Abstract
Acute myeloid leukemia (AML) is a common myelogenous malignancy in adults that is often characterized by disease relapse. The pathophysiological mechanism of AML has not yet been elucidated. The present study aimed to identify the crucial microRNAs (miRNAs/miRs) and target genes in AML, and to uncover the potential oncogenic mechanism of AML. miRNA and mRNA expression-profiling microarray datasets were downloaded from the Gene Expression Omnibus database. Differential expression analysis was performed and a regulatory network between miRNAs and target genes was constructed. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes pathway enrichment analyses were used to predict the biological functions of the differentially expressed genes. Reverse transcription-quantitative polymerase chain reaction analysis was employed to verify the expression levels of miRNAs and target genes in AML patient samples. A total of 86 differentially expressed miRNAs and 468 differentially expressed mRNAs between AML and healthy blood samples were identified. In total, 47 miRNAs and 401 mRNAs were found to be upregulated, and 39 miRNAs and 67 mRNAs were found to be downregulated in AML. A total of 223 miRNA-target genes pairs were subjected to the construction of a regulatory network. Differentially expressed target genes were significantly enriched in the Wnt signaling pathway (hsa04310), melanogenesis (hsa04916) and pathways in cancer (hsa05200). Significantly differentially expressed miRNAs and genes, including hsa-miR-155, hsa-miR-192, annexin A2 (ANXA2), frizzled class receptor 3 (FZD3), and pleomorphic adenoma gene 1 (PLAG1), may serve essential roles in AML oncogenesis. Overall, hsa-miR-155, hsa-miR-192, ANXA2, FZD3 and PLAG1 may be associated with the development of AML via the involvement of the Wnt signaling pathway, melanogenesis and other cancer-associated signaling pathways.
Keywords: acute myeloid leukemia, differential expression, microRNA, target genes, regulatory network, reverse transcription-quantitative polymerase chain reaction
Introduction
Leukemia is one of the 10 leading causes of cancer-associated mortality in China; in 2011 there were 27,907 mortalities in men and 19,708 mortalities in women from leukemia (1). The four types of Leukemia are acute lymphocytic leukemia, chronic lymphocytic leukemia, acute myeloid leukemia (AML) and chronic myeloid leukemia. AML accounts for ~80% of cases of acute leukemia in adults (2).
AML is a highly heterogeneous leukemia associated with excessive progenitor cell proliferation and a differentiation block for cell-cycle arrest. AML is often caused by karyotypic abnormalities, including chromosomal translocations, deletions and inversions (3,4). Etiological factors driving AML development remain unclear, but lifestyle and environmental exposures, including obesity and smoking, are reported to be associated with the disease (5).
The French-American-British (FAB) and World Health Organization (WHO) systems are the two main AML classification systems. The FAB system classifies AML into subtypes M0-M7 according to the cell type from which AML develops and the degree of maturation of the cells (6). According to the 2008 WHO Classification, AML are classified into six subgroups: AML with recurring genetic abnormalities, AML with myelodysplasia-related changes, therapy-related myeloid neoplasms, not otherwise specified AML, myeloid proliferations related to down syndrome and blastic plasmacytic dendritic cell neoplasms, with diagnosis performed according to morphology, cytochemistry, immunophenotype, genetics and clinical features (7).
Karyotypic abnormalities and genetic mutations are associated with AML progression and prognosis. Translocation of chromosomes 15 and 17 [t(15;17)], t(8;21) or inversion of chromosome 16 is predictive of a relatively good prognosis (8), whereas deletion of chromosome 7, deletion of 5q or >3 chromosomal abnormalities is predictive of a poor prognosis in AML patients (9,10). Fms-like tyrosine kinase 3-internal duplication (FLT3-ITD) and nucleophosmin (NPM1) are the two most commonly mutated genes in AML patients. Mutations to NPM1 occur in 50% of AML patients, whereas mutations to FLT3-ITD occur in 30%. FLT3-ITD, KIT proto-oncogene receptor tyrosine kinase and brain and acute leukemia, cytoplasmic gene mutations have a negative impact on AML prognosis (11,12), while NPM1 and CCAAT/enhancer binding protein-α have a positive impact on prognosis (12–14).
At present, the pathogenic mechanism of AML is unclear. Acute promyelocytic leukemia (APL) is an M3 subtype of AML according to the FAB classification system. Overexpression of microRNA (miRNA/miR)-125a decreases APL NB4 cell proliferation, the inhibition of cell cycle progression and the promotion of cell apoptosis by targeting the ErbB pathway in APL (15). miR-150 expression induces the myeloid differentiation of human acute leukemia cells and normal hematopoietic progenitors. In AML patient samples and cell lines, miR-150 expression is low or absent, which contributes to the blocking of myeloid differentiation in acute leukemia cells (16).
The aim of the present study was to identify featured target genes of significantly differentially expressed miRNAs in AML by comparing AML samples with healthy ones, and analyzing the correlation of miRNA-target genes. Candidate target genes identified by these approaches may provide the groundwork for the elucidation of the mechanism of AML. However, further investigation of the potential function of these genes in the treatment of AML is required.
Materials and methods
Transcriptomics datasets
In the Gene Expression Omnibus (GEO; http://ncbi.nlm.nih.gov/geo/) (17), only the studies comparing AML and healthy blood were assessed. A total of 6 studies were assessed in which the global profile of gene expression was measured in AML patients' blood samples, with accession numbers GSE48558, GSE35008, GSE35010, GSE24395, GSE17054 and GSE51908. The details of studies, including the platform, number of cases, controls, year and author, were extracted and assessed.
Data processing and identification of differentially expressed miRNAs and mRNAs
Raw expression datasets were downloaded from the GEO and the raw datasets were preprocessed by log2 transformation and Z-score normalization. Limma, which is a linear model for microarray data analysis, was utilized to analyze the differentially expressed miRNAs and mRNAs between the AML and healthy control samples (18). A false discovery rate (FDR) of <0.05 was set as the threshold of differentially expressed miRNAs and mRNAs.
miRNA target gene prediction
Targets genes for differentially expressed miRNAs were predicted via miRTarBase (http://mirtarbase.mbc.nctu.edu.tw/). Over 50,000 miRNA-target interactions in the miRTarBase database have been validated by experiments such as reporter assays, western blotting or microarray experiments with overexpression or knockdown of miRNAs (19,20).
Construction of regulatory miRNA-mRNA networks
The miRNA-mRNA interaction network of differentially expressed miRNA and mRNA was visualized using Cytoscape (http://cytoscape.org) (21). This software presents the regulation between miRNA and mRNA as two-dimensional network with nodes and edges, which represent miRNA-target gene associations.
Functional enrichment analysis of the differentially expressed target genes
To obtain the functions of differentially expressed targeted genes, Gene Ontology (GO) terms (22) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (23) pathways were enriched using GOEAST (http://omicslab.genetics.ac.cn/GOEAST) (24) and GeneCodis (http://genecodis.cnb.csic.es/analysis), respectively (25). P<0.01 and FDR <0.05 were set as the thresholds of significance for GO terms and KEGG pathway analysis.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The blood samples were collected from 3 males with AML treated in Qilu Hospital of Shandong University (Shandong, China) in 2015, with a mean age of 45.6 years. In addition, 3 normal blood samples were also included with corresponding gender and age. Total RNA of fresh blood samples were extracted by TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's instructions. Use of these samples was approved by the Ethics Committee of Qilu Hospital of Shandong University (Jinan, China). The SuperScript III Reverse Transcription kit (Invitrogen; Thermo Fisher Scientific, Inc.) was used to synthesize the cDNA according to the manufacturer's instructions. RT-qPCR was performed using Power SYBR Green PCR Master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) on the Applied Biosystems 7500 (Applied Biosystems; Thermo Fisher Scientific, Inc.). The RT-qPCR cycling conditions were 1 cycle of 95°C for 10 min, followed by 45 cycles of 95°C for 15 sec and 60°C for 60 sec. The miRcute miRNA First-Strand cDNA kit (Tiangen Biotech Co., Ltd., Bejing, China) and the miRcute miRNA qPCR Detection kit (Tiangen Biotech Co., Ltd.) were used for miRNA expression level detection. The RT-qPCR cycling conditions for miRNA were 1 cycle of 94°C for 2 min, followed by 45 cycles of 94°C for 20 sec and 60°C for 34 sec. U6 small nuclear RNA and β-actin was used as internal controls for miRNA and mRNA detection, respectively. The relative expression of target genes was calculated using the 2−ΔΔCq method (26). At least three independent experiments were performed. The PCR primers used were as follows: hsa-miR-155 forward, 5′-TAATGCTAATCGTGATAGGGGT-3′ and reverse, GTGCAGGGTCCGAGGT; hsa-miR-192 forward, 5′-TGACCTATGAATTGACAGCC-3′ and reverse, GTGCAGGGTCCGAGGT; frizzled class receptor 3 (FZD3) forward, 5′-TCTCCTCTTAGCTGGCATTATATCC-3′ and reverse, 5′-GCAGCGTTCTTGTATCCACGTT-3′; and Annexin A2 (ANXA2) forward, 5′-AGAATCATGGTCTCCCGCAGTG-3′ and reverse, 5′-TCCACCACACAGGTACAGCAGC-3′.
Statistical analysis
RT-qPCR experimental data was expressed as the mean ± standard deviation. Statistical significance was evaluated using an unpaired Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
Differentially expressed miRNAs and mRNAs in AML
A total of 5 mRNA and 1 miRNA expression profiles datasets, including 137 AML and 84 healthy samples were downloaded from the GEO, normalized and processed (Table I) (27–31). Differentially expressed genes between AML and normal samples, including 86 miRNAs and 468 mRNAs, were screened with a threshold of FDR<0.05. Of the 86 miRNAs, 47 were upregulated and 39 were downregulated in AML samples compared with the normal samples; of the 468 mRNAs, 401 were upregulated and genes 67 were downregulated. The top 10 upregulated and downregulated miRNAs are shown in Table II (the full list of differentially expressed miRNAs and mRNAs is not shown).
Table I.
Characteristics of mRNA and miRNA expression profiling of the acute myeloid leukemia.
| A, mRNA expression profiling | ||||
|---|---|---|---|---|
| Author, year | Gene expression omnibus ID | Platform | Samples, H:P | (Refs.) |
| Civin et al, 2013 | GSE48558 | GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array [transcript (gene) version] | 49:18 | (27) |
| Barreyro et al, 2012 | GSE35008 | GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array | 16:12 | (28) |
| Barreyro et al, 2012 | GSE35010 | GPL6244 [HuGene-1_0-st] Affymetrix Human Gene 1.0 ST Array | 16:15 | (28) |
| Kikushige et al, 2010 | GSE24395 | GPL6106 Sentrix Human-6 v2 Expression BeadChip | 5:12 | (29) |
| Majeti R et al, 2009 | GSE17054 | GPL570 [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array | 4:9 | (30) |
| B, miRNA expression profiling | ||||
| Author, year | Gene expression omnibus ID | Platform | Samples, H:P | (Refs.) |
| Tan YS et al, 2013 | GSE51908 | GPL8786 [miRNA-1_0] Affymetrix miRNA Array | 47:18 | (31) |
H, healthy subject; P, AML patient; miRNA, microRNA.
Table II.
Significantly differentially expressed miRNAs (top 10).
| miRNA | P-value | Log (fold-change) |
|---|---|---|
| Upregulated miRNAs | ||
| hsa-miR-432 | 9.93×10−12 | 1.66 |
| hsa-miR-126 | 7.44×10−10 | 1.57 |
| hsa-miR-10a | 4.35×10−8 | 1.55 |
| hsa-miR-130a | 3.39×10−11 | 1.54 |
| hsa-miR-34a | 2.05×10−14 | 1.43 |
| hsa-miR-181d | 2.32×10−13 | 1.3 |
| hsa-miR-181a* | 6.65×10−10 | 1.3 |
| hsa-miR-551b* | 3.27×10−8 | 1.17 |
| hsa-miR-501-5p | 1.17×10−8 | 1.08 |
| hsa-miR-125b | 6.04×10−5 | 1.06 |
| Downregulated miRNAs | ||
| hsa-miR-192 | 6.74×10−7 | −1.12 |
| hsa-miR-29b-1* | 2.75×10−8 | −1.1 |
| hsa-miR-194 | 1.66×10−5 | −1.1 |
| hsa-miR-31 | 2.98×10−3 | −1.05 |
| hsa-miR-26b | 6.59×10−8 | −0.971 |
| hsa-miR-628-3p | 6.31×10−4 | −0.755 |
| hsa-miR-30e | 2.84×10−4 | −0.715 |
| hsa-miR-29b | 1.53×10−4 | −0.664 |
| hsa-miR-200c | 3.06×10−5 | −0.635 |
| hsa-miR-21 | 3.96×10−3 | −0.605 |
miRNA/miR, microRNA.
Construction of miRNA-mRNA regulatory networks
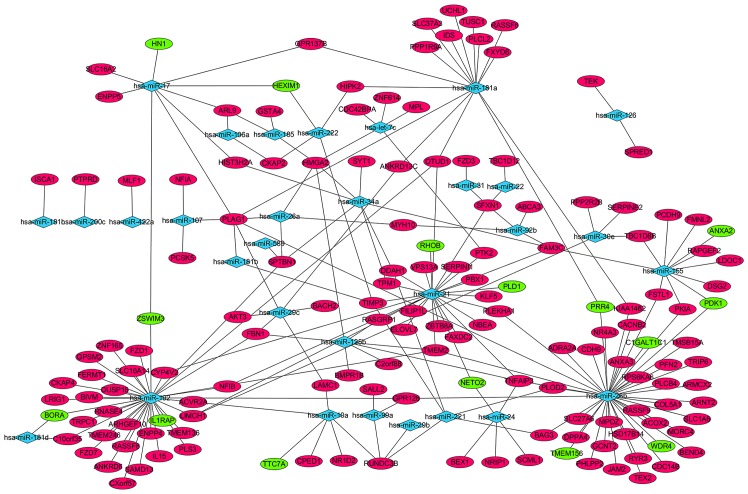
The miRTarBase database was used to predict the target genes of the 47 upregulated and 39 downregulated miRNAs in AML; 223 miRNA-target gene pairs, including 31 differentially expressed miRNAs and 153 target genes, were visualized using Cytoscape software (Fig. 1). A total of 55 differentially expressed miRNAs, including hsa-miR-29b-1* and hsa-miR-194, were not displayed in the network, as the 55 differentially expressed miRNAs were not available in miRTarBase database (data not shown). hsa-miR-26b, hsa-miR-192, hsa-miR-21, hsa-miR-181a and hsa-miR-155 regulated 43, 25, 26, 15 and 11 targets, respectively, and displayed the highest connectivity. Pleomorphic adenoma gene 1 (PLAG1), high-mobility group AT-hook 2, RUN-domain-containing 3B, transmembrane protein 2, TNF-α induced protein 3 and family with sequence similarity 3 member C, which were regulated by 7, 5, 4, 4, 4 and 4 miRNAs, respectively, were the mRNAs with the highest connectivity (Fig. 1).
Figure 1.
miRNA-target gene regulatory network of acute myeloid leukemia. Circular nodes represent target genes and diamond nodes represent miRNAs. Green nodes represent downregulation, red nodes represent upregulation. Solid lines indicate regulatory associations between the miRNAs and target genes. miRNA/miR, microRNA.
Functional analysis of miRNA target genes
GO classification and KEGG pathway analyses were used to obtain the biological functions of miRNA target genes, including biological process, cellular component, molecular function and signaling pathway. The threshold of GO classification was set as P<0.01. Negative regulation of blood coagulation (GO:0030195, P=1.83×10−24), negative regulation of hemostasis (GO:1900047, P=1.83×10−24) and negative regulation of coagulation (GO:0050819, P=2.65×10−23) were the most significantly enriched target genes of biological processes; sarcolemma (GO:0042383, P=1.85×10−29), Schmidt-Lanterman incisure (GO:0043220, P=1.80×10−25) and myelin sheath adaxonal region (GO:0035749, P=5.91×10−25) were the most significantly enriched target genes of the cellular component; and phospholipase inhibitor activity (GO:0004859, P=1.14×10−44), lipase inhibitor activity (GO:0055102, P=3.76×10−43) and calcium-dependent phospholipid binding (GO:0005544, P=5.77×10−41) were the most significantly enriched target genes of the molecular function (Table III).
Table III.
GO annotation of differentially expressed microRNA target genes in acute myeloid leukemia samples (top 15).
| GO ID | GO Term | Count | P-value |
|---|---|---|---|
| Biological process | |||
| GO:0030195 | Negative regulation of blood coagulation | 21 | 1.83×10−24 |
| GO:1900047 | Negative regulation of hemostasis | 21 | 1.83×10−24 |
| GO:0050819 | Negative regulation of coagulation | 21 | 2.65×10−23 |
| GO:0042730 | Fibrinolysis | 17 | 1.90×10−22 |
| GO:0040023 | Establishment of nucleus localization | 16 | 2.55×10−22 |
| GO:0051961 | Negative regulation of nervous system development | 14 | 2.70×10−22 |
| GO:0051964 | Negative regulation of synapse assembly | 14 | 2.70×10−22 |
| GO:0030198 | Extracellular matrix organization | 35 | 6.31×10−21 |
| GO:0043062 | Extracellular structure organization | 35 | 6.58×10−21 |
| GO:0051241 | Negative regulation of multicellular organismal process | 40 | 2.08×10−20 |
| GO:0001525 | Angiogenesis | 35 | 2.86×10−20 |
| GO:0060252 | Positive regulation of glial cell proliferation | 15 | 3.64×10−20 |
| GO:0030320 | Cellular monovalent inorganic anion homeostasis | 14 | 3.68×10−20 |
| GO:0030644 | Cellular chloride ion homeostasis | 14 | 3.68×10−20 |
| GO:0055064 | Chloride ion homeostasis | 14 | 3.68×10−20 |
| Cellular component | |||
| GO:0042383 | Sarcolemma | 33 | 1.85×10−29 |
| GO:0043220 | Schmidt-Lanterman incisure | 18 | 1.80×10−25 |
| GO:0035749 | Myelin sheath adaxonal region | 17 | 5.91×10−25 |
| GO:0043218 | Compact myelin | 18 | 2.95×10−23 |
| GO:0005925 | Focal adhesion | 30 | 1.69×10−21 |
| GO:0005924 | Cell-substrate adherens junction | 30 | 3.04×10−21 |
| GO:0030055 | Cell-substrate junction | 30 | 1.30×10−20 |
| GO:0070161 | Anchoring junction | 32 | 1.09×10−17 |
| GO:0005912 | Adherens junction | 31 | 1.73×10−17 |
| GO:0043209 | Myelin sheath | 18 | 2.16×10−15 |
| GO:0019897 | Extrinsic to plasma membrane | 18 | 2.01×10−13 |
| GO:0019898 | Extrinsic to membrane | 18 | 4.02×10−10 |
| GO:0030054 | Cell junction | 40 | 1.61×10−09 |
| GO:0014704 | Intercalated disc | 14 | 2.10×10−09 |
| GO:0044291 | Cell-cell contact zone | 14 | 3.13×10−09 |
| Molecular function | |||
| GO:0004859 | Phospholipase inhibitor activity | 29 | 1.14×10−44 |
| GO:0055102 | Lipase inhibitor activity | 29 | 3.76×10−43 |
| GO:0005544 | Calcium-dependent phospholipid binding | 35 | 5.77×10−41 |
| GO:0030234 | Enzyme regulator activity | 79 | 1.58×10−23 |
| GO:0004857 | Enzyme inhibitor activity | 43 | 1.15×10−22 |
| GO:0005509 | Calcium ion binding | 65 | 2.23×10−20 |
| GO:0005546 | Phosphatidylinositol-4,5-bisphosphate binding | 18 | 2.28×10−20 |
| GO:0005543 | Phospholipid binding | 53 | 7.37×10−19 |
| GO:1901981 | Phosphatidylinositol phosphate binding | 19 | 2.81×10−17 |
| GO:0008289 | Lipid binding | 56 | 1.54×10−15 |
| GO:0043548 | Phosphatidylinositol 3-kinase binding | 14 | 6.81×10−14 |
| GO:0008092 | Cytoskeletal protein binding | 51 | 1.35×10−13 |
| GO:0017137 | Rab GTPase binding | 17 | 1.55×10−13 |
| GO:0004713 | Protein tyrosine kinase activity | 25 | 1.43×10−12 |
| GO:0035091 | Phosphatidylinositol binding | 22 | 2.75×10−11 |
GO, Gene Ontology.
In total, 148 of the 153 differentially expressed miRNA target genes were enriched in the KEGG database. The Wnt signaling pathway (FDR=8.70×10−4), melanogenesis (FDR=8.70×10−4) and pathways in cancer (FDR=1.60×10−3) were the most significantly enriched pathways in KEGG analysis, with the criteria of FDR<0.05 (Table IV).
Table IV.
KEGG pathway enrichment analysis of differentially expressed microRNA target genes in acute myeloid leukemia (top 15).
| KEGG ID | KEGG term | Count | FDR | Genes |
|---|---|---|---|---|
| hsa04310 | Wnt signaling pathway | 4 | 8.70×10−4 | FZD7, PLCB4, FZD1, FZD3 |
| hsa04916 | Melanogenesis | 4 | 8.70×10−4 | FZD7, PLCB4, FZD1, FZD3 |
| hsa05200 | Pathways in cancer | 8 | 1.60×10−3 | FZD7, AKT3, FZD1, LAMC1, FZD3, PTK2, ARNT2, PLD1 |
| hsa05146 | Amoebiasis | 4 | 2.65×10−3 | PLCB4, LAMC1, PTK2, COL5A1 |
| hsa05222 | Small cell lung cancer | 3 | 2.90×10−3 | AKT3, LAMC1, PTK2 |
| hsa04010 | MAPK signaling pathway | 6 | 3.04×10−3 | DUSP16, RASGRP1, RPS6KA6, RAPGEF2, AKT3, CACNB2 |
| hsa05217 | Basal cell carcinoma | 3 | 3.59×10−3 | FZD7, FZD1, FZD3 |
| hsa04724 | Glutamatergic synapse | 4 | 4.75×10−3 | SLC1A6, PLCB4, TRPC1, PLD1 |
| hsa04530 | Tight junction | 4 | 5.01×10−3 | JAM2, MYH10, AKT3, MPDZ |
| hsa04630 | Jak-STAT signaling pathway | 4 | 8.08×10−3 | IL15, AKT3, MPL, SPRED1 |
| hsa04060 | Cytokine-cytokine receptor interaction | 5 | 9.00×10−3 | IL15, BMPR1B, MPL, IL1RAP, ACVR2A |
| hsa04660 | T-cell receptor signaling pathway | 3 | 1.57×10−2 | RASGRP1, AKT3, PDK1 |
| hsa04510 | Focal adhesion | 4 | 1.61×10−2 | AKT3, LAMC1, PTK2, COL5A1 |
| hsa04722 | Neurotrophin signaling pathway | 3 | 2.02×10−2 | RPS6KA6, AKT3, PDK1 |
| hsa05145 | Toxoplasmosis | 3 | 2.12×10−2 | AKT3, LAMC1, PDK1 |
KEGG, Kyoto Encyclopedia of Genes and Genomes; FDR, false discovery rate.
RT-qPCR validation of differentially expressed miRNAs and target genes
To validate the microarray analysis data, the levels of significant differentially expressed miRNA and target genes were quantified by RT-qPCR in three AML blood samples and three normal blood samples. hsa-miR-155 was significantly (P<0.05) upregulated in AML compared with that in the normal samples, and the target gene ANXA2 was significantly downregulated in AML (Fig. 2A). FZD3 was significantly upregulated in the three AML samples compared with the normal samples (P<0.01; Fig. 2B). The present study identified hsa-miR-192 as a downregulated miRNA in AML, although the expression level was not found to be significantly different in AML by RT-qPCR validation (Fig. 2C).
Figure 2.
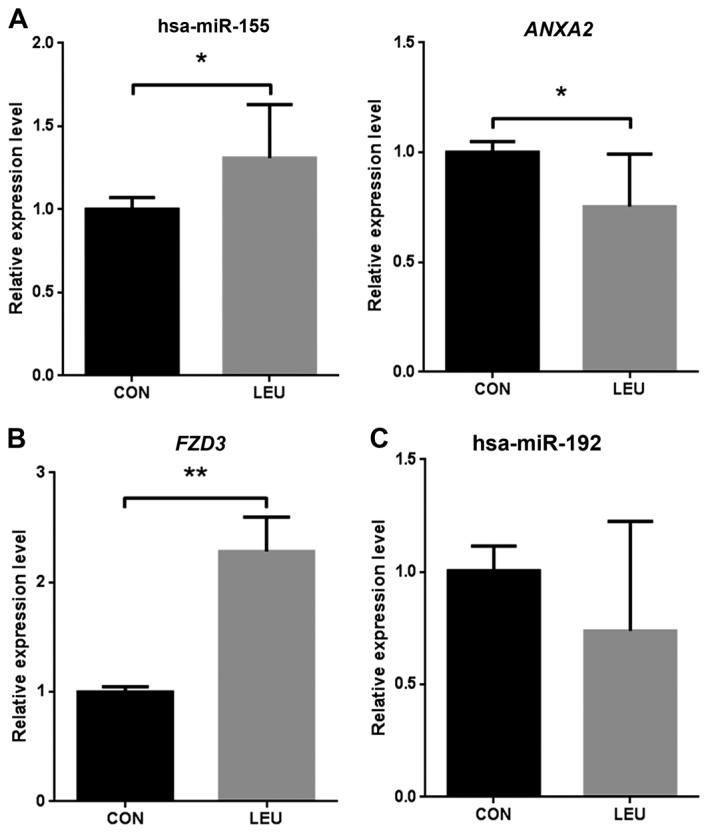
Verification of miRNA and target gene expression levels in AML and normal controls, as determined by reverse transcription-quantitative polymerase chain reaction. (A) hsa-miR-155 and ANXA2 expression levels in AML patients and healthy controls. (B) FZD3 expression levels in AML patients and healthy controls. (C) hsa-miR-192 expression levels in AML patients and healthy controls. *P<0.05, **P<0.01. miRNA/miR, microRNA; AML, acute myeloid leukemia; ANXA2, annexin A2; FZD3, frizzled class receptor 3; CON, healthy control patient blood samples; LEU, AML patient blood samples.
Discussion
In the present study, hsa-miR-155 was one of the five miRNAs with the highest connectivity with target genes, targeting 11 differentially expressed mRNAs (Fig. 1), and was significantly upregulated in AML. In the present study, ANXA2 was predicted as a putative target gene of hsa-miR-155. RT-qPCR validated that hsa-miR-155 was significantly upregulated and ANXA2 was significantly downregulated in AML (Fig. 2A), which is in accordance with the bioinformatics analysis. The fact that hsa-miR-155 was upregulated in AML was consistent with the results of a previous study (32). Mounting evidence identifies hsa-miR-155 as having an oncogenic role, generating AML; overexpression of hsa-miR-155 causes myeloproliferation with cell cell-cycle arrest (33,34). High expression of hsa-miR-155 is associated with a poor outcome in AML patients, which has been observed in numerous AML patients via sequencing studies and miRNA expression analyses (35–37). Additionally, hsa-miR-155 is reported to contribute to the metastasis of various solid tumors, including colorectal carcinoma (38), oral squamous cell carcinoma (39) and renal cell carcinoma (40). ANXA2 is a target gene of hsa-miR-155 and its downregulation is associated with a poor AML patient prognosis, based on gene expression profile analysis (41). hsa-miR-155 upregulation and ANXA2 downregulation may be potential biomarkers for the clinical evaluation of AML prognosis.
Through KEGG analysis, FZD3 was found to be enriched in four signaling pathways, including the Wnt signaling pathway, melanogenesis, pathways in cancer and basal cell carcinoma. The Wnt signaling pathway was the most significantly enriched pathway in AML (Table IV). Higher expression of FZD3 was detected in three AML patients compared with that in the normal control, as determined by RT-qPCR (Fig. 2B), which was consistent with the bioinformatics analysis. FZD3 is a member of the frizzled gene family, which also includes FZD1 and FZD7, and functions as a receptor for the canonical Wnt/β-catenin signaling pathway. Overactivation of the Wnt signaling pathway contributes to tumorigenesis (42,43). According to the present study, the Wnt signaling pathway was essential for AML progression and oncogenicity. CXXC finger protein 5, which is frequently deleted in AML, inhibits the Wnt pathway and leukemic cell proliferation (44). Activation of the Wnt/β-catenin pathway mediates transformation of AML progenitor cells and results in impaired myelomonocytic differentiation (45,46). The FZD3/Wnt signaling pathway may therefore be important in AML pathogenesis.
In the present study, hsa-miR-192 was the most significantly downregulated miRNA and regulated 25 target genes in AML (Fig. 1). miR-192 downregulation is associated with cell cycle progression, cell growth, apoptosis and proliferation of solid tumors (47,48). Overexpression of miR-192 induces apoptotic death in bladder cancer cells, increases the proportion of cells in the G0/G1 phase and decreases the proportion of cells in the S phase compared with a control (47). Curcumin is a traditional Chinese medicine extracted from turmeric that inhibits non-small cell lung cancer cell (NSCLC) cell proliferation and induces NSCLC cell apoptosis through the upregulation of miR-192-5p and the suppression of the phosphoinositide-3 kinase/protein kinase B signaling pathway (47,48). In the present study, hsa-miR-192 was downregulated in AML (Fig. 2C), suggesting that it may also serve a key role in AML cell apoptosis and proliferation.
PLAG1 was targeted by 7 miRNAs, meaning it had the highest connectivity of the mRNAs in the miRNA-mRNA network (Fig. 1). The PLAG family consists of 3 members (PLAG1, PLAGL1 and PLAGL2), each with a highly conserved zinc finger structure that allows them to function as transcription factors to recognize DNA and/or RNA (49). PLAG1 serves an oncogenic role in AML, cooperating with CBF-SMMHC to induce AML tumorigenesis (50). The results of the present study revealed that PLAG1 was upregulated in AML.
In summary, a miRNA-mRNA regulatory network was constructed based on differentially expressed miRNAs and target genes in AML. In this network, a number of miRNAs and target genes that may play important roles in AML, such as hsa-miR-155, hsa-miR192, ANXA2, FZD3 and PLAG1, were identified. These results indicated that the Wnt signaling pathway, melanogenesis and pathways in cancer may be involved in the pathogenesis of AML. An miRNA-target gene regulatory network was constructed in AML using bioinformatic tools. A number of miRNAs and mRNAs that are potentially important for AML tumorigenesis were identified. However, the mechanism behind the associations between miRNA, mRNA and miRNA-mRNA involved in AML progression and development requires further investigation.
Acknowledgements
The present study was supported by a grant from the Program of Jining Science and Technology Development Plan (grant no, 2015-57-102).
References
- 1.Chen W, Zheng R, Zeng H, Zhang S. The updated incidences and mortalities of major cancers in China, 2011. Chin J Cancer. 2015;34:507–507. doi: 10.1186/s40880-015-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cripe LD. Adult acute leukemia. Curr Probl Cancer. 1997;21:1–64. doi: 10.1016/S0147-0272(97)80006-2. [DOI] [PubMed] [Google Scholar]
- 3.Marcucci G, Haferlach T, Döhner H. Molecular genetics of adult acute myeloid leukemia: Prognostic and therapeutic implications. J Clin Oncol. 2011;29:475–486. doi: 10.1200/JCO.2010.30.2554. [DOI] [PubMed] [Google Scholar]
- 4.Deguchi K, Gilliland DG. Cooperativity between mutations in tyrosine kinases and in hematopoietic transcription factors in AML. Leukemia. 2002;16:740–744. doi: 10.1038/sj.leu.2402500. [DOI] [PubMed] [Google Scholar]
- 5.Finn L, Sproat L, Heckman MG, Jiang L, Diehl NN, Ketterling R, Tibes R, Valdez R, Foran J. Epidemiology of adult acute myeloid leukemia: Impact of exposures on clinical phenotypes and outcomes after therapy. Cancer Epidemiol. 2015;39:1084–1092. doi: 10.1016/j.canep.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 7.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, Harris NL, Le Beau MM, Hellström-Lindberg E, Tefferi A, Bloomfield CD. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: Rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 8.Marcucci G, Mrózek K, Ruppert AS, Maharry K, Kolitz JE, Moore JO, Mayer RJ, Pettenati MJ, Powell BL, Edwards CG, et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): A Cancer and Leukemia Group B study. J Clin Oncol. 2005;23:5705–5717. doi: 10.1200/JCO.2005.15.610. [DOI] [PubMed] [Google Scholar]
- 9.Stone RM. Prognostic factors in AML in relation to (ab)normal karyotype. Best Pract Res Clin Haematol. 2009;22:523–528. doi: 10.1016/j.beha.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Schanz J, Haase D. Cytogenetic features in myelodysplastic syndromes. 2014 doi: 10.1007/s00277-008-0483-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Care RS, Valk PJM, Goodeve AC, Abu-Duhier FM, Geertsma-Kleinekoort WM, Wilson GA, Gari MA, Peake IR, Löwenberg B, Reilly JT. Incidence and prognosis of c-KIT and FLT3 mutations in core binding factor (CBF) acute myeloid leukaemias. Br J Haematol. 2003;121:775–777. doi: 10.1046/j.1365-2141.2003.04362.x. [DOI] [PubMed] [Google Scholar]
- 12.Langer C, Radmacher MD, Ruppert AS, Whitman SP, Paschka P, Mrózek K, Baldus CD, Vukosavljevic T, Liu CG, Ross ME, et al. High BAALC expression associates with other molecular prognostic markers, poor outcome, and a distinct gene-expression signature in cytogenetically normal patients younger than 60 years with acute myeloid leukemia: A Cancer and Leukemia Group B (CALGB) study. Blood. 2008;111:5371–5379. doi: 10.1182/blood-2007-11-124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preudhomme C, Sagot C, Boissel N, Cayuela JM, Tigaud I, de Botton S, Thomas X, Raffoux E, Lamandin C, Castaigne S, et al. Favorable prognostic significance of CEBPA mutations in patients with de novo acute myeloid leukemia: A study from the acute leukemia French association (ALFA) Blood. 2002;100:2717–2723. doi: 10.1182/blood-2002-03-0990. [DOI] [PubMed] [Google Scholar]
- 14.Döhner K, Tobis K, Ulrich R, Fröhling S, Benner A, Schlenk RF, Döhner H. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: A study of the acute myeloid leukemia study group Ulm. J Clin Oncol. 2002;20:3254–3261. doi: 10.1200/JCO.2002.09.088. [DOI] [PubMed] [Google Scholar]
- 15.Ufkin ML, Peterson S, Yang X, Driscoll H, Duarte C, Sathyanarayana P. miR-125a regulates cell cycle, proliferation, and apoptosis by targeting the ErbB pathway in acute myeloid leukemia. Leuk Res. 2014;38:402–410. doi: 10.1016/j.leukres.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris VA, Zhang A, Yang T, Stirewalt DL, Ramamurthy R, Meshinchi S, Oehler VG. MicroRNA-150 expression induces myeloid differentiation of human acute leukemia cells and normal hematopoietic progenitors. PLoS One. 2013;8:e75815. doi: 10.1371/journal.pone.0075815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgar R, Domrachev M, Lash AE. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diboun I, Wernisch L, Orengo CA, Koltzenburg M. Microarray analysis after RNA amplification can detect pronounced differences in gene expression using limma. BMC Genomics. 2006;7:252. doi: 10.1186/1471-2164-7-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu SD, Lin FM, Wu WY, Liang C, Huang WC, Chan WL, Tsai WT, Chen GZ, Lee CJ, Chiu CM, et al. miRTarBase: A database curates experimentally validated microRNA-target interactions. Nucleic Acids Res. 2011;39:D163–D169. doi: 10.1093/nar/gkq1107. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu SD, Tseng YT, Shrestha S, Lin YL, Khaleel A, Chou CH, Chu CF, Huang HY, Lin CM, Ho SY, et al. miRTarBase update 2014: An information resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2014;42:D78–D85. doi: 10.1093/nar/gkt1266. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng L, Lin H, Hu Y, Wang J, Yang Z. Gene function prediction based on the Gene Ontology hierarchical structure. PLoS One. 2014;9:e107187. doi: 10.1371/journal.pone.0107187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Q, Wang XJ. GOEAST: A web-based software toolkit for Gene Ontology enrichment analysis. Nucleic Acids Res. 2008;36:W358–W363. doi: 10.1093/nar/gkn276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmona-Saez P, Chagoyen M, Tirado F, Carazo JM, Pascual-Montano A. GENECODIS: A web-based tool for finding significant concurrent annotations in gene lists. Genome Biol. 2007;8:R3. doi: 10.1186/gb-2007-8-1-r3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Cramer-Morales K, Nieborowska-Skorska M, Scheibner K, Padget M, Irvine DA, Sliwinski T, Haas K, Lee J, Geng H, Roy D, et al. Personalized synthetic lethality induced by targeting RAD52 in leukemias identified by gene mutation and expression profile. Blood. 2013;122:1293–1304. doi: 10.1182/blood-2013-05-501072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barreyro L, Will B, Bartholdy B, Zhou L, Todorova TI, Stanley RF, Ben-Neriah S, Montagna C, Parekh S, Pellagatti A, et al. Overexpression of IL-1 receptor accessory protein in stem and progenitor cells and outcome correlation in AML and MDS. Blood. 2012;120:1290–1298. doi: 10.1182/blood-2012-01-404699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki Y, Akashi K. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7:708–717. doi: 10.1016/j.stem.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Majeti R, Becker MW, Tian Q, Lee TL, Yan X, Liu R, Chiang JH, Hood L, Clarke MF, Weissman IL. Dysregulated gene expression networks in human acute myelogenous leukemia stem cells. Proc Natl Acad Sci USA. 2009;106:3396–3401. doi: 10.1073/pnas.0900089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan YS, Kim M, Kingsbury TJ, Civin CI, Cheng WC. Regulation of RAB5C is important for the growth inhibitory effects of MiR-509 in human precursor-B acute lymphoblastic leukemia. PLoS One. 2014;9:e111777. doi: 10.1371/journal.pone.0111777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Havelange V, Stauffer N, Heaphy CC, Volinia S, Andreeff M, Marcucci G, Croce CM, Garzon R. Functional implications of microRNAs in acute myeloid leukemia by integrating microRNA and messenger RNA expression profiling. Cancer. 2011;117:4696–4706. doi: 10.1002/cncr.26096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forrest AR, Kanamori-Katayama M, Tomaru Y, Lassmann T, Ninomiya N, Takahashi Y, de Hoon MJ, Kubosaki A, Kaiho A, Suzuki M, et al. Induction of microRNAs, mir-155, mir-222, mir-424 and mir-503, promotes monocytic differentiation through combinatorial regulation. Leukemia. 2010;24:460–466. doi: 10.1038/leu.2009.246. [DOI] [PubMed] [Google Scholar]
- 34.O'Connell RM, Rao DS, Chaudhuri AA, Boldin MP, Taganov KD, Nicoll J, Paquette RL, Baltimore D. Sustained expression of microRNA-155 in hematopoietic stem cells causes a myeloproliferative disorder. J Exp Med. 2008;205:585–594. doi: 10.1084/jem.20072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcucci G, Maharry KS, Metzeler KH, Volinia S, Wu YZ, Mrózek K, Nicolet D, Kohlschmidt J, Whitman SP, Mendler JH, et al. Clinical role of microRNAs in cytogenetically normal acute myeloid leukemia: miR-155 upregulation independently identifies high-risk patients. J Clin Oncol. 2013;31:2086–2093. doi: 10.1200/JCO.2012.45.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chuang MK, Chiu YC, Chou WC, Hou HA, Chuang EY, Tien HF. A 3-microRNA scoring system for prognostication in de novo acute myeloid leukemia patients. Leukemia. 2015;29:1051–1059. doi: 10.1038/leu.2014.333. [DOI] [PubMed] [Google Scholar]
- 37.Zhi F, Cao X, Xie X, Wang B, Dong W, Gu W, Ling Y, Wang R, Yang Y, Liu Y. Identification of circulating microRNAs as potential biomarkers for detecting acute myeloid leukemia. PLoS One. 2013;8:e56718. doi: 10.1371/journal.pone.0056718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qu YL, Wang HF, Sun ZQ, Tang Y, Han XN, Yu XB, Liu K. Up-regulated miR-155-5p promotes cell proliferation, invasion and metastasis in colorectal carcinoma. Int J Clin Exp Pathol. 2015;8:6988–6994. [PMC free article] [PubMed] [Google Scholar]
- 39.Baba O, Hasegawa S, Nagai H, Uchida F, Yamatoji M, Kanno NI, Yamagata K, Sakai S, Yanagawa T, Bukawa H. MicroRNA-155-5p is associated with oral squamous cell carcinoma metastasis and poor prognosis. J Oral Pathol Med. 2016;45:248–255. doi: 10.1111/jop.12351. [DOI] [PubMed] [Google Scholar]
- 40.Merhautova J, Hezova R, Poprach A, Kovarikova A, Radova L, Svoboda M, Vyzula R, Demlova R, Slaby O. miR-155 and miR-484 are associated with time to progression in metastatic renal cell carcinoma treated with sunitinib. Biomed Res Int. 2015;2015:941980. doi: 10.1155/2015/941980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park MH, Cho SA, Yoo KH, Yang MH, Ahn JY, Lee HS, Lee KE, Mun YC, Cho DH, Seong CM, Park JH. Gene expression profile related to prognosis of acute myeloid leukemia. Oncol Rep. 2007;18:1395–1402. [PubMed] [Google Scholar]
- 42.Conacci-Sorrell M, Zhurinsky J, Ben-Ze'ev A. The cadherin-catenin adhesion system in signaling and cancer. J Clin Invest. 2002;109:987–991. doi: 10.1172/JCI0215429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 44.Kühnl A, Valk PJ, Sanders MA, Ivey A, Hills RK, Mills KI, Gale RE, Kaiser MF, Dillon R, Joannides M, et al. Downregulation of the Wnt inhibitor CXXC5 predicts a better prognosis in acute myeloid leukemia. Blood. 2015;125:2985–2994. doi: 10.1182/blood-2014-12-613703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Krivtsov AV, Sinha AU, North TE, Goessling W, Feng Z, Zon LI, Armstrong SA. The Wnt/beta-catenin pathway is required for the development of leukemia stem cells in AML. Science. 2010;327:1650–1653. doi: 10.1126/science.1186624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simon M, Grandage VL, Linch DC, Khwaja A. Constitutive activation of the Wnt/beta-catenin signalling pathway in acute myeloid leukaemia. Oncogene. 2005;24:2410–2420. doi: 10.1038/sj.onc.1208431. [DOI] [PubMed] [Google Scholar]
- 47.Ye M, Zhang J, Zhang J, Miao Q, Yao L, Zhang J. Curcumin promotes apoptosis by activating the p53-miR-192-5p/215-XIAP pathway in non-small cell lung cancer. Cancer Lett. 2015;357:196–205. doi: 10.1016/j.canlet.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 48.Jin H, Qiao F, Wang Y, Xu Y, Shang Y. Curcumin inhibits cell proliferation and induces apoptosis of human non-small cell lung cancer cells through the upregulation of miR-192-5p and suppression of PI3K/Akt signaling pathway. Oncol Rep. 2015;34:2782–2789. doi: 10.3892/or.2015.4258. [DOI] [PubMed] [Google Scholar]
- 49.Kas K, Voz ML, Hensen K, Meyen E, Van de Ven WJ. Transcriptional activation capacity of the novel PLAG family of zinc finger proteins. J Biol Chem. 1998;273:23026–23032. doi: 10.1074/jbc.273.36.23026. [DOI] [PubMed] [Google Scholar]
- 50.Landrette SF, Kuo YH, Hensen K, van Waalwijk van Doorn-Khosrovani Barjesteh S, Perrat PN, Van de Ven WJ, Delwel R, Castilla LH. Plag1 and Plagl2 are oncogenes that induce acute myeloid leukemia in cooperation with Cbfb-MYH11. Blood. 2005;105:2900–2907. doi: 10.1182/blood-2004-09-3630. [DOI] [PubMed] [Google Scholar]