Abstract
Background
The efficacy of and overall survival associated with metastatic castration-resistant prostate cancer (CRPC) treatments rely on patients' consistent adherence to the recommended dosage regimens.
Objectives
To evaluate treatment patterns and patient adherence to abiraterone acetate or enzalutamide therapy in real-world practice, and to examine the factors that may be associated with medication dose reduction in patients with metastatic CRPC.
Methods
Retrospective analyses were conducted using the Truven Health MarketScan research databases among patients with metastatic CRPC who initiated treatment with abiraterone acetate or enzalutamide between October 1, 2012, and December 31, 2014 (index date). The patients were followed for up to 12 months, and their baseline characteristics were assessed during the 6 months before the index date. Medication adherence was measured at 3, 6, 9, and 12 months postindex using medication possession ratios (MPRs), and dose reduction was measured using refill gaps and relative dose intensity over the entire observation period. Kaplan-Meier survival analyses and Cox proportional hazards models were used to assess the association between the initial treatment and the risk for dose reduction.
Results
The study included 2591 and 807 patients who initiated treatment with abiraterone acetate and enzalutamide, respectively. At 6, 9, and 12 months postindex, the patients who initiated abiraterone acetate had higher MPRs than the patients who initiated enzalutamide. In addition, the patients who initiated abiraterone acetate had lower Kaplan-Meier rates of dose reduction across 4 measurements for dose reduction. All hazard ratios for treatment (abiraterone acetate vs enzalutamide) were significantly lower than 1 (range, 0.57–0.80), indicating a lower risk for dose reduction associated with abiraterone acetate.
Conclusion
Patients who initiated abiraterone acetate therapy had higher medication adherence and lower risk for dose reduction than those who initiated enzalutamide therapy. Improved medication adherence may be associated with longer duration of treatment, which in turn may lead to better survival. Further research is needed to assess the potential effect of medication adherence on the overall survival of patients with metastatic CRPC.
Keywords: abiraterone acetate therapy, dose reduction, enzalutamide therapy, medication adherence, medication possession ratio, metastatic castration-resistant prostate cancer, proportional hazards, survival, treatment patterns
Prostate cancer is a substantial cause of cancer-related morbidity and mortality in the United States.1 Approximately 1 of 7 men will be diagnosed with prostate cancer during his lifetime, and approximately 1 of 39 men will die of prostate cancer in the United States.2 Castration-resistant prostate cancer (CRPC) is defined by disease progression, despite androgen-deprivation therapy, and may present as a constant rise of prostate-specific antigen in serum levels, the progression of pre-existing disease, the appearance of new metastases, or any combination of the 3.3 Once CRPC has spread to other organs or tissues in the body, such as in bone or in the bladder, it is referred to as metastatic CRPC. In recent years, treatment options for advanced prostate cancer have expanded. Prolonged survival rates, pain reduction, and improvement in quality of life have been achieved by using new drugs, such as docetaxel, cabazitaxel, abiraterone acetate, enzalutamide, and sipuleucel-T.3
KEY POINTS
-
▸
Treatment options for prostate cancer are expanding and can prolong survival and improve quality of life, but lack of medication adherence remains a concern.
-
▸
This retrospective study compares real-world treatment patterns and adherence to abiraterone acetate versus enzalutamide in patients with metastatic CRPC.
-
▸
Compared with patients who initiated enzalutamide therapy, initiating abiraterone acetate treatment led to better medication adherence, reflected in higher medication possession ratios (MPRs), greater proportions of MPRs of ≥80%, and lower proportions of medication nonpersistence.
-
▸
Patients who initiated abiraterone acetate therapy were less likely to have dose reduction than those initiating enzalutamide therapy.
-
▸
Overall, the initial treatment choice, previous use of a corticosteroid, and the presence of a CNS condition during follow-up are potential predictors for dose reduction in this patient population.
Abiraterone acetate is an androgen biosynthesis inhibitor that was approved in 2011 by the US Food and Drug Administration (FDA) to be used in combination with prednisone as a treatment for patients with metastatic CRPC after previous docetaxel treatment.4,5 Enzalutamide, which works by blocking the androgen receptor pathway, was approved by the FDA for the treatment of metastatic CRPC in 2012. Both medications are now approved for a broader indication comprising patients with metastatic CRPC.5,6
Patient medication adherence is critical for effective treatment. Patients who underuse their prescribed medication are likely to experience worsening of their primary condition and the emergence of new comorbid conditions.7 Dose reductions or discontinuation of a drug, which can lead to a lack of patient adherence to treatment protocols, may have a major impact on patient health and on the healthcare system as a whole. This study compares the utilization of and adherence to abiraterone acetate or enzalutamide in real-world practice in patients with metastatic CRPC, and examines the potential factors that may be associated with medication dose reduction in patients with metastatic CRPC.
Methods
This study used a retrospective longitudinal design. In the main analysis, patients with prostate cancer who initiated abiraterone acetate or enzalutamide therapy between September 1, 2012, and December 31, 2014, were selected to form the study population. The index date was defined as the date of initiation of abiraterone acetate or enzalutamide therapy after September 1, 2012 (ie, right after the FDA approval date for enzalutamide) in a patient who had no evidence of previous use of abiraterone acetate or enzalutamide. Patients were included in the study if they had at least 6 months of continuous eligibility before the index date (ie, the baseline period) and at least 1 diagnosis of prostate cancer (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] 185.xx) during the period of continuous eligibility.
The observation period spanned from the index date until health plan disenrollment or the end of data availability, whichever occurred first. For time-to-event analysis, follow-up was censored by the end of the 12-month follow-up. Patients were classified into 2 study cohorts, the abiraterone acetate cohort and the enzalutamide cohort, based on their initial treatment. Because abiraterone acetate was approved for metastatic CRPC in 2011, sensitivity analyses that also included patients initiating abiraterone acetate before September 1, 2012, were conducted.
Data Sources
This study used the Truven Health MarketScan Research databases from January 2010 to December 2014, including separate databases (ie, the Commercial Claims and Encounters database, and the Medicare Supplemental and Coordination of Benefits database) to cover all age-groups; these databases contain claims from employers, health plans, and public organizations. The data in the database comprise service-level claims for inpatient and outpatient healthcare services and outpatient prescription drugs. All census regions are represented in the databases, but the South and North Central (Midwest) regions are predominant.
Outcomes and Statistical Analysis
To evaluate the treatment and adherence patterns in the use of abiraterone acetate or enzalutamide, medication possession ratio (MPR) and nonpersistence were assessed for all patients at 3, 6, 9, and 12 months after the index date. MPR was defined as the sum of the days of supply of the medication, divided by the number of days between the first fill and the last refill plus the days of supply of the last refill.8 When prescription fills overlapped (ie, the start date of the refill started before the end of the previous fill's supply), an adjustment was made such that the prescription start date of the next fill was reset to be the day after the previous fill ended. The proportion of patients with an MPR of ≥80% was also reported. Nonpersistence was defined as a gap of more than 60 days between 2 adjacent refills or between the end of the last refill and the end of the evaluation period.
Dose reduction was assessed using 4 definitions, including (1) a relative dose intensity (RDI) of <85%, where RDI was calculated as the ratio of the delivered dose intensity (ie, dose per unit of time, which was calculated by dividing the total delivered dose by the period over which the total dose was measured) to the standard dose intensity as recommended in the prescribing information of abiraterone acetate or enzalutamide9; (2) an RDI of <80%; (3) a refill gap of ≥30 days, where a refill gap was defined as the number of days between a fill plus the days of supply of that fill and the subsequent refill; and (4) a refill gap of ≥60 days. The event date was defined as the date of the last day of supply of the fill before a dose reduction event. Patients who did not have a dose reduction event were censored at the earlier date between their last day of supply from the last refill and the end of the observation period.
Descriptive statistics were used to report the patient and clinical characteristics at baseline and treatment patterns during the observation period. Means, standard deviations (SDs), and medians were used to describe continuous variables; frequencies and percentages were reported for categorical variables. Comparisons between the 2 study cohorts were conducted using the chi-square test for categorical variables and the 2-sided Student's t-test or Wilcoxon rank sum test for continuous variables, depending on whether the variable was normally distributed.
Kaplan-Meier survival analyses were conducted to illustrate the differences in the distribution of time to dose reduction between patients initiating abiraterone acetate therapy and patients initiating enzalutamide therapy. Kaplan-Meier cumulative probabilities of dose reduction were reported at 3, 6, 9, and 12 months after the index date and were compared between the 2 groups using log-rank tests.
Cox proportional hazards models were used to assess the association between dose reduction (ie, evaluated using the 4 aforementioned definitions) and the initial treatment, as well as other demographic or clinical factors. We conducted 3 Cox proportional hazards models, and reported the hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs).
Model 1 only included the initial treatment (abiraterone acetate vs enzalutamide); model 2 included the initial treatment (abiraterone acetate vs enzalutamide) and potential baseline confounders; and model 3 included the initial treatment (abiraterone acetate vs enzalutamide), potential baseline confounders, and 2 time-dependent covariates that were measured during the observation period.
The baseline confounders included age, region, year of index date, Quan-Charlson comorbidity index at baseline, central nervous system (CNS) conditions at baseline, comorbidities at baseline (ie, diabetes, hypertension, cardiovascular disease, and depression), selected malignant neoplasms at baseline, prostate cancer treatments such as chemotherapy treatments (ie, docetaxel, cabazitaxel, and other chemotherapies), hormonal treatments, sipuleucel-T, radium 223, and corticosteroids (ie, prednisone, dexamethasone, and other corticosteroids). The 2 time-dependent covariates were the presence of a CNS condition diagnosis and the use of medications that may interact with abiraterone acetate or with enzalutamide and were determined according to the prescribing information for the 2 drugs.5,6
Results
A total of 2591 patients who initiated abiraterone acetate therapy and 807 patients who initiated enzalutamide therapy were included in the study population. Compared with patients who initiated enzalutamide, patients who initiated abiraterone acetate were older (mean ± SD, abiraterone acetate vs enzalutamide, 73.8 ± 10.5 years vs 72.8 ± 10.8 years; P = .02), less likely to have previously received chemotherapy (aabiraterone acetate vs enzalutamide, 11.5% vs 20.0% for docetaxel; 1.6% vs 4.1% for cabazitaxel; and 14.4% vs 25.2% for other chemotherapies; all P <.001), and more likely to have previously received corticosteroids (abiraterone acetate vs enzalutamide, 54.8% vs 42.6%; P <.0001) at baseline (Table 1, available at www.AHDBonline.com).
Table 1.
Demographic and Clinical Characteristics of Patients with Metastatic CRPC Who Initiated Abiraterone Acetate or Enzalutamide Therapy
| Characteristic | Abiraterone acetate cohort (N = 2591) | Enzalutamide cohort (N = 807) | P valuea |
|---|---|---|---|
| Age, mean ± SD [median] | 73.8 ± 10.5 [75.0] | 72.8 ± 10.8 [73.0] | .0216b |
| Age category, yrs, N (%) | |||
| 35–44 | 3 (0.1) | 2 (0.2) | .3408 |
| 45–54 | 69 (2.7) | 36 (4.5) | .0100b |
| 55–64 | 546 (21.1) | 171 (21.2) | .9435 |
| 65–74 | 669 (25.8) | 222 (27.5) | .3408 |
| ≥75 | 1304 (50.3) | 376 (46.6) | .0638 |
| Region, N (%) | |||
| Northeast | 617 (23.8) | 165 (20.4) | .0472b |
| North Central | 706 (27.2) | 217 (26.9) | .8416 |
| South | 789 (30.5) | 276 (34.2) | .0450b |
| West | 464 (17.9) | 136 (16.9) | .4923 |
| Unknown | 15 (0.6) | 13 (1.6) | .0046b |
| Year of index date, N (%) | |||
| 2012 | 418 (16.1) | 127 (15.7) | .7892 |
| 2013 | 1400 (54.0) | 280 (34.7) | <.0001b |
| 2014 | 773 (29.8) | 400 (49.6) | <.0001b |
| Payment type, N (%) | |||
| Medicare | 1994 (77.0) | 607 (75.2) | .3078 |
| Commercial | 597 (23.0) | 200 (24.8) | .3078 |
| Plan type, N (%) | |||
| Preferred provider organization | 1135 (43.8) | 354 (43.9) | .9758 |
| Comprehensive | 896 (34.6) | 280 (34.7) | .9521 |
| Health maintenance organization | 315 (12.2) | 68 (8.4) | .0034b |
| Point of service | 101 (3.9) | 42 (5.2) | .1065 |
| Consumer-driven health plan | 53 (2.0) | 18 (2.2) | .7484 |
| High-deductible health plan | 33 (1.3) | 12 (1.5) | .6434 |
| Exclusive provider organization | 5 (0.2) | 1 (0.1) | 1.0000 |
| Capitated or partially capitated point of service | 4 (0.2) | 2 (0.2) | .6325 |
| Unknown | 49 (1.9) | 30 (3.7) | .0026b |
| Physician specialty, N (%) | |||
| Oncology/hematology | 1239 (47.8) | 388 (48.1) | .8973 |
| Urology | 315 (12.2) | 97 (12.0) | .9167 |
| Internist/family practitioner/PCP | 784 (30.3) | 250 (31.0) | .6977 |
| Other specialty/unknown | 253 (9.8) | 72 (8.9) | .4773 |
| Quan-Charlson comorbidity index, mean ± SD [median] | 6.0 ± 2.3 [6.0] | 6.1 ± 2.3 [6.0] | .3794 |
| Baseline comorbidities, N (%) | |||
| Diabetes | 587 (22.7) | 211 (26.1) | .0411b |
| Depression | 135 (5.2) | 59 (7.3) | .0247b |
| Hypertension | 1316 (50.8) | 396 (49.1) | .3933 |
| Cardiovascular disease | 1004 (38.7) | 317 (39.3) | .7867 |
| Any CNS conditionsc | 848 (32.7) | 280 (34.7) | .3000 |
| Malignant neoplasms, N (%) | |||
| Lip, oral cavity, and pharynx | 3 (0.1) | 3 (0.4) | .1500 |
| Digestive organs and peritoneum | 89 (3.4) | 29 (3.6) | .8299 |
| Respiratory and intrathoracic organs | 59 (2.3) | 18 (2.2) | .9380 |
| Bone, connective tissue, skin, and breast | 214 (8.3) | 55 (6.8) | .1846 |
| Genitourinary organs (excluding 185.xx) | 151 (5.8) | 42 (5.2) | .5041 |
| Other and unspecified sites | 1932 (74.6) | 613 (76.0) | .4250 |
| Eye | 3 (0.1) | 0 (0.0) | 1.0000 |
| Brain | 9 (0.3) | 2 (0.2) | 1.0000 |
| Nervous system | 9 (0.3) | 3 (0.4) | 1.0000 |
| Thyroid glands and related structures | 5 (0.2) | 3 (0.4) | .4044 |
| Endocrine glands and related structures | 1 (0.0) | 0 (0.0) | .4186 |
| Other ill-defined sites | 17 (0.7) | 6 (0.7) | .7915 |
| Secondary malignant neoplasm and unspecified lymph nodes | 264 (10.2) | 101 (12.5) | .0624 |
| Secondary malignant neoplasm of respiratory and digestive sites | 197 (7.6) | 65 (8.1) | .6747 |
| Secondary malignant neoplasm of other specified sites | 1781 (68.7) | 559 (69.3) | .7761 |
| Malignant neoplasm without specification of site | 316 (12.2) | 116 (14.4) | .1048 |
| Lymphatic and hematopoietic tissues | 68 (2.6) | 22 (2.7) | .8752 |
| Prostate cancer treatments, N (%) | |||
| Chemotherapy | |||
| Docetaxel | 299 (11.5) | 161 (20.0) | <.0001b |
| Cabazitaxel | 42 (1.6) | 33 (4.1) | <.0001b |
| Other chemotherapy | 372 (14.4) | 203 (25.2) | <.0001b |
| Hormonal treatmentsd | 2236 (86.3) | 658 (81.5) | .0009b |
| Sipuleucel-T | 150 (5.8) | 62 (7.7) | .0521 |
| Radium 223 | 0 (0.0) | 0 (0.0) | — |
| Corticosteroids | 1419 (54.8) | 344 (42.6) | <.0001b |
| Prednisone | 1145 (44.2) | 221 (27.4) | <.0001b |
| Dexamethasone | 446 (17.2) | 219 (27.1) | <.0001b |
| Other corticosteroids | 416 (16.1) | 126 (15.6) | .7645 |
NOTE: Characteristics were evaluated during the 6-month baseline period.
P value was calculated using chi-square tests for categorical variables and t-tests for continuous variables.
Indicates P <.05.
CNS conditions include amnesia/memory impairment, anxiety, ataxia, cognitive disorders, confusion, convulsions, disturbance in attention, dizziness, falls, fatigue/asthenia, hallucinations, headaches, insomnia, pain, paresthesia, and seizures.
Hormone therapy includes LhRH/GnRH, estrogens, antiandrogens, and adrenal androgen blockers.
CNS indicates central nervous system; CRPC, castration-resistant prostate cancer; GnRH, gonadotropin-releasing hormone; LhRH, luteinizing hormone-releasing hormone; PCP, primary care provider; SD, standard deviation.
Treatment Patterns
Table 2 presents a comparison in the treatment patterns between patients with metastatic CRPC who received abiraterone acetate and those who received enzalutamide. The patients who initiated abiraterone acetate therapy had a longer exposure to treatment, which was calculated as the difference between the day of the first fill and the last day of supply from the last refill for a given treatment (abiraterone acetate vs enzalutamide, mean ± SD, 7.5 ± 6.1 months vs 6.3 ± 5.9 months; P <.0001) relative to the patients who initiated enzalutamide.
Table 2.
Treatment Patterns in Patients with Metastatic CRPC Who Initiated Abiraterone Acetate or Enzalutamide Therapy During the Observation Period
| Treatment parameters | Abiraterone acetate cohort (N = 2591) | Enzalutamide cohort (N = 807) | P valuea |
|---|---|---|---|
| Observation period, mo, mean ± SD [median] | 11.0 ± 7.4 [9.6] | 8.6 ± 7.1 [6.6] | <.0001b |
| Patients with an observation period, N (%) | |||
| ≥3 mo | 2188 (84.4) | 576 (71.4) | <.0001b |
| ≥6 mo | 1745 (67.3) | 432 (53.5) | <.0001b |
| ≥9 mo | 1373 (53.0) | 319 (39.5) | <.0001b |
| ≥12 mo | 1032 (39.8) | 219 (27.1) | <.0001b |
| Exposure to treatment, mo, mean ± SD [median]c | 7.5 ± 6.1 [5.7] | 6.3 ± 5.9 [4.0] | <.0001b |
| Fills, N, mean ± SD [median] | 6.7 ± 5.4 [5.0] | 5.4 ± 4.7 [4.0] | <.0001b |
| Days of supply per fill, mean ± SD [median] | 33.5 ± 12.4 [30.0] | 32.7 ± 11.2 [30.0] | .0765 |
| Medication possession ratiod | |||
| Medication possession ratio, mean ± SD [median] | |||
| 3 mo | 0.97 ± 0.08 [1.00] | 0.97 ± 0.08 [1.00] | .0666 |
| 6 mo | 0.96 ± 0.09 [1.00] | 0.94 ± 0.12 [1.00] | .0222b |
| 9 mo | 0.95 ± 0.10 [1.00] | 0.92 ± 0.15 [1.00] | <.0001b |
| 12 mo | 0.95 ± 0.11 [1.00] | 0.92 ± 0.16 [1.00] | .0330b |
| Medication possession ratio ≥80%, N (%) | |||
| 3 mo | 2082 (95.2) | 541 (93.9) | .2319 |
| 6 mo | 1631 (93.5) | 383 (88.7) | <.0001b |
| 9 mo | 1272 (92.6) | 270 (84.6) | <.0001b |
| 12 mo | 946 (91.7) | 187 (85.4) | .0039b |
| Nonpersistence, N (%) | |||
| 3 mo | 160 (7.3) | 51 (8.9) | .2151 |
| 6 mo | 406 (23.3) | 118 (27.3) | .0780 |
| 9 mo | 517 (37.7) | 141 (44.2) | .0307b |
| 12 mo | 516 (50.0) | 119 (54.3) | .2435 |
P value was calculated using chi-square tests for categorical variables and t-tests for continuous variables.
Indicates P <.05.
Exposure to treatment was calculated as the number of days between the first fill and the last refill plus the days of supply of the last refill.
If prescription fills overlapped (the start date of the refill started before the end of the previous fill's supply), an adjustment was made such that the prescription start date of the following fill was reset to be the day after the previous fill ended.
CRPC indicates castration-resistant prostate cancer; SD, standard deviation.
Regarding adherence to medication, compared with patients who initiated enzalutamide, the patients who initiated abiraterone acetate had greater MPRs and, accordingly, higher proportions of MPRs of ≥80% (at 6, 9, and 12 months after the index date, respectively; all comparisons had P <.01). In addition, patients who initiated abiraterone acetate therapy had lower proportions of medication nonpersistence, although only the difference for the comparison at 9 months was statistically significant (Table 2).
Dose Reduction
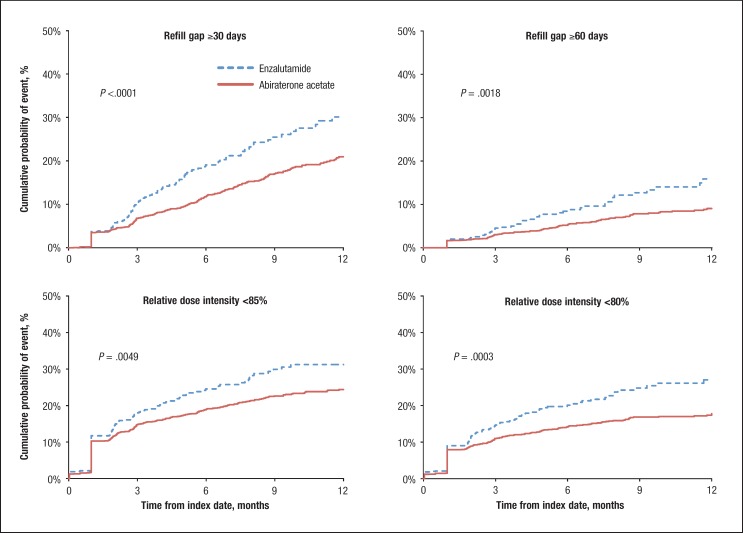
The Kaplan-Meier estimates of cumulative hazards for dose reduction during a 12-month follow-up indicated that patients who initiated abiraterone acetate therapy had lower rates of dose reduction than patients who initiated enzalutamide therapy (overall log-rank test P <.01 across all 4 definitions for dose reduction; Figure 1). The differences in cumulative rates of dose reduction between treatment groups began to develop 3 months after treatment initiation and were significant (all P <.01) after 9 months of the index date.
Figure 1. Cumulative Hazards for Dose Reduction in Patients Initiating Abiraterone Acetate or Enzalutamide During 12-Month Follow-Up Period.
The cumulative event rates at 12 months postindex in patients who initiated abiraterone acetate were 20.9%, 9.1%, 24.4%, and 17.8% based on the 4 definitions for dose reduction, respectively (ie, a refill gap ≥30 days, a refill gap ≥60 days, RDI <85%, and RDI <80%), which were systematically lower than those observed in patients who initiated enzalutamide (30.1%, 15.9%, 31.2%, and 24.8%; all P <.01).
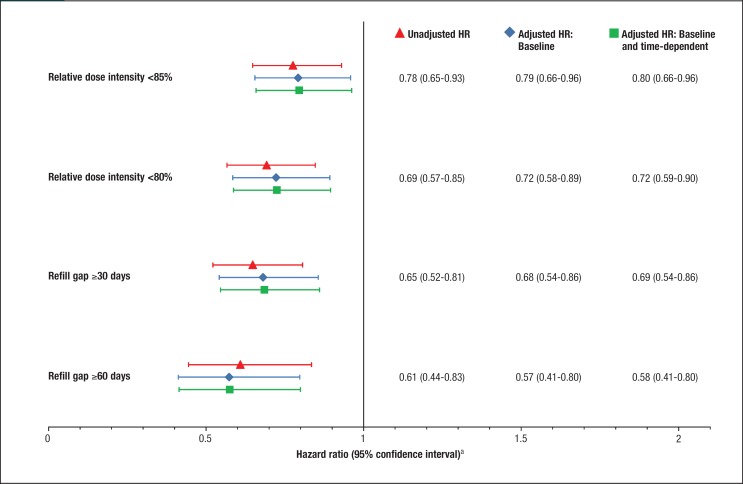
Consistently, the results from the Cox proportional hazards models suggested that patients who initiated abiraterone acetate had a lower risk for dose reduction than patients who initiated enzalutamide, with all the crude and adjusted HRs for treatment (abiraterone acetate vs enzalutamide) significantly lower than 1 (Figure 2).
Figure 2. Hazard Ratios Associated with Dose Reduction in Patients Initiating Abiraterone Acetate or Enzalutamide During a 12-Month Follow-Up Period.
The multivariate Cox model analysis results for dose reduction measured with RDI <85% suggest that patients were less likely to have a dose reduction if they initiated abiraterone acetate (vs enzalutamide, HR, 0.80; 95% CI, 0.66–0.96), were middle-aged (ie, 55–64 years vs ≥75 years, HR, 0.73; 95% CI, 0.58–0.92), were from the Northeastern United States (vs unknown region, HR, 0.48; 95% CI, 0.23–1.00), were receiving prednisone before the initiation of abiraterone acetate or enzalutamide (HR, 0.83; 95% CI, 0.69–0.99), or were without a diagnosis for a CNS condition during follow-up (HR, 0.70; 95% CI, 0.56–0.86). Similar predictors were found when the other definitions for dose reduction were used in the analysis.
By including all patients who received abiraterone acetate in the sensitivity analyses, similar results to those observed in the main analyses (ie, including only patients who initiated abiraterone acetate or enzalutamide therapy after September 1, 2012) regarding treatment patterns, time to dose reduction, and predictors of dose reduction were observed.
Discussion
This observational study evaluated the treatment patterns and dose reduction associated with abiraterone acetate and enzalutamide therapy in patients with metastatic CRPC based on a large claims database. The results suggest that fewer patients who initiated abiraterone acetate therapy had dose reduction during follow-up than those who initiated enzalutamide therapy. The Kaplan-Meier survival curves further illustrate that the lower risk for dose reduction in patients who initiated abiraterone acetate therapy (vs enzalutamide) became noticeable at 3 months of follow-up and were more pronounced during longer follow-up periods.
Two multivariate Cox proportional hazards models were used to estimate the association between the initial treatment with either agent and the risk for dose reduction. Both models indicated that initiating abiraterone acetate therapy was associated with a lower risk for dose reduction than initiating therapy with enzalutamide in patients with metastatic CRPC. Consistent results were obtained using different definitions for dose reduction, indicating that the study results are robust regarding the measurement used for dose reduction.
Moreover, the results show that the initiation of abiraterone acetate, the use of prednisone before abiraterone acetate or enzalutamide treatment initiation, and not being diagnosed with a CNS condition during follow-up were associated with a lower risk for dose reduction. Furthermore, compared with patients who initiated abiraterone acetate therapy, a larger proportion of patients who initiated enzalutamide therapy received chemotherapy previously; this disparity, however, did not have an impact on the difference in risk for dose reduction between the 2 study cohorts.
Our study results for medication adherence to abiraterone acetate therapy are consistent with the MPRs reported by Lafeuille and colleagues, who also used the Truven Health Analytics MarketScan database in their analyses.10 Although that study was conducted during an earlier period (ie, December 2010-August 2012), which was before the FDA approval of enzalutamide, the MPR findings on abiraterone acetate therapy of the 2 studies are very similar (with a mean MPR of approximately 95% across all assessment time points), suggesting that patients who initiated abiraterone acetate therapy had consistently high medication adherence over time.
The higher rate of dose reduction in patients who initiated enzalutamide therapy (vs abiraterone acetate) observed in our study may be in part explained by the increased risk for fatigue associated with enzalutamide that was reported in clinical trials.11–14 These clinical trials for comparing abiraterone acetate and enzalutamide with placebo among chemotherapy-naïve or postchemotherapy patients with metastatic CRPC showed that, in general, abiraterone acetate and enzalutamide had comparable (with a few exceptions) safety profiles relative to placebo.11–14 The exceptions included abiraterone acetate being associated with higher rates of mineralocorticoid-related adverse events (including fluid retention, hypertension, and hypokalemia), and enzalutamide being associated with higher rates of fatigue, diarrhea, and hot flashes compared with the corresponding placebo groups.11–14
The phase 1/2 study of enzalutamide also reported that the most common adverse event with enzalutamide was dose-dependent fatigue, which resolved after dose reduction.15 By contrast, abiraterone acetate was shown to be associated with a significant improvement in fatigue intensity in patients with metastatic CRPC who had previously received docetaxel.16 Therefore, in our current study, patients who initiated enzalutamide therapy might have had fatigue events, which in turn might have resulted in decisions related to dose reduction during the treatment period.
Moreover, the higher rate of dose reduction observed in patients who initiated enzalutamide versus abiraterone acetate therapy might also be driven by the recommendations for patient management listed in the prescribing information for the 2 medications. The prescribing information for enzalutamide recommends that patients may reuse enzalutamide at the same dose or at a reduced dose after experiencing a grade 3 or worse toxicity or an intolerable side effect.6 The prescribing information for abiraterone acetate recommends considering dose reduction in patients with baseline moderate hepatic impairment or in patients who have hepatotoxicity during treatment.5
However, regardless of whether the dose reduction resulted from a patient nonadherence to therapy, patients not receiving their medications on time, or other clinical reasons (ie, the medication was stopped or reduced to manage toxicity), our study showed that compared with patients who initiated abiraterone acetate therapy, patients who initiated enzalutamide therapy had greater reductions of the drug's dosage than we would expect based on the prescribing information.
To our knowledge, this is the first study to assess and compare the treatment patterns of and adherence to abiraterone acetate and enzalutamide in real-world settings. The use of different measures for medication adherence, the use of alternative definitions for dose reduction, and the assessment of adherence at different time points during follow-up contribute to the robustness of our study's results.
Limitations
As with all claims databases, the Truven Health MarketScan research databases are subject to billing inaccuracies or the incomplete coding of diagnoses. Because no information was available in pharmacy claims to assess whether the medication was taken as prescribed, the results of this study may overestimate the actual consumption of the medications.
In addition, because of the limitations in ICD-9-CM coding specificity, the verification of a metastatic CRPC diagnosis was not possible.
Moreover, as with all observation studies, our results may be subject to residual confounding factors becasue of unmeasured confounding factors, such as the potential difference in patients' disease states that may confound the observed association between treatments for metastatic CRPC and dose reduction.
Nevertheless, health insurance claims data remain a valuable source of information, because they constitute a fairly valid large sample of patients' characteristics and outcomes in a real-world setting.
Conclusions
This retrospective study showed that patients who initiated abiraterone acetate therapy had greater medication adherence and a lower risk for dose reduction compared with patients who initiated enzalutamide therapy.
The attempt to identify potential predictors for dose reduction should motivate further investigation in this area. In addition, as high-deductible health plans are becoming more pervasive, and the costs associated with the treatment of metastatic CRPC are high, future studies may be conducted to assess the potential impact of patients' out-of-pocket expenses on medication adherence to treatments of metastatic CRPC. Because treatment with abiraterone acetate or with enzalutamide can prolong survival in patients with metastatic CRPC, and longer duration of treatment may be associated with longer survival, our findings suggest that patients who initiate abiraterone acetate may have a better survival rate, lower rates of healthcare resource utilization, and lower healthcare costs than patients who initiate enzalutamide therapy. Future studies should be conducted to confirm these hypotheses.
Source of Funding
This study was funded by Janssen Scientific Affairs, LLC.
Author Disclosure Statement
Dr Behl is an employee and stockholder of Johnson & Johnson. Dr Ellis is an employee of Janssen Scientific Affairs, and a stockholder of Johnson & Johnson. Mr Pilon, Dr Xiao, and Mr Lefebvre are employees of Analysis Group, which received a research grant from Janssen Scientific Affairs.
Contributor Information
Ajay S. Behl, Director of US Oncology Economics and Outcomes Research, Janssen Scientific Affairs, Titusville, NJ.
Lorie A. Ellis, Director of US Rheumatology Economics and Outcomes Research, Janssen Scientific Affairs.
Dominic Pilon, Manager, Groupe d'analyse, Montréal, Québec, Canada.
Yongling Xiao, Associate, Groupe d'analyse.
Patrick Lefebvre, Managing Principal, Groupe d'analyse.
References
- 1. Kohli M, Tindall DJ. New developments in the medical management of prostate cancer. Mayo Clin Proc. 2010; 85:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Cancer Society. What are the key statistics about prostate cancer? 2017. www.cancer.org/cancer/prostate-cancer/about/key-statistics.html. Accessed July 26, 2017.
- 3. Saad F, Hotte SJ. Guidelines for the management of castrate-resistant prostate cancer. Can Urol Assoc J. 2010; 4:380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ang JE, Olmos D, de Bono JS. CYP17 blockade by abiraterone: further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br J Cancer. 2009; 100:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zytiga (abiraterone acetate) tablets [prescribing information]. Horsham, PA: Janssen Pharmaceutical Companies; April 2017. [Google Scholar]
- 6. Xtandi (enzalutamide) capsules [prescribing information]. Northbrook, IL: Astellas Pharma US; October 2016. [Google Scholar]
- 7. Jensen GA, Li Y. Long-run health effects of cost-related non-adherence to prescribed medications among adults in late midlife. J Pharm Health Serv Res. 2012; 3:85–93. [Google Scholar]
- 8. Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005; 11:449–457. [PubMed] [Google Scholar]
- 9. Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003; 21:4524–4531. [DOI] [PubMed] [Google Scholar]
- 10. Lafeuille MH, Grittner AM, Lefebvre P, et al. Adherence patterns for abiraterone acetate and concomitant prednisone use in patients with prostate cancer. J Manag Care Spec Pharm. 2014; 20:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ryan CJ, Smith MR, de Bono JS, et al; for the COU-abiraterone acetate-302 Investigators. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013; 368:138–148. Erratum in: N Engl J Med. 2013; 368:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Bono JS, Logothetis CJ, Molina A, et al; for the COU-abiraterone acetate-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011; 364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beer TM, Armstrong AJ, Rathkopf DE, et al; for the PREVAIL Investigators. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014; 371:424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scher HI, Fizazi K, Saad F, et al; for the AFFIRM Investigators. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012; 367:1187–1197. [DOI] [PubMed] [Google Scholar]
- 15. Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet. 2010; 375:1437–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sternberg CN, Molina A, North S, et al. Effect of abiraterone acetate on fatigue in patients with metastatic castration-resistant prostate cancer after docetaxel chemotherapy. Ann Oncol. 2013; 24:1017–1025. [DOI] [PubMed] [Google Scholar]