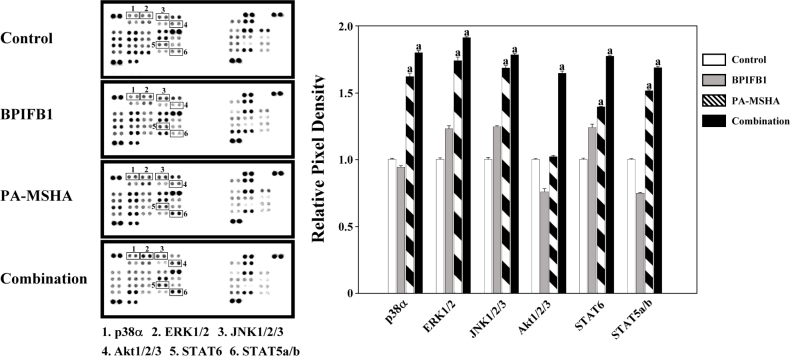
To detect possible protein kinases involved in the signaling transduction of PA-MSHA-BPIFB1 induction, a proteome profiler antibody array was used to evaluate the phosphorylation levels of relative important kinases such as ERK1/2, p38, AKT1. As shown in Figure 3, PA-MSHA treatment triggered the phosphorylation of ERK1/2, p38α, JNK1/2/3, AKT1/2/3, and STATs. The PA-MSHA-BPIFB-1treatment enhanced this effect significantly. Furthermore, we found that BPIFB1 treatment alone did not affect the changes for the kinases. Because ERK1/2, p38α, and JNK1/2/3 are the important members of the MAPK protein kinase family and AKT1/2/3 is an important component of PI-3K signaling pathway, these results demonstrate that the activation of MAPK, STAT, and PI-3K pathways is involved in the PA-MSHA-BPIFB1 induction that controls the secretion of targeting cytokines in the downstream.
Figure 3.
Phosphorylation of kinases was involved in PA-MSHA-BPIFB1 treatment.
Approximately 1×107 cells with PA-MSHA-BPIFB1treatment were solubilized in lysis buffer. 300 ug of prepared cell lysates and antibodiesconjugated membrane were incubated. After keeping with horseradish peroxidase-conjugated streptavidin, the membranes intensity was acquired using chemiluminescence and pixel densities can be analyzed using Gelpro Analyzer software. Densities were measured as a percentage of the positive controls included on each membrane. Data representative results derived from a minimum of 3 independent experiments. a: p < 0.05 compared with none PA-MSHA and BPIFB1 treatment.
3.4. PA-MSHA-BPIFB1induction increases the production of inflammatory cytokines but not that of IL-4
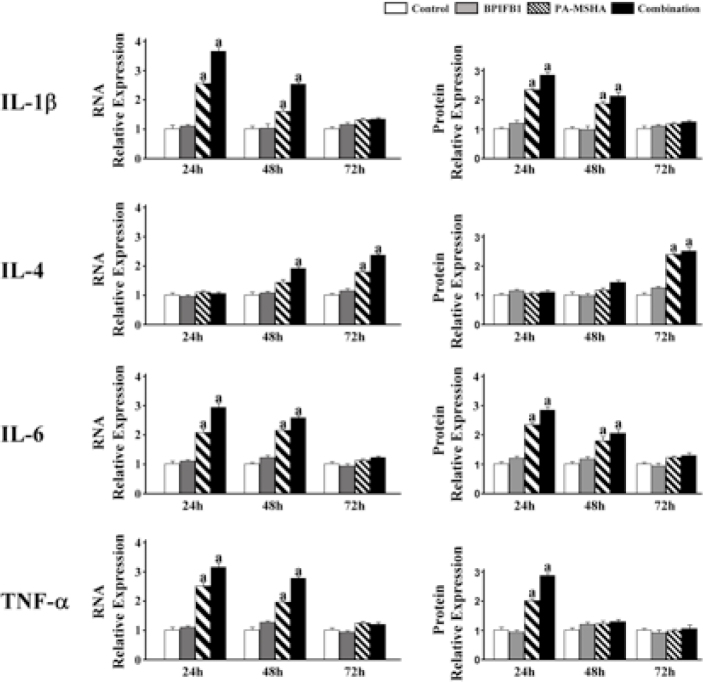
To further validate the effect of PA-MSHA-BPIFB1 induction on cytokine secretion, we examined the mRNA and protein expression of some inflammatory cytokines with PA-MSHA and BPIFB1 treatment. We found that the expression levels of various cytokines changed significantly depending on treatment time. 24 hours and 48 hours incubation of PA-MSHA and BPIFB1 vigorously increased mRNA and protein levels of the inflammatory cytokines IL-1β, IL-6 and TNF-α. At 72 hours incubation with PA-MSHA and BPIFB1, this was no longer visible. Furthermore, although mRNA levels of TNF-α were significantly elevated at 24 and 48 hours incubation, the changes did not appear to be associated with synchronization in the levels of corresponding proteins, suggesting a hysteresis of TNF protein function. Finally, we observed that the IL-4 expression did not keep pace with other cytokines with the treatment of PA-MSHA and BPIFB1. 24 hours and 48 hours incubation of PA-MSHA and BPIFB1 could not increase the expression in mRNA and protein levels of IL-4, but the levels were changed significantly with 72-hour treatment (Figure 4). Together, the data indicate that PA-MSHA-BPIFB1 combination increases the production of inflammatory cytokines but not that of IL-4.
Figure 4.
PA-MSHA-BPIFB1 treatment increased the productions of inflammatory cytokines.
Differentiated THP-1 cells were incubated with PA-MSHA and BPIFB1 protein as described above. The mRNA and protein productions of IL-1β, IL-4, IL-6, and TNF-α cytokines were assayed by real-time PCR and western blot. Data representative results derived from a minimum of 3 independent experiments. a: p<0.05 compared with none PA-MSHA and BPIFB1 treatment.
4. Discussion
As a novel candidate member of host defense compounds in the oral and upper airways epithelium, BPIFB1 protein may share the functions with BPI [14]. Our data demonstrated that, without LPS participation, BPIFB1 alone does not show any direct stimulating effects on cytokine secretion, whereas in combination with PA-MSHA, it resulted in a higher production of tumor necrosis factor-α (TNF-α). TNF-α, a pro-inflammatory cytokine, is involved in a variety of physiological regulations of the body and can launch the inflammatory response to infection. This suggests that PA-MSHA might have ability similar to LPS to initiate the inflammatory reaction and that BPIFB1 may accept the stimulation signal from PA-MSHA to participate in the process.
The inflammatory reactions trigged by LPS require the mediation of some specific proteins on the cell surface. Cluster of differentiation 14 (CD14) is an important transmitter in the process. Wright et al have reported that CD14 can form a ternary complex to concentrate LPS response and transfer the signals for LPS-induced inflammation [15]. When we treated the cells with PA-MSHA and BPIFB1, our findings verified that PA-MSHA treatment can induce the expression of CD14 and stimulate subsequent downstream cytokine secretion. Furthermore, the activation was significantly enhanced by addition of BPIFB1, although BPIFB1 alone did not have the same inductive activity.
However, more evidences indicate that CD14 is differs from other trans-membrane signal receptors by far. It can not communicate the signals directly within the cells because it lacks trans-membrane and intracellular domains; thus, it needs other receptors to present signals in the cytoplasm [16-17].
TLR4 is a member of TLRs with a leucine-rich sequence and intracellular region. It has the typical structural features of the interleukin-1 receptor (IL-1R) superfamily. Other studies have confirmed that TLR4 is a LPS-derived signal transduction molecule that plays an important role in mediating the activation of LPS signaling. Therefore, we wanted to explore whether TLR4 can act as the signaling entity for PA-MSHA and initiate the TLR4 signaling cascade into the cytoplasm [18-20]. Our findings showed that the expression level of TLR4 was increased when treated with PA-MSHA and BPIFB1. Furthermore, there was also a significant increase of myeloid differentiation factor 88 (MyD88) in the combination treatment. However, when the TLR4 pathway was blocked by a TLR4 inhibitor TAK-242, no receptor expression and cytokine secretion were detected. Given that MyD88 is associated with the Toll/interleukin-1 receptor (TIR) domain of TLR4, it culminates in the activation of nuclear factor- kappa B (NFKB), a transcription factor that mediates the activation of numerous inflammatory cytokines and chemokines [21-23]. Combining the results, we indicate that the presence of CD14/TLR4/MyD88 is crucial for immune response to PA-MSHA-BPIFB1 induction.
Because there is little known about the interaction of different pathways involved in PA-MSHA-BPIFB1 induction we used a proteome profiler antibody array to evaluate the phosphorylation levels of several specific kinases. We found that the phosphorylation of ERK1/2, p38α, JNK1/2/3, AKT1/2/3 and STATs was induced by PA-MSHA treatment. The PA-MSHA-BPIFB1 combination enhanced this effect significantly. ERK1/2 kinase is essential for regulation of the transcription factors in the nucleus by transmitting extracellular signals to the nucleus [24]. Also, p38 is responsible to stress stimuli such as cytokines [25]. PI3K can phosphorylate Akt, which is an important induction component for NF-κB activity [26]. Our results confirm the concept that activation of TLR4 response to the PA-MSHA-BPIFB1 induction triggered the rapid recruitment of different factors, stimulated various intracellular signaling pathways including MAPK, STAT and PI-3K phosphorylation, and thereby activated downstream transcription factors and secretion of targeting cytokines.
Cytokines are secreted by cells that can regulate growth and development and participate in the inflammatory response. They are divided into two types: pro-inflammatory cytokines and anti-inflammatory cytokines [27-28]. Presently, the cytokines with pro-inflammatory functions are IL-1β, IL-6 and TNF-α; IL-4 is an anti-inflammatory cytokine [29-31]. The functional cytokine network is a central element in the homeostasis of the immune response, and its alteration may lead to an abnormal immune response [32]. Therefore, we screened the content changes of the cytokines under the combination treatment. When we assessed productions of the pro-inflammatory cytokines, we found that the production of IL-1β, IL-6 and TNF-α were markedly increased with 24 hours or 48 hours incubation with PA-MSHA and BPIFB1, but that this process was no longer visible at 72-hour incubation. The results were consistent with other experiments [33]. In contrast, the anti-inflammatory cytokine IL-4 was not induced at 24 hours post-incubation and only had little content at 48-hour treatment, whereas it significantly induced a large increase at 72-hour incubation. This suggests that these differences in cytokine profile should be reflected in coordinating the inflammatory response with regulatory or inhibitory activities in PA-MSHA and BPIFB1 treatment.
5. Conclusion
Our data demonstrated that BPIFB1 can accept the stimulation from PA-MSHA, activate the CD14/TLR4/MyD88 complex, and stimulate the secretions of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α. Meanwhile, the gradual release of anti-inflammatory cytokines induced by PA-MSHA-BPIFB1 can also neutralize and regulate the degree of inflammatory response, so the host can effectively begin an innate immune process to resist environmental risks and also control the inflammatory response in an appropriate and adjustable manner. Although the detailed mechanism of PA-MSHA-BPIFB1 combination is still unknown, our observations indicate that CD14/TLR4/MyD88 is responsible for the transduction of PA-MSHA-BPIFB1 signaling. Future studies need to clarify the specific molecules comprising the signaling pathways and identify novel therapeutic approaches for PA-MSHA and BPIFB1 that contribute to innate immunity.
Acknowledgement
The work is supported by the Research Foundation of Shenyang Science and Technology Bureau (F14-181-1-00).
Footnotes
Authors’ contribution: WZ and CX participated in the design of the study; WZ, ZD and BY performed experiments; WZ and CX analyzed data; WZ wrote the manuscript; WZ and CX reviewed the manuscript.
Conflict of interest: Authors state no conflict of interest.
Reference
- [1].Diamond G, Beckloff N, Ryan LK. Host defense peptides in the oral cavity and the lung: similarities and differences. J. Dent. Res. 2008;87(10):915–927. doi: 10.1177/154405910808701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lundegaard C, Lund O, Kesmir C, Brunak S, Nielsen M. Modeling the adaptive immune system: predictions and simulations. Bioinformatics. 2007;23(24):3265–3275. doi: 10.1093/bioinformatics/btm471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Saferali A, Obeidat M, Bérubé JC, Lamontagne M, Bossé Y, Laviolette M, Hao K, Nickle DC, Timens W, Sin DD, Postma DS, Strug LJ, Gallins PJ, Paré PD, Bingle CD, Sandford AJ. Polymorphisms associated with expression of BPIFA1/BPIFB1 and lung disease severity in cystic fibrosis. Am J Respir Cell Mol Biol. 2015;53(5):607–614. doi: 10.1165/rcmb.2014-0182OC. [DOI] [PubMed] [Google Scholar]
- [4].Wheeler TT, Hood K, Oden K, McCracken J, Morris CA. Bovine parotid secretory protein: structure, expression and relatedness to other BPI (bactericidal/permeability-increasing protein)-like proteins. Biochem Soc Trans. 2003;31:781–784. doi: 10.1042/bst0310781. [DOI] [PubMed] [Google Scholar]
- [5].Bingle CD, Craven CJ. PLUNC: a novel family of candidate host defence proteins expressed in the upper airways and nasopharynx. Hum. Mol. Genet. 2002;11(8):937–943. doi: 10.1093/hmg/11.8.937. [DOI] [PubMed] [Google Scholar]
- [6].Bingle CD, Craven CJ. Comparative analysis of the PLUNC (palate, lung and nasal epithelium clone) protein families. Biochem. Soc. Trans. 2003;31:806–809. doi: 10.1042/bst0310806. [DOI] [PubMed] [Google Scholar]
- [7].Gao J, Ohlmeier S, Nieminen P, Toljamo T, Tiitinen S, Kanerva T, Bingle L, Araujo B, Rönty M, Höyhtyä M, Bingle CD, Mazur W, Pulkkinen V. Elevated sputum BPIFB1 levels in smokers with chronic obstructive pulmonary disease: a longitudinal study. Am J Physiol Lung Cell Mol Physiol. 2015;309(1):L17–26. doi: 10.1152/ajplung.00082.2015. [DOI] [PubMed] [Google Scholar]
- [8].Zhang Y, Wang H, Li Y, Chen K, Ye J, Liao X, Chen Y, Ran W. The Pseudomonas aeruginosa Mannose Sensitive Hamemagglutination Strain (PA-MSHA) Induces a Th1-Polarizing Phenotype by Promoting Human Dendritic Cells Maturation. Indian J Microbiol. 2014;54(2):163–169. doi: 10.1007/s12088-013-0436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu XF, Wang L, Qu Y, Zhong DW, Miao XY, Yao HL. Effect of the PA-MSHA vaccine on septic serum-induced inflammatory response. Mol Med Rep. 2013;7(4):1350–1354. doi: 10.3892/mmr.2013.1337. [DOI] [PubMed] [Google Scholar]
- [10].Hou J, Liu Y, Liu Y, Shao Y. The MSHA strain of Pseudomonas aeruginosa activated TLR pathway and enhanced HIV-1 DNA vaccine immunoreactivity. PLoS One. 2012;7(10):e47724. doi: 10.1371/journal.pone.0047724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li T, Yang L, Fu SJ, Xiao EL, Yuan X, Lu JZ, Ma BL, Shi TK, Wang ZP. Subcutaneous Injections of the Mannose-Sensitive Hemagglutination Pilus Strain of Pseudomonas aeruginosa Stimulate Host Immunity, Reduce Bladder Cancer Size and Improve Tumor Survival in Mice. Cell Biochem Biophys. 2015;73(1):245–252. doi: 10.1007/s12013-015-0611-y. [DOI] [PubMed] [Google Scholar]
- [12].Jia L, Wang C, Kong H, Yang J, Li F, Lv S, Xu G. Effect of PA-MSHA vaccine on plasma phospholipids metabolic profiling and the ratio of Th2/Th1 cells within immune organ of mouse IgA nephropathy. J Pharm Biomed Anal. 2007;43(2):646–654. doi: 10.1016/j.jpba.2006.07.040. [DOI] [PubMed] [Google Scholar]
- [13].Jiang MX, Hong X, Liao BB, Shi SZ, Lai XF, Zheng HY, Xie L, Wang Y, Wang XL, Xin HB, Fu M, Deng KY. Expression profiling of TRIM protein family in THP1-derived macrophages following TLR stimulation. Sci Rep. 2017;(7):42781. doi: 10.1038/srep42781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nashida T, Yoshimura K, Yoshie S, Mizuhashi F, Shimomura-Kuroki J. Upregulation of Bpifb1 expression in the parotid glands of non-obese diabetic mice. Oral Dis. 2016;22(1):46–52. doi: 10.1111/odi.12377. [DOI] [PubMed] [Google Scholar]
- [15].Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- [16].Schröder NW, Opitz B, Lamping N, Michelsen KS, Zähringer U, Göbel UB, Schumann RR. Involvement of lipopolysaccharide binding protein, CD14, and Toll-like receptors in the initiation of innate immune responses by Treponema glycolipids. J Immunol. 2000;165(5):2683–2693. doi: 10.4049/jimmunol.165.5.2683. [DOI] [PubMed] [Google Scholar]
- [17].Ruiz-Alcaraz AJ, Tapia-Abellán A, Fernández-Fernández MD, Tristán-Manzano M, Hernández-Caselles T, Sánchez-Velasco E, Miras-López M, Martínez-Esparza M, García-Peñarrubia P. A novel CD14high CD16high subset of peritoneal macrophages from cirrhotic patients is associated to an increased response to LPS. Mol Immunol. 2016;72:28–36. doi: 10.1016/j.molimm.2016.02.012. [DOI] [PubMed] [Google Scholar]
- [18].Zhang H, Tay PN, Cao W, Li W, Lu J. Integrin-nucleated Toll-like receptor (TLR) dimerization reveals subcellular targeting of TLRs and distinct mechanisms of TLR4 activation and signaling. FEBS. Lett. 2002;532(1-2):171–176. doi: 10.1016/s0014-5793(02)03669-4. [DOI] [PubMed] [Google Scholar]
- [19].Lee HK, Dunzendorfer S, Tobias PS. Cytoplasmic domainmediated dimerizations of toll-like receptor 4 observed by beta-lactamase enzyme fragment complementation. J. Biol. Chem. 2004;279(11):10564–10574. doi: 10.1074/jbc.M311564200. [DOI] [PubMed] [Google Scholar]
- [20].O’Neill LA. Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr. Top. Microbiol. Immunol. 2002;270:47–61. [PubMed] [Google Scholar]
- [21].Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- [22].O’Neill LA, Dunne A, Edjeback M, Gray P, Jefferies C, Wietek C. MyD88: adapter proteins involved in signal transduction by Toll-like receptors. J. Endotoxin. Res. 2003;9(1):55–59. doi: 10.1179/096805103125001351. [DOI] [PubMed] [Google Scholar]
- [23].Sugiyama K, Muroi M, Kinoshita M, Hamada O, Minai Y, Sugita-Konishi Y, Kamata Y, Tanamoto K. NF-κB activation via MyD88-dependent Toll-like receptor signaling is inhibited by trichothecene mycotoxin deoxynivalenol. J Toxicol Sci. 2016;41(2):273–279. doi: 10.2131/jts.41.273. [DOI] [PubMed] [Google Scholar]
- [24].Soares-Silva M, Diniz FF, Gomes GN, Bahia D. The Mitogen-Activated Protein Kinase (MAPK) Pathway: Role in Immune Evasion by Trypanosomatids. Front Microbiol. 2016;7:183. doi: 10.3389/fmicb.2016.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Pan W, Yu H, Huang S, Zhu P. Resveratrol Protects against TNF-α-Induced Injury in Human Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced Repression of NF-KB and p38 MAPK. PLoS One. 2016;11(1):e0147034. doi: 10.1371/journal.pone.0147034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J. Immunol. 2003;33(3):597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- [27].Mizgerd JP, Spieker MR, Doerschuk CM. Early response cytokines and innate immunity: essential roles for TNF receptor 1 and type I IL-1 receptor during Escherichia coli pneumonia in mice. J. Immunol. 2001;166(6):4042–4048. doi: 10.4049/jimmunol.166.6.4042. [DOI] [PubMed] [Google Scholar]
- [28].Mizgerd JP, Lupa MM, Hjoberg J, Vallone JC, Warren HB, Butler JP, Silverman ES. Roles for early response cytokines during Escherichia coli pneumonia revealed by mice with combined deficiencies of all signaling receptors for TNF and IL-1. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2004;286(6):L1302–1310. doi: 10.1152/ajplung.00353.2003. [DOI] [PubMed] [Google Scholar]
- [29].Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J. Immunol. 2005;175(11):7530–7535. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stewart AG, Beart PM. Inflammation: maladies, models, mechanisms and molecules. Br J Pharmacol. 2016;173(4):631–634. doi: 10.1111/bph.13389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sharma P, Stallknecht DE, Murphy MD, Howerth EW. Expression of interleukin-1 beta and interleukin-6 in white-tailed deer infected with Epizootic Hemorrhagic Disease virus. Vet Ital. 2015;51(4):283–288. doi: 10.12834/VetIt.556.2637.1. [DOI] [PubMed] [Google Scholar]
- [32].Szylberg Ł, Janiczek M, Popiel A, Marszałek A. Expression of COX-2, IL-1β, TNF-α and IL-4 in epithelium of serrated adenoma, adenoma and hyperplastic polyp. Arch Med Sci. 2016;12(1):172–178. doi: 10.5114/aoms.2016.57594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Jurisic V, Srdic-Rajic T, Konjevic G, Bogdanovic G, Colic M. TNF-α induced apoptosis is accompanied with rapid CD30 and slower CD45 shedding from K-562 cells. J Membr Biol. 2011;239(3):115–122. doi: 10.1007/s00232-010-9309-7. [DOI] [PubMed] [Google Scholar]